Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Covalently linked rotaxane encapsulation of a benzil core for consistent organic room-temperature phosphorescence across diverse solvents
Sotaro Shimadaa,
Yoichi Masui *a,
Hiromichi V. Miyagishi†
*a,
Hiromichi V. Miyagishi†
 a,
Susumu Tsuda
a,
Susumu Tsuda b,
Hiroshi Masai‡
b,
Hiroshi Masai‡
 ac and
Jun Terao
ac and
Jun Terao *a
*a
aGraduate School of Arts and Sciences, The University of Tokyo 3-8-1, Komaba, Meguro-ku, 153-8902, Tokyo, Japan. E-mail: cterao@g.ecc.u-tokyo.ac.jp
bDepartment of Chemistry, Osaka Dental University Hirakata, Osaka 573–1121, Japan
cPRESTO, Japan Science and Technology Agency 4-1-8, Honcho, Kawaguchi, Saitama, 332-0012, Japan
First published on 6th January 2026
Abstract
We achieved room-temperature phosphorescence (RTP) of benzil, an aromatic diketone, in diverse solvents, as well as in amorphous solid states, by encapsulating the core with covalently linked cyclodextrins and introducing bulky capping moieties to effectively suppress intramolecular motions.
Phosphorescence, characterized by its long-lived emission, has attracted significant interest for applications, such as in organic electroluminescence devices1a,b and bioimaging.2a,b While the presence of oxygen typically quenches phosphorescence and is therefore undesirable for emission applications, this sensitivity can be exploited in some cases for oxygen sensing.3a,b Thus, phosphorescent materials present a wide range of potential applications, but a common challenge associated with them is achieving sufficiently high emission efficiency.
One possible approach is to facilitate intersystem crossing (ISC), which is often achieved by introducing heavy atoms that enhance spin–orbit coupling.4 However, these approaches raise concerns about cost and environmental impact, motivating alternative strategies. Benzil and benzophenone are classic heavy-atom-free organic phosphors.5a–e In particular, benzil, an aromatic diketone, is known to show weak room-temperature phosphorescence (RTP) in carefully deoxygenated solutions,6 due to the ISC facilitated by its adjacent carbonyl groups. However, organic phosphorescence in solution is susceptible to nonradiative deactivation through intramolecular motion because the emission lifetime is longer than fluorescence.7 Strategies, such as cooling to liquid-nitrogen temperature,8a,b crystallization, amorphization to restrict intramolecular rotation,9a–d and embedding in polymer matrices10a–c can enhance phosphorescence, but suppressing intramolecular motion in solution remains challenging. In solid-state systems, homopolymers or copolymers possessing a high density of functional units, typically aromatic backbones, are regarded as promising candidates for thin-film and coating applications.11a,b However, in such cases, another challenge emerges: close packing of the aromatic moieties enhances π–π interactions, promoting nonradiative deactivation and reducing emission efficiency.12
Encapsulating an emissive unit within a well-defined cage is an effective strategy to suppress intramolecular motion and thereby reduce nonradiative decay. Rebek Jr. and co-workers demonstrated that a dimeric capsule formed via hydrogen bonding between two tetraimide units in nonpolar solvents could encapsulate benzil and exhibit RTP in mesitylene13 (Fig. 1A, a → b). However, these hydrogen-bonded cages may dissociate in polar media, thereby limiting their broad applicability.
 | ||
| Fig. 1 Methods of realizing organic RTP in (A) previous work and (B) this work. (a: unencapsulated structure, b: encapsulated structure without capping, c: encapsulated structure with capping). | ||
Here, we employ the linked rotaxane strategy previously developed by our group14a–c (Fig. 1B). This design suppresses shuttling in the rotaxane structure, which has been used for molecular protection,15,16 thereby enabling precise protection of the emissive core. Furthermore, the directionality of the cyclic molecule can be restricted to a single direction.17 First, we synthesized a non-encapsulated structure X-unenc (a) in which a benzil core was covalently linked to two permethylated α-cyclodextrins (PM α-CDs), which are known to be soluble in a wide range of solvents. Heating this construct in polar solvents such as MeOH/H2O induced a transformation into an encapsulated pseudo-rotaxane X-enc (b). For covering a wider range compared to that when using one CD, two CDs were used, which were linked to benzil for precise protection. As a result, X-enc is expected to exhibit RTP in solvents. However, the conversion between X-unenc and X-enc is known to be reversible,17 with X-enc slowly reverting to X-unenc in nonpolar solvents owing to the influence of solvent polarity. Subsequent end capping with bulky stopper molecules via click reaction yields the permanently locked encapsulated structure X-enc-cap (c). This design ensures that high solubility is maintained in diverse solvents while the encapsulated state of the benzil core is firmly preserved, thereby enabling phosphorescence in multiple solvents. Furthermore, we extended this strategy to polymers via alternating copolymerization with a diazide linker, yielding phosphorescent materials in which each emissive unit was appropriately and permanently encapsulated. Therefore, this linked rotaxane approach provides a rational design concept for suppressing intramolecular motion, offering a promising pathway to organic RTP in both molecular and polymeric systems.
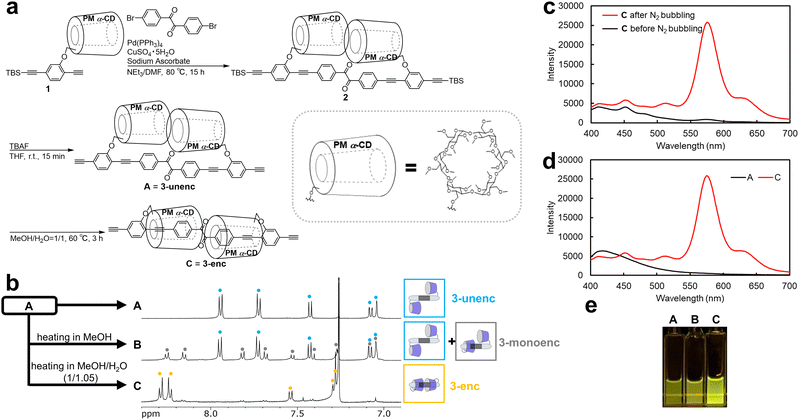
As an initial step, 3-enc and 3-unenc were selected to examine the effect of the rotaxane-type encapsulation on benzil phosphorescence (Fig. 2a). Compound 1 was synthesized according to the literature.18 Sonogashira coupling of 4,4′-dibromobenzil and 1 yielded 2, and subsequent deprotection of the tert-butyldimethylsilyl (TBS) groups yielded A. We have previously found that linked rotaxane precursors initially exist in the unencapsulated state when synthesized in less polar solvents, and convert to the encapsulated state as a thermodynamically stable form upon heating in polar solvents.17 Accordingly, encapsulation was attempted by heating A in MeOH/H2O at 60 °C for 3 h, affording C. 1H nuclear magnetic resonance (NMR) spectroscopy in CDCl3 (Fig. 2b) confirmed that A consisted of a single component, identified as 3-unenc. In contrast, the spectrum of C indicated that 3-unenc had completely disappeared, and the product was composed entirely of 3-enc. Downfield shifts of the aromatic protons in 3-enc compared with 3-unenc suggested the progress of the encapsulation.19 When the encapsulation was carried out in pure MeOH, a less polar condition, the product B was composed mainly of 3-unenc. As these species can be mutually converted and have the same molecular weight, the minor component was assigned as 3-monoenc, in which only one of the CDs is encapsulated, while the other remains unencapsulated.
Subsequently, the emission spectrum of C (3-enc) was examined in methanol. After 30 min of nitrogen bubbling, the sample exhibited a broad band in the 400–550 nm region with peaks at 411, 451, 512 nm, and an intense peak at 573 nm. In contrast, before nitrogen bubbling, only a broad band in the 400–550 nm region was observed and the 573 nm peak was absent (Fig. 2c). The emission of the benzil molecule was attributed to fluorescence at 430 and 505 nm at room temperature and to phosphorescence at 523 nm at 77 K by Bhattacharya et al.20 Therefore, the 573 nm emission can be assigned to phosphorescence, namely RTP. In contrast, the emission spectrum of A obtained under the same conditions showed no phosphorescence peak (Fig. 2d). These results indicate that encapsulation is essential for benzil phosphorescence. The phosphorescence intensities of A, B, and C were also measured in methanol after nitrogen bubbling for 30 min: C showed the highest intensity, while A showed the lowest (Fig. 2e). In summary, while 3-enc was synthesized and shown to exhibit RTP, its solvent-dependent unencapsulation could not be suppressed, highlighting the limitations of this system.
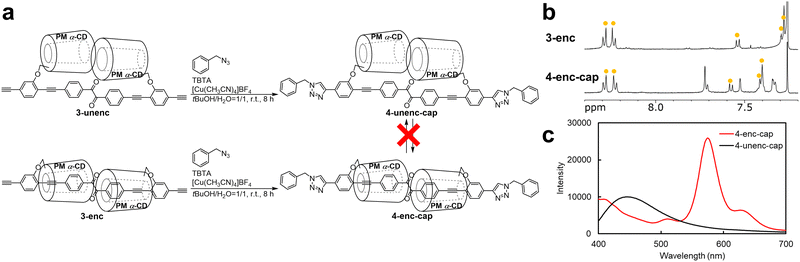
To overcome this limitation, bulky end-capping moieties were introduced into 3-enc and 3-unenc via a click reaction with benzyl azide, affording 4-enc-cap and 4-unenc-cap, respectively (Fig. 3a). The emission properties of 4-enc-cap and 4-unenc-cap were examined in solvents at room temperature. In chloroform, the emission spectrum of 4-enc-cap after nitrogen bubbling for 30 min displayed a peak at 573 nm (Fig. 3b), which disappeared upon subsequent oxygen bubbling, confirming its phosphorescent origin. In contrast, 4-unenc-cap showed no corresponding peak under the same conditions (Fig. 3c). Furthermore, the emission spectra of 4-enc-cap exhibited phosphorescence regardless of the solvent used. In these measurements, the fluorescence maxima red-shifted in polar solvents (Fig. S22), indicating positive solvatochromism arising from the structural relaxation of the excited singlet state.14a In contrast, the phosphorescence maximum wavelength was 575 nm in toluene, 574 nm in chloroform, 573 nm in acetonitrile and 575 nm in dimethyl sulfoxide, remaining nearly constant across solvents. This is likely because the triplet state is less sensitive to solvent polarization.21 In addition, the phosphorescence quantum yield of 4-enc-cap was 4.7% in toluene, 6.8% in chloroform, 5.1% in acetonitrile and 3.8% in dimethyl sulfoxide. In other words, 4-enc-cap exhibited RTP in diverse solvents, including polar solvents, as the end-capping moieties permanently lock the encapsulated structure. In addition, 4-enc-cap exhibited phosphorescence in the amorphous state with a yield of 2.8%. These results confirm that 4-enc-cap retains RTP in both solvents and the amorphous state.
Using this encapsulated structure as a design motif, we synthesized a phosphorescent polymer with a high density of emissive units. Polymers are attractive as practical device materials because of their superior processability and thermodynamic stability compared with low-molecular-weight compounds. Conventional polymers, primarily conjugated, often suffer from strong π–π stacking interactions, which promote aggregation-caused quenching. In contrast, the steric bulk of the encapsulated units effectively suppressed interchain aggregation, allowing the benzil moieties to maintain their phosphorescent properties, even in the condensed phases. Based on this concept, we designed polymer 5-enc as shown in Fig. 4a. The click polymerization between 3-enc/3-unenc and 4,4′-bis(azidomethyl)biphenyl, which was synthesized according to the literature,22 yielded 5-enc/5-unenc. The progress of polymerization was confirmed by size-exclusion chromatography (Fig. S23 and S24). The phosphorescence properties of 5-enc and 5-unenc were examined in solution at room temperature. After nitrogen bubbling for 30 min, a phosphorescence peak at 573 nm was clearly observed only for 5-enc (Fig. 4b), demonstrating that encapsulation was essential for RTP emission. The phosphorescence maximum wavelength of 5-enc closely matched that of 4-enc-cap, suggesting that both species have a comparable conjugation length. The phosphorescence quantum yield of 5-enc was 4.0% in chloroform and 2.9% in the film state, confirming that efficient emission was retained, even in the condensed phase. These results indicate that encapsulation effectively suppresses nonradiative deactivation arising from π–π interactions, highlighting the potential of this polymer as a phosphorescent film, optical information storage material, or security ink.10a–c
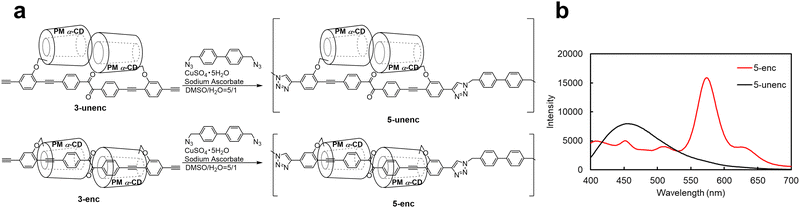 | ||
| Fig. 4 (a) Synthetic procedure of 5-unenc and 5-enc. (b) Emission spectra of 5-enc and 5-unenc in chloroform after nitrogen bubbling for 30 min (excitation: 365 nm). | ||
In conclusion, we have demonstrated an effective strategy for achieving RTP from a benzil core in diverse solvents by using cyclodextrins for encapsulation and introducing capping moieties to lock the encapsulated structure. As a result, the encapsulated and capped benzil (4-enc-cap) exhibited phosphorescence in solvents regardless of their polarity. In addition, this molecule exhibited phosphorescence both in the amorphous state and as a polymer (5-enc). These results demonstrate that the present design concept is effective for realizing organic RTP in multiple physical states, including diverse solvents, and suggest that further molecular designs based on this approach will broaden the range of organic RTP materials.
This work was supported by JSPS Research Fellows, JSPS KAKENHI Grant Numbers 25K08607 and 25H00892, the Toshiaki Ogasawara Memorial Foundation, Murata Science and Education Foundation, the Canon Foundation, and the Uehara Memorial Foundation.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article are included in the supplementary information (SI). The supplementary information includes detailed experimental procedures, characterization data, and additional figures supporting this study. See DOI: https://doi.org/10.1039/d5cc06466j.References
- (a) X. Peng, P. Zou, J. Zeng, X. Wu, D. Xie, Y. Fu, D. Yang, D. Ma, B. Z. Tang and Z. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202405418 CrossRef PubMed; (b) Y. Meng, W. Liu, Z. Liu, M. Gao, M. Fang, J. Yang, D. Ma and Z. Li, ACS Appl. Mater. Interfaces, 2024, 16, 60658 CrossRef PubMed.
- (a) G. Yin, J. Zhou, W. Lu, L. Li, D. Liu, M. Qi, B. Z. Tang, P. Théato and T. Chen, Adv. Mater., 2024, 36, 2311347 CrossRef; (b) Y. Zhao, J. Yang, C. Liang, Z. Wang, Y. Zhang, G. Li, J. Qu, X. Wang, Y. Zhang, P. Sun, J. Shi, B. Tong, H.-Y. Xie, Z. Cai and Y. Dong, Angew. Chem., Int. Ed., 2024, 63, e202317431 CrossRef.
- (a) A. Tomkeviciene, A. Dabulienė, T. Matulaitis, M. Guzauskas, V. Andruleviciene, J. V. Grazulevicius, Y. Yamanaka, Y. Yano and T. Ono, Dyes Pigm., 2019, 170, 107605 CrossRef; (b) H. Kurokawa, H. Ito, M. Inoue, K. Tabata, Y. Sato, K. Yamagata, S. Kizaka-Kondoh, T. Kadonosono, S. Yano, M. Inoue and T. Kamachi, Sci. Rep., 2015, 5, 10657 CrossRef PubMed.
- Y. Tani, K. Miyata, E. Ou, Y. Oshima, M. Komura, M. Terasaki, S. Kimura, T. Ehara, K. Kubo, K. Onda and T. Ogawa, Chem. Sci., 2024, 15, 10784 Search PubMed.
- (a) D. J. Morantz and A. J. C. Wright, J. Chem. Phys., 1971, 54, 692 CrossRef; (b) M. Mukai, S. Yamaguchi and N. Hirota, J. Phys. Chem., 1992, 96, 9328 Search PubMed; (c) K. K. Das and D. Majumdar, J. Mol. Struct., 1993, 288, 55 CrossRef; (d) W. Z. Yuan, X. Y. Shen, H. Zhao, J. W. Y. Lam, L. Tang, P. Lu, C. Wang, Y. Liu, Z. Wang, Q. Zheng, J. Z. Sun, Y. Ma and B. Z. Tang, J. Phys. Chem. C, 2010, 114, 6090 Search PubMed; (e) M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081 RSC.
- L. Flamigni, F. Barigelletti, S. Dellonte and G. Orlandi, J. Photochem., 1983, 21, 237 CrossRef.
- S. Hirata, Adv. Opt. Mater., 2017, 5, 1700116 CrossRef.
- (a) D. B. Clapp, J. Am. Chem. Soc., 1939, 61, 523 CrossRef; (b) D. Olness and H. Sponer, J. Chem. Phys., 1963, 38, 1779 Search PubMed.
- (a) M. S. Kwon, Y. Yu, C. Coburn, A. W. Phillips, K. Chung, A. Shanker, J. Jung, G. Kim, K. Pipe, S. R. Forrest, J. H. Youk, J. Gierschner and J. Kim, Nat. Commun., 2015, 6, 8947 CrossRef PubMed; (b) Y. Gong, Y. Tan, H. Li, Y. Zhang, W. Yuan, Y. Zhang, J. Sun and B. Z. Tang, Sci. China: Chem., 2013, 56, 1183 Search PubMed; (c) D. Li, F. Lu, J. Wang, W. Hu, X. M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916 Search PubMed; (d) L. Bian, H. Shi, X. Wang, K. Ling, H. Ma, M. Li, Z. Cheng, C. Ma, S. Cai, Q. Wu, N. Gan, X. Xu, Z. An and W. Huang, J. Am. Chem. Soc., 2018, 140, 10734 CrossRef PubMed.
- (a) Z. Yin, M. Gu, H. Ma, X. Jiang, J. Zhi, Y. Wang, H. Yang, W. Zhu and Z. An, Angew. Chem., Int. Ed., 2021, 60, 2058 CrossRef; (b) C. Zhou, T. Xie, R. Zhou, C. O. Trindle, Y. Tikman, X. Zhang and G. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 17209 CrossRef CAS PubMed; (c) H. A. Al-Attar and A. P. Monkman, Adv. Funct. Mater., 2012, 22, 3824 CrossRef CAS.
- (a) G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747 Search PubMed; (b) T. Ogoshi, H. Tsuchida, T. Kakuta, T. Yamagishi, A. Taema, T. Ono, M. Sugimoto and M. Mizuno, Adv. Funct. Mater., 2018, 29, 1707369 Search PubMed.
- N. Gan, H. Shi, Z. An and W. Huang, Adv. Funct. Mater., 2018, 28, 1802657 Search PubMed.
- M. R. Ams, D. Ajami, S. L. Craig, J.-S. Yang and J. Rebek, Jr., J. Am. Chem. Soc., 2009, 131, 13190 Search PubMed.
- (a) S. Shimada, H. V. Miyagishi, H. Masai, Y. Masui and J. Terao, Bull. Chem. Soc. Jpn., 2022, 95, 163 Search PubMed; (b) H. V. Miyagishi, H. Masai and J. Terao, Chem. – Asian J., 2020, 15, 1890 CrossRef CAS PubMed; (c) H. V. Miyagishi, H. Masai and J. Terao, Chem. – Eur. J., 2022, 28, e202103175 CrossRef CAS.
- S. Anderson, T. D. W. Claridge and H. L. Anderson, Angew. Chem., Int. Ed. Engl., 1997, 36, 1310 CrossRef CAS.
- M. R. Craig, T. D. W. Claridge, M. G. Hutchings and H. L. Anderson, Chem. Commun., 1999, 1537 RSC.
- H. Masai, J. Terao, T. Fujihara and Y. Tsuji, Chem. – Eur. J., 2016, 22, 6624 CrossRef CAS PubMed.
- S.-Y. Chou, H. Masai, S. Tsuda and J. Terao, Chem. – Asian J., 2019, 14, 1667 CrossRef CAS PubMed.
- T. Fujimoto, Y. Sakata and T. Kaneda, Chem. Commun., 2000, 2143 Search PubMed.
- B. Bhattacharya, B. Jana, D. Bose and N. Chattopadhyay, J. Chem. Phys., 2011, 134, 044535 CrossRef PubMed.
- K. Bhattacharya and M. Chowdhury, J. Photochem., 1986, 33, 61 CrossRef.
- M. Pascu, A. Ruggi, R. Scopelliti and K. Severin, Chem. Commun., 2013, 49, 45 Search PubMed.
Footnotes |
| † Current address: Department of Chemistry, Faculty of Science, Hokkaido University, Kita-10 Nishi-8 Kita-ku, Sapporo 060-0810, Japan. |
| ‡ Current address: Department of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-8654, Japan. |
| This journal is © The Royal Society of Chemistry 2026 |