Open Access Article
Open Access ArticleReactivity of gold and copper acetylide with a secondary borane
Ragene A. Thornton,
Kirk French,
Kevin Shuford and
Caleb D. Martin
and
Caleb D. Martin *
*
Baylor University, Department of Chemistry and Biochemistry, One Bear Place #97348, Waco, TX 76798, USA. E-mail: caleb_d_martin@baylor.edu
First published on 10th December 2025
Abstract
Gold(I) triphenylphosphine phenylacetylide reacts with bis(1-methyl-ortho-carboranyl)borane to give a hydroboration product that is best described as an η2-1-borataallene gold complex. Contrarily, the analogous reaction with the copper acetylide undergoes transmetalation to furnish an alkynylborate with the CuPPh3+ moiety bound to the alkyne and a hydride bridging boron to copper. The compounds were analyzed by single crystal X-ray diffraction and DFT computations that rationalize the unusual bonding arrangements.
The hydroboration reaction is a versatile synthetic tool to introduce functionalization on unsaturated carbon–carbon bonds with anti-Markovnikov selectivity.1 The boryl group in hydroboration products is a convenient handle that can be oxidized to a variety of functional groups or used as a building block for coupling reactions.2 Borane (BH3) is an effective hydroboration reagent, however, it bears three reactive B–H bonds, thus secondary boranes offer more control with the reactivity of the borane tuned by the two other substituents on boron.3 Secondary boranes featuring chelating π-donors increase stability but reduce reactivity that typically necessitates a catalyst.4 Electron withdrawing groups increase the electrophilicity at boron and enhance hydroboration reactivity.5 Fluoroaryl groups have been very effective and recently, it has been demonstrated that ortho-carborane substituents give rise to potent organoborane Lewis acids.6
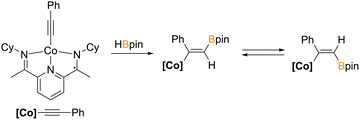
The first catalyzed hydroboration reactions were with rhodium and since then, other late transition metals have become prominent.7 In recent years, gold and copper catalysts have demonstrated high efficacy in the hydroboration of alkynes.8 In copper catalyzed hydroborations of terminal alkynes, copper acetylide species are proposed intermediates in the catalytic cycle.9 Despite this, direct stoichiometric reactions between gold or copper acetylides and secondary boranes have not been reported. In fact, the only stoichiometric reaction reported of a metal acetylide with a secondary borane is that of a bis(imino)pyridine ligated cobalt(II) acetylide with HBPin that furnished E and Z 1,1-hydroboration products, where the hydrogen and boryl group are on the same carbon (Fig. 1).10 In addition to the hydroboration catalysis, it is known that electron rich metal centers proximal to main group Lewis acidic centers, including tricoordinate boranes, readily engage in Z-type interactions.11 In this work, we investigate the reactivity of a gold and copper acetylide with the extremely potent hydroboration reagent, bis(1-methyl-ortho-carboranyl)borane (HBMeoCb2, MeoCb = 1-methyl-ortho-carborane)12 to determine whether typical hydroboration reactivity occurs, Z-type bonding, or a different outcome.
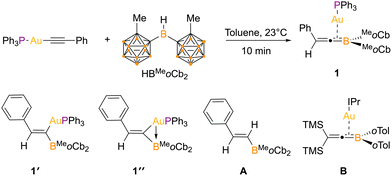
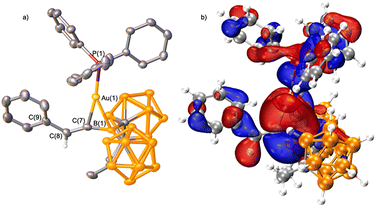
The equimolar reaction of Ph3PAuCCPh and HBMeoCb2 in toluene at 23 °C was monitored by NMR spectroscopy (Fig. 2). After 30 minutes, a 11B{1H} NMR resonance was observed at 25.0 ppm, shifted upfield from HBMeoCb2 (70.9 ppm) and in the 31P NMR spectrum, a resonance at 41.7 ppm is observed, shifted slightly from Ph3PAuCCPh (44.7 ppm) with complete consumption of the two starting materials. Upon work up, the new species was isolated in 57% yield. X-ray diffraction of crystals grown from vapor diffusion of n-pentane into a concentrated toluene solution revealed a product consistent with a syn-1,2-hydroboration reaction with the substituents introduced according to the polarity of the acetylide. The boryl group is introduced on the α-carbon of Ph3PAuCCPh and the hydrogen to the β-carbon (1, Fig. 3a).
Close analysis of the structure reveal that the structure of 1 deviates from an idealized vinyl borane from a syn-1,2-hydroboration (1′). While the bond distance between the formerly acetylide carbons, C(7) to C(8), is 1.331(5) Å and consistent with a double bond, the C(7) to B(1) bond length is shorter than a single bond at 1.497(9) Å, lying in the range of a borataalkene.13a However, the C(8)-C(7)-B(1) bond angle of 155.2(4)° is considerably perturbed from an idealized trigonal planar geometry at C(7). Comparing this angle in 1 to the analogous complex with a proton in place of gold, E-PhHC = CHBMeoCb2 (A: 128.8(6)°) reveals considerable expansion. Based on these metrical parameters, two other bonding descriptors were considered that would impact this angle, a vinyl borane that features a gold to boron Z-type interaction (1″) and an η2-coordinated 1-boratallene gold complex. The Au–B distance of 2.436(1) Å is in the range of both a Z-type (2.26 to 2.59 Å) or an η2-boratalkene interaction (2.29 to 2.42 Å).11g,13
DFT analysis reveals a gold to carbon Wiberg bond index (WBI) less than unity of 0.68 and from gold to boron at 0.41. The B(1) to C(7) WBI is 1.29, considerably higher than that of the analogous complex with a proton in place of gold (A, WBI = 0.91). Combined with a WBI of 1.81 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, these results indicate the best descriptor is a Au-borataallene complex. The gold center interacts stronger with the central carbon atom of the borataallene fragment than the boron [Au–B = 2.436(1) Å and Au–C = 2.135(4) Å], in line with the polarized nature of Au-borataalkene systems.14 The NPA charge at B(1) is positive at 0.651, attributed to the electron withdrawing power of the carborane substituents. The central carbon, C(7) has an NPA charge of −0.530, consistent with the polarization of a borataalkene while the gold atom bears a positive charge of 0.454, in line with boratalkene π-complexation to AuPPh3+. Analysis of the frontier orbitals revealed the HOMO−1 features a bonding interaction between the gold center and the BC π-bond (Fig. 3b).
C bond, these results indicate the best descriptor is a Au-borataallene complex. The gold center interacts stronger with the central carbon atom of the borataallene fragment than the boron [Au–B = 2.436(1) Å and Au–C = 2.135(4) Å], in line with the polarized nature of Au-borataalkene systems.14 The NPA charge at B(1) is positive at 0.651, attributed to the electron withdrawing power of the carborane substituents. The central carbon, C(7) has an NPA charge of −0.530, consistent with the polarization of a borataalkene while the gold atom bears a positive charge of 0.454, in line with boratalkene π-complexation to AuPPh3+. Analysis of the frontier orbitals revealed the HOMO−1 features a bonding interaction between the gold center and the BC π-bond (Fig. 3b).
In the literature, borataallene structures are rare. There have been four 1-borataallenes reported with two being this year and only one 2-boratallene.15 Regarding borataallene metal complexes, only one exists, a gold 1-borataallene complex reported by Yamashita and co-workers (B) that features ortho-tolyl groups on boron, two trimethylsilyl groups on the allenic carbon, and the IPr carbene on gold (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene).16 Their method of preparation involved reacting IPrAu-B(o-toyl)2 with bis(trimethylsilyl)acetylene. The solid-state structures of 1 and B are similar with the pertinent Au–B, Au–C, B–C, and C–C distances close to or within error of measurement of each other [Au–B = 2.430(4) vs. 2.441(4) Å, Au–C = 2.135(4) vs. 2.164(4) Å, B–C = 1.469(6) vs. 1.509(6) Å, and C–C = 1.331(5) vs. 1.338(5) Å] despite featuring very different substituents on the allenic carbon and boron atoms as well as a different ancillary ligand on gold. Interestingly, neither 1 or B were generated from the free 1-borataallene and are distinct synthetic routes.
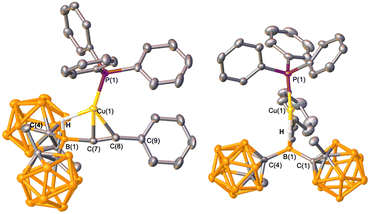
Treatment of Ph3PCuCCPh with HBMeoCb2 in toluene at 23 °C and examining the 11B{1H} NMR spectrum after 30 minutes revealed a resonance upfield from the cluster boron region at −18.5 ppm, very different than the boryl resonance for 1 at 25.0 ppm (Fig. 4). This peak became a doublet in the 11B NMR spectrum, consistent with the hydride retained on boron. The 31P NMR resonance is at −1.4 ppm, slightly shifted from Ph3PCuCCPh at −4.6 ppm. Obtaining a single crystal X-ray diffraction structure reveals the product as the copper-alkyne π-complex, 2, with the HBMeoCb2 moiety covalently bound to the α-carbon (Fig. 5). The borane underwent transmetalation with copper to furnish the borate functionalized alkyne and CuPPh3+ migrated to the alkyne. In the 1H NMR spectrum, the hydrogen in the B–H–Cu unit is in the region of the carborane cluster B–H atoms. Conducting the reaction with DBMeoCb2 and analyzing the 2H NMR spectrum reveals a peak at 2.38 ppm corresponding to the bridging deuteride.17 To determine if 2 would isomerize to give a hydroboration product, the toluene solution of 2 was stirred for 16 h at 23 °C but no change was observed by 31P or 11B NMR spectroscopy. Heating at 80 °C for 72 hours gave an indiscernible mixture.
The CC bond in 2 is 1.218(2) Å and the FT-IR CC stretching frequency is 2211 cm−1, both in the range of alkynes (CC bond lengths range 1.20 to 1.22 Å and FT-IR frequencies lie between 2100 and 2260 cm−1). The copper atom is equidistant to the two carbon atoms [Cu(1)–C(1) 2.0037(14) Å and Cu(1)–C(2) 2.0083(16) Å] and the bond angles about the two alkyne carbon atoms are close to linear [C(8)–C(7)–B(1) 165.11(15)° and C(7)–C(8)–C(9) 165.37(18)°]. The sum of the C–B–C bond angles about B(1) is 343.11(18)°, consistent with a distorted tetrahedral environment with a hydride bound to boron. In the mass spectrum, a peak was observed in the cation mode for CuPPh3+ (m/z = 325.0185) and in the negative mode for the alkynylborate fragment (m/z = 427.4581).
The isolated species 2 resembles cobalt hydride alkyne π-complexes that have been proposed as catalytic intermediates in cobalt-mediated alkyne hydroboration reactions with HBPin by Chirik as well as by Grutzmacher and Hu.10,18 Their cobalt complex reacted with an equivalent of terminal alkyne to release the vinyl borane hydroboration product and regenerate the metal acetylide. Given this, 2 was subjected to stoichiometric reactions with terminal alkynes (phenylacetylene and trimethylsilyl acetylene) and heated in toluene to 80 °C for 16 h, however no reaction was observed. The high hydride affinity of the bis(1-methyl-ortho-carboranyl)boryl unit rationalizes the trapping of 2.6h The mechanism to form 2 could be via a hydroboration sequence involving metalate shift of the CuPPh3 moiety or through a direct transmetalation. Copper acetylides are more susceptible to transmetalation than gold acetylides, that may be the root of the differing reactivity.19
The Cu–C WBIs approach 0.5 that is in line with π-complexation and reveal a slightly stronger bond to the borate functionalized carbon of the alkyne [C(7)–Cu = 0.41, C(8)–Cu = 0.33]. The computed NPA charges show significant cationic charge on copper (+0.83) and slight negative charges on the alkyne carbon atoms, with the borate carbon being higher in magnitude [C(7) (−0.29) and C(8) (−0.07)]. Regarding the hydride, the B–H interaction is computed to be much stronger than the Cu–H interaction (WBI = 0.69 vs. 0.20) but consistent with the bridged hydride. The NPA on the central boron atom is +0.19, attributed to the strong electron withdrawing ability of the carborane substituents and the “hydride” is 0.00, indicative of the contacts to copper and boron.
This work demonstrates that HBMeoCb2 reacts with Ph3PAuCCPh and Ph3PCuCCPh to give two distinct products. The gold complex is best described as an η2-coordinated 1-boratallene gold complex while the copper species is an alkynylborate complexed to CuPPh3+ via the alkyne. The former is a rare example of a 1-boratallene or a borataalkene unit complexed to gold. The copper species features a bridging hydride between boron and the metal that is reminiscent of proposed intermediates in metal catalyzed hydroborations. The resilient B-H interaction is attributed to the high hydride affinity of the bis(ortho-carboranyl)boryl moiety and provides insight into unique reactivity of secondary boranes with CC triply bonded species.
R. A. T. carried out the synthetic experiments and the single crystal X-ray diffraction analyses. K. A. F. and K. L. S. performed the DFT calculations and composed the related text. C. D. M. conceived and supervised the project. R. A. T. and C. D. M. wrote the manuscript with edits from K. A. F. and K. L. S.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, NMR spectra, computational details, and X-ray crystallographic data. See DOI: https://doi.org/10.1039/d5cc06957b.CCDC 2491533 and 2491534 contain the supplementary crystallographic data for this paper.20a,b
Acknowledgements
We are grateful to the National Science Foundation (Awards No. 2349851-C. D. M. and 2147956-K. L. S.) for their generous support of this work. The authors thank Baylor University High Performance Research Computing services for their allocation of computational resources on the Kodiak HPC cluster. Manjur Akram is acknowledged for preliminary observations.References
- (a) H. C. Brown, R. Liotta and C. G. Scouten, J. Am. Chem. Soc., 1976, 98, 5297–5301 CrossRef; (b) H. C. Brown, R. Liotta and L. Brener, J. Am. Chem. Soc., 1977, 99, 3427–3432 CrossRef; (c) G. Zweifel and H. C. Brown, Org. React., 1963, 13, 1–54 Search PubMed; (d) G. Wilkinson, F. G. A. Stone and E. W. Abel, Comprehensive organometallic chemistry: the synthesis, reactions, and structures of organometallic compounds, Pergamon Press, 1982 Search PubMed; (e) H. C. Brown, in Advances in Organometallic Chemistry, ed. F. G. A. Stone and R. West, Academic Press, 1973, vol. 11, pp. 1–20 Search PubMed; (f) H. C. Brown, Science, 1980, 210, 485–492 CrossRef PubMed.
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef PubMed; (b) J. X. Qiao and P. Y. S. Lam, Synthesis, 2011, 829–856 CrossRef; (c) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef.
- (a) T. Clark and P. V. R. Schleyer, J. Organomet. Chem., 1978, 156, 191–202 CrossRef; (b) J. M. Clay and E. Vedejs, J. Am. Chem. Soc., 2005, 127, 5766–5767 CrossRef.
- (a) D. P. Zobernig, B. Stöger, L. F. Veiros and K. Kirchner, ACS Catal., 2024, 14, 12385–12391 CrossRef; (b) H. Braunschweig, R. D. Dewhurst and A. Schneider, Chem. Rev., 2010, 110, 3924–3957 CrossRef; (c) G. A. Chotana, B. A. Vanchura II, M. K. Tse, R. J. Staples, J. Robert, E. Maleczka and M. R. Smith III, Chem. Commun., 2009, 5731–5733 RSC; (d) B. A. Vanchura II, S. M. Preshlock, P. C. Roosen, V. A. Kallepalli, R. J. Staples, J. R. E. Maleczka, D. A. Singleton and M. R. Smith III, Chem. Commun., 2010, 46, 7724–7726 RSC; (e) M. R. Smith III, R. Bisht, C. Haldar, G. Pandey, J. E. Dannatt, B. Ghaffari, R. E. Jr. Maleczka and B. Chattopadhyay, ACS Catal., 2018, 8, 6216–6223 CrossRef.
- (a) S. Ranasinghe, Y. Li, M. E. Andrews, M. O. Akram, R. A. Thornton and C. D. Martin, Chem. Commun., 2025, 61, 10182–10185 RSC; (b) D. J. Parks, R. E. von, H. Spence and W. E. Piers, Angew. Chem., Int. Ed. Engl., 1995, 34, 809–811 CrossRef.
- (a) L. Xiang, J. Wang, A. Matler and Q. Ye, Chem. Sci., 2024, 15, 17944–17949 RSC; (b) K. Vashisth, S. Dutta, M. O. Akram and C. D. Martin, Dalton Trans., 2023, 52, 9639–9645 RSC; (c) X. Tan, X. Wang, Z. H. Li and H. Wang, J. Am. Chem. Soc., 2022, 144, 23286–23291 CrossRef; (d) L. Xiang, A. Matler, L. Tan and Q. Ye, Dalton Trans., 2024, 53, 11655–11658 RSC; (e) K. M. Lee, J. O. Huh, T. Kim, Y. Do and M. H. Lee, Dalton Trans., 2011, 40, 11758–11764 RSC; (f) J. O. Huh, H. Kim, K. M. Lee, Y. S. Lee, Y. Do and M. H. Lee, Chem. Commun., 2010, 46, 1138–1140 RSC; (g) K. C. Song, H. Kim, K. M. Lee, Y. S. Lee, Y. Do and M. H. Lee, Dalton Trans., 2013, 42, 2351–2354 RSC; (h) M. O. Akram, C. D. Martin and J. L. Dutton, Inorg. Chem., 2023, 62, 13495–13504 CrossRef; (i) M. O. Akram, J. R. Tidwell, J. L. Dutton and C. D. Martin, Angew. Chem., Int. Ed., 2022, 61, e202212073 CrossRef; (j) V. Nori, F. Pesciaioli, A. Sinibaldi, G. Giorgianni and A. Carlone, Catalysts, 2021, 12, 5 CrossRef; (k) J. Wang, L. Xiang, X. Liu, A. Matler, Z. Lin and Q. Ye, Chem. Sci., 2024, 15(13), 4839–4845 RSC; (l) C. Zhang, X. Liu, J. Wang and Q. Ye, Angew. Chem., Int. Ed., 2022, 61, 36 Search PubMed; (m) C. Zhang, J. Wang, W. Su, Z. Lin and Q. Ye, J. Am. Chem. Soc., 2021, 143, 8552–8558 CrossRef; (n) L. Xiang, J. Wang, I. Krummenacher, K. Radacki, H. Braunschweig, Z. Lin and Q. Ye, Chem. – Eur. J., 2023, 29, e202301270 CrossRef PubMed; (o) J. Kahlert, L. Böhling, A. Brockhinke, H.-G. Stammler, B. Neumann, L. M. Rendina, P. J. Low, L. Weber and M. A. Fox, Dalton Trans., 2015, 44, 9766–9781 RSC; (p) S. Yruegas, J. C. Axtell, K. O. Kirlikovali, A. M. Spokoyny and C. D. Martin, Chem. Commun., 2019, 55, 2892–2895 RSC; (q) M. Diab, K. Jaiswal, D. Bawari and R. Dobrovetsky, Isr. J. Chem., 2023, 63, e202300010 CrossRef; (r) C. Zhang, J. Wang, Z. Lin and Q. Ye, Inorg. Chem., 2022, 61(45), 18275–18284 CrossRef; (s) L. Xiang, J. Wang, I. Krummenacher, K. Radacki, H. Braunschweig, Z. Lin and Q. Ye, Chem. – Eur. J., 2023, 29, e202301270 CrossRef PubMed; (t) H. Wang and Z. Xie, Organometallics, 2021, 40(22), 3819–3824 CrossRef; (u) C. Dai, Y. Huang and J. Zhu, Organometallics, 2022, 41(12), 1480–1487 CrossRef; (v) M. Faizan, D. Behera, M. Chakraborty and R. Pawar, ChemPhysChem, 2024, 25, e202400647 CrossRef; (w) L. Zhang and Z. Xie, Chem. Sci., 2021, 12, 1745–1749 RSC; (x) M. Zhong, J. Zhang and Z. Xie, Chem. Sci., 2024, 15, 18048–18051 RSC.
- (a) S. J. Geier, C. M. Vogels, J. A. Melanson and S. A. Westcott, Chem. Soc. Rev., 2022, 51, 8877–8922 RSC; (b) M. Haberberger and S. Enthaler, Chem. – Asian J., 2013, 8, 50–54 CrossRef PubMed; (c) H. Wadepohl, U. Arnold, U. Kohl, H. Pritzkow and A. Wolf, J. Chem. Soc. Dalton Trans., 2000, 20, 3554–3565 RSC; (d) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179–1191 CrossRef; (e) D. Männig and H. Nöth, Angew. Chem., Int. Ed. Engl., 1985, 24, 878–879 CrossRef.
- (a) D. Hemming, R. Fritzemeier, S. A. Westcott, W. L. Santos and P. G. Steel, Chem. Soc. Rev., 2018, 47, 7477–7494 RSC; (b) M. Zhong, Y. Gagné, T. O. Hope, X. Pannecoucke, M. Frenette, P. Jubault and T. Poisson, Angew. Chem., 2021, 133, 14619–14624 CrossRef; (c) I. G. R. Juliani Costa, P. R. Batista, M. T. de Oliveira and A. A. C. Braga, ACS Org. Inorg. Au, 2025, 5, 181–193 CrossRef PubMed; (d) Q. Wang, S. E. Motika, N. G. Akhmedov, J. L. Petersen and X. Shi, Angew. Chem., Int. Ed, 2014, 53, 5418–5422 CrossRef; (e) G. Wang, Y. Wang, Z. Li, H. Li, M. Yu, M. Pang and X. Zhao, Org. Lett., 2022, 24, 9425–9430 CrossRef.
- (a) E. A. Romero, R. Jazzar and G. Bertrand, J. Organomet. Chem., 2017, 829, 11–13 CrossRef; (b) X. Liu, W. Ming, A. Friedrich, F. Kerner and T. B. Marder, Angew. Chem., Int. Ed., 2020, 59, 304–309 CrossRef PubMed; (c) Y. Gao, Z. Q. Wu and K. M. Engle, Org. Lett., 2020, 22(13), 5235–5239 CrossRef PubMed.
- J. V. Obligacion, J. M. Neely, A. N. Yazdani, I. Pappas and P. J. Chirik, J. Am. Chem. Soc., 2015, 137, 5855–5858 CrossRef PubMed.
- (a) A. Amgoune and D. Bourissou, Chem. Commun., 2010, 47, 859–871 RSC; (b) H. Braunschweig and R. D. Dewhurst, Dalton Trans., 2010, 40, 549–558 RSC; (c) J. S. Jones and F. P. Gabbaï, Acc. Chem. Res., 2016, 49, 857–867 CrossRef; J. S. Jones, C. R. Wade and F. P. Gabbaï, Angew. Chem., Int. Ed., 2014, 53, 8876–8879 Search PubMed; (d) H. Braunschweig and M. Colling, Coord. Chem. Rev., 2001, 223, 1–51 CrossRef; (e) W. Huynh, J. W. Taylor, W. H. Harman and M. P. Conley, Dalton Trans., 2021, 50, 14855–14863 RSC; (f) J. W. Taylor, A. McSkimming, M.-E. Moret and W. H. Harman, Angew. Chem., Int. Ed., 2017, 56, 10413–10417 CrossRef PubMed; (g) H. Kameo and H. Nakazawa, Chem. – Asian J., 2013, 8, 1720–1734 CrossRef CAS PubMed; (h) F. Krämer, M. Radius, H. Berberich, I. Fernández and F. Breher, Chem. Commun., 2022, 58, 3905–3908 RSC; (i) F.-G. Fontaine, J. Boudreau and M.-H. Thibault, Eur. J. Inorg. Chem., 2008, 5439–5454 CrossRef CAS; (j) C. R. Wade, B. L. Murphy, S. Bedajna and F. P. Gabbaï, Organometallics, 2024, 43, 1785–1788 CrossRef CAS PubMed; Y.-H. Lo and F. P. Gabbaï, Angew. Chem., Int. Ed., 2019, 58, 10194–10197 Search PubMed; (k) D. You, H. Yang, S. Sen and F. P. Gabbaï, J. Am. Chem. Soc., 2018, 140, 9644–9651 CrossRef CAS PubMed; (l) G. Bouhadir and D. Bourissou, Chem. Soc. Rev., 2016, 45, 1065–1079 RSC; (m) G. Bouhadir and D. Bourissou, Chem. Soc. Rev., 2016, 45, 1065–1079 RSC.
- M. O. Akram, J. Tidwell, J. L. Dutton and C. D. Martin, Angew. Chem., Int. Ed., 2023, 62, e202307040 CrossRef CAS PubMed.
- (a) M. Eaton, Y. Zhang and S.-Y. Liu, Chem. Soc. Rev., 2024, 53, 1915–1935 RSC; (b) Y. Li, Y.-H. Shen, A. M. Esper, J. R. Tidwell, A. S. Veige and C. D. Martin, Dalton Trans., 2023, 52, 668–674 RSC; (c) A. Simonneau, F. Jaroschik, D. Lesage, M. Karanik, R. Guillot, M. Malacria, J.-C. Tabet, J.-P. Goddard, L. Fensterbank, V. Gandon and Y. Gimbert, Chem. Sci., 2011, 2, 2417–2422 RSC; (d) S. Bontemps, G. Bouhadir, K. Miqueu and D. Bourissou, J. Am. Chem. Soc., 2006, 128, 12056–12057 CrossRef CAS PubMed; (e) A. Ueno, K. Watanabe, C. G. Daniliuc, G. Kehr and G. Erker, Chem. Commun., 2019, 55, 4367–4370 RSC; (f) D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400–6441 CrossRef CAS.
- (a) N. A. Phillips, R. Y. Kong, A. J. P. White and M. R. Crimmin, Angew. Chem., Int. Ed., 2021, 60, 12013–12019 CrossRef CAS; (b) T. A. Bartholome, A. Kaur, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Angew. Chem., Int. Ed., 2020, 59, 11470 CrossRef CAS PubMed; (c) G. K. Wisofsky, K. Rojales, X. Su, T. A. Bartholome, A. Molino, A. Kaur, D. J. D. Wilson, J. L. Dutton and C. D. Martin, Inorg. Chem., 2021, 60(24), 18981–18989 CrossRef CAS PubMed.
- (a) M. O. Akram, K. A. French, K. L. Shuford and C. D. Martin, J. Am. Chem. Soc., 2025, 147, 33120–33126 CrossRef CAS PubMed; (b) M. Pilz, J. Allwohn, P. Willershausen, W. Massa and A. Berndt, Angew. Chem., Int. Ed. Engl., 1990, 29, 1030–1032 CrossRef; (c) R. Bertermann, H. Braunschweig, C. K. L. Brown, A. Damme, R. D. Dewhurst, C. Hörl, T. Kramer, I. Krummenacher, B. Pfaffinger and K. Radacki, Chem. Commun., 2014, 50, 97–99 RSC; (d) C. Kojima, K.-H. Lee, Z. Lin and M. Yamashita, J. Am. Chem. Soc., 2016, 138, 6662–6669 CrossRef CAS PubMed.
- A. Suzuki, L. Wu, Z. Lin and M. Yamashita, Angew. Chem., Int. Ed., 2021, 60, 21007–21013 CrossRef CAS PubMed.
- A. Begum, M. O. Akram and C. D. Martin, Dalton Trans., 2025, 54, 5664–5667 RSC.
- J. Wen, Y. Huang, Y. Zhang, H. Grützmacher and P. Hu, Nat. Commun., 2024, 15, 2208 CrossRef CAS PubMed.
- (a) S. Díez-González, Adv. Organomet. Chem., 2016, 66, 93–146 CrossRef; (b) B. Ranieri, I. Escofet and A. M. Echavarren, Org. Biomol. Chem., 2015, 13(26), 7103–7118 RSC; (c) I. Meana, P. Espinet and A. C. Albéniz, Organometallics, 2014, 33(1), 1–7 CrossRef CAS; (d) L. Zhang, W. Zhang, D. Li, R. Yang and Z. Xia, iScience, 2023, 27(1), 108531 CrossRef PubMed; (e) D. V. Partyka, M. Zeller, A. D. Hunter and T. G. Gray, Angew. Chem., Int. Ed., 2006, 45, 8188–8191 CrossRef CAS PubMed.
- (a) CCDC 2491533: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2pmn11; (b) CCDC 2491534: Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2pmn22.
| This journal is © The Royal Society of Chemistry 2026 |