Open Access Article
Open Access ArticleActinide complexes with Wells–Dawson polyoxometalates (part 2): californium
Ian Colliard * and
Gauthier J.-P. Deblonde
* and
Gauthier J.-P. Deblonde *
*
Lawrence Livermore National Laboratory, Livermore, California, USA. E-mail: Colliard1@LLNL.gov; Deblonde1@LLNL.gov
First published on 14th January 2026
Abstract
We report the first example of a polyoxometalate (POM) compound containing californium. Using a Wells–Dawson POM ligand, K17Cf(P2W17O61)2·2H2O was synthesized and characterized. Californium is the heaviest element ever crystalized with a POM. Significant solubility differences among the POM's f-element complexes were observed, enabling selective precipitation with separation factors rivaling liquid–liquid extraction.
Californium represents the last frontier for “bulk” chemical synthesis. All Cf isotopes are radioactive, and its only industrial application (neutron sources for reactor startup, well logging, etc.) involves 252Cf, which only has a half-life of 2.6 years.1 It is estimated that between 1966 and 2019, a cumulative total of ∼10 grams of 252Cf have been produced at the High Flux Isotope Reactor (Oak Ridge, TN, USA), across 78 irradiation campaigns.2 Its isotope used for chemical research, 249Cf, has a half-life of 351 years but it does not benefit from dedicated production campaigns due to the lack of commercial use. As such, it is recovered only in minute quantities and just a few milligrams or less are available to the chemistry community.3 The scarcity of Cf source materials explains the remarkably low number of known Cf compounds. Since its discovery4 in 1950, only 13 coordination compounds of Cf have been isolated and structurally characterized, including the one reported here (Table S1).
The first single crystal structure of a Cf compound, Cf(IO3)3, was reported in 2006 by Sykora et al.5 Four years later, the structure of the Cf3+ aqua ion, isolated as a triflate salt, was reported.6 In a remarkable multi-year experimental campaign, a team led by Prof. Thomas Albrecht and co-workers, synthesized and characterized most of the Cf compounds known to date. Since 2014, these authors synthesized and characterized 10 californium complexes (Table S1), including a Cf(III) borate,7 dipicolinate,8 squarate-oxalate,7 metallocene,9 thiocarbamate,10 and other Cf(III) complexes with organic ligands.11–13 Albrecht's team also reported in 2023 the first, as thus far only, Cf(II) compound: Cf(18-crown-6)I2. Despite these recent and significant progress in Cf chemistry, we noted that no polyoxometalate (POM) compound containing Cf has ever been isolated.
We here bridge this gap by reporting the first Cf-POM compound. Using the Wells–Dawson POM ligand P2W17O6110− (P2W17), we isolated K17Cf(P2W17O61)2·2H2O via aqueous synthesis. The present study complements a companion paper14 on the analogous Am3+ and Cm3+ compounds, K17Am(P2W17O61)2·12H2O and K17Cm(P2W17O61)2·8H2O (Fig. 1). This combined dataset gives an unprecedent perspective on Cf chemistry given the isostructural and isomorphic nature of these heavy actinide compounds.
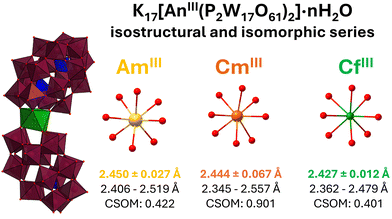 | ||
| Fig. 1 Structure of K17Cf(P2W17O61)2·2H2O and its analogues K17Am(P2W17O61)2·12H2O and K17Cm(P2W17O61)2·8H2O. Am3+ forms two structure types with P2W17.14 Average An–O distances, bond range, and Continuous Symmetry operation Measure (CSoM, as defined by Nielsen & Sørensen15) are indicated at the bottom. Potassium counterions and water molecules are omitted for clarity. Color code: W: Maroon. P: Blue. O: Red. Am: Yellow. Cm: Orange. Cf: Green. See Fig. S1–S3 for additional views of the structures. | ||
Prior to this study, hardly any information had been published on californium-POM complexes, even in terms of spectroscopic data (whether in solution or solid-state). To the best of our knowledge, only a handful and studies had been published on Cf-POMs; most during the USSR era and some data was translated and relayed in the English literature. In 1976, Sapryskin et al. reported16 that POMs, notably P2W17, affect the redox potentials of actinides. Subsequent studies yielded experimental values for the redox potentials of [AnIV(P2W17)2]16−/[AnIII(P2W17)2]17− (An = Np, Pu, Am, Cm, Bk)17,18 and an estimated value for [CfIV(P2W17)2]16−/[CfIII(P2W17)2]17−.17,19 A 1998 experimental study investigated the temperature effects on the reduction of Am4+, Cm4+, Bk4+, and Cf4+ in the presence of P2W17, yielding ΔG, ΔH, and ΔS values. The authors also reported the kinetic trend, showing that Cf4+ is the fastest to reduce among the [AnIV(P2W17)2]16− complexes (Cf4+ > Cm4+ > Bk4+ > Am4+).20 These redox results have been tabulated in two related EXAFS and cyclic voltammetry studies on the P2W17 actinide complexes (Th, U, Np, Pu, Am) by Antonio and co-workers in 2003.21,22 Except for these electrochemistry-focused studies, no structural or spectroscopic data has been published on Cf-POMs.
In an effort to obtain structural information on heavy actinide chemistry, we previously leveraged the unique properties of POMs, to study Am3+ and Cm3+. We found that the high molecular weight of POMs (e.g., 1207 g mol−1 for W5O186−, 2677 g mol−1 for PW11O397−, 4163 g mol−1 for P2W17O397−) and their controllable solubility properties allows for crystallizing Am-POM and Cm-POM complexes while using only ∼2–10 µg of the actinide isotope.23–26 We recently extended these studies to Np4+ and Pu4+.27–29 We had thus far focused on smaller POM structures such as the Weakley-Peacock ([M(W5O18)2]x−) and Keggin complexes ([M(XW11O39)2]x−, with X = B3+, Si4+, P5+, Ge4+, Ga3+). Using the heavier POM30 P2W17 allowed for scaling down ever further the synthesis. We reliably obtained single crystals from just ∼300 nanograms of actinide, verified with Am3+, Cm3+, and Cf3+. The synthesis was not attempted with Bk but some of its properties can be extrapolated (vide infra).
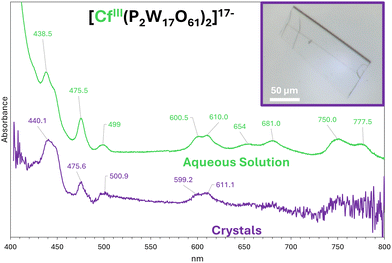
The formation of the [CfIII(P2W17)2]17- complex (abbreviated CfIII(P2W17)2) was first confirmed in aqueous solution via UV-vis-NIR. The main absorbance bands of the complex are shown in Fig. 2. Nine Cf(III)-specific bands were observed in the 420–800 nm region, plus an intense absorbance below 420 nm that is due to the POM itself. The Cf(III)-specific bands are consistent with absorbance spectra of Cf(III) complexes with organic chelators published by Albrecht and co-workers.7,12,13 Based on their nomenclature, the observed line groups are: group G (438.5 nm), group F (475.5 and 499 nm), group E (600.5, 610.0, 654.0, and 681.0 nm), and group D (750.0 and 777.5 nm).
Upon addition of excess KCl, single crystals appear after ∼4 weeks of slow evaporation/concentration at room temperature (Fig. 2). The K17[CfIII(P2W17)2]·2H2O compound crystallizes in the monoclinic space group P21/n, with a volume of 15045.9 Å (Tables S2 and S3). The synthesis was done with 0.3 to 0.5 µg of 249Cf per sample. Comparing the absorbance spectra complex in aqueous solution versus in the crystals (Fig. 2), minimal peak shifting is seen, indicating that the obtained Cf complex in the solid state generally has the same structural metrics as in solution. In a companion paper,14 we report the analogous systems with Am3+ and Cm3+. Two different structures were obtained for Am (monoclinic K17[AmIII(P2W17O61)2]·12H2O and triclinic K17Am(P2W17O61)2·42.5H2O) while Cm only yields monoclinic K17Cm(P2W17O61)2·8H2O. Consistent with this apparent trend along the heavy actinide series, Cf only crystalizes as the monoclinic phase. The absorbance spectrum for AmIII(P2W17)2 in solution also matches exactly the spectrum of its monoclinic phase (but not its triclinic phase), similar to what is observed for monoclinic CfIII(P2W17)2. These results indicate that polymorphisms in these systems can impact the electronic properties of the actinides and the analysis of spectroscopic properties warrants cautions when relying on specific structural data. We also obtained an analogous structure of PrIII(P2W17)2 that had not been reported (triclinic – Table S2) to provide more clarity on the impact of triclinic versus monoclinic distortions in these P2W17 complexes. Pr3+ is now the only LnIII(P2W17)2 for which the triclinic and monoclinic phases have been characterized, and this confirms that the monoclinic phase leads to elongated bonds (2.47 vs. 2.43 Å for Pr. Fig. S4).
Taken together, the Am, Cm and Cf complexes represent one of the first isostructural and isomorphic series for Am, Cm and Cf coordination compounds. Crystals that share the same structure, yet not necessarily the same composition or unit cell, are often claimed to be isostructural. Herein, the LnIII/AnIII(P2W17)2 (along with a AnIV(P2W17)2 series previously reported31) are isostructural in that all share the same kind of complexes. The varying structural metrics previously extracted conform with this definition, providing comparisons between crystals. However, due to the identification of polymorphic phases for these P2W17 structures, the compounds can now be more rigorously categorized in isomorphic crystal series. Isomorphous crystals have, (1) same unit cell and space group, and (2) same atom type and positions, except for replacement of one or more atom. In other words, these crystals not only share the same unit cell (a = 12.4 Å, b = 23.5 Å, c = 51.9 Å, a = y = 90, b = 89.4, and V = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 Å3), but the same formula with very minor differences (hydration and or metal replacement i.e., Am3+, Cm3+, or Cf3+).
050 Å3), but the same formula with very minor differences (hydration and or metal replacement i.e., Am3+, Cm3+, or Cf3+).
This more rigorous classification of the structures now allows for more accurate and meaningful comparisons. For example, comparing the unit cell for the isomorphic AmIII(P2W17)2, CmIII(P2W17)2, and CfIII(P2W17)2 reveals a small contraction in all unit cell dimensions. When comparing the structural metrics previously defined for POM complexes (bending, stretching, twisting),26,32 there is a small but consistent shifts: AnIII–O bonds contract from 2.450 Å for Am, to 2.444 Å for Cm, and 2.424 Å for Cf. The P1-An-P1′ bending angles are nearly identical for the three structures: 169.1° (Am), 169.1° (Cm), and 168.9° (Cf). The second bending angle, P2-An-P2′, is also very consistent across the three structures: 138.5° (Am) to 138.2° (Cm) to 138.2° (Cf). However, the complex seems to twist slightly more as the actinide gets smaller, with the P1-AnO8-P1′ increasing from 1.0° (Am), to 1.3° (Cm), to 2.0° (Cf). Since these complexes exhibit the same unit cell and space group, any structural changes can thus be attributed to the impact of the actinide.
Based on an empirical correlation between the structures of Keggin complexes ([AnIV(PW11O39)2]10−) and AnO2 oxides, we recently proposed29 a set of values for An(IV)–O bond distances in POMs, including AmIV–O (2.331 Å), CmIV–O (2.324 Å), BkIV–O (2.312 Å), and CfIV–O (2.304 Å). The set of Wells–Dawson structures obtained here allows for proposing an equivalent set of values for An(III)–O bonds in POMs as the missing value for BkIII(P2W17)2 can be fairly extrapolated from the ionic radius trend (Fig. 3): AmIII–O (2.450 Å), CmIII–O (2.444 Å), BkIII–O (2.436 Å), and CfIIII–O (2.427 Å).
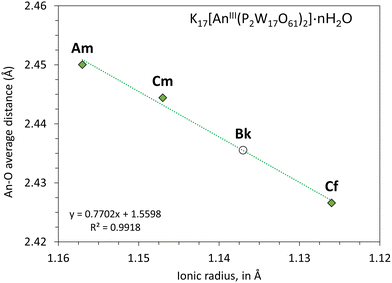 | ||
| Fig. 3 Average actinide(III)–oxygen bond distance in the Wells–Dawson complexes. The Am, Cm, and Cf points are from the single crystal XRD structures (monoclinic phase) of K17An(P2W17O61)2·nH2O. Bk point extrapolated from the linear trendline. Ionic radii are from Lundberg and Persson (CN = 9).33 The same extrapolated BkIII–O distance is obtained when considering the Shannon34 ionic radii (available for CN = 6) – see Fig. S5. | ||
In our attempts to crystallize the AnIII(P2W17)2 compounds, we eventually tested our protocol with lanthanide(III) and noticed significant solubility differences among the complexes. As shown in Fig. S6, when exposed to different concentrations of K+ counterions, some LnIII(P2W17)2 precipitate before others. Separation factors of ∼3 to ∼10 were measured between Ho, Pr, and Nd (Fig. S7), without particular optimization. It appears that lanthanides could be separated via selective precipitation upon complexation to P2W17 followed by addition of counterions in aqueous solution of the LnIII(P2W17)2 complexes. This proposed method requires more development but could be a readily different way of separating rare earth elements, relative to the current industrial methods that are based on solvent extraction.35 Selective precipitation methods for separation of Ln3+ have been proposed recently but these methods are based on complexes with organic chelators.36,37 To the best of our knowledge a method based on POMs and counterions has not been proposed before.
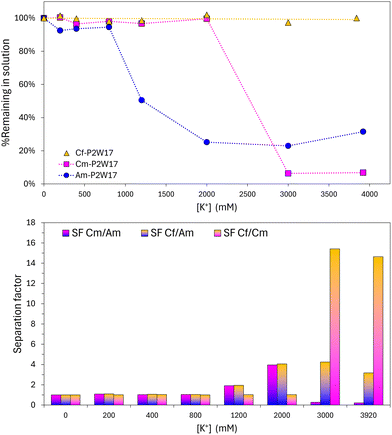
In line with these observations on the LnIII(P2W17)2 complexes, we noticed the same kind of trend with actinides, with the CfIII(P2W17)2 complex requiring much longer evaporation/concentration to yield crystals. In a controlled series with varied KCl concentration added to AmIII(P2W17)2, CmIII(P2W17)2, CfIII(P2W17)2, we observed precipitation of Am and Cm but Cf remained completely soluble, even at K+ concentration as high a 4 M. Fig. 4 shows the concentrations of Am, Cm, Cf remaining in solution at equilibrium. Such solubilities differences represent a potentially simple, yet efficient, leverage to separate heavy actinides.
The calculated separation factors from the solubility curves reach 15 for Cf/Cm and 4.0 for both Cm/Am and Cf/Cm. These results highlight the significant solubility differences among the K17An(P2W17O61)2·nH2O compounds. Such separation factors among heavy actinides are on par or higher with selectivity obtained with some of the most recent liquid–liquid extraction processes developed for separating Am3+ and Cm3+. Jensen et al. obtained a selectivity Am/Cm of 4.1 using a liquid–liquid extraction system comprised of both an organic extractant and a water-soluble Am3+-selective complexant (“bp18c62−”, also known as “macropa”).38 Miguirditchian and co-workers recently39 developed Am3+-selective stripping agent (“H4TPAEN”) to back-extract Am following the co-extraction of Am3+ and Cm3+ by the organic extractant TODGA. The observed Am/Cm separation factors in this case were in the range 3.1–4.2.
In conclusion, this study presents the first structural and spectroscopic characterization of a Cf-POM complex. When put in perspective with the corresponding compounds with Ln3+, Am3+, and Cm3+ it is clear that the formation of two different phases (e.g., monoclinic versus triclinic) has a non-negligible impact on the bonding, electronic properties, and solubility properties. From a structural standpoint, the CfIII(P2W17)2 and CmIII(P2W17)2 compounds appear similar, forming only the monoclinic phase. AmIII(P2W17)2 yields both the monoclinic and triclinic phases, akin to some of the lanthanide complexes. The isomorphous and isostructural series of CfIII(P2W17)2, CmIII(P2W17)2, and AmIII(P2W17)2 (monoclinic phase) exhibits predictable contraction of the AnIII–O bonds and similar bending, yet different twisting angles. The solubility property of these compounds seems to be the most impacted by the nature of the f-element it contains. While the results presented here are exploratory and require further optimization, it appears that solubility differences in POM/f-element/counterion systems could be exploited for a different generation of separation processes for f-elements. Efforts are underway to expand on the present findings.
This material is based upon work supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Heavy Element Chemistry program at Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. Release number: LLNL-JRNL-2014895.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article has been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc07083j.CCDC 2514973 and 2514979 contain the supplementary crystallographic data for this paper.40a,b
References
- D. Hartanto, D. Chandler, H. Green, J. Whan Bae, K. M. Burg, Y. Robert and C. Sizemore, Ann. Nucl. Energy, 2025, 211, 110960 CrossRef CAS.
- S. M. Robinson, D. E. Benker, E. D. Collins, J. G. Ezold, J. R. Garrison and S. L. Hogle, Radiochim. Acta, 2020, 108, 737–746 CrossRef CAS.
- J. B. Roberto, C. W. Alexander, R. A. Boll, J. D. Burns, J. G. Ezold, L. K. Felker, S. L. Hogle and K. P. Rykaczewski, Nucl. Phys. A, 2015, 944, 99–116 Search PubMed.
- S. G. Thompson, K. Street, A. Ghiorso and G. T. Seaborg, Phys. Rev., 1950, 78, 298–299 Search PubMed.
- R. E. Sykora, Z. Assefa, R. G. Haire and T. E. Albrecht-Schmitt, Inorg. Chem., 2006, 45, 475–477 CrossRef CAS.
- C. Apostolidis, B. Schimmelpfennig, N. Magnani, P. Lindqvist-Reis, O. Walter, R. Sykora, A. Morgenstern, E. Colineau, R. Caciuffo, R. Klenze, R. G. Haire, J. Rebizant, F. Bruchertseifer and T. Fanghänel, Angew. Chem., Int. Ed., 2010, 49, 6343–6347 CrossRef CAS.
- N. Brenner, J. M. Sperling, T. N. Poe, C. Celis-Barros, K. Brittain, E. M. Villa, T. E. Albrecht-Schmitt and M. J. Polinski, Inorg. Chem., 2020, 59, 9384–9395 CrossRef CAS.
- S. K. Cary, M. Vasiliu, R. E. Baumbach, J. T. Stritzinger, T. D. Green, K. Diefenbach, J. N. Cross, K. L. Knappenberger, G. Liu, M. A. Silver, A. E. DePrince, M. J. Polinski, S. M. V. Cleve, J. H. House, N. Kikugawa, A. Gallagher, A. A. Arico, D. A. Dixon and T. E. Albrecht-Schmitt, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- C. A. P. Goodwin, J. Su, L. M. Stevens, F. D. White, N. H. Anderson, J. D. Auxier, T. E. Albrecht-Schönzart, E. R. Batista, S. F. Briscoe, J. N. Cross, W. J. Evans, A. N. Gaiser, A. J. Gaunt, M. R. James, M. T. Janicke, T. F. Jenkins, Z. R. Jones, S. A. Kozimor, B. L. Scott, J. M. Sperling, J. C. Wedal, C. J. Windorff, P. Yang and J. W. Ziller, Nature, 2021, 599, 421–424 Search PubMed.
- S. K. Cary, J. Su, S. S. Galley, T. E. Albrecht-Schmitt, E. R. Batista, M. G. Ferrier, S. A. Kozimor, V. Mocko, B. L. Scott, C. E. Van Alstine, F. D. White and P. Yang, Dalton Trans., 2018, 47, 14452–14461 RSC.
- S. S. Galley, S. A. Pattenaude, C. A. Gaggioli, Y. Qiao, J. M. Sperling, M. Zeller, S. Pakhira, J. L. Mendoza-Cortes, E. J. Schelter, T. E. Albrecht-Schmitt, L. Gagliardi and S. C. Bart, J. Am. Chem. Soc., 2019, 141, 2356–2366 CrossRef CAS.
- J. M. Sperling, E. Warzecha, C. J. Windorff, B. E. Klamm, A. N. Gaiser, M. A. Whitefoot, F. D. White, T. N. Poe and T. E. Albrecht-Schönzart, Inorg. Chem., 2020, 59, 10794–10801 CrossRef CAS.
- Z. Bai, N. B. Beck, B. Scheibe, J. M. Sperling, A. Weiland, M. Ruf, J. P. Brannon, B. M. Rotermund, D. Gomez Martinez and T. E. Albrecht-Schönzart, J. Am. Chem. Soc., 2024, 146, 7822–7830 CrossRef CAS PubMed.
- I. Colliard and G. Deblonde, Chem. Comm., 2026 10.1039/D5CC06999H.
- V. R. M. Nielsen and T. J. Sørensen, Nat. Commun., 2025, 16, 11122 CrossRef CAS.
- A. S. Saprykin, V. P. Shilov, V. I. Spitsyn and N. N. Krot, Dokl. Akad. Nauk SSSR; (USSR).
- G. A. Timofeev, V. M. Chistyakov, E. A. Erin, A. A. Baranov and V. V. Kopytov, Soviet Radiochemistry, 1986, 28, 156–161 Search PubMed.
- E. A. Erin, A. A. Baranov, A. Y. Volkov, V. M. Chistyakov and G. A. Timofeev, Radiochemistry, 2001, 43, 350–353 CrossRef CAS.
- A. A. Baranov, G. A. Simakin, V. N. Kosyakov, E. A. Erin, V. V. Kopytov, G. A. Timofeev and A. G. Rykov, Soviet Radiochemistry, 1981, 23, 104–106 Search PubMed.
- E. A. Erine, A. A. Baranov, A. Y. Volkov, V. M. Chistyakov and G. A. Timofeev, J. Alloys Compd., 1998, 271–273, 782–785 CrossRef CAS.
- M.-H. Chiang, L. Soderholm and M. R. Antonio, Eur. J. Inorg. Chem., 2003, 2929–2936 Search PubMed.
- M.-H. Chiang, C. W. Williams, L. Soderholm and M. R. Antonio, Eur. J. Inorg. Chem., 2003, 2663–2669 CrossRef CAS.
- I. Colliard, J. R. I. Lee, C. A. Colla, H. E. Mason, A. M. Sawvel, M. Zavarin, M. Nyman and G. J.-P. Deblonde, Nat. Chem., 2022, 14, 1357–1366 Search PubMed.
- I. Colliard and G. J.-P. Deblonde, JACS Au, 2024, 4, 2503–2513 CrossRef CAS PubMed.
- I. Colliard and G. J.-P. Deblonde, Chem. Commun., 2024, 60, 5999–6002 RSC.
- I. Colliard and G. J.-P. Deblonde, J. Am. Chem. Soc., 2025, 147, 14455–14467 CrossRef CAS PubMed.
- I. Colliard, V. Stavila and G. J.-P. Deblonde, Chem. Commun., 2025, 61, 11858–11861 RSC.
- A. Hastings, I. Colliard, D. Kaseman, C. Colla and G. Deblonde, Inorg. Chem., 2025, 64(37), 18925–18937 Search PubMed.
- J. Bustos, I. Colliard, V. Stavila, D. C. Kaseman, C. A. Colla, M. Nyman and G. J.-P. Deblonde, Inorg. Chem., 2025 DOI:10.1021/acs.inorgchem.5c03627.
- W. J. Randall, D. K. Lyon, P. J. Domaille and R. G. Finke, Inorganic Syntheses, John Wiely & Sons, Inc, 1998, vol. 32, pp. 242–268 Search PubMed.
- M. N. Sokolova, A. M. Fedosseev, G. B. Andreev, N. A. Budantseva, A. B. Yusov and P. Moisy, Inorg. Chem., 2009, 48, 9185–9190 CrossRef CAS PubMed.
- I. Colliard and G. J.-P. Deblonde, Inorg. Chem., 2024, 63, 16293–16303 CrossRef CAS PubMed.
- D. Lundberg and I. Persson, Coord. Chem. Rev., 2016, 318, 131–134 Search PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef.
- T. Cheisson and E. J. Schelter, Science, 2019, 363, 489–493 CrossRef CAS PubMed.
- Y. Gao, G. L. Licup, N. P. Bigham, D. C. Cantu and J. J. Wilson, Angew. Chem., 2024, 136, e202410233 CrossRef.
- R. F. Higgins, K. P. Ruoff, A. Kumar and E. J. Schelter, Acc. Chem. Res., 2022, 55, 2616–2627 Search PubMed.
- M. P. Jensen, R. Chiarizia, I. A. Shkrob, J. S. Ulicki, B. D. Spindler, D. J. Murphy, M. Hossain, A. Roca-Sabio, C. Platas-Iglesias, A. de Blas and T. Rodríguez-Blas, Inorg. Chem., 2014, 53, 6003–6012 CrossRef CAS.
- C. Marie, P. Kaufholz, V. Vanel, M.-T. Duchesne, E. Russello, F. Faroldi, L. Baldini, A. Casnati, A. Wilden, G. Modolo and M. Miguirditchian, Solvent Extr. Ion Exch., 2019, 37, 313–327 CrossRef CAS.
- (a) CCDC 2514973: Experimental Crystal Structure Determination, 2025 DOI:10.25505/fiz.icsd.cc2qf15d; (b) CCDC 2514979: Experimental Crystal Structure Determination, 2025 DOI:10.25505/fiz.icsd.cc2qf1cl.
| This journal is © The Royal Society of Chemistry 2026 |