 Open Access Article
Open Access ArticleSilver clusters embedded within zeolites for innovative (bio)sensing platforms
Cecilia García-Guzmán
 a,
Stefano Cinti
a,
Stefano Cinti
 *bcd,
Eduardo Coutino-Gonzalez
*bcd,
Eduardo Coutino-Gonzalez
 *e and
Eden Morales-Narváez
*e and
Eden Morales-Narváez
 *f
*f
aCentro de Investigaciones en Óptica (CIO), A. C., Loma Del Bosque 115, Lomas Del Campestre, León, 37150, Guanajuato, Mexico
bDepartment of Pharmacy, University of Naples “Federico II”, 80131, Naples, Italy. E-mail: stefano.cinti@unina.it
cSbarro Institute for Cancer Research and Molecular Medicine, Center for Biotechnology, College of Science and Technology, Temple University, Philadelphia, PA 19122, USA
dBioelectronics Task Force at University of Naples Federico II, Via Cinthia 21, Naples, 80126, Italy
eInteruniversity Microelectronics Center (IMEC), Kapeldreef 75, 3001, Leuven, Belgium. E-mail: eduardo.coutinogonzalez@imec.be
fUniversidad Nacional Autónoma de México, Centro de Física Aplicada y Tecnología Avanzada, Querétaro, 76230, Mexico. E-mail: eden@fata.unam.mx
First published on 13th January 2026
Abstract
Silver clusters embedded within zeolites combine the molecular-like optical and electrochemical properties of sub-nanometric clusters with the structural robustness, porosity, and ion-exchange capacity of crystalline frameworks. Their unique features, such as tunable emission with high quantum yields, large Stokes shifts, redox activity, electrocatalytic behavior, and sensitivity to external stimuli, enable versatile sensing capabilities. These composites have already demonstrated remarkable versatility, enabling optical detection of water vapor, volatile amines, oxygen, and biomolecules, as well as electrochemical sensing of hydrogen peroxide, tryptophan, and chloride ions. Their integration into immunosensors, optical hydration switches, and electrochemical paper-based strips highlights their disruptive potential for portable and wearable devices. However, key challenges remain, including controlled nucleation, tailoring excitation–emission properties, and ensuring stability after biorecognition functionalization and device integration. This review discusses the structure–property relationships that govern the optical and electrochemical responses of silver clusters embedded within zeolites, summarizes recent advances in (bio)sensing applications, and outlines the challenges that must be overcome for their translation into scalable, robust, and sustainable devices. With continued progress in material design, surface functionalization, and integration within microfluidic and data analysis platforms, silver cluster–zeolite composites hold strong promise to evolve from laboratory prototypes into portable technologies for diagnostics and environmental monitoring, displaying significant promises for next-generation point-of-care and wearable sensors.
Introduction
Next-generation (bio)sensing platforms are redefining how we detect biomolecules and environmental contaminants, aiming for high stability, sensitivity, and selectivity in cost-effective and sustainable formats. The integration of functional materials with exceptional photophysical and electrochemical properties is central to this progress, which enables conceptually innovative (bio)sensing platforms.1 Among the strategies driving this transformation, silver clusters have emerged as versatile functional materials that combine unique optical and electrochemical properties with tunable chemical interactions.2,3These clusters have enabled innovative sensing approaches that were previously difficult to achieve. For instance, DNA-templated silver clusters can be used to detect cancer-associated miRNAs, such as miRNA-21, in human serum at nanomolar concentrations using fluorescence resonance energy transfer (FRET), offering a minimally invasive route for early disease detection.4 Similarly, polyethyleneimine-stabilized silver clusters allow rapid, colorimetric and fluorometric detection of metabolites such as ascorbic acid directly in urine, demonstrating the potential for point-of-care diagnostics.5 Silver clusters have also been applied in environmental monitoring, achieving micromolar detection limits for metal ions like Cu2+, Ni2+, and Fe2+ in tap and wastewater, providing rapid and practical tools for public health and ecological safety.6 Besides, the integration of silver clusters into portable devices and wearable sensors represents a potential breakthrough in the field of biosensors. To achieve this, new host templates have been explored to enhance stability and facilitate their incorporation into portable devices. Building on these concepts, silver clusters confined into inorganic templates, such as zeolites, have demonstrated remarkable (bio)sensing potential, offering improved stability, high luminescence quantum yields, electrical conductivity, biocompatibility, and facile surface functionalization. Moreover, zeolite-supported silver clusters can respond sensitively to external stimuli, with detectable luminescence changes enabling wash-free, rapid, and cost-effective analyte detection,1,7 positioning zeolite-confined silver clusters on organic and inorganic templates such as DNA, polymers and metal organic frameworks (MOFs), among others.
Compared with other nanomaterials commonly used for (bio)sensing, such as gold nanoparticles8 and semiconductor quantum dots,9 silver clusters stand out for their sub-nanometric dimensions, molecular-like electronic structure, and biocompatibility.10 Their small size minimizes cytotoxicity and enables interaction with biomolecules at the molecular level, while their discrete energy levels confer tunable luminescence and redox properties not attainable in larger nanoparticles.11 Furthermore, when confined within zeolites, these clusters exhibit remarkable stability against aggregation and photobleaching, ensuring long-term operation under physiological and environmental conditions.12
Herein, we highlight recent advances in the optical and electrochemical properties of silver clusters stabilized in zeolite templates, discussing how zeolite frameworks stabilize and modulate these properties. A particular emphasis is made on their structure–property relationships and implications for (bio)sensing. Then, recent advances in chemical sensing, biosensing and the integration of silver–cluster zeolite composites into portable optical and electrochemical devices are highlighted. Finally, the challenges and opportunities associated with the integration of these materials into portable, robust, selective and sustainable biosensing devices are presented, offering a forward-looking view on their potential to transform healthcare diagnostics and environmental monitoring.
Sub-nanometric silver clusters in zeolites: structure, properties, and implications for (bio)sensing
Sub-nanometric silver clusters (<2 nm), composed of a few to several hundred atoms, exhibit molecular-like behavior due to their size being comparable to the Fermi wavelength. Their conduction and valence bands consist of discrete energy levels separated by a band gap,13 endowing them with unique physical and chemical properties such as electrocatalytic activity,7 photoluminescence with large Stokes shifts,14 tunable emission15 and responsiveness to environmental stimuli,16 making them highly attractive for (bio)sensing applications. These clusters are often referred to as “super-atoms” due to their high symmetry, super-atomic orbitals, and predictable electronic structures in the gas phase, where environmental perturbations are minimized. Their physicochemical properties include high conductivity and strong photoluminescence.17,18 Importantly, these properties are size-dependent; smaller clusters (subnanometer to 1–2 nm) exhibit enhanced surface reactivity and catalytic potential, while larger clusters (few to several nanometers) offer greater thermodynamic stability and bonding energy.19 Theoretical and experimental studies have predicted optimal crystal structures and orbital configurations, driving research into how these parameters influence cluster behavior.20–22However, due to their high surface energy and environmental reactivity, metallic clusters tend to aggregate into nanoparticles. To prevent this, stabilization strategies involving protective ligands or templates are commonly employed.23 Among inorganic templates, zeolites stand out for their structural and chemical versatility. Zeolites are three-dimensional aluminosilicate frameworks composed of tetrahedral units, where partial substitution of Si4+ by Al3+ generates a negative framework charge balanced by extra-framework cations such as Na+, K+, and Ca2+, among other monovalent and divalent cations.24 The Si/Al ratio governs key properties including reactivity and hydrophilicity.25 Variations in the crystal structure and pore size, determined by the arrangement of tetrahedral units, enable the stabilization of diverse sub-nanometric metallic clusters.24,26 Cluster size and type are influenced by the dimensions of the crystalline cavities, atomic arrangement within the framework, and the exchange affinity of the extra-framework cations.27 Furthermore, zeolite porosity acts as a molecular sieve, imparting selectivity and enabling targeted interactions with analytes based on size and chemical properties.
To date, ion exchange remains the most widely used method for synthesizing silver clusters in zeolites and other inorganic frameworks, owing to its simplicity and cost-effectiveness. In this approach, natural or synthetic zeolites are suspended in a silver cation solution, enabling the exchange of extra-framework cations (e.g., Na+, K+, Ca2+, and Mg2+) through competitive affinity. Subsequent reduction of the silver ions, via thermal treatment,28 UV irradiation,29 or X-ray exposure,30 yields silver clusters with high quantum yields and notable catalytic activity. However, this method offers limited control over cluster size, as the ion-exchange capacity restricts silver incorporation, leading to preferential formation in superficial zeolite cavities.
Optoelectrochemical properties of silver clusters in zeolites: dynamics, modulation, and sensing potential
Silver clusters, owing to quantum confinement and molecular-like behavior, exhibit a rich array of optical and electrochemical properties, including multiple absorption bands, photoluminescence, and charge transfer.21 Their excited-state dynamics span from femtoseconds to milliseconds, with interactions in the surrounding media playing a critical role in shaping electronic states.31Photoluminescence arises from transitions such as d-orbital excitations at shorter wavelengths and s → p transitions at longer wavelengths, depending on the cluster's net charge. Upon photon absorption, relaxation occurs via several pathways: ultrafast decay (fs–ps) through internal conversion and vibrational relaxation; intermediate decay (ps–ns) from emissive states; and long-lived decay (µs–ms) involving intersystem crossing, electron–hole recombination, or trap states.31–33
The local electronic environment strongly influences intersystem crossing, particularly through overlap between the triplet and P-type singlet states.16,31 Water molecules coordinated within the zeolite framework and nearby atoms enhance luminescence by generating degenerate singlet and triplet excited states.34,35 Water molecules within the zeolite cavities play a central role in modulating emission behavior, either enhancing or quenching luminescence depending on their coordination to the clusters. Controlled dehydration, achieved through thermal treatment, or exclusion by small cations such as Li+, which hinder cavity entry, can shift emission wavelengths and modify luminescence lifetimes by altering electrostatic fields and electronic coupling between cluster atoms and degenerate states.32,36 Silver atoms within the clusters interact strongly with framework oxygen atoms, forming partial covalent bonds, while weaker interactions occur with cavity water molecules residing in the zeolite cavities.32,34 These interactions facilitate electron-transfer processes that underpin the characteristic luminescence behavior of the clusters. The nature of charge-compensating cations further modulates these effects: smaller cations (Li+ and K+) impose steric hindrance, limiting water coordination and leading to blue-shifted emission, whereas larger cations (Na+) allow greater hydration and red-shifted or quenched emission.34
Collectively, these findings reveal that the optoelectrochemical response of silver clusters in zeolites is dynamically tunable through hydration control and cation selection. Such structural and environmental sensitivity makes these materials ideal candidates for responsive optical sensing platforms, where variations in moisture, ionic composition, or molecular adsorption can be transduced into measurable luminescence signals.
Beyond water interactions, emission can be tuned across the visible spectrum by introducing ions or electron-rich molecules into zeolite pores or surfaces, which modulate the cluster's electronic environment.1,35,37,38 The zeolite framework structure also plays a central role in modulating the photoluminescence quantum yield (PLQY). For instance, silver clusters within Faujasite (FAU) zeolites have achieved the highest reported PLQY (97.4%), while silver clusters into Linde Type A (LTA) zeolites exhibit a low PLQY; substituting Na+ with Li+ in LTA dramatically increases the PLQY to 83%, accompanied by a redshift from blue to green/yellow emission, in addition to minimized photoblinking and photobleaching in the presence of Li+ cations.32,34,39–41 Table 1 shows the design levers for silver–cluster zeolite composites in sensing.
| Design lever | Contribution | Photophysical response | Sensing implication | Ref. |
|---|---|---|---|---|
| External quantum efficiencies (EQEs). | ||||
| Framework type | Controls cavity size, topology, and Ag cluster site distribution | FAU → high EQE, nanosecond decay | FAU → high-brightness sensors | 42 and 43 |
| LTA → low EQE, hundreds of micro and nano second decays | LTA → high environmental sensitivity | |||
| Si/Al ratio | Modulates the framework charge and number of Ag+ sites | Lower Si/Al → red shift + quenching Higher Si/Al → stronger emission | Low Si/Al → high sensitivity but lower stability High Si/Al → stable sensing | 42 |
| Extra-framework cations (Li+, Na+, and K+) | Alters the local electric field, polarizability, and cluster stabilization | Small cations (Li+ and K+) → blue shift | Cation engineering for brightness | 39 and 43 |
| Larger cations (Na+) → red shift + quenching | Wavelength tuning | |||
| Li+ in LTA increases external quantum efficiencies. | ||||
| Hydration state | H2O modifies cluster coordination and electronic coupling. | Fully hydrated → yellow emission Partially hydrated (2–17% water content) → blue/green emission | Wavelength tuning | 31 and 39 |
| 1% water content → blue emission | Humidity sensing | |||
| Surface chemistry/functionalization | Modification of the electronic environment | Amines: Ag–N → blue shift | Sensing by changes in the emission | 1 and 16 |
| Specificity to target analytes | Biomolecules → red shift | |||
Another important property of silver clusters confined within zeolite frameworks is their remarkable charge transfer capability, enabling efficient electron exchange between the confined clusters and surrounding redox species such as analytes or mediators in electrochemical systems.44 For instance, silver clusters embedded in FAU zeolite have been shown to exhibit enhanced electron-exchange-transfer and improved conductivity due to strong Ag–framework interactions and partial charge delocalization.31 The porous structure of the zeolite further facilitates analyte adsorption and local preconcentration, thereby increasing the electroactive surface area and improving electrode–analyte interactions.7
These synergistic effects, combining the redox activity of silver clusters (Ag+/Ag0) cycling with the ion-exchange and confinement properties of zeolites, lead to measurable improvements in the charge transfer resistance, catalytic current, and sensitivity in electrochemical assays.7 Consequently, silver cluster–zeolite composites act as efficient electron mediators and electrocatalytic platforms, bridging molecular-like redox behavior with robust solid-state conductivity, essential for next-generation (bio)sensing technologies.
Expanding (bio)sensing applications of silver cluster–zeolite composites
A reversible hydration–dehydration sensor was later developed using the LTA zeolite, demonstrating on–off luminescence switching. In the hydrated state, silver clusters exist as Ag4(H2O)42+, coordinated with water molecules. Upon thermal treatment, dehydration drives the conversion of Ag4(H2O)42+ into larger Ag6(O4R)142+ clusters, where silver atoms establish coordination with the framework oxygen atoms (O4R groups) instead of water ligands. This structural rearrangement alters the local electronic environment and governs the observed emission shift. This reversible transformation, governed by the equation: Ag4(H2O)42+ + 2Ag+ ↔ Ag6(O4R)142+ + 2X+, modulates the emission wavelength and intensity through changes in Ag–O bonding and electron-transfer dynamics.34,39,49 Such reversible switching at the atomic level, non-commonly observed in other luminescent nanomaterials, exemplifies how zeolite-embedded clusters are redefining stability and reusability in chemical sensing.
Building on the luminescence responsiveness of silver clusters, strong coordination bonds such as Ag–N can outcompete Ag–O interactions with the zeolite framework or water molecules, thereby locally redistributing electron density and triggering measurable emission changes.16 A reversible amine gas sensor was developed using silver cluster–zeolite composites. Exposure to triethylamine gas quenched luminescence and induced a blue shift (530 nm to 480 nm), attributed to the formation of Ag–N coordination bonds that disrupt Ag–O interactions. Subsequent exposure to acetic acid vapor restored the original emission by reversing the coordination and reestablishing Ag–O bonds.50
A dual-mode oxygen sensor was also demonstrated using silver clusters in FAU zeolites. Initially orange-emitting clusters, generated under a H2 atmosphere, transitioned to a weak cyan-emitting state upon exposure to O2 gas. This change, driven by triplet–triplet interactions and slow diffusion of oxygen into zeolite channels, altered the electron density of the luminescent center and shortened the emission lifetime. The original state was restored under vacuum and H2 conditions.51 This is a relevant example of how zeolite confinement provides reversible and multi-signal response in sensing.
Beyond luminescence, silver clusters in zeolites have enabled hybrid optical–electrochemical sensing platforms. Their small band gap facilitates hole injection and efficient electron–hole recombination, functioning as charge traps or donor centers.44 While zeolites are inherently non-conductive, their porous structure enhances electrochemical performance by retaining the analytes and promoting electron transfer in the presence of silver clusters.18 An electrocatalytic sensor for hydrogen peroxide (H2O2) was developed using silver nanocomposites in NaY zeolite, incorporated into a carbon paste electrode. Amperometric measurements in phosphate buffer solution (PBS) revealed enhanced current response due to improved charge transfer and diffusion control.52 A more complex two-step system was later designed for tryptophan (Trp) detection. Silver clusters first undergo electrochemical oxidation (Ag → Ag+), followed by complexation with Trp to form Ag(Trp)23−, facilitating electron transfer during Trp oxidation. Compared to conventional electrodes, this system offered improved sensitivity and cost-efficiency, attributed to the nanometric size of silver clusters and zeolite mesoporosity.53
A sophisticated dual-mode sensor for sulfadiazine detection has also been reported. Optical enhancement occurred via charge transfer, as absorption of the sulfadiazine band aligned with the silver cluster band gap in franzinite zeolites. The molecule altered the HOMO–LUMO configuration of the clusters, reducing the band gap and increasing fluorescence. The electrochemical performance was also improved, with silver clusters increasing the active sites and reducing the resistance. Compared to unmodified electrodes, this setup achieved a 2.6-fold increase in the electroactive surface area and an approximately 2-fold increase in the charge transfer rate, enabling ultra-trace detection across two linear ranges.54
Altogether, these examples illustrate how embedding silver clusters within zeolite frameworks transforms chemical sensing. Silver cluster–zeolite composites uniquely couple molecular-like optical transitions with the robustness and porosity of crystalline frameworks. This synergy enables reversible switching, dual-mode readouts (optical–electrochemical), and ultra-trace detection, features rarely attainable in traditional sensor platforms. Zeolites provide a high level of control over sensor performance by tuning the emission through host–guest chemistry, and enhancing electrochemical charge transfer, demonstrating that silver cluster–zeolite composites are redefining how chemical sensing can be implemented, obtaining highly tunable, multifunctional, and robust sensing platforms.
Detection is based on the sensitivity of silver clusters to local surrounding media. Upon formation of an antibody–analyte immunocomplex on the zeolite surface, the luminescence intensity of the silver clusters is modulated. The proposed mechanism involves an antenna-like donor–acceptor effect analogous to the system reported for chromophores confined within zeolite channels,55,56 in which guest molecules act as light-harvesting donors that transfer excitation energy to terminal “stopcock” acceptors at the crystal surface through short-range dipole–dipole coupling. Efficient interaction in such architectures requires nanometric donor–acceptor separations (typically within 2–6 nm) and appropriate spectral overlap.
In the present silver cluster–zeolite immunosystem, the silver clusters serve as luminescent donors and the immunocomplex acts as a non-luminescent acceptor that modulates the donor's emission upon analyte binding. Analyte recognition alters the electronic environment of the acceptor, facilitating energy transfer and triggering a measurable luminescence change. This mechanism supports a rapid (5 to 20 min), wash-free detection strategy with demonstrated potential in complex matrices such as human serum.1
Altogether, these advances highlight how embedding silver clusters within zeolites might result in the enhancement of biosensors; in fact, while conventional biosensing platforms, often require washing, labelling, and/or multiple signal amplification steps, on the other hand silver cluster–zeolite composites inherently combine strong luminescence, redox activity, and facile surface functionalization. This synergy enables fast, selective, and wash-free detection in complex media, paving the way for portable and minimally invasive diagnostic devices and redefining the path toward next-generation healthcare diagnostics. Fig. 1 summarizes different mechanisms for (bio)chemical sensors using zeolites embedding silver clusters.
 | ||
| Fig. 1 Different mechanisms for (bio)chemical sensors using zeolites embedding silver clusters. (A) Wavelength shift and luminescence quenching. Adapted with permission from ref. 50 Copyright 2023, American Chemical Society. (B) Colorimetric response and luminescence quenching. Adapted with permission from ref. 47 Copyright 2020, Elsevier. (C) Emission switching and life-time reduction. Adapted with permission from ref. 51 Copyright 2024, American Chemical Society. (D) Formation of the coordination complex and electron transfer. Adapted with permission from ref. 53 Copyright 2024, Springer Nature. (E) Electron-exchange-transfer (optical and electrochemical responses). Adapted with permission from ref. 54 Copyright 2024, Elsevier. (F) Wavelength emission modulation. Adapted from ref. 49 Copyright 2016, The authors, published by JoVE licensed under the license indicated (G) Luminescence enhancement by the antenna effect. Adapted from ref. 1 Copyright 2023, The authors, published by Angewandte licensed under the license indicated. | ||
More recently, the first portable and disposable electrochemical device incorporating silver clusters in zeolite composites was introduced. In this system, the working electrode surface of electrochemical strips was modified with zeolites embedding silver clusters, enhancing analyte immobilization, electrode–analyte interaction, and electron transfer. Two detection mechanisms were evaluated through the (i) redox reactivity where the oxidative–reductive potential of silver clusters was demonstrated through chloride ion detection. In the presence of Cl−, Ag0 is oxidized to Ag+, forming AgCl, a key reaction in environmental and biomedical analysis; and (ii) electrocatalysis, in this approach the proof of concept was tested on two substrates, filter paper and polyester. Fig. 2, illustrates the advances in the development of portable biosensor devices using silver clusters embedded within zeolites.
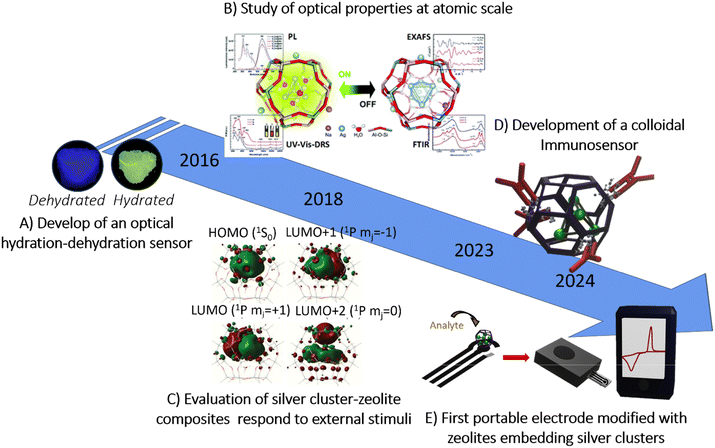 | ||
| Fig. 2 Advances in the development of portable biosensor devices using silver clusters embedded into zeolites. (A) Development of an optical hydration–dehydration sensor. Adapted from ref. 49 Copyright 2016, The authors, published by Journal of Visualized Experiments (JoVE) licensed under the license indicated. (B) Study of optical properties at the atomic scale. Adapted with permission from ref. 34 Copyright 2018, Royal Society of Chemistry. (C) Evaluation of silver cluster–zeolite composites response to external stimuli. Adapted with permission from ref. 31 Copyright 2018, Science. (D) Development of a colloidal immunosensor. Adapted from ref. 1 Copyright 2023, The authors, published by Angewandte under the license indicated. (E) First portable electrode modified with zeolites embedding silver clusters. Adapted with permission from ref. 7 Copyright 2024, The authors, published by American Chemical Society under the license indicated. | ||
Filter paper strips offered lower detection limits due to higher porosity, while polyester strips provided greater sensitivity through more uniform electrode printing. The electrocatalytic activity was further evaluated for glucose detection using glucose oxidase. The electrochemical reduction of H2O2, generated by the enzymatic reaction, showed enhanced sensitivity and lower detection limits compared to unmodified strips, attributed to improved electron transfer and analyte interaction. This work establishes a foundation for developing portable (bio)sensors by integrating silver cluster–zeolite composites, improving both portability and response time.7 However, despite these advancements, practical applications still face significant limitations in terms of stability and property control following integration into miniaturized platforms. The following section discusses the key challenges and future directions that must be addressed to enable a successful transition from early prototypes to robust, portable (bio)sensing technologies.
From prototypes to practical devices: challenges, outlook and future directions
The transition of silver cluster–zeolite composites from laboratory prototypes to practical portable devices requires overcoming challenges that range from specific material-level limitation to broad technological barriers. Addressing these systematically will be key to transforming silver cluster–zeolite composites from proof-of-concept into robust platforms for portable (bio)sensing. For instance, achieving precise control of their optoelectrochemical properties and their successful integration into stable and miniaturized devices remain a critical challenge that must be addressed.At the structural level, zeolite architecture and surface chemistry strongly influence analyte–cluster interactions. Tailoring pore sizes (micro, meso, or hybrid structures) and morphologies, such as nanozeolites, could increase sensitivity and selectivity by enhancing analyte selectivity access to active sites.
At the material-specific level, control over their optoelectrochemical properties, including quantum yields, excitation–emission wavelengths, and charge transfer efficiency, remains a major challenge, directly affecting biosensor sensitivity and detection limits. The commonly used ion-exchange method produces silver clusters with variable sizes. Achieving atomically precise cluster nucleation and growth under well-defined conditions could improve sensitivity and reproducibility. In parallel, strategies to tune excitation–emission wavelengths would have a significant impact on various applications. Currently, excitation typically occurs around 300 nm (250–320 nm), a range where common substrates like glass, polyester and polystyrene exhibit high absorption, limiting excitation efficiency. Importantly, photodamage (when exposure to prolonged irradiation on these wavelengths) leading local heating, photo-oxidation of the zeolite framework, or degradation of nearby biomolecules should also be experimentally considered. Moreover, for biomedical applications, red/infrared emission is essential to minimize absorption by biological tissues. Potential approaches include bimetallic cluster formation (i.e. with gold) or the incorporation of functional molecules into zeolite cavities near the clusters.16 Both strategies remain largely unexplored and require further theoretical and experimental investigation to understand how molecular environments modulate the photophysics of metallic clusters. In addition, biofouling of zeolite surfaces or Ag+ leaching or cytotoxicity could be an issue in biomedical applications; however, surface passivation, antifouling coatings, red-shift strategies, and on-chip optical filtering could be considered to address the aforementioned challenges.
Surface functionalization is crucial for specificity. While antibodies have been demonstrated as effective biorecognition elements,1 a broader exploration of biomolecules, such as aptamers, enzymes, or engineered proteins, as well as diverse attachment strategies could expand the scope of detectable analytes and improve device versatility. When integrating these biomolecules, it is important to consider their limited accessibility to the microporous interior of zeolites and the potential steric hindrance that may arise at the external surface, as these factors can influence the efficiency of analyte recognition and the kinetics of the sensing response. The use of nanozeolite particles can help mitigate these constraints by increasing the external surface area and improving the accessibility and orientation of immobilized biomolecules, thereby broadening the range of viable biorecognition architectures for silver–cluster-based sensing interfaces. At the same time, the underlying detection mechanisms are not yet fully understood, and further research is needed to elucidate these mechanisms across different classes of analytes.
At the broadest level, stability and reproducibility remain pressing issues. Although silver cluster–zeolite composites demonstrate intrinsic stability, systematic studies evaluating their performance after biomolecular functionalization and incorporation into sensing platforms are still lacking. Such studies are essential to assess device longevity and operational robustness under real-world conditions. For commercial deployment, scalable, reproducible, and cost-effective synthesis methods are also critical. While zeolite synthesis has already been scaled successfully, equivalent efforts for silver cluster–zeolite composites, particularly those functionalized for biosensing, remain underdeveloped.
Device-level integration represents some of the major challenges. Embedding silver cluster–zeolite composites into microfluidic or lateral-flow systems could enable rapid and low-cost point-of-care testing. The use of biodegradable polymers as substrates would support sustainable fabrication by reducing the environmental burden and manufacturing costs. However, integration must be achieved without compromising the intrinsic luminescence and/or electrochemical properties, ensuring stable bonding with the device, maintaining effective analyte interaction. Substrate compatibility is also essential, as many device materials may interfere with efficient excitation–emission collection, making it necessary to select UV-transparent substrates (e.g., quartz) or incorporate suitable waveguides/filters to ensure proper optical delivery and read-out.
Finally, incorporating advanced data analysis, particularly through machine learning algorithms, could significantly enhance the interpretation of complex optical and electrochemical signals, improve sensitivity and enable real-time diagnostics.57,58 Fig. 3 summarized the challenges of the integration of silver cluster–zeolite composites into a portable device.
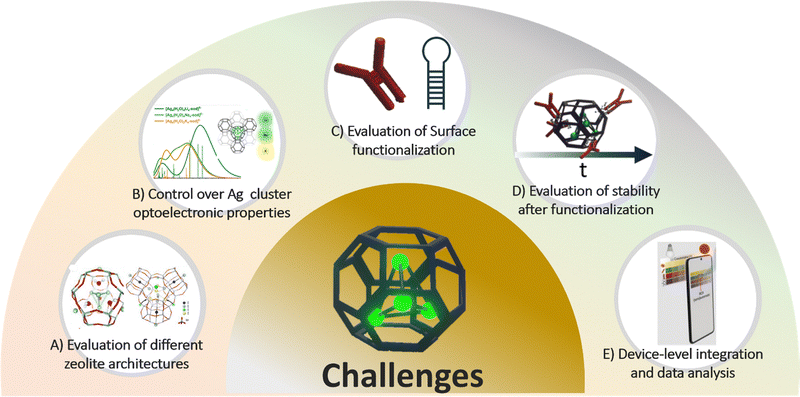 | ||
| Fig. 3 Challenges of the integration of silver cluster–zeolite composites into a portable device. (A) Evaluation of different zeolite architectures. Adapted with permission from ref. 28 Copyright 2016, American Chemical Society and ref. 31 Copyright 2018, Science. (B) Control over Ag cluster optoelectrochemical properties. Adapted with permission from ref. 59 Copyright 2024, American Chemical Society. (C) Evaluation of surface functionalization. (D) Evaluation of the stability and reproducibility after functionalization. Adapted from ref. 1 Copyright 2023, the authors, published by Angewandte under the license indicated. (E) Device-level integration and data analysis incorporation. Adapted with permission from ref. 57 Copyright 2025, Elsevier. | ||
By addressing these challenges and opportunities, silver cluster–zeolite composites could evolve from proof-of-concept demonstrations into scalable, robust, and sustainable sensing platforms. This would unlock their full potential for healthcare diagnostics and environmental monitoring, combining sensitivity, selectivity, and sustainability in a compact format. We believe that future research will rely on strategies that advance both material performance and device integration.
Author contributions
C. G. G., S. C., E. C.-G. and E. M.-N wrote, reviewed and edited the original manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the highlights of this overview are available within the article.Acknowledgements
C. G.-G. thanks SECIHTI for the PhD fellowship. E. C.-G. gratefully acknowledges the financial support from the FWO through the research stay grant V502522N. E. M.-N. acknowledges support from the Dirección General de Asuntos del Personal Académico de la Universidad Nacional Autónoma de México (grant PAPIIT-IT100124). E. M.-N. also acknowledges support from the Fundación Marcos Moshinsky UNAM (Cátedra Moshinsky 2024) and SECIHTI (grant MADTEC-2025-M-137). E. M.-N. and S. C. acknowledge financial support from MAECI-AMEXCID (Joint Research Proposals for the Years 2023–2025, DPC01225-7/2024).References
- C. García-Guzmán, E. Morales-Narváez and E. Coutino-Gonzalez, Angew. Chem., Int. Ed., 2023, e202307718 Search PubMed.
- M. Retout, L. Amer, W. Yim, M. N. Creyer, B. Lam, D. F. Trujillo, J. Potempa, A. J. O’Donoghue, C. Chen and J. V. Jokerst, ACS Nano, 2023, 17, 17308–17319 CrossRef PubMed.
- J. Sakai, S. Biswas, T. Irie, H. Mabuchi, T. Sekine, Y. Niihori, S. Das and Y. Negishi, Nanoscale, 2023, 15, 12227–12234 Search PubMed.
- V. Nasirian, M. Shamsipur, F. Molaabasi, K. Mansouri, M. Sarparast, V. Salim, A. Barati and S. Kashanian, Sens. Actuators, B, 2020, 308, 127673 CrossRef.
- Y. Chen and R. G. Compton, Chemosensors, 2023, 11, 297 CrossRef.
- D. Ungor, R. Bélteki, K. Horváth, O. Dömötör and E. Csapó, Int. J. Mol. Sci., 2022, 23 Search PubMed.
- C. García-Guzmán, A. Raucci, E. Coutino-Gonzalez, E. Morales-Narváez and S. Cinti, Anal. Chem., 2024, 96, 17915–17921 CrossRef PubMed.
- D. Prakashan, N. S. Shrikrishna, M. Byakodi, K. Nagamani and S. Gandhi, J. Med. Virol., 2023, 95, e28416 CrossRef PubMed.
- F. Gao, C. Liu, Y. Yao, C. Lei, S. Li, L. Yuan, H. Song, Y. Yang, J. Wan and C. Yu, Biosens. Bioelectron., 2022, 199, 113892 CrossRef PubMed.
- E. Coutiño-Gonzalez, W. Baekelant, J. A. Steele, C. W. Kim, M. B. J. Roeffaers and J. Hofkens, Acc. Chem. Res., 2017, 50, 2353–2361 CrossRef PubMed.
- H. Nasrollahpour, B. J. Sánchez, M. Sillanpää and R. Moradi, ACS Appl. Nano Mater., 2023, 6, 12609–12672 CrossRef.
- E. Coutino-Gonzalez, M. Roeffaers and J. Hofkens, in Dye. Photoactive Mol. Microporous Syst., 2020, pp. 75–103 Search PubMed.
- I. Díez and R. H. A. Ras, Few-Atom Silver Clusters as Fluorescent Reporters, Advanced Fluorescence Reporters in Chemistry and Biology II: Molecular Constructions, Polymers and Nanoparticles, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 307–332 Search PubMed.
- Y. Chai, W. Shang, W. Li, G. Wu, W. Dai, N. Guan and L. Li, Adv. Sci., 2019, 6, 1900299 CrossRef PubMed.
- J. Yuan, Q. Li, C. Yue, N. Wang, P. Li and H. Li, J. Mater. Chem. C, 2023, 11, 17080–17086 RSC.
- B. Chan, Phys. Chem. Chem. Phys., 2021, 23, 1984–1993 RSC.
- F. Baletto, J. Phys.: Condens. Matter, 2019, 31, 113001 Search PubMed.
- H. Hirai, S. Ito, S. Takano, K. Koyasu and T. Tsukuda, Chem. Sci., 2020, 11, 12233–12248 Search PubMed.
- H. Berry, 2023. Preprint (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-3716517/v1].
- T. Nishimura, T. Toba, G. Sakane and T. Ishii, Crystals, 2022, 12 Search PubMed.
- X. Kang, H. Chong and M. Zhu, Nanoscale, 2018, 10, 10758–10834 Search PubMed.
- X. Wu and Y. Sun, J. Nanopart. Res., 2017, 19, 201 CrossRef.
- T. M. Soini and N. Rösch, Phys. Chem. Chem. Phys., 2015, 17, 28463–28483 RSC.
- B. Jha and D. N. Singh, Basics of Zeolites, in Fly Ash Zeolites: Innovations, Applications, and Directions, ed. B. Jha and D. N. Singh, Springer Singapore, Singapore, 2016, pp. 5–31 Search PubMed.
- A. Walcarius, Electroanalysis, 1996, 8, 971–986 Search PubMed.
- X. Zeng, X. Hu, H. Song, G. Xia, Z.-Y. Shen, R. Yu and M. Moskovits, Microporous Mesoporous Mater., 2021, 323, 111262 Search PubMed.
- X. Xv, S. Ye, L. Pan, P. Lin, H. Liao and D. Wang, Materials, 2022, 15, 7431 Search PubMed.
- T. Altantzis, E. Coutino-Gonzalez, W. Baekelant, G. T. Martinez, A. M. Abakumov, G. Van Tendeloo, M. B. J. Roeffaers, S. Bals and J. Hofkens, ACS Nano, 2016, 10, 7604–7611 CrossRef PubMed.
- M. El-Roz, I. Telegeiev, N. E. Mordvinova, O. I. Lebedev, N. Barrier, A. Behilil, M. Zaarour, L. Lakiss and V. Valtchev, ACS Appl. Mater. Interfaces, 2018, 10, 28702–28708 CrossRef.
- O. Fenwick, E. Coutiño-Gonzalez, F. Richard, S. Bonacchi, W. Baekelant, D. de Vos, M. B. J. Roeffaers, J. Hofkens and P. Samorì, Small, 2020, 16, 2002063 CrossRef PubMed.
- D. Grandjean, E. Coutiño-Gonzalez, N. T. Cuong, E. Fron, W. Baekelant, S. Aghakhani, P. Schlexer, F. D’Acapito, D. Banerjee, M. B. J. Roeffaers, M. T. Nguyen, J. Hofkens and P. Lievens, Science, 2018, 361, 686–690 Search PubMed.
- E. Fron, S. Aghakhani, W. Baekelant, D. Grandjean, E. Coutino-Gonzalez, M. Van der Auweraer, M. B. J. Roeffaers, P. Lievens and J. Hofkens, J. Phys. Chem. C, 2019, 123, 10630–10638 Search PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 Search PubMed.
- S. Aghakhani, D. Grandjean, W. Baekelant, E. Coutiño-Gonzalez, E. Fron, K. Kvashnina, M. B. J. Roeffaers, J. Hofkens, B. F. Sels and P. Lievens, Nanoscale, 2018, 10, 11467–11476 RSC.
- G. Romolini, J. A. Steele, J. Hofkens, M. B. J. Roeffaers and E. Coutino-Gonzalez, Adv. Opt. Mater., 2021, 9, 2100526 Search PubMed.
- E. Johan, Y. Kanda, N. Matsue, Y. Itagaki and H. Aono, J. Lumin., 2019, 213, 482–488 CrossRef.
- J. Yu, S. Ye, H. Liao, X. Xv and D. Wang, ACS Appl. Nano Mater., 2021, 4, 13692–13699 CrossRef.
- W. Baekelant, S. Aghakhani, E. Coutino-Gonzalez, K. Kennes, F. D’Acapito, D. Grandjean, M. Van der Auweraer, P. Lievens, M. B. J. Roeffaers, J. Hofkens and J. A. Steele, J. Phys. Chem. Lett., 2018, 9, 5344–5350 CrossRef PubMed.
- E. Coutino-Gonzalez, W. Baekelant, D. Grandjean, M. B. J. Roeffaers, E. Fron, M. S. Aghakhani, N. Bovet, M. Van der Auweraer, P. Lievens, T. Vosch, B. Sels and J. Hofkens, J. Mater. Chem. C, 2015, 3, 11857–11867 Search PubMed.
- G. De Cremer, E. Coutiño-Gonzalez, M. B. J. Roeffaers, D. E. De Vos, J. Hofkens, T. Vosch and B. F. Sels, Chem. Phys. Chem., 2010, 11, 1627–1631 Search PubMed.
- W. Baekelant, S. Aghakhani, E. Fron, C. Martin, C. Woong-Kim, J. A. Steele, T. De Baerdemaeker, F. d’Acapito, D. Chernysov, M. van der Auweraer, P. Lievens, D. Grandjean, M. Roeffaers, J. Hofkens and E. Coutino-Gonzalez, J. Mater. Chem. C, 2019, 7, 14366–14374 Search PubMed.
- E. Coutino-Gonzalez, M. B. J. Roeffaers, B. Dieu, G. De Cremer, S. Leyre, P. Hanselaer, W. Fyen, B. Sels and J. Hofkens, J. Phys. Chem. C, 2013, 117, 6998–7004 Search PubMed.
- G. De Cremer, E. Coutiño-Gonzalez, M. B. J. Roeffaers, B. Moens, J. Ollevier, M. Van der Auweraer, R. Schoonheydt, P. A. Jacobs, F. C. De Schryver, J. Hofkens, D. E. De Vos, B. F. Sels and T. Vosch, J. Am. Chem. Soc., 2009, 131, 3049–3056 Search PubMed.
- K. Kennes, E. Coutino-Gonzalez, C. Martin, W. Baekelant, M. B. J. Roeffaers and M. Van der Auweraer, Adv. Funct. Mater., 2017, 27, 1606411 CrossRef.
- M. Rálek, P. Jírů, O. Grubner and H. Beyer, Collect. Czech. Chem. Commun., 1962, 27, 142–146 CrossRef.
- D. Brühwiler, R. Seifert and G. Calzaferri, J. Phys. Chem. B, 1999, 103, 6397–6399 CrossRef.
- D. Yao, Y. Wang and H. Li, Sens. Actuators, B, 2020, 305, 127451 CrossRef.
- J. Yu, S. Ye, X. Xv, L. Pan, P. Lin, H. Liao and D. Wang, Nanomaterials, 2022, 12, 3215 CrossRef PubMed.
- E. Coutino-Gonzalez, W. Baekelant, B. Dieu, M. B. J. Roeffaers and J. Hofkens, JoVE, 2016, e54674 Search PubMed.
- Q. Li, X. Tian, J. Yuan, D. Zhao, Y. Wang and H. Li, Inorg. Chem., 2023, 62, 2430–2439 CrossRef PubMed.
- L. Sun, G. Romolini, B. Dieu, D. Grandjean, M. Keshavarz, E. Fron, F. D’Acapito, M. B. J. Roeffaers, M. Van der Auweraer and J. Hofkens, Adv. Opt. Mater., 2024, 2400784 CrossRef.
- H. Ramezani, S. N. Azizi and S. R. Hosseini, Sens. Actuators, B, 2017, 248, 571–579 CrossRef.
- S.-F. Mousavi, M. Alimoradi, A. Shirmardi and V. Zare-Shahabadi, J. Porous Mater., 2020, 27, 1505–1514 CrossRef.
- X. Zhu, Z. Li, F. Fang, E. Yifeng, P. Chen, L. Li and K. Qian, Anal. Chim. Acta, 2023, 1276, 341619 CrossRef PubMed.
- G. Calzaferri, S. Huber, H. Maas and C. Minkowski, Angew. Chem., Int. Ed., 2003, 42, 3732–3758 CrossRef PubMed.
- G. Calzaferri, Langmuir, 2012, 28, 6216–6231 CrossRef PubMed.
- A. Horta-Velázquez, G. Ramos-Ortiz and E. Morales-Narváez, Biosens. Bioelectron., 2025, 273, 117089 CrossRef PubMed.
- D. L. Mancera-Zapata, C. Rodríguez-Nava, F. Arce and E. Morales-Narváez, Anal. Chem., 2024, 96, 13756–13761 Search PubMed.
- L. Pan, S. Ye, R. Huang, M. Wang, T. Zheng, H. Zhang and D. Wang, Inorg. Chem., 2024, 63, 24867–24875 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |




