Open Access Article
Open Access ArticleInhibition of glycoprotein biosynthesis in the pathogenic bacterium Helicobacter pylori by masked carbohydrate phosphonates
Ebrahim
Soleimani†
a,
Aniqa
Chowdhury†
b,
Jian-She
Zhu
a,
Elisa
Ospanow
a,
Karen D.
Moulton
b,
Danielle H.
Dube
 *b and
David L.
Jakeman
*b and
David L.
Jakeman
 *ac
*ac
aDepartment of Chemistry, Dalhousie University, Halifax, Nova Scotia B3H 4J3, Canada. E-mail: David.jakeman@dal.ca
bDepartment of Chemistry and Biochemistry, Bowdoin College, Maine, USA. E-mail: ddube@bowdoin.edu
cCollege of Pharmacy, Dalhousie University, Halifax, Nova Scotia B3H 4R2, Canada
First published on 1st December 2025
Abstract
The glycan-rich surface of Helicobacter pylori plays a critical role in host–pathogen interactions and represents a promising target for therapeutic intervention. We report the synthesis and biological evaluation of a masked bis(pivaloyloxymethyl) phosphonate analogue of α-D-glucose 1-phosphate designed to inhibit glycoprotein biosynthesis in H. pylori. This prodrug strategy enhances bacterial uptake by neutralizing the phosphonate's dianionic charge, potentially enabling intracellular esterase-mediated release of the active phosphonate. Using metabolic oligosaccharide engineering (MOE) with Ac4GlcNAz, we demonstrate that the masked phosphonate exhibits dose-dependent inhibition of glycoprotein biosynthesis, whereas unmasked and cyclic phosphonate analogues show minimal activity. These findings highlight the potential of masked phosphonates as chemical tools for probing bacterial glycosylation and as leads for novel antibacterial agents.
Bacteria rely on their glycan-rich surface coats to mediate host interactions, promote colonization, and resist drug entry.1 Importantly, bacterial glycans differ structurally from those found in mammalian cells and play crucial roles in pathogenesis by aiding immune evasion and promoting tissue adhesion.2 Targeting bacterial glycosylation, therefore, offers a promising strategy to develop selective antibacterial therapies that minimize harm to the host. Bacterial glycan biosynthesis pathways, including those involved in glycoprotein and lipopolysaccharide assembly, lack direct genetic labelling strategies, necessitating the use of complementary chemical approaches for structural and functional analysis.3,4
Metabolic oligosaccharide engineering (MOE)5 is an enabling analytical technique used to monitor glycan assembly in both mammalian and bacterial cells.6,7 Its application in bacteria has yielded important insights into glycoprotein biosynthesis in select pathogenic species. In particular, Helicobacter pylori is an aetiological factor for gastric cancer and peptic ulcer disease,8,9 that is particularly amenable to MOE (Fig. 1).10–13 Metabolic labelling of glycoproteins in H. pylori with the azidosugar N-azidoacetylglucosamine (GlcNAz) led to the discovery of specific small molecules capable of modulating glycoprotein biosynthesis in H. pylori, including benzyl14 or thioglycosides,15 however, the concentrations necessary to effect observable changes in glycoprotein biosynthesis remain relatively high, in the millimolar regime. These high concentrations likely reflect, in part, restricted uptake across the bacterial envelope.16 This permeability barrier remains a significant impediment to developing glycan-targeted antibacterial agents.17
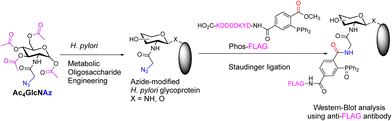 | ||
| Fig. 1 Metabolic Oligosaccharide Engineering in H. pylori.11 Ac4GlcNAz = N-azidoacetyltetraacetylglucosamine. The acetyl esters increase lipophilicity of the azidocarbohydrate to enhance uptake whereupon esters are cleaved. | ||
Other pathogenic bacteria have remained recalcitrant towards utilization of MOE to observe glycoprotein biosynthesis, potentially due to the lack of metabolic processing of GlcNAz. Nitz and co-workers have recently explored a mutant E. coli strain over-expressing an anomeric kinase, to determine the fate of GlcNAz in E. coli with a view to demonstrating incorporation of GlcNAz into peptidoglycan (PGN), poly-β-1,6-N-acetylglucosamine (PNAG), lipopolysaccharide (LPS) or enterobacterial common antigen (ECA), with the highest levels identified in PNAG.18 In addition to the lack of MOE in a broad swathe of pathogenic bacteria, there are relatively few reports of small molecules capable of modulating glycoprotein biosynthesis in bacteria. Successes in this arena have focused on high-throughput library screens19,20 to identify inhibitors of bacterial monosaccharide biosynthesis that subsequently impede downstream glycoprotein construction.
To expand the approaches available to inhibit bacterial glycoprotein biosynthesis, we were inspired by successes in using carbohydrate phoshonate-based inhibitors in mammalian systems21 and C-glycosides in bacterial systems.22 Carbohydrate biosynthesis pathways utilize monosaccharide 1-phosphates, which are subsequently transformed into activated sugar nucleotides and transferred by glycosyltransferases onto target substrates.23 Sugar phosphate analogues that gain access to bacterial cells and interfere with enzymes involved in glycan processing offer an attractive route to inhibit glycan biosynthesis. Phosphonates are non-hydrolysable analogues of phosphates where the carbon–oxygen–phosphorus (COP) linkage is replaced with a direct carbon–phosphorus (CP) linkage. Thus, phosphonates have long garnered interest as probe compounds for phosphoryl transfer or enzyme systems where phosphate binding is invoked.24–28 The charged nature of a phosphonate often limits the uptake of compounds into cells and as a consequence, prodrug or masked phosphonate approaches have been devised to facilitate cellular uptake,29–31 including clinically approved phosphorus-containing drugs.30,32,33 Isosteric phosphonate analogues of α-D-glucose 1-phosphate have been demonstrated to be substrates for thymidylyltransferases (E.C. 2.7.7.24), an enzyme class responsible for condensing sugar 1-phosphates with nucleoside triphosphates.34–36 More recently, subsequent enzymes within the corresponding pathway have turned over the modified sugar nucleotide.37 Delivery of the phosphonate into bacteria could potentially interfere with sugar nucleotide formation, and subsequently with glycosyltransferase activities necessary for glycoprotein biosynthesis. However, cellular activities of these phosphonates have not been reported.
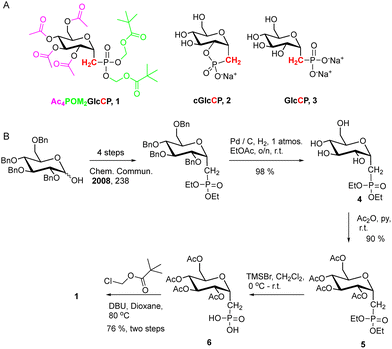
Here, we sought to couple a prodrug-based strategy to increase bioavailability of carbohydrate phosphonates and a cell-based screen to detect their biological activity. We hypothesized that masked carbohydrate phosphonates would disrupt glycoprotein biosynthesis in H. pylori. To test this hypothesis, a masked phosphonate synthesis was undertaken to install an acyloxymethyl ester upon the phosphonate, a prodrug functionality that is designed to undergo cleavage upon bacterial uptake.38 The effects of the masked phosphonate Ac4POM2GlcCP (1), possessing no net negative charge, a structurally related cyclic phosphonate cGlcCP (2),39 possessing a single net negative charge, and the unmasked phosphonate GlcCP (3), possessing a dianionic charge, on glycoprotein biosynthesis in wild-type H. pylori were investigated using MOE. The effects of charge on the phosphonic acid, coupled to masking of the hydrophilic hydroxyl groups, were expected to influence the ability of each compound to traverse the bacterial cell envelope.
The preparation of the masked bis(pivaloyloxymethyl, POM) derivative (Ac4POM2GlcCP, 1) was accomplished in eight steps (Scheme 1B). The synthesis commenced with the preparation, over four steps, of the known diethyl tetrabenzyl phosphonate, from commercially available 2,3,4,6-tetra-O-benzyl-D-glucopyranose, as disclosed previously.34 The benzyl ethers were removed using catalytic hydrogenation with palladium hydroxide at atmospheric hydrogen pressure to deliver 4 in 98% yield, and the exposed hydroxyl functionality acetylated using acetic anhydride/pyridine, and the product (5) isolated after chromatography in 90% yield. Next, the diethyl phosphonate functionality was cleaved using trimethylsilyl bromide to furnish the free phosphonic acid (6). This material was dried and used directly without purification for the next step, whereupon it was refluxed with chloromethyl pivalate in dioxane and the product (1) isolated after chromatography in 76%. Switching the order of hydrogenolysis and ethyl ester cleavage was not satisfactory: the POM derivative with free hydroxyl substituents around the carbohydrate was unstable, and hydrolyzed over a series of days (data not shown).
The effect of phosphonates 1–3 on H. pylori glycoprotein biosynthesis was probed using MOE with GlcNAz (Fig. 1).11–15,40 In this approach, the azide functionality is incorporated into newly synthesized glycoproteins and provides a handle for tagging using appropriate antibody reagents for western blot analysis to interrogate glycoprotein biosynthesis. The GlcNAz is delivered as the lipophilic ester derivative, Ac4GlcNAz, since the presence of the acetyl functionality improves cellular uptake, in an analogous fashion to the incorporation of the POM functionality on the phosphorus(V) functionality. Thus, H. pylori was grown using standard approaches11 in the presence of Ac4GlcNAz (0.5 mM) together with increasing micromolar concentrations of 1–3. After 3–4 days growth, the H. pylori were harvested and lysed, and lysates were standardized to equivalent protein concentrations prior to treatment with Phos-FLAG reagent to visualize azide-labelled glycoproteins by western blot with anti-FLAG-HRP antibody. Treatment of bacteria with the azide-free negative control sugar peracetylated N-acetylglucosamine (Ac4GlcNAc) captures azide-independent signal and benchmarks the lowest amount of signal expected in the absence of azide-labeled glycoprotein biosynthesis. By contrast, treatment of bacteria with Ac4GlcNAz (0.5 mM) without addition of 1–3 captures uninhibited azide-labeled glycoprotein biosynthesis and benchmarks the highest amount of signal expected in the absence of inhibition (Fig. 2). Treatment of H. pylori with 1 (100–500 µM) alongside Ac4GlcNAz (0.5 mM) led to a substantial reduction in the azide-labeled glycoprotein fingerprint, corresponding to inhibition of glycoprotein biosynthesis in H. pylori when treated with 1 (Fig. 2). By contrast, the cyclic phosphonate 2 demonstrated minimal effects upon glycoprotein biosynthesis over the same concentration range. This result reveals that the cyclic phosphonate ester with a single anionic charge does not affect glycoprotein biosynthesis. Insightfully, 3, the isosteric phosphonate analogue of α-D-glucose 1-phosphate, where the phosphonate functionality has not been masked and the dianionic charge is present, also failed to provide significant glycoprotein biosynthesis inhibition. Even millimolar concentrations of 2 or 3 failed to substantially inhibit glycoprotein biosynthesis (Fig. S10, SI), whereas dose-dependent inhibition of 1 was observed at low micromolar concentrations (Fig. S10, SI). Treatment of H. pylori with either phosphonate 1 or 3 did not appreciably impact growth of bacteria relative to untreated controls (Fig. S11, SI), suggesting that glycoprotein biosynthesis inhibition was not due to compound toxicity. These data clearly demonstrate the importance of masking polar moieties (the phosphonate dianionic charge and the hydroxyl groups) with cleavable functionality to effect glycan biosynthesis. It should be noted, however, that the derivative of 3 with the hydroxyl groups acetylated was not prepared and evaluated, therefore, the precise role of acetylation on inhibitor activity cannot be detangled from the role of masking the anionic charge. The masked phosphonate likely facilitates uptake of 1 across the lipophilic membrane of H. pylori, whereupon the ester functionalities (both POM and acetyl) are anticipated to be hydrolysed leading to the presence of 3 within the bacterial cell, where it exerts a substantial effect upon glycoprotein biosynthesis.
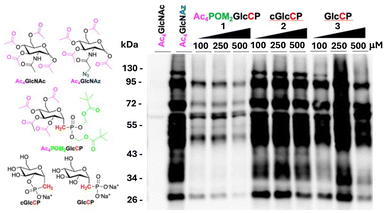 | ||
| Fig. 2 Inhibition of glycoprotein biosynthesis in H. pylori by phosphonates 1–3. Coomassie stain (SI, Fig. S10D) demonstrated equivalent protein loading for all samples. | ||
The mechanism of action of compound 1 remains to be elucidated. There are multiple targets within the H. pylori amenable to inhibition after intracellular hydrolysis of the acetyl and POM functionality in 1 to form 3, including: (i) acting as an inhibitor of the enzymes responsible for sugar nucleotide biosynthesis, (ii) acting as a substrate for sugar nucleotide biosynthesis where the non-hydrolysable phosphonate functions as a non-hydrolysable glycosyltransferase donor sugar, (iii) acting as a glycosyltransferase surrogate acceptor substrate for glycoprotein biosynthesis, and (iv) acting as an inhibitor of GlcNAz incorporation, and thereby having no effect on H. pylori glycoprotein biosynthesis, although given the lack of the 2-N-acetyl functionality in 3, this remains a remote possibility.
Investigations into the use of other prodrug forms may further enhance bacterial uptake of 3, given the diversity of physicochemical and biological properties demonstrated using a wider array of acyloxy prodrugs in other systems.41
The first synthesis of masked bis(POM) phosphonate (1) has been completed, and 1 demonstrated significant inhibition of glycoprotein biosynthesis in H. pylori. 1 is charge-neutral, with ester functionalities masking both the phosphonate dianionic charge and the carbohydrate hydroxyl substituents. The corresponding phosphonate that lacked esterification (3) resulted in substantially less significant inhibition, providing evidence for the importance of masking the anionic charge in order to elicit bioactivity in bacteria. The cyclized phosphonate (2) had the least effect on glycoprotein biosynthesis.
Bacterial glycoproteins and glycan biosynthesis remain relevant antibiotic targets. The discovery that the masked phosphonate potently modulates glycoprotein biosynthesis sets the stage for future enquiries into the mechanism of action of masked phosphonates and their effects upon pathogenic bacteria. Such studies will be reported in the course of our research.
Conceptualisation: DLJ, DHD. Funding acquisition DLJ, DHD. Investigation ES, JSZ, AC, EO, KDM. Project administration: DLJ, DHD. Supervision: DLJ, DHD, KDM. Writing – original drafts, review and editing: ES, JSZ, AC, EO, KDM, DHD, DLJ.
This work was supported, in part, by funding from NSERC and CIHR to D. L. J., by funding from the National Science Foundation #2247752 to D. H. D., by a Maine Space Grant Consortium fellowship to A. C., and by an Institutional Development Award from the National Institutes of Health (grant P20GM103423).
Conflicts of interest
There are no conflicts to declare.Data availability
The materials, methods and data supporting this article have been included as part of the supplementary information (SI). See DOI: https://doi.org/10.1039/d5cc05452d.References
- F. Y. Y. Tan, C. M. Tang and R. M. Exley, Trends Biochem. Sci., 2015, 40, 342–350 CrossRef CAS PubMed.
- L. Yakovlieva, J. A. Fülleborn and M. T. C. Walvoort, Front. Microbiol., 2021, 12, 745702 CrossRef PubMed.
- M. T. Moghadam, Z. Chegini, A. Khoshbayan, I. Farahani and A. Shariati, Curr. Mol. Med., 2021, 21, 549–561 CAS.
- M. Kufleitner, L. M. Haiber and V. Wittmann, Chem. Soc. Rev., 2023, 52, 510–535 RSC.
- J. A. Prescher, D. H. Dube and C. R. Bertozzi, Nature, 2004, 430, 873–877 CrossRef CAS PubMed.
- P.-A. Gilormini, A. R. Batt, M. R. Pratt and C. Biot, Chem. Sci., 2018, 9, 7585–7595 RSC.
- D. H. Dube and C. R. Bertozzi, Curr. Opin. Chem. Biol., 2003, 7, 616–625 CrossRef CAS PubMed.
- S. Suerbaum and P. Michetti, N. Engl. J. Med., 2002, 347, 1175–1186 CrossRef CAS PubMed.
- H. Li, T. Liao, A. W. Debowski, H. Tang, H.-O. Nilsson, K. A. Stubbs, B. J. Marshall and M. Benghezal, Helicobacter, 2016, 21, 445–461 CrossRef CAS PubMed.
- E. L. Clark, M. Emmadi, K. L. Krupp, A. R. Podilapu, J. D. Helble, S. S. Kulkarni and D. H. Dube, ACS Chem. Biol., 2016, 11, 3365–3373 CrossRef CAS PubMed.
- M. B. Koenigs, E. A. Richardson and D. H. Dube, Mol. BioSyst., 2009, 5, 909 RSC.
- K. D. Moulton, A. P. Adewale, H. A. Carol, S. A. Mikami and D. H. Dube, ACS Infect. Dis., 2020, 6, 3247–3259 CrossRef CAS PubMed.
- K. Champasa, S. A. Longwell, A. M. Eldridge, E. A. Stemmler and D. H. Dube, Mol. Cell. Proteomics, 2013, 12, 2568–2586 CrossRef CAS PubMed.
- D. A. Williams, K. Pradhan, A. Paul, I. R. Olin, O. T. Tuck, K. D. Moulton, S. S. Kulkarni and D. H. Dube, Chem. Sci., 2020, 11, 1761–1774 RSC.
- I. D. L. Quintana, A. Paul, A. Chowdhury, K. D. Moulton, S. S. Kulkarni and D. H. Dube, ACS Infect. Dis., 2023, 9, 2025–2035 CrossRef CAS PubMed.
- M. F. Richter, B. S. Drown, A. P. Riley, A. Garcia, T. Shirai, R. L. Svec and P. J. Hergenrother, Nature, 2017, 545, 299–304 CrossRef CAS PubMed.
- M. S. Butler, W. Vollmer, E. C. A. Goodall, R. J. Capon, I. R. Henderson and M. A. T. Blaskovich, ACS Infect. Dis., 2024, 10, 3440–3474 CrossRef CAS PubMed.
- A. Eddenden, M. K. Dooda, Z. A. Morrison, A. Shankara Subramanian, P. L. Howell, J. M. Troutman and M. Nitz, ACS Chem. Biol., 2024, 19, 69–80 CrossRef CAS PubMed.
- R. Ménard, I. C. Schoenhofen, L. Tao, A. Aubry, P. Bouchard, C. W. Reid, P. Lachance, S. M. Twine, K. M. Fulton, Q. Cui, H. Hogues, E. O. Purisima, T. Sulea and S. M. Logan, Antimicrob. Agents Chemother., 2014, 58, 7430–7440 CrossRef PubMed.
- J. W. De Schutter, J. P. Morrison, M. J. Morrison, A. Ciulli and B. Imperiali, J. Med. Chem., 2017, 60, 2099–2118 CrossRef CAS PubMed.
- J. G. Allen, M. Mujacic, M. J. Frohn, A. J. Pickrell, P. Kodama, D. Bagal, T. San Miguel, E. A. Sickmier, S. Osgood, A. Swietlow, V. Li, J. B. Jordan, K.-W. Kim, A.-M. C. Rousseau, Y.-J. Kim, S. Caille, M. Achmatowicz, O. Thiel, C. H. Fotsch, P. Reddy and J. D. McCarter, ACS Chem. Biol., 2016, 11, 2734–2743 CrossRef CAS PubMed.
- C.-H. Hsu, M. Schelwies, S. Enck, L.-Y. Huang, S.-H. Huang, Y.-F. Chang, T.-J. R. Cheng, W.-C. Cheng and C.-H. Wong, J. Org. Chem., 2014, 79, 8629–8637 CrossRef CAS PubMed.
- K. Hashimoto, S. Goto, S. Kawano, K. F. Aoki-Kinoshita, N. Ueda, M. Hamajima, T. Kawasaki and M. Kanehisa, Glycobiology, 2006, 16, 63R–70R CrossRef CAS PubMed.
- R. Engel, Chem. Rev., 1977, 77, 349–367 CrossRef CAS.
- G. P. Horsman and D. L. Zechel, Chem. Rev., 2017, 117, 5704–5783 CrossRef CAS PubMed.
- E. De Clercq and G. Li, Clin. Microbiol. Rev., 2016, 29, 695–747 CrossRef PubMed.
- A. J. Wiemer and D. F. Wiemer, in Phosphorus Chemistry I: Asymmetric Synthesis and Bioactive Compounds, ed. J.-L. Montchamp, Springer International Publishing, Cham, 2015, pp. 115–160 Search PubMed.
- Y. Jin, D. Bhattasali, E. Pellegrini, S. M. Forget, N. J. Baxter, M. J. Cliff, M. W. Bowler, D. L. Jakeman, G. M. Blackburn and J. P. Waltho, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12384–12389 CrossRef CAS PubMed.
- S. Freeman and K. C. Ross, in Progress in Medicinal Chemistry, ed. G. P. Ellis and D. K. Luscombe, Elsevier, 1997, vol. 34, pp. 111–147 Search PubMed.
- U. Pradere, E. C. Garnier-Amblard, S. J. Coats, F. Amblard and R. F. Schinazi, Chem. Rev., 2014, 114, 9154–9218 CrossRef CAS PubMed.
- S. J. Hecker and M. D. Erion, J. Med. Chem., 2008, 51, 2328–2345 CrossRef CAS PubMed.
- E. W. Petrillo, in Biotechnology: Pharmaceutical Aspects, ed. V. J. Stella, R. T. Borchardt, M. J. Hageman, R. Oliyai, H. Maag and J. W. Tilley, Springer, New York, NY, 2007, vol. 5(Pt. 2), pp. 551–558 Search PubMed.
- S. A. Ranadive, A. X. Chen and A. T. M. Serajuddin, Pharm. Res., 1992, 9, 1480–1486 CrossRef CAS PubMed.
- S. A. Beaton, M. P. Huestis, A. Sadeghi-Khomami, N. R. Thomas and D. L. Jakeman, Chem. Commun., 2009, 238–240 RSC.
- S. M. Forget, D. Bhattasali, V. C. Hart, T. S. Cameron, R. T. Syvitski and D. L. Jakeman, Chem. Sci., 2012, 3, 1866–1878 RSC.
- W. A. Barton, J. Lesniak, J. B. Biggins, P. D. Jeffrey, J. Jiang, K. R. Rajashankar, J. S. Thorson and D. B. Nikolov, Nat. Struct. Biol., 2001, 8, 545–551 CrossRef CAS PubMed.
- S. M. Forget, J.-S. Zhu, B. Rowland and D. L. Jakeman, ACS Catal., 2025, 15, 13898–13903 CrossRef CAS.
- J. P. Krise and V. J. Stella, Adv. Drug Delivery Rev., 1996, 19, 287–310 CrossRef CAS.
- J.-S. Zhu, K. M. Stiers, E. Soleimani, B. R. Groves, L. J. Beamer and D. L. Jakeman, J. Org. Chem., 2019, 84, 9627–9636 CrossRef CAS PubMed.
- P. Luong, A. Ghosh, K. D. Moulton, S. S. Kulkarni and D. H. Dube, ACS Infect. Dis., 2022, 8, 889–900 CrossRef CAS PubMed.
- U. Singh, G. Pawge, S. Rani, C.-H. C. Hsiao, D. F. Wiemer and A. J. Wiemer, ACS Med. Chem. Lett., 2024, 15, 1771–1777 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to the research. |
| This journal is © The Royal Society of Chemistry 2026 |