Open Access Article
Open Access ArticleA fast method based on programmed temperature vaporization-mass spectrometry for the quantification of 17α-ethinylestradiol and drospirenone in contraceptive formulations
Pablo Álvarez Alonso†
,
Iria González-Mariño† ,
Miguel del Nogal Sánchez
,
Miguel del Nogal Sánchez *,
Ana Ballester-Caudet
*,
Ana Ballester-Caudet ,
Encarnación Rodríguez-Gonzalo
,
Encarnación Rodríguez-Gonzalo and
José Luis Pérez Pavón
and
José Luis Pérez Pavón
Departamento de Química Analítica, Nutrición y Bromatología. Facultad de Ciencias Químicas, Universidad de Salamanca, 37008 Salamanca, Spain. E-mail: mns@usal.es; Fax: +34-923-294483
First published on 23rd December 2025
Abstract
A rapid method has been developed for the determination of 17α-ethinylestradiol and drospirenone in contraceptive formulations. The method is based on the direct coupling of a programmed temperature vaporizer inlet to a quadrupole mass spectrometer via a deactivated fused silica tube (10 m × 0.18 mm) that is maintained at 275 °C throughout the entire analysis. The inlet is equipped with a baffled glass liner coated with Siltek™ and the injection is performed in split mode (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). The data acquisition time was only 1.0 min per sample, allowing for the high-throughput quantification of active principles in contraceptive pills in minimal time. The goal is to apply this strategy as a screening tool in quality control processes, limiting the use of the more costly and time-consuming chromatographic methods to those pills showing anomalous results, thus optimizing laboratory resources. Sample treatment involved a fast ultrasound-assisted solid–liquid extraction, and both sample preparation and instrumental conditions were optimized. The determination of the active principles was performed using the standard addition method. Accuracy, expressed as recovery percentage relative to the mass of active principle declared by the manufacturer, varied between 80% and 120%. Intra- and inter-day precision were adequate, with values of relative standard deviation (% RSD) equal to or less than 9% and 11%, respectively. Furthermore, the sustainability profile of the proposed rapid method was evaluated using the HEXAGON algorithm, which demonstrated that the PTV-MS method contributes to sustainable development by benefiting both the environment and society.
10). The data acquisition time was only 1.0 min per sample, allowing for the high-throughput quantification of active principles in contraceptive pills in minimal time. The goal is to apply this strategy as a screening tool in quality control processes, limiting the use of the more costly and time-consuming chromatographic methods to those pills showing anomalous results, thus optimizing laboratory resources. Sample treatment involved a fast ultrasound-assisted solid–liquid extraction, and both sample preparation and instrumental conditions were optimized. The determination of the active principles was performed using the standard addition method. Accuracy, expressed as recovery percentage relative to the mass of active principle declared by the manufacturer, varied between 80% and 120%. Intra- and inter-day precision were adequate, with values of relative standard deviation (% RSD) equal to or less than 9% and 11%, respectively. Furthermore, the sustainability profile of the proposed rapid method was evaluated using the HEXAGON algorithm, which demonstrated that the PTV-MS method contributes to sustainable development by benefiting both the environment and society.
1. Introduction
Oral contraceptive pills are one of the most preferred methods of contraception. Their effectiveness relies on the combined action of two primary components, an estrogen and a progestogen, which together inhibit ovulation and alter both the cervical mucus and the uterine lining to block the sperm. Particularly, the combination of the synthetic estrogen 17α-ethinylestradiol and the progestin drospirenone (6β,7β:15β,16β-dimethylene-3-oxo-17α-pregn-4-ene-21,17-carbolactone) is being commercialized in two different dosage strategies: 3 mg of drospirenone with 30 µg of 17α-ethinylestradiol, administered for 21 consecutive days followed by a 7-day break; and 3 mg of drospirenone with 20 µg of 17α-ethinylestradiol, administered for 24 days followed by a 4-day break.1–3To accurately quantify the concentrations of these active principles in pharmaceutical products, most analytical methods rely on liquid chromatography (LC) with optical detection, either using a UV absorbance detector alone4–6 or in series with a fluorescence detector to measure 17α-ethinylestradiol.7 Although LC with spectrophotometric detection has been widely used, mass spectrometry (MS), coupled to either LC or gas chromatography (GC), has also been applied for the determination of drospirenone or 17α-ethinylestradiol, along with other hormones, in biological and environmental samples: hair,8 blood,9 urine,10 water,11–14 and sediments.15 Recently, our group developed the first method based on GC-MS for the simultaneous quantification of these two synthetic hormones in contraceptive formulations.16 Unlike most GC-MS methods developed for the separation and detection of hormones, no derivatization was applied: analytes were extracted from the pills and the extract was directly injected into the GC system. This approach reduced the use of organic (often toxic) reagents, sample handling, and overall analysis times. Specifically, sample extraction was performed in 15 min (plus 10 min for centrifugation) and the chromatographic analysis was completed in 7.4 min (plus 2.4 min to adjust the GC oven and injector to the initial temperatures for the next run).
The development of rapid analytical methods is a key strategy to reduce analysis costs and optimize laboratory resources. To this end, non-separative techniques based on the direct introduction of the sample into a mass spectrometer are increasingly used as screening tools. By bypassing the time-consuming step of chromatographic separation, these strategies can provide results within minutes, being highly suitable for high-throughput analysis in different analytical fields. In the pharmaceutical field, they can be used for the rapid quantification of active principles in quality control laboratories. However, in some cases, a chemometric treatment of the data is necessary to extract the relevant chemical information from the recorded signal profiles.17–19
In this line, herein we propose a fast MS-based method for the quantification of 17α-ethinylestradiol and drospirenone in contraceptive formulations. It is based on the direct coupling of a programmed temperature vaporizer (PTV) inlet to a quadrupole mass spectrometer via a deactivated fused silica tube (10 m × 0.18 mm) maintained at 275 °C throughout the entire analysis. Interaction with the stationary phase is removed, since there is no chromatographic column, and the total run time is 1.0 min (plus 2.8 min to adjust the injector to the initial temperatures for the next run). To correct matrix effects, quantification is performed using a three-point standard addition approach. The goal is to apply this methodology as a screening tool in the pharmaceutical industry. Quantification of active principles can be preliminary performed by the high-throughput approach, and only when anomalous results –defined as concentrations deviating by more than 20% from the declared value, in accordance with the criteria set by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)20– samples are subjected to a second, confirmatory analysis by GC-MS.16 This two-tiered approach ensures that most samples are processed using only the rapid screening method, the slower, more expensive confirmatory one is reserved for the limited number of samples where the active ingredient content falls outside the specified range. Consequently, this screening-confirmatory scheme provides a more efficient and sustainable workflow for the quality control of pharmaceuticals. The figures of merit, including chemical and health risks, environmental impact, and economic cost, were assessed using the HEXAGON metric tool.21 This tool was employed to evaluate not only environmental and safety aspects, but also the cost-benefit relationship. The arithmetic mean (Sav) of the 0–4 eco-scale, as defined by the HEXAGON tool, was calculated to compare the proposed fast MS-based method with its chromatographic counterpart.
2. Experimental
2.1. Chemicals and standard solutions
Drospirenone (C24H30O3) and 17α-etinhylestradiol (C20H24O2) were supplied by Supelco (Bellefonte, Pennsylvania, USA). Ethyl acetate and methanol were provided by Sigma-Aldrich (Steinheim, Germany). The purity of all the chemicals was equal to or higher than 98%. Stock solutions (1000 mg L−1 for 17α-ethinylestradiol and 5000 mg L−1 for drospirenone) were prepared in ethyl acetate and stored at 4 °C until use. Standard solutions containing both analytes were prepared in ethyl acetate by taking different volumes from the stock solutions described above.2.2. Samples
Contraceptive formulations from three different brands (samples a–c) were purchased at a local pharmacy in Salamanca (Spain). According to the information provided by the manufacturers, all three formulations contain 3.00 mg of drospirenone per tablet. Regarding 17α-ethinylestradiol, samples a and b contain 0.02 mg, and sample c contains 0.03 mg.2.3. Sample treatment: solid–liquid extraction
The extraction of the active ingredients was performed by ultrasound-assisted solid–liquid extraction. Each tablet was placed in a 10 mL glass centrifuge tube; 1.0 mL of methanol was added, and the tube was immersed in an ultrasonic bath for 3.0 min. The resulting extract was diluted to a final volume of 4.0 mL with ethyl acetate and centrifuged at 4500 rpm (1815×g) for 5 min. Finally, an aliquot of the supernatant was transferred to a glass vial for injection into the PTV-MS system. Spiked samples were prepared following the previous protocol but, after extraction, the required volume of standard solutions containing the analytes was added. Finally, suspensions were brought up to a final volume of 4.0 mL with ethyl acetate.2.4. Instrumental configuration: PTV-MS
The vial with the extract was placed in an MPS2 Multi-Purpose Sampler (Gerstel, Mülheim an der Ruhr, Germany). The injection was performed with a programmed temperature vaporizer (PTV) inlet (CIS-4, Gerstel, Baltimore, MD) equipped with a Siltek™ coated baffled glass liner (71 mm × 2 mm I.D., Gerstel, CIS-4). One microliter of the sample was injected in split mode (ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) applying a split flow of 20 mL min−1. Helium N50 (99.999% pure, air liquid) was used as carrier gas at a constant flow rate of 2 mL min−1. The PTV was maintained at an initial temperature of 130 °C for 0.1 min and subsequently increased at 12 °C s−1 to a final temperature of 350 °C, which was maintained for 0.6 min. The total run time was 1.0 min per sample. However, 2.8 additional minutes were needed to cool the PTV to its initial temperature (130 °C), bringing the total time between injections to 3.8 min. This cooling process was performed with CO2 (air liquid) and occurred concurrently with the syringe cleaning process. Considering this, the interval between injections was 3.8 min. An Agilent 6890 GC system was equipped with an ultimate plus deactivated fused silica tubing (10 m × 0.18 mm) from J&W Scientific (Folsom, CA, USA) as interface between the PTV inlet and the mass spectrometer (HP 5973 N). The chromatographic oven was maintained at a constant temperature of 275 °C throughout the entire analysis.
10) applying a split flow of 20 mL min−1. Helium N50 (99.999% pure, air liquid) was used as carrier gas at a constant flow rate of 2 mL min−1. The PTV was maintained at an initial temperature of 130 °C for 0.1 min and subsequently increased at 12 °C s−1 to a final temperature of 350 °C, which was maintained for 0.6 min. The total run time was 1.0 min per sample. However, 2.8 additional minutes were needed to cool the PTV to its initial temperature (130 °C), bringing the total time between injections to 3.8 min. This cooling process was performed with CO2 (air liquid) and occurred concurrently with the syringe cleaning process. Considering this, the interval between injections was 3.8 min. An Agilent 6890 GC system was equipped with an ultimate plus deactivated fused silica tubing (10 m × 0.18 mm) from J&W Scientific (Folsom, CA, USA) as interface between the PTV inlet and the mass spectrometer (HP 5973 N). The chromatographic oven was maintained at a constant temperature of 275 °C throughout the entire analysis.
The detector was a quadrupole mass spectrometer (HP 5973 N) with an inert ion source operating in electron-ionization mode (70 eV as ionization voltage). The ion source and quadrupole temperatures were set at 230 °C and 150 °C, respectively. For compound identification, data acquisition was performed in full scan mode (2.11 scan per s). The m/z range was 50–400 amu and the solvent delay was set at 0.11 min. For the quantification of the active principles, the selected ion monitoring (SIM) mode was used recording three m/z ratios per compound with a dwell time of 30 ms per m/z ratio (Table 1).
| Acquisition mode | m/z ratio | q/Q | ||
|---|---|---|---|---|
| SCAN | 50–400 | Scan per s: 2.11 | ||
| SIM | 17α-Ethinylestradiol | 160 | 3.3 | Dwell time: 30 ms |
| 213 | 3.8 | |||
| 296 | — | |||
| Drospirenone | 255 | 4.5 | ||
| 256 | 1.9 | |||
| 366 | — |
2.5. Data analysis
Data acquisition was accomplished with the Enhanced ChemStation software from Agilent Technologies (Agilent Technologies, Santa Clara, CA, USA).3. Results and discussion
3.1. Profile signal and mass spectra
The signal profile of a standard solution containing 17α-ethinylestradiol (7.5 mg L−1) and drospirenone (750 mg L−1) is shown in Fig. 1a, with the first peak corresponding to 17α-ethinylestradiol and the second one to drospirenone. This rapid separation, achieved in less than one minute in a deactivated fused silica tube, is possible due to the significant difference in their boiling points: 457 °C for 17α-ethinylestradiol and 552 °C for drospirenone (values calculated using the Advanced Chemistry Development (ACD/Labs) Software (© 1994–2025 ACD/Labs)). This strategy leverages differences in volatility, causing the compounds to enter the gaseous phase at different rates, like in a distillation process. While such separation could be considered a disadvantage in a non-separative method intended to produce a single co-eluting peak, it offers a key advantage: it simplifies the selection of quantification ions. By separating the analytes in time, it increases the number of unique m/z ratios available for each compound, avoiding mutual interferences. Consequently, each analyte can be quantified using simple univariate calibration models. This is especially useful in this case, since the two compounds share several m/z ratios.The mass spectra of the two compounds studied are shown in Fig. 1b and c. The most abundant m/z ratios are 213 and 296 for 17α-ethinyestradiol, and 255 and 366 for drospirenone. Notably, every major m/z ratio from 17α-ethinylestradiol also appears in the mass spectrum of drospirenone. However, the molecular ion of this heavier compound (m/z 366) is exclusive to drospirenone and, therefore, it was selected for its quantification. For 17α-ethinylestradiol, the molecular ion (m/z 296) was also selected as quantifier ion (Q). The most abundant one (m/z 213) was shared by some unknown excipients in real pills, what led us to acquire a third ion (m/z 160) for confirmatory purposes (qualifier ion, q). Qualifier-to-Quantifier (q/Q) ratios were obtained for both standards and pill extracts. Except for 213/296, ratio deviations in real samples were maintained below 15%.
3.2. Sample treatment optimization
Following the procedure described by Peña et al.,16 analytes were extracted into 1.0 mL of methanol by ultrasound assistance. To reduce the analysis times reported in previous works,4,6,7,16 we compared extraction times of 3 and 15 min. To this end, eight tablets from two different pharmaceutical products were extracted by ultrasound assistance for 3 and 15 min, and the peak areas of the primary quantification ions were measured. Since no significant differences in analyte signal were observed between the two extraction times, the shorter 3-min duration was selected. In addition, the centrifugation time was reduced from 10 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 to 5 min, with no differences been observed.
16 to 5 min, with no differences been observed.
As concluded in our previous study,16 17α-ethinylestradiol is partially degraded to estrone (C18H22O2) when dissolved in pure methanol and exposed to the high operating temperatures of the PTV. While this degradation does not occur in ethyl acetate, this solvent is not able to extract 17α-ethinylestradiol from the solid pill and, thus, the use of methanol as extracting solvent is required.16 To avoid the subsequent degradation within the injector, different dilutions of the methanolic extract (1.0 mL) in ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were tested. Estrone formation was inhibited at a 1
4) were tested. Estrone formation was inhibited at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dilution and beyond. However, the 1
2 dilution and beyond. However, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dilution ratio was selected to prolong the liner's lifespan without excessively diluting the extract. Analyte stability over time in both standards and pill extracts had already been addressed in our previous study.16 Since no relevant degradation was observed in any case, the stability assessment was not repeated here.
3 dilution ratio was selected to prolong the liner's lifespan without excessively diluting the extract. Analyte stability over time in both standards and pill extracts had already been addressed in our previous study.16 Since no relevant degradation was observed in any case, the stability assessment was not repeated here.
3.3. Optimization of PTV-MS instrumental conditions
Since the analyte concentrations in the extracts is sufficiently high, the selected injection mode was split with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. A classical hot split injection at 250 °C was not feasible because it caused 17α-ethinylestradiol to co-elute with the solvent peak. To delay the peak of 17α-ethinylestradiol and separate it from the solvent front, various initial injection temperatures ranging from 70 to 230 °C were tested. A compromise temperature of 130 °C was selected, as it effectively separated the first peak from the solvent peak; the use of lower temperatures unnecessarily lengthened the analysis time. This initial temperature was maintained for 0.1 min and then ramped at the maximum rate allowed by the instrument configuration (12 °C s−1) to the maximum temperature allowed by the liner manufacturer (see below).
10. A classical hot split injection at 250 °C was not feasible because it caused 17α-ethinylestradiol to co-elute with the solvent peak. To delay the peak of 17α-ethinylestradiol and separate it from the solvent front, various initial injection temperatures ranging from 70 to 230 °C were tested. A compromise temperature of 130 °C was selected, as it effectively separated the first peak from the solvent peak; the use of lower temperatures unnecessarily lengthened the analysis time. This initial temperature was maintained for 0.1 min and then ramped at the maximum rate allowed by the instrument configuration (12 °C s−1) to the maximum temperature allowed by the liner manufacturer (see below).
Four different liners were tested: two packed liners (one with glass wool and the other with Tenax TA™) and two empty baffled liners (one deactivated and the other coated with Siltek™). The liner filled with Tenax™ was discarded because it retained the analytes completely (no signal observed even when working at the maximum temperature allowed, 350 °C). The glass wool liner and the deactivated empty baffled liner provided good results, but their maximum operating temperature of 275 °C was insufficient to achieve an adequate cleaning between injections. Therefore, the SiltekTM-coated empty liner, whose high maximum temperature of 350 °C allows for a thorough cleaning between injections, was selected. To demonstrate the absence of carryover effects, Fig. S1 of the SI displays the Extracted Ion Chromatogram (EIC) at m/z 296 and m/z 366 of a solvent solution injected before and right after the analysis of a pill extract.
3.4. Calibration, validation and determination of active principles in contraceptive samples
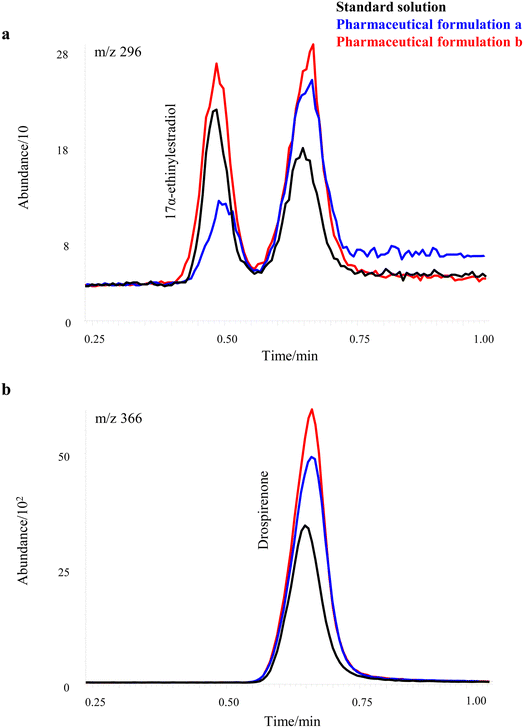
Initially, the possibility of using external standard calibration to quantify the analytes in different commercial products was considered. To this end, the signals from the drug extracts were compared with those of standard solutions prepared at the theoretical concentrations in the extract according to the information provided by the manufacturers. However, this approach revealed significant discrepancies that ranged from 48% to 190%.Fig. 2a shows the profile signal of the m/z ratio 296 (molecular ion of 17α-ethinylestradiol) in two commercial samples and in a standard solution containing 17α-ethinylestradiol and drospirenone at the expected theoretical concentration in the extracts of the pharmaceutical products (5 and 750 mg L−1 for 17α-ethinylestradiol and drospirenone, respectively). The first peak corresponds to 17α-ethinylestradiol and the second one to the contribution of drospirenone to the m/z ratio 296. Note that its signal is of the same order as that of 17α-ethinylestradiol due to the important difference in concentrations that exist in the commercial drugs even when the abundance of this ratio in the mass spectrum of drospirenone is less than 3%. Fig. 2b shows the profile signal of the m/z ratio 366 (molecular ion of drospirenone) in the same samples of Fig. 2a. In this case, only one peak is obtained since this m/z ratio is unique to drospirenone.
To account for the matrix effect, calibration was performed based on a three-point standard addition approach. The first level corresponded to the concentration of the active principles in the pills without any addition, while the other two levels are shown in Table 2. Each sample was measured in triplicate. The resulting calibration curves showed good linearity and no lack of fit. Table 2 shows the results obtained for each commercial product, along with their accuracy, expressed as percentage of recovery relative to the value declared by the manufacturer. Recovery values varied, in all cases, between 80% and 120%. According to the International Council for Harmonization (ICH), the content of an active ingredient must fall within this 80 to 120% range for method validation.20 Therefore, the results confirm that the proposed method satisfies the ICH criteria for accuracy. Although the existence of matrix effects forced the quantification via the standard addition method, the impact on overall throughput is negligible in a quality-control environment since a single three-point calibration curve can be applied to an entire commercial batch, thereby streamlining the workflow of routine analysis. Under these conditions, only the measurement of each individual sample is required, since the calibration points obtained through standard addition can be prepared in advance and used for all samples within the same batch.
| Calibration | Prediction | |||||
|---|---|---|---|---|---|---|
| 17α-Ethinylestradiol | Drospirenone | 17α-Ethinylestradiol | Drospirenone | |||
| Standard addition (mg L−1) | µg/tablet | Accuracy (% recovery) | µg/tablet | Accuracy (% recovery) | ||
| Sample a | 0–5.0–10.0 | 0–400–800 | 24 ± 4 | 120 | (28 ± 4) × 102 | 93 |
| Sample b | 0–5.0–10.0 | 0–400–800 | 16 ± 4 | 80 | (28 ± 8) × 102 | 93 |
| Sample c | 0–7.5–15.0 | 0–400–800 | 31 ± 2 | 103 | (28 ± 1) × 102 | 93 |
A statistical evaluation was performed to compare the concentrations obtained by this method to those corresponding to the chromatographic method in commercial pharmaceutical samples. As the determinations were performed on paired samples, the analysis incorporated the combined uncertainty associated with each measurement to assess the difference between mean concentrations. In all cases, the observed differences were below the 95% confidence threshold, indicating that the concentrations reported by the chromatographic method and the rapid method were not significantly different. These findings support the analytical equivalence of both approaches for the quantification of the studied analytes in commercial drug products.
To evaluate precision, five tablets of pharmaceutical product b were analysed. Intra-day precision was assessed on a single day, while inter-day precision was determined repeating the analysis on two consecutive days. In both cases, precision was expressed as the relative standard deviation (RSD) calculated from the peak area of each analyte's molecular ion. RSD was satisfactory with values equal to or less than 9% for intra-day precision (9% for 17α-ethinylestradiol and 5% for drospirenone) and equal to or less than 11% for inter-day precision (11% for 17α-ethinylestradiol and 5% for drospirenone).
The limits of detection (LOD) and quantification (LOQ) were calculated from a non-spiked pill extract as the analyte concentration providing a signal-to-noise ratio of 3 (LOD) and 10 (LOQ). They were 0.9 and 3.0 mg L−1, LOD and LOQ, respectively, for 17α-ethinylestradiol, and 2.2 and 7.3 mg L−1 for drospirenone.
Selectivity was addressed by analysing inactive pills (“resting period pills”) and active pills of the same commercial brands and overlapping the EICs at m/z 296 (for 17α-ethinylestradiol) and at m/z 366 (for drospirenone). As shown in Fig. S2 of the SI, no signals were observed in the first case, demonstrating the appropriate discrimination between active principles and potentially co-occurring interfering excipients.
Finally, the robustness of the method was assessed by performing slight modifications to the main experimental variables (initial injection temperature and time). No significant differences were observed, demonstrating that the developed method is robust. RSD values ranged from 1.5 to 2.2% when the temperature was changed and 1.1% when the initial time was changed. The data are shown in Table S1 (SI).
3.5. Comparison with other methods in the bibliography
Table 3 compiles the times and analytical figures of other published methods for the simultaneous quantification of 17α-ethinylestradiol and drospirenone in contraceptive formulations. As can be seen, a substantial reduction in the run time is achieved: 1.0 min versus 7–8 min per sample.4,6,7,16 When accounting for the additional time needed to cool the injector to the initial temperature (see Section 2.4 and Fig. 3), this difference is reduced but still remarkable: 3.8 min (this method) versus 9.8 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 or versus times between 8 and 9 min.4,6,7 The latter, LC-based studies do not specify the exact interval between injections; however, it is likely to take at least one additional minute to fill the LC-injector loop. This implies that a maximum of seven extracts can be analyzed per hour, compared to the sixteen extracts that can be processed with the methodology developed in this study. Focusing only on the sample treatment, ours is also the shortest: 8 min compared to 10 min
16 or versus times between 8 and 9 min.4,6,7 The latter, LC-based studies do not specify the exact interval between injections; however, it is likely to take at least one additional minute to fill the LC-injector loop. This implies that a maximum of seven extracts can be analyzed per hour, compared to the sixteen extracts that can be processed with the methodology developed in this study. Focusing only on the sample treatment, ours is also the shortest: 8 min compared to 10 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4,6 (excluding the time needed for filtration, not specified), 25 min,16 or 35 min
4,6 (excluding the time needed for filtration, not specified), 25 min,16 or 35 min![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (excluding again the time needed for filtration). Analytical figures were, in all cases, comparable (Table 3).
7 (excluding again the time needed for filtration). Analytical figures were, in all cases, comparable (Table 3).
| Ref. | Sample treatment | Determination | Accuracya (%R) | LOQb (µg mL−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Technique | Time (min) | Technique | Run time (min) | 17α-Ethinylestradiol | Drospirenone | 17α-Ethinylestradiol | Drospirenone | |
| a Average values of the recovery rates provided by authors.b LOQ: instrumental limits of quantification (calculated from calibration curves or standard solutions) in all works but the current one: overall limit of quantification calculated from a pill extract.c FD: fluorescence detection. | ||||||||
| 4 | Ultrasound-assisted SLE (10 min) + filtration | 10 | LC-UV | 8.0 | 100 | 100 | 0.028 | 9.50 |
| 6 | Ultrasound-assisted SLE (10 min) + dilution | 10 | LC-UV | 7.0 | 100 | 100 | 0.00087 | 0.308 |
| 7 | Ultrasound-assisted SLE (20 min) + centrifugation (15 min) + filtration + dilution | 35 | LC-UV-FDc (in series) | 7.0 | 101 | 100 | 0.06 | 14.80 |
| 16 | Ultrasound-assisted SLE (15 min) + dilution + centrifugation (10 min) | 25 | GC-MS | 7.4 | 106 | 93 | 0.82 | 22 |
| This work | Ultrasound-assisted SLE (3 min) + dilution + centrifugation (5 min) | 8 | MS | 1.0 | 101 | 93 | 3.0 | 7.3 |
 | ||
Fig. 3 Comparison between the times needed for the sample treatment and instrumental analysis with the chromatographic method developed![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1616 and with the non-chromatographic method developed in this work. 1616 and with the non-chromatographic method developed in this work. | ||
A schematic diagram of the times needed for the sample treatment and instrumental analysis of our two developed methods (the screening method-this work, and the chromatographic confirmatory method16) is shown in Fig. 3. As mentioned, the largest time reduction is achieved in the run time (1.0 min versus 7.4 min) but also the sample treatment time is reduced considerably.
3.6. Sustainability assessment of the PTV-MS method versus GC-MS
The sustainability profile of the PTV-MS method was evaluated using the HEXAGON pictogram,21 which integrates analytical performance, health and safety, environmental impact, and cost-effectiveness. This comprehensive approach provides penalization scores across key parameters, with lower scores indicating better adherence to Green Analytical Chemistry (GAC) principles.22 As shown in Table 4, PTV-MS consistently yielded lower penalization scores than GC-MS when analyzing the aforementioned active ingredients in contraceptive tablets. These improvements mainly come from streamlined sample preparation and significantly shorter analysis time, enabled by the optimized ultrasound-assisted extraction and centrifugation steps that reduced solvent consumption and treatment time.Fig. 4a illustrates the most substantial differences, with instrumental runtime decreasing from 7.4 minutes (GC-MS) to 1 minute (PTV-MS). This reduction increased sample throughput, from six to sixteen extracts per hour, and lowered energy consumption, improving analytical efficiency. Calibration was also simplified through a three-point standard addition approach, avoiding the ten-level internal standard method required for GC-MS and reducing penalties linked to calibration and quality control.
 | ||
| Fig. 4 Sustainability assessment of PTV-MS versus GC-MS. (a) Penalty points to analytical figures of merit that are time-dependent. (b) Overall scores of the variables from the HEXAGON tool. | ||
Scores related to health and safety aspects reflected the lower solvent volumes used in PTV-MS, which reduced both operator exposure and waste generation. Additional penalties for GC-MS accounted for its reliance on carrier gases such as helium and carbon dioxide. Regarding environmental impact, quantified in terms of CO2 equivalents based on electricity consumption,23 PTV-MS exhibited markedly lower carbon footprint (0.36 kg CO2 per run versus 1.61 kg CO2 per run for GC-MS), largely due to shorter runtime and reduced cooling requirements. Cost assessment also advantaged PTV-MS owing to minimized solvent use, energy consumption and the absence of a gas chromatographic column. These combined factors enhance the overall sustainability of the PTV-MS method. As presented in Fig. 4b through the HEXAGON pictogram and eco-scale values (Sav),24 PTV-MS achieved lower average score (1.71 vs. 2.43 for GC-MS), confirming its applicability as a more sustainable alternative for pharmaceutical analysis.
4. Conclusions
A rapid and robust PTV-MS method has been developed and validated for the simultaneous quantification of 17α-ethinylestradiol and drospirenone in contraceptive formulations. Data acquisition time is 1.0 min, although additional time is necessary to prepare the PTV-MS for the next injection. In total, 16 samples can be analysed per hour, which makes this approach exceptionally well-suited for the high-throughput quantification of active principles in the pharmaceutical industry. Both sample preparation and instrumental parameters were optimized to minimize analysis times while ensuring the reliable quantification of both analytes. Although the existence of matrix effects forced the quantification via the standard addition method, the impact on overall throughput is negligible in a quality control setting, since a single three-point calibration curve can be applied to an entire commercial batch, streamlining the routine analysis workflow.The main advantage of the proposed methodology over conventional approaches is the significant reduction in overall analysis times (including sample treatment and PTV-MS measurement times) while maintaining comparable levels of accuracy and precision. Furthermore, in this case both analytes are quantified simultaneously in a single run (of only 1.0 min) and using the same detector. While conventional GC-MS methods typically involve time-consuming and costly processes, the proposed screening method represents a more sustainable, cost-effective, and efficient alternative, positioning it as a useful tool for routine screening in the pharmaceutical industry. This is evidenced by its lower score (1.71) on the HEXAGON eco-scale metric, compared to the corresponding confirmatory GC-MS method (2.43), highlighting its superior sustainability over conventional chromatographic techniques.
Author contributions
Pablo Álvarez Alonso: methodology, validation, formal analysis, investigation. Iria González Mariño: conceptualization, methodology, validation, investigation, writing – review & editing, supervision. Miguel del Nogal Sánchez: conceptualization, methodology, validation, formal analysis, investigation, writing – review & editing, supervision. Ana Ballester-Caudet: formal analysis, writing – original draft. Encarnación Rodriguez-Gonzalo: conceptualization, methodology, resources, writing – review & editing, supervision. Jose Luís Pérez Pavón: conceptualization, methodology, resources, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting the findings of this study are available within the article.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ay01770j.
Acknowledgements
This work was financially supported by the Spanish Ministry of Science, Innovation and Universities (project No. PID2021–127679NB-I00).References
- C. Batukan and I. I. Muderris, Efficacy of a new oral contraceptive containing drospirenone and ethinyl estradiol in the long-term treatment of hirsutism, Fertil. Steril., 2006, 85, 436, DOI:10.1016/j.fertnstert.2005.07.1311.
- H. Blode, W. Wuttke, W. Loock, G. Röll and R. Heithercher, A 1-year pharmacokinetic investigation of a novel oral contraceptive containing drospirenone in healthy female volunteers, Eur. J. Contracept. Reprod. Health Care, 2000, 5, 256, DOI:10.1080/13625180008500407.
- P. Rosenbaum, W. Schmidt, F. M. Helmerhorst, W. Wuttke, W. Rossmanith, F. Freundl, K. Thomas, M. Grillo, A. Wolf and R. Heithecker, Inhibition of ovulation by a novel progestogen (drospirenone) alone or in combination with ethinylestradiol, Eur. J. Contracept. Reprod. Health Care, 2000, 5, 16, DOI:10.1080/13625180008500376.
- E. M. Seifeldeen, M. A. Etman and H. Y. Aboul-Enein, Simultaneous determination of four hormonal compounds in oral contraceptive tablet formulations by high performance liquid chromatography, J. Liq. Chromatogr. Relat. Technol., 2016, 39, 134, DOI:10.1080/10826076.2015.1137002.
- S. Talath and S. Dhaneshwar, A simple and rapid validated stability indicating HPLC method for the determination of drospirenone in a pharmaceutical product, Indo Am. J. Pharm. Res., 2017, 7, 7401 CAS.
- C. Praveen, M. K. Ranganath and P. Divakar, Method development and validation for simultaneous estimation of ethinyl estradioland drospirenone and forced degradation behavior by HPLC in combined dosage form, Pharm. Anal. Acta, 2013, 4, 231, DOI:10.4172/2153-2435.1000231.
- V. Silva, A. A. G. Galdos, C. M. A. Mothe, M. B. Pallastrelli, M. S. Prado, A. K. Singh, E. Kedor-Hackmann and M. M. Santoro, Simultaneous determination of ethinyl estradiol and drospirenone in oral contraceptive by high performance liquid chromatography, Braz. J. Pharm. Sci., 2013, 49, 521, DOI:10.1590/S1984-82502013000300013.
- A. A. Durant, C. A. Fente, C. M. Franco, B. I. Vázquez and A. Cepeda, Gas chromatography-tandem mass spectrometry determination of 17alpha-ethinylestradiol residue in the hair of cattle. Application to treated animals, J. Agric. Food Chem., 2002, 50, 436, DOI:10.1021/jf010834g.
- N. Idota, M. Kobayashi, D. Miyamori, Y. Kakiuchi and H. Ikegaya, Drospirenone detected in postmortem blood of a young woman with pulmonary thromboembolism: A case report and review of the literature, Leg. Med., 2015, 17, 109, DOI:10.1016/j.legalmed.2014.10.001.
- X.-Y. Xiao and D. McCalley, Quantitative analysis of estrogens in human urine using gas chromatography/negative chemical ionisation mass spectrometry, Rapid Commun. Mass Spectrom., 2000, 14, 1991, DOI:10.1002/1097-0231(20001115)14:21<1991::AID-RCM125>3.0.CO;2-H.
- E. Mirmont, A. Boeuf, M. Charmel, S. Vaslin-Reimann, B. Lalere, O. Laprevote and S. Lardy-Fontan, Development and implementation of an analytical procedure for the quantification of natural and synthetic steroid hormones in whole surface waters, J. Chromatogr. B, 2021, 1175, 122732, DOI:10.1016/j.jchromb.2021.122732.
- A. Gonzalez, J. Avivar and V. Cerda, Estrogens determination in wastewater samples by automatic in-syringe dispersive liquid-liquid microextraction prior silylation and gas chromatography, J. Chromatogr. A, 2015, 1413, 1, DOI:10.1016/j.chroma.2015.08.031.
- J. G. Ronderos-Lara, H. Saldarriaga-Noreña, M. A. Murillo-Tovar and J. Vergara-Sánchez, Optimization and application of a GC-MS method for the determination of endocrine disruptor compounds in natural water, Separations, 2018, 5, 33, DOI:10.3390/separations5020033.
- H. G. J. Mol, S. Sunarto and O. M. Steijger, Determination of endocrine disruptors in water after derivatization with N-methyl-N-(tert-butyldimethyltrifluoroacetamide) using gas chromatography with mass spectrometric detection, J. Chromatogr. A, 2000, 879, 97, DOI:10.1016/S0021-9673(00)00124-2.
- R. Liu, J. L. Zhou and A. Wilding, Microwave-assisted extraction followed by gas chromatography-mass spectrometry for the determination of endocrine disrupting chemicals in river sediments, J. Chromatogr. A, 2004, 1038, 19, DOI:10.1016/j.chroma.2004.03.030.
- J. Peña, I. González-Mariño and J. L. Pérez Pavón, Ultrasound-assisted extraction, followed by gas chromatography-mass spectrometry for the simultaneous quantification of ethinyl estradiol and drospirenone in contraceptive formulations, Molecules, 2023, 18, 4978, DOI:10.3390/molecules28134978.
- A. M. Casas-Ferreira, M. del Nogal Sánchez, J. L. Pérez Pavón and B. Moreno Cordero, Non-separative mass spectrometry methods for non-invasive medical diagnostic based on volatile organic compounds: A review, Anal. Chim. Acta, 2019, 1045, 10, DOI:10.1016/j.aca.2018.07.005.
- A. M. Casas-Ferreira, M. del Nogal Sánchez, E. Rodríguez-Gonzalo and J. L. Pérez Pavón, Non-separative determination of isomeric polycyclic aromatic hydrocarbons by electrospray Ag (I) cationization mass spectrometry and multivariate calibration, Microchem. J., 2022, 183, 108072, DOI:10.1016/j.microc.2022.108072.
- I. González-Mariño, A. M. Casas-Ferreira, M. del Nogal Sánchez and J. L. Pérez Pavón, Use of a guard column coupled to mass spectrometry as a fast semi-quantitative methodology for the determination of plasticizer metabolites in urine, J. Chromatogr. A, 2023, 1690, 463788, DOI:10.1016/j.chroma.2023.463788.
- European Medicines Agency International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), ICH Harmonized Guideline on Validation of Analytical Procedures, 2024 Search PubMed.
- A. Ballester-Caudet, P. Campíns-Falcó, B. Pérez, R. Sancho, M. Lorente, G. Sastre and C. González, A new tool for evaluating and/or selecting analytical methods: summarizing the information in a hexagon, TrAC, Trends Anal. Chem., 2019, 118, 538, DOI:10.1016/j.trac.2019.06.015.
- M. Sajid and J. Płotka-Wasylka, Green analytical chemistry metrics: A review, Talanta, 2022, 238, 123046, DOI:10.1016/j.talanta.2021.123046.
- J. Jiménez, L. De la Cruz, J. Carballo, and A. Doménech, Enfoques metodológicos para el cálculo de la Huella de Carbono, OSE Estudios Gráficos Europeos S.A., Spain, 2011 Search PubMed.
- P. M. Nowak, P. Kocielniak, M. Tobiszewski, A. Ballester-Caudet and P. Campíns-Falcó, Overview of the three multicriteria approaches applied to a global assessment of analytical methods, TrAC, Trends Anal. Chem., 2020, 133, 116065, DOI:10.1016/j.trac.2020.116065.
Footnote |
| † Both authors have equally contributed to this work and the two should be considered as first authors. |
| This journal is © The Royal Society of Chemistry 2026 |