Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid synthesis of SrRuO3 using supercritical water fluid with improved oxygen evolution activity
Yumeng Lia,
Takaaki Toriyamab,
Tomokazu Yamamotob,
Yasukazu Murakamibc,
Hirotaka Ashitanid,
Shogo Kawaguchi d,
Yoshiki Kubotae,
Hiroshi Kitagawa
d,
Yoshiki Kubotae,
Hiroshi Kitagawa *a and
Kohei Kusada
*a and
Kohei Kusada *afgh
*afgh
aDivision of Chemistry, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan. E-mail: kitagawa@kuchem.kyoto-u.ac.jp; kusada.kohei.236@m.kyushu-u.ac.jp
bThe Ultramicroscopy Research Center, Kyushu University, Fukuoka 819-0395, Japan
cDepartment of Applied Quantum Physics and Nuclear Engineering, Graduate School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
dJapan Synchrotron Radiation Research Institute (JASRI), Hyogo 679-5198, Japan
eDepartment of Physics, Graduate School of Science, Osaka Metropolitan University, Osaka 558-8585, Japan
fThe HAKUBI Center for Advanced Research, Kyoto University, Kyoto 606-8502, Japan
gInstitute of Advanced Study, Kyushu University, Fukuoka 816-8580, Japan
hFaculty of Engineering Sciences, Kyushu University, Fukuoka 816-8580, Japan
First published on 23rd December 2025
Abstract
Perovskite oxides are typically synthesised using hydrothermal and sol–gel methods, which require long reaction times and can result in broadened nucleation. Herein, we report a rapid, one-step facile synthesis of SrRuO3 using supercritical water fluid that offers improved oxygen evolution reaction activity compared with the conventional batch hydrothermal method.
The development of rapid, controllable, and scalable synthesis routes for metal oxides remains challenging in materials chemistry. Conventional sol–gel and hydrothermal methods typically require hours to days at elevated temperatures and sometimes harsh alkaline conditions.1–3 Hydrothermal batch synthesis, in particular, exhibits broadened nucleation and growth owing to long heat-up and cool-down times.4 Moreover, post-synthetic calcination is typically required for both methods to improve crystallinity.2,3
Supercritical-water-flow synthesis provides a fundamentally different synthesis method. When water is heated above its critical point (374 °C, 22.1 MPa), its dielectric constant decreases, whereas its diffusivity and ionic product increase. This results in an exceptionally reactive medium.4–6 In a continuous-flow reactor, cold precursor solutions combine rapidly with supercritical water, while the abrupt change in solvent properties promotes near-instantaneous supersaturation, nucleation, and crystallisation within a residence time of only a few seconds.4,7,8 While other rapid synthesis approaches such as microwave-assisted and solution-combustion methods can reduce reaction times to the order of minutes or seconds, they often encounter issues such as non-uniform heating and difficulty in controlling reaction temperature.9,10 In contrast, supercritical-flow offers precise temperature and mixing control through the flow rate and reactor geometry, thus ensuring reproducibility and scalability.4,11,12 Additionally, its continuous operation enables a straightforward scaling up by merely increasing the amount of the precursor solution. Beyond speed and scalability, the non-equilibrium reaction conditions of this process impart qualities that are not typically accessible in slow, conventional syntheses. Supercritical flow can yield materials with subtle structural or chemical features that differ from those synthesised through gradual thermal growth, including possible variations in oxidation state and oxygen content.4 These non-equilibrium effects of supercritical water highlight that this method not only crystallises oxides rapidly but also enables access to metastable states and structures that can offer functional advantages.
Supercritical-water-flow synthesis is a promising method for synthesising perovskite oxides, whose flexible framework readily accommodates diverse cation combinations and oxygen stoichiometries.13 Among their many applications (e.g. electrocatalysis and spintronics), we focus on oxygen evolution reaction (OER) catalysis, i.e. the rate-limiting step in water electrolysis,14 where the tunable electronic structure and oxygen vacancy of perovskites are relevant.15 As a model system, we selected strontium ruthenate (SrRuO3) owing to its relatively high theoretically predicted OER activeness and structural flexibility,16,17 which render it a natural platform for investigating possible differences in electronic structure and electrochemical performance between supercritical conditions with rapid crystallisation and the conventional batch-hydrothermal synthesis. Additionally, SrRuO3 cannot be easily synthesised via the hydrothermal method and reports regarding its batch synthesis are scarce. Herein, we report the first successful synthesis of crystalline SrRuO3 under supercritical water flow, which yielded nanocrystals with distinct OER activity compared with batch-hydrothermal analogues.
SrRuO3 was synthesised via both the supercritical-water-flow (f-SrRuO3) and batch-hydrothermal (b-SrRuO3) methods. For the flow synthesis, 50 mL of a metal precursor solution was prepared by dissolving 0.42 mmol each of RuCl3·xH2O and Sr(NO3)2 in deionised water, while another 50 mL of 0.3 M NaOH solution was prepared separately. During the operation of the flow reactor, deionised water was pumped at a rate of 80 mL min−1, pressurised to 25 MPa, and heated to 376 °C to generate supercritical water. The precursor and NaOH solutions were fed into the reactor at a rate of 5 mL min−1 and mixed with the supercritical stream (Fig. S1). Nucleation and crystallisation occurred during the flow from the mixer to the chiller prior to being quenched in the chiller. The resulting suspension was collected and then the precipitates were separated via centrifugation and washed with deionised water. Subsequently, they were vacuum dried and then treated with 0.02 M HCl to remove impurities, followed by rewashing with deionised water and vacuum drying to obtain f-SrRuO3. For batch-hydrothermal synthesis, 0.42 mmol of each of KRuO4 and Sr(NO3)2 were dissolved in 50 mL of saturated NaOH solution and sealed in a Teflon-lined autoclave. Subsequently, the mixture was heated to 175 °C at 2 °C min−1 and reacted for 24 h. The product was collected, washed with deionised water, and vacuum dried to obtain b-SrRuO3. Average yield for supercritical flow synthesis is 13 mg before HCl washing and 3 mg after washing, while the typical average yield is 6 mg for batch synthesis.
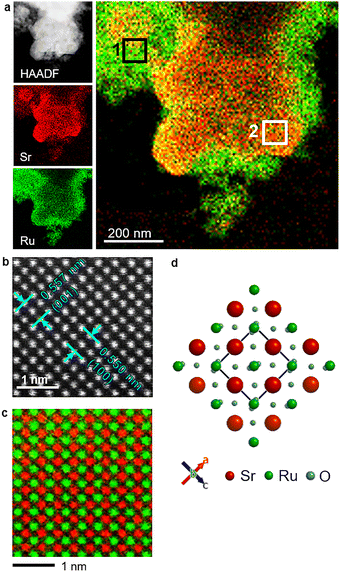
The phase purity and crystallographic structure of the as-synthesised samples were verified via powder X-ray diffraction (XRD). XRD provided direct evidence of the crystal structure as well as quantitative lattice parameters using Rietveld refinement. The diffraction patterns of both f-SrRuO3 and b-SrRuO3 were indexed to the orthorhombic perovskite structure of SrRuO3 (COD #96-153-3614), as shown in Fig. S2, thus indicating that both have adopted the expected perovskite phase while TEM images in Fig. S3 shows the agglomerated particles. Rietveld refinement of the obtained patterns (Fig. 1) further revealed subtle lattice distortions. Compared with the values reported for bulk SrRuO3 (a = 5.5684 Å, b = 7.8452 Å, and c = 5.5320 Å),18 f-SrRuO3 showed a slight contraction in the length of a axis and increase in length of b and c axis while, b-SrRuO3 exhibited larger length values in all three a, b and c axes. Such anisotropic changes may have been induced by the different synthesis methods used (calcination and sintering in Bansal et al.'s study18 vs. batch hydrothermal and flow synthesis without calcination in our study). To complement these bulk structural results, the microstructure and elemental distribution were examined using scanning transmission electron microscopy (STEM) coupled with energy-dispersive X-ray spectroscopy (STEM-EDX). Elemental maps of Sr and Ru show that both f-SrRuO3 and b-SrRuO3 (Fig. 2a and Fig. S4) contain two distinguishable regions: a uniform crystalline region containing both Sr and Ru, which corresponds to SrRuO3, and a Ru-rich amorphous region. The Ru-rich region is attributed to a minor impurity phase, which may partially originate from non-stoichiometric (hydro)oxides, as it contains both Sr and Ru (Table S1). Atomic-resolution high-angle annular dark-field (HAADF) STEM imaging and EDX mapping of f-SrRuO3 (Fig. 2b and c) provided confirmation at the atomic scale of the perovskite arrangement. The image showed the expected sublattices composed of Sr and Ru, respectively and is consistent with the ideal SrRuO3 structure viewed along the [010] direction (Fig. 2d). For the crystalline region, the lattice spacings of the (001) and (100) planes (0.557 and 0.550 nm, respectively) were extracted from the HAADF image in Fig. 2b, and the values were in good agreement with those obtained from Rietveld refinement. This indicates that the local structure imaged via HAADF is consistent with the long-range atomic order confirmed by Rietveld refinement, thus confirming the formation of a well-ordered perovskite lattice, even within the sub-second crystallisation timeframe of the supercritical-flow synthesis.
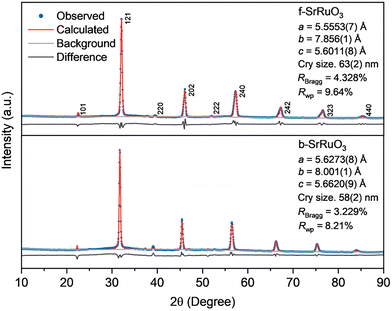 | ||
| Fig. 1 XRD patterns and fitting results by Rietveld refinement of f-SrRuO3 and b-SrRuO3 (λ = 1.54 Å). | ||
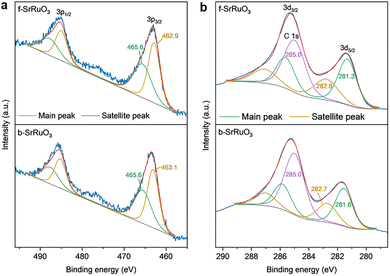
To analyse the electronic states of Sr and Ru, X-ray photoelectron spectroscopy (XPS) was performed for b-SrRuO3 and f-SrRuO3 and the XPS spectra were calibrated by setting the C 1s peak at 285.0 eV.19 The Sr 3d XPS result (Fig. S5) showed that both samples were in the Sr2+ state with peak positions (Table S2) similar to previously reported values of 132.2-133.7 eV.20 In the Ru 3p spectra for b-SrRuO3 and f-SrRuO3 (Fig. 3a), the peak position of 3p3/2 for b-SrRuO3 at 463.3 eV was similar to the previously reported value of the main peak, i.e. approximately 463.2 eV,20,21 and was consistent with the Ru4+ oxidation-state characteristic of stoichiometric SrRuO3. However, the peak position for f-SrRuO3 is downshifted by 0.4 eV, thus indicating a slightly reduced state from Ru4+. Measurements of the Ru 3d region reinforced this observation. In the Ru 3d measurement, a slight upshift of 0.3 eV was observed in the 3d5/2 peak for b-SrRuO3 when compared with the previously reported value of approximately 281.3 eV,22,23 whereas f-SrRuO3 has a similar value (Fig. 3b). However, when the peak positions of f-SrRuO3 and b-SrRuO3 were compared, a downshift in the peak positions of f-SrRuO3 was again observed. This result reaffirms the possibility of partial Ru reduction in f-SrRuO3. One possible reason for this is oxygen deficiency in the perovskite phase. Another possibility for the charge state reduction is the presence of non-stoichiometric (hydro)oxides that might be present in the amorphous region, as observed in STEM, which might favour the partial reduction of Ru from stoichiometric Ru4+.
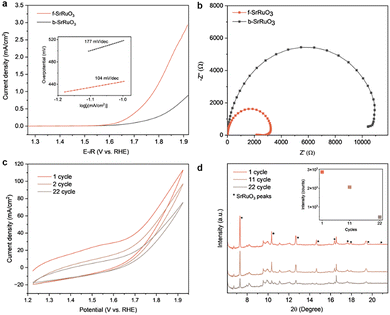
Having confirmed the structural integrity and oxidation state of f-SrRuO3 and b-SrRuO3, OER measurements were performed on both samples to evaluate whether the rapidly synthesised f-SrRuO3 maintained a similar OER activity to that of b-SrRuO3, which was synthesised via a more conventional route. All measurements were performed in 1 M KOH using a standard three-electrode system (details of the procedure in SI). Linear sweep voltammetry (LSV) revealed a pronounced activity enhancement for f-SrRuO3, where it delivered a current density and a mass activity approximately three times higher than that of b-SrRuO3 at the highest measured potential of 1.92 V vs. RHE (Fig. 4a and Fig. S8). Additionally, the corresponding Tafel slope of 104 mV/dec was smaller than that of the batch-synthesised sample (177 mV dec−1), thus indicating that f-SrRuO3 was more responsive to changes in the applied potential. The electrochemical impedance (EIS) data further support these findings. The plot presented in Fig. 4b shows that f-SrRuO3 possesses a smaller charge-transfer resistance (Rct) (Table S4) than b-SrRuO3. As the surface area used can affect the apparent current density, we further normalised the LSV curves to the electrochemically active surface area (ECSA), which was determined from the double-layer capacitance obtained via cyclic voltammetry (CV) at various scan rates (Fig. S6). As shown in Fig. S7, even after this normalisation, f-SrRuO3 retained a higher current density than that by b-SrRuO3, thus indicating a higher intrinsic activity of the catalytically active sites in f-SrRuO3.This result combined with the larger calculated ECSA for f-SrRuO3 (Table S4) suggests that the enhanced OER current density of f-SrRuO3 arises from a dual contribution: a higher intrinsic activity of the active sites and an increased density of accessible active sites. One possible contributor to this increase in catalytic activity may be the change in the electronic structure of Ru, which can increase the electrical conductivity and exposure of active sites in perovskite oxides, as previously reported in the literature.15,24 Specifically, prior studies on Ru-based oxides such as RuO2 have shown that a reduction in Ru oxidation state can enhance *OOH adsorption and decrease the energy barrier for the rate-determining *O to *OOH step and thereby lower the OER overpotential.25 The slightly reduced Ru in f-SrRuO3 may have induced analogous electronic effects that promoted *OOH formation, and thus provide a possible explanation for its enhanced OER activity. Another possibility is that the minor impurity phase could have had a positive effect on OER activity. However, as the impurity content is low, the main contributor to the enhanced OER activity is still likely SrRuO3. The durability of the catalysts was investigated via cyclic voltammetry (CV) cycling. Fig. S9 shows that both b-SrRuO3 and f-SrRuO3 exhibited a gradual decrease in current density upon repeated cycling. However, the decline was less pronounced for f-SrRuO3, thus indicating the slightly improved stability of f-SrRuO3 in alkaline OER conditions. To investigate the origin of the activity loss, operando XRD measurement during the CV cycling of f-SrRuO3 was conducted at SPring-8 BL13XU (Fig. S10).26 The results were consistent with those of the stability test, and the current density decreased progressively with increasing cycling (Fig. 4c). The simultaneously recorded XRD patterns revealed that the characteristic peaks of SrRuO3 decreased steadily and was almost undetectable after 22 cycles, indicating a progressive structural breakdown (Fig. 4d). STEM-EDX map of the post-cycled electrode catalyst (from the stability test) has also provided support of compositional change (Fig. S11). Regions containing only Sr were detected and implies the dissolution of Ru from the perovskite structure. This observation suggests that the decrease in current density is due to Ru dissolution, which is consistent with previous studies linking the limited alkaline stability of SrRuO3 to Ru leaching.15,27 Some possible strategies to improve SrRuO3 stability would be A- and/or B-site doping. A-site substitution can modify lattice structure, oxygen vacancy content and B-site oxidation states to improve stability28 while B-site doping directly tune M–O covalency, mitigating a stability limitation of ABO3 perovskites.29 Collectively, these approaches could provide a rational basis for stabilizing SrRuO3 under OER conditions.
In summary, we demonstrated the rapid synthesis of SrRuO3 via supercritical-water-flow synthesis with a crystallisation time of less than 1 s and under much milder alkaline conditions compared with those of batch-hydrothermal synthesis. The resulting f-SrRuO3 sample was crystalline with slightly reduced Ru species, as inferred from XPS measurements. Electrochemical measurements further showed that f-SrRuO3 exhibited higher OER activity than the batch-hydrothermal sample. These results indicate that supercritical water flow synthesis is a viable method for preparing perovskite oxides, thus facilitating the synthesis of perovskites customised to different reactions by varying the metal combinations.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc06643c.Acknowledgements
We acknowledge support from JST SPRING grant (number JPMJSP2110), JSPS KAKENHI grant (numbers JP25K01622, JP20H05623, JP25H01669, and 25H00804), JST FOREST JPMJFR221P and the ENEOS Hydrogen Trust Fund. This work was partially supported by the Demonstration Project of Innovative Catalyst Technology for Decarbonization through Regional Resource Recycling, Ministry of the Environment, Government of Japan, and Kyushu University Inamori Frontier Program. Synchrotron XRD measurements were performed at beamline BL13XU in SPring-8 under proposal number 2024B2058. STEM analyses were supported by Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (Proposal Numbers JPMXP12225KU0003 and JPMXP12225KU0004).References
- T. Ye, Z. Dong, Y. Zhao, J. Yu, F. Wang, L. Zhang and Y. Zou, Dalton Trans., 2011, 40, 2601–2606 RSC.
- X. Xue and B. Li, Nanomaterials, 2025, 15, 472 CrossRef CAS.
- E. Danks, S. R. Hall and Z. Schnepp, Mater. Horiz., 2016, 3, 91–112 RSC.
- J. A. Darr, J. Zhang, N. M. Makwana and X. Weng, Chem. Rev., 2017, 117, 11125–11238 CrossRef CAS.
- J. Becker, P. Hald, M. Bremholm, J. S. Pedersen, J. Chevallier, S. B. Iversen and B. B. Iversen, ACS Nano, 2008, 2, 1058–1068 CrossRef CAS PubMed.
- T. Adschiri, Y.-W. Lee, M. Goto and S. Takami, Green Chem., 2011, 13, 1380–1390 RSC.
- S. Hanabata, K. Kusada, T. Yamamoto, T. Toriyama, S. Matsumura, S. Kawaguchi, Y. Kubota, Y. Nishida, M. Haneda and H. Kitagawa, J. Am. Chem. Soc., 2024, 146, 181–186 CrossRef CAS PubMed.
- Q. Li, L. Liu, Z. Wang and X. Wang, Nanomaterials, 2022, 12, 668 Search PubMed.
- T. Sajini and J. Joseph, RSC Sustainability, 2025, 3, 4911–4935 RSC.
- A. Varma, A. S. Mukasyan, A. S. Rogachev and K. V. Manukyan, Chem. Rev., 2016, 116, 14493–14586 CrossRef CAS PubMed.
- A. A. Chaudhry, S. Haque, S. Kellici, P. Boldrin, I. Rehman, F. A. Khalid and J. A. Darr, Chem. Commun., 2006, 2286–2288 RSC.
- D. Malarde, I. D. Johnson, I. J. Godfrey, M. J. Powell, G. Cibin, R. Quesada-Cabrera, J. A. Darr, C. J. Carmalt, G. Sankar, I. P. Parkin and R. G. Palgrave, J. Mater. Chem. C, 2018, 6, 11731–11739 RSC.
- L.-B. Liu, C. Yi, H.-C. Mi, S. L. Zhang, X.-Z. Fu, J.-L. Luo and S. Liu, Electrochem. Energy Rev., 2024, 7, 14 CrossRef CAS PubMed.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- J.-W. Zhao, Y. Li, D. Luan and X. W. (David) Lou, Sci. Adv., 2024, 10, eadq4696 CrossRef CAS.
- I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- M. Cuoco and A. Di Bernardo, APL Mater., 2022, 10, 090902 CrossRef CAS.
- C. Bansal, H. Kawanaka, R. Takahashi and Y. Nishihara, J. Alloys Compd., 2003, 360, 47–53 CrossRef CAS.
- P. Swift, Surf. Interface Anal., 1982, 4, 47–51 CrossRef CAS.
- Q. Li, D. Wang, Q. Lu, T. Meng, M. Yan, L. Fan, Z. Xing and X. Yang, Small, 2020, 16, 1906380 CrossRef CAS.
- Y. K. Wakabayashi, S. Kaneta-Takada, Y. Krockenberger, K. Takiguchi, S. Ohya, M. Tanaka, Y. Taniyasu and H. Yamamoto, AIP Adv., 2021, 11, 035226 CrossRef.
- I. Rodríguez-García, D. Galyamin, L. Pascual, P. Ferrer, M. A. Peña, D. Grinter, G. Held, M. Abdel Salam, M. Mokhtar, K. Narasimharao, M. Retuerto and S. Rojas, J. Power Sources, 2022, 521, 230950 CrossRef.
- S. A. Lee, S. Oh, J.-Y. Hwang, M. Choi, C. Youn, J. W. Kim, S. H. Chang, S. Woo, J.-S. Bae, S. Park, Y.-M. Kim, S. Lee, T. Choi, S. W. Kim and W. S. Choi, Energy Environ. Sci., 2017, 10, 924–930 RSC.
- H. Arandiyan, S. S. Mofarah, Y. Wang, C. Cazorla, D. Jampaiah, M. Garbrecht, K. Wilson, A. F. Lee, C. Zhao and T. Maschmeyer, Chem. – Eur. J., 2021, 27, 14418–14426 CrossRef PubMed.
- C. Zhou, L. Li, Z. Dong, F. Lv, H. Guo, K. Wang, M. Li, Z. Qian, N. Ye, Z. Lin, M. Luo and S. Guo, Nat. Commun., 2024, 15, 9774 CrossRef.
- S. Kawaguchi, S. Kobayashi, H. Yamada, H. Ashitani, M. Takemoto, Y. Imai, T. Hatsui, K. Sugimoto and O. Sakata, J. Synchrotron Radiat., 2024, 31, 955–967 CrossRef PubMed.
- S. H. Chang, N. Danilovic, K.-C. Chang, R. Subbaraman, A. P. Paulikas, D. D. Fong, M. J. Highland, P. M. Baldo, V. R. Stamenkovic, J. W. Freeland, J. A. Eastman and N. M. Markovic, Nat. Commun., 2014, 5, 4191 CrossRef.
- Y. Liu, H. Huang, L. Xue, J. Sun, X. Wang, P. Xiong and J. Zhu, Nanoscale, 2021, 13, 19840–19856 RSC.
- R. Xiao, Y. Liu, X. Jiang, C. Xing, X. Qi, X. Wang and A. Cabot, Chem. Eng. J., 2025, 525, 169987 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |