Open Access Article
Open Access ArticleElectronic coupling in a synthetic model of the Cu–cofactor unit of the PM state in cytochrome c oxidase
Sang Tae Lee a,
Christopher G. Bailey
a,
Christopher G. Bailey bc,
Daniel A. Santos
bc,
Daniel A. Santos a,
Mohan Bhadbhade
a,
Mohan Bhadbhade d,
Heejin Kim
d,
Heejin Kim *e and
Dong Jun Kim
*e and
Dong Jun Kim *a
*a
aSchool of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: dongjun.kim@unsw.edu.au
bSchool of Physics, The University of Sydney, Sydney, NSW 2006, Australia
cSydney Nano, The University of Sydney, Sydney, NSW 2006, Australia
dMark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW 2052, Australia
eResearch Center for Materials Analysis, Korea Basic Science Institute, Daejeon 34133, Republic of Korea. E-mail: heejinkim@kbsi.re.kr
First published on 3rd January 2026
Abstract
A hydroquinone–imidazole–Cu(II) complex reproduces the Tyr–His–Cu unit of cytochrome c oxidase. Laser-induced EPR and DFT reveal a semiquinone radical electronically isolated from Cu(II) by orthogonal orbital alignment. The weak magnetic exchange underscores how geometry governs coupling, providing insight into transient states of metalloenzymes and their synthetic analogues.
Energy conversion in biological systems is driven by orchestrated sequences of electron and proton transfer events. This process is driven by cytochrome c oxidase (CcO), the terminal respiratory enzyme that catalyses the four-electron reduction of dioxygen to water while translocating protons across a membrane. This dual function underlies the generation of the electrochemical gradient used for adenosine triphosphate synthesis, with CcO accounting for the majority of energy conserved through oxidative metabolism. At its core, the binuclear centre (BNC), comprising heme a3 and CuB, coordinates redox transformations and proton pumping.1–3
Within the catalytic sequence, the PM intermediate arises immediately following O–O bond cleavage, preceding proton transfer steps that yield water.4–10 The histidine–tyrosine (His–Tyr) crosslink, first identified in crystallographic studies of bovine CcO, remains a distinctive feature of the active site.1 A wide range of synthetic strategies have sought to reproduce this crosslink and explore its role in redox chemistry, yielding insights but often limited by instability.11–27 Raman and crystallographic studies provided clear evidence for a ferryl–oxo FeIV = O unit accompanied by a CuII centre and a tyrosyl radical on the covalently crosslinked His–Tyr residue.28,29 This finding reframed the question of how the three redox-active sites—FeIV, CuII, and Tyr˙—communicate electronically. Magnetic exchange between CuII and the tyrosyl radical within PM has been the focus of significant recent progress. Computational modelling with spectroscopic interpretation by Schaefer and co-workers30 suggested that the Cu–Tyr pair could exhibit ferromagnetic coupling under certain geometries. More recently, high-resolution magnetic circular dichroism measurements by José et al.31 established that PM is best described as a three-spin system, FeIV = O (S = 1), CuII (S = ½), and Tyr˙ (S = ½), with overall antiferromagnetic behaviour. Their analysis assigned specific pairwise couplings within this triad, including antiferromagnetic exchange between Cu and Tyr. However, because these couplings are defined in the context of the full Fe–Cu–Tyr system, the intrinsic Cu–Tyr interaction cannot be disentangled from Fe-mediated superexchange or protein-imposed geometry. This leaves open the question of how these two centres interact in isolation.
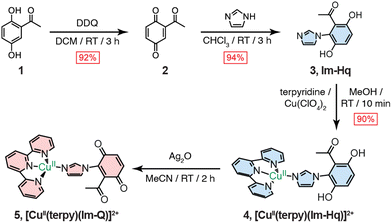
Here, we report a hydroquinone–imidazole–Cu(II) complex as a synthetic subunit analogue of the Tyr–His–CuB subunit in CcO that provides a platform for characterising the intrinsic electronic coupling between Cu(II) and the cofactor. Using laser-induced electron paramagnetic resonance (EPR) spectroscopy, we detect the formation of a stable semiquinone-radical coordinated to Cu(II), in a geometry that density functional theory (DFT) calculations indicate yields orthogonal spin densities, resulting in weak exchange. This result aligns with earlier interpretations of minimal Cu–Tyr coupling in model systems,30 while complementing the three-spin topology determined in the intact PM state.31 By resolving the behaviour of the Cu–cofactor, this platform provides a mechanistic reference point for understanding how protein constraints modulate exchange in CcO.
Binding of O2 to the reduced binuclear centre gives the oxy intermediate (A).32,33 Subsequent O–O bond cleavage, driven by proton and electron transfer from the His–Tyr cross linked residue, produces the PM state, formulated as an Fea3(IV) = O, CuB(II)–OH, TyrO˙ triad.34 The computational study of Blomberg indicates that, in this PM state, the TyrO˙ unit accepts electron density and carries substantial spin density in a phenoxyl-type π system centred on the oxygen atom.35 To capture this local electronic structure we use the Im-Hq/Im-Sq platform. Deprotonation and one-electron oxidation of Im-Hq give an O centred semiquinone (Im-Sq) that behaves as a phenoxyl radical strongly coupled to Cu(II) through the imidazole–aryl framework, providing a synthetic model for the TyrO˙–CuB(II) fragment in the PM state.
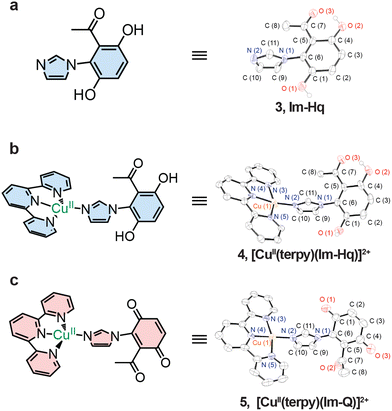
Synthetic analogues of the His–Tyr cofactor have conventionally relied on phenol scaffolds to mimic the tyrosyl radical, yet these constructs are often limited by oxidative instability.13,30,36–39 To overcome this, we employed a 2-acetyl-1,4-hydroquinone to generate an imidazole–hydroquinone (Im-Hq) ligand (Fig. S1). Coordination to Cu(II)–terpyridine afforded [CuII(terpy)(Im-Hq)]2+, with in situ oxidation yielding the quinone analogue [CuII(terpy)(Im-Q)]2+ (Fig. 1). Yields and characterisation data are provided in the SI.
Single-crystal X-ray diffraction of Im-Hq, [CuII(terpy)(Im-Hq)]2+, and [CuII(terpy)(Im-Q)]2+ revealed a progressive geometric evolution across the redox series (Fig. 2). Oxidation of the hydroquinone to quinone was marked by C–O bond contraction (1.360 → 1.206 Å), shortening of the N(imidazole)–C bond (1.415 → 1.396 Å), and elongation of the Cu–N(imidazole) bond (1.854 → 1.967 Å). The most notable trend was in the dihedral angle between the aromatic and imidazole planes, decreasing from 88.7° in the free ligand to 74.0° in [CuII(terpy)(Im-Hq)]2+ and 48.2° in [CuII(terpy)(Im-Q)]2+. This systematic reduction highlights how redox chemistry reorganises the geometry of this motif, potentially underpinning conformational control in the native His–Tyr crosslink of CcO. As dihedral angle directly governs orbital overlap, it provides a quantifiable parameter linking redox events to electronic coupling and frames the interpretation of subsequent EPR studies.
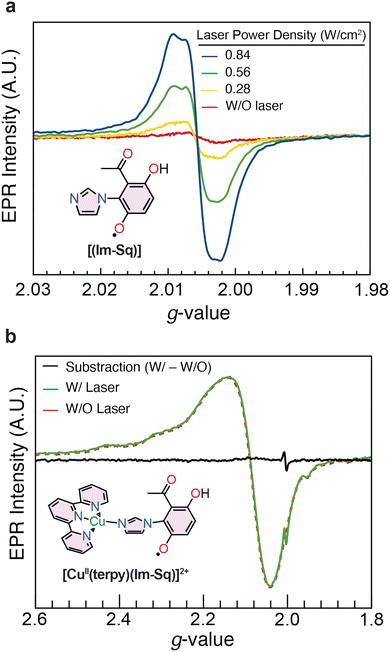
In order to probe radical formation and the electronic structure for [CuII(terpy)(Im-Hq)]2+ and its oxidised analogue [CuII(terpy)(Im-Sq)]2+, we performed EPR spectroscopy under laser excitation. Triethylamine (3 equiv.) was added prior to measurement to facilitate semiquinone generation at 100 K without inducing full deprotonation. UV-vis spectra recorded before and after triethylamine addition showed no significant shift (Fig. S2), confirming that the hydroquinone remains protonated under ambient conditions. Samples were flash-frozen in liquid nitrogen and analysed by EPR at 100 K under variable laser excitation. The quinone complex, [CuII(terpy)(Im-Q)]2+ (5), is used only to establish the redox-dependent structural trend and is not an intermediate in the laser-induced generation of the semiquinone radical.
Laser-irradiated EPR spectra of Im-Hq (Fig. 3a) reveal the formation of a semiquinone-radical, with a g-value of 2.0057, characteristic of a phenoxyl-type radical localised near the oxygen atom. This value lies within the range reported for tyrosyl radicals (g ≈ 2.005–2.0058) and suggests that the spin density remains largely confined to the aromatic ring system.40 The electronic structure inferred from this g-value is consistent with a scenario in which the unpaired electron is localised within the semiquinone π-system with minimal delocalisation onto adjacent groups. As will be discussed in the DFT section below, this localisation likely arises from a twisted geometry between the semiquinone and imidazole rings, limiting π conjugation and orbital overlap.
In the [CuII(terpy)(Im-Hq)]2+ complex (Fig. 3b), the EPR spectra features characteristic of an elongated octahedral-square pyramidal Cu(II) coordination environment.41 Upon laser excitation, a new radical signal with g = 2.0050 emerges, while the Cu(II) peak (g|| = 2.26, g⊥ = 2.08) remains unaltered. Subtraction of the pre-excitation spectrum confirms independent generation of the radical signal. The slightly lower g-value compared to the free cofactor reflects subtle differences in the local environment, but the absence of major spectral changes indicates minimal spin delocalisation into the ligand or metal orbitals. Spectral simulations using EasySpin yielded comparable fits for both ferromagnetic (FM) and antiferromagnetic (AFM) coupling models, indicating the exchange interaction near the resolution limit of the technique.
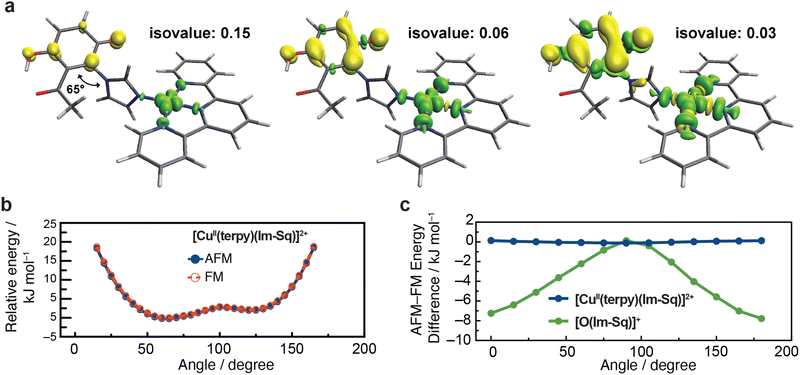
To further examine the electronic arrangement, we performed DFT calculations on the semiquinone–imidazole–Cu(II) ([CuII(terpy)(Im-Sq)]2+) complex, incorporating acetonitrile as the solvent. Spin density maps in Fig. 4a visualised at different isovalues of 0.15, 0.06, and 0.03, together spin population analysis (Fig. S3), reveal that the up-spin density is concentrated on the Cu(II) centre and its four planar nitrogen ligands, while the down-spin density is largely confined to the π system of the semiquinone. It indicates that both the Cu(II) and semiquinone centres have weak spin interactions with the bridging imidazole. The spin communication between Cu(II) and the imidazole ligand occurs through σ-type interactions between Cu(II) dx2–y2 orbital and the lone pair of the imidazole nitrogen. These specific orbitals are orthogonal to the π system, which in turn restricts spin interaction between the Cu(II) and imidazole. On the other hand, between the semiquinone and imidazole ligand, the spin localisation is associated with a twisted geometry (dihedral angle ∼65°) between their planes, which limits π overlap and through-space conjugation. These spin and geometry arrangements yield a weak spin coupling between the Cu(II) and semiquinone centres, consistent with the EPR observations.
DFT scans of the dihedral angle between the semiquinone and imidazole support the above interpretation. When rotating the angle (Fig. 4b), the spin interaction between the two moieties varies, but the aforementioned orbital orthogonality between the Cu(II) and imidazole restricts the spin coupling over the entire complex. This leads to only ∼0.2 kJ mol−1 energy difference between AFM and FM states across the range (blue line in Fig. 4c). To examine the impact of the dihedral angle, we next explored a system in which Cu(II) was replaced by an oxygen-centred radical (Fig. S4), i.e., [O-(Im-Sq)]+, as the O˙ has an excess electron in p-orbital and can make π overlap with the imidazole depending on a dihedral angle. In a sharp contrast to the [CuII(terpy)(Im-Sq)]2+ case, [O-(Im-Sq)]+ system exhibits a strong angle-dependent energy profile. The energy difference between AFM and FM states is ∼8 kJ mol−1 (green line in Fig. 4c) at two endpoints, where the π systems are aligned, while it approaches zero at orthogonal geometries (around 90°), where orbital overlap is suppressed. This result confirms that the dihedral angle primarily acts as a structural gate that keeps the spin coupling weak. In the protein context, modest conformational changes at the His–Tyr motif could therefore modulate proton-coupled electron transfer at the PM state without altering the underlying oxidation states of the metals and cofactor. In this light, the His–Tyr motif in CcO may function as a conformationally regulated electron–proton transfer gateway. Together with the EPR results, these findings demonstrate that orthogonal orbital symmetry electronically isolates the Cu(II)–cofactor pair, providing a subunit-level complement to the three-spin topology of PM.
In summary, we have developed a hydroquinone–imidazole–Cu(II) complex as a structurally defined analogue of the Tyr–His–Cu subunit in CcO. Structural analysis revealed redox-dependent flattening of the ligand framework, while laser-induced EPR and DFT studies demonstrated that the semiquinone radical and Cu(II) centre remain electronically decoupled by orthogonal orbital alignment. The small AFM–FM energy differences (<0.2 kJ mol−1) underscore the intrinsically weak magnetic exchange within this Cu–cofactor pair. These findings provide a subunit-level perspective that complements multi-spin descriptions of the PM state and highlight dihedral angle and orbital orientation as design parameters for controlling coupling in redox cofactors. Beyond enzymatic modelling, this approach offers opportunities to control spin topology, enabling architecturally programmed proton-coupled electron transfer in responsive, bioinspired systems.
The authors acknowledge support from the Australian Research Council (DE210101618) and the Korea Basic Science Institute (C512310).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this study are available in the supplementary information (SI) of this article. Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc05355b.CCDC 2432274 (3), 2432390 (4), and 2432275 (5) contain the supplementary crystallographic data for this paper.42a–c
References
- S. Yoshikawa, K. Shinzawa-Itoh, R. Nakashima, R. Yaono, E. Yamashita, N. Inoue, M. Yao, M. J. Fei, C. P. Libeu, T. Mizushima, H. Yamaguchi, T. Tomizaki and T. Tsukihara, Science, 1998, 280, 1723–1729 CrossRef CAS.
- C. Ostermeier, A. Harrenga, U. Ermler and H. Michel, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 10547–10553 CrossRef CAS PubMed.
- T. Soulimane, G. Buse, G. P. Bourenkov, H. D. Bartunik, R. Huber and M. E. Than, EMBO J., 2000, 19, 1766–1776 CrossRef CAS.
- A. Shimada, T. Tsukihara and S. Yoshikawa, Front. Chem., 2023, 11, 1108190 CrossRef CAS.
- I. Ishigami, R. G. Sierra, Z. Su, A. Peck, C. Wang, F. Poitevin, S. Lisova, B. Hayes, F. R. Moss, S. Boutet, R. E. Sublett, C. H. Yoon, S.-R. Yeh and D. L. Rousseau, Nat. Commun., 2023, 14, 5752 CrossRef CAS.
- M. Wikström and V. Sharma, Biochim. Biophys. Acta, 2018, 1859, 692–698 CrossRef.
- F. Kolbe, S. Safarian, Ż. Piórek, S. Welsch, H. Müller and H. Michel, Nat. Commun., 2021, 12, 6903 CrossRef CAS.
- D. L. Rousseau, I. Ishigami and S.-R. Yeh, J. Inorg. Biochem., 2025, 262, 112730 CrossRef CAS.
- R. Paduvari, R. Arekal and D. M. Somashekara, Int. J. Biol. Macromol., 2025, 309, 142773 CrossRef CAS.
- V. Sharma, K. D. Karlin and M. Wikström, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 16844–16849 CrossRef CAS.
- D. G. Lonnon, S. T. Lee and S. B. Colbran, J. Am. Chem. Soc., 2007, 129, 5800–5801 Search PubMed.
- J.-G. Liu, Y. Naruta and F. Tani, Chem. – Eur. J., 2007, 13, 6365–6378 CrossRef CAS PubMed.
- J. P. Collman, N. K. Devaraj, R. A. Decréau, Y. Yang, Y.-L. Yan, W. Ebina, T. A. Eberspacher and C. E. D. Chidsey, Science, 2007, 315, 1565–1568 CrossRef CAS PubMed.
- J. P. Collman, R. A. Decréau, Y. Yan, J. Yoon and E. I. Solomon, J. Am. Chem. Soc., 2007, 129, 5794–5795 CrossRef CAS PubMed.
- J.-G. Liu, Y. Naruta, F. Tani, T. Chishiro and Y. Tachi, Chem. Commun., 2004, 120–121 RSC.
- K. Kamaraj, E. Kim, B. Galliker, L. N. Zakharov, A. L. Rheingold, A. D. Zuberbühler and K. D. Karlin, J. Am. Chem. Soc., 2003, 125, 6028–6029 Search PubMed.
- J. A. Cappuccio, I. Ayala, G. I. Elliott, I. Szundi, J. Lewis, J. P. Konopelski, B. A. Barry and Ó. Einarsdóttir, J. Am. Chem. Soc., 2002, 124, 1750–1760 CrossRef CAS.
- K. M. McCauley, J. M. Vrtis, J. Dupont and W. A. van der Donk, J. Am. Chem. Soc., 2000, 122, 2403–2404 CrossRef CAS.
- J. P. Collman, Z. Wang, M. Zhong and L. Zeng, J. Chem. Soc., Perkin Trans. 1, 2000, 1217–1222 Search PubMed.
- M. Aki, T. Ogura, Y. Naruta, T. H. Le, T. Sato and T. Kitagawa, J. Phys. Chem. A, 2002, 106, 3436–3444 CrossRef CAS.
- M. Voicescu, Y. El Khoury, D. Martel, M. Heinrich and P. Hellwig, J. Phys. Chem. B, 2009, 113, 13429–13436 CrossRef CAS.
- F. Himo, L. Noodleman, M. R. A. Blomberg and P. E. M. Siegbahn, J. Phys. Chem. A, 2002, 106, 8757–8761 CrossRef CAS.
- R. P. Pesavento, D. A. Pratt, J. Jeffers and W. A. van der Donk, Dalton Trans., 2006, 3326–3337 Search PubMed.
- S. B. Colbran and M. N. Paddon-Row, J. Biol. Inorg. Chem., 2003, 8, 855–865 CrossRef CAS.
- Z. He, S. B. Colbran and D. C. Craig, Chem. – Eur. J., 2003, 9, 116–129 CrossRef CAS.
- J. P. Collman, R. A. Decréau and C. J. Sunderland, Chem. Commun., 2006, 3894–3896 RSC.
- K. D. Karlin and E. Kim, Chem. Lett., 2004, 33, 1226–1231 CrossRef CAS.
- I. Ishigami, A. Lewis-Ballester, A. Echelmeier, G. Brehm, N. A. Zatsepin, T. D. Grant, J. D. Coe, S. Lisova, G. Nelson, S. Zhang, Z. F. Dobson, S. Boutet, R. G. Sierra, A. Batyuk, P. Fromme, R. Fromme, J. C. H. Spence, A. Ros, S.-R. Yeh and D. L. Rousseau, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 3572–3577 CrossRef CAS.
- K. Oda, T. Ogura, E. H. Appelman and S. Yoshikawa, FEBS Lett., 2004, 570, 161–165 CrossRef CAS PubMed.
- A. W. Schaefer, A. C. Roveda, Jr., A. Jose and E. I. Solomon, J. Am. Chem. Soc., 2019, 141, 10068–10081 CrossRef CAS PubMed.
- A. Jose, A. W. Schaefer, A. C. Roveda, W. J. Transue, S. K. Choi, Z. Ding, R. B. Gennis and E. I. Solomon, Science, 2021, 373, 1225–1229 Search PubMed.
- M. I. Verkhovsky, J. E. Morgan and M. Wikstroem, Biochemistry, 1994, 33, 3079–3086 CrossRef CAS.
- A. Sucheta, I. Szundi and Ó. Einarsdóttir, Biochemistry, 1998, 37, 17905–17914 CrossRef.
- M. Karpefors, P. Ädelroth, A. Namslauer, Y. Zhen and P. Brzezinski, Biochemistry, 2000, 39, 14664–14669 CrossRef PubMed.
- M. R. A. Blomberg, Biochemistry, 2016, 55, 489–500 CrossRef.
- A. Offenbacher, K. N. White, I. Sen, A. G. Oliver, J. P. Konopelski, B. A. Barry and Ó. Einarsdóttir, J. Phys. Chem. B, 2009, 113, 7407–7417 CrossRef.
- Y. Nagano, J.-G. Liu, Y. Naruta, T. Ikoma, S. Tero-Kubota and T. Kitagawa, J. Am. Chem. Soc., 2006, 128, 14560–14570 CrossRef PubMed.
- D. A. Proshlyakov, M. A. Pressler and G. T. Babcock, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 8020–8025 CrossRef PubMed.
- K. N. White, I. Sen, I. Szundi, Y. R. Landaverry, L. E. Bria, J. P. Konopelski, M. M. Olmstead and Ó. Einarsdóttir, Chem. Commun., 2007, 3252–3254 RSC.
- E. Kim, E. E. Chufán, K. Kamaraj and K. D. Karlin, Chem. Rev., 2004, 104, 1077–1134 CrossRef PubMed.
- C.-C. Su and C.-B. Li, Polyhedron, 1994, 13, 825–834 Search PubMed.
- (a) CCDC 2432274: Experimental Crystal Structure Determination, 2026 DOI:10.5517/ccdc.csd.cc2mmzgq; (b) CCDC 2432390: Experimental Crystal Structure Determination, 2026 DOI:10.5517/ccdc.csd.cc2mn36m; (c) CCDC 2432275: Experimental Crystal Structure Determination, 2026 DOI:10.5517/ccdc.csd.cc2mmzhr.
| This journal is © The Royal Society of Chemistry 2026 |