Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterizing noninvasively conservation status of historical wet collections using spatially offset Raman spectroscopy
Wren Montgomery *a,
Chelsea McKibbin
*a,
Chelsea McKibbin a,
Ana Blancob,
Sam Walkerb,
James Maclaine
a,
Ana Blancob,
Sam Walkerb,
James Maclaine a,
Roberto Portela Miguez
a,
Roberto Portela Miguez a,
Patrick Campbell
a,
Patrick Campbell a,
Robert Stokesb,
Pavel Matousek
a,
Robert Stokesb,
Pavel Matousek c and
Sara Mosca
c and
Sara Mosca *cd
*cd
aDepartment of Science, Natural History Museum, Cromwell Road, London SW7 5BD, UK. E-mail: wren.montgomery@gmail.com
bAgilent Technologies LDA UK, Becquerel Avenue, Didcot OX11 0RA, UK
cCentral Laser Facility, Research Complex at Harwell, STFC Rutherford Appleton Laboratory, UKRI, Harwell Campus, OX11 0QX, UK. E-mail: sara.mosca@stfc.ac.uk
dDepartment of Physics and Astronomy, University of Exeter, Exeter EX4 4QL, UK
First published on 24th December 2025
Abstract
Fluid-preserved specimens are central to the scientific and cultural value of natural history collections, yet their conservation is challenged by chemical and physical instabilities of both the specimens and their preservation media. Here we report the application of handheld spatially offset Raman spectroscopy (SORS) to noninvasively characterize historical specimens from their conservation status perspective. This goes beyond previously reported basic determination of the major constituents of preservation fluids by providing detailed chemical information on minor dissolved components, such as lipids, protein fragments, and residual fixation products, as well as organic deposits on container walls. This provides insight into fluid degradation, leakage, and specimen-fluid interactions of sealed wet collection items. Furthermore, we demonstrate the capability of directly probing specimen composition. All measurements were performed in situ without opening containers, demonstrating the robustness and versatility of SORS for comprehensive monitoring wet collection status under museum conditions and offering curators actionable insights into degradation processes and long-term collection integrity.
Introduction
The study of specimens preserved in fluid media is essential to both the scientific value and cultural significance of natural history collections, providing a unique opportunity for research and long-term biodiversity documentation. However, the maintenance of fluid collections involve a number of preservation challenges linked to various degradation processes that compromise both specimen integrity and the preservation fluids themselves.1,2 One of the main challenges in managing fluid-preserved collections is the lack of consistent documentation regarding the preservatives used, which complicates efforts to maintain specimens and increases the risk of causing damage when topping up solutions. Over time, fluids may change composition due to interactions with the specimen, container or storage condition. Common problems include preservative fluid evaporation3 from poor environmental conditions and suboptimal containers, especially in alcohol solutions where ethanol concentration can decline rapidly.4 Additionally, evaporation also leads to loss of fluid volume and potential exposure of tissues to air, inducing microbial growth.1 In addition, the main fluid may undergo chemical changes such as acidification through oxidation; for example, formaldehyde or ethanol can oxidize, forming formic or acetic acid that can impact the specimen status.5 Specimens may also undergo discoloration, shrinkage,6 or swelling and leaching.7–9 These can be related to various biochemical mechanisms. For example, paraformaldehyde precipitation can produce cloudiness or surface coatings on specimens. Other biological compounds, such as lipids, proteins, pigments, and blood, often leach from specimens into the surrounding solution,10 producing turbidity and further chemical interaction with the fluid (e.g. yellow-red discoloration).5 Deposits of organic material can also adhere to the inner container walls, sometimes solidifying after evaporation or with temperature fluctuations. These deposits can also provide information on the components leaching from sample into solution. These processes not only alter the preservation chemistry and can potentially and irreversibly damage the specimen integrity but also introduce contamination and reduce the potential for downstream analyses that require sample extraction. Conventional methods, such as gas chromatography-mass spectroscopy or density measurements, provide detailed compositional information2,11 but require fluid sampling, which risks fluid loss, contamination, and operator exposure to toxic vapours, including formaldehyde. By contrast, Raman spectroscopy offers a powerful non-invasive alternative,5 delivering molecularly specific information directly through sealed containers. In particular, spatially offset Raman spectroscopy (SORS)12 enables the effective suppression of the fluorescence and Raman signals from the container itself,13–15 allowing access to the chemical fingerprint of the fluid and its interaction with the specimen. In our previous studies, we have demonstrated that handheld SORS combined with multivariate analysis can non-invasively classify the principal components of the preservation fluids ethanol, methanol, glycerol, and formaldehyde, as well as their mixtures, both in controlled laboratory settings13 and in situ on historic samples in original jars at the Natural History Museum, London.16 In this study we demonstrate the potential of SORS to provide non-invasive insight into the current conservation status of wet collections. We show that minor dissolved components, such as fats or degradation products released from specimens; organic deposits on inner container walls resulting from specimen leakage or fluid evaporation; and signals from the specimens themselves, can be detected along with the main fluid composition, which offer additional insight into specimen-fluid interactions. We present characteristic examples of each case, measured directly from historic jars at the Natural History Museum, London, thereby demonstrating that handheld SORS can provide curators with useful information on fluid composition and possible indicators of specimen degradation. This capability marks a step change from preservation fluid classification alone towards integrated, more holistic monitoring of preservation and conservation status of wet collections.Materials and methods
Historical fluid-preserved samples
Raman measurements were performed in situ at the Natural History Museum, London, UK (Fig. 1A). Four historic fluid-preserved specimens were selected to represent typical case studies, the challenges highlighted in the introduction, such as fat release, dissolution of minor components into the fluid, sediment accumulation (e.g. formation of degradation products on container walls), and the specimen itself. These examples are particularly relevant to the conservation and analytical study of museum wet collections. The samples analyzed included: (E1) a sample collected by Charles Darwin during the second voyage of HMS Beagle (1831–1836); (E2) a mid-20th century specimen from the former Wellcome Trust collection; (E3) a mixed fish sample from the museum's “Tank Room,” from the late 20th century; and (E4) experimental preservation material of uncertain provenance and composition, dating from the early- to mid-20th century. A summary of the samples is provided in Table 1. | ||
| Fig. 1 (A) NHM London, UK, (B) schematic SORS measurement for in situ analysis of preservation fluid, and (C) example SORS spectra processing. | ||
| Label | Specimen | Primary fluid | Residual Raman bands (cm−1) | Further components | Comments |
|---|---|---|---|---|---|
| E1 | Gobius lineatus, holotype, 2nd ‘Beagle’ voyage | EtOH 50–60% | • 1023 | • MeOH | Clear solution, measurement far away from specimen |
| 1110, 1266, 1444, 1463 | Fats, lipids17 | ||||
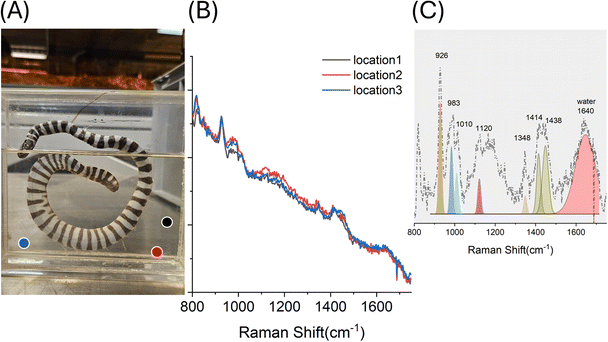
| E2 | Snake, herpetology, former Wellcome | Formaldehyde 4–5% | • 926, 1348, 1414, 1438 | • Potassium acetate18 | Clear solution, measurement far away from specimen |
| ∼1640 | Water | ||||
| 983, 1010, (weak) | • Proteins/amino acids19 (e.g. phenylalanine)20 | ||||
| • 1120 (weak) | Fats | ||||
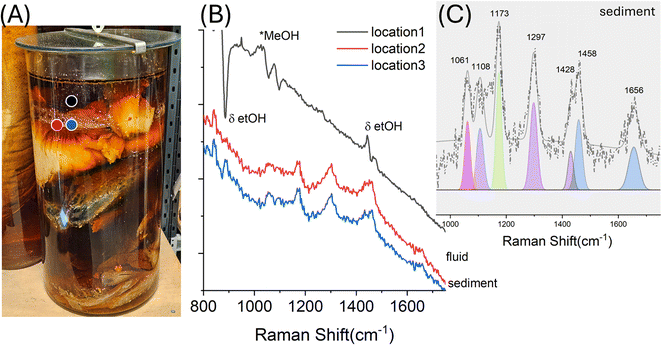
| E3 | Uncatalogued fish parts | EtOH 70% | Deposit, 1060, 1108, 1297, 1428, 1458, 1656 | • Lipids, unsaturated fatty acids17 | Intense dark fluid, measurements performed on fluid and white deposit on internal wall |
| • 1173 and 1656 | • Proteins, collagen (e.g. tyrosine21) | ||||
| • In fluid, 1023 | MeOH | ||||
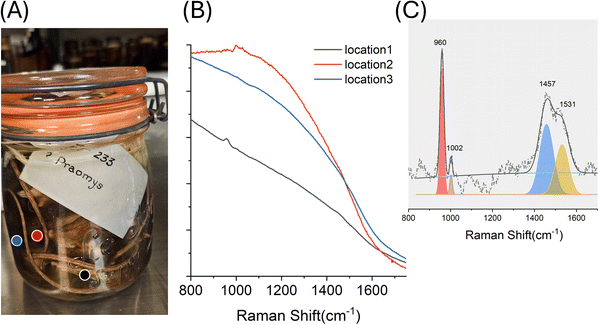
| E4 | Praomys, Dalton's mouse, Mammals | Formaldehyde 4%, MeOH 1.5% | • 960 | • Ca3(PO4)2 (ref. 22) | Fluid intense dark colour, one measurement probed specimen |
| 1002 | Phenylalanine23 | ||||
| 1457, 1531 | • Collagen/amino acid (e.g. lysine and tryptophan)20 |
Instrument and measurement protocol
A schematic of the measurement configuration is shown in Fig. 1B. For each container, three separate SORS measurements were acquired using a handheld SORS device (Resolve, Agilent Technologies, Oxfordshire, UK) equipped with an 830 nm laser (maximum output power: 475 mW). The instrument was operated in “through-barrier” analysis mode, enabling the collection of both zero and offset-displacement spectra. The total acquisition time per sampling location was 25 s, comprising 5 s for the zero-displacement measurement (1 s × 5 accumulations) and 20 s for the offset measurement (2 s × 10 accumulations) recorded with a 5.5 mm spatial displacement. All measurements were performed in situ at the Natural History Museum. To minimize interference from ambient light, specimen jars were covered with a black cloth during Raman acquisition.Data analysis
The internally calibrated zero- and offset-displacement Raman spectra were extracted and processed using a semi-automated analysis pipeline developed in MATLAB (R2019b) with further analysis in OriginPro (2018b). Following the procedure described in our previous work,13,16 the glass/container contribution (zero-displacement spectrum) was subtracted from the corresponding offset spectrum to isolate the preservation fluid signature (SORS spectrum).Each SORS spectrum was then compared to the appropriate reference standard fluid (e.g., ethanol 50–70%, formaldehyde 4–5%, see Table 1), and subtraction of the reference spectrum was performed to enhance minor spectral contributions. The residual Raman spectra were subsequently analysed by Gaussian curve fitting to identify and characterize additional components. Representative data subtraction is shown in Fig. 1C.
Results and discussion
Fat dissolved in fluid
Minor specimen components dissolved in preservation fluids are valuable indicators of degradation and fluid instability. Their detection provides insight into ongoing chemical changes within collections, informing curatorial decisions and supporting remedial conservation. The first example demonstrates the capability of SORS to detect lipids dissolved in preservation fluid. Raman spectra from sample E1 (Fig. 2B and C) show residual methanol peaks (1023 cm−1, compatible with industrial methylated spirit (IMS) in the main fluid) and additional bands consistent with lipids.17 Specifically, strong C–C stretching (1110 cm−1) and C–H deformation bands (1444, 1463 cm−1) confirm the presence of lipidic material in the solution (Fig. 2C). Moreover, the band at 1266 cm−1 suggests the presence of unsaturated fatty acids17 (likely omega-3 lipids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are common in fish tissue). Notably, repeated measurements from three different spatial positions yielded consistent spectra (Fig. 2B), indicating that these lipid signatures arise from the fluid itself rather than local surface deposits. This demonstrates the ability of SORS to identify molecular markers of specimen leakage directly through sealed containers.Other components dissolved in fluid
In addition to lipids, other minor solutes may be detected in preservation fluids, reflecting residual fixation agents, proteins, or degradation products. Identifying such compounds is important for reconstructing preservation histories and assessing ongoing chemical processes. Sample E2 (snake specimen, clear fluid) provides an illustrative case (Fig. 3). Measurements were acquired away from the specimen in order to characterize the preserving fluid composition only, and the dominant formaldehyde contribution was subtracted during processing (see Methods – Data Analysis). The resulting spectra revealed reproducible minor Raman bands across three independent repetitions, confirming the presence of dissolved components rather than localized contamination on wall surface (Fig. 3B). Processed spectra and Gaussian fitting (Fig. 3C) highlight bands at 926, 1348, 1414, and 1438 cm−1, characteristic of potassium acetate, a common component in Kaiserling I fixation protocols, assumed to have leached from the specimen. This could indicate that the specimen was originally fixed in Kaiserling I and subsequently transferred to formalin for storage, or alternatively, that it remains preserved in a Kaiserling-based solution. Additional weak bands (∼983 and 1010 cm−1) suggest the presence of protein fragments or free amino acids (e.g. phenylalanine), while the feature around 1120 cm−1 together with the band at 1438 cm−1 may indicate the presence of lipids. However, precise identification of these bands is not straightforward, as their interpretation is complicated by the fluorescence background, weak signals, and possible interactions between amino acid residues and residual formaldehyde. This factor may lead to intensity fluctuations and further complicate the assignment of the observed spectral features.24 Additionally, the study by Domanski et al.2 provides valuable context, demonstrating comparable analytical challenges in assessing chemical changes within long-term preservation fluids and reinforcing the need for further systematic investigations in this field. A broad water band at ∼1640 cm−1 was also observed.Deposits on internal container walls
Beyond dissolved species, solid deposits on container walls can also provide markers of degradation processes. Such residues often result from evaporation, fluid refilling, or temperature fluctuations, leading to the precipitation of organic material from the specimen. In sample E3, a historic jar showing dark discoloration and visible white sediment (Fig. 4A), SORS measurements revealed that the deposit was primarily lipidic in nature (Fig. 2B, location 2 and 3). Raman spectra showed characteristic bands of fatty acids, including strong C–H vibrations (1426, 1456 cm−1), C–C stretching (1061 cm−1), and methylene wagging/twisting (1297 cm−1). The presence of unsaturated fatty acids was indicated by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch at 1656 cm−1, consistent with long-chain polyunsaturated fatty acids (PUFAs). Additional Raman features at 1173 cm−1 suggest residual contributions from fish skin proteins/collagen (e.g. tyrosine21). Collectively, these findings indicate that the white solid represents a precipitated mixture of lipids and biomolecules, likely rendered insoluble by fluctuations in ethanol concentration due to evaporation and repeated fluid replacement. Fluctuations in storage temperature conditions may also have contributed to or accelerated these precipitation processes (see Fig. 1C). Importantly, SORS enabled the characterization of this deposit without opening the jar, providing important insight into the degradation status of both the specimen and the preservation environment. Additionally, Raman spectra from location 1 in E3 (Fig. 4B, solid black line) show residual methanol peaks in the preservation fluid (1023 cm−1) compatible with presence of IMS in the preservation liquid.
C stretch at 1656 cm−1, consistent with long-chain polyunsaturated fatty acids (PUFAs). Additional Raman features at 1173 cm−1 suggest residual contributions from fish skin proteins/collagen (e.g. tyrosine21). Collectively, these findings indicate that the white solid represents a precipitated mixture of lipids and biomolecules, likely rendered insoluble by fluctuations in ethanol concentration due to evaporation and repeated fluid replacement. Fluctuations in storage temperature conditions may also have contributed to or accelerated these precipitation processes (see Fig. 1C). Importantly, SORS enabled the characterization of this deposit without opening the jar, providing important insight into the degradation status of both the specimen and the preservation environment. Additionally, Raman spectra from location 1 in E3 (Fig. 4B, solid black line) show residual methanol peaks in the preservation fluid (1023 cm−1) compatible with presence of IMS in the preservation liquid.
Specimen – bone and organic component
Finally, SORS can also be used to probe the specimen directly, providing molecular insight into tissue composition and its interaction with preservation fluids. This extends the application of SORS from basic preservation fluid assessment to non-invasive monitoring of specimen integrity. Sample E4 (darkened fluid, Fig. 5) illustrates this potential. Measurements collected from three distinct positions revealed different contributions depending on the probed region. Location 1 (Fig. 5B, black line; Fig. 5C) shows a clear bone signature, with a strong 960 cm−1 band assigned to calcium phosphate (carbonated hydroxyapatite), and additional peaks indicating the organic matrix, including collagen. These include the phenylalanine band (1002 cm−1) and other collagen-amino acid-related features19 at 1457 cm−1 (e.g. lysine) and 1531 cm−1 (e.g. tryptophan), in solution20 which may show relative intensity variation due to cross-linking induced by formaldehyde.24–26 Location 2 spectra were dominated by residual phenoxetol presence (998 and 1027 cm−1) over a strong fluorescence background, reflecting measurements taken primarily within the fluid. Location 3 had a strong fluorescence background, consistent with organic degradation products such as residual blood or leaked biomolecules of fluorescence coming from the specimen itself (e.g. hairs). The variability in these spectra demonstrates the capacity of SORS to probe both fluid and specimen chemistry non-invasively, offering a richer picture of the conservation state.Conclusions
This study demonstrates, for the first time, the application of handheld Spatially Offset Raman Spectroscopy (SORS) for the in situ characterization of conservation status of historic preservation fluids and specimens in sealed museum containers. We have shown that SORS can detect minor dissolved components (e.g. lipids, protein fragments, residual fixation salts), characterize organic deposits on container walls, and directly probe specimen composition through the fluid. These capabilities provide critical markers of fluid degradation, specimen leakage, and preservation history. Importantly, all measurements were conducted non-invasively, without opening the containers demonstrating the robustness of the method under real museum conditions. This not only minimizes the risk of specimen handling but also reduces potential exposure of staff to unknown or hazardous substances within the containers. Some of the spectral features observed may be due to unknown interactions or undocumented interventions that occurred over the long historical lifetime of these specimens, underscoring the need for future controlled mock-up studies and accelerated ageing experiments to better understand these processes. This expands the use of Raman spectroscopy in heritage science from fluid classification to comprehensive monitoring of wet collections, offering actionable insights into both fluid chemistry and specimen integrity. By enabling the identification of degradation markers and documenting fluid-specimen interactions, handheld SORS emerges as a portable analytical tool for in situ preventive conservation. Its adoption can help curators detect early signs of fluid or specimen deterioration, prioritize interventions, and safeguard the historical and scientific value of collections over time.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Data openly available in STFC public repository eDATA27 available at the following link https://doi.org/10.5286/edata/956.Supplementary information (SI): photograph of resolve SORS unit during in situ measurements. See DOI: https://doi.org/10.1039/d5ay01654a.
Acknowledgements
We thank Agilent Technologies for the loan of the Resolve instrument. We would like to thank the NHM Life Science curators: Jeff Streicher, David Gower, Louise Tomsett and Simon Loader, for generously sharing their knowledge, specimens, fluids, and jars, to help facilitate this work.References
- J. E. Simmons, Fluid Preservation, Rowman & Littlefield Publishers, Lanham, 2014 Search PubMed.
- J. Domański, A. Janczura, M. Wanat, K. Wiglusz, M. Grajzer, J. E. Simmons, Z. Domagała and J. C. Szepietowski, J. Anat., 2023, 243, 148–166 CrossRef.
- E. Braker, Fluid Collection Monitoring, https://spnhc.org/fluid-collection-monitoring/, 2025.
- A. J. Van Dam, Collect. Forum, 2000, 14, 78–92 Search PubMed.
- S. Cersoy, V. Rouchon, O. Belhadj, J. Cuisin and M. Herbin, Collect. Forum, 2020, 34, 53–72 Search PubMed.
- L. Allington-Jones and C. McKibbin, Collect. Forum, 2017, 31, 53–69 Search PubMed.
- S. J. Moore, Biol. Curators Gr. Newsl., 1994, 6, 44–45 Search PubMed.
- J. E. Simmons, in Preventive Conservation: Collection Storage, ed. L. Elkin and C. A. Norris, Society for the Preservation of Natural History, 2019, pp. 491–509 Search PubMed.
- J. Carter, J. Simmons, O. Crimmen and D. Neumann, Best Practices in the Preservation and Management of Fluid-Preserved Biological Collections, Society for the Preservation of Natural History Collections, 2022 Search PubMed.
- S. Moore, Biol. Curat., 2002, 1, 44–46 Search PubMed.
- D. W. Von Endt, Collect. Forum, 1994, 10, 10–19 Search PubMed.
- S. Mosca, C. Conti, N. Stone and P. Matousek, Nat. Rev. Methods Primers, 2021, 1, 21 CrossRef CAS.
- S. Mosca, W. Montgomery, C. McKibbin, R. Stokes, C. Conti and P. Matousek, ACS Omega, 2025, 10, 8658–8664 CrossRef CAS PubMed.
- S. Banerjee, S. Mosca, I. Legge, B. Gangadharan, J. Walsby-Tickle, B. Y. Arman, R. Stokes, T. Bharucha, M. Deats, H. A. Merchant, J. McCullagh, N. Zitzmann, C. Caillet, P. N. Newton and P. Matousek, J. Pharm. Biomed. Anal., 2025, 265, 6 CrossRef.
- S. Mosca, Q. Lin, R. Stokes, T. Bharucha, B. Gangadharan, R. Clarke, L. G. Fernandez, M. Deats, J. Walsby-Tickle, B. Y. Arman, S. R. Chunekar, K. D. Patil, S. Gairola, K. Van Assche, S. Dunachie, H. A. Merchant, R. Kuwana, A. Maes, J. McCullagh, C. Caillet, N. Zitzmann, P. N. Newton and P. Matousek, Vaccine, 2023, 41, 6960–6968 CrossRef.
- A. Blanco, W. Montgomery, W. Sam, C. McKibbin, R. Stokes, P. Matousek and S. Mosca, ACS Omega, 2025 DOI:10.1021/acsomega.5c09045.
- K. Czamara, K. Majzner, M. Z. Pacia, K. Kochan, A. Kaczor and M. Baranska, J. Raman Spectrosc., 2015, 46, 4–20 CrossRef CAS.
- R. L. Frost and J. T. Kloprogge, J. Mol. Struct., 2000, 526, 131–141 CrossRef CAS.
- P. T. C. Freire, F. M. Barboza, J. A. Lima, F. E. A. Melo and J. M. Filho, in Raman Spectroscopy of Amino Acid Crystals – Raman Spectroscopy and Applications, InTech, 2017, pp. 205–220 Search PubMed.
- G. Zhu, X. Zhu, Q. Fan and X. Wan, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2011, 78, 1187–1195 CrossRef PubMed.
- M. Połomska, L. Kubisz, J. Wolak and D. Hojan-Jezierska, Appl. Sci., 2021, 11, 8358 CrossRef.
- T. Buchwald, K. Niciejewski, M. Kozielski, M. Szybowicz, M. Siatkowski and H. Krauss, J. Biomed. Opt., 2012, 17, 017007 CrossRef PubMed.
- Y. Ishimaru, Y. Oshima, Y. Imai, T. Iimura, S. Takanezawa, K. Hino and H. Miura, Molecules, 2018, 23, 1–14 CrossRef PubMed.
- B. Metz, G. F. A. Kersten, P. Hoogerhout, H. F. Brugghe, H. A. M. Timmermans, A. De Jong, H. Meiring, J. Ten Hove, W. E. Hennink, D. J. A. Crommelin and W. Jiskoot, J. Biol. Chem., 2004, 279, 6235–6243 CrossRef CAS PubMed.
- J. J. A. G. Kamps, R. J. Hopkinson, C. J. Schofield and T. D. W. Claridge, Commun. Chem., 2019, 2, 126 CrossRef.
- E. A. Hoffman, B. L. Frey, L. M. Smith and D. T. Auble, J. Biol. Chem., 2015, 290, 26404–26411 CrossRef CAS PubMed.
- S. Mosca and P. Matousek, Residual SORS spectra of Historical Wet Collections, https://edata.stfc.ac.uk/items/c21269d9-792f-4d5f-bbcc-3b2060f38a63.
| This journal is © The Royal Society of Chemistry 2026 |