Core–shell hydrogel microspheres with sequential drug release and magnetothermal synergy for drug-resistant ovarian cancer
Peinan
Yin
a,
Anamaria
Brozovic
 b,
Wei
Zhang
b,
Wei
Zhang
 *a and
Chengwei
Wu
a
*a and
Chengwei
Wu
a
aState Key Laboratory of Structural Analysis, Optimization and CAE Software for Industrial Equipment, School of Mechanics and Aerospace Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: wei.zhang@dlut.edu.cn
bDivision of Molecular Biology, Ruđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia
First published on 4th November 2025
Abstract
Ovarian cancer (OC) is one of the most fatal malignant tumors of the female reproductive system, and its high recurrence rate in advanced stages and drug resistance severely limit the efficacy of current treatment methods. The molecular mechanisms of drug resistance are complex and remain incompletely understood. Previous studies have attempted to enhance treatment sensitivity by co-delivering antitumor drugs with inhibitors of drug resistance-associated factors. However, these approaches often suffer from inadequate therapeutic efficacy and poor precision due to the inability to precisely control the sequential release of the two agents. To address this, this study designed and constructed a core–shell hydrogel microsphere (MSs) system with both sequential release and magnetothermal synergy functions to effectively intervene in drug-resistant OC. In this system, the shell layer is loaded with the DYRK1B inhibitor AZ191, which is released preferentially to disrupt drug-resistant signaling pathways and sensitize tumor cells. Subsequently, the core layer releases cisplatin to achieve sustained killing of tumor cells. In addition, magnetic nanoparticles embedded in the core can be heated to 42–46 °C under an alternating magnetic field, inducing thermosensitive apoptosis and enhancing cisplatin efficacy. This approach holds promise as a non-invasive alternative to traditional hyperthermic intraperitoneal chemotherapy (HIPEC). In vitro drug release experiments demonstrated that AZ191 exhibited rapid release within the first three hours with a cumulative release of approximately 26%, whereas cisplatin showed minimal early release (∼5%) followed by a markedly accelerated release. In vitro antitumor studies confirmed that the combined chemo-hyperthermia treatment using the core–shell MSs produced the most effective inhibitory effect on drug-resistant OC cells, reducing cell viability to 21% after 48 h, significantly outperforming either chemotherapy or hyperthermia alone. This strategy enables a “resistance-reversal first, precision-killing later” treatment model, offering a novel and effective solution for the treatment of drug-resistant OC.
1. Introduction
Ovarian cancer (OC) remains one of the most lethal malignancies affecting the female reproductive system. Globally, it ranks third in incidence among gynecological cancers but has consistently held the highest mortality rate. According to GLOBOCAN 2022, there were an estimated 324![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 398 new cases and 206
398 new cases and 206![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 839 deaths from OC worldwide in 2022.1 Due to the lack of specific symptoms during the early stages, OC is often diagnosed at an advanced stage, which significantly reduces patient survival. The 5-year survival rates for stage III and IV OC are approximately 37% and 25%, respectively.2 The current standard treatment for advanced OC consists of cytoreductive surgery followed by platinum-based chemotherapy, typically the combination of carboplatin and paclitaxel.3 However, the majority of advanced-stage patients experience disease recurrence within 18 months, eventually leading to chemotherapy resistance and limited further treatment options.4
839 deaths from OC worldwide in 2022.1 Due to the lack of specific symptoms during the early stages, OC is often diagnosed at an advanced stage, which significantly reduces patient survival. The 5-year survival rates for stage III and IV OC are approximately 37% and 25%, respectively.2 The current standard treatment for advanced OC consists of cytoreductive surgery followed by platinum-based chemotherapy, typically the combination of carboplatin and paclitaxel.3 However, the majority of advanced-stage patients experience disease recurrence within 18 months, eventually leading to chemotherapy resistance and limited further treatment options.4
Dual-specificity tyrosine-phosphorylation-regulated kinase 1B (DYRK1B) has been identified as a critical regulatory factor in mediating the development of chemoresistance in tumor cells.5 It facilitates drug resistance through multiple mechanisms, including the evasion of cell cycle arrest,6–8 adaptation to oxidative stress,9,10 enhancement of DNA repair efficiency,11,12 and modulation of the tumor immune microenvironment.13 Given its central involvement in these resistance-associated pathways, DYRK1B has emerged as a promising molecular target for overcoming chemoresistance and enhancing therapeutic efficacy. AZ191, a novel and highly selective small-molecule inhibitor of DYRK1B, exhibits potent inhibitory activity and holds strong potential for reversing drug resistance.14,15
To improve outcomes in drug-resistant OC, the combined use of hyperthermia and chemotherapy has gained increasing attention. Hyperthermic intraperitoneal chemotherapy (HIPEC), a local intraoperative delivery technique involving heated chemotherapeutic agents, has shown some clinical benefit by enhancing drug penetration and cytotoxicity at elevated temperatures.16 However, HIPEC is also associated with several clinical limitations, including a high risk of infection, abdominal pain, intestinal obstruction, thrombosis, and peritoneal adhesions due to catheterization.17
Hydrogels, three-dimensional crosslinked networks composed of hydrophilic polymers, exhibit excellent biocompatibility, tunable physicochemical properties, and high water content, making them highly suitable for drug delivery applications.18,19 Encapsulation of anticancer agents such as cisplatin, oxaliplatin, and paclitaxel within hydrogels for intraperitoneal administration has been widely studied, demonstrating significant tumor suppression in OC models.20–26 However, in drug-resistant OC, the therapeutic efficacy of conventional chemotherapy remains markedly compromised. Recent approaches have focused on polymer-based co-delivery systems incorporating both antitumor agents and inhibitors of drug resistance-associated factors to restore drug sensitivity in drug-resistant OC.27–30 Although these dual-delivery systems have shown encouraging outcomes, their limited ability to achieve temporally controlled and sequential release of multiple agents often results in suboptimal therapeutic synergy and reduced precision in overcoming chemoresistance.
To address these challenges, we designed the core–shell hydrogel microspheres (MSs) system featuring dual functions: sequential drug release and magnetothermal synergy to overcome drug resistance in OC. As illustrated in Fig. 1a, the outer shell, composed of chitosan (CS) crosslinked with genipin (Ge), is loaded with the DYRK1B inhibitor AZ191, which is rapidly released to suppress DYRK1B activity and sensitize resistant tumor cells. The inner core, formed by crosslinking with Ca2+-alginate, encapsulates cisplatin together with magnetic nanoparticles (MNPs). This inner matrix enables delayed cisplatin release, while the embedded MNPs generate localized hyperthermia (42–45 °C) under exposure to an alternating magnetic field (AMF), thereby promoting thermally induced apoptosis and enhancing cisplatin efficacy (Fig. 1b). Collectively, this strategy offers a non-invasive alternative to HIPEC, enabling a therapeutic model of “resistance-reversal first, precision-killing later”.
 | ||
| Fig. 1 Design and mechanism of core–shell MSs for drug-resistant OC therapy. (a) Core–shell structure and crosslinking strategy. (b) Mechanism of sequential drug release and synergistic therapy. | ||
2. Materials and methods
2.1. Materials
Iron chloride hexahydrate (FeCl3·6H2O, ≥99.0%), acetic acid (≥99.5%), and sodium hydroxide (NaOH, ≥96.0%) were procured from Tianjin Kemiou Chemical Reagent Co., Ltd, China. Magnesium chloride hexahydrate (MgCl2·6H2O, ≥98.0%) and cobalt chloride hexahydrate (CoCl2·6H2O, ≥99.0%) were purchased from Damao Chemical Reagent Factory, China. Zinc chloride (ZnCl2, ≥98.0%) and chitosan (CS, 80.0–95.0% degree of deacetylation, DD) were supplied by Sinopharm Chemical Reagent Co., Ltd, China. Ethanol (≥99.7%) was obtained from Tianjin Fuyu Fine Chemical Co., Ltd, China. Sodium alginate (SA, viscosity 1.05–1.15 Pa s) was provided by Tianjin Berens Biotechnology Co., Ltd, China. Calcium chloride anhydrous (CaCl2, ≥99.9%), genipin (Ge, ≥98.0%), and cis-diammineplatinum dichloride (cisplatin, Pt = 65.0%) were obtained from Shanghai Aladdin Biochemical Technology Co. Ltd, China. AZ191 was purchased from Selleck. Phosphate-buffered saline (PBS) was brought from Beijing Solarbio Science & Technology Co., Ltd. Dulbecco's minimum essential medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco, USA. Cell Counting Kit-8 (CCK-8) was supplied by ApexBio, USA. Calcein acetoxymethyl ester (Calcein AM) and propidium iodide (PI) were sourced from Jiangsu KeyGEN BioTECH Co., Ltd, China. All the materials were used without further purification. The human ovarian cancer cells (SKOV3), human ovarian epithelial cells (IOSE-80), and mouse ovarian cancer cells (ID8), and mouse fibroblasts (NIH/3T3) cells were obtained from Procell Life Science & Technology Co., Ltd.2.2. Preparation of MNPs
MNPs were prepared via a hydrothermal synthesis approach.31 Specifically, 8.92 g FeCl3·6H2O, 0.67 g MgCl2·6H2O, 0.79 g CoCl2·6H2O, and 1.35 g ZnCl2 were completely dissolved in 80 mL deionized water to form a transparent metal salt solution. Separately, 18 g NaOH was dissolved in 150 mL deionized water to prepare the NaOH solution. Under magnetic stirring at room temperature, the NaOH solution was slowly introduced dropwise into the metal salt solution, resulting in the formation of a precursor mixture. This precursor was then transferred into a 500 mL sealed autoclave and subjected to hydrothermal treatment at 300 °C for 6 h with continuous stirring. Upon completion, the autoclave was allowed to cool slowly to room temperature. The resulting MNPs suspension was collected, repeatedly rinsed with deionized water and ethanol until a neutral pH was reached, and finally dried at 80 °C for 8 h to obtain the final MNPs.2.3. Preparation of core–shell MSs
0.2 g SA was dissolved in 20 mL deionized water to form a SA solution (1% w/v) under magnetic stirring (500 rpm, 25 °C) for 6 h. Then, 4 mg cisplatin and 0.4 g MNPs were added to the 20 mL SA solution followed by ultrasonication for 10 min to homogenize the mixture, called solution A. Solution A was subsequently atomized through a spray nozzle (1.6 MPs pressure) into CaCl2 solution (5% w/v). After 30 min for completing ionic crosslinking at 25 °C, the resulting core MSs were collected and sieved to obtain cores with particle sizes ranging from 100 to 300 μm. Separately, 0.4 g Ge was dissolved in 30 mL of 75% ethanol under ultrasonication for 10 min. The sieved core MSs were immersed in the Ge solution for 12 h at 25 °C in a dark environment.0.2 g CS was dissolved in 20 mL (1% v/v) acetic acid under magnetic stirring (500 rpm, 25 °C) for 6 h. Meanwhile, 5 mg AZ191 was dissolved in 0.2 mL ethanol via ultrasonication for 10 min and then added to the CS solution (1% w/v) under stirring to form solution B. The core MSs were washed thoroughly with deionized water and immersed in solution B, followed by mechanical stirring (60 rpm, 25 °C) for 24 h. Afterward, the resulting core–shell MSs were rinsed with deionized water and freeze-dried under vacuum for 24 h. The dry core–shell MSs are stored away from light.
2.4. Characterization
The morphology of the MNPs was analyzed by transmission electron microscope (TEM, JEM-F200) and energy-dispersive X-ray spectroscopy (EDS) was performed to determine elemental composition. The magnetization curves were recorded on a vibrating sample magnetometer (VSM, LakeShore-7400S) at room temperature with an AMF of ±18 kOe. Thermogravimetric analysis (TGA, Q-500) was conducted from 30–600 °C at a heating rate of 10 °C min−1 in a nitrogen atmosphere. The Curie temperature was determined from the mass-temperature curves recorded by TGA. The morphology of the core–shell MSs was analyzed using a fluorescence microscope (Nikon ECLIPSE Ti2-U) and scanning electron microscopy (SEM, FEI Quanta 200). The focused ion beam–scanning electron microscopy (FIB-SEM, Helios 5Hydra UX) was used to precisely section the cross-sections of the core–shell MSs, followed by cross-sectional imaging and EDS mapping to investigate the elemental distribution. The zeta potential of the core MSs and core–shell MSs was measured at a temperature of 25 °C on a Zetasizer Nano ZS90 fitted with the zeta potential cell (DTS1060). The analysis of the functional group was conducted by Fourier transform infrared spectroscopy (FTIR, Nicolet 6700). The amount of drug release in vitro was tested by Ultraviolet–visible spectrophotometer (UV-Vis spectrophotometer, UV 1800). The mechanical properties of core–shell MSs were analyzed by the Atomic force microscope (AFM, NanoWizard4XP). The fiber-optic thermometer (Optocon FotempTrafo FTT0100) was employed to record the change in temperature of the core–shell MSs suspension.2.5. The stability and internal structure of core–shell MSs
The mechanical stability of the core–shell MSs was evaluated using AFM to measure the elastic modulus in both dry state and after immersion in release medium for 0, 3, 6, and 24 h. Additionally, SEM images were acquired at 0 h and 24 h to visualize morphological changes during the immersion process.The surface charge and stability of the MNPs and core–shell MSs were analyzed by measuring their zeta potentials using a Zeta potential analyser.
To mimic the intraperitoneal environment, artificial peritoneal fluid was prepared according to a published protocol.32,33 A total of 40 mg of dry microspheres were dispersed in 10 mL of the prepared fluid and incubated at 37 °C with gentle shaking (100 rpm) to simulate physiological motion. Samples were collected on day 10 and day 25, freeze-dried, and weighed to determine the remaining dry mass. The surface morphology of the core–shell MSs at each time point was further examined by SEM.
The internal distribution of MNPs within the core–shell MSs was further investigated using FIB-SEM. The Ga+ ion beam was employed to precisely mill the core–shell MSs and expose cross-sections without mechanical artifacts. Subsequent EDS mapping was performed on the cross-sections to identify the spatial distribution of elements.
2.6. In vitro drug release
The standard curves of AZ191 and cisplatin in phosphate-buffered saline (PBS, pH 7.4) containing Tween 80 (0.5% w/v) were obtained using an Ultraviolet–visible spectrophotometer (UV-Vis spectrophotometer, UV 1800). The UV absorption spectra of AZ191 and cisplatin were scanned in the range of 200–400 nm to determine their absorption characteristic peaks. Subsequently, solutions of different concentrations for AZ191/cisplatin were prepared, and their absorbance values were measured at the respective absorption maxima to construct the standard curves.The drugs were encapsulated into the core–shell MSs by an embedding method. To determine the drug loading capacity of the core–shell MSs, 40 mg of dried core–shell MSs were thoroughly ground and placed into a dialysis bag (molecular weight cutoff: 1 kDa). The dialysis bag was immersed in PBS containing Tween 80 (0.5% w/v) and incubated at 37 °C under constant stirring. At a predetermined time, aliquots of the release medium were withdrawn and replaced with fresh medium. The drug concentrations in the collected media were determined using UV-Vis spectrophotometry. When the cumulative release of both drugs reached a stable plateau, the released amount was considered as the drug loading content of the core–shell MSs. The drug loading efficiency (LE) is calculated by eqn (1).
 | (1) |
The in vitro drug release behavior of the core–shell MSs was further evaluated. Briefly, 40 mg of dried core–shell MSs were dispersed in 3 mL PBS containing Tween 80 (0.5% w/v), and incubated in a water bath at 37 °C with shaking at 100 rpm, under light-protected conditions. At predetermined time intervals, 2 mL of release medium was withdrawn and replaced with 2 mL of fresh medium. All collected samples were temporarily protected from light, filtered through a 0.42 μm membrane filter, and analyzed by UV-Vis spectrophotometry to measure the absorbance. The accumulated drug release percentage (Q) is defined according to eqn (2).
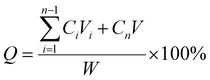 | (2) |
To optimize and validate the controllability of the system, the heating profiles and corresponding drug release behaviors of the suspensions of core–shell MSs were examined under different magnetic field strengths. Specifically, 40 mg core–shell MSs were dispersed in 3 mL of release medium, and subjected to AMF of 100, 400, and 500 Oe, respectively. The temperature rise of the suspension and the associated drug release curves were continuously recorded. A 3 mL release medium without core–shell MSs under identical magnetic field conditions was used as a control to exclude the influence of ambient temperature fluctuations.
2.7. In vitro cytotoxicity assay
The in vitro biocompatibility of core–shell MSs was tested using NIH/3T3 and IOSE-80 cells respectively. Core–shell MSs unloaded with antitumor drugs at concentrations of 20 and 40 mg mL−1 were subjected to ultrasonic dispersion and disinfection prior to cell experiments. The core–shell MSs were first immersed in 75% ethanol for 12 h for preliminary sterilization. After centrifugation, the core–shell MSs were collected and resuspended in culture medium, followed by ultraviolet irradiation for 24 h to ensure complete sterilization. NIH/3T3 and IOSE-80 cells were seeded at a density of 1 × 104 cells per well in 24-well Transwell plates. After a 12 h incubation to allow for cell attachment, the culture medium was refreshed, and the sterilized core–shell MSs were added to the upper chambers of the Transwell plates. Cell viability was assessed at 24, 48, and 72 h using the Cell Counting Kit-8 (CCK-8) assay. The cells were incubated with the pure culture medium without core–shell MSs served as the control group, and the pure culture medium was measured as the blank group. All experiments were conducted in quadruplicate. The cells were stained with Calcein AM and PI to perform a live/dead assay. The percentage of viable cells was calculated according to eqn (3). | (3) |
2.8. In vitro antitumor activity
The evaluation of the in vitro antitumor effect was conducted using 24-well transwell plates. The experiment was divided into four groups: control, Chemotherapy (Chemo), Hyperthermia (HT), Combined Hyperthermia + Chemotherapy (HT + Chemo). The SKOV3 cells (2 × 104 cells per well) were seeded in the lower plate with the addition of 1 mL culture medium in all four groups. After incubation for 12 h, the same amounts of core–shell MSs were placed in the upper chamber of Chemo, HT, and HT + Chemo. The core–shell MSs in Chemo and HT + Chemo were loaded with both MNPs and agents (cisplatin + AZ191), whereas the core–shell MSs in HT contained only MNPs. No additional treatment was carried out in the upper chambers of the control group. The culture plates of HT and HT + Chemo were exposed to AMF of 400 Oe at 100 kHz for 45 min at the predetermined time. After incubation, a CCK-8 assay was performed to quantify the cell viability. Each experiment was repeated four times. SKOV3 cells were stained with Calcein AM and PI to observe the live/dead assay.2.9. In vivo cytotoxicity assay
To further investigate the systemic safety and in vivo biocompatibility of the prepared core–shell MSs, healthy female C57BL/6 mice (4 weeks, n = 6) were intraperitoneally injected with core–shell MSs unloaded with antitumor drugs (Group2). The healthy mice without any operations were Group 1. The intraperitoneal route was selected to mimic the microenvironment relevant to potential clinical administration and to maximize direct exposure of abdominal organs to the biomaterial. After 20 days of observation without additional interventions, all animals were sacrificed, and major organs including heart, liver, spleen, lung, kidney, and small intestine were harvested. These tissues were subjected to hematoxylin–eosin (H&E) staining to examine overall histopathological alterations. The rationale for H&E staining was to comprehensively evaluate organ integrity, including potential signs of inflammation, fibrosis, vascular congestion, necrosis, or structural disruption, thereby providing insight into both acute and chronic physiological responses to the implanted core–shell MSs.2.10. In vivo antitumor activity
To establish a subcutaneous ovarian cancer model, ID8 cells were harvested during the logarithmic growth phase and resuspended in DMEM at a density of 1 × 107 cells per mL. The C57BL/6 mouse (4 weeks, female) was inoculated subcutaneously with 100 μL of the cell suspension. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Dalian University of Technology and approved by the Animal Ethics Committee of Dalian University of Technology. All procedures in animal experiments were strictly carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of China. After 7 days, when palpable tumors had formed, mice were randomly divided into four groups (n = 5 per group): a control group (control) without treatment; a chemotherapy group (Chemo) receiving intratumoral cisplatin (0.5 mg per mouse, dose converted from clinical human body surface area34); a hyperthermia group (HT) treated with core–shell MSs unloaded with antitumor drugs under an AMF (400 Oe); and a combined therapy group (HT + Chemo) treated with core–shell MSs followed by AMF exposure (400 Oe). Treatments were performed on days 7, 12, and 17 post-inoculation. On day 20, all mice were sacrificed, and tumors were excised for further analysis. Tumor volume (V) was calculated using the eqn (4). | (4) |
3. Results and discussion
3.1. Preparation and characterization of MNPs
The morphology of the MNPs was characterized using TEM. As shown in Fig. 2a, the MNPs exhibit a uniform rhombic shape with an average particle size of approximately 18.5 nm. EDS analysis (Fig. 2b) indicated the presence of Fe, Mg, Co, and Zn metal elements, which is consistent with the components used in the synthesis, confirming the accuracy of the preparation formulation. To determine the Curie temperature, thermogravimetric analysis was performed under a static magnetic field (Fig. 2c). The normalized mass of the MNPs gradually increased with temperature. Eventually, it stabilized at unity, indicating that the magnetization of the MNPs progressively decreased to zero as the temperature increased. The temperature corresponding to the maximum of the first derivative of the thermogravimetric curve was defined as the Curie temperature, which was determined to be 145.8 °C, following the method reported in ref. 35. Magnetic properties were further evaluated by measuring the magnetization curve of the MNPs (Fig. 2d). The saturation magnetization was found to be 53.86 emu g−1. The absence of a noticeable hysteresis loop indicates that the MNPs exhibit superparamagnetic behavior.3.2. Preparation and characterization of core–shell MSs
The optical microscope images of core MSs and core–shell MSs are shown in Fig. 3a. It can be observed that in bright field mode, the core MSs exhibit regular spherical shapes with an average particle size of 233.1 μm (Fig. 3b). Additionally, it is evident that they contain distinct black nanoparticles inside. In contrast, the core–shell MSs have an average diameter of 129.9 μm (Fig. 3b), showing a significant reduction in size. This process can be immediately observed after adding core to the CS solution, thus ruling out the possibility that it is caused by long-term Ge cross-linking with CS. The immediate shrinkage of the core–shell MSs can be primarily attributed to dehydration caused by the formation of a complex coacervate-like structure between the oppositely charged hydrophilic polymer network and the free polymer.36Fluorescence imaging revealed that the SA core exhibited no detectable fluorescence under excitation wavelengths, indicating the absence of intrinsic fluorescent properties. In contrast, the core–shell MSs displayed strong and distinguishable fluorescence upon excitation at 488 nm and 543 nm, emitting green and red signals, respectively. Notably, the fluorescence signals were localized predominantly at the outer region of the core–shell MSs, suggesting that the emission originated from the shell layer. This fluorescence behavior is attributed to the formation of fluorescent complexes between CS and Ge, which are known to produce characteristic emissions in the visible range upon crosslinking.37 These results confirm the successful encapsulation of the core MSs by the CS outer layer in the core–shell MSs.
The core–shell MSs were characterized by FTIR (Fig. 3c). Fig. 3d shows the magnified region at 1000–2000 cm−1 in the infrared spectrum. As shown in Fig. 3d (1), the FTIR spectrum of CS exhibits a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak at 1658 cm−1, corresponding to the residual acetyl groups, and an N–H bending vibration peak of the amino groups at 1594 cm−1. In contrast, the spectrum of the CS–Ge crosslinked product shows a distinct absorption band at 1560 cm−1, which is attributed to the coupled vibration of N–H bending and C–N stretching in the newly formed amide bonds, indicating the successful formation of covalent cross-linking between CS and Ge. In the Fig. 3d (2), the absorption band at 1400 cm−1 corresponds to the symmetric stretching vibration of carboxylate groups (–COO−). After crosslinking, a shoulder peak appears at this position, indicating the presence of –COO− groups in different chemical environments. This is attributed to the coordination of –COO− with Ca2+via various binding modes (e.g., monodentate, bidentate, or bridging coordination), which results in distinct symmetric stretching frequencies.38,39 The Fig. 3d (3) is the FTIR spectra of the core–shell MSs and the physical mixture of the CS–Ge with the SA–Ca2+. In the physically mixed sample, the band at 1628 cm−1 is assigned to the asymmetric stretching vibration of –COO− groups. In contrast, this peak shifts to 1601 cm−1 in the spectrum of the core–shell MSs. This red shift is caused by electrostatic interactions between the –COO− groups in the SA and the –NH3+ groups in the CS. The attraction from positively charged –NH3+ reduces the electron density around –COO−, lowering the force constant of the C
O stretching vibration peak at 1658 cm−1, corresponding to the residual acetyl groups, and an N–H bending vibration peak of the amino groups at 1594 cm−1. In contrast, the spectrum of the CS–Ge crosslinked product shows a distinct absorption band at 1560 cm−1, which is attributed to the coupled vibration of N–H bending and C–N stretching in the newly formed amide bonds, indicating the successful formation of covalent cross-linking between CS and Ge. In the Fig. 3d (2), the absorption band at 1400 cm−1 corresponds to the symmetric stretching vibration of carboxylate groups (–COO−). After crosslinking, a shoulder peak appears at this position, indicating the presence of –COO− groups in different chemical environments. This is attributed to the coordination of –COO− with Ca2+via various binding modes (e.g., monodentate, bidentate, or bridging coordination), which results in distinct symmetric stretching frequencies.38,39 The Fig. 3d (3) is the FTIR spectra of the core–shell MSs and the physical mixture of the CS–Ge with the SA–Ca2+. In the physically mixed sample, the band at 1628 cm−1 is assigned to the asymmetric stretching vibration of –COO− groups. In contrast, this peak shifts to 1601 cm−1 in the spectrum of the core–shell MSs. This red shift is caused by electrostatic interactions between the –COO− groups in the SA and the –NH3+ groups in the CS. The attraction from positively charged –NH3+ reduces the electron density around –COO−, lowering the force constant of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and thereby decreasing the vibrational frequency. Additionally, the restricted vibrational freedom of –COO− due to electrostatic binding leads to peak broadening and a further shift to lower wavenumbers.
O bond and thereby decreasing the vibrational frequency. Additionally, the restricted vibrational freedom of –COO− due to electrostatic binding leads to peak broadening and a further shift to lower wavenumbers.
EDS analysis (Fig. 3e) demonstrated the presence of Fe, Mg, Co, Zn, and N elements. The detection of characteristic elements (Fe, Mg, Co, Zn) originating from the core MNPs in the EDS spectrum of the core–shell MSs surface is likely due to the intrinsic probing depth of EDS analysis, which allows signals not only from the outermost layer but also from the underlying core. In addition, the presence of N element can be attributed to the amino groups of the chitosan shell, further confirming the successful coating of the chitosan layer.
3.3. The stability and internal structure of core–shell MSs
The mechanical properties of core–shell MSs after immersion in PBS for different durations were evaluated using AFM, as shown in Fig. 4a. The schematic inset illustrates the principle of elastic modulus measurement via AFM. During indentation, the displacement of the probe and the interaction force between the probe and the core–shell MSs surface are recorded, from which the elastic modulus can be calculated.40 The initial elastic modulus of the vacuum-dried core–shell MSs was approximately 10.08 MPa. As the immersion time increased, the modulus gradually decreased, reaching 350 kPa after 24 h. SEM images taken before and after immersion revealed no significant morphological changes, suggesting that the reduction in stiffness was due to water uptake rather than structural degradation.The zeta potential values of the MNPs, core MSs, and core–shell MSs are shown in Fig. 4b. Zeta potential measurements revealed that the MNPs exhibited a surface potential of −9.11 ± 0.95 mV, indicating a slightly negative surface charge. This negative charge is likely attributed to the alkaline conditions employed during the co-precipitation process, which introduced hydroxyl (–OH) functional groups on the particle surface. The core MSs exhibit a surface charge of −21.95 ± 5.66 mV, attributed to the negatively charged –COO− of SA. As expected, after coating with a CS shell whose –NH3+ are positively charged, the zeta potential of the core–shell MSs shifts to +12.21 ± 5.75 mV. These results further confirmed the successful deposition of the CS shell onto the core MSs.
The core–shell MSs demonstrated significant time-dependent degradation. SEM observations indicated that the core–shell MSs exhibited surface cracking and structural damage by day 10, and by day 25, further degradation was evident, with smaller fragments observed (Fig. 4c). The quantitative analysis showed that the remaining dry weight of the core–shell MSs was approximately 84% of the initial mass on day 10 and decreased to around 35% by day 25, indicating substantial mass loss over this period. These results suggest that the core–shell MSs are biodegradable.
To further characterize the elemental distribution within the core–shell MSs, the cross-sections of core–shell MSs were prepared using FIB-SEM (schematic diagram in Fig. 4d) and analyzed by EDS. During FIB-SEM sectioning, the core–shell MSs cross-sections were not perfectly smooth and displayed slight edge curling and porous features. This phenomenon is attributed to the soft nature of the hydrogel matrix and the ion-beam milling conditions: (i) localized swelling, shrinkage, or thermal effects may occur under high vacuum and Ga+ ion bombardment, causing edge deformation, and (ii) excessive ion-beam energy can induce localized over-milling and transient thermal damage, resulting in micro-voids or surface erosion. Such structural changes are commonly observed in soft hydrogel materials during FIB processing and do not indicate intrinsic defects of the core–shell MSs. The results revealed that Fe, Mg, Co, and Zn, the characteristic elements of the MNSs, were homogeneously distributed throughout the cross-sections of core–shell MSs, indicating uniform dispersion. Such uniform distribution is expected to ensure consistent magnetic responsiveness and thermal properties across the core–shell MSs. In addition, Ca ions were also detected with uniform distribution, consistent with the core layer structure formed through crosslinking of SA with Ca2+, further confirming the uniformity of crosslinking and the structural integrity of the core–shell MSs. These findings not only validate the successful fabrication of the core–shell MSs but also provide a solid foundation for their subsequent drug loading and controlled release applications.
3.4. In vitro drug release
Fig. 5a presents the standard curves of AZ191 and cisplatin in the release medium (PBS with 0.5% w/v Tween 80), which were used for quantitative analysis of drug release. The LE of AZ191 and cisplatin in core–shell MSs were measured and calculated to be 1.88% and 5.02%, respectively. The UV characteristic absorption peaks for AZ191 and cisplatin are 210 and 245 nm, respectively.As shown in Fig. 5b, the cumulative release profile of AZ191 from the outer shell generally follows a typical free diffusion, with a high release rate during the first 3 h, gradually leveling off at 12 h, and reaching a cumulative release of approximately 58% at 24 h. In contrast, the release of cisplatin from the inner core exhibits a slow–fast–slow non-linear trend: less than 5% was released at 3 h, significantly lower than the 26% release of AZ191 at 3 h. The release rate of cisplatin began to increase after 3 h, gradually leveling off at 18 h, and ultimately achieving a cumulative release of 63% at 24 h. This sequential release behavior is attributed to the distinct structural features of the core–shell MSs and the differences in release mechanisms. AZ191, located in the outer shell, is primarily released via free diffusion. In contrast, cisplatin is encapsulated within the hydrogel core and must undergo two stages for release: water penetration and swelling of the inner core and diffusion of the drug through the shell layer into the surrounding medium. Due to the additional barrier of the shell, the release of cisplatin is delayed relative to AZ191, resulting in a clear sequential release profile.
The magnetothermal heating and drug release behaviors of the core–shell MSs were further evaluated under AMF fields of 250, 400, and 500 Oe to assess their controllability (Fig. 5c). The RT curve increased slightly, from 30 °C to 31.2 °C. This was due to the heat generated by the coil itself, causing the ambient temperature to rise. To evaluate the thermal response and drug release behavior of the system under different magnetic conditions, the heating profiles of core–shell MSs suspensions were measured at 250, 400, and 500 Oe. At 250 Oe, the core–shell MSs exhibited a slow temperature rise, reaching a stable value of 43.8 °C after approximately 45 min. Increasing the field strength to 400 Oe markedly enhanced the heating rate, with the suspension rapidly reaching a stable temperature of 44.6 °C within 15 min, which lies within the clinically accepted safe window for magnetic hyperthermia capable of inducing tumor cell death without damaging normal tissues.31,41 Further increasing the field to 500 Oe accelerated the heating rate even more and resulted in a slightly higher final temperature of 46.1 °C. Given minor experimental variations and differences in heat-dissipation efficiency, a fluctuation of about 1–2 °C is within the normal range of experimental deviation. Therefore, the final stable temperatures of the core–shell MSs under different magnetic field strengths are not significant. Considering that the heating rate generated by 250 Oe is relatively low and 500 Oe is close to the operational limit of the equipment, 400 Oe was selected as the optimal condition in all subsequent magnetic thermal experiments, achieving a balance between heating efficiency and experimental safety.
The drug release profiles followed a similar trend, showing accelerated release with increasing magnetic field strength (Fig. 5d). This trend correlates with the enhanced heating efficiency observed at higher fields, where more rapid local heating promotes polymer chain mobility and transient pore expansion within the hydrogel matrix, facilitating drug diffusion. These findings demonstrate that both the heating efficiency and drug release rate of the core–shell MSs can be precisely regulated by adjusting the magnetic field intensity, highlighting the system's excellent magnetothermal responsiveness and controllability for safe, chemo-hyperthermia therapy.
3.5. In vitro cytotoxicity assay
The viability of NIH/3T3 and IOSE-80 cells incubated with core–shell MSs at varying concentrations (0, 20, and 40 mg mL−1) for 24, 48, and 72 h was assessed to evaluate the biocompatibility of the core–shell MSs (Fig. 6). As shown in Fig. 6a and c, an increase in green fluorescence intensity over time indicates active cell proliferation, suggesting high cell viability. The viability of NIH/3T3 cells treated with 20 mg mL−1 of core–shell MSs was 92.3%, 100.5%, and 93.5% at 24, 48, and 72 h, respectively. Even at a higher concentration of 40 mg mL−1, the cell viability remained at 95.4%, 91.4% and 90.3% after 24, 48, and 72 h of incubation, respectively, showing no significant cytotoxicity (Fig. 6b). The cell viability of IOSE-80 cells co-cultured with core–shell MSs also indicated a similar trend to that of NIH/3T3 cells (Fig. 6c and d). At 20 mg mL−1 concentration, the cell viability at 24 h remained above approximately 99%, with no significant difference compared to the control group. Upon prolonged incubation for 48 and 72 h, cell viabilities were still maintained at high levels (93.2% and 93.5%, respectively), and no evident cytotoxic effects were observed (Fig. 6d). When the concentration of core–shell MSs cocultured with cells is higher, the cells still maintain a relatively high activity (Fig. 6c). These findings confirm that the prepared core–shell MSs possess favorable biocompatibility, ensuring safety for potential further in vitro and in vivo applications.3.6. In vitro antitumor activity
For the measurement of the in vitro antitumor activity of newly prepared core–shell MSs in different treatment protocols, the human ovarian cancer SKOV3 cell line was used. This cell line is characterized by its resistance to several cytotoxic drugs, including cisplatin.42,43 The timeline of the in vitro antitumor experiment is illustrated in Fig. 7a. For the HT group, an optical fiber thermometer was inserted into the culture medium to monitor the temperature under AMF (Fig. 7b). To prevent any contamination caused by exposing the culture plate to a non-sterile environment, an additional plate loaded with the same quantity of core–shell MSs but without seeded cells was used exclusively for temperature measurement.The viability of SKOV3 cells subjected to different treatments is shown in Fig. 7c. In the Chemo group, cell viability decreased to 70.3% after 24 h of treatment. However, only a slight improvement in therapeutic efficacy was observed at 48 h, with cell viability further reduced to just 62.6%. This limited effect is attributed to the passive diffusion-based release of AZ191 and cisplatin from the core–shell MSs, where the majority of drug release occurs within the first 24 h. During the subsequent 24 h, the release rate dropped sharply, resulting in diminished therapeutic impact.
The core–shell MSs in the HT group were loaded with self-regulating MNPs. Upon exposure to an AMF, the local temperature in the culture plate increased to 42–46 °C, which falls within the therapeutic range for inducing tumor cell death while sparing normal tissues. Consequently, the viability of SKOV3 cells at 48 h was significantly reduced to 32.6%.
As expected, the combined HT + Chemo group exhibited the most potent antitumor effect among all groups, with cell viability dropping to 21.0% at 48 h. This enhanced efficacy is attributed to the sustained release of AZ191 and cisplatin from the core–shell MSs for chemotherapy, in conjunction with the thermal effect that further promotes tumor cell death. Notably, the rising temperatures also helped accelerate the release of the remaining drugs inside the core–shell MSs, thereby achieving a synergistic chemo-thermal tumor-killing effect. The live/dead fluorescence images in Fig. 6e further substantiate these findings.
3.7. In vivo antitumor and cytotoxicity assay
The therapeutic outcomes of the different treatment groups were evaluated in the subcutaneous ovarian cancer model. The treatment timeline is illustrated in Fig. 8a. Tumor growth curves showed that the control group exhibited rapid tumor progression, with final tumor volumes reaching approximately 111 mm3. The chemo and HT group significantly reduced tumor growth compared to the control group, with final tumor volumes of about 81 and 40 mm3, respectively. Notably, the combined HT + Chemo group demonstrated the most pronounced antitumor effect, with tumor volumes reduced to 12 mm3, significantly smaller than those in both the Chemo and HT group. Representative images of excised tumors (Fig. 8b) visually confirmed the therapeutic efficacy, consistent with quantitative tumor volume analysis (Fig. 8d). Body weight monitoring (Fig. 8c) revealed no significant differences among groups, indicating good systemic tolerance of the treatments. Together, these results demonstrate that the AZ191/cisplatin-loaded core–shell MSs, in combination with magnetic hyperthermia, effectively suppressed tumor growth in vivo and provided superior therapeutic outcomes compared with single-modality treatments.Histological examination revealed that all major organs from the core–shell MSs-treated mice maintained normal tissue architecture without observable inflammatory infiltration, necrosis, or other pathological alterations, as shown in Fig. 8e. The heart exhibits intact, neatly arranged myocardial fibers with well-preserved nuclear morphology. The liver demonstrates well-preserved hepatic lobule architecture, with hepatocytes interconnected in cord-like formations. The spleen displays normal white and red pulp structures. No alveolar damage is observed in the lungs. Renal medullary cells show regular, centrally located nuclei with pale cytoplasm. The small intestine reveals intact villous structures without epithelial cell desquamation. No inflammatory infiltration was observed in any of the above specimens. These results demonstrate that the core–shell MSs exhibit good in vivo biocompatibility and do not induce obvious systemic toxicity under the tested conditions.
4. Conclusions
This study successfully developed a core–shell MSs system with dual functions of sequential release and magnetothermal synergy, providing a novel and efficient therapeutic strategy for the treatment of advanced drug-resistant OC. The system prioritizes the release of the AZ191, DYRK1B inhibitor, effectively reducing the drug resistance of OC tumor cells followed by the release of cisplatin to achieve precise tumor killing. Additionally, exposure to an external AMF activates the MNPs within the core to generate a controllable localized thermal effect, which enhances the chemotherapeutic synergy, triggers thermally induced apoptosis in tumor cells, and markedly improves overall therapeutic efficacy, thereby demonstrating strong potential for clinical translation.Nevertheless, certain challenges remain before clinical application can be fully realized.
(1) Scalability: the current fabrication of core–shell MSs involves multiple procedures, including spray formation, sieving, and post-processing steps. Future efforts should focus on integrating microfluidic or continuous synthesis technologies to streamline the preparation process, enhance production efficiency.
(2) Potential long-term effects: although MNPs have exhibited good biocompatibility both in vitro and in vivo, their long-term metabolism, biodistribution, and possible accumulation in normal tissues warrant further investigation to ensure biosafety.
Overall, this work establishes a promising foundation for the development of next-generation, multifunctional hydrogel-based delivery systems for drug-resistant OC therapy, while also identifying the key directions for their future optimization and translational advancement.
Author contributions
Peinan Yin: Writing – original draft, methodology, investigation. Anamaria Brozovic: Writing – review & editing. Wei Zhang: Writing – review & editing, project administration. Chengwei Wu: Validation.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.Acknowledgements
This work is supported by Fundamental Research Funds for the Central Universities of China (DUT24YG208) and the National Key Research and Development Project of China (2022YFE0115400, 2022YFB400350). TEM data were obtained using equipment maintained by Instrumental Analysis Center, Dalian University of Technology.References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA-Cancer J. Clin., 2024, 74, 229–263 CrossRef
.
- S. C. Chauhan, D. Kumar and M. Jaggi, Mucins in ovarian cancer diagnosis and therapy, J. Ovarian Res., 2009, 2, 21 CrossRef PubMed
.
- J. Spiliotis, E. Halkia, E. Lianos, N. Kalantzi, A. Grivas, E. Efstathiou and S. Giassas, Cytoreductive Surgery and HIPEC in Recurrent Epithelial Ovarian Cancer: A Prospective Randomized Phase III Study, Ann. Surg. Oncol., 2014, 22, 1570–1575 CrossRef PubMed
.
- G. C. Jayson, E. C. Kohn, H. C. Kitchener and J. A. Ledermann, Ovarian cancer, Lancet, 2014, 384, 1376–1388 CrossRef PubMed
.
- M. Ems, A. Brichkina and M. Lauth, A safe haven for cancer cells: tumor plus stroma control by DYRK1B, Oncogene, 2025, 44, 341–347 CrossRef CAS
.
- X. Deng, S. E. Mercer, S. Shah, D. Z. Ewton and E. Friedman, The Cyclin-dependent Kinase Inhibitor p27Kip1 Is Stabilized in G0 by Mirk/dyrk1B Kinase, J. Biol. Chem., 2004, 279, 22498–22504 CrossRef CAS PubMed
.
- Y. Zou, D. Z. Ewton, X. Deng, S. E. Mercer and E. Friedman, Mirk/dyrk1B Kinase Destabilizes Cyclin D1 by Phosphorylation at Threonine 288, J. Biol. Chem., 2004, 279, 27790–27798 CrossRef CAS PubMed
.
- W. Becker, A wake-up call to quiescent cancer cells – potential use of DYRK1B inhibitors in cancer therapy, FEBS J., 2017, 285, 1203–1211 CrossRef
.
- X. Deng, D. Z. Ewton and E. Friedman, Mirk/Dyrk1B Maintains the Viability of Quiescent Pancreatic Cancer Cells by Reducing Levels of Reactive Oxygen Species, Cancer Res., 2009, 69, 3317–3324 CrossRef CAS
.
- X. B. Deng, S. E. Mercer, C. Y. Sun and E. Friedman, The normal function of the cancer kinase Mirk/dyrk1B is to reduce reactive oxygen species, Genes Cancer, 2014, 5, 22 CrossRef CAS PubMed
.
- C. Dong, K. L. West, X. Y. Tan, J. Li, T. Ishibashi, C.-H. Yu, S. M. H. Sy, J. W. C. Leung and M. S. Y. Huen, Screen identifies DYRK1B network as mediator of transcription repression on damaged chromatin, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 17019–17030 CrossRef CAS PubMed
.
- C. Dong, L. An, C.-H. Yu and M. S. Y. Huen, A DYRK1B-dependent pathway suppresses rDNA transcription in response to DNA damage, Nucleic Acids Res., 2021, 49, 1485–1496 CrossRef CAS
.
- A. Brichkina, M. Ems, R. Suezov, R. Singh, V. Lutz, F. S. R. Picard, A. Nist, T. Stiewe, J. Graumann, M. Daude, W. E. Diederich, F. Finkernagel, H.-R. Chung, D. K. Bartsch, K. Roth, C. Keber, C. Denkert, M. Huber, T. M. Gress and M. Lauth, DYRK1B blockade promotes tumoricidal macrophage activity in pancreatic cancer, Gut, 2024, 73, 1684–1701 CrossRef CAS
.
- N. Kokkorakis, M. Zouridakis and M. Gaitanou, Mirk/Dyrk1B Kinase Inhibitors in Targeted Cancer Therapy, Pharmaceutics, 2024, 16, 528 CrossRef CAS PubMed
.
- A. L. Ashford, D. Oxley, J. Kettle, K. Hudson, S. Guichard, S. J. Cook and P. A. Lochhead, A novel DYRK1B inhibitor AZ191 demonstrates that DYRK1B acts independently of GSK3β to phosphorylate cyclin D1 at Thr286, not Thr288, Biochem. J., 2013, 457, 43–56 CrossRef PubMed
.
- W. J. van Driel, S. N. Koole, K. Sikorska, J. H. Schagen van Leeuwen, H. W. R. Schreuder, R. H. M. Hermans, I. H. J. T. de Hingh, J. van der Velden, H. J. Arts, L. F. A. G. Massuger, A. G. J. Aalbers, V. J. Verwaal, J. M. Kieffer, K. K. Van de Vijver, H. van Tinteren, N. K. Aaronson and G. S. Sonke, Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer, N. Engl. J. Med., 2018, 378, 230–240 CrossRef CAS
.
- S. N. Koole, W. J. van Driel and G. S. Sonke, Hyperthermic intraperitoneal chemotherapy for ovarian cancer: The heat is on, Cancer, 2019, 125, 4587–4593 CrossRef CAS
.
- B. Chen and J. Liu, Advancements in Hydrogel-Based Therapies for Ovarian Cancer: A Review, Cell Biochem. Biophys., 2024, 83, 87–108 CrossRef PubMed
.
- P. Yin, C. Wei, A. Brozovic, C. Wu and W. Zhang, Chitosan/polyvinyl alcohol-based magnetic hydrogel microspheres with controlled retention and regulated drug release for intravesical instillation, Int. J. Biol. Macromol., 2025, 287, 138412 CrossRef CAS PubMed
.
- E. J. Cho, B. Sun, K.-O. Doh, E. M. Wilson, S. Torregrosa-Allen, B. D. Elzey and Y. Yeo, Intraperitoneal delivery of platinum with in situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer, Biomaterials, 2015, 37, 312–319 CrossRef CAS
.
- B. Sun, M. S. Taha, B. Ramsey, S. Torregrosa-Allen, B. D. Elzey and Y. Yeo, Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals, J. Controlled Release, 2016, 235, 91–98 CrossRef CAS
.
- G. Xu, B. Li, T. Wang, J. Wan, Y. Zhang, J. Huang and Y. Shen, Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system, RSC Adv., 2018, 8, 21229–21242 RSC
.
- K. Yamaguchi, O. Hiraike, H. Iwaki, K. Matsumiya, N. Nakamura, K. Sone, S. Ohta, Y. Osuga and T. Ito, Intraperitoneal Administration of a Cisplatin-Loaded Nanogel through a Hybrid System Containing an Alginic Acid-Based Nanogel and an In Situ Cross-Linkable Hydrogel for Peritoneal Dissemination of Ovarian Cancer, Mol. Pharm., 2021, 18, 4090–4098 CrossRef CAS
.
- X. Han, G. Li, S. You, M. Shen, Y. Xu, H. Yang, C. Lu, M. Zhang, J. Fang, Q. Zhou and Q. Yao, Injectable bio-multifunctional hyaluronic acid-based hydrogels loaded with poly ADP-ribose polymerase inhibitors for ovarian cancer therapy, Int. J. Biol. Macromol., 2024, 270, 132275 CrossRef CAS PubMed
.
- J. V. Lim, M. R. Nepacina and Y.-C. Hsu, The study of designing a controlled drug release using oxaliplatin-loaded hydrogel for ovarian cancer treatment, J. Taiwan Inst. Chem. Eng., 2024, 163, 105326 CrossRef CAS
.
- M. J. Jo, M. S. Yoon, S. Y. Kim, J. M. Lee, S. J. Kang, C.-W. Park, J.-S. Kim, J.-H. Yoon and D. H. Shin, A combination formulation of TPGS micelles loaded with paclitaxel and olaparib and a pH-thermosensitive hydrogel for treating peritoneal metastasis and drug-resistant ovarian cancer, J. Pharm. Invest., 2025, 55, 303–319 CrossRef CAS
.
- Q. Gou, L. Liu, C. Wang, Q. Wu, L. Sun, X. Yang, Y. Xie, P. Li and C. Gong, Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer, Colloids Surf., B, 2015, 126, 26–34 CrossRef CAS PubMed
.
- N. Wang, T. He, Y. Shen, L. Song, L. Li, X. Yang, X. Li, M. Pang, W. Su, X. Liu, Q. Wu and C. Gong, Paclitaxel and Tacrolimus Coencapsulated Polymeric Micelles That Enhance the Therapeutic Effect of Drug-Resistant Ovarian Cancer, ACS Appl. Mater. Interfaces, 2016, 8, 4368–4377 CrossRef CAS
.
- W. Zheng, M. Li, Y. Lin and X. Zhan, Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer, Biomed. Pharmacother., 2018, 108, 565–573 CrossRef CAS
.
- C. He, D. Liu and W. Lin, Self-assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer, Biomaterials, 2015, 36, 124–133 CrossRef CAS
.
- R. Yang, X. Yu, H. Li, C. Wang, C. Wu, W. Zhang and W. Guo, Effect of Mg doping on magnetic induction heating of Zn–Co ferrite nanoparticles, J. Alloys Compd., 2021, 851, 156907 CrossRef CAS
.
- M. R. Marques, R. Loebenberg and M. Almukainzi, Simulated biological fluids with possible application in dissolution testing, Dissolution Technol., 2011, 18, 15–28 CrossRef CAS
.
- J. Skach, I. Slamborova, P. Hromadka, P. Exnar and R. Gurlich, Surface treatment of artificial implants with hybrid nanolayers: results of antibacterial tests, leachates and scanning electron microscope analysis, Ann. Surg. Treat. Res., 2024, 107, 108–119 CrossRef CAS PubMed
.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2007, 22, 659–661 CrossRef PubMed
.
- W. Zhang, X. Zuo, Y. Niu, C. Wu, S. Wang, S. Guan and S. R. P. Silva, Novel nanoparticles with Cr3+ substituted ferrite for self-regulating temperature hyperthermia, Nanoscale, 2017, 9, 13929–13937 RSC
.
- J. Gong, C. C. L. Schuurmans, A. M. V. Genderen, X. Cao, W. Li, F. Cheng, J. J. He, A. López, V. Huerta, J. Manríquez, R. Li, H. Li, C. Delavaux, S. Sebastian, P. E. Capendale, H. Wang, J. Xie, M. Yu, R. Masereeuw, T. Vermonden and Y. S. Zhang, Complexation-induced resolution enhancement of 3D-printed hydrogel constructs, Nat. Commun., 2020, 11, 1267 CrossRef CAS
.
- F.-L. Mi, Synthesis and Characterization of a Novel Chitosan–Gelatin Bioconjugate with Fluorescence Emission, Biomacromolecules, 2005, 6, 975–987 CrossRef CAS PubMed
.
- L. Fuks, D. Filipiuk and M. Majdan, Transition metal complexes with alginate biosorbent, J. Mol. Struct., 2006, 792–793, 104–109 CrossRef CAS
.
- P. Tordi, F. Ridi, P. Samorì and M. Bonini, Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials, Adv. Funct. Mater., 2024, 35, 2416390 CrossRef
.
- W. Sun, P. Yin, C. Wang, Y. Ren, X. Han, C. Wu and W. Zhang, Determination of the elastic modulus of adherent cells using spherical atomic force microscope probe, J. Mater. Sci., 2021, 56, 18210–18218 CrossRef CAS
.
- B. Hildebrandt, P. Wust, O. Ahlers, A. Dieing, G. Sreenivasa, T. Kerner, R. Felix and H. Riess, The cellular and molecular basis of hyperthermia, Crit. Rev. Oncol. Hematol., 2002, 43, 33–56 CrossRef PubMed
.
- H. Morimoto, S. Yonehara and B. Bonavida, Overcoming Tumor Necrosis Factor and Drug Resistance of Human Tumor Cell Lines by Combination Treatment with Anti-Fas Antibody and Drugs or Toxins, Cancer Res., 1993, 53, 2591–2596 CAS
.
- H. Morimoto, J. T. Safrit and B. Bonavida, Synergistic effect of tumor necrosis factor-alpha- and diphtheria toxin-mediated cytotoxicity in sensitive and resistant human ovarian tumor cell lines, J. Immunol., 1991, 147, 2609–2616 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |







