Supramolecular self-assembled peptide scaffolds for fluorescence enhancement and delayed emission
Sowbhick
Patra
ab and
Goutam
Ghosh
 *ab
*ab
aCentre for Nano and Soft Matter Sciences (CeNS), Shivanapura, Dasanapura Hobli, Bengaluru, 562162, India. E-mail: gghosh@cens.res.in
bAcademy of Scientific and Innovation Research (AcSIR), Ghaziabad, 201002, India
First published on 26th December 2025
Abstract
Luminescent organic materials are increasingly important for applications ranging from bioimaging and sensing to optoelectronics and phototherapy. Their performance depends on controlling excited-state dynamics, yet organic luminophores face intrinsic limitations such as weak spin–orbit coupling, aggregation-induced quenching, and oxygen sensitivity. Conventional strategies such as crystallization, polymer encapsulation, and host–guest assembly can improve fluorescence, thermally activated delayed fluorescence (TADF), and room-temperature phosphorescence (RTP), but often lack biocompatibility, adaptability, or aqueous stability. Peptide-based supramolecular assemblies are emerging as versatile alternatives, offering modularity, biodegradability, and the ability to create ordered nanostructures through hydrogen bonding (H-bonding), π–π stacking, hydrophobic interactions, and electrostatics. These assemblies generate confined and tunable microenvironments that suppress non-radiative losses, stabilize triplet states, and protect excitons from quenching, thereby enabling efficient fluorescence, long-lived RTP, and oxygen-tolerant TADF. In this review, we highlight recent advances in peptide–luminophore co-assemblies that enhance emission efficiency and stability under biological conditions. We discuss molecular design principles, mechanistic insights, and representative examples across fluorescence, RTP, and TADF systems and outline future directions in predictive peptide design, stability engineering, and multifunctional applications. Overall, peptide supramolecular scaffolds show great promise as next-generation platforms for development of efficient and versatile luminescent materials.
1. Introduction
The development of luminescent organic materials has received growing attention due to their diverse applications in bioimaging,1–5 biosensing,6–8 photodynamic therapy,9–11 optoelectronics,12–14 and information storage.15–17 In particular, fluorescence, TADF, and RTP have attracted significant interest.18,19 Fluorescence provides bright and fast emission but often suffers from quenching in aggregated or aqueous environments.20 TADF allows harvesting of both singlet and triplet excitons, yet it is highly sensitive to oxygen and molecular motions.21 RTP, with its long emission lifetime and large Stokes shift, is highly useful for time-resolved imaging and sensing.22,23 However, achieving stable RTP materials in soft or biological systems remains difficult.24,25 Traditionally, inorganic luminescent materials such as rare-earth complexes, transition-metal phosphors, and quantum dots have been employed to overcome these issues.26–29 While inorganic systems demonstrate strong emission and long lifetimes, their major limitations include toxicity, poor biodegradability, and high cost of synthesis. These drawbacks restrict their practical use in biological environments. In contrast, organic-based luminescent systems are more environmentally friendly, tunable, and cost-effective due to advances in molecular design and supramolecular chemistry.30–32 However, organic luminophores face their own intrinsic challenge: weak spin–orbit coupling, which hampers efficient inter system crossing (ISC) and limits triplet harvesting.33Beyond intrinsic molecular structures, supramolecular self-assembly plays a decisive role in governing excited-state dynamics and emission efficiency. Through controlled intermolecular organization, self-assembled architectures precisely regulate intermolecular spacing, restrict intramolecular motions, and create confined microenvironments. These collective effects profoundly modulate both radiative and non-radiative decay pathways, thereby dictating the overall luminescence behavior. Consequently, rational control over supramolecular self-assembly has emerged as a central design principle for high-performance luminescent systems.
Efficient luminescence requires the fulfilment of several key criteria, including effective population of triplet states via intersystem crossing (ISC) and reverse intersystem crossing (RISC), enhanced radiative decay from both singlet and triplet excited states, and suppression of non-radiative pathways arising from molecular vibrations, solvent collisions, and oxygen quenching.34 Supramolecular self-assembly provides a versatile platform to address these requirements by imposing structural rigidity, enhancing electronic coupling, and shielding excited states from external quenchers. In this context, diverse supramolecular strategies such as crystallization, polymer embedding, host–guest interactions, hydrogen and halogen bonding, and aggregation-induced emission (AIE)35–38 have proven highly effective in optimizing excited-state processes and achieving superior luminescence performance. These strategies create rigid and confined environments that protect excitons and enhance emission efficiency. Crystalline materials often lack solubility and are difficult to process in aqueous media. Polymer-doped systems and host–guest complexes may involve synthetic complexity, limited biodegradability, and potential cytotoxicity. Moreover, many of these strategies are unable to adapt dynamically to complex biological surroundings. These limitations highlight the need for soft, biocompatible, and programmable scaffolds that can operate efficiently under aqueous and physiological conditions.
Recently, peptide-based supramolecular assemblies have emerged as a promising alternative platform for luminescent systems.39,40 Peptides offer intrinsic advantages, including biocompatibility, biodegradability, and structural programmability, which will help in advanced biological applications.30,41–44 However, their potential in enhancing and regulating luminescence has been relatively less explored in delayed emission systems compared to conventional organic or polymer-based systems. Their rich noncovalent interactions, such as H-bonding, electrostatics, hydrophobic collapse, π–π stacking, and van der Waals forces, can drive the formation of ordered nanostructures, including nanofibers, vesicles, nanotubes, and hydrogels.42,45 Such assemblies provide confined microenvironments that suppress non-radiative decay, stabilize emissive states, and facilitate exciton interactions. Importantly, peptide sequences can be rationally designed to incorporate aromatic residues to enhance stacking, charged residues for improved binding, or bioactive motifs to impart recognition ability.45–48 This tunability allows peptides to act not only as scaffolds but also as active regulators of emission processes, enhancing fluorescence, stabilizing long-lived triplet states for RTP, and protecting TADF cores from oxygen quenching.
In this review, we summarize recent progress on peptide-based supramolecular scaffolds to enhance the luminescence properties of the system. We first discuss the general photophysical mechanisms of different luminescence processes. Then we will discuss the mechanistic insight of luminescence enhancement. Next, we highlight representative examples where peptides enhance emission efficiency and stability in aqueous and biological environments. Finally, we provide perspectives on the challenges and opportunities for peptide assemblies in advanced applications in biology and optoelectronics.
2. General process of luminescence
Luminescence arises when a molecule absorbs light and subsequently emits photons as it relaxes back to the ground state. Upon photon absorption, an electron is promoted from the ground state (S0) to an excited singlet state (S1 or Sn). Within femtoseconds, the molecule undergoes vibrational relaxation to reach the lowest vibrational level of S1, from which multiple relaxation pathways can occur. Radiative decay from S1 to S0 produces fluorescence, while nonradiative transitions such as internal conversion (IC) or intersystem crossing (ISC) transfer energy to the triplet state (T1). From T1, phosphorescence can occur as the molecule returns to the ground state, giving rise to long-lived emission useful for time-resolved or phosphorescence imaging.The triplet state also enables a range of functional processes: interaction with molecular oxygen can generate reactive oxygen species (1O2), forming the basis of photodynamic therapy (PDT), where the nonradiative decay contributes to localized heating, which underpins photothermal and photoacoustic imaging;49,50 and radiative transitions from singlet states are exploited for fluorescence-based sensing and bioimaging. Their ability to emit light with high sensitivity and spatial precision allows real-time monitoring of biological phenomena from cellular dynamics to disease detection. Although conventional fluorescent dyes and proteins have long been used for these purposes, their reliance on singlet excitons limits quantum efficiency and often leads to photobleaching and short lifetimes. These challenges have driven the development of advanced exciton management strategies, including TADF and RTP, which enable efficient utilization of both singlet and triplet excitons for next-generation luminescent materials.
2.1. Fluorescence
Fluorescence, which originates when an excited electron relaxes radiatively from the singlet excited state (S1) to the ground state (S0), is one of the most widely utilized photophysical processes in both materials science and biology (Scheme 1). Typically occurring on the nanosecond timescale, fluorescence offers the advantage of rapid and highly sensitive detection, making it central to conventional imaging, sensing, and diagnostic platforms.52–56 However, despite its widespread use, fluorescence suffers from several inherent limitations that restrict its performance in biological applications. One of the most critical drawbacks is photobleaching, the irreversible degradation of fluorophores upon prolonged light irradiation. This not only reduces signal intensity over time but also limits long-term tracking of biological events.57,58 Additionally, fluorescence signals often exhibit low signal-to-noise ratios in complex biological environments, where background noise competes with the probe emission. A major contributor to this issue is tissue autofluorescence, the intrinsic emission of biological molecules such as flavins, collagen, or nicotinamide adenine dinucleotide hydrogen (NADH) when excited by light.59,60 Because autofluorescence also occurs on the nanosecond timescale, it overlaps directly with probe emission, making it difficult to distinguish true signals from background. Furthermore, the inherently short lifetime of fluorescence limits opportunities for temporal separation of probe emission from background noise. This restriction complicates deep-tissue imaging and reduces sensitivity in low-concentration detection. Collectively, these drawbacks, such as photobleaching, interference from autofluorescence, and short emission lifetimes, highlight the need for alternative luminescence mechanisms that can overcome the intrinsic limitations of conventional fluorescence in biological systems.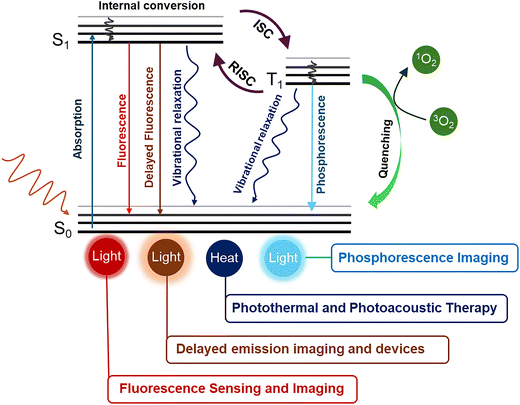 | ||
| Scheme 1 Schematic representation of the general photophysical processes in luminescent systems. Adapted with modification.51 Copyright 2020, The Royal Society of Chemistry. | ||
2.2. Phosphorescence and room temperature phosphorescence (RTP)
The emission process in phosphorescence materials involves multiple photophysical steps that distinguish them from conventional fluorescence (Scheme 1). Upon photoexcitation, molecules in the ground state (S0) absorb photons and are promoted to higher-energy singlet states (Sn, n ≥ 1). These excited species quickly undergo vibrational relaxation and internal conversion, dissipating excess energy as heat until reaching the lowest singlet excited state (S1). From S1, two major competing pathways occur: radiative relaxation to S0, observed as fluorescence or ISC to populate the triplet manifold (Tn). Subsequent relaxation to the lowest triplet state (T1) allows radiative decay back to S0, producing phosphorescence. Because the T1 to S0 transition is spin-forbidden, phosphorescence lifetimes are much longer than fluorescence, typically in the millisecond-to-second range. In conventional organic systems, phosphorescence is typically observed only at low temperatures or within rigid matrices, where non-radiative decay and oxygen quenching are suppressed. However, when such long-lived triplet emission is achieved under ambient conditions, it is specifically termed RTP.61,62 This distinction emphasizes the material's ability to stabilize triplet excitons and sustain phosphorescence emission without cryogenic support, representing a significant advancement for practical applications in sensing, imaging, and optoelectronics. Achieving efficient RTP in organic materials has historically been challenging, as triplet states are highly susceptible to non-radiative decay and quenching by molecular motion or oxygen. Early studies on crystalline carbazole and dibenzothiophene revealed that structural rigidity and controlled intermolecular interactions can stabilize triplet excitons and suppress quenching.63,64 In the biological field, the long-lived emission of RTP materials offers unique advantages over conventional fluorescence.65–68 Most notably, delayed emission enables time-gated imaging, where signals are collected after short-lived tissue autofluorescence has decayed, thereby enhancing sensitivity and the signal-to-noise ratio. The persistent afterglow of RTP materials also reduces the need for continuous excitation, lowering photodamage and improving compatibility with living systems. Despite these benefits, challenges remain, particularly in achieving efficient and stable RTP in aqueous or oxygen-rich biological environments. Emerging supramolecular strategies, including peptide-based scaffolds, offer promising solutions by providing rigid, protective microenvironments that stabilize triplet states and extend luminescence lifetimes under physiological conditions.69,702.3. Thermally activated delayed fluorescence (TADF)
TADF has emerged as a powerful strategy to overcome the inherent limitations of conventional fluorescence, particularly the inefficient use of triplet excitons. In traditional fluorescent materials, only a small portion of the excited states, called singlet excitons, produces light. Most of the excited states, about 75%, are triplet excitons, which usually lose their energy as heat instead of emitting light.71 This limits the brightness and efficiency of normal fluorescence, making it hard to get strong and long-lasting emission, especially for applications like biological imaging. TADF materials circumvent this loss by engineering a very small energy gap between the lowest singlet and triplet states. At ambient temperatures, thermal energy enables the triplet excitons to undergo reverse intersystem crossing (RISC) back to the singlet manifold, where they can emit as delayed fluorescence (Scheme 1). This recycling of otherwise wasted excitons significantly enhances emission efficiency and quantum yield. In biological contexts, TADF offers several distinct advantages: the delayed emission allows separation from short-lived tissue autofluorescence,72 thereby improving the signal-to-noise ratio and imaging contrast; the longer-lived emission supports time-gated detection and advanced biosensing.73 when combined with biocompatible carriers such as peptide-based supramolecular assemblies or nanoparticles, TADF luminophores can achieve improved solubility, stability, and cellular uptake.74 Collectively, these properties make TADF an attractive approach for long-lived, high-contrast bioimaging, precise biosensing, and multifunctional theranostic applications.Thus, while fluorescence represents the most direct emission pathway, RTP and TADF have emerged as two powerful approaches to make better use of triplet excitons. RTP does so by stabilizing the triplet state for long-lived phosphorescence, whereas TADF actively converts triplets back to singlets to boost delayed fluorescence. Both mechanisms address the same underlying challenge: how to minimize exciton loss and maximize the number of photons emitted per absorbed photon. Rational molecular design through control of spin–orbit coupling, rigidity, host–guest interactions, or supramolecular assembly can determine whether triplet excitons will vanish, glow faintly as RTP, or be recycled efficiently as TADF.
In summary, the evolution from fluorescence alone to incorporating RTP and TADF reflects a broader shift in luminescent material design: moving from simply achieving light emission to engineering pathways that control exciton populations, ultimately driving quantum yields closer to unity for both fundamental research and advanced applications.
3. Factors for luminescence enhancement
Enhancing the luminescence of fluorescent, TADF, and phosphorescent systems is a fundamental objective in the design of advanced optical materials. The efficiency and lifetime of emission in these systems are often limited by non-radiative decay pathways, including intramolecular rotations, vibrations, and quenching by environmental factors such as oxygen or solvent molecules.75–78 To overcome these limitations, strategies that modulate the molecular environment and control the spatial arrangement of luminophores have been widely employed. Increasing molecular rigidity can suppress non-radiative processes and improve quantum yields, while precise control over intermolecular interactions, such as π–π stacking, H-bonding, and hydrophobic interactions, can stabilize excited states and influence energy transfer. Achieving efficient luminescence in biological systems is challenging because biomolecular environments are inherently dynamic, flexible, and rich in quenchers like water and oxygen. These factors promote non-radiative decay and destabilize excited states, making it difficult to sustain long-lived or high-efficiency emission.79–81 Creating confined or heterogeneous microenvironments, including polarity gradients or restricted volumes, can further protect excited states from quenching, extend emission lifetimes, and facilitate delayed luminescence phenomena such as TADF or RTP. Collectively, these approaches provide a versatile toolkit for tuning photophysical properties and optimizing luminescence performance across diverse molecular systems. Although these strategies are widely explored in small-molecule and polymer-based systems, their application in biological environments remains limited due to issues such as poor biocompatibility, synthetic complexity, and instability in aqueous media.3.1. Non-covalent interactions
The luminescence properties of molecules are strongly influenced by their spatial arrangement, which is governed by a variety of intermolecular interactions. Key interactions include H-bonding, π–π stacking, hydrophobic interactions, and electrostatic forces, all of which can modulate emission intensity, wavelength, and lifetime by affecting molecular packing and the local environment (Fig. 1).82–84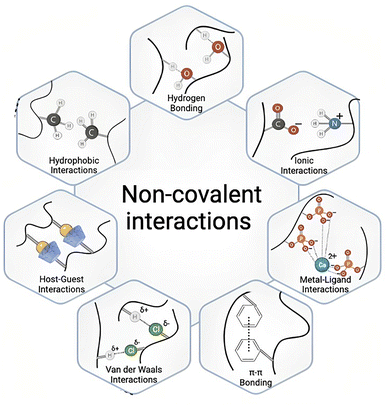 | ||
| Fig. 1 Schematic representation of various non-covalent interactions that govern supramolecular assembly and enhance luminescence by stabilizing excited states and suppressing nonradiative decay. Adapted with permission.94 Copyright 2022, Wiley-VCH. | ||
However, while these strategies have been extensively explored in synthetic supramolecular gels and polymer matrices, their integration into biologically relevant scaffolds remains relatively limited.
3.2. Crystallization and rigidity
Crystallization and molecular rigidity are crucial factors in enhancing luminescence efficiency in organic and hybrid systems. In the crystalline state, molecules adopt ordered packing that restricts intramolecular rotations and vibrations, which can follow primary nonradiative decay pathways, thereby promoting radiative recombination of excitons. This rigidification effect suppresses energy loss through thermal relaxation and facilitates efficient fluorescence, TADF, and RTP (Fig. 2).95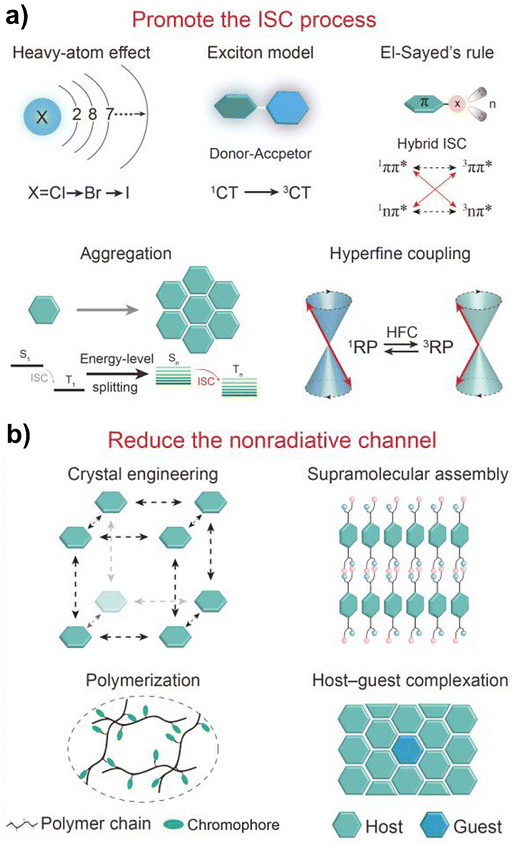 | ||
| Fig. 2 Strategies to enhance luminescence efficiency: (a) promoting ISC to increase triplet exciton population, and (b) suppressing non-radiative decay through molecular rigidity, crystallization, host–guest confinement, or supramolecular assembly. Adapted with permission.95 Copyright 2022, American Chemical Society. | ||
Rigid crystalline environments also stabilize excited triplet states by reducing dynamic quenching from molecular motion and external oxygen diffusion. For instance, crystalline carbazole and dibenzothiophene derivatives exhibit persistent RTP due to dense packing that isolates triplet excitons from quenchers.96 Similarly, crystalline benzil and aromatic carbonyl compounds show enhanced phosphorescence lifetimes compared to their amorphous counterparts.97
Overall, crystallization and rigidity act synergistically to boost luminescence by minimizing nonradiative losses and stabilizing emissive states. Rational molecular design focusing on rigid backbones, π–π stacking, and supramolecular ordering continues to be a key strategy for developing high-performance luminescent organic materials.
3.3. Heavy-atom effect on luminescence
Incorporating heavy atoms such as bromine, iodine, sulfur, or transition metals (e.g., Ir, Pt, and Ru) is a powerful strategy to enhance luminescence efficiency in fluorescent, TADF, and RTP systems. Heavy atoms promote spin–orbit coupling (SOC), which facilitates ISC from the singlet (S1) to triplet states (T1), thereby increasing the population of triplet excitons (Fig. 2). This process enables bright phosphorescence and long-lived delayed emission, crucial for efficient triplet harvesting in TADF materials. For example, halogenated luminophores such as 4-bromo-N-phenylcarbazole98,99 and dibenzothiophene100,101 derivatives exhibit enhanced RTP and TADF due to localized SOC effects, while organometallic emitters like Ir(ppy)3102,103 and Ru(bpy)32+ complexes104 display strong phosphorescence with high quantum yields and tunable lifetimes from microseconds to milliseconds. The heavy-atom effect also stabilizes excited states and suppresses non-radiative decay pathways, improving emission intensity and lifetime stability.Building on these findings, modern triplet state harvesting design strategies generally focus on two approaches: (a) promoting ISC to maximize triplet generation and (b) minimizing non-radiative decay through molecular rigidity, crystallization, confinement in host–guest systems, or supramolecular assembly (Fig. 2)
However, despite these photophysical benefits, heavy-atom incorporation poses several challenges for biological applications. Metal-based complexes often suffer from poor water solubility, photoinstability, and high cytotoxicity, which can trigger oxidative stress or disrupt intracellular redox balance. Heavy-halogenated organic luminophores also tend to exhibit limited biocompatibility and bioavailability. Furthermore, excessive SOC may lead to emission quenching or compromised color purity under physiological conditions. To address these issues, researchers are developing hybrid strategies such as encapsulation within peptide-based supramolecular scaffolds, which retain SOC-driven luminescence while improving solubility, stability, and biocompatibility.
4. Enhanced emission by supramolecular self-assembled peptide scaffolds
Conventional strategies to enhance luminescence, such as encapsulation in polymers, nanoparticles, or crystalline matrices, often face challenges including poor biocompatibility, limited control over chromophore organization, and inefficient cellular uptake. Peptides offer a compelling solution for designing advanced luminescent materials due to their inherent ability to self-assemble into well-defined nanostructures with tunable rigidity, polarity, and functionality.40,105,106 Their modular design allows precise incorporation of hydrophobic, aromatic, or charged residues, which govern key interactions such as π–π stacking, H-bonding, and local polarity, ultimately controlling excited-state behavior and emission properties. Beyond their structural versatility, peptides combine biocompatibility, biodegradability, and functional adaptability, enabling the integration of recognition motifs for targeted delivery, sensing, and imaging applications. Their ordered scaffolds can restrict non-radiative motions, protect triplet states from quenching, and facilitate energy transfer, making them excellent candidates for enhancing fluorescence, phosphorescence, and TADF. Despite these advantages, peptide-based luminescent systems remain surprisingly underexplored, with most research still dominated by conventional organic or polymeric molecules. This gap highlights an exciting opportunity: by harnessing the structural precision and tunable functionality of peptides, it is possible to develop next-generation luminescent materials that combine high photophysical performance with biocompatibility and targeted functionality.4.1. Peptide-mediated fluorescence enhancement
Fluorescence efficiency in peptides is often hindered by aggregation-caused quenching (ACQ) and solvent-induced non-radiative decay. ACQ occurs when chromophores aggregate, leading to π–π stacking interactions that facilitate non-radiative energy transfer between molecules, thereby dissipating energy as heat rather than light.107 Additionally, interactions with polar solvents can induce vibrational and rotational motions in the excited-state chromophores, providing alternative pathways for energy dissipation through non-radiative processes.108 These phenomena collectively reduce the overall fluorescence efficiency of peptide-based systems. To overcome these challenges, Chen et al. developed a self-assembly locking strategy inspired by the metal-binding mechanism in a Green fluorescent protein (GFP) mutant (BFPms1) (Fig. 3a), which can enhance fluorescence efficiency through bioinspired supramolecular self-assembly.109 They synthesized cyclic(L-histidine-D-histidine) (CHH) peptides and introduced Zn(II) ions to form CHH–Zn nanostructures (Fig. 3b). This coordination restricted molecular motion, reduced energy loss, and increased the quantum yield to over 70%, comparable to inorganic quantum dots. Structural analysis confirmed that Zn(II) coordinated with imidazole groups, forming stable, bright fluorescent assemblies. The self-assembly process involved Zn(II) binding to CHH to form a Zn-centered nucleus, which was further stabilized by surrounding NO3− molecules, forming a CHH–Zn(II) nucleus enclosed by a CHH–NO3− shell. The CHH–Zn nanostructures were applied in optoelectronic devices. As an emissive layer in LEDs and OLEDs, they produced bright green light with high luminous efficiency (Fig. 3c and d). They also explored biomedical applications by developing a “self-encapsulation” strategy. CHH–Zn nanocarriers effectively delivered the anticancer drug epirubicin into HeLa cells while enabling real-time fluorescence monitoring of drug release (Fig. 3e). Two-photon fluorescence lifetime imaging microscopy (FLIM) with phasor analysis revealed time-dependent epirubicin release and nuclear accumulation, indicated by decreasing fluorescence lifetimes across subcellular compartments. This research demonstrated that bioinspired peptide self-assembly could lead to highly fluorescent nanomaterials with applications in optoelectronics and drug delivery. The findings opened new possibilities for developing eco-friendly, multifunctional materials for advanced technologies.109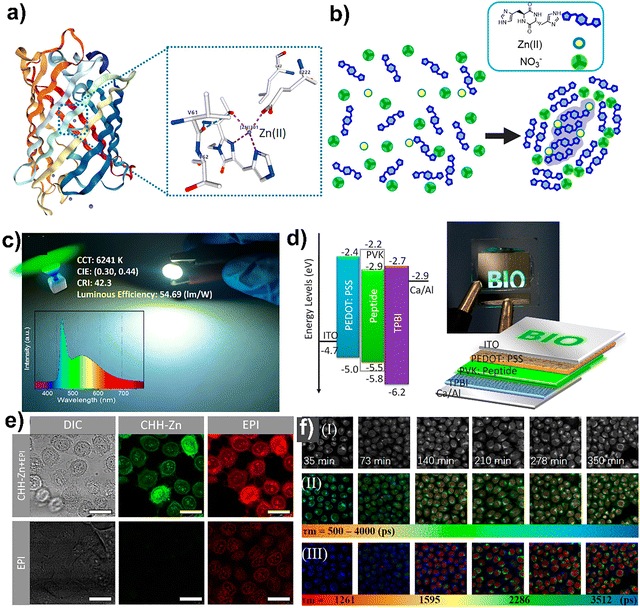 | ||
| Fig. 3 (a) BFPms1 overall structure and its coordination geometry with Zn(II). (b) Scheme of the self-assembly mechanism of CHH, Zn(II), and NO3−. (c) CHH–Zn phosphor enabled a green LED (Inset: emission spectrum). (d) OLED structure, energy diagram, and operation photograph. (e) Confocal fluorescence images of HeLa cells treated with CHH–Zn+ epirubicin and epirubicin alone. Adapted with permission.109 Copyright 2020, American Chemical Society. | ||
Expanding this concept, Perrier and co-workers presented an artificial light-harvesting system based on supramolecular peptide nanotubes in aqueous media, which can offer a promising solution to challenges like poor solubility and aggregation-induced fluorescence quenching seen in traditional artificial photosynthetic systems.110 They have designed three cyclic peptide–polymer conjugates PYR-CP-PEG (pyrene donor), NTI-CP-PEG (naphthalene monoimide intermediate), and Cy3-CP-PEG (cyanine3 acceptor), which can spontaneously self-assemble into well-ordered nanotubes, ensuring precise chromophore alignment and minimizing quenching effects, Fig. 4(a). These nanostructures facilitated an efficient stepwise FRET mechanism, with energy cascading from pyrene to naphthalene monoimide to cyanine3, achieving 95% energy transfer efficiency and maintaining a high fluorescence quantum yield of 30% Fig. 4(b). They showed that this system had tunable emission across the visible spectrum, with a specific combination producing white-light emission and a 29.9% quantum yield, making it promising for optoelectronics and bioimaging (Fig. 4c and d). This study offered a scalable and water-compatible approach for artificial photosynthesis, but factors like long-term stability, environmental durability, and practical device integration still need to be studied. This work advanced supramolecular self-assembly for bio-inspired light-harvesting, leading to new optical materials, photonic devices, and energy transfer systems.110
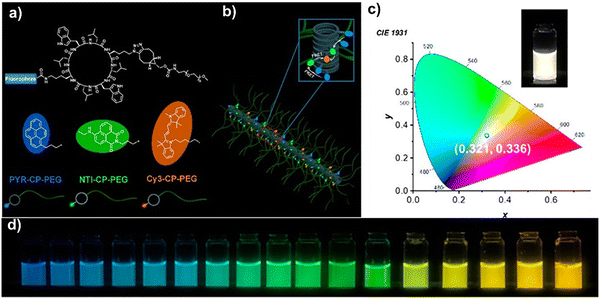 | ||
| Fig. 4 Artificial light-harvesting systems based on supramolecular peptide nanotubes in water. (a) Chemical structures of three fluorophore–cyclic peptide–polymer conjugates. (b) Schematic illustration of the peptide nanotube assembly functioning as an artificial light-harvesting system. (c) White light emission with CIE coordination. (d) Different emission colors at different PYR-CP-PEG/NTI-CP-PEG/Cy3-CP-PEG ratios. Adapted with permission.110 Copyright 2020, American Chemical Society. | ||
Perrier and co-workers previously reported an artificial light-harvesting system based on supramolecular peptide nanotubes in aqueous media, addressing challenges such as poor solubility and aggregation-induced fluorescence quenching common in traditional artificial photosynthetic systems.110 Building on this concept, they developed ultrabright supramolecular fluorophores, marking a significant advancement in overcoming the intrinsic limitations of conventional dyes, including ACQ, low stability, and restricted performance in aqueous environments.111 In the present study, they introduced supra-fluorophores, which are fluorescent supramolecular assemblies derived from cyclic peptide–fluorophore–polymer conjugates. These conjugates leveraged the self-assembly of cyclic peptides into ordered nanotubes, creating confined and rigid microenvironments that stabilized excited states and suppressed non-radiative decay. This structural design effectively prevented detrimental π–π stacking interactions while enabling strong and stable fluorescence in water (Fig. 5a). The modular nature of these assemblies allowed incorporation of different fluorophores, generating versatile artificial light-harvesting systems. Within the peptide nanotube scaffold, donor–acceptor pairs were positioned at controlled distances, which facilitated efficient Förster resonance energy transfer (FRET) and mimicked natural photosynthetic complexes (Fig. 5b). These assemblies showed high fluorescence quantum yields and exceptional brightness per volume, with values reaching up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 m−1 cm−1 nm−3 (Fig. 5c). The system worked well for a wide range of fluorophore families such as cyanines, xanthenes, and BODIPYs. Notably, near-infrared dyes like cyanine 7 (Cy7) and indocyanine green (ICG), which usually suffer from low brightness and poor stability, also showed up to 20-fold improvement in brightness and photostability when incorporated into this system. A particularly feature of these supra-fluorophores was their ultrahigh brightness and photostability under aqueous conditions. The peptide–polymer hybrid design provided both solubility and biocompatibility, which were essential for applications in bioimaging, sensing, and phototherapy. These supra-fluorophores were also successfully employed for bioimaging in live cells and as fluorescent inks, demonstrating their practical potential (Fig. 5d and e).111
060 m−1 cm−1 nm−3 (Fig. 5c). The system worked well for a wide range of fluorophore families such as cyanines, xanthenes, and BODIPYs. Notably, near-infrared dyes like cyanine 7 (Cy7) and indocyanine green (ICG), which usually suffer from low brightness and poor stability, also showed up to 20-fold improvement in brightness and photostability when incorporated into this system. A particularly feature of these supra-fluorophores was their ultrahigh brightness and photostability under aqueous conditions. The peptide–polymer hybrid design provided both solubility and biocompatibility, which were essential for applications in bioimaging, sensing, and phototherapy. These supra-fluorophores were also successfully employed for bioimaging in live cells and as fluorescent inks, demonstrating their practical potential (Fig. 5d and e).111
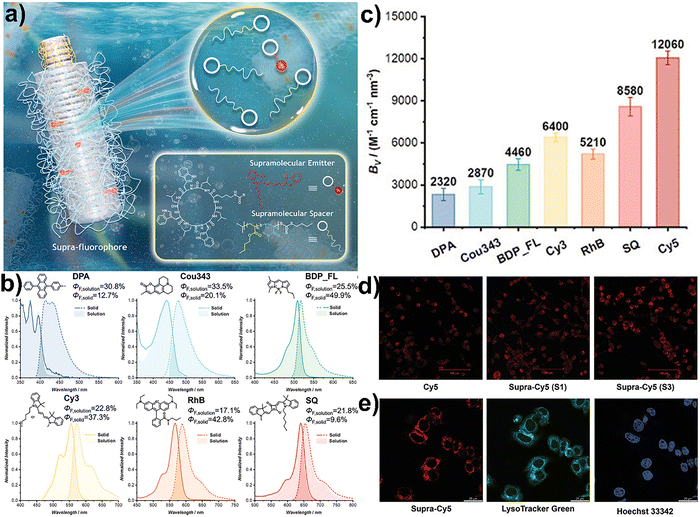 | ||
| Fig. 5 (a) Cartoon illustration of the construction of supra-fluorophores through the co-assembly of supramolecular emitters and supramolecular spacers in aqueous media. (b) Optical properties and practical applications of supra-fluorophores, showing their emission spectra, bright fluorescence in solution and solid states. (c) Summary of maximum brightness values (BV) of supra-fluorophores. (d) Confocal images of cancer cells incubated with supra-fluorophores. (e) Intracellular localization experiment showing supra-fluorophore (red), LysoTracker Green (green), and Hoechst (blue) channels. Adapted with permission.111 Copyright 2024, Wiley-VCH. | ||
4.2. Peptide-mediated room temperature phosphorescence (RTP)
Apart from fluorescence, RTP could also be achieved in aqueous media using supramolecular peptide assemblies. Song and co-workers demonstrated that peptides can play a crucial role in promoting RTP in water. They developed a simple and universal strategy based on cyclic peptide–diblock copolymer conjugates that self-assembled into cylindrical nanostructures with a rigid hydrophobic microdomain.70 These nanostructures can effectively protect organic phosphors from water and oxygen, enabling long-lived phosphorescence at room temperature. By covalently attaching phosphorescent dyes to the peptide units and co-assembling them with the scaffold, they created stable RTP materials in water (Fig. 6a and b). Their method worked across a wide range of organic phosphors, proving its versatility (Fig. 6c). Furthermore, by introducing fluorescent acceptors, they built phosphorescence resonance energy transfer (PRET) systems with tunable afterglow emission and high energy transfer efficiency (Fig. 6d and e). These assemblies also showed excellent stability in various biological media and performed well in live-cell imaging, making them promising for biological and sensing applications (Fig. 6f). This study highlighted the power of peptide-based supramolecular design in expanding the possibilities of aqueous phosphorescent materials.70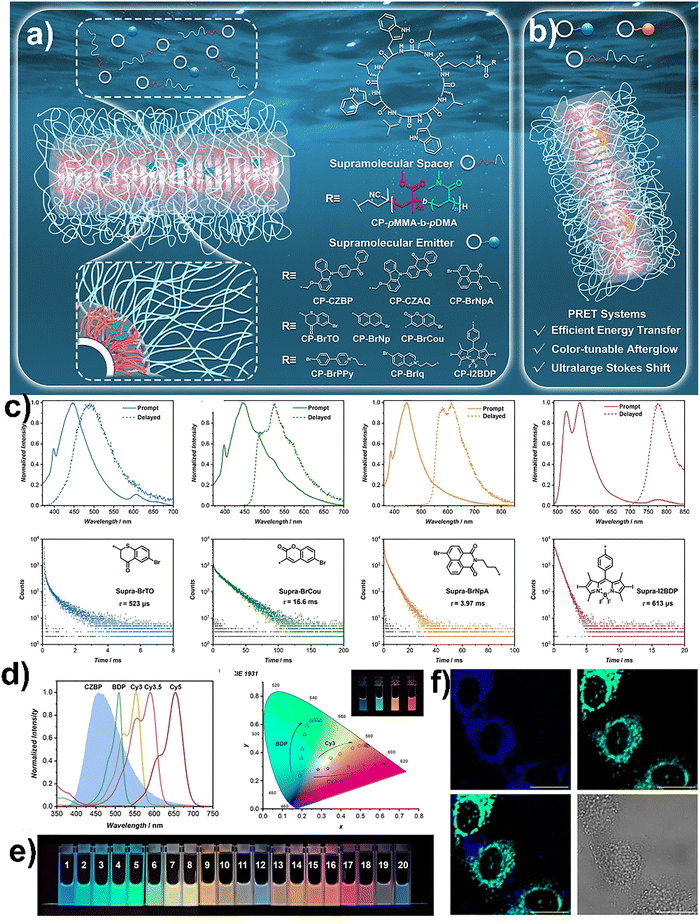 | ||
| Fig. 6 Cartoon illustration of (a) aqueous RTP materials constructed from cyclic peptide-based supramolecular scaffolds and (b) supramolecular PRET systems with color-tunable afterglow emission. (c) Photoluminescence properties of supramolecular RTP materials in water under N2, showing emission spectra and lifetime decay profiles of different assemblies. (d) Color-tunable afterglow emission via PRET and phosphorescence spectrum of supramolecular emitter with absorption spectra of acceptors and CIE diagram. (e) Photograph under UV light showing tunable afterglow emission colors by varying molar ratios of the cyclic peptide with different phosphorescent molecules. (f) Confocal images showing intracellular localization to LysoTracker Green and bright-field channels. Adapted with permission.70 Copyright 2024, Wiley-VCH. | ||
Phosphorescent organic semiconductors such as platinum octaethylporphyrin (PtOEP) are widely used in optoelectronics and sensing due to strong ISC and long-lived emission. However, their translation into biological applications has been limited. Kang et al. reported a strategy that couples PtOEP with a peptide aptamer to achieve selective protein recognition and simultaneous enhancement of phosphorescence intensity and lifetime.69 Using a reprecipitation method, PtOEP was hybridized with the A17 peptide (YCAYYSPRHKTTF), which specifically binds heat shock protein 70 (HSP70) (Fig. 7a). Structural characterization confirmed that the peptide integration preserved PtOEP's crystallinity and emission properties while providing biological recognition functionality. Remarkably, upon HSP70 binding, the PtOEP-A17 assemblies exhibited a 125% increase in phosphorescence intensity and lifetime prolongation from 115 ns to 153 ns (Fig. 7b, e and f). These enhancements, rare in biological environments, are attributed to suppression of non-radiative relaxation pathways and reduced oxygen quenching caused by peptide–protein interactions. Control experiments with bovine serum albumin and nucleolin demonstrated minimal enhancement, confirming selectivity toward HSP70 (Fig. 7c and d). Furthermore, solid-state films of PtOEP-A17 assemblies retained the protein-induced enhancements, underscoring their potential for device integration. This study demonstrated that protein–peptide interactions can be harnessed to stabilize triplet states and boost phosphorescence in hybrid assemblies. Beyond fundamental insights, the findings suggest applications in persistent luminescence imaging, selective biosensing, and diagnostic platforms. By combining a classic OLED phosphor with a biological recognition unit, Kang et al. effectively bridged optoelectronic materials and biotechnology, expanding the utility of phosphorescent emitters into biomedical fields.69
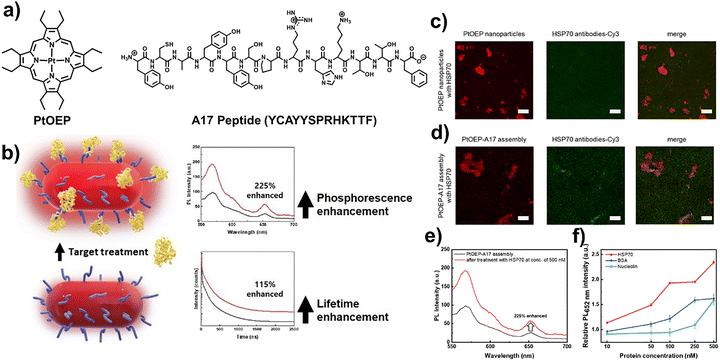 | ||
| Fig. 7 (a) Molecular structures of PtOEP and the A17 peptide. (b) Phosphorescence enhancement of PtOEP–A17 assembly induced by HSP70 binding. (c) CLSM images of PtOEP nanoparticles after treatment with HSP70, Cy3-conjugated HSP70 antibodies, and the merged image. (d) CLSM images of PtOEP–A17 assembly after treatment with HSP70, Cy3-conjugated HSP70 antibodies, and merged channels, acquired with 555 nm excitation. (e) PL spectra of PtOEP–A17 assembly before and after treatment with 500 nM HSP70 (510 nm excitation, solution phase). (f) Relative PL intensity at 652 nm after treatment with HSP70, BSA, and nucleolin at different concentrations. Adapted with permission.69 Copyright 2024, Elsevier B.V. | ||
4.3. Peptide-assisted thermally activated delayed fluorescence (TADF)
Fluorescent probes are indispensable for biological imaging, but conventional dyes often suffer from short lifetimes and poor signal-to-noise ratios due to cellular autofluorescence.72 Long-lived luminescent systems such as TADF molecules offer an attractive alternative, as their delayed emission enables time-gated detection. However, TADF molecules are not stable under normal cellular conditions, because their triplet excited states are easily quenched by oxygen or lost through intramolecular vibrations, while their hydrophobicity often leads to aggregation in aqueous media.112–114 Encapsulation within nanoparticles (NPs) has emerged as an effective strategy to overcome these issues, since aggregation can restrict intramolecular vibration and the NP matrix can shield the triplet state from oxygen. By co-assembling with amphiphilic molecules or polymers, hydrophobic luminophores can be dispersed in aqueous solutions and maintain long-lived luminescence for time-gated or fluorescence lifetime imaging. Yet, many such NPs still suffer from slow cellular uptake and limited biocompatibility, highlighting the need for simpler and more efficient delivery strategies. Zhu et al. introduced an elegant solution by employing an amphiphilic cell-penetrating peptide (CPP), F6G6(rR)3R2, to assemble water-dispersible nanoparticles (NPs) with three representative TADF emitters (Fig. 8a).74 The CPP consists of hydrophobic hexaphenylalanine, a flexible glycine linker, and an arginine-rich cell-penetrating domain. This design allows hydrophobic TADF molecules – 4CzIPN, NAI-DPAC, and BTZ-DMAC to self-assemble into well-dispersed NPs. These NPs exhibit red-shifted steady-state and delayed emission spectra with microsecond lifetimes, confirming efficient TADF through RISC (Fig. 8b–g). Notably, their delayed fluorescence persists under aerobic conditions, indicating effective protection of triplet states within the peptide–NP matrix. Biological evaluation revealed rapid cellular uptake, low cytotoxicity, and strong intracellular emission. Importantly, time-gated fluorescence imaging demonstrated that autofluorescence could be eliminated, yielding high-contrast signals from the long-lived TADF NPs. HeLa cells incubated with peptide-TADF NPs showed bright delayed emission (0.05–2 µs gate), in clear contrast to short-lived background fluorescence (Fig. 8h–p). This work demonstrated a versatile and generalizable strategy for delivering hydrophobic luminophores into cells using CPPs via noncovalent interactions. By combining high permeability, biocompatibility, and microsecond-scale delayed emission, the system advances time-resolved luminescence imaging. Beyond fluorophores, this amphiphilic CPP design may be extended to drug delivery or theranostic platforms, where controlled intracellular transport and long-lived optical signals are desirable.74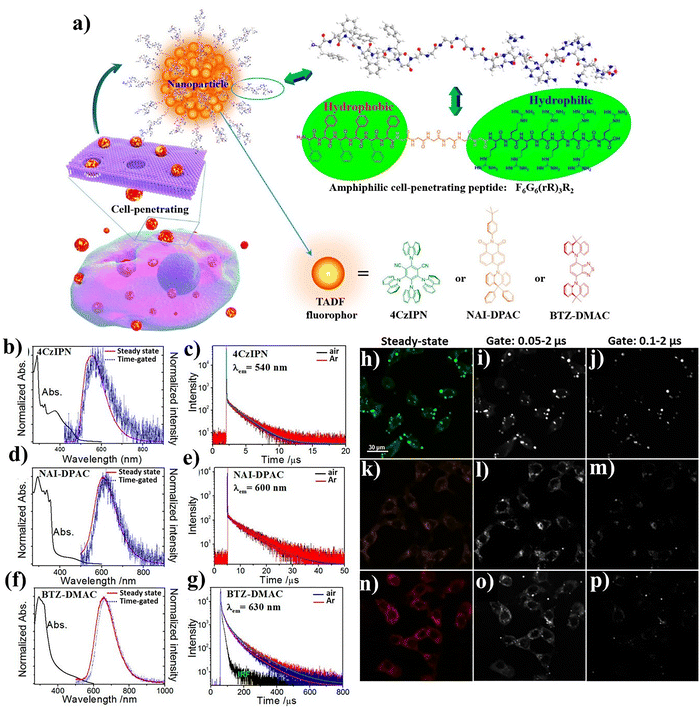 | ||
| Fig. 8 (a) Schematic of cell-penetrating NPs formed by self-assembly of the amphiphilic peptide [F6G6(rR)3R2] with TADF molecules. (b), (d) and (f) Absorption, steady-state, and time-gated emission spectra of TADF NP dispersions (λex = 405 nm, delay = 20 µs). (c), (e) and (g) Luminescence decay curves of TADF NP dispersions. CLSM images of HeLa cells incubated with peptide-functionalized 4CzIPN (h) and (k), NAI-DPAC (i) and (l), and BTZ-DMAC (j) and (m) NPs for 5 min (h)–(j) and 15 min (k)–(m). CLSM images of HeLa cells after incubation with naked 4CzIPN (n), NAI-DPAC (o), and BTZ-DMAC (p) particles for 5 min. Adapted with permission.74 Copyright 2018, American Chemical Society. | ||
Following their earlier studies on peptide-assisted TADF nanoprobes,74 Zhu et al. further advanced the field by developing highly efficient red TADF nanoparticles for real-time, in vivo time-resolved luminescence imaging. In this work, they introduced a host–guest assembly strategy in which the guest molecule 2,6-bis[4-(diphenylamino)phenyl]anthraquinone (TPAAQ) was doped into a 4,4′-bis(carbazol-9-yl)biphenyl (CBP) host matrix through a nanoprecipitation method (Fig. 9a–c).115 Optimal host–guest interactions within CBP suppressed π–π stacking of TPAAQ, resulting in extended luminescence lifetimes exceeding 0.1 ms and a high photoluminescence quantum yield (PLQY) of up to 35% (Fig. 9d). Incorporation of a cell-penetrating peptide (CPP) during assembly enhanced cellular internalization and improved TADF signal intensity (Fig. 9e–g). The CPP-functionalized nanoparticles exhibited bright red emission (λem ≈ 610–650 nm) and prolonged decay profiles, enabling high-contrast lifetime imaging in HepG2 cells (Fig. 9h). Using a cost-effective chopper-based time-gated luminescence microscope, they achieved rapid image acquisition without the need for complex streak or intensified cameras. In vivo imaging of zebrafish confirmed the suppression of tissue autofluorescence and clear detection of delayed luminescence with lifetimes of 0.1–0.2 ms (Fig. 9i). This work demonstrates how rational host–guest molecular design and CPP-assisted delivery can overcome the limitations of low-efficiency organic emitters, opening new opportunities for low-cost, real-time bioimaging using long-lived organic TADF probes under physiological conditions.115
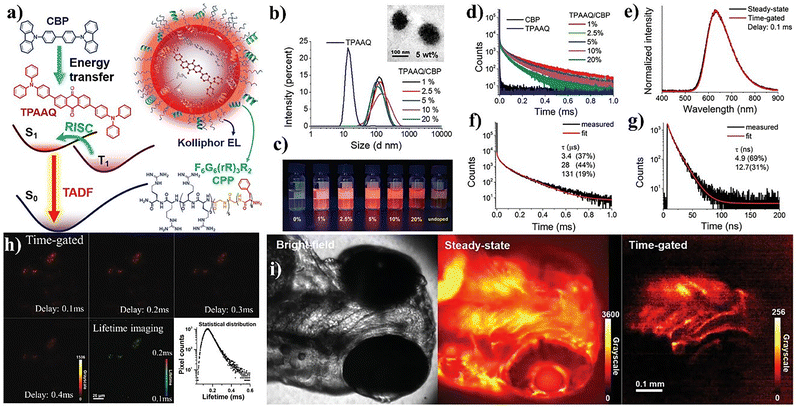 | ||
| Fig. 9 (a) Schematic illustration of host–guest assembled TADF nanoparticles. (b) DLS and TEM images showing uniform morphology. (c) Photographs of nanoparticle solutions under UV light. (d) Steady-state emission spectra of nanoparticle dispersions. (e) Normalized emission spectra and (f) and (g) luminescence decay curves of the nanoparticle dispersion of TPAAQ/CBP/CPP. (h) Time-gated and lifetime luminescence imaging of HepG2 cells incubated with TPAAQ/CBP/CPP nanoparticles (λex = 365 nm, gated time = 0.2 ms). (i) Bright-field, steady-state, and time-gated luminescence images of zebrafish incubated with TPAAQ/CBP/CPP nanoparticles (λex = 365 nm, gated time = 0.2 ms). Adapted with permission.115 Copyright 2023, Wiley-VCH. | ||
In another example, Chu et al. demonstrated how rigid α-helical polypeptide scaffolds can effectively enhance TADF for high-contrast bioimaging.116 Instead of modifying the luminophore itself, they focused on engineering the molecular environment, which shows that conformational rigidity of the host polymer greatly influences emission behavior and lifetime. Star-shaped amphiphilic block copolypeptides were synthesized through N-carboxyanhydride polymerization using poly(amidoamine) (PAMAM) dendrimers as initiators. The α-helical poly(γ-benzyl-L-glutamate) (PBLG) segment formed a stiff hydrophobic core, while the hydrophilic poly(L-lysine) corona ensured aqueous dispersibility (Fig. 10a). Covalent incorporation of a naphthalimide–acridine (ND) TADF dye yielded ∼50 nm unimolecular micelles exhibiting bright orange emission (>600 nm) with microsecond-scale delayed fluorescence, even under oxygenated conditions. The polypeptide-TADF nanoprobes with an enantiomerically pure α-helical core were termed L-PTN, while a flexible-core analogue, DL-PTN, was also synthesized for comparison (Fig. 10b and c). The rigid α-helical conformation restricted molecular motion and prevented aggregation-caused quenching, thereby reducing nonradiative decay and enhancing radiative efficiency (Fig. 10d). Compared to flexible random-coil analogues, these helical nanoprobes displayed nearly three-fold stronger emission and two orders longer lifetimes. Further structural optimization, such as tuning dye density and polymer branching, can show that spatial separation of fluorophores and π–π stacking among aromatic side chains were crucial for maximizing emission performance. For imaging applications, the L-PTN nanoprobes delivered outstanding signal-to-background ratios (SBR), as their long-lifetime orange emission minimized interference from short-lived autofluorescence. Time-resolved detection (50 µs delay) further suppressed background signals, enabling clear cellular visualization (Fig. 10e) and excellent in vivo tissue penetration with high imaging contrast (Fig. 10f–h). This study showed how structural rigidity and polymer conformation can be leveraged to fine-tune TADF properties, offering a powerful route toward next-generation, long-lifetime luminescent probes for biological imaging.116
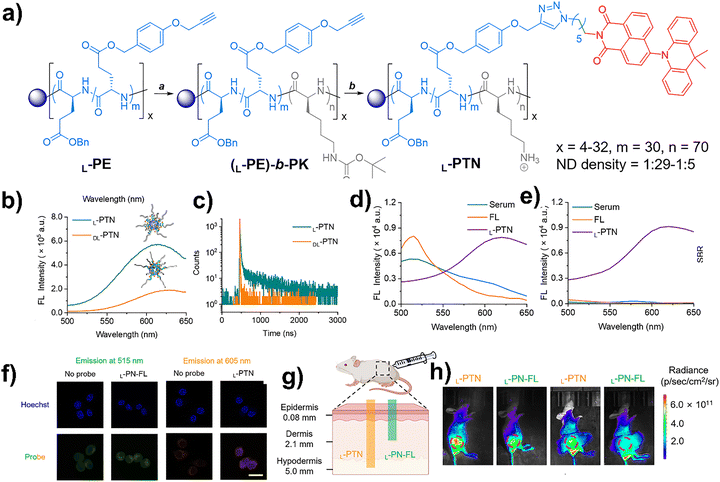 | ||
| Fig. 10 (a) Synthetic routes to polypeptide-based TADF (L-PTN) nanoprobes. (b) Emission spectra and (c) decay profiles of L- and DL-PTN nanoprobes at [ND] = 8 µM in water (λex = 350 nm). (d) and (e) Emission spectra of serum autofluorescence, fluorescein (FL), and L-PTN (d) under prompt and (e) time-resolved (50 µs delay) analysis (λex = 350 nm), showing background suppression. (f) CLSM images of RAW 264.7 cells with/without nanoprobes; emission collected at 515 nm (green, FL) and 605 nm (orange, L-PTN) under 488 nm excitation. Enhanced contrast and tissue penetration of L-PTN nanoprobes for in vivo imaging. (g) Illustration of deeper tissue penetration of orange-emitting L-PTN vs. green-emitting L-PN-FL (FL-based polypeptide nanoprobes). (h) In vivo fluorescence of mice post subcutaneous injection under normal or 6 mm tissue-covered conditions. Adapted with permission.116 Copyright 2025, American Chemical Society. | ||
We have summarised representative peptide-based supramolecular assemblies and their key photophysical parameters, including emission type wavelength, lifetime and quantum yield, highlighting how self-assembly enhances fluorescence, RTP and TADF in aqueous and biological environments (Table 1).
| Luminophore system | Peptide scaffold/assembly type | Emission type | Emission max (nm) | Lifetime (τ) | Quantum yield (Φ) | Medium | Key role of self-assembly | Ref. |
|---|---|---|---|---|---|---|---|---|
| NR: Not reported. | ||||||||
| CHH-Zn(II) nanostructurs | Cyclic His peptide coordinated with Zn(II) | Fluorescence | ∼520 | 1.3 ns and 1.89 ns | >70% | Aqueous | Coordination-driven rigidity restricts molecular motion and suppresses non-radiative decay | 109 |
| PYR–CP–PEG/NTI–CP–PEG/Cy3–CP–PEG | Cyclic peptide nanotubes | Fluorescence (FRET) | Tunable and white light | 3 ns to 67 ns | ∼30% | Aqueous | Ordered nanotube assembly enables controlled chromophore spacing and efficient energy transfer | 110 |
| Supra-fluorophores | Cyclic peptide–polymer nanotubes | Fluorescence | Visible–NIR | 0.5 ns to 1.6 ns | 34% | Aqueous | Confined rigid nanotube microenvironment prevents ACQ and stabilizes excited states | 111 |
| Organic phosphor–peptide conjugates | Cyclic peptide-based cylindrical assemblies | RTP | 520–790 | 100 µs–750 µs | 31% | Aqueous | Hydrophobic rigid microdomains shield triplet states from oxygen and water | 70 |
| PtOEP-A17 peptide hybrid | Peptide aptamer–phosphor assembly | RTP | ∼652 | 115–153 ns | 125% | Aqueous/solid | Peptide–protein binding suppresses non-radiative relaxation and oxygen quenching | 69 |
| CPP-TADF nanoparticles | Amphiphilic cell-penetrating peptide NPs | TADF | 550–650 | 1 µs–100 µs | 12% | Aqueous | Peptide-assisted confinement protects triplet states and enables RISC under aerobic conditions | 74 |
| TPAAQ/CBP/CPP NPs | Host–guest + CPP assembly | TADF | 610–650 | 0.1–0.2 ms | ∼35% | Aqueous/in vivo | Host–guest packing suppresses π–π stacking and the peptide enhances cellular uptake | 115 |
| L-PTN polypeptide nanoprobes | α-helical polypeptide micelles | TADF | >600 | ∼2 µs | NR | Aqueous/biological | Helical rigidity restricts motion and enhances delayed emission with high SBR | 116 |
5. Conclusion and future outlook
In this review, we have focused on the use of peptide-based supramolecular scaffolds as versatile platforms for enhancing luminescence, including fluorescence, RTP, and TADF, particularly in biologically relevant environments. Peptides offer unique advantages due to their biocompatibility, biodegradability, modularity, and self-assembly capabilities, allowing precise control over molecular interactions, chromophore orientation, and local microenvironments, which are the key factors for efficient emission. By incorporating hydrophobic, aromatic, or charged residues, peptide assemblies can finely tune π–π stacking, H-bonding, and polarity, enabling the control of excited-state dynamics and minimizing energy loss.117Despite these advantages, peptide-based luminescent systems remain underexplored, particularly for biomedical applications. Challenges include aggregation-caused quenching, solvent-induced non-radiative decay, limited stability under physiological conditions, and the empirical nature of sequence design. Rational and predictive design of peptide sequences remains a major focus for future research. Advanced computational tools such as molecular dynamics simulations, machine learning, and artificial intelligence could guide the design of peptide scaffolds with optimized energy transfer, exciton migration, and delayed emission.40
Stability in complex environments is another critical concern. Peptide assemblies are often sensitive to pH, ionic strength, temperature, or enzymatic degradation, which can reduce luminescence efficiency or limit biological applications. Future strategies could include cyclizing the peptides, adding non-natural amino acids, or combining peptides with polymers to keep their versatile design while making them more stable (Scheme 2).
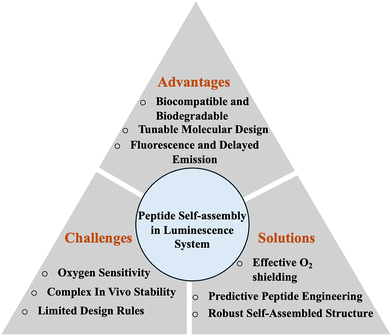 | ||
| Scheme 2 Overview of advantages, challenges and solutions for peptide-based supramolecular luminescent systems highlighting future outlook. | ||
Expanding the range of compatible chromophores is also crucial. Many advanced luminophores, including near-infrared dyes, phosphorescent organometallic complexes, or TADF molecules, face quenching, poor solubility, or aggregation issues in aqueous or cellular environments. Peptide scaffolds can offer hydrophobic protection, structural rigidity, and controlled chromophore orientation, extending the utility of peptide assemblies for bioimaging, optoelectronics, and artificial photosynthetic systems.
Additionally, peptides provide opportunities for development of multifunctional and stimuli-responsive systems. Incorporating recognition motifs, catalytic sites, or stimuli-responsive units can enable the formation of assemblies that respond to pH changes, enzymatic activity, or light, allowing dynamic control of emission and the integration of imaging, sensing, and therapeutic functions within a single platform.
Scalability and device integration remain important considerations. Laboratory-scale peptide–luminophore assemblies show promising optical properties, but translation into practical devices or commercial applications will require reproducible self-assembly, standardized protocols, robust purification, and long-term stability. Hybrid approaches combining peptides with polymers or inorganic supports may help bridge this gap.
In conclusion, peptide-mediated self-assembly represents a highly adaptable and tunable approach to controlling luminescence, combining molecular precision with biocompatibility. By addressing challenges in sequence design, stability, chromophore compatibility, and integration into devices or biological systems, future research can unlock the full potential of peptides as scaffolds for advanced photonic, optoelectronic, and biomedical applications. This review highlights the promising role of peptides in creating next-generation luminescent materials that are not only high-performing but also multifunctional and biologically relevant.
Author contributions
GG conceptualized the topic of the review. GG and SP contributed to surveying the literature and writing processes.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
SP acknowledges CeNS for the fellowship. GG thanks the Ramanujan Fellowship (file no. RJF/2022/000002), SERB (now ANRF), Government of India and CeNS (Project no.: CeNSitize-5-GG) for the funding support.References
- Y. Su, B. Yu, S. Wang, H. Cong and Y. Shen, Biomaterials, 2021, 271, 120717 CrossRef CAS PubMed.
- Y. Huang, Y. Zhang, F. Huo, Y. Wen and C. Yin, Sci. China: Chem., 2020, 63, 1742–1755 CrossRef CAS.
- J. Wang, C. Yin and F. Huo, Analyst, 2025, 150, 220–239 RSC.
- M. Asgher, S. A. Qamar, M. Sadaf and H. M. N. Iqbal, Biomed. Phys. Eng. Express, 2020, 6, 012003 CrossRef PubMed.
- U. Ghosh, N. Kumar and G. Ghosh, in Pharmaceutical Applications of Supramolecules, ed. N. Goel, N. Kumar, Springer, Cham, 2022, pp. 273–299 Search PubMed.
- K. Wu, Y. Zheng, R. Chen, Z. Zhou, S. Liu, Y. Shen and Y. Zhang, Biosens. Bioelectron., 2023, 223, 115031 CrossRef CAS PubMed.
- D. Deng, Y. Chang, W. Liu, M. Ren, N. Xia and Y. Hao, Biosensors, 2023, 13(8), 773 CrossRef CAS.
- J. B. Kaushal, P. Raut and S. Kumar, Biosensors, 2023, 13(11), 976 CrossRef CAS.
- J. Zou, Z. Yin, P. Wang, D. Chen, J. Shao, Q. Zhang, L. Sun, W. Huang and X. Dong, Chem. Sci., 2018, 9, 2188–2194 RSC.
- L. Li, Y. Chen, W. Chen, Y. Tan, H. Chen and J. Yin, Chin. Chem. Lett., 2019, 30, 1689–1703 CrossRef CAS.
- L. Sun, J. Wang, B. Yang, X. Wang, G. Yang, X. Wang, Y. Jiang, T. Wang and J. Jiang, RSC Adv., 2021, 11, 10061–10074 RSC.
- J. Yang, D. Yan and T. S. Jones, Chem. Rev., 2015, 115, 5570–5603 CrossRef PubMed.
- O. Ostroverkhova, Chem. Rev., 2016, 116, 13279–13412 Search PubMed.
- R. Iftikhar, F. Z. Khan and N. Naeem, Mol. Diversity, 2024, 28, 271–307 CrossRef PubMed.
- M. He, C. Ding, H. Guo and Q. Li, React. Mater., 2024, 2, e20240014 Search PubMed.
- G. Jiang, Y. Song, X. Guo, D. Zhang and D. Zhu, Adv. Mater., 2008, 20, 2888–2898 CrossRef.
- B. J. Cafferty, A. S. Ten, M. J. Fink, S. Morey, D. J. Preston, M. Mrksich and G. M. Whitesides, ACS Cent. Sci., 2019, 5, 911–916 CrossRef.
- Y. Xiong, J. Gong, J. Liu, D. Wang, H. Wu, Z. Zhao, M. Fang, Z. Li, D. Wang and B. Z. Tang, J. Mater. Chem. C, 2022, 10, 10009–10016 RSC.
- L. Zhan, Z. Chen, S. Gong, Y. Xiang, F. Ni, X. Zeng, G. Xie and C. Yang, Angew. Chem., Int. Ed., 2019, 58, 17651–17655 CrossRef PubMed.
- N. G. Zhegalova, S. He, H. Zhou, D. M. Kim and M. Y. Berezin, Contrast Media Mol. Imaging, 2014, 9, 355–362 Search PubMed.
- F. Di Maiolo, D. K. A. Phan Huu, D. Giavazzi, A. Landi, O. Racchi and A. Painelli, Chem. Sci., 2024, 15, 5434–5450 Search PubMed.
- Y. Hou, G. Jiang, J. Gong, R. Sha and J. Wang, Chem. Res. Chin. Univ., 2021, 37, 73–82 Search PubMed.
- H. Sun, Y. Xiao, Y. He, X. Wei, J. Zou, Y. Luo, Y. Wu, J. Zhao, V. K.-M. Au and T. Yu, Chem. Sci., 2025, 16, 5299–5309 RSC.
- H. Sun and L. Zhu, Aggregate, 2023, 4, e253 CrossRef.
- S. Datta and J. Xu, ACS Appl. Bio Mater., 2023, 6, 4572–4585 CrossRef PubMed.
- Y. Zhang, Y. Huang, R. Miao and H. Chen, Small Struct., 2023, 4, 2300157 CrossRef.
- L. Su, X. Fan, T. Yin, H. Wang, Y. Li, F. Liu, J. Li, H. Zhang and H. Xie, Adv. Opt. Mater., 2020, 8, 1900978 CrossRef.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef PubMed.
- J. Liu, A. M. Kaczmarek and R. Van Deun, Chem. Soc. Rev., 2018, 47, 7225–7238 RSC.
- F. Sheehan, D. Sementa, A. Jain, M. Kumar, M. Tayarani-Najjaran, D. Kroiss and R. V. Ulijn, Chem. Rev., 2021, 121, 13869–13914 CrossRef.
- S. Narayanan, A. A. Kongasseri and S. J. George, Chem. Sci., 2025, 16, 15781–15795 RSC.
- S. K. Panda, A. De and S. Banerjee, Photochem. Photobiol., 2024, 100, 796–829 CrossRef PubMed.
- T. Pope, J. Eng, A. Monkman and T. J. Penfold, J. Chem. Theory Comput., 2024, 20, 1337–1346 CrossRef PubMed.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- X. Y. Lou and Y. W. Yang, Aggregate, 2020, 1, 19–30 CrossRef.
- T. Wu, J. Huang and Y. Yan, Cell Rep. Phys. Sci., 2022, 3, 100771 CrossRef.
- H. T. Le, C. V. Stanley and A. Goto, Polym. Chem., 2024, 15, 2873–2882 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef.
- M. Z. Cai, Z. Wen, H.-Z. Li, Y. Yang, J.-X. Liang, Y.-S. Liao, J.-Y. Wang, L.-Y. Wang, N.-Y. Zhang, K. Kamei, H.-W. An and H. Wang, Biosens. Bioelectron., 2025, 276, 117255 CrossRef.
- X. Zhong, Y. Xie, Y. Chen, Y. Lu and M. Hou, Int. J. Nanomed., 2025, 10751–10770 CrossRef PubMed.
- D. Tesauro, A. Accardo, C. Diaferia, V. Milano, J. Guillon, L. Ronga and F. Rossi, Molecules, 2019, 24, 351 CrossRef.
- T. N. Das, A. Ramesh, A. Ghosh, S. Moyra, T. K. Maji and G. Ghosh, Nanoscale Horiz., 2025, 10, 279–313 RSC.
- U. Ghosh and G. Ghosh, in Pharmaceutical Applications of Supramolecules, ed. N. Goel and N. Kumar, Springer, Cham, 2022, pp. 241–271 Search PubMed.
- A. Ramesh, T. N. Das, T. K. Maji and G. Ghosh, Chem. Sci., 2024, 15, 16355–16366 RSC.
- G. Ghosh, K. K. Kartha and G. Fernández, Chem. Commun., 2021, 57, 1603–1606 RSC.
- S. V. R. Jonnalagadda, E. Ornithopoulou, A. A. Orr, E. Mossou, V. Trevor Forsyth, E. P. Mitchell, M. W. Bowler, A. Mitraki and P. Tamamis, Mol. Syst. Des. Eng., 2017, 2, 321–335 RSC.
- Q. Li, W. Chao and L. Qiu, Amino Acids, 2025, 57, 25 CrossRef.
- X. Fan, Y. Sato, Y. Shiraki and S. Nishizawa, Anal. Sci., 2024, 40, 609–614 CrossRef.
- S. Patra, R. Gowdihalli Nagaraj, S. Moyra and G. Ghosh, Chem. Phys. Chem., 2025, 26, e202500150 CrossRef.
- M. Abbas, Q. Zou, S. Li and X. Yan, Adv. Mater., 2017, 29, 1605021 CrossRef.
- G. Feng, G. Q. Zhang and D. Ding, Chem. Soc. Rev., 2020, 49, 8179–8234 RSC.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef.
- H. Szmacinski and J. R. Lakowicz, Sens. Actuators, B, 1995, 29, 16–24 CrossRef.
- H. Zhu, J. Fan, J. Du and X. Peng, Acc. Chem. Res., 2016, 49, 2115–2126 CrossRef PubMed.
- Y. Lin, Z. Yang, R. J. Lake, C. Zheng and Y. Lu, Angew. Chem., Int. Ed., 2019, 58, 17061–17067 CrossRef.
- W. Zheng, Z. Zhou, T. Zhu, Z. Zou, Q. Shan, Q. Huang, G. Wang and Y. Wang, Microchem. J., 2025, 210, 112968 CrossRef.
- J. A. Stibbe, P. Hoogland, F. B. Achterberg, D. R. Holman, R. S. Sojwal, J. Burggraaf, A. L. Vahrmeijer, W. B. Nagengast and S. Rogalla, Mol. Imaging Biol., 2023, 25, 18–35 CrossRef PubMed.
- N. W. Nkune, K. Moloudi, B. P. George and H. Abrahamse, RSC Adv., 2025, 15, 22267–22284 RSC.
- Y. Jiang and K. Pu, Chem. Rev., 2021, 121, 13086–13131 CrossRef PubMed.
- J. Zhu, L. Zhao, W. An and Q. Miao, Chem. Soc. Rev., 2025, 54, 1429–1452 RSC.
- K. Xiao, S. Sun, J. Xu and X. Ma, Chin. J. Chem. Eng., 2025, 86, 87–103 CrossRef.
- C. Li, M. Wu, Z. Zhao, J. W. Y. Lam, B. Xu and B. Z. Tang, Chem, 2025, 11, 102654 Search PubMed.
- S. Yuan, Q. Sun, Y. Wang, L. Yue, J. Ma, Y. Zhang, H. Zhang, S. Xue and W. Yang, J. Mater. Chem. C, 2021, 9, 8302–8307 RSC.
- S. Ravi, S. Karthikeyan, M. Pannipara, A. G. Al-Sehemi, D. Moon and S. P. Anthony, CrystEngComm, 2024, 26, 1773–1778 RSC.
- X. Huang, Y. Zhang, G. Li, T. Wang, P. Sun, J. Shi, B. Tong, J. Zhi, Z. Li, Z. Cai and Y. Dong, ChemPhotoChem, 2025, 9, e202400315 CrossRef CAS.
- S. Peng, X. Cai, Q. Zhang, Y. Sun, L. Zheng, Q. Cao and Y. Shi, Mater. Chem. Front., 2025, 9(22), 3245–3263 RSC.
- Y. Yang, Q. Li and Z. Li, Mater. Chem. Front., 2025, 9, 744–753 RSC.
- Z. Zhan, S. Li, L. Shen, Z. Cheng, X. Men and H. Chen, Adv. Mater. Technol., 2025, 10, e01366 CrossRef.
- M. J. Kang, Y. H. Cho, S. Kim and D. J. Ahn, Int. J. Biol. Macromol., 2024, 266, 131195 CrossRef CAS.
- R. Feng, X. Yan, Y. Sang, X. Liu, Z. Luo, Z. Xie, Y. Ke and Q. Song, Angew. Chem., Int. Ed., 2025, 64, e202421729 CrossRef CAS PubMed.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Appl. Phys. Lett., 2011, 98, 083302 CrossRef.
- Y. Xia, M. Zheng, X. Li, K. Song and Z. Teng, iScience, 2025, 28, 113526 CrossRef CAS PubMed.
- Y. Sun, L. Wu, L. Zhu, G. V. Baryshnikov, F. Zhang and X. Li, Small Methods, 2025, 9, 2400982 CrossRef CAS PubMed.
- Z. Zhu, D. Tian, P. Gao, K. Wang, Y. Li, X. Shu, J. Zhu and Q. Zhao, J. Am. Chem. Soc., 2018, 140, 17484–17491 CrossRef CAS PubMed.
- Z. Xie, L. Ma, K. E. deKrafft, A. Jin and W. Lin, J. Am. Chem. Soc., 2010, 132, 922–923 CrossRef CAS PubMed.
- L. W. Lo, C. J. Koch and D. F. Wilson, Anal. Biochem., 1996, 236, 153–160 CrossRef CAS PubMed.
- M. A. Haidekker and E. A. Theodorakis, J. Biol. Eng., 2010, 4, 11 CrossRef PubMed.
- X. Hu, D. Wang, Y. Wang, Y. Wang and S. Zhang, J. Chem. Phys., 2024, 160, 154302 CrossRef CAS PubMed.
- H. Chen, Y. Zhang, J. Shan, M. Dong, Z. Qian, A. Lv, H.-J. Qian, H. Ma, Z. An, L. Gu and W. Huang, Angew. Chem., Int. Ed., 2025, 64, e202500610 CrossRef CAS PubMed.
- J. Maillard, K. Klehs, C. Rumble, E. Vauthey, M. Heilemann and A. Fürstenberg, Chem. Sci., 2021, 12, 1352–1362 RSC.
- X. F. Wang, H. Xiao, P. Z. Chen, Q. Z. Yang, B. Chen, C.-H. Tung, Y.-Z. Chen and L.-Z. Wu, J. Am. Chem. Soc., 2019, 141, 5045–5050 CrossRef CAS PubMed.
- C. Yin, Z.-A. Yan and X. Ma, Chem. Commun., 2023, 59, 13421–13433 RSC.
- Q. Li and L. Blancafort, Chem. Commun., 2013, 49, 5966–5968 RSC.
- S. Suzuki, S. Sasaki, A. S. Sairi, R. Iwai, B. Z. Tang and G. Konishi, Angew. Chem., Int. Ed., 2020, 59, 9856–9867 CrossRef CAS PubMed.
- M. S. Tsai, S.-Y. Tsai, Y.-F. Huang, C. L. Wang, S. S. Sun and J. S. Yang, Chem. – Eur. J., 2020, 26, 5942–5945 CrossRef CAS PubMed.
- L. Yang, Y. Yu, J. Zhang, F. Ge, J. Zhang, L. Jiang, F. Gao and Y. Dan, Chem. – Asian J., 2015, 10, 1215–1224 CrossRef CAS PubMed.
- P. Singh, L. S. Mittal and K. Kumar, Chem. Commun., 2025, 61, 10505–10521 RSC.
- L. Shi, C. Nguyen, M. Daurat, N. Richy, C. Gauthier, E. Rebecq, M. Gary-Bobo, S. Cammas-Marion, O. Mongin, C. O. Paul-Roth and F. Paul, Cancers, 2022, 14, 2358 CrossRef CAS PubMed.
- D. Li, F. Lu, J. Wang, W. Hu, X.-M. Cao, X. Ma and H. Tian, J. Am. Chem. Soc., 2018, 140, 1916–1923 CrossRef CAS PubMed.
- Z. Y. Zhang, Y. Chen and Y. Liu, Angew. Chem., Int. Ed., 2019, 58, 6028–6032 CrossRef CAS PubMed.
- C. I. C. Crucho, J. Avó, A. M. Diniz, S. N. Pinto, J. Barbosa, P. O. Smith, M. N. Berberan-Santos, L.-O. Pålsson and F. B. Dias, Front. Chem., 2020, 8, 00404 CrossRef CAS.
- A. J. Gillett, A. Pershin, R. Pandya, S. Feldmann, A. J. Sneyd, A. M. Alvertis, E. W. Evans, T. H. Thomas, L.-S. Cui, B. H. Drummond, G. D. Scholes, Y. Olivier, A. Rao, R. H. Friend and D. Beljonne, Nat. Mater., 2022, 21, 1150–1157 CrossRef CAS PubMed.
- J. Fan, H. Liu, Y. Song, L. Lin, C.-K. Wang, Y. Xu and X. Zhao, J. Mater. Chem. C, 2024, 12, 6114–6130 RSC.
- J. Omar, D. Ponsford, C. A. Dreiss, T.-C. Lee and X. J. Loh, Chem. – Asian J., 2022, 17, e202200081 CrossRef CAS PubMed.
- H. Shi, W. Yao, W. Ye, H. Ma, W. Huang and Z. An, Acc. Chem. Res., 2022, 55, 3445–3459 CrossRef CAS PubMed.
- R. Kabe and C. Adachi, Nature, 2017, 550, 384–387 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- G. Liu, L. Yue, Y. Wang, S. Xue, Q. Sun and W. Yang, Chem. Eng. J., 2024, 498, 155171 CrossRef CAS.
- J. Wang, S. Hu, Y. Wu, Y. Zhang, Y. Tao and Y. Tao, Dyes Pigm., 2025, 240, 112822 CrossRef CAS.
- J. S. Ward, R. S. Nobuyasu, M. A. Fox, J. A. Aguilar, D. Hall, A. S. Batsanov, Z. Ren, F. B. Dias and M. R. Bryce, J. Org. Chem., 2019, 84, 3801–3816 CrossRef CAS PubMed.
- C. Fabregat, R. Bujaldón, J. Garcia-Amorós, D. Volyniuk, M. Ghasemi, J. V. Grazulevicius and D. Velasco, Dyes Pigm., 2025, 243, 113053 CrossRef CAS.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- M. S. Mehata, Y. Yang, Z. J. Qu, J. S. Chen, F. J. Zhao and K.-L. Han, RSC Adv., 2015, 5, 34094–34099 RSC.
- Y. Sun, Z. M. Hudson, Y. Rao and S. Wang, Inorg. Chem., 2011, 50, 3373–3378 CrossRef CAS PubMed.
- B. Wang, L. Wang, C. Chen, Y. Zhang, J. Gao, K. Lu, C. Yan, G. Nan and Y. Li, ChemistrySelect, 2023, 8, e202302216 CrossRef CAS.
- T. Li, X.-M. Lu, M.-R. Zhang, K. Hu and Z. Li, Bioact. Mater., 2022, 11, 268–282 CAS.
- A. Aarzoo, K. Saita, M. Kobayashi, T. Tsuneda, T. Taketsugu and R. K. Roy, Phys. Chem. Chem. Phys., 2025, 27, 12593–12602 RSC.
- M. F. Pignataro, M. G. Herrera and V. I. Dodero, Molecules, 2020, 25, 4854 CrossRef CAS PubMed.
- Y. Chen, A. A. Orr, K. Tao, Z. Wang, A. Ruggiero, L. J. W. Shimon, L. Schnaider, A. Goodall, S. Rencus-Lazar, S. Gilead, I. Slutsky, P. Tamamis, Z. Tan and E. Gazit, ACS Nano, 2020, 14, 2798–2807 CrossRef CAS PubMed.
- Q. Song, S. Goia, J. Yang, S. C. L. Hall, M. Staniforth, V. G. Stavros and S. Perrier, J. Am. Chem. Soc., 2021, 143, 382–389 CrossRef CAS PubMed.
- Y. Lei, Y. Wang, S. K. Hill, Z. Cheng, Q. Song and S. Perrier, Adv. Mater., 2024, 36, 2401346 CrossRef CAS PubMed.
- X. Xiong, L. Zheng, J. Yan, F. Ye, Y. Qian and F. Song, RSC Adv., 2015, 5, 53660–53664 RSC.
- A. Vaasa, K. Ligi, S. Mohandessi, E. Enkvist, A. Uri and L. W. Miller, Chem. Commun., 2012, 48, 8595–8597 RSC.
- X. Xiong, F. Song, J. Wang, Y. Zhang, Y. Xue, L. Sun, N. Jiang, P. Gao, L. Tian and X. Peng, J. Am. Chem. Soc., 2014, 136, 9590–9597 CrossRef CAS PubMed.
- Z. Zhu, Z. Luo, Y. Q. Xie, Y. Sun, L. Xu and Q. Wu, Adv. Funct. Mater., 2024, 34, 2313701 CrossRef CAS.
- Y. Chu, X. Jin, G. Ji, P. Li, S. Xiao, W. Wang and Z. Song, ACS Nano, 2025, 19, 680–690 CrossRef CAS PubMed.
- N. J. Sinha, M. G. Langenstein, D. J. Pochan, C. J. Kloxin and J. G. Saven, Chem. Rev., 2021, 121, 13915–13935 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |


