Open Access Article
Open Access ArticlePlasmonic nanopore to monitor in-pore chemistry
Weihong Wanga,
Shukun Wengb,
Ali Douakibc,
German Lanzavecchiabc,
Yanqiu Zoud,
Qifei Ma a,
Huaizhou Jine,
Roman Krahne
a,
Huaizhou Jine,
Roman Krahne b,
Shangzhong Jin*a,
Makusu Tsutsui
b,
Shangzhong Jin*a,
Makusu Tsutsui *f and
Denis Garoli
*f and
Denis Garoli *abc
*abc
aCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou 310018, China. E-mail: jinsz@cjlu.edu.cn; denis.garoli@unimore.it
bIstituto Italiano di Tecnologia, Via Morego 30, 16136, Genova, Italy
cDip. di Scienze e Metodi dell'Ingegneria, Università di Modena e Reggio Emilia, via Amendola 2, 42122, Reggio Emilia, Italy
dState Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou 310027, China
eKey Laboratory of Quantum Precision Measurement, College of Physics, Zhejiang University of Technology, Hangzhou, China
fSANKEN, The University of Osaka, Ibaraki 567-0047, Osaka, Japan. E-mail: makusu32@sanken.osaka-u.ac.jp
First published on 14th January 2026
Abstract
Reliable control of solid-state nanopore gating remains challenging. Voltage-controlled metal phosphate reactions enable reversible pore blocking via cyclic precipitation and dissolution driven by cation transport. Negative bias blocks ion flow, while polarity reversal restores conductance, forming a nanofluidic diode. Here we show how plasmonic nanopores allow in-pore SERS measurements, directly confirming the localized chemistry and enabling high-resolution monitoring of nanoscale processes.
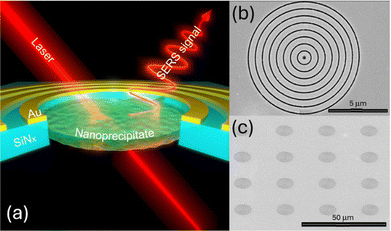
Over the past two decades, solid-state nanopore technology has been the focus of extensive research, particularly aimed at creating artificial systems that mimic biological nanopores, i.e. transmembrane proteins capable of regulating the passage of individual molecules and ions.1–4 While the primary effort has traditionally centered on sequencing applications,5–9 the scope of interest in nanopore systems has now expanded considerably. Among various research directions, the use of solid-state nanopores for constructing nanofluidic circuits, such as ionic transistors and diodes, has recently attracted significant attention.2,10–12 To develop a nanofluidic ionic system that mimics the behavior of a diode, precise control over the nanopore's geometry and surface charges is essential.13,14 In this context, a novel strategy for fabricating solid-state nanopores exhibiting diode-like properties, characterized by exceptionally high ion current rectification (ICR), has been recently demonstrated by Tsutsui et al.15 This method relies on the so-called “in-pore chemistry,” where two solutions interact at the interface between the cis and trans reservoirs of the nanopore chamber, an interface effectively defining the nanopore volume itself. In particular, this can be possible by using a thin Si3N4 membrane including a nanopore connecting two distinct ionic reactant solutions for precise confinement of the ion electromigration. These ions are capable of inducing electrically tunable metal phosphate precipitation/dissolution in the pore. This can be applied to the preparation of advanced nanofluidic devices including diodes and memristors representing a significant plus with respect to the conventional nano-scale systems.16–18 A similar method has been more recently reported by Siwy et al., where the cycling precipitation and dissolution inside the nanopore are used to investigate ionic memory effects.19 To improve our understanding on the reported “in-pore chemistry”,15 here we investigated the chemical reaction inside the nanopore using optical spectroscopy. Considering that the reaction takes place in a very small volume (with a nanopore's diameter of 200 nm and thickness of 50 nm, the volume is of the order of 10−3 fL), the chemical reaction can be monitored only using a highly localized label free spectroscopy. In particular, Surface Enhanced Raman Spectroscopy (SERS) represents a valuable technique where the electromagnetic field can be squeezed in the nanopore volume thanks to a proper design of a plasmonic nanopore.4,20 Therefore, in this work we replaced the Si3N4 nanopore previously used15,21 with a plasmonic nanopore designed as a thin gold layer on a Si3N4 membrane where the nanopore is surrounded by a bulls-eye grating with a period matching the excitation of the surface plasmon polaritons at the laser wavelength used for the excitation. As extensively proved in literature,20,22–28 this structure enables a strong near-field enhancement inside the nanopore, therefore facilitating measurements of the vibrational spectra during the precipitation/dissolution reactions. The bulls-eye integrated plasmonic nanopores were fabricated by means of focused ion beam (FIB) milling in a 50 nm thick Si3N4 membrane supported on a Si wafer (See SI for details). An initial pore's diameter of about 200 nm was prepared.15 The sample was then modified with an additional thin bilayer of Ti/Au (5/25 nm) and a successive FIB sculpturing was used to prepare a bull-eye grating with a grating period matching the surface plasmon polariton wavelength (λSPP) for an incident light at 532 nm (see SI for details on the electromagnetic field confinement produced by this structure).29,30 Fig. 1 shows an illustration of the process and reports SEM images of the fabricated sample.
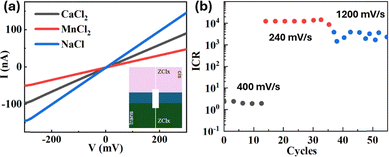
As a first step we characterize the electrical properties of the nanopore to verify if our design comprising the Ti/Au layers results in the desired diode-like response. Here the metallic layers can, in principle, act as a floating electrode under the external applied field.31 Therefore, we first measured the current from the nanopore applying a transmembrane voltage (Vb) with a scan rate (rscan) of 60 mV s−1 in phosphate-buffered saline containing 1.37 M NaCl at pH 7.4 (hereafter, NaCl solution refers to the PBS unless otherwise denoted). In all our experiments we observed a perfect ohmic behavior with a conductance of 0.5 µS (Fig. 2a), consistent with Maxwell–Hall's model (see SI for details) for dpore of about 200 nm and solution resistivity (ρNaCl) of 0.08 Ωm. The same set of measurements have been performed replacing PBS respectively with CaCl2 and MnCl2 (concentration 2 mol L−1) on both sides of the microfluidic chamber. These two solutions demonstrated to be very effective in the nanoprecipitation process that will be discussed later. As can be seen from Fig. 2a, also in these cases, symmetric configurations on the cis and trans sides produce linear ohmic I–V response, as also demonstrated in a recent publication by Tsutsui et al.32 The nanopore's conductances were, 0.33 µS and 0.15 µS, respectively for CaCl2 and MnCl2. A completely different response is expected when switching from a symmetric to asymmetric configurations of cis and trans sides of the chamber. In particular, using PBS at the trans side and filling the cis compartment with CaCl2 or MnCl2 salts (same concentrations used before) resulted in significant asymmetry in the Iion–Vb characteristics. CaCl2 and MnCl2 exhibited strong suppression of the ionic current at Vb < 0. In the case of CaCl2, the rectification ratio ICR = |Imax/Imin| reached over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Fig. 2b), where Imax and Imin are Iion at Vb = 0.6 V and −0.6 V, respectively. On the contrary, in the case of MnCl2, the ICR reached a value up to 105 for a scan rate of 200 mV s−1 considering in this second case Imax and Imin as Iion at Vb = 1 V and −1 V, respectively (see Fig. S1). We note that, in both the cases, relatively small Vb values reach very high ICR values, and consequently, with higher Vb values, the saturation of the current range that can be measured in the nanopore reader was reached (max measurable I = 200 nA). These ICR values are more than an order of magnitude higher than the state-of-the-art systems based on nanofluidic diodes.33–35 The rectification behavior depends on the voltage scan speed (SI – Fig. S1 and S2). For CaCl2, the maximum rectification is observed at a scan rate of 240 mV s−1, where the ionic current at negative Vb values remains very low and stable, indicating an almost complete suppression of the translocation of high-concentration cations and anions under negative bias. For both CaCl2 and MnCl2, increasing the scan rate markedly diminishes the diode-like response, resulting in lower ICR values (see Fig. S2). Nevertheless, the diode-like behavior can always be recovered by decreasing the scan rate, as shown in Fig. S1. This ionic rectification cannot be justified considering concentration polarization or ion selectivity or electroosmotic flow.15,32,34,35
000 (Fig. 2b), where Imax and Imin are Iion at Vb = 0.6 V and −0.6 V, respectively. On the contrary, in the case of MnCl2, the ICR reached a value up to 105 for a scan rate of 200 mV s−1 considering in this second case Imax and Imin as Iion at Vb = 1 V and −1 V, respectively (see Fig. S1). We note that, in both the cases, relatively small Vb values reach very high ICR values, and consequently, with higher Vb values, the saturation of the current range that can be measured in the nanopore reader was reached (max measurable I = 200 nA). These ICR values are more than an order of magnitude higher than the state-of-the-art systems based on nanofluidic diodes.33–35 The rectification behavior depends on the voltage scan speed (SI – Fig. S1 and S2). For CaCl2, the maximum rectification is observed at a scan rate of 240 mV s−1, where the ionic current at negative Vb values remains very low and stable, indicating an almost complete suppression of the translocation of high-concentration cations and anions under negative bias. For both CaCl2 and MnCl2, increasing the scan rate markedly diminishes the diode-like response, resulting in lower ICR values (see Fig. S2). Nevertheless, the diode-like behavior can always be recovered by decreasing the scan rate, as shown in Fig. S1. This ionic rectification cannot be justified considering concentration polarization or ion selectivity or electroosmotic flow.15,32,34,35
On the contrary, the diode-like behaviour of the nanopore system is due to the formation of a solid precipitate via Vb-derived reactions that occluded the space for the electromigration of ions through the pores. Hence, we are observing (a scan rate dependent) precipitation reactions between divalent cations with the phosphoric acid in the electrolyte buffer to form metal phosphates. This can be understood considering the components in our chamber. The main ionic components of PBS in water are: Na+, Cl−, HPO42− and H2PO4− (the phosphate buffer pair). A divalent chloride compound in aqueous solution dissolves as: XCl2 (s) → X2+ (aq) + 2Cl− (aq), where X represents here Ca and Mn. Calcium and Manganese ions (X2+) are known to react with phosphate ions to form insoluble phosphate salts, with a pH dependent reaction:
| 3X2+ + 2PO43− → X3(PO4)2 | (1) |
In PBS, most phosphate is present as HPO42− or H2PO4−, but under physiological pH, there is still sufficient PO43− to trigger partial precipitation of salts phosphate (especially hydroxyapatite or amorphous X–P compounds). Therefore, the following reaction most likely happens at the interface between cis and trans sides:
| 3XCl2 +2Na3PO4 →X3(PO4)2 +6NaCl | (2) |
This reaction is strongly pH dependent.15 For this reason the experiments reported here used PBS at physiological pH.
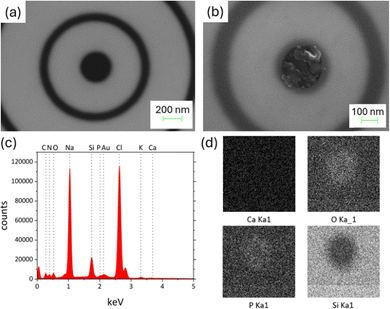
Hence, the electrical response observed in our nanopores is ascribed to a solid-phase precipitate that clogs the aperture of the pore where the two solutions are mixed. The possibility to drive this reaction by external electrical potential is fundamental to provide the diode-like response and the significant ICR values reported. Fig. 3 reports the characterizations performed on the CaCl2 system using the electron microscopy combined with energy dispersion spectroscopy (EDS).
The morphological and compositional characterizations of the nanopore were done at the two stages corresponding to Imax and Imin, i.e. when the external potential was Vb = 0.6 V and −0.6 V, respectively. Fig. 3a and b clearly illustrate what happens inside the nanopore using the plasmonic configuration studied here. Additional tests using bare Si3N4 nanopores (reported in SI – note #2), confirmed the precipitation and dissolution reactions. To note, these micrographs can be obtained stopping the reaction at the specific Vb and resulted to be reproducible after multiple cycles. EDS maps revealed the presence of oxygen and phosphor inside the nanopore's cavity, as expected, even if the sensitivity of the technique is probably not enough to obtain convincing results.
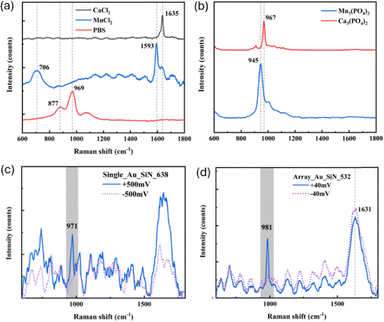
We then performed optical spectroscopic analyses by means of Raman spectroscopy. The goal was to obtain the spectrum from the nanopore's cavity in order to monitor the localized chemical reaction. Before to do it, the Raman spectra of the individual compounds were collected preparing them as dry droplet, in the case of PBS (i.e. we let the PBS droplet to dry on a solid-state substrate), and as dry powders, in the cases of CaCl2 and MnCl2. Fig. 4a reports the obtained vibration signatures where it is possible to observe how for PBS three peaks dominates, corresponding to 877 cm−1, 979 cm−1 and 1077 cm−1, respectively. They are associated with the phosphate (PO43−) buffer system, specifically the vibrations of the hydrogen phosphate (HPO42−) and dihydrogen phosphate (H2PO4−) ions.36 In the cases of CaCl2 and MnCl2, on the contrary, only a main peak at around 1600 cm−1 appeared. This can be associated with the H–O–H bending vibration of the water of crystallization.37 In the nanopore system we are not dealing with salts powders, but we are interested to monitor the precipitation and dissolution processes driven by the external applied electrical potential. Therefore we also collected the Raman signatures of the precipitated salts as reported in eqn (1). To do that, we mixed the compounds using the same concentrations and pH conditions used in the nanopore experiments and, after letting them dry, we collected the spectra. In both the cases (Ca3(PO4)2 and Mn3(PO4)2), we can observe the presence of a main peak at around 960 cm−1, clearly corresponding to the vibration of the phosphate group.38,39 A minor broad peak at around 1600 cm−1 can be also detected, as expected, to be associated with the H–O–H bending.37,40 Having a clear view on the vibrational signatures of the chemicals involved in our reactions, it is possible to proceed with the Raman experiments on the nanopore systems. Considering that this reactions have been firstly demonstrated in a Si3N4 pore, we performed the precipitation/dissolution processes focusing the laser over the nanopore directly prepared in a bare Si3N4 (the fabrication included both single nanopore and an array of nanopores in order to increase the reaction volume (see SI)). Cycling them applying external electrical potential as discussed above and in ref. 15 we can obtain the precipitation and dissolution processes. Considering the required integration time for the Raman acquisitions (tens of seconds), it was not possible to detect the dynamics of the precipitation reaction. On the contrary, stopping the reaction at Vb = 0.6 V and −0.6 V, we know that the nanopore(s) will be completely open and completely clogged, respectively (as illustrated in SI – Fig. S3). Important to note, the Raman measurements were done using a transparent microfluidic chip compatible with the simultaneous electrical and optical measurements, as demonstrated in ref. 20. This enables the monitoring of the reaction inside the nanopore. In order to avoid the background signal from the PBS solution in the trans volume, we excited the pore from the cis side where only the chloride salts are present. Testing both a single nanopore and an array of 16 pores it was not possible to detect convincing spectra to be ascribed to the precipitated salts. This is because the volume of detection is spatially limited by the nanopore's diameter. While with the dry precipitates we can collect the signals from large area and large volume samples, here it has not been possible to obtain enough photons from the fL volume of the excited materials. This is the main motivation for the choice of the plasmonic nanopore. In fact, replicating the experiment replacing the bare Si3N4 pore with the single plasmonic nanopore (illustrated in Fig. 1b) it was easily possible to collect the SERS signatures from the individual nanopore in the two main configurations (open and clogged) using CaCl2. As can be seen in Fig. 4c, there are clear differences in the Raman spectra associated with open and close pore. In particular, switching from the open to the close configuration, the peak at around 970 cm−1 appears, confirming the formation of the phosphate precipitate. While the other spectral regions are not changed, we also observed and increased intensity for the peak at around 1600 cm−1, associated with the H–O–H bending. Performing the same experiment by using MnCl2, it resulted to be more challenging to collect the signatures from a single plasmonic nanopore. Therefore, in order to increase the detection volume, we increased the laser spot size and we explored a configuration comprising 16 nanopores in array (Fig. 1c). In this case, it has been again possible to clearly detect the peak associated to the phosphate salts precipitation, presents only in the spectrum related to the clogged pore, while it always disappeared switching to the open pore configuration.
In conclusion, this study demonstrates the advantages of a plasmonic nanopore platform for probing chemical processes at the nanoscale. By confining intense electromagnetic fields within an attoliter-scale pore, the system enables high spatiotemporal resolution for tracking localized reactions via ionic current measurements. Simultaneously, surface-enhanced Raman spectroscopy (SERS) generated at the plasmonic hotspot provides molecular vibrational information, yielding chemical fingerprints of reactants and products. The combination of label-free electrical and optical readouts allows real-time, in situ monitoring of dynamic nanoscale processes that are difficult to access using conventional techniques.
Author contributions
W. W. performed the electrical and spectroscopic characterization, S. W., A. D. and G. L. prepared the samples, Y. Z., Q. M. and H. J. supported the data analysis, R. K. provided the access to the lab, S. J., M. T., and D. G. conceived and supervised the work.Conflicts of interest
There are no conflicts to declare.Data availability
Data for this article, including SEM micrograph, electrical measurements and Raman spectroscopy data are available on request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc07127e.
Acknowledgements
We are grateful for continued financial support from National Key Research and Development Project of China (No. 2023YFF0613603), National Natural Science Foundation of China (No. 22202167), Provincial Science and Technology Plan Project: Micro and Nano Preparation and Photoelectronic Detection (No. 03014/226063). D. G. thank the HORIZON-MSCADN-2022: DYNAMO, grant Agreement 101072818. A part of this work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers 22H01926, 22H01410, and 24K21715. M. Tsutsui acknowledges support from Kansai Research Foundation for Technology Promotion.References
- D. G. Haywood, A. Saha-Shah, L. A. Baker and S. C. Jacobson, Anal. Chem., 2015, 87, 172–187 CrossRef CAS PubMed.
- S. Zhang, J. Wang, A. Yaroshchuk, Q. Du, P. Xin, M. L. Bruening and F. Xia, J. Am. Chem. Soc., 2024, 146(16), 11036–11042 CAS.
- E. Angeli, L. Repetto, G. Firpo and U. Valbusa, Curr. Opin. Electrochem., 2021, 29, 100754 CrossRef CAS.
- W. Li, J. Zhou, N. Maccaferri, R. Krahne, K. Wang and D. Garoli, Anal. Chem., 2022, 94, 503–514 CrossRef CAS PubMed.
- Y.-L. Ying, Z.-L. Hu, S. Zhang, Y. Qing, A. Fragasso, G. Maglia, A. Meller, H. Bayley, C. Dekker and Y.-T. Long, Nat. Nanotechnol., 2022, 17, 1136–1146 Search PubMed.
- T. Zhang, H. Li, M. Jiang, H. Hou, Y. Gao, Y. Li, F. Wang, J. Wang, K. Peng and Y.-X. Liu, J. Genet. Genomics, 2024, 51, 1361–1374 CrossRef CAS PubMed.
- J. Ritmejeris, X. Chen and C. Dekker, Nat. Rev. Bioeng., 2024, 3, 303–316 CrossRef.
- A. Dorey and S. Howorka, Nat. Chem., 2024, 16, 314–334 Search PubMed.
- Z. Zhang, J. Sha, G. Qin, D. Chen, W. Si, Q. Wang and L. Liu, Small Methods, 2025, e01603 CrossRef CAS PubMed.
- X. Zhang, Y. Dai, J. Sun, J. Shen, M. Lin and F. Xia, Anal. Chem., 2024, 96, 2277–2285 CrossRef CAS PubMed.
- B. Yameen, M. Ali, R. Neumann, W. Ensinger, W. Knoll and O. Azzaroni, J. Am. Chem. Soc., 2009, 131, 2070–2071 CrossRef CAS PubMed.
- G. Pérez-Mitta, J. S. Tuninetti, W. Knoll, C. Trautmann, M. E. Toimil-Molares and O. Azzaroni, J. Am. Chem. Soc., 2015, 137, 6011–6017 CrossRef PubMed.
- A. J. Storm, J. H. Chen, X. S. Ling, H. W. Zandbergen and C. Dekker, Nat. Mater., 2003, 2, 537–540 CrossRef CAS PubMed.
- I. Yanagi, T. Ishida, K. Fujisaki and K. Takeda, Sci. Rep., 2015, 5, 14656 CrossRef CAS PubMed.
- M. Tsutsui, W.-L. Hsu, C. Hsu, D. Garoli, S. Weng, H. Daiguji and T. Kawai, Nat. Commun., 2025, 16, 1089 CrossRef CAS PubMed.
- P. Ramirez, S. Portillo, J. Cervera, S. Nasir, M. Ali, W. Ensinger and S. Mafe, J. Chem. Phys., 2024, 160, 044701 CrossRef CAS PubMed.
- J. Cervera, S. Portillo, P. Ramirez and S. Mafe, Phys. Fluids, 2024, 36, 047129 CrossRef CAS.
- R. Yang, Y. Balogun, S. Ake, D. Baram, W. Brown and G. Wang, J. Am. Chem. Soc., 2024, 146, 13183–13190 Search PubMed.
- A. D. Cho, A. Wawrzkiewicz-Jałowiecka, C. E. P. Dewi, S. Tang, D. Cain, E. Cao, C. Martens, T. E. Schäffer, J. Cervera, P. Ramirez, S. Mafe and Z. S. Siwy, J. Am. Chem. Soc., 2025, 147(51), 47559–47572 CrossRef CAS PubMed.
- A. Douaki, S. Weng, G. Lanzavecchia, A. Sapunova, A. Stuber, G. Nanni, N. Nakatsuka, M. Tsutsui, K. Yokota, R. Krahne and D. Garoli, Adv. Opt. Mater., 2025, 13, 2402189 Search PubMed.
- M. Tsutsui, W. L. Hsu, D. Garoli, A. Douaki, Y. Komoto, H. Daiguji and T. Kawai, Nat. Commun., 2026, in press Search PubMed.
- G. W. Kim, J.-H. Ko, D. J. Park, S. S. Choi, H. Kim and S. B. Choi, J. Korean Phys. Soc., 2018, 73, 1698–1702 CrossRef.
- T. Mahinroosta and S. M. Hamidi, Appl. Phys. A: Mater. Sci. Process., 2022, 128, 1043 Search PubMed.
- D. Komisar, S. Kumar, Y. Kan, C. Wu and S. I. Bozhevolnyi, ACS Photonics, 2021, 8, 2190–2196 CrossRef CAS.
- P. Zilio, G. Parisi, D. Garoli, M. Carli and F. Romanato, Opt. Lett., 2014, 39, 4899 CrossRef PubMed.
- C. R. Crick, P. Albella, H.-J. Kim, A. P. Ivanov, K.-B. Kim, S. A. Maier and J. B. Edel, ACS Photonics, 2017, 4, 2835–2842 CrossRef CAS.
- J. D. Spitzberg, A. Zrehen, X. F. Van Kooten and A. Meller, Adv. Mater., 2019, 31, 1900422 CrossRef PubMed.
- C. R. Crick, P. Albella, B. Ng, A. P. Ivanov, T. Roschuk, M. P. Cecchini, F. Bresme, S. A. Maier and J. B. Edel, Nano Lett., 2015, 15, 553–559 CrossRef CAS PubMed.
- T. Nagasue, T. Shinohara, S. Hasegawa, K. Imura and K. Tawa, Opt. Express, 2022, 30, 7526 CrossRef CAS PubMed.
- A. Ghaffari, S. Kashani, J. Li, P. Gkoupidenis, R. Riehn and Q. Gu, ACS Nanosci. Au, 2025, 5, 306–313 CrossRef CAS PubMed.
- C. Yang, P. Hinkle, J. Menestrina, I. V. Vlassiouk and Z. S. Siwy, J. Phys. Chem. Lett., 2016, 7, 4152–4158 CrossRef CAS PubMed.
- M. Tsutsui, K. Yokota, W.-L. Hsu, Y. Komoto, D. Garoli, H. Daiguji and T. Kawai, ACS Nano, 2025, 19(48), 41076–41085 CrossRef CAS PubMed.
- Q. Liu, Y. Liu, B. Lu, Y. Wang, Y. Xu, J. Zhai and X. Fan, RSC Adv., 2020, 10, 7377–7383 RSC.
- H. Daiguji, Y. Oka and K. Shirono, Nano Lett., 2005, 5, 2274–2280 CrossRef CAS PubMed.
- E. C. Yusko, R. An and M. Mayer, ACS Nano, 2010, 4, 477–487 CrossRef CAS PubMed.
- M. D. Fontana, K. Ben Mabrouk and T. H. Kauffmann, in Spectroscopic Properties of Inorganic and Organometallic Compounds, ed. J. Yarwood, R. Douthwaite and S. Duckett, Royal Society of Chemistry, Cambridge, 2013, vol. 44, pp. 40–67 Search PubMed.
- L. M. Uriarte, J. Dubessy, P. Boulet, V. G. Baonza, I. Bihannic and P. Robert, J. Raman Spectroscopy, 2015, 46, 822–828 CrossRef CAS.
- S. Zhai, X. Wu and E. Ito, J. Raman Spectroscopy, 2010, 41, 1011–1013 CrossRef CAS.
- P. N. De Aza, C. Santos, A. Pazo, S. De Aza, R. Cuscó and L. Artús, Chem. Mater., 1997, 9, 912–915 CrossRef CAS.
- S. Quillard, C. Mellier, R. Gildenhaar, J. Hervelin, P. Deniard, G. Berger and J. M. Bouler, Key Eng. Mater., 2011, 493–494, 225–230 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |