Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adhesive tape for spatially resolved and sensitive detection of lead in dust using XRF
Ornella Joseph a,
Vikrant Jandev
a,
Vikrant Jandev b,
Zhutian Zhang
b,
Zhutian Zhang c,
Darbie Kwon
c,
Darbie Kwon b,
Brighten Cho
b,
Brighten Cho b,
Devena Sammanasub,
Alyssa Wicks
b,
Devena Sammanasub,
Alyssa Wicks b and
Marya Lieberman
b and
Marya Lieberman *b
*b
aDepartment of Chemistry, Earlham College, Richmond, IN, USA. E-mail: ojoseph2@alumni.nd.edu
bDepartment of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN, USA. E-mail: vjandev@nd.edu; dkwon3@alumni.nd.edu; bcho2@alumni.nd.edu; awicks@alumni.nd.edu; mlieberm@nd.edu
cDepartment of Natural and Quantitative Sciences, Holy Cross College, Notre Dame, IN, USA. E-mail: zzhang@hcc-nd.edu
First published on 21st November 2025
Abstract
A method for rapidly and quantitatively measuring lead (Pb) in dust has been developed. Different types of wiping materials were tested. Due to its adhesive properties and flatness when folded, painter's tape effectively picks up dust and, when coupled with portable X-ray fluorescence (XRF) provides a rapid and sensitive measurement of Pb in dust. The tape can pick up approximately 0.095 g of dust, which is comparable to the amount of dust present in one square foot of a house that is vacuumed once a month (0.100 g). Dust in an area of 1 square foot was wiped up and a calibration curve was constructed by analyzing the folded tape on XRF (in ppm) versus the amount of Pb on the wipe that was determined by digestion and ICP-OES (in µg). Validation of this method revealed that when tested at each of the current EPA action levels for Pb dust (5 µg for floors, 40 µg for windowsills and 100 µg for window troughs), each of the false positive rates (FPR) was below 15% and each of the false negative rates (FNR) was below 5%. Thus, at these lower Pb levels, Painter's tape with XRF measurement could allow for spatially-resolved, rapid determination of Pb in dust on site, which has been a long-standing need.
Introduction
Pb-containing dust from deteriorated Pb-based paints and contaminated soil is the primary contributor to Pb poisoning among children.1,2 Studies have shown that even low blood Pb levels can have significant health impacts on children.3 In the United States, parents are encouraged to have children's blood Pb tested at 12 and 24 months, and if an elevated blood Pb level (greater than 3.5 µg dL−1) is detected, the home receives a state-funded Pb inspection and risk assessment (LIRA) conducted by certified Pb risk assessors.4Pb risk assessors visit the home with a portable X-ray fluorescence (XRF) analyzer to measure Pb in paint on-site. Since each XRF run takes a maximum of 2 minutes, a large variety of painted surfaces can be tested with XRF during a single home visit. However, Pb in dust is not tested on-site, but rather, dust from floors and windowsills is collected using the standardized wipe method adopted by the U.S. Environmental Protection Agency (EPA) and the U.S. Department of Housing and Urban Development (HUD).5 This standardized wipe method uses the entirety of a moist wipe to pick up the dust in an area of one square foot (ft2). The sample is then sent to a lab where the wipe is digested, and Pb concentration is determined by Flame or Graphite Furnace Atomic Absorption Spectroscopy (FAAS or GF-AAS) or Inductively Coupled Optical Emission Spectroscopy (ICP-OES).6
While the laboratory method is highly accurate in determining Pb in dust, it can be expensive and does not support determining remedial action on site. This necessitates a second visit by the Pb risk assessors or deferring remedial action to the residents. Researchers have proposed XRF testing of sieved vacuum cleaner dust for identifying the overall Pb hazard in the home.7 However, there remains a need for an analytical method that gives a spatially resolved and sensitive quantification of Pb when present at lower µg levels in dust.
The current EPA Dust Lead Action Level (DLAL) for risk assessment and Pb abatement work is 5 µg ft−2 for floors, 40 µg ft−2 for windowsills, and 100 µg ft−2 for window troughs. Moreover, the EPA also proposes a Dust Lead Reportable Level (DLRL) for home residents, to be any value above zero µg ft−2, recognizing that there is no safe dust Pb level for children.8,9
Currently, the EPA has approved 2 color tests for the detection of Pb paint;10 the Leadcheck™ test kit which is based on the formation of pink Pb rhodizonate, now produced and distributed by Luxfer Magtech (previously manufactured by 3M); and D-lead which is based on the formation of brown/gray/black Pb sulfide. However, no color test has been approved for the detection of Pb in dust. Additionally, the approved color tests for Pb in paint have a significantly higher detection limit of 1 mg cm−2 (approximately 930![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 µg ft−2) which prevents the color tests from being applicable to the detection of Pb in dust. Other commercially available rhodizonate color tests (Lead Check Swabs) have proved to be ineffective to reliably flag dust containing Pb below 1000 µg ft−2 (false negative rate is greater than 10%).11 This leaves a gap in testing methods for residents, or renovators who could benefit from a rapid and reliable home test for Pb in dust. Furthermore, in instances like occupational exposure sites, the main route of Pb poisoning is through ingestion of dust.12 Therefore, to take precautionary steps in the absence of an XRF, it would be beneficial, at minimum, to have a color test that reliably detects Pb below 1000 µg ft−2.
000 µg ft−2) which prevents the color tests from being applicable to the detection of Pb in dust. Other commercially available rhodizonate color tests (Lead Check Swabs) have proved to be ineffective to reliably flag dust containing Pb below 1000 µg ft−2 (false negative rate is greater than 10%).11 This leaves a gap in testing methods for residents, or renovators who could benefit from a rapid and reliable home test for Pb in dust. Furthermore, in instances like occupational exposure sites, the main route of Pb poisoning is through ingestion of dust.12 Therefore, to take precautionary steps in the absence of an XRF, it would be beneficial, at minimum, to have a color test that reliably detects Pb below 1000 µg ft−2.
In November 2023, a novel perovskite color test for Pb was reported.13 In this color test, a Pb-containing substrate, when sprayed with methylammonium bromide, instantly forms Pb halide perovskite crystals, which when exposed to 365 nm UV light, display green fluorescence. The reagent can be sprayed onto a surface, making it well suited for field screening, particularly in homes or environments where rapid Pb detection is needed.
Helmbrecht et al.13 reported a limit of detection for Pb at 1 ng mm−2 (approximately 93 µg ft−2) in spiked diatomaceous earth. A second recent study using the same reagent, reports a limit of detection of 500 ppm for Pb in paint.14 However, this test has not been directly tested on Pb-containing house dust. In this paper, we examine the utility of this test by spraying the Pb dust containing tape and detecting green fluorescence by eye as well as by using a phone image.
Other studies have used wet wipes with XRF detection as a screening test for Pb in dust.15–17 However, the sensitivity of the data was limited due to the absorption of excitation X-rays and scattering of fluorescent X-rays by water18 and the precision was affected by the heterogeneous distribution of Pb on the wipe.19 This required drying of the wipe and the testing of multiple spots.20–22 Moreover, since the penetration depth of X-rays is limited to a few millimeters, Pb wipes that have been folded thin, with XRF testing of a single spot, have been proposed.23 This paper explores the use of adhesive wipes (Painter's tape) to pick up dust. The tape is subsequently folded thin to facilitate a single XRF measurement spot.
Materials
For lab-generated dust, certified reference material (CRM) 0.1% Pb in paint chips (High-Purity Standards), silica (ICN Biomedicals GmbH), and sieved vacuum cleaner dust samples (250 µm sieve), were used. In addition, unsieved vacuum cleaner dust was obtained from 3 households. The wipes tested were sourced from a national grocery chain and online retailers. The Painter's tape used in this study was of the brands STIKK and ULine and consisted of blue crepe paper tape with a medium-tack adhesive which ensured that it only picked up dust and did not remove paint. The manufacturer reports a performance temperature range of 10–90 °C with outdoor exposure of up to a week.The perovskite color test kit was purchased from Lumetallix b.v., a company based in the Netherlands (product stock keeping unit number: L-01). The test reagent is reported to have a shelf-life of 6–24 months.24
Instrumentation
To digest wipes for ICP-OES analysis, concentrated trace metal grade nitric acid (VWR, 67% w/w) and a microwave digester (CEM Mars 6, North Carolina, USA) were used. ICP-OES data were gathered using a PerkinElmer Optima 8000 (Connecticut, USA) instrument (measurement mode: radial; Pb emission wavelength: 220.353 nm; internal standard: 1 ppm yttrium; matrix: 5% w/w trace metal grade nitric acid). X-Ray Fluorescence (XRF) Spectroscopy was performed using a handheld SciAps X-100 (Massachusetts, USA) XRF analyzer (excitation source: 40 kV electron tube with rhodium anode; acquisition time: 20 seconds with the “beam 2” setting; detector: 20 mm2 silicon drift detector; calibration mode: soil, Pb emission line: Lβ at 12.61 keV).Methods
XRF attenuation distance of fluorescent X-rays from the sample
A finely ground and homogeneous powder containing approximately 500 ppm Pb was placed in an XRF sample cup and placed directly on the XRF reading window and the reading was recorded. To determine the attenuation distance for the fluorescent X-rays from the sample to reach the detector, cut sheets of printing paper (0.10 mm each in thickness) were placed one at a time, between the XRF and the sample to gradually increase the distance until the XRF reading was below the detection level of 3 ppm.Initial screening of dust wipes
However, for moistened (water or alcohol-containing) wipes, the mass of dust picked up was determined from the difference in the mass of the moist wipe on a weigh boat originally, and the mass of the weigh boat after wiping and allowing the moisture to evaporate.
Adhesive Painter's tape as the wipe of choice
Painter's tape (from a 3.0-inch-wide roll) was mounted on a transparent film and cut such that the dimensions of the tape were 3.0 × 1.5 in2. The tape was then carefully peeled off the transparent film backing and used to pick up dust in a 1 ft2 area (Fig. 1A) by repeatedly sticking the tape to collect the dust on the surface. When the tape was covered in dust, it was folded three times (until a 0.75 in2 square shape was obtained) and bagged (Fig. 1B). Each bagged wipe was taped on the XRF analyzer and both sides of the bagged wipe were measured with the XRF analyzer facing upwards (Fig. 1C). The higher of the two XRF readings was recorded due to the intensity of fluorescent X-rays decreasing with increasing distance of the Pb-containing sample from the XRF detector (SI Fig. S3).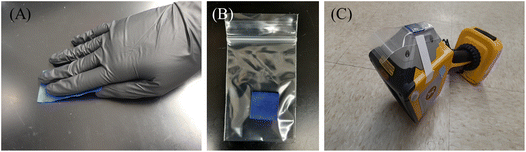 | ||
| Fig. 1 Using the Painter's tape to pick up dust (A), folding the tape three times to localize the dust in a small area (B), and securing the folded and bagged tape over the XRF reading window (C). | ||
In a separate experiment, two Painter's tapes containing 10 µg and 40 µg of Pb respectively, were measured with XRF for a total of 12 times on each side, by sequentially moving locations of the folded and bagged tape over the central section of the XRF reading window. The data are given in the SI Excel file.
Painter's tape XRF calibration curve and validation study
For the construction of a calibration curve and subsequent validation study, lab-generated (N = 9) and pre-existing field dust samples (N = 118) were wiped by 15 different users. For lab-generated dust, a mass of 0.100 g each of sieved vacuum-cleaner dust samples was weighed out and manually dispersed on areas of 1 ft2. For collection of dust from field sites, a variety of wooden, concrete and tiled floor and windowsill surfaces were sampled from two homes (built before 1978 and containing Pb paint, N = 61) and three churches (with stained glass windows containing Pb came, N = 57). At each surface, an area of approximately 1 ft2 was sampled using Painter's tape and both sides of the tape were measured using XRF with the higher of the two XRF readings recorded. The actual amount of Pb on the tape was thereafter determined by microwave digestion followed by ICP-OES. Data from half of the samples (N = 64) were used to generate a calibration curve and the other half of samples (N = 63) were used to validate the method.The exact amount of Pb on the wipe was thereafter determined by ICP-OES where the tape was first digested with 10 mL of concentrated nitric acid in a microwave digester (200 °C for 15 minutes). The digestate was then diluted to 50.0 mL, filtered (Whatman Grade 1 qualitative filter paper), and analyzed with ICP-OES. Blank corrections were applied to the value obtained from ICP-OES to account for any Pb contamination from the Painter's tape, microwave digestion vessels, and reagents. ICP-OES Pb values between 0.4 and 0.9 µg were replaced with 0.9 µg which is the limit of detection for this analysis method on ICP-OES. The calibration and validation data are provided in the SI Excel file.
Statistical analysis
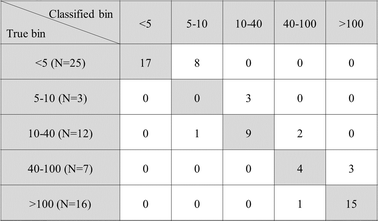
For the calibration data, a plot of the logarithm of the XRF reading versus the logarithm of the actual amount of Pb contained on the tape (obtained via digestion of tape followed by ICP-OES, and blank and LOD correction) was constructed and used as the calibration curve (Fig. 2). For the calibration line, the 95% confidence and prediction intervals were calculated.26For the validation data, a plot of predicted Pb versus actual Pb was constructed (Fig. 3). The current EPA action levels (5 µg ft−2 for floors, 40 µg ft−2 for windowsills, and 100 µg ft−2 for window troughs) were each considered as thresholds, and the FPR and FNR at each threshold were calculated. Additionally, a truth table was constructed to evaluate what types of errors in classification were made. For example, how often samples that are in one class might be erroneously classified as being from the neighboring class.
Perovskite color test for Pb detection
Painter's tape that had been used to pick up dust was sprayed with the Lumetallix test reagent (1% w/w methyl ammonium bromide in isopropyl alcohol)13 and allowed to air-dry for 15 minutes. If Pb was present, the Pb methylammonium bromide perovskite formed would fluoresce in green when exposed to UV light (365 nm). Painter's tape was illuminated with the UV flashlight provided in the manufacturer's kit, and images were captured in a lightbox using a mobile phone camera (iPhone 14 Pro).Results and discussion
Painter's tape as the wipe of choice
Among the wipes tested (SI Fig. S1A), Painter's tape was among the wipes that picked up the highest amount of house dust (SI Fig. S1B) while giving a measurable XRF reading for 10 µg ft−2 Pb with a lower standard deviation (SI Fig. S1C). The significant XRF reading can be attributed to the dryness of the adhesive wipe and the flatness due to which it can be folded to match the XRF reading window of a 1 cm2 surface area and 1.2 mm depth. Beyond an attenuation depth of 1.2 mm of stacked paper, the intensity of the XRF reading from a Pb-containing sample is reduced to 1/e, where e ≈ 2.718. Thus, at a distance beyond 1.2 mm, the XRF reading from the sample would be less than 37% of its original value (SI Fig. S3).Further experiments tested the efficiency of different dimensions of Painter's tape for picking up dust (SI Fig. S2). Of the dimensions tested, the dimension of 3.0 × 1.5 in2 proved to be the most effective given that the XRF reading was high (indicating high sensitivity) and triplicate measurements had a lower deviation (indicating less variability). The experiment for sequentially analyzing different locations on both sides of folded Painter's tape confirms that one side consistently gave higher Pb readings with a lower relative standard deviation, confirming the choice of measuring each side once, and then using the higher of the two XRF readings (SI Excel file).
Painter's tape XRF calibration curve and validation study
From the calibration curve (Fig. 2), the equation of the fitted line was y = (0.80 ± 0.05)x + (1.24 ± 0.09), where y is the logarithm of the XRF reading and x is the logarithm of the ICP-OES corrected value of the digested tape. Considering that the prediction intervals are significantly wide, this method is not suitable for quantitation, but rather for screening.The limit of detection (LOD) was calculated using the formula LOD = 3.143 × SD,27 where 3.143 corresponds to the single-tailed 99th percentile t-statistic for 6 degrees of freedom, and SD corresponds to the standard deviation of 7 replicate determinations of low concentration Pb-containing tape samples (approximately 0.7 µg ft−2). The calculated LOD was 4.3 µg ft−2. To confirm detection at low Pb levels, a raw XRF spectrum for a tape with 10 µg Pb and a raw XRF spectrum for a blank tape were obtained (SI Fig. S4). At the Pb emission energy of 12.61 keV, it is evident that a significantly higher XRF signal intensity was obtained from the tape with 10 µg Pb compared to the blank tape.
For the validation study 63 dust samples were randomly selected. The equation of the calibration curve (from Fig. 2) was used to convert the XRF reading to a blank-corrected ICP-OES reading (predicted Pb) and then plotted against the specific sample's actual blank-corrected ICP-OES reading (actual Pb) (Fig. 3).
When a threshold Pb level in dust was set at 5 µg ft−2 (current EPA action level for floors), a false positive rate (FPR) of 13% (8/63) and no false negatives were obtained. For a threshold of 40 µg ft−2 (current EPA action level for windowsills), an FPR of 3% (2/63) and no false negatives were obtained. For a threshold of 100 µg ft−2 (current EPA action level for window troughs), an FPR of 5% (3/63) and a FNR of 2% (1/63) were obtained. This data has been summarized in a truth table (Fig. 4) to assess the degree of correct and incorrect classification based on bins. Examining the bin that the samples were misclassified into shows that samples misclassified at a threshold of 5 µg ft−2 were classified as being between 5 and 10 µg ft−2, indicating that the classification was only slightly off. Examining the truth table for the other bins also reveals that the misclassified samples fell into neighboring bins and not far off bins, indicating that the misclassification was only slightly off.
A reporting limit of at least 80% of the current 5 µg ft−2 EPA action level for floors (4 µg ft−2) is required of laboratories certified under the US National Lead Laboratory Accreditation Program (NLLAP).28 When a threshold Pb level in dust was set at 4 µg ft−2 with this method, a false positive rate (FPR) of 8% (5/63) and no false negatives were obtained.
The main strengths of the Painter's tape coupled with XRF method are that it is rapid, can be performed on site, has good robustness (across different surface types, dust types, sample collectors, and XRF analysts), and provides spatially resolved results. However, the dust content on different surfaces can be variable and can exceed the maximum amount that can be picked up by the wipe (approximately 0.095 g). Furthermore, the mass of dust that can be picked up by the wipe could be variable depending on the volume of dust. Thus, surfaces with large volumes of dust could give false negative results. Additionally, the XRF's active reading window captures only a fraction of the folded tape, and depending on the area tested, could give variable readings, resulting in a greater confidence interval for each value.
The ability to test a sample in under a minute is a considerable improvement in testing time over ICP-OES and FAAS which can take days to yield results. Furthermore, other XRF test methods require either drying and sieving of vacuum cleaner dust or drying of the Ghost Wipe containing the dust, whereas the Painter's tape allows for direct measurement. A comparison of the proposed XRF method with existing methods for testing Pb in dust is given in the SI (Table S1).
Given these strengths and weaknesses, the XRF method is proposed as a screening test, rather than a quantitative analysis method. This method shows promise for Pb abatement use cases as a screening method preceding clearance. However, further field work will be needed in homes undergoing abatement operations to validate this hypothesis.
Perovskite color test for Pb detection
The perovskite color reagent, purchased from Lumetallix Inc. was sprayed on Painter's tape that contained dust and visualized under UV light. Researchers have previously reported on the selectivity and suitability of the perovskite color test for the detection of Pb in a variety of samples including diatomaceous earth,13 paint14 and gunshot residue.29Images of the tape obtained under UV light were analyzed with ImageJ image analysis software. Each image's color was split into red, green, and blue channels and the green channel intensity was selected using the threshold range of 152 to 255. Then, a circle with a constant area (circle diameter = 1000 px) was drawn on the image for analysis. A circular region was chosen because the UV flashlight used had a circular head and produced even illumination across a circular area. Images of the tape, along with the % green area values and the corresponding actual Pb contained on the tape for a subset of the sprayed samples, are shown in Fig. 5.
In the absence of image analysis software, naked eye reading can be performed. While the perovskite color test does not give false positives for most elements, dust can contain fibers, chemicals such as laundry brighteners, and minerals with native fluorescence.30 Although the colors are often different, this native fluorescence can confuse observers who are not familiar with the expected color. Therefore, in this study, when the tape containing dust was observed with the naked eye, except for thread-like structures showing green fluorescence, all other observable green areas were considered positive for Pb. Of the 20 samples with Pb levels below 100 µg ft−2, there were four instances in which the three analysts disagreed on the visibility of green fluorescence.
However, for Pb levels above 100 µg, all analysts unanimously agreed that green fluorescence was clearly visible in all samples. At Pb levels above 100 µg, green fluorescence was consistently visible either due to the presence of large and clearly visible paint chip(s) or significant amounts of fine Pb dust from oxidized Pb came in stained glass windows31 (Fig. 6).
Therefore, the perovskite color test consistently indicates Pb when present as paint chips or large amounts of fine particles (>100 µg ft−2) in dust. Thus, when an XRF analyzer is unavailable, and especially when simplicity and portability are priorities, the perovskite color test provides a convenient method for visualizing high Pb levels.
Currently available Pb dust analyses such as ICP-OES, FAAS or even XRF involve high initial (instrument) cost and require formal training before use, limiting their accessibility. On the other hand, currently available at-home tests involve minimum cost, but suffer from high rates of false positive and false negative results with LOD at 1000 µg ft−2. A comparison of the proposed perovskite color test with existing methods for testing Pb in dust has been given in the SI (Table 1).
The perovskite color test, although in the early stages of exploration for Pb dust, is presented with the goal of sharing preliminary data. In future work, optimizing factors such as (1) reagent formulation and application method, (2) the reaction time before imaging, (3) the use of adhesive wipes that are of a different color and/or have lower background fluorescence, (4) the use of different wiping motions for efficient dust dispersion on the wipe and (5) the use of a more intense UV light for viewing with higher sensitivity, will be investigated.
Conclusion
Adhesive tape was an effective tool to pick up dust. Using XRF and an appropriate calibration curve, the method has a LOD of 4.3 µg ft−2 and a false positive rate of 11% at a threshold of 5 µg ft−2. Additionally, dust collected on Painter's tape sprayed with methylammonium bromide and visualized under UV light provides a reliable visual identification when Pb is present in dust above 100 µg ft−2.Thus, XRF detection can serve as an on-site screening method, while the perovskite color detection could be used to visualize gross contamination of dust with Pb (greater than 100 µg ft−2), especially when simplicity and accessibility are priorities. These tests could also increase the impact of a LIRA since the risk assessor would be able to carry out on-site testing and point out remedial action while in the resident's home, which has been a long-standing need in Pb hazard mitigation.
Author contributions
Ornella Joseph: conceptualization, methodology, investigation, formal analysis, visualization, data curation, writing – original draft; Vikrant Jandev: methodology, investigation, data curation, writing – review and editing; Zhutian Zhang: methodology, investigation, formal analysis, writing – review and editing; Darbie Kwon: methodology, investigation, writing – review and editing; Brighten Cho: methodology, investigation; Devena Sammanasu: investigation; Alyssa Wicks: investigation, and writing – review and editing; Marya Lieberman: conceptualization, funding acquisition, methodology, investigation, formal analysis, supervision, writing – review and editing.Conflicts of interest
ML and OJ are listed as co-inventors on a provisional patent for the painter's tape method described in this manuscript. The other authors have no relevant financial or non-financial interests to disclose.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: data from different types of wiping materials, XRF attenuation data, raw XRF spectra for low Pb and blank samples, and a comparison of the developed methods with other analytical methods reported for analysis of lead in dust. A spreadsheet contains replicate measurements, calibration, and validation data as described in the methods and results section. See DOI: https://doi.org/10.1039/d5ay01275a.Acknowledgements
This research was funded by the US Department of Housing and Urban Development (HUD), grant number INLTS0017-20. The opinions expressed in this study are the authors' alone, and do not represent the US Department of HUD's opinions. The authors would like to thank the Center for Environmental Science and Technology (CEST) at the University of Notre Dame for providing use of the microwave digester and ICP-OES instrument. The authors express their gratitude to Rachel Roller who offered the idea of using Painter's tape as an adhesive wipe. Thanks also go out to Megan Zhang and Janine Mbianda for work done in the preliminary stages of this project, John Ramos for providing sieved vacuum cleaner dust, Tom Neltner for feedback, and Rachel Roller for testing the new wipe. Last, but not least, gratitude is extended to Graham Peaslee and the members of the Notre Dame Lead Innovation Team (ND LIT) for the many insightful discussions.References
- B. P. Lanphear, et al., The contribution of lead-contaminated house dust and residential soil to children's blood lead levels. A pooled analysis of 12 epidemiologic studies, Environ. Res., 1998, 79(1), 51–68, DOI:10.1006/ENRS.1998.3859.
- I. Thornton, J. Watt, D. Davies, A. Hunt, J. Cotter-Howells and D. Johnson, Lead contamination of UK dusts and soils and implications for childhood exposure: An overview of the work of the Environmental Geochemistry Research Group, Imperial College, London, England 1981-1992, Environ. Geochem. Health, 1994, 16(4), 113 CrossRef CAS PubMed.
- A. Menke, P. Muntner, V. Batuman, E. K. Silbergeld and E. Guallar, Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults, Circulation, 2006, 114(13), 1388–1394, DOI:10.1161/CIRCULATIONAHA.106.628321.
- CDC, “Recommended Actions Based on Blood Lead Levels”, Accessed: May 03, 2024. [Online]. Available: https://www.cdc.gov/nceh/lead/advisory/acclpp/actions-blls.htm.
- EPA, “Lead Dust Sampling Technician Field Guide (brochure)”, Accessed: May 03, 2024. [Online]. Available: https://www.epa.gov/sites/default/files/documents/ldstguide.pdf.
- CDC and NIOSH, “LEAD in Surface Wipe Samples: METHOD 9100”, Accessed: May 03, 2024. [Online]. Available: https://www.cdc.gov/niosh/docs/2003-154/pdfs/9100.pdf.
- M. Dietrich, L. R. Wood, J. T. Shukle, A. Herrmann and G. M. Filippelli, Contributory science reveals insights into metal pollution trends across different households and environmental media, Environ. Res. Lett., 2023, 18, 034013, DOI:10.1088/1748-9326/acbaad.
- EPA, “Hazard Standards and Clearance Levels for Lead in Paint, Dust and Soil (TSCA Sections 402 and 403)”, https://www.epa.gov/lead/hazard-standards-and-clearance-levels-lead-paint-dust-and-soil-tsca-sections-402-and-403#:%7E:text=EPA's-new-clearance-levels-are-the-dust%2Dlead-clearance-levels.
- I. N. Y. Doyi, C. F. Isley, N. S. Soltani and M. P. Taylor, Human exposure and risk associated with trace element concentrations in indoor dust from Australian homes, Environ. Int., 2019, 133, 105125, DOI:10.1016/J.ENVINT.2019.105125.
- Lead Test Kits | US EPA, Accessed: Jun. 27, 2025. [Online]. Available: https://www.epa.gov/lead/lead-test-kits Search PubMed.
- K. S. Korfmacher and S. Dixon, Reliability of spot test kits for detecting lead in household dust, Environ. Res., 2007, 104, 241–249, DOI:10.1016/j.envres.2007.02.001.
- J. P. Gorce and M. Roff, Hand self-wiping protocol for the investigation of lead exposure in the workplace, J. Occup. Environ. Hyg., 2015, 12(10), 699–707, DOI:10.1080/15459624.2015.1043052.
- L. Helmbrecht, S. W. Van Dongen, A. Van Der Weijden, C. T. Van Campenhout and W. L. Noorduin, Direct Environmental Lead Detection by Photoluminescent Perovskite Formation with Nanogram Sensitivity, Environ. Sci. Technol., 2023, 57, 20494–20500, DOI:10.1021/acs.est.3c06058.
- A. van Geen, et al., Lead-based paint detection using perovskite fluorescence and X-ray fluorescence, Anal. Chim. Acta, 2024, 1307, 342618, DOI:10.1016/J.ACA.2024.342618.
- M. Harper, T. S. Hallmark and A. A. Bartolucci, A comparison of methods and materials for the analysis of leaded wipes, J. Environ. Monit., 2002, 4(6), 1025–1033, 10.1039/b208456m.
- M. Tighe, et al., Validation of a screening kit to identify environmental lead hazards, Environ. Res., 2020, 181, 108892, DOI:10.1016/J.ENVRES.2019.108892.
- D. A. Sterling, R. D. Lewis, D. A. Luke, and B. N. Shadel, “A portable X-ray fluorescence instrument for analyzing dust wipe samples for lead: Evaluation with field samples”, in Environmental Research, Academic Press Inc., 2000, pp. 174–179, DOI:10.1006/enrs.2000.4058.
- L. Ge, W. Lai and Y. Lin, Influence of and correction for moisture in rocks, soils and sediments on in situ XRF analysis, X-Ray Spectrom., 2005, 34(1), 28–34, DOI:10.1002/XRS.782.
- A. Wicks, et al., Validation of a low-cost lead hazard screening kit for the home environment, Integr. Environ. Assess. Manage., 2024, 00, 0–1, DOI:10.1002/ieam.4934.
- A. B. Obeng, et al., Validity of a portable X-ray fluorescence device for analyzing field dust wipe samples for lead, Int. J. Environ. Sci. Technol., 2022, 19(11), 10625–10636, DOI:10.1007/s13762-021-03898-8.
- United States Environmental Protection Agency, “Environmental Technology Verification Report Lead in Dust Wipe Measurement Technology NITON LLC X-Ray Fluorescence Spectrum Analyzer, XLt 700 Series Oak Ridge National Laboratory”, 2003, [Online]. Available: http://www.niton.com/.
- C. Ibañez-Del Rivero, C. A. Wheeler, K. L. Fry and M. P. Taylor, Portable X-ray fluorescence spectrometry: a cost-effective method for analysing trace metals in deposited dust, Anal. Methods, 2024, 16(29), 5038–5048, 10.1039/d4ay00368c.
- Inc. XRF Research, “Dust Wipe”, Accessed: May 03, 2024, [Online]. Available: https://www.xrfresearch.com/dust-wipe/.
- FAQ - Lumetallix”, Accessed: Oct. 01, 2025, [Online]. Available: https://www.lumetallix.com/faq/.
- C. Lanzerstorfer, Variations in the composition of house dust by particle size, J. Environ. Sci. Health, Part A, 2017, 52(8), 770–777, DOI:10.1080/10934529.2017.1303316.
- C. Zaiontz, “Plots of Regression Intervals | Real Statistics Using Excel”, Accessed: Oct. 16, 2025. [Online]. Available: https://real-statistics.com/regression/confidence-and-prediction-intervals/plots-regression-confidence-prediction-intervals/.
- United States Environmental Protection Agency, “Definition and Procedure for the Determination of the Method Detection Limit, Revision 2”, Accessed: Sep. 30, 2025. [Online]. Available: https://www.epa.gov/sites/default/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf.
- United States Environmental Protection Agency, “Final Revisions to the National Lead Laboratory Accreditation Program (NLLAP)”, Accessed: Aug. 01, 2025. [Online]. Available: https://www.federalregister.gov/documents/2024/10/23/2024-24558/final-revisions-to-the-national-lead-laboratory-accreditation-program-nllap-notice-of-availability.
- K. Adelberg, A. van der Weijden, L. Helmbrecht, D. Blaauw, A. C. van Asten and W. L. Noorduin, Perovskite-based photoluminescent detection of lead particles in gunshot residue, Forensic Sci. Int., 2025, 370, 112415, DOI:10.1016/J.FORSCIINT.2025.112415.
- Z. A. VanOrman and L. Nienhaus, Kitchen Spectroscopy: Shining a (UV) Light on Everyday Objects, Matter, 2020, 2(6), 1348, DOI:10.1016/J.MATT.2020.04.026.
- M. García-Heras, M. A. Villegas, J. M. A. Caen, C. Domingo and J. V. García-Ramos, Patination of historical stained windows lead cames from different European locations, Microchem. J., 2006, 83(2), 81–90, DOI:10.1016/J.MICROC.2006.03.001.
| This journal is © The Royal Society of Chemistry 2026 |