Open Access Article
Open Access ArticleAssessing fluoride concentrations in Iowa's groundwater and drinking water: implications for public health and water management
Darrin A.
Thompson
 *ab,
Sophie M.
Pierce
e,
John C.
Flunker
c,
Daniel W.
Gilles
d,
Rick
Langel
e,
Abdul
Quraishi
b,
Alex
Sukalski
b,
Steven M.
Levy
cf,
David M.
Cwiertny
*ab,
Sophie M.
Pierce
e,
John C.
Flunker
c,
Daniel W.
Gilles
d,
Rick
Langel
e,
Abdul
Quraishi
b,
Alex
Sukalski
b,
Steven M.
Levy
cf,
David M.
Cwiertny
 bgh and
Keith E.
Schilling
e
bgh and
Keith E.
Schilling
e
aDepartment of Occupational and Environmental Health, University of Iowa, College of Public Health, Iowa City, Iowa, USA. E-mail: Darrin-thompson-1@uiowa.edu
bCenter for Health Effects of Environmental Contamination, University of Iowa, USA
cDepartment of Epidemiology, College of Public Health, University of Iowa, USA
dIIHR, College of Engineering, University of Iowa, USA
eIowa Geological Survey, University of Iowa, USA
fDepartment of Preventive and Community Dentistry, College of Dentistry, University of Iowa, USA
gDepartment of Civil and Environmental Engineering, College of Engineering, University of Iowa, USA
hDepartment of Chemistry, College of Liberal Arts and Sciences, University of Iowa, USA
First published on 22nd September 2025
Abstract
This study investigates the occurrence and distribution of fluoride in Iowa's groundwater and drinking water. Fluoride, added to community water supplies to prevent dental caries, can pose health risks at high concentrations. The U.S. Public Health Service recommends an optimal fluoride concentration of 0.7 mg L−1, while the EPA sets a maximum contaminant level (MCL) at 4 mg L−1 and a secondary MCL at 2 mg L−1. This research analyzes fluoride data from various sources, including the Iowa Department of Natural Resources and the US Geological Survey, covering 9011 raw groundwater samples from 1931 and 2017 and 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 treated drinking water samples from 1934 to 2021. Fluoride concentrations in Iowa's groundwater ranged from <0.1 mg L−1 to 11.2 mg L−1, with an average of 0.65 mg L−1 and a median of 0.35 mg L−1. Approximately 69% of untreated raw source groundwater samples fell below the recommended 0.7 mg L−1, while 7% exceeded the secondary MCL of 2 mg L−1. Higher fluoride levels are associated with deeper wells and specific aquifers, such as the Cambrian-Ordovician and Mississippian. Treated public drinking water showed an average fluoride concentration of 0.87 mg L−1, indicating a higher average of 0.24 mg L−1 (mean) compared to untreated groundwater due to fluoridation practices. Fluoride concentrations in treated water peaked between 1980 and 1999, then declined slightly after 2000 and more so when systems began aligning with the 2015 recommendation to lower the optimal level to 0.7 mg L−1. This pattern reflects how regulatory guidance and water source management have influenced fluoride levels over time. This study highlights significant regional variability in fluoride levels, influenced by aquifer lithology, well depth, and water chemistry. Anthropogenic sources also contribute to fluoride concentrations. The findings underscore the need for tailored water management strategies to balance the benefits of fluoridation with the risks of excessive fluoride intake. This research provides valuable insights for public health agencies, water suppliers, and residents, aiming to optimize fluoride levels in Iowa's drinking water to ensure safety and efficacy.
280 treated drinking water samples from 1934 to 2021. Fluoride concentrations in Iowa's groundwater ranged from <0.1 mg L−1 to 11.2 mg L−1, with an average of 0.65 mg L−1 and a median of 0.35 mg L−1. Approximately 69% of untreated raw source groundwater samples fell below the recommended 0.7 mg L−1, while 7% exceeded the secondary MCL of 2 mg L−1. Higher fluoride levels are associated with deeper wells and specific aquifers, such as the Cambrian-Ordovician and Mississippian. Treated public drinking water showed an average fluoride concentration of 0.87 mg L−1, indicating a higher average of 0.24 mg L−1 (mean) compared to untreated groundwater due to fluoridation practices. Fluoride concentrations in treated water peaked between 1980 and 1999, then declined slightly after 2000 and more so when systems began aligning with the 2015 recommendation to lower the optimal level to 0.7 mg L−1. This pattern reflects how regulatory guidance and water source management have influenced fluoride levels over time. This study highlights significant regional variability in fluoride levels, influenced by aquifer lithology, well depth, and water chemistry. Anthropogenic sources also contribute to fluoride concentrations. The findings underscore the need for tailored water management strategies to balance the benefits of fluoridation with the risks of excessive fluoride intake. This research provides valuable insights for public health agencies, water suppliers, and residents, aiming to optimize fluoride levels in Iowa's drinking water to ensure safety and efficacy.
Environmental significanceThis study evaluates fluoride levels in Iowa's untreated groundwater and treated drinking water, revealing significant regional variation. While fluoridation raises average fluoride concentrations to beneficial levels for dental health, about 7% of groundwater samples exceed safe thresholds. The findings underscore the need for tailored water management strategies to balance the benefits of fluoridation with the risks of excessive fluoride exposure. This research provides valuable insights for public health agencies, water suppliers, and residents, aiming to optimize fluoride levels in Iowa's drinking water to ensure safety and efficacy. |
1. Introduction
Community water fluoridation has been employed to improve human dental health in the United States since the mid-1900s.1 Fluoride reduces the prevalence and severity of tooth decay in adults and children.2 It is added to toothpaste and mouthwashes and consumed in small quantities present in many foods and beverages.2 Community water fluoridation is recommended by most public health agencies and advocacy groups including the U.S. Public Health Service (USPHS),2 World Health Organization (WHO),3 American Dental Association,4 and American Academy of Pediatrics.5 It is widely recognized as the most efficient, equitable, and cost-effective/cost-saving method of delivering fluoride to the general population.6 An estimated 400 million people in 25 countries, such as Australia, Brazil, Canada, Chile, Ireland, Malaysia, United Kingdom, the United States, and Vietnam, receive drinking water with added fluoride.Deficient fluoride concentrations can contribute to a higher occurrence of tooth decay, while excess fluoride concentration is linked to irreversible dental or skeletal fluorosis.2,3 Some associations with other health effects also have been reported.7–13 Fluoride exposure greater than 1.5 mg L−1 (ref. 14) during early pregnancy has been associated with adverse fetal outcomes, including miscarriages and stillbirths,12,15 preterm and low birth weight infants,16,17 neurological malformations,12 and maternal anemia.12,18 Although fluoride ingestion has been proposed as a risk factor for osteosarcoma, a type of bone cancer, the best available studies do not support an association between fluoride levels in drinking water and an increased risk of osteosarcoma.19–24 Available evidence also suggests that children and teenagers may be more susceptible to fluoride exposure compared to adults.25 For instance, neurocognitive deficits in children aged 3 to 4 years have also been observed,7,12,26 but these results should be interpreted with caution, as significant concerns regarding the quality of these studies including methodological, data integrity variability in study quality and potential biases have been noted by experts.27,28 Importantly, most of these studies have focused on populations with fluoride exposures substantially higher than those typically provided by U.S. water supplies.24 Despite these reports of adverse health effects, fluoridation is widely considered one of the great public health successes of the 20th century.29
The ideal range for fluoride concentrations in drinking water is relatively narrow. In 2015, the U.S. Public Health Service (PHS) lowered its recommended fluoride concentration in drinking water from a range of 0.7 to 1.2 milligrams per liter (mg L−1) to a single, optimal level. The prior recommendation was put in place in 1962.30 The U.S. Public Health Service (PHS) now recommends an optimal fluoride concentration of 0.7 mg L−1 in drinking water to prevent tooth decay with minimal risk of fluorosis.2,30,31 Due to the health risks associated with long-term exposure to high fluoride concentrations, the U.S. Environmental Protection Agency (USEPA) has set an enforceable maximum contaminant level (MCL) at 4 mg L−1 with a secondary MCL (SMCL) of 2 mg L−1.32 The World Health Organization recommends a guideline limit of 1.5 mg L−1 with an optimal range between 0.5–1.0 mg L−1.3
Globally, it has been estimated that as many as 330 million people are exposed to fluoride concentrations over 1.5 mg L−1.33 In the United States, over 522![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people on domestic wells are potentially exposed to concentrations above the EPA's 2 mg L−1 SMCL.34 For community water systems (CWS), from 2016–2021, the CDC's Water Fluoridation Reporting System (WFRS) reported that only 0.01% of population-weighted monthly fluoride measurements (person-months) exceeded this SMCL.35
000 people on domestic wells are potentially exposed to concentrations above the EPA's 2 mg L−1 SMCL.34 For community water systems (CWS), from 2016–2021, the CDC's Water Fluoridation Reporting System (WFRS) reported that only 0.01% of population-weighted monthly fluoride measurements (person-months) exceeded this SMCL.35
Fluoride concentrations in natural waters are generally in the range of 0.1–12 mg L−1, but substantial variation exists across water supplies in different areas.36 Fluoride concentrations in groundwater above the WHO guideline occur in parts of Africa, China, India, Iran, and North and South America.25,34,36–45 In the United States, most of the aquifers with fluoride concentrations exceeding the EPA MCL and SMCL are found in the western United States.34 In 2020, McMahon analyzed data from 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 wells, finding that more than 85% of fluoride concentrations were below the 0.7 mg L−1 oral-health benchmark with an estimated 3 million people in the country served with water containing F concentrations >0.7 mg L−1.34 In comparison, fluoride concentrations in Iowa's surface and groundwater are relatively low, like much of the eastern US, with low risk of excess fluoride levels. However, there is a significant range of fluoride concentrations observed in different Iowa source waters, which are highly regionalized.
000 wells, finding that more than 85% of fluoride concentrations were below the 0.7 mg L−1 oral-health benchmark with an estimated 3 million people in the country served with water containing F concentrations >0.7 mg L−1.34 In comparison, fluoride concentrations in Iowa's surface and groundwater are relatively low, like much of the eastern US, with low risk of excess fluoride levels. However, there is a significant range of fluoride concentrations observed in different Iowa source waters, which are highly regionalized.
Aquifer lithology, including the presence of F-bearing minerals such as fluorite and apatite, is a key determinant of fluoride in drinking water, as fluoride is mostly retained in minerals in an aqueous environment.44 High levels of fluoride in groundwater typically are found in aquifers with acidic igneous basement rocks, volcanic and geothermal rocks, as well as derived from sedimentary deposits and metamorphic rocks.33,34 Chemical and other characteristics of the water including low pH, low calcium concentrations, high Total Dissolved Solids (TDS) concentration, higher temperatures, long groundwater residence times, and greater well depth are all factors associated with higher fluoride concentrations.33,34,45–47 Additionally, anthropogenic sources such as superphosphate fertilizer, pesticides, and industrial waste also can influence fluoride concentrations in water.34,48–50
Community water fluoridation first began in Iowa in 1951.51 By 1966 half of the state's population received water containing adjusted or dosed fluoridation in line with USPHS recommendations.51 As of 2022, nearly 89% of Iowans, or 2.3 million people, had access to fluoridated water.52,53 Only 69.9% had access to optimally fluoridated water.52 Some drinking water systems have naturally sufficient fluoride concentrations, while others have elevated fluoride concentrations and blend with water from other sources to maintain compliance with regulatory standards and optimize fluoride levels for public health.44,54 The studies by McMahon and DeSimone34,55,56 suggest significant variability in F− concentrations in Iowa waters, with concentrations ranging from 0.1 to 4 mg L−1. However, variation in fluoride levels across the state, particularly in source groundwater compared to finished drinking water, has not been evaluated or discussed.34 For water providers, it is important to understand the base concentration of fluoride present in their water sources to make evidence-based decisions regarding fluoridation. This paper aims to characterize the occurrence and distribution of fluoride in Iowa's source and finished drinking water to better inform decision-making by water suppliers, public health agencies, and the residents of Iowa.
2. Materials and methods
2.1 Data
This study analyzed fluoride data from several sources. First, groundwater (n = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128) data collected between 1931 and 2017 were obtained from the Iowa Department of Natural Resources' (IDNR) (n = 4029) database AQuIA57 and the US Geological Survey's (USGS) National Water Information System (NWIS) (n = 6099) database.58 These data represent fluoride concentrations in raw source waters from public water systems and monitoring wells; private wells were not included in this analysis. Sample records were coded by aquifer lithology or aquifer age, so aquifer codes were condensed, and samples re-classified by aquifer age into seven groups: Alluvial, Quaternary, Cretaceous, Mississippian, Devonian, Silurian, and Cambrian-Ordovician. Samples that were listed as coming from multiple aquifers, an unknown aquifer, or an unidentifiable source were excluded from this analysis. A total of 1117 samples were excluded due to data errors or because the correct lithology or aquifer could not be identified, leaving a total sample set of 9011.
128) data collected between 1931 and 2017 were obtained from the Iowa Department of Natural Resources' (IDNR) (n = 4029) database AQuIA57 and the US Geological Survey's (USGS) National Water Information System (NWIS) (n = 6099) database.58 These data represent fluoride concentrations in raw source waters from public water systems and monitoring wells; private wells were not included in this analysis. Sample records were coded by aquifer lithology or aquifer age, so aquifer codes were condensed, and samples re-classified by aquifer age into seven groups: Alluvial, Quaternary, Cretaceous, Mississippian, Devonian, Silurian, and Cambrian-Ordovician. Samples that were listed as coming from multiple aquifers, an unknown aquifer, or an unidentifiable source were excluded from this analysis. A total of 1117 samples were excluded due to data errors or because the correct lithology or aquifer could not be identified, leaving a total sample set of 9011.
Another compiled set of groundwater samples, which included data from the IDNR and USGS,59 was used to examine the relationships between fluoride concentration and other key parameters such as hardness, turbidity, pH, and well depth using Spearman correlation coefficients (Table S1). While this dataset consisted of over 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points, the number of results for various parameters varied significantly. As a result, this dataset was only used for correlation analysis because these parameters were not available with the primary data set.
000 data points, the number of results for various parameters varied significantly. As a result, this dataset was only used for correlation analysis because these parameters were not available with the primary data set.
Fluoride levels in finished drinking water samples (n = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280) from CWS were obtained from the Center for Health Effects of Environmental Contamination (CHEEC) at the University of Iowa. Iowans receive treated drinking water from 1801 CWS. These systems consist of a mixture of CWS (n = 1076), non-transient non-CWS (n = 113), and transient non-CWS (n = 612).60 Non-transient and transient systems represent water systems such as schools, factories, gas stations, campgrounds, and rest stops that either provide water to at least 25 people for a minimum of 6 months or where people do not remain for long periods of time.61 Alternatively, a CWS is defined as a system that provides water for human consumption that has at least 15 service connections or serves at least 25 people at least 60 days during the year.61 This study examined finished water samples from CWSs. Data from 891 of the 1076 were identified with fluoride concentrations reported for post-treatment samples from 1934 to 2021. CHEEC maintains a database of historical community water data on water sources (ground or surface water), treatments used, populations served, and water quality.62
280) from CWS were obtained from the Center for Health Effects of Environmental Contamination (CHEEC) at the University of Iowa. Iowans receive treated drinking water from 1801 CWS. These systems consist of a mixture of CWS (n = 1076), non-transient non-CWS (n = 113), and transient non-CWS (n = 612).60 Non-transient and transient systems represent water systems such as schools, factories, gas stations, campgrounds, and rest stops that either provide water to at least 25 people for a minimum of 6 months or where people do not remain for long periods of time.61 Alternatively, a CWS is defined as a system that provides water for human consumption that has at least 15 service connections or serves at least 25 people at least 60 days during the year.61 This study examined finished water samples from CWSs. Data from 891 of the 1076 were identified with fluoride concentrations reported for post-treatment samples from 1934 to 2021. CHEEC maintains a database of historical community water data on water sources (ground or surface water), treatments used, populations served, and water quality.62
2.2 Statistical analysis
Fluoride concentration data used in this study were obtained from IDNR and USGS, both of which are reputable sources that utilize certified laboratories and standardized procedures. Although the publicly available datasets did not include metadata for inter-laboratory comparisons or detailed QA/QC documentation, prior work by the USGS has described the analytical methods and quality assurance protocols used in their water quality monitoring programs.34 Due to the absence of standardized metadata across all sources and time periods, we were unable to provide a comprehensive range of detection limits or confirm consistency in analytical methods between laboratories. This limitation is acknowledged and should be considered when interpreting long-term trends in fluoride concentrations.Descriptive statistics were calculated to summarize fluoride concentrations by year, aquifer, and water type (Tables 1 and 2). The distributions of continuous data and their natural log transformations were examined for normality using the Shapiro–Wilk test. Median, maximum, and minimum were reported to account for skewed distributions. Non-detects were historically recorded as zero and retained as such to maintain consistency across the long temporal span of the dataset. Mean values and other summary statistics were calculated using these zero values, reflecting historical reporting practices in Iowa's water monitoring records. The strength and direction of association between pairs of continuous variables were measured using Spearman's rank correlation analyses (Tables S1). Non-parametric tests, Wilcoxon Rank-Sum Tests and Kruskal–Wallis Tests, as appropriate, were used to compare differences in fluoride concentrations. Fluoride concentrations were compared to the USPHS recommended concentration of 0.7 mg L−1, the US EPA's MCL and SMCL, and the WHO's recommendations as benchmarks. Values above these standards were considered elevated for the purposes of this study.2,3,31,32 Fluoride values below the USPHS recommendation were considered low and, if used as source water, should be considered for adjustment to the optimal water fluoride level. Values above the 2 mg L−1 and 4 mg L−1 EPA limits would be of concern due to the increased risk of dental fluorosis.
| Mean | Std dev. | 95% CI | Median | Min | 25% | 75% | Max | N | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0.65 | 0.74 | 0.63 | 0.66 | 0.35 | 0.00 | 0.23 | 0.75 | 11.20 | 9011 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Aquifer type | |||||||||||
| Alluvial | 0.27 | 0.21 | 0.26 | 0.28 | 0.25 | 0.00 | 0.20 | 0.30 | 3.30 | 1958 | <0.0001 |
| Cambrian-Ordovician | 1.18 | 0.94 | 1.13 | 1.22 | 1.10 | 0.00 | 0.40 | 1.60 | 11.20 | 1755 | |
| Cretaceous | 0.66 | 0.50 | 0.56 | 0.76 | 0.55 | 0.00 | 0.35 | 0.80 | 3.00 | 99 | |
| Devonian | 0.86 | 0.78 | 0.81 | 0.91 | 0.60 | 0.00 | 0.30 | 1.00 | 6.00 | 1088 | |
| Mississippian | 0.94 | 1.03 | 0.88 | 1.00 | 0.50 | 0.00 | 0.30 | 1.20 | 9.00 | 1117 | |
| Quaternary | 0.39 | 0.29 | 0.38 | 0.41 | 0.35 | 0.00 | 0.25 | 0.45 | 3.00 | 2098 | |
| Silurian | 0.38 | 0.38 | 0.36 | 0.41 | 0.30 | 0.00 | 0.20 | 0.40 | 3.00 | 896 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Landform* | |||||||||||
| Des Moines Lobe | 0.84 | 0.89 | 0.80 | 0.88 | 0.40 | 0.00 | 0.30 | 1.10 | 9.00 | 2024 | <0.0001 |
| East-Central Iowa Drift Plain | 0.30 | 0.22 | 0.27 | 0.34 | 0.25 | 0.00 | 0.20 | 0.40 | 1.27 | 155 | |
| Iowa-Cedar Lowland | 0.35 | 0.5 | 0.21 | 0.5 | 0.23 | 0.05 | 0.15 | 0.30 | 3.00 | 49 | |
| Iowan Surface | 0.60 | 0.6 | 0.57 | 0.62 | 0.40 | 0.00 | 0.20 | 0.80 | 8.70 | 1998 | |
| Loess Hills | 0.31 | 0.19 | 0.27 | 0.36 | 0.30 | 0.00 | 0.25 | 0.35 | 1.60 | 66 | |
| Mississippi River Alluvial Plain | 0.20 | 0.24 | 0.17 | 0.22 | 0.15 | 0.00 | 0.10 | 0.20 | 3.20 | 399 | |
| Missouri River Alluvial Plain | 0.51 | 0.47 | 0.42 | 0.59 | 0.37 | 0.00 | 0.30 | 0.40 | 2.80 | 123 | |
| Northwest Iowa Plains | 0.47 | 0.35 | 0.45 | 0.50 | 0.40 | 0.00 | 0.30 | 0.50 | 2.80 | 752 | |
| Paleozoic Plateau | 0.36 | 0.42 | 0.31 | 0.41 | 0.25 | 0.00 | 0.20 | 0.40 | 3.30 | 297 | |
| Southern Iowa Drift Plain | 0.71 | 0.82 | 0.68 | 0.74 | 0.35 | 0.00 | 0.25 | 1.00 | 11.2 | 3148 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Distance from Manson impact structure | |||||||||||
| <50 km | 0.81 | 0.74 | 0.76 | 0.85 | 0.45 | 0.00 | 0.30 | 1.10 | 6.50 | 1039 | <0.0001 |
| 51–150 km | 0.63 | 0.75 | 0.60 | 0.65 | 0.35 | 0.00 | 0.25 | 0.60 | 9.00 | 3707 | |
| 151–250 km | 0.64 | 0.72 | 0.62 | 0.67 | 0.35 | 0.00 | 0.20 | 0.81 | 11.20 | 2629 | |
| >250 km | 0.59 | 0.75 | 0.56 | 0.63 | 0.30 | 0.00 | 0.15 | 0.70 | 5.30 | 1636 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Well depth | |||||||||||
| <20 m | 0.30 | 0.20 | 0.29 | 0.31 | 0.25 | 0.00 | 0.20 | 0.39 | 2.70 | 1526 | <0.0001 |
| 21–50 m | 0.34 | 0.29 | 0.33 | 0.35 | 0.30 | 0.00 | 0.20 | 0.40 | 3.60 | 2537 | |
| 51–100 m | 0.54 | 0.51 | 0.52 | 0.56 | 0.35 | 0.00 | 0.25 | 0.70 | 7.00 | 2481 | |
| 101–150 m | 1.29 | 1.02 | 1.25 | 1.33 | 1.10 | 0.00 | 0.50 | 1.80 | 11.20 | 2467 | |
| Untreated groundwater (n = 9011) | Treated public drinking water (n = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280) 280) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Med | Min | 25% | 75% | Max | Mean | Med | Min | 25% | 75% | Max | p-Value | |
| Total | 0.65 | 0.35 | 0.00 | 0.23 | 0.75 | 11.20 | 0.87 | 0.86 | 0.00 | 0.56 | 1.05 | 8.60 | <0.0001 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||
| Year | |||||||||||||
| ≤ 1959 | 0.80 | 0.50 | 0.00 | 0.25 | 1.00 | 9.00 | 0.54 | 0.30 | 0.00 | 0.25 | 0.50 | 4.60 | |
| 1960–1979 | 0.68 | 0.38 | 0.03 | 0.25 | 0.80 | 11.20 | 0.90 | 0.60 | 0.10 | 0.30 | 1.10 | 8.60 | |
| 1980–1999 | 0.47 | 0.30 | 0.00 | 0.20 | 0.45 | 5.30 | 0.98 | 0.96 | 0.00 | 0.67 | 1.12 | 8.00 | |
| ≥ 2000 | 0.44 | 0.28 | 0.05 | 0.22 | 0.43 | 4.70 | 0.83 | 0.83 | 0.00 | 0.60 | 1.01 | 4.30 | |
SAS 9.4 (SAS Institute, Cary, NC) was used to perform the statistical analyses. Geographic data for boundaries of municipalities, locations of private wells, Iowa landforms and other geological features were obtained from Iowa Geospatial Data (https://geodata.iowa.gov/). Maps of Iowa fluoride concentrations were developed using kriging interpolation of private well and mean finished water provider locations. Kriging is a spatial interpretation method that fills in gaps between observations. Kriging also weights the influence of nearby observations using a variogram of spatial correlation of nearby points to minimize prediction error.63 The mean fluoride concentration for each finished water source was calculated by averaging its collected sample concentrations. Geographic mapping was conducted using ArcGIS Pro (version 3.2.1, ESRI, Redlands, California, USA).
3. Results and discussion
3.1 Fluoride in untreated raw source groundwater
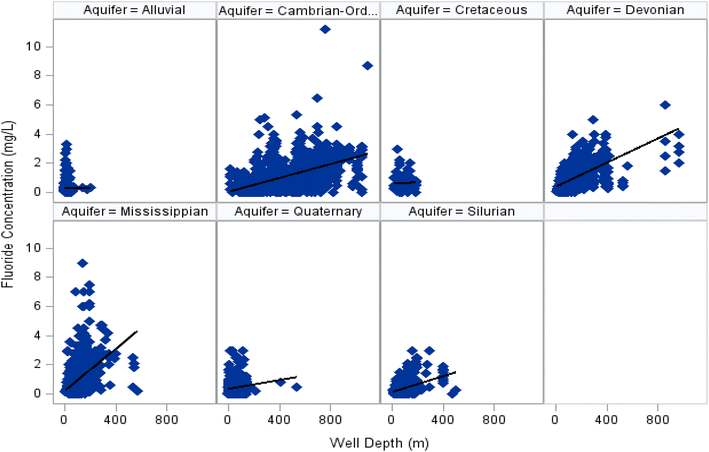
Descriptive statistics for groundwater F− concentrations in each aquifer are given in Table 1. Fluoride concentrations across all aquifers ranged from <0.1 mg L−1 to 11.2 mg L−1, with a median value of 0.35 mg L−1 and mean of 0.65 mg L−1 (95% CI 0.63–0.66). Broadly speaking, fluoride concentrations in Iowa groundwater were low. In this study, 69% of all untreated groundwater samples were below the recommended fluoride level for public dental health (0.7 mg L−1), while only 0.4% were above the EPA's MCL of 4 mg L−1 and 7% were above the SMCL of 2 mg L−1. Similarly, 88% of all groundwater samples were below the WHO's guideline limit of 1.5 mg L−1.Fluoride concentrations were significantly different by aquifer type (Kruskal Wallis, p ≤ 0.0001). Alluvial, Quaternary, and Silurian aquifers wells had the lowest levels of fluoride with average concentrations of 0.27 (95% CI 0.26–0.28), 0.39 (95% CI 0.38–0.41), and 0.38 (95% CI 0.36–0.41), respectively. Their median concentrations ranged from 0.25–0.35 mg L−1. Over ninety-nine percent of all samples collected from these types of aquifers had concentrations below 2 mg L−1. Wells from Cretaceous and Devonian aquifers were generally closer to 0.7 mg L−1 with mean values of 0.66 mg L−1 (95% CI 0.56–0.76) and 0.86 mg L−1 (95% CI 0.81–0.91), respectively. Twelve percent of samples from these two aquifers exceeded the EPA's SMCL.
Higher fluoride concentrations were found in Mississippian and Cambrian-Ordovician wells, with mean concentrations of 0.94 (95% CI 0.88–1.00) and 1.18 (95% CI 1.13–1.22) mg L−1, respectively. The maximum concentration detected was 11.20 mg L−1. Both aquifers had concentrations below 0.7 mg L−1 in nearly half of their wells, while 16% of Mississippian and 18% of Cambrian-Ordovician wells had concentrations above the SMCL. Less than 2% of samples from these two aquifers exceeded the EPA's MCL. In Iowa, the Cambrian Ordovician is the only aquifer where concentrations regularly exceed both EPA and WHO recommendations. Concentrations (Fig. 1 and Table S2) within the Cambrian-Ordovician (p = 0.052), Cretaceous (p = 0.452), and Silurian (p = 0.371) aquifers have not varied significantly over time since 1931, whereas greater variability was observed over time in the Alluvial, Devonian, Mississippian, and Quaternary aquifers (p = <0.05).
In addition to well depth, previous analysis has shown correlations between fluoride concentration and environmental and water quality factors like pH, TDS concentration, and mean annual precipitation.34,57 Spearman tests (Table S1) indicated strong statistically significant (p < 0.0001) correlations between fluoride concentration and several water quality analytes or characteristics. High concentrations of fluoride correlated (P < 0.05) positively with well depth, pH, calcium, hardness, potassium, and sodium and correlated negatively with barium, carbon dioxide, dissolved oxygen, manganese, and tritium. Deeper aquifers, such as the Cambrian-Ordovician, are generally highly mineralized because of long groundwater residence times and may have increased fluorite solubility because they are warmer. The positive associations, therefore, likely reflects increasing mineralization and as aquifers become deeper. On the other hand, low TDS waters are found in groundwater recently recharged (shallow and carbonate aquifers). In particular, the negative associations with tritium suggest that fluoride concentrations are related to groundwater age.64,65 Tritium is a radioactive isotope of hydrogen with a half-life of about 12.3 years.64,65 Its presence in groundwater is primarily due to atmospheric nuclear testing in the mid-20th century, which significantly increased tritium levels in precipitation.64,65 Higher levels of tritium suggest that the groundwater likely was recharged after 1953.64,65
These findings align closely with associations found between fluoride concentrations and aquifer type. The average depth across all aquifers was 143 meters (m) (469 feet (ft)), but depths varied considerably by aquifer (Fig. 2). Average well depths ranged from 15 m (49 ft) for alluvial wells to 475 m (1558 ft) for Cambrian-Ordovician wells. The highest groundwater F− concentrations in Iowa were found in deeper wells of the Devonian (mean depth = 125 m, 410 ft), Cambrian-Ordovician, and Mississippian (100 m, 328 ft) aquifers. Conversely, lower concentrations were observed in the Alluvial, Quaternary (34 m, 112 ft), Cretaceous (99 m, 325 ft), and Silurian (105 m, 344 ft) aquifers.
Groundwater fluoride levels are determined largely by the presence of fluorine-bearing minerals and the solubility of fluorite. The positive correlation found in this study between F− concentration and well depth indicates that deeper groundwater is higher in fluoride due to presence of F-bearing minerals, residence time, or increased fluorite solubility. Median fluoride concentrations are highest in volcanic rocks (0.4 mg L−1) followed by sandstone (0.3 mg L−1), sandstone-carbonate rocks (0.3 mg L−1), carbonate rocks (0.2 mg L−1), and shale (0.2 mg L−1).34 Elevated F− levels found in Devonian, Cambrian-Ordovician and Mississippian wells may be related to the presence of sandstone and carbonates in these aquifers. Fluorite solubility increases with temperature, and as deep groundwater is warmed by geothermal heating gradients, deeper groundwaters may have increased fluorite solubility causing higher F− concentrations. Groundwater temperature in the Cambrian-Ordovician aquifer ranges from 60–80 °F.
Alluvial wells in Iowa have low levels of fluoride, and this is likely due to infiltration of rainfall with low concentrations of F− to recharge areas. While position in the groundwater-flow system can influence water–rock interactions and contribute to the variability in F− levels across aquifers, regional groundwater flowpaths are not well-defined at the state scale.66 According to the Iowa Geological Survey, flow is generally downward and toward nearby rivers, with no consistent lateral direction.67 In Iowa, fluoride concentrations appear to be more strongly associated with groundwater age than with flow direction. For example, in the Cambrian-Ordovician aquifer, water flows from northwest to southeast, yet fluoride concentrations increase from northeast to southwest, consistent with the age gradient of the water.68 Position in the groundwater-flow system will impact the dynamics of water–rock interactions and this likely has bearing on the wide range of F− levels seen in most aquifers. Globally, surface water fluoride is generally low and rarely at levels with potential to be detrimental to human health, except in crystalline basement aquifers, volcanic areas, geothermal areas, and areas with significant evapotranspiration.69,70 Iowa surface waters are low in fluoride, with a mean value of 0.3 mg L−1 – similar to levels seen in surficial aquifers. Fluoride can also be anthropogenic in origin in groundwater and surface water, coming from industrial wastewater or chemical spills.34,48–50
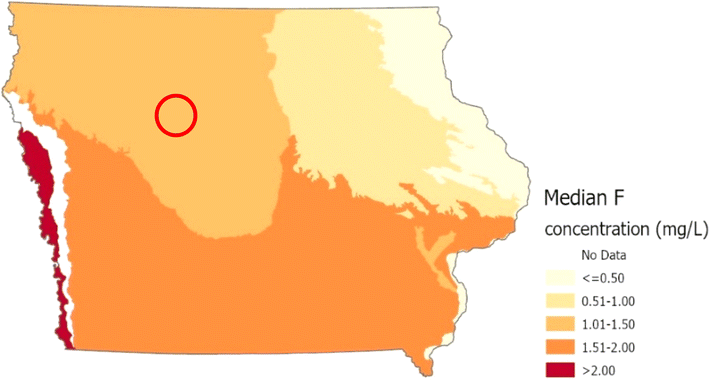
Iowa is composed of several distinct landforms (Fig. 3). Different aquifers are present within each landform. The local geology and quantity of water needed determines which aquifer is utilized for water supplies by CWSs. Fluoride concentrations in groundwater vary significantly by landform region (Kruskal Wallis, p ≤ 0.0001). The Des Moines Lobe had the highest concentrations with an average of 0.84 mg L−1 (95% CI 0.80–0.88), while the Mississippi River Alluvial Plain had the lowest average of 0.20 mg L−1 (95% CI 0.17–0.22). The geology associated with these landform regions likely accounts for the observed fluoride variability. The aquifers utilized by CWSs in the Des Moines Lobe include the Mississippian, Devonian, and Manson Impact Crater. This paper documents higher mean fluoride concentrations in the Mississippian aquifer. High fluoride concentrations have been documented historically in the Devonian aquifer.71 The Manson Impact Crater, which has some of Iowa's highest fluoride concentrations in groundwater, is contained exclusively in the Des Moines Lobe.72 CWSs primarily use shallow alluvial wells in the Mississippi River Alluvial Plain. The groundwater in these wells is geologically “young,” lacking substantial time to dissolve fluoride from the geological materials that constitute the aquifer.
The Cambrian-Ordovician aquifer illustrates the connection between landforms and aquifers. The Cambrian-Ordovician aquifer is inclined in a southwesterly direction under Iowa and is present under all landform regions. Wells in this aquifer in the northeast Iowa are shallower and utilize younger water than those in southwest Iowa. Median fluoride concentrations increase from the northeast to southwest Iowa (Fig. 4). This confirms associations found among fluoride concentrations, well depth, and age of groundwater.
The high F− concentrations seen in the Manson Impact Structure (MIS) are likely due to dissolution of biotite and low Ca2+ concentrations, which can allow for elevated F− levels.57,72 The MIS is a 37-km diameter crater formed about 74 million years ago located in northwest Iowa near the city of Manson. It lies buried under the southeast corner of Pocahontas County and extends into portions of three adjoining counties – Calhoun, Humboldt, and Webster.72 The MIS features an outer ring of down-dropped rock blocks, a central peak of crystalline rocks lifted from deep below, and a crater moat filled with resurge material called Phanerozoic-Clast Breccia (PCB).72 The one area of the crater that is the most dependable source of water is the central peak.72
A reconnaissance study of the groundwater supply in Iowa published in 2015 found that groundwater from the central peak aquifer contained high concentrations of fluoride (max = 10.0 mg L−1).72 Schilling et al.72 also reported that fluoride concentrations in Manson City wells from 1912 to 2010 ranged from 4.0 to 4.7 mg L−1, exceeding the EPA's MCL for drinking water.
In the current study, we found that proximity to the MIS was a significant predictor of fluoride concentrations in groundwater. Concentrations within 50 km of the MIS were significantly higher (Kruskal Wallis, p ≤ 0.0001) than wells further away, with an average concentration of 0.81 (95% CI 0.76–0.85) compared to <0.64 mg L−1. This suggests that the MIS plays an important role, at least within north-central Iowa, in influencing fluoride concentrations.
3.2 Fluoride in treated drinking water
The variation in fluoride levels seen in Iowa source water presents a challenge for drinking water utilities across the state (Fig. 5). Historically, central and southeastern Iowa have reported higher levels of fluoride compared to the rest of the state. Within the past 20 years, these geospatial trends are primarily a result of aquifer use, with central Iowa relying primarily on the Mississippian aquifer and being closer to the MIS. Alternatively, southeast Iowa water systems have primarily used the very old and deep Cambrian-Ordovician aquifer for water. | ||
| Fig. 5 Average concentrations of fluoride in untreated groundwater and treated, finished drinking water from all sources from community water systems in Iowa. | ||
Average fluoride concentrations in treated drinking water from all sources were 0.87 mg L−1 (95% CI: 0.86–0.87), compared to 0.65 mg L−1 (95% CI: 0.63–0.66) in untreated groundwater (Table 2). The median concentration in treated drinking water (0.86 mg L−1) was more than twice that of untreated groundwater (0.35 mg L−1), suggesting that fluoridation practices have raised fluoride levels by approximately 0.24 mg L−1 (mean) to 0.51 mg L−1 (median). Historically, fluoride concentrations in treated drinking water reached as high as 8.6 mg L−1 (1979) and 8.0 mg L−1 (1982), while the highest levels in untreated groundwater were 9.4 mg L−1 (1995) and 11.2 mg L−1 (1974), likely reflecting localized geologic conditions.
Looking at temporal trends (Table 2 and Fig. 6), before 1959 the mean fluoride concentration in drinking water was 0.54 mg L−1 (95% CI: 0.50–0.59). After the adoption of widespread fluoridation in Iowa in 1966,51 fluoride concentrations in treated drinking water increased significantly, while concentrations in untreated groundwater sources declined sharply—likely due to reduced reliance on naturally fluoridated groundwater and a shift toward centralized water treatment. From 1960 to 1979, the average fluoride concentration in drinking water rose to 0.90 mg L−1 (95% CI: 0.86–0.93), and between 1980 and 1999, it peaked at 0.98 mg L−1 (95% CI: 0.96–1.00). These elevated levels likely reflect efforts by CWS to align with earlier public health recommendations to maintain fluoride concentrations near 1.0 mg L−1 for the prevention of dental caries. After 2000, the mean concentration declined slightly to 0.83 mg L−1 (95% CI: 0.83–0.84), indicating a gradual shift in practice as systems began aligning with the 2015 U.S. Public Health Service recommendation to lower the optimal fluoride concentration to 0.7 mg L−1. After 2000, the mean concentration declined slightly to 0.83 mg L−1 (95% CI: 0.83–0.84). Much of this change occurred after 2015, indicating a shift in practice as systems began aligning with the more recent U.S. Public Health Service recommendation to lower the optimal fluoride concentration to 0.7 mg L−1. In fact, after 2015, the average concentrations in treated drinking water declined to 0.64 mg L−1 from 2016 to 2021 compared to 0.89 mg L−1 from 2000 to 2015.
Since 2000, 99% of fluoride measurements in treated drinking water (n = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215 samples) have remained below the EPA's Secondary Maximum Contaminant Level (SMCL) of 2.0 mg L−1. However, 285 samples from 56 systems exceeded the SMCL, and only one sample exceeded the EPA's Maximum Contaminant Level (MCL) at 4.3 mg L−1. Most of these exceedances (79%) were reported prior to the U.S. Public Health Service's recommendation to reduce fluoride concentrations in drinking water from 1.0 to of 0.7 mg L−1 in 2015. Only 23 CWS, serving a total population of over 27
215 samples) have remained below the EPA's Secondary Maximum Contaminant Level (SMCL) of 2.0 mg L−1. However, 285 samples from 56 systems exceeded the SMCL, and only one sample exceeded the EPA's Maximum Contaminant Level (MCL) at 4.3 mg L−1. Most of these exceedances (79%) were reported prior to the U.S. Public Health Service's recommendation to reduce fluoride concentrations in drinking water from 1.0 to of 0.7 mg L−1 in 2015. Only 23 CWS, serving a total population of over 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people, have exceeded the SMCL since 2015. Eighteen of these systems are in the Des Moines Lobe region of in north-central Iowa. Over the entire 87-year period analyzed, only 76 treated water samples exceeded 4.0 mg L−1. The changes in fluoride concentrations in treated drinking water over this time period reflect efforts by community water systems to maintain fluoride at optimal levels—initially around 1.0 mg L−1, and later adjusted to 0.7 mg L−1 following the 2015 recommendation.2,30,31 This pattern clearly illustrates how regulatory guidance and water source management have influenced fluoride levels in drinking water over time.
000 people, have exceeded the SMCL since 2015. Eighteen of these systems are in the Des Moines Lobe region of in north-central Iowa. Over the entire 87-year period analyzed, only 76 treated water samples exceeded 4.0 mg L−1. The changes in fluoride concentrations in treated drinking water over this time period reflect efforts by community water systems to maintain fluoride at optimal levels—initially around 1.0 mg L−1, and later adjusted to 0.7 mg L−1 following the 2015 recommendation.2,30,31 This pattern clearly illustrates how regulatory guidance and water source management have influenced fluoride levels in drinking water over time.
Water from CWSs typically come from three types of sources: groundwater (GW), surface water (SW), or groundwater under the influence of surface water (GU).60 Groundwater is the primary source of water for over 90% of Iowa's water systems.41 From 2010 to 2021, the fluoride concentrations in drinking water from Iowa's CWSs varied across the three primary water sources (Table 3). Systems using surface water (SW) had the highest average fluoride concentrations in treated, finished drinking water at 0.75 mg L−1 (95% CI 0.74–0.77), with a maximum concentration of 1.80 mg L−1. CWSs reliant on groundwater (GW) had the next highest fluoride level in drinking water with a mean of 0.70 mg L−1 (95% CI 0.69–0.71) and a peak of 3.60 mg L−1, with the broader range between mean and maximum values indicating more variability (Table S3 and Fig. S1). CWSs using groundwater under the influence of surface water (GU) had the lowest mean fluoride concentration in drinking water, averaging 0.61 mg L−1 (95% CI: 0.57–0.65), with a maximum observed value of 1.32 mg L−1.
| Treated, finished water source | Mean | Med | Min | 25% | 75% | Max | p-Value |
|---|---|---|---|---|---|---|---|
| Groundwater under influence of surface water | 0.61 | 0.60 | 0.00 | 0.49 | 0.75 | 1.32 | <0.0001 |
| Groundwater | 0.70 | 0.68 | 0.00 | 0.50 | 0.84 | 3.60 | |
| Surface water | 0.75 | 0.73 | 0.04 | 0.63 | 0.83 | 1.80 |
These differences highlight the variability in fluoride levels depending on the water source, and appreciating these differences across sources will be important for public health protection and water management. Despite this variability, median concentrations across the state have been near the USPHS recommendation, with most testing results also within the WHO's guideline limit of 1.5 mg L−1.
4. Conclusions
This study provides a comprehensive analysis of fluoride concentrations in Iowa's groundwater and treated drinking water, highlighting the implications for public health and water management. The findings reveal significant variability in fluoride levels across different aquifers, regions and source water types, influenced by both natural and anthropogenic factors.34,48–50Fluoride concentrations in Iowa's groundwater range widely, with a median value of 0.35 mg L−1 and a maximum of 11.2 mg L−1 across the time span of nearly 90 years. In this study, 69% of all untreated groundwater samples were below the recommended fluoride level for public dental health (0.7 mg L−1), while only 0.4% were above the EPA's MCL of 4 mg L−1 and 7% were above the SMCL of 2 mg L−1. Similarly, 88% of all groundwater samples were below the WHO's guideline limit of 1.5 mg L−1. Higher fluoride levels were found predominantly in deeper wells and specific aquifers, such as the Cambrian-Ordovician and Mississippian, which are characterized by the presence of fluoride-bearing minerals and longer groundwater residence times. Historically, central and southeastern Iowa have reported higher levels of fluoride compared to the rest of the state.
The analysis of treated, finished drinking water reveals an average historical fluoride concentration of 0.87 mg L−1, indicating that CWS in Iowa have generally succeeded in implementing fluoridation practices aligned with public health goals. Historical trends show that fluoride concentrations in treated water peaked between 1980 and 1999, with an average of 0.98 mg L−1, reflecting the earlier recommendation to maintain fluoride levels near 1.0 mg L−1 to prevent dental caries.2,30,31 Since 2000, the mean concentration has declined slightly to 0.83 mg L−1, consistent with a broader shift in practice following the 2015 U.S. Public Health Service recommendation to lower the optimal fluoride concentration to 0.7 mg L−1.2,30,31 These changes in treated drinking water reflect deliberate efforts by water systems to maintain fluoride at levels considered both effective and safe—first targeting 1.0 mg L−1, and later adjusting downward in response to updated scientific guidance. Notably, 99% of fluoride measurements in treated water (n = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215 samples) since 2000 have remained below the EPA's Secondary Maximum Contaminant Level (SMCL) of 2.0 mg L−1. However, 285 samples since 2000 from 56 systems exceeded the SMCL, and one sample surpassed the EPA's Maximum Contaminant Level (MCL) of 4.0 mg L−1, raising concerns about the potential for dental fluorosis. Most of these exceedances were found in the Des Moines Lobe region of in north-central Iowa. This pattern clearly illustrates how regulatory guidance and water source management have shaped fluoride levels in drinking water over time.
215 samples) since 2000 have remained below the EPA's Secondary Maximum Contaminant Level (SMCL) of 2.0 mg L−1. However, 285 samples since 2000 from 56 systems exceeded the SMCL, and one sample surpassed the EPA's Maximum Contaminant Level (MCL) of 4.0 mg L−1, raising concerns about the potential for dental fluorosis. Most of these exceedances were found in the Des Moines Lobe region of in north-central Iowa. This pattern clearly illustrates how regulatory guidance and water source management have shaped fluoride levels in drinking water over time.
The study underscores the importance of understanding the regional variability in fluoride concentrations to inform water management strategies for both community water systems and private wells. Although, the study focused on data from CWSs, nearly 8% of Iowans, over 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people rely on private well water for their drinking water.73 The responsibility for delivering safe drinking water falls on property owners.74 Factors such as aquifer lithology, well depth, water chemistry, and anthropogenic sources contribute to the observed differences in fluoride levels. For instance, deeper wells in the Cambrian-Ordovician and Mississippian aquifers tend to have higher fluoride concentrations due to the presence of fluoride-bearing minerals and increased solubility at greater depths. Additionally, the use of superphosphate fertilizers, pesticides, and industrial waste can elevate fluoride levels in groundwater.
000 people rely on private well water for their drinking water.73 The responsibility for delivering safe drinking water falls on property owners.74 Factors such as aquifer lithology, well depth, water chemistry, and anthropogenic sources contribute to the observed differences in fluoride levels. For instance, deeper wells in the Cambrian-Ordovician and Mississippian aquifers tend to have higher fluoride concentrations due to the presence of fluoride-bearing minerals and increased solubility at greater depths. Additionally, the use of superphosphate fertilizers, pesticides, and industrial waste can elevate fluoride levels in groundwater.
While this study focused on fluoride concentrations in community water systems (CWSs), the findings may also have relevance for private well users, particularly in regions where naturally elevated fluoride levels have been observed. The results underscore the importance of regular testing for fluoride in areas with known geochemical vulnerability. Although blending water from different sources is a common practice among some CWSs to manage fluoride levels, this approach may be less feasible for private well owners. In such cases, routine testing can help identify potentially problematic sources of exposure. With appropriate government support, private well owners could explore practical mitigation strategies, such as accessing water from less vulnerable aquifers or installing point-of-use treatment systems. Given the health concerns associated with excessive fluoride intake, it is essential to balance the benefits of community water fluoridation with the potential risks. The study's findings highlight the need for regionally tailored water management practices. In areas with naturally high fluoride levels, blending or treatment may be necessary to reduce concentrations to safe levels, while regions with low fluoride levels may benefit from adjusted fluoridation to achieve optimal concentrations for dental health. The study also emphasizes the role of ongoing monitoring and data collection in managing fluoride levels in drinking water. By maintaining comprehensive databases and regularly updating water quality information, public health agencies and water suppliers can make informed decisions to protect the health of Iowa's residents. The use of advanced statistical and geospatial analysis techniques, as demonstrated in this study, can further enhance the understanding of water fluoride distributions and guide effective water management strategies.
In conclusion, this study provides valuable insights into the occurrence and distribution of fluoride in Iowa's groundwater and drinking water. The findings underscore the need for region-specific water management practices to optimize fluoride levels, ensuring the dental caries-preventive benefits of community water fluoridation, while minimizing the risks of excessive exposure. By leveraging the study's results, public health agencies, water suppliers, and residents can work together to achieve safe and effective fluoride concentrations in Iowa's drinking water.
Conflicts of interest
There are no conflicts of interest to declare.Data availability
The datasets analyzed for this study are available from the corresponding author on reasonable request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5va00189g.
Acknowledgements
Funding for this research was provided by the University of Iowa's Center for Health Effects of Environmental Contamination through its funding from the State of Iowa and the Iowa Department of Natural Resources. In memory of R. William (Bill) Field, mentor and friend.References
- Center for Disease Control and Prevention, Community Water Fluoridation, 2020, https://www.cdc.gov/fluoridation/about/index.html.
- U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation, U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries, Publ. Health Rep., 2015, 130(4), 318–331, DOI:10.1177/003335491513000408.
- World Health Organization, Preventing Disease through Healthy Environments, Inadequate or Excess Fluoride: A Major Public Health Concern, 2019, https://iris.who.int/handle/10665/329484.
- American Dental Association, Current Policies, 2021, https://www.ada.org/about/governance/current-policies#fluoridation.
- M. B. Clark, R. L. Slayton, Section on Oral Health, A. Segura, S. Boulter, M. B. Clark, R. Gereige, D. Krol, W. Mouradian, R. Quinonez and F. Ramos-Gomez, Fluoride use in caries prevention in the primary care setting, Pediatrics, 2014, 134(3), 626–633, DOI:10.1542/peds.2014-1699.
- Centers for Disease Control And Prevention, Achievements in public health, 1900-1999: fluoridation of drinking water to prevent dental caries, MMWR, 1999, vol. 48, pp. 933–940, https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4841a1.htm.
- R. Green, B. Lanphear, R. Hornung, D. Flora, E. A. Martinez-Mier, R. Neufeld, P. Ayotte, G. Muckle and C. Till, Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada, JAMA Pediatr., 2019, 173(10), 940–948, DOI:10.1001/jamapediatrics.2019.1729.
- S. K. Jha, V. K. Mishra, D. K. Sharma and T. Damodaran, Fluoride in the environment and its metabolism in humans, Rev. Environ. Contam. Toxicol., 2011, 211, 121–142, DOI:10.1007/978-1-4419-8011-3_4.
- S. Srivastava and S. J. S. Flora, Fluoride in drinking water and skeletal fluorosis: a review of the global impact, Curr. Environ. Health Rep., 2020, 7, 140–146, DOI:10.1007/s40572-020-00270-9.
- F. A. Dar and S. Kurella, Fluoride in drinking water: An in-depth analysis of its prevalence, health effects, advances in detection and treatment, Mater. Today: Proc., 2024, 102, 349–360, DOI:10.1016/j.matpr.2023.05.645.
- Y. Singh Solanki, M. Agarwal, A. B. Gupta, S. Gupta and P. Shukla, Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review, Sci. Total Environ., 2022, 807, 150601, DOI:10.1016/j.scitotenv.2021.150601.
- L. D. Goyal, D. K. Bakshi, J. K. Arora, A. Manchanda and P. Singh, Assessment of fluoride levels during pregnancy and its association with early adverse pregnancy outcomes, J. Fam. Med. Prim. Care, 2020, 9(6), 2693–2698, DOI:10.4103/jfmpc.jfmpc_213_20.
- C. Anirban, M. K. Adak, A. Mukherjee, P. Dhak, J. Khatun and D. Dhak, A critical review on geochemical and geological aspects of fluoride belts, fluorosis and natural materials and other sources for alternatives to fluoride exposure, J. Hydrol., 2019, 574, 333–359, DOI:10.1016/j.jhydrol.2019.04.033.
- C. Goodman, M. Hall, R. Green, R. Hornung, E. A. Martinez-Mier, B. Lanphear and C. Till, Maternal fluoride exposure, fertility and birth outcomes: the MIREC cohort, Environ. Adv., 2022, 7, 100135, DOI:10.1016/j.envadv.2021.100135.
- V. K. Moghaddam, M. Yousefi, A. Khosravi, M. Yaseri, A. H. Mahvi, M. Hadei, A. A. Mohammadi, Z. Robati and A. Mokammel, High concentration of fluoride can be increased risk of abortion, Biol. Trace Elem. Res., 2018, 185(2), 262–265 CrossRef CAS PubMed.
- M. Diouf, D. Cisse, C. M. M. Lo, M. Ly, D. Faye and O. Ndiaye, Pregnant women living in areas of endemic fluorosis in Senegal and low birthweight newborns: case-control study, Rev. Epidemiol. Sante Publique, 2012, 60(2), 103–108, DOI:10.1016/j.respe.2011.09.009.
- M. Gurumurthy Sastry, S. Mohanty, A. V. Bhongir, A. K. Mishra and P. Rao, Association of higher maternal serum fluoride with adverse fetal outcomes, Int. J. Med. Publ. Health, 2011, 1(2), 13, DOI:10.5530/ijmedph.2.2011.4.
- A. K. Susheela, R. Gupta and N. K. Mondal, Anaemia in adolescent girls: An intervention of diet editing and counselling, Natl. Med. J. India, 2016, 29(4) Search PubMed , https://fluoridealert.org/wp-content/uploads/susheela-2016.pdf.
- F. M. Kim, C. Hayes, S. L. Burgard, H.-D. Kim, R. N. Hoover, National Osteosarcoma Etiology Group, C. W. Douglass and D. Couper, A case-control study of fluoridation and osteosarcoma, J. Dent. Res., 2020, 99(10), 1157–1164, DOI:10.1177/0022034520919385.
- F. M. Kim, C. Hayes, P. L. Williams, G. M. Whitford, K. J. Joshipura, R. N. Hoover, C. W. Douglass and National Osteosarcoma Etiology Group, An assessment of bone fluoride and osteosarcoma, J. Dent. Res., 2011, 90(10), 1171–1176, DOI:10.1177/0022034511418828.
- B. Karen, R. G. Feltbower, R. C. Parslow, P. W. James, B. Gómez Pozo, C. Stiller, T. J. Vincent, P. Norman, P. A. Mckinney and M. F. Murphy, Is fluoride a risk factor for bone cancer? Small area analysis of osteosarcoma and Ewing sarcoma diagnosed among 0–49-year-olds in Great Britain, 1980–2005, Int. J. Epidemiol., 2014, 43(1), 224–234, DOI:10.1093/ije/dyt259.
- P. A. Natalie, T. S. Napier and J. F. Villanacci, Fluoride exposure in public drinking water and childhood and adolescent osteosarcoma in Texas, Cancer Causes Control, 2016, 27, 863–868, DOI:10.1007/s10552-016-0759-9.
- M. Levy and B.-S. Leclerc, Fluoride in drinking water and osteosarcoma incidence rates in the continental United States among children and adolescents, Cancer Epidemiol., 2012, 36(2), e83–e88, DOI:10.1016/j.canep.2011.11.008.
- M. M. Sheila, E. D. Vanable, M. H. Mcguire, J. A. Buckwalter and C. W. Douglass, Is there a link between fluoridated water and osteosarcoma?, J. Am. Dent. Assoc., 1991, 122(4), 38–45, DOI:10.14219/jada.archive.1991.0149.
- S. Ali, M. Baboo Agarwal, S. Verma, R. Islam, R. Kumar Deolia, S. Singh, J. Kumar, A. A. Mohammadi, M. Kumar Gupta and M. Fattahi, Variability of groundwater fluoride and its proportionate risk quantification via Monte Carlo simulation in rural and urban areas of Agra district, India, Sci. Rep., 2023, 13(1), 18971 CrossRef CAS PubMed.
- K. W. Taylor, S. E. Eftim, C. A. Sibrizzi, R. B. Blain, K. Magnuson, P. A. Hartman, A. A. Rooney and J. R. Bucher, Fluoride exposure and children’s IQ scores: a systematic review and meta-analysis, JAMA Pediatr., 2025, 179(3), 282–292, DOI:10.1001/jamapediatrics.2024.5542.
- S. M. Levy, Caution Needed in Interpreting the Evidence Base on Fluoride and IQ, JAMA Pediatr., 2025, 179(3), 231–234, DOI:10.1001/jamapediatrics.2024.5539.
- B. J. Matthew, J. Heathers and D. R. Grimes, Major Flaws in Taylor et al.'s (2025) Meta-analysis on Fluoride Exposure and Children's IQ Scores, 2025, DOI:10.31219/osf.io/zhm54_v3.
- Center for Disease Control and Prevention, Community Water Fluoridation – One of the 10 Greatest Public Health Achievements of the 20th Century, 2015, https://blogs.cdc.gov/pcd/2015/04/23/community-water-fluoridation-one-of-the-10-greatest-public-health-achievements-of-the-20th-century/.
- Center for Disease Control and Prevention, Timeline for Community Water Fluoridation, 2024, https://www.cdc.gov/fluoridation/timeline-for-community-water-fluoridation/index.html.
- L. K. Barker, K. K. Duchon, S. Lesaja, V. A. Robison and S. M. Presson, Adjusted fluoride concentrations and control ranges in 34 states: 2006–2010 and 2015, J.–Am. Water Works Assoc., 2017, 109(8), 13–25, DOI:10.5942/jawwa.2017.109.0095.
- United States Environmental Protection Agency, Secondary Drinking Water Standards: Guidance for Nuisance Chemicals, 2022, https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals.
- J. Podgorski and M. Berg, Global analysis and prediction of fluoride in groundwater, Nat. Commun., 2022, 13(1), 4232, DOI:10.1038/s41467-022-31940-x.
- P. B. Mcmahon, C. J. Brown, T. D. Johnson, K. Belitz and B. D. Lindsey, Fluoride occurrence in United States groundwater, Sci. Total Environ., 2020, 732, 139217, DOI:10.1016/j.scitotenv.2020.139217.
- T. J. Boehmer, Community water fluoridation levels to promote effectiveness and safety in oral health—United States, 2016–2021.MMWR Morbidity and Mortality Weekly Report, 2023, vol. 72, https://www.cdc.gov/mmwr/volumes/72/wr/mm7222a1.htm Search PubMed.
- W. Mike Edmunds and P. L. Smedley, Fluoride in natural waters, Essentials of Medical Geology: Revised Edition, 2012, p. 311–336, DOI:10.1007/978-94-007-4375-5_13.
- R. Fuge, Fluorine in the environment, a review of its sources and geochemistry, Appl. Geochem., 2019, 100, 393–406, DOI:10.1016/j.apgeochem.2018.12.016.
- K. Vivian, P. Bhattacharya, F. Mtalo, J. Mtamba and A. Ahmad, Fluoride occurrence in groundwater systems at global scale and status of defluoridation–state of the art, Groundw. Sustain. Dev., 2019, 9, 100223, DOI:10.1016/j.gsd.2019.100223.
- M. D. Gupta, V. Singh, P. Rajwanshi, M. Agarwal, K. Rai, S. Srivastava, R. Shrivastav and S. Dass, Groundwater quality assessment of Tehsil Kheragarh, Agra (India) with special reference to fluoride, Environ. Monit. Assess., 1999, 59, 275–285, DOI:10.1023/A:1006117604763.
- R. Tekle-Haimanot, Z. Melaku, H. Kloos, C. Reimann, W. Fantaye, L. Zerihun and K. Bjorvatn, The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley, Sci. Total Environ., 2006, 367(1), 182–190, DOI:10.1016/j.scitotenv.2005.11.003.
- B. Laura, M. G. Garcia, G. L. Bia, Y. V. Stupar, P. Le Coustumer and P. J. Depetris, Mechanisms of fluoride release in sediments of Argentina's central region, Sci. Total Environ., 2013, 443, 245–255, DOI:10.1016/j.scitotenv.2012.10.093.
- C. Wu, X. Wu, C. Qian and G. Zhu, Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China, Appl. Geochem., 2018, 98, 404–417, DOI:10.1016/j.apgeochem.2018.10.016.
- P. Li, X. He, Y. Li and G. Xiang, Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: a case study of Tongchuan, Northwest China, Expo. Health, 2019, 11(2), 95–107, DOI:10.1007/s12403-018-0278-x.
- O. Selinus, Essentials of Medical Geology: Revised Edition, 2013, DOI:10.1007/978-94-007-4375-5.
- Y. Mahmood, V. K. Moghaddam, S. M. Nasab, R. Nabizadeh, M. Hadei, A. Zarei, F. B. Asghari and A. A. Mohammadi, Northwest of Iran as an endemic area in terms of fluoride contamination: a case study on the correlation of fluoride concentration with physicochemical characteristics of groundwater sources in Showt, Desalination Water Treat., 2019, 155, 183–189 CrossRef.
- M. Vithanage and P. Bhattacharya, Fluoride in the environment: sources, distribution and defluoridation, Environ. Chem. Lett., 2015, 13, 131–147, DOI:10.1007/s10311-015-0496-4.
- Y. Wang, J. Li, T. Ma, X. Xie, Y. Deng and Y. Gan, Genesis of geogenic contaminated groundwater: As, F and I, Crit. Rev. Environ. Sci. Technol., 2021, 51(24), 2895–2933, DOI:10.1080/10643389.2020.1807452.
- G. M. Hannah, R. Van De Graaff, A. T. Mikkonen, B. O. Clarke, R. Dasika, C. J. Wallis and S. M. Reichman, Environmental and anthropogenic influences on ambient background concentrations of fluoride in soil, Environ. Pollut., 2018, 242, 1838–1849, DOI:10.1016/j.envpol.2018.07.083.
- B. Karthikeyan and E. Lakshmanan, Fluoride in groundwater: causes, implications and mitigation measures, Fluoride Properties, Applications and Environmental Management, 2011, vol. 1, pp. 111–136, DOI:10.1007/978-3-031-77247-4_11.
- E. Shaji, K. v. Sarath, M. Santosh, P. k. Krishnaprasad, B. k. Arya and M. S. Babu, Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures, Geosci. Front., 2024, 15(2), 101734 CrossRef CAS.
- W. C. Maurer, Fluoridation in Iowa, Iowa Heritage Illustrated, 2005, vol. 86, https://pubs.lib.uiowa.edu/ihi/article/id/1164/download/pdf/.
- Iowa Health and Human Services, Community Water Fluoridation, 2024, https://hhs.iowa.gov/programs/programs-and-services/dental-and-oral-health/fluoridation.
- Iowa Department of Health and Human Services, Public Water Use Data, 2024, https://hhs.iowa.gov/data/environment/drinking-water/use.
- Iowa Department of Health and Human Services, Fluoridation FAQs & Resources, 2025, https://hhs.iowa.gov/health-prevention/dental-oral-health/community-water-fluoridation/faqs-resources.
- L. A. Desimone, Quality of water from domestic wells in principal aquifers of the United States, 1991–2004, US Geological Survey, 2009, p. , p. 139, https://pubs.usgs.gov/circ/circ1332/ Search PubMed.
- A. D. Leslie, P. B. Mcmahon and M. R. Rosen, The Quality of Our Nation's Waters: Water Quality in Principal Aquifers of the United States, 1991–2010, 2015, DOI:10.3133/cir1360.
- Iowa Department of Natural Resources, AQuIA, 2022, https://programs.iowadnr.gov/aquia/ Search PubMed.
- National Water Quality Monitoring Council, Water Quality Portal, 2022, https://www.waterqualitydata.us/ Search PubMed.
- Iowa Geospatial Data, Historic Well Water Quality, Iowa, 1905–2019, 2020, https://geodata.iowa.gov/documents/b4fe83dc08984c08844cb5577ad1b9e9/about Search PubMed.
- Iowa Department of Natural Resources, Drinking Water Portal - Summary of Public Water Supply (PWS) Information, 2025, https://programs.iowadnr.gov/iowadrinkingwater/Summary/PwsSummaryCharts.
- United States Environmental Protection Agency, Information about Public Water Systems, 2024, https://www.epa.gov/dwreginfo/information-about-public-water-systems.
- University of Iowa Center for Health Effects of Environmental Contamination, Environmental Databases, 2025, https://cheec.uiowa.edu/data/environmental-databases Search PubMed.
- Esri, How Kriging Works, 2025, https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-analyst/how-kriging-works.htm.
- United States Geological Survey, 3H/3He Dating Background, 2016, https://water.usgs.gov/lab/3h3he/background/.
- United States Geological Survey, Groundwater age-dating simplified, 2019, https://www.usgs.gov/news/groundwater-age-dating-simplified.
- J. H. Oh, D.-C. Koh, H.-S. Seo, N. Koh and S. W. Kim, Evaluating major element hydrogeochemistry and fluoride occurrence in groundwater of crystalline bedrock aquifers and associated controlling factors of Eumseong basin area, South Korea, Environ. Geochem. Health, 2025, 47(2), 43 CrossRef CAS.
- Iowa Geological Survey, Groundwater Modeling, 2025, https://iowageologicalsurvey.uiowa.edu/research/groundwater-modeling.
- P. G. Olcott, W. J. Danchuk, C. M. Bebow, C. J. Wipperfurth and G. D. Latzke, Ground Water Atlas of the United States: Iowa, Michigan, Minnesota, and Wisconsin. Segment 9, US Geological Survey, 1992 Search PubMed.
- V. Mariappan Santhi, D. Periasamy, M. Perumal, P. M. Sekar, V. Varatharajan, D. Aravind, K. Senthilkumar, S. T. Kumaran, S. Ali and S. Sankar, The Global Challenge of Fluoride Contamination: A Comprehensive Review of Removal Processes and Implications for Human Health and Ecosystems, Sustainability, 2024, 16(24), 11056, DOI:10.3390/su162411056.
- S. Ahmad, R. Singh, T. Arfin and K. Neeti, Fluoride contamination, consequences and removal techniques in water: a review, Environ. Sci. Adv., 2022, 1(5), 620–661, 10.1039/D1VA00039J.
- F. R. Twenter and R. W. Coble, The Water Story in Central Iowa, Iowa Geological Survey Water Atlas, 1965, https://www.iihr.uiowa.edu/igs/publications/uploads/WA-01.pdf Search PubMed.
- Keith E. Schilling, R. R. Anderson, D. W. Peate, J. A. Dorale and E. C. Alexander, Mining unique soft old water within the Manson Impact Structure, Iowa (USA), Hydrogeol. J., 2015, 23(1), 95, DOI:10.1007/s10040-014-1193-2.
- A. Staudt, Rural Drinking Water Survey Shows Significant Nitrate Risk for Many Iowans, 2022, https://www.extension.iastate.edu/news/rural-drinking-water-survey-shows-significant-nitrate-risk-many-iowans.
- United States Environmental Protection Agency, Private Drinking Water Wells, 2025, https://www.epa.gov/privatewells.
| This journal is © The Royal Society of Chemistry 2026 |