Open Access Article
Open Access ArticleMorphochemical information on microplastic fibers found in edible tissue of local commercial fishes from the South China Sea and the Straits of Malacca for potential human consumption†
Yusof Shuaib
Ibrahim
abc,
Nur Izzati
Abd Razak
ab,
Nur Sakinah
Roslan
ab,
Ku Mohd Kalkausar Ku
Yusof
ab,
Ahmad Ammarluddin
Mohd Ali
ab,
Nor Fatihah
Omar
b,
Chingakham
Chinglenthoiba
d,
Nurul Najihah
Mohamad
e and
Sabiqah Tuan
Anuar
 *ab
*ab
aMicroplastic Research Interest Group (MRIG), Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia. E-mail: sabiqahanuar@umt.edu.my
bFaculty of Science and Marine Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia
cInstitute of Oceanography and Environment (INOS), Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia
dCentre for Research Impact & Outcome, Chitkara University, Punjab, India
eFisheries Research Institute, Batu Maung, Penang, Malaysia
First published on 14th May 2025
Abstract
Plastic debris is widely dispersed in the marine environment, posing a growing environmental concern due to microplastic pollution. Microplastics (less than 5 mm in size) form through plastic fragmentation and include fibers, fragments, films, pellets and foams. These particles may harm marine ecosystems, as ingestion of microplastics can disturb marine life. This study investigates and characterizes the presence of microplastics in commercial fish tissues from the South China Sea (SCS) and the Straits of Malacca (SOM). A total of 80 individuals from four species (Rastrelliger kanagurta – Indian mackerel, Atule mate – yellowtail scad, Decapterus punctatus – round scad, and Pampus argenteus – silver pomfret) were examined. The results showed a high abundance of fiber-shaped microplastic in all tissues samples (mean 8.95 particles per ind.). The presence of microplastics in samples collected from the SCS (10.28 particles per ind.) was higher than in those from the SOM (7.63 particles per ind.). Microplastic was found in higher concentrations in pelagic fish than in benthic fish. The dominant color of the microplastic was black, constituting 39% (SCS) and 45% (SOM). The smallest average size of microplastics in the fish tissues was 0.04 mm, which is from South China Sea fish. Scanning electron microscopy (SEM) images indicate different surface characteristics of the microplastics as a result of environmental exposure. Microplastics were associated with polyethylene (PE), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polycarbonate (PC), polyamide (PA), polypropylene (PP), polyester, rayon and poly(vinyl methyl ketone). The estimated daily intakes (EDIs) of microplastic through fish consumption are between 1.129 and 1.582 microplastics per capita. Overall, the data show that microplastics are widely distributed in commercial marine fish from Malaysian waters, which could contribute to human exposure through fish intake.
Environmental significanceThis article reports the assessment of microplastic presence in tissues of commercial fishes from the South China Sea and the Straits of Malacca – both important complex water regions and water resources known for port areas, shipping channels, and fishery activities. While existing studies suggest that microplastics predominantly reside in the gastrointestinal tracts of fish, the identification of microplastics in fish flesh tissues, which are consumed by humans, reveals potential pathways for exposure. The elucidation of Estimated Daily Intake (EDI) provides useful information on microplastics and their potential risk in humans and could serve as a reference for other species and ecological implications. This is in line with the UNSDG’s and UN Ocean Decade’s call for an extensive monitoring program pertaining to marine plastics and microplastics. |
1. Introduction
Plastic is a synthetic or semisynthetic material produced from organic polymers that is industrially made from petrochemicals and used in a wide range of applications.1 Small pieces of plastic that are less than 5 mm in size are called microplastics.2,3 Their spread in marine environments has raised global alarm, with recent estimates suggesting that over 14 million tons of microplastics reside on the ocean floor.4 This presence of plastic debris in the ocean might have negative impacts on marine life, such as causing physical interference and being ingested by organisms, while the small plastic particles can affect marine organisms through respiration, absorption, gastric obstruction, physiological effects, chemical transfer, or trophic transfer, as well as causing the threat of economic issues, especially for fisheries.5 These particles vary in shape, size, and density, influencing their distribution across marine environments: buoyant fibers accumulate in surface waters, while denser fragments sink to the benthic zone.6 This vertical dispersion intensifies ecological risks, as microplastics infiltrate all trophic levels. For instance, filter feeders like zooplankton ingest nanoplastics,7 while larger fish consume microplastics directly or through contaminated prey,8 leading to bioaccumulation of toxic additives (e.g., phthalates, flame retardants) and adsorbed pollutants (e.g., heavy metals, Polychlorinated Biphenyls, PCBs).5 To quantify human exposure to microplastics through seafood consumption, researchers employ the Estimated Daily Intake (EDI) metric, which calculates the average daily ingestion of contaminants (e.g., microplastics) per kilogram of body weight.9,10In an aquatic environment, microplastics are extremely effective at adsorbing persistent organic pollutants that are already present in the water. When a fish swallows a microplastic particle, it may also swallow the toxicant, resulting in a direct harm to fish.11 However, the risk of consuming contaminated microplastics may not be greater than the risk of consuming contaminated natural prey, because the concentration of the same toxicants associated with microplastics is much lower than the concentration already absorbed and/or adsorbed by the prey item. Furthermore, ingestion of microplastics is unlikely to increase exposure (and thus risk) to fish, at least in the case of hydrophobic organic toxicants associated with microplastics. This is based on hydrophobic-organic-chemical transfer rates from microplastic to organism, simulated desorption rates in artificial gut fluids, and bioaccumulation potential.12 However, other chemicals associated with microplastics (such as metals and persistent organic pollutants) may have an additive effect on fish ingestion and thus increase the risk.
Seafood, particularly fish, is a primary source of protein for the Malaysian population, and its consumption plays a vital and important part of the diet, especially for the coastal communities where fish also represent important sources of income and economic activity, particularly with Malaysia being regarded as a maritime country with a total 4675 km length of coastline (ranked 29th in the world). However, fish consumption has been suspected as one of major pathways for human exposure to microplastics, as the presence of plastic debris in the ocean might cause bioaccumulation and biomagnification of microplastics in aquatic organisms including fish. Moreover, studies have shown that the widespread marine plastic and microplastic pollution has led to the ingestion of microplastics by various marine species, with potential of translocation of microplastics from the digestive system into other tissues.13 Marine organisms, especially fish, have been reported to contain microplastics in their tissues and gastrointestinal tract, and are ingested through the consumption of contaminated food and primary sources.14–16 Therefore, consumption of seafood, particularly fish, may serve as an important source of microplastic exposure in coastal nations such as Malaysia. Hence, this study significantly investigates and characterizes microplastic presence in the edible tissues of commercial fishes collected from South China Sea (SCS) and Straits of Malacca (SOM) waters using physical and chemical analysis. This includes the elucidation of Estimated Dietary Intake (EDI) of microplastics from selected species. The selected species, namely Indian mackerel (Rastrelliger kanagurta), yellowtail scad (Atule mate), round scad (Decapterus punctatus), and silver pomfret (Pampus argenteus), were chosen based on their high abundance and are the commercial fishes that are commonly consumed in Malaysia.17 This study will show the quantity and morphochemical characteristics of microplastic present in the edible tissue of fish and will provide insights into the potential risk and impact on human health through seafood consumption.
2. Methods
2.1. Sample collections
A total of 80 individual fish from four species of commercial fish (fish per species, n = 10) for human consumption purposes were locally collected from either a fish jetty or fresh fish market. 40 individuals each were collected from the fresh fish market in Jeti Nelayan Pantai Siring, Merlimau (Straits of Malacca) and the local market at Tok Jembal beach in Terengganu (South China Sea). Each of the species collected from these Malaysian waters was chosen by taking into account their weight and size (Table 1). The selection of species is based on their abundance and they are the commercial fishes that are commonly consumed by lots of people in Malaysia.17 Fish were bought and transferred to the Microplastic Research Interest Group (MRIG) laboratory at Universiti Malaysia Terengganu (UMT).| Sampling area | Common name | Species | Habitat | Weight (g) | Length (cm) | Number of individuals | Microplastic abundance (particles per ind.) | Average size of microplastics (mm) |
|---|---|---|---|---|---|---|---|---|
| South China Sea (SCS) Pantai Tok Jembal, Terengganu | Silver pomfret | Pampus argentus | Benthic | 124 (±21.3) | 20.72 (±1.2) | 10 | 8.8 | 0.056 |
| Yellowtail scad | Atule mate | Pelagic | 138 (±26.0) | 22.23 (±1.5) | 10 | 10.8 | 0.074 | |
| Indian mackerel | Rastrelliger kanagurta | Epipelagic neritic | 142 (±28.4) | 21.63 (±0.8) | 10 | 11.2 | 0.112 | |
| Round scad | Decapterus punctatus | Neritic | 131 (±22.2) | 20.21 (±0.6) | 10 | 10.3 | 0.086 | |
| Straits of Malacca (SOM) Pantai Siring Melaka | Silver pomfret | Pampus argentus | Benthic | 87 (±10.0) | 21.85 (±3.0) | 10 | 6.7 | 0.047 |
| Yellowtail scad | Atule mate | Pelagic | 114 (±25.0) | 22.38 (±0.9) | 10 | 12.7 | 0.076 | |
| Indian mackerel | Rastrelliger kanagurta | Epipelagic neritic | 117 (±5.0) | 22.38 (±2.2) | 10 | 6.2 | 0.063 | |
| Round scad | Decapterus punctatus | Neritic | 136 (±25.0) | 22.85 (±1.8) | 10 | 4.9 | 0.109 |
2.2. Extraction
Muscle tissues were collected by cutting and removing them from the bones and skins of the samples. The dissection procedures of the samples were carried out by using a knife and metal scalpel. The minced fish tissue samples (approximately 5–15 g) were weighed and then digested by adding 250 mL of 10 M potassium hydroxide (KOH, Sigma Aldrich, Malaysia) to each 500 mL beaker. The samples were subsequently incubated at 40 °C with continuous stirring at 60 rpm for 48 hours until complete digestion, following a previous study.18–20 Digestates were filtered through 0.45 μm Whatman cellulose nitrate filter membranes using a vacuum pump (Gast vacuum pump, DOA-P504-BN) with a filter funnel connected. The filter membranes containing the samples were then dried at room temperature in a glass dissector and stored for the visual identification of particles.2.3. Visual sorting of microplastics and physical analysis
Large-size microplastics on filter membranes were observed using the naked eye for five minutes to 10 minutes before being analyzed using a binocular stereomicroscope (Wincom Binocular S-30, Olympus, Japan) at various magnifications (10×–80×) connected with a DinoEye digital eyepiece for more clear observation of small-size microplastics. The microplastics were photographed and sorted based on morphological characteristics, which were shape and color, then transferred into glass vials labelled according to the specific shape and color. During the sorting process, a hot-needle test was carried out in order to distinguish between plastic materials and non-plastic materials according to physical analysis guidelines.21 The amount of microplastics sorted was recorded in the data sheet prepared. The data then was tabulated, and a graph was plotted accordingly. All data underwent thorough statistical analysis and interpretation using R-Studio software (version 2024.04.2, R Development, USA) to ensure reliability and accuracy. Additionally, a detailed image of the surface morphology of the microplastics was analyzed using a scanning electron microscope (SEM JEOL JSM-636 OLA, JEOL Ltd, Tokyo, Japan). In brief, the microplastic samples were mounted on aluminum stubs, coated with a thin layer of gold, and then visualized at various magnifications to evaluate the surface topography at 16 kV emission.2.4. Chemical analysis via micro-FTIR and pyrolysis-GC/MS
Fourier-transform infrared microscopic spectroscopy (Thermo Nicolet iN10 MX FTIR Microscope, ThermoFisher Scientific) using a MCT detector was used to analyze individual microplastic particles (>500 μm in size) in each sample for polymer identification in the reflectance mode to minimize the interference and instrumental background. This analysis was conducted in a spectral range of 4000–600 cm−1 with 4 cm−1 resolution and a rate of 64 scans for every sample. Each sample was prepared on a silicon membrane (diameter 1 μm, size 10 mm × 10 mm) before it was filtered. The membrane filter was placed on the μ-FTIR microscope stage for measurement. Spectroscopic analysis was performed based on OMNIC Picta Particle Wizard (ThermoFisher Scientific) in order to locate the micro-particles and determine the distribution of microplastics across the membrane filter. The instrument automatically collects the spectrum for each particle, and searches the data against the polymer spectral library.Meanwhile, for particle sizes less than 500 μm, pyrolysis-GC/MS was carried out according to the previous work done,22 with some optimization carried out for the calibration curves of the polymer standard. In brief, the microplastic sample was filtered using an aluminum oxide mesh filter and underwent derivatization using 4 mg CaCO3 and 5 mL tetramethylammonium hydroxide (TMAH) solution (25% in methanol) prior to the analysis. The temperature program for the pyrolysis furnace was set up at 400 °C and maintained for 10 min. For the GC/MS analysis, the split mode was used at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the initial GC temperature was set at 40 °C (2 min), before it was increased at 20 °C min−1 to 280 °C (10 min), followed by another increment at 40 °C min−1 to 320 °C (15 min). The MS was programmed in a full scan mode in the range of 29 to 350 amu, with a scan rate of 4 scans per s with similar main characteristic ions. The external calibration curve was prepared using a series of polymer standards, including polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polycarbonate (PC), polymethylmethylacrelate (PMMA), and polyamide (PA).
1, and the initial GC temperature was set at 40 °C (2 min), before it was increased at 20 °C min−1 to 280 °C (10 min), followed by another increment at 40 °C min−1 to 320 °C (15 min). The MS was programmed in a full scan mode in the range of 29 to 350 amu, with a scan rate of 4 scans per s with similar main characteristic ions. The external calibration curve was prepared using a series of polymer standards, including polyethylene (PE), polypropylene (PP), polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), polycarbonate (PC), polymethylmethylacrelate (PMMA), and polyamide (PA).
2.5. Contamination prevention and quality assurance (QA)
To prevent cross-contamination, all sample handling was conducted in a closed clean room at the MRIG laboratory during sample processing and analysis. Prior to use, all glassware and apparatus was rinsed with filtered deionized water, and the workspace was sterilized with 80% ethanol. Only glass and stainless-steel apparatus is used during sample preparation and analysis. A sealed glass box was used to prevent airborne contamination during physical sorting. A procedural blank was included throughout the process, from sampling activity to sample identification. These controls were systematically validated using microscopic observation and chemical analysis. To minimize fiber contamination, only white cotton lab coats were worn.2.6. Estimated daily and annual human intake of microplastic through fish consumption
Ingestion of microplastics through fish consumption can be calculated using the widely accepted intake index approach,9,10,23 ensuring consistency with previous research. Estimated daily intake (EDI) measures human exposure to microplastics per day by using the average number of microplastics per g (items per g) in fish muscle (C), as quantified in this study, to provide realistic exposure estimates. Meanwhile, the average daily fish consumption (IR) for Malaysians,24 who reported a daily per capita consumption of 122 g of fish for a Malaysian adult with an average body weight (BW) of 62 kg based on data collected from 2675 respondents.17 The EDI and the corresponding annual intake index (EAI) are determined using the following equations: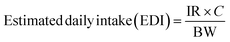 | (1) |
| Estimated annual intake (EAI) = EDI × 365 days | (2) |
Following established environmental health risk assessments, a range of EDI values was presented rather than a single estimate. Additionally, the calculated EDI values were also compared with published microplastic studies to ensure alignment with existing data. Many published studies of calculated EDI through fish have been reviewed worldwide, providing a robust basis for assessing potential human exposure to microplastics.10,25
3. Results and discussion
3.1. Abundance of microplastics
Overall, 80 fish were analyzed in this study, with 40 individuals each from the SCS and SOM (Table 1). The total microplastic count in both sampling areas was 716 microplastics particles (8.95 particles per individual), with 411 microplastic particles from the SCS (10.28 particles per ind.) and 305 microplastic particles from the SOM (7.63 particles per ind.). For the SCS, Indian mackerel (Rastrelliger kanagurta) showed the highest abundance of microplastics found in the tissues at 11.2 particles per ind.; meanwhile, yellowtail scad (Atule mate) presented the most prevalent microplastic abundance from the SOM (12.7 particles per ind.). Fig. 1 shows that for the SCS, Indian mackerel ingested the most microplastics, followed by yellowtail scad, round scad and silver pomfret. Among SOM samples, yellowtail scad exhibited the largest amount of microplastics, then silver pomfret, Indian mackerel and round scad (49 particles). In the term of gram-basis, the ranges of microplastic particles found in the fish tissues are 0.54–0.71 particles per g in SCS samples, and 0.43–0.91 particles per g in SOM samples. A normality test was done, and the data was normally distributed (p > 0.5). Other than that, a two-way ANOVA was conducted to examine the effects of fish species and water region on microplastic abundance. The results show that neither fish species nor water region had a statistically significant effect on the abundance of microplastics in fish. The p-values for both factors were greater than 0.05, indicating that there is no statistically significant difference in microplastic abundance between different fish species or water regions. This suggests that microplastic contamination in fish is relatively consistent across species and locations within the given abundance.The different sizes for each fish species could be the reason for the variation in microplastics found in the fish tissue from both study areas. In this study, the size of fish collected from the South China Sea waters is bigger than the ones from the Straits of Malacca. In general, fish length and body condition were the most important factors influencing microplastic ingestion,14 whereby the presence of microplastics in gut contents was found to be more closely linked to fish length rather than prey type. In this study, we found that the fish specimens with better body condition (body mass and size/length measurement), for instance as in samples from the SOM (size range: 21.85 ± 3.0–22.85 ± 1.8 cm), had lower levels of microplastics, compared to the samples from the SCS (size range: 20.21 ± 0.06–22.23 ± 1.5 cm), indicating that better body condition was beneficial. Because the condition factor is used to assess a fish’s overall health, it stands to reason that better body conditions would result in lower ingestions. Table 1 shows that microplastic was found in higher concentrations in pelagic fish than in benthic fish. However, no conclusive results in terms of trophic variables and feeding habits have been discovered in our findings, since we did not infer a complete taxonomic description of fish diet. Additionally, recent studies of microplastic presence in aquatic organisms, particularly in fishes, have mostly focussed on the digestive tract, whereas little investigation has been carried out focusing on the flesh tissues of the fish. The occurrence of microplastic in marine organisms, especially fishes, might be due to smaller species being prey for bigger species. For instance, one of the types of fish sampled in this study, the Indian mackerel (Rastrelliger kanagurta), feeds on macroplankton and zooplankton as its main food source, which have been reported to ingest microplastics.7,26 Moreover, microplastics have a proven ability to be translocated into tissues of marine organisms, such as fishes, from the digestive system of the marine organisms.8 Given the fact that all fish species selected in this present study are commercial fishes that are commonly consumed by lots of people in Malaysia,17 and surely often consumed as a whole, there might be a possibility for the translocation of a significant amount of microplastics into the bodies of the consumers. These issues have also raised concerns about the safety of seafood products. Previous studies in the region have investigated the extent of microplastic ingestion in fish, highlighting the influence of environmental conditions, pollution sources, and species-specific feeding behaviors (Table 2). High and moderate microplastic levels were recorded in fish (either in tissue, the GIT or both) from the Pulau Rambut, Jakarta Bay (7–26 particles per ind.), Mersing and Pantai Remis, Johor (7.08 particles per ind.), Skudai River, Johor (1.08 ± 1.77 items per ind.), and from various places across the South China Sea and Straits of Malacca (1.7–6.7 items per ind.), showing similar trends to that found in the study.
| Study site | Sample matrix | Abundance of microplastics | Shape of microplastics | Colour of microplastics | Size of microplastics | Type of polymer | References |
|---|---|---|---|---|---|---|---|
| Pulau Rambut, Jakarta bay | Fish | 110 particles (7–26 particles per individual) | Films, fibers | Transparent (57.35%), blue (26.4%), black (10%), red (6.4%) | 0.10–1.0 mm | N/A | 27 |
| Jember Regency, Indonesia | Fish | N/A | Fibers, fragments, granules, filaments | N/A | N/A | N/A | 28 |
| Various locations across the SCS and SOM | Fish | 673 particles (1.7–6.7 particles per individual) | Fibers (93.02%), fragments (6.69%), films (0.3%) | Black (69.24%), blue (16.34%), whitish (8.92%) | <0.2–2 mm | Rayon, PA, PE, PET, PVC, PU, PC, PTFE, PMMA | 29 |
| Tanjung Penyabung, Mersing and Pantai Remis, Johor | Fish | 1118 particles (7.08 particles per individuals) | Fibres (80.2%), fragments (17.7%) filaments (3.1%). | Blue (31.9%), black (31.1%), red (19.5%), grey (12.3%), and others (5.2%) | 0.06–0.1 mm (4%), 0.1–0.5 mm (36.3%), 0.5–1.0 mm (31.9%), 1.0–5.0 mm (27.7%) | PE PP, ABS, PS, PET | 30 |
| Northwest Peninsular Malaysia | Fish | 5.0–6.5 items per individual | Fragments (49.5%), fibres (41.9%), pellets (7.6%) and films (0.9%) | N/A | N/A | N/A | 31 |
| Skudai River, Johor | Fish | 1.08 ± 1.77 particles per individual | Film (43.28%), fragments (28.36%), fibers (20.9%) and foam (2.99%) | Blue (42.19%) followed by white (26.56%), red (21.88%), black (7.81%) and yellow (1.56%) | 0.05–5.0 mm | N/A | 32 |
| Pantai Tok Jembal (SCS) and Pantai Siring (SOM) | Fish | 716 particles (8.95 particles per individual) | Fibers (99.65%), foams (0.35%) | Black (45%), transparent (26%), red (16%), brown (6%), and others (7%). | 0.05–0.1 mm (4.8%), 0.1–0.5 mm (46.4%), 0.5–1.0 mm (40.7%), 1.0–5.0 mm (8.1%) | PE, PP, PVC, PA, PET, PC | This study |
3.2. Characteristics of microplastics
The size distribution of microplastics in fish tissues from the study area showed the highest percentage in smaller-sized microplastics (<0.50 mm, 52%). In the size range of 0.05–0.10 mm, the highest concentrations were found in round scad (39%) and silver pomfret (33%) samples from the SOM, compared to Indian mackerel (47%) and yellowtail scad (27%) from the SCS, as depicted in Fig. 2. Meanwhile, microplastics ranging from 0.10–0.50 mm were most prevalent in round scad (42%) in the SOM, compared to Indian mackerel (32%) from the SCS. Larger microplastics (>0.50 mm) were predominantly found in yellowtail scad and round scad from the SOM, as well as in round scad and silver pomfret from the SCS. Indian mackerel, an epipelagic and neritic species, inhabits areas where the surface water temperature is at least 17 °C. This species forms schools based on size, and adults primarily feed on macroplankton and zooplankton, such as larval shrimp and fish, as reported by the Food and Agriculture Organization of the United Nations (FAO) in the work of Fischer & Whitehead (1974).33 Therefore, it is not surprising that this species dominated the smaller size range of microplastics in the colder SCS waters, taking them in as a food either from smaller prey or directly from the surface water. Similar microplastic size ranges were previously reported in waters offshore of Terengganu and various places across the SCS.29,34 The high incidence of microplastics in the tissues of round scad in the SOM area is likely attributed to its ecological behaviour as a neritic species. While commonly inhabiting the seabed in shallow waters, round scad also frequents pelagic zones near the surface, increasing its exposure to microplastic-contaminated environments. The prevalence of smaller microplastics is concerning, as these tiny particles are more easily ingested and can accumulate in fish tissues, potentially affecting their health and posing risks to human consumers.35 | ||
| Fig. 2 Percentage size of microplastics based on species: inner circle (South China Sea) vs. outer circle (Strait of Malacca). | ||
Fig. 3 illustrates the predominant colors of microplastics found in the fish tissue. In the SCS, the most abundant color is black at 39%, followed by transparent (36%), red (9%), brown (5%), and others (11%). Similarly, for fish from the SOM, black is also the most common at 45%, followed by transparent (26%), red (16%), brown (6%), and others (7%). “Other” refers to varieties of colors, such as blue, green, pink, yellow, and purple, that account for a minor portion of the microplastic abundances. Respectively, fibers (Fig. 4) were the most commonly found type of microplastics in all sampled commercial fish from the study, accounting for 99.65%, followed by foam at only 0.35% (Table 2). Most fibers identified in this study were black in both samples, although transparent was also dominant, as discussed earlier. Microplastics within the size range of 0.5–5.0 mm appeared primarily black, and for the smaller size category of 0.05–0.1 mm, black was also predominant. It is important to note that no fragments, films, or pellets were found in the tissues from either sampling areas, suggesting the improbability of these shapes of plastics translocating into the flesh of the fish. As previously mentioned in Table 1, the fish from the South China Sea contained the highest abundance of microplastic particles in their tissues. This is likely due to tourism and anthropogenic activities in the waters off Terengganu, which feature beautiful beaches that attract tourists annually, as suggested previously.36 Uncontrolled littering may result in plastic waste being carried by marine water waves during high and low tides, which is then ingested by fish. Additionally, effluents from heavy manufacturing industries present in these waters, such as the textile industry, as well as fishing activities using synthetic gear and marine ropes, contribute to the high number of fiber microplastics, as fibers are a primary component in textile production. In the Straits of Malacca, the sampling site is related to a major international waterway in Malaysia. As a result, this area is expected to have a high concentration of plastic debris floating in the marine water.37 Fiber microplastics, which can be found ubiquitously as marine contaminants, statistically constitute up to 90% of the global microplastic concentration.16 These microplastics are persistent and known for their slow degradability and high durability; hence, they do not degrade easily. Similarly, fibers emerged as the most dominant (93.02%) form from various places in SCS and SOM, 80.2% in Mersing and Pantai Remis, Johor, and 41.9% in Northwest Peninsular Malaysia (Table 2). This trend is consistent with findings from other global studies, where synthetic fibers often shed from clothing, fishing nets, and ropes dominate microplastic pollution.38 However, other studies reported the prevalence of fragments and films among the microplastics in fish, particularly in the GIT, for instance in the samples collected in Northwest Peninsular Malaysia,31 Skudai,32 and Pulau Rambut, Jakarta Bay.27 These patterns suggest that microplastic sources range from synthetic textiles to degraded plastic packaging and industrial waste.39
 | ||
| Fig. 3 Percentages of microplastic counts according to color: inner circle (South China Sea) vs. outer circle (Strait of Malacca). | ||
 | ||
| Fig. 4 Microscopy images of microplastics found in fish species, namely fibers with different colours: (a) blue, (b) red, (c) black and (d) transparent. | ||
This study investigates the occurrence and distribution of microplastics in four commercially significant fish species—Atule mate (Selaroides leptolepis), Indian mackerel (Rastrelliger kanagurta), silver pomfret (Pampus argenteus), and round scad (Decapterus punctatus)—collected from two major marine ecosystems, the Strait of Malacca (SOM) and the South China Sea (SCS). The findings reveal a predominance of microplastics within the 0.10–0.5 mm size range, with an average abundance of 39.63 particles per fish across all species. This suggests that smaller microplastics are more bioavailable and susceptible to ingestion due to their high environmental mobility and prolonged suspension in the water column. In contrast, larger microplastics (1.0–5.0 mm) were significantly less prevalent (mean = 6.88 particles per fish), likely due to selective egestion, size-related ingestion constraints, or differential deposition dynamics. These findings align with previous studies indicating that smaller microplastics exhibit higher environmental persistence and an increased potential for trophic transfer, thereby amplifying their ecological risks. A comparative analysis of microplastic ingestion between the two marine regions, conducted using Welch’s t-test, revealed no statistically significant differences (p > 0.05) in total microplastic ingestion between fish sampled from the Strait of Malacca and those from the South China Sea. This suggests that regional variability does not substantially influence microplastic ingestion, likely due to the role of large-scale hydrodynamic processes, including oceanic currents and water mixing, in facilitating the widespread dispersion of microplastics. However, a key limitation of this study is the absence of direct measurements of oceanographic parameters, such as seawater conductivity, pH, temperature, gradient salinity, total dissolved solids, total suspended solids, and deposition velocity to substantiate this hypothesis, since this study was initiated as a preliminary method in order to understand the microplastic distribution in the SOM and SCS.36,40,41 Despite this constraint, the size distribution of ingested microplastics provides valuable insight into their environmental behaviour, highlighting that finer microplastic fibers remain suspended for longer durations and pose a greater risk of ingestion and bioaccumulation than their larger counterparts.26
Inter-species variation in microplastic ingestion was assessed using one-way ANOVA, which identified a statistically significant difference in the ingestion of 0.5–1.0 mm microplastics among species (p = 0.017). Post hoc Tukey’s honestly significant difference (HSD) analysis revealed that silver pomfret (SOM) exhibited significantly lower ingestion of 0.5–1.0 mm microplastics compared to round scad (SCS) (p < 0.05). This discrepancy is likely attributable to species-specific feeding strategies and habitat preferences, as pelagic filter feeders such as round scad demonstrate a higher propensity for ingesting suspended microplastics compared to benthic-feeding species such as silver pomfret. The absence of significant differences in the ingestion of other microplastic size fractions (p > 0.05) suggests a relatively uniform exposure across species, reinforcing the notion that microplastics are a ubiquitous contaminant within the marine environment.
The dominance of smaller-sized microplastics (0.10–0.5 mm) in fish gastrointestinal tracts raises significant concerns regarding their potential for trophic transfer, whereby microplastics ingested by lower trophic organisms are progressively transferred to higher-order consumers, including humans. Due to their small size, these microplastics exhibit a greater likelihood of systemic translocation, wherein particles pass through biological membranes and accumulate in tissues, posing risks of oxidative stress, inflammatory responses, and endocrine disruption.35 Furthermore, smaller microplastics, particularly fibers, serve as effective vectors for persistent organic pollutants (POPs), heavy metals, and hydrophobic contaminants, which may increase their toxicological impact upon ingestion.42 The findings of this study underscore the disproportionate risk posed by fine microplastic fibers, given their higher ingestion rates and environmental persistence. These results emphasize the necessity for stringent regulatory measures to mitigate microplastic pollution and advocate for continued research into the bioaccumulation and biomagnification of microplastics within aquatic food webs. Future investigations should incorporate polymer-specific characterization, hydrodynamic modelling, and in vivo toxicological assessments to provide a comprehensive understanding of the ecological and human health implications of microplastic contamination.
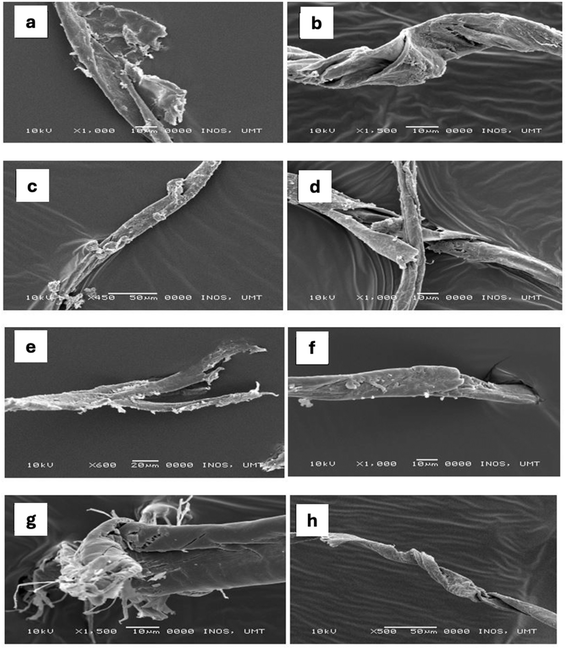
3.3. Surface morphology from SEM
Scanning electron microscopy (SEM) was employed to examine the surface morphologies of the microplastics. This method effectively distinguished plastics from other inorganic materials, such as fish scales, shells, silica and ceramic flakes, with high accuracy and minimal identification error. Fiber-shape microplastics were selected for SEM analysis due to their prevalence in the fish tissues, where it is seen that they are prone to breakage caused by twisting or pulling forces, as observed in all the fish samples. The SEM images revealed microplastics with rough surfaces and cracks, which were attributed to photodegradation, mechanical degradation and biological degradation processes. These processes can occur simultaneously in the environment, collectively contributing to the fragmentation and changes of microplastics over time. These findings are consistent with previous studies on microplastics retrieved from biological and environmental samples.43,44 For instance, photodegradation, driven by UV radiation from sunlight, causes the breakdown of polymer chains in microplastics, leading to alteration in surface textures and size distributions.45 Additionally, mechanical degradation could be induced by physical forces such as wave action or abrasion, which subsequently will fragment these particles into smaller pieces, and modify their shape and surface characteristics, as evidenced in the SEM images.46,47 Biological degradation facilitated by microorganisms can also play a significant role in altering the morphology of microplastics through mechanisms such as biofilm formation and enzymatic activity, which promote further fragmentation.48,49 However, the SEM images in this current study showed no notable evidence of biofilm formation, likely because the microplastic particles were extracted from fish tissue rather than directly from the digestive tract of fish, where biofilms are more commonly observed, as reported elsewhere.2,15 The colonization and growth of the gut’s microorganisms, including bacteria, fungi, and algae, could enhance surface roughness and alter the chemical properties of microplastics, thereby increasing their susceptibility to photodegradation and mechanical degradation.50,51The images also revealed aged microplastics, characterized by uneven and rough surfaces, leading to an increase in surface area and reaction rates. The enhanced surface areas will further promote interactions between microplastics and marine contaminants, organic matter, or microorganisms and biofoulants. It is notable that the degree of surface roughness varied among the observed samples, indicating differing levels of environmental exposure. Additionally, the cracks on microplastic surfaces caused by the different degradation processes could result in increased surface-to-volume ratio, hence providing a possible mechanism for the enriched accumulation of persistent organic pollutants (POPs) on microplastic surfaces.52 It is worth mentioning here that the surface morphology of microplastics in aquatic environments is greatly influenced by various environmental parameters, including salinity, temperature, pH, and biological interactions.53–55 For instance, salinity plays a critical role in shaping the surface characteristics of microplastics by modulating their physical and chemical interactions in aquatic systems.56 Due to monsoonal changes in the region, the salinity in the SCS and SOM varied seasonally, whereby the recorded average salinity of the SOM was lower than that of the SCS.57 High salinity levels can induce aggregation of microplastics, altering their surface morphology and promoting biofilm formation, as evidenced in Fig. 5 and reported elsewhere.58,59 This also affects the flotation and transportation of microplastics, which indirectly effects their exposure to fish species inhabiting different water column zones, such as pelagic, neritic, and epipelagic neritic regions.
3.4. Chemical analysis via micro-FTIR and pyrolysis-GC/MS
Microplastics larger than 0.5 mm (>500 μm) were chemically analyzed for polymer identification using micro-FTIR. Particles from the SOM samples showed a confident match of over 60% with polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET or polyester), PA (polyamide or nylon) and rayon according to the polymer spectral library (ESI†). Meanwhile, particles selected from SCS samples were verified as PE, PP, PET, PA, rayon, polyvinyl alcohol (PVA) and PVMK (polyvinyl methyl ketone) (ESI†). Subsequently, for microplastics smaller than 0.5 mm (<500 μm), chemical analysis was conducted using analytical pyrolysis-GC/MS, which revealed the concentrations of PE (50–60 ppm), PET (60 ppm), PVC (60–70 ppm), PC (90 ppm) and polyamide (40–60 ppm), but not PP, which was quantified at trace levels (i.e., below the calibration limit of quantitation), as listed in Table 2. The polymers were identified and quantified based on twelve individual polymer standard calibration curves, and unlike FTIR, pyrolysis-GC/MS does not show semi-synthetic rayon polymer.While FTIR is able to identify a wide range of polymers, pyrolysis-GC/MS allows the chemical structure of the microplastic to be fragmented, facilitating simultaneous identification and quantification of microplastics through comparison with fragment ions of polymer-specific degradation products in the library. For example, in the analysis, 1,13-tetradecadiene, capronitrile, and 2,4-dimethylheptene were used as unique degradation fragments for PE, PA and PP, respectively. However, due to the limitations of the full-scan mode employed in the analysis, the more sensitive single-ion monitoring (SIM) GC/MS mode is recommended for future studies. The SIM mode targets specific fragment characteristic of each polymer, thus enabling more detailed quantitative analysis, especially in complex sample matrices.22,60
Among all analyzed tissue samples, PE, PET and PP were the most predominant polymers, in agreement with the FTIR results for larger microplastic sizes. These polymers are commonly associated with single-use plastic, packaging and bottles, as well as textile productions (synthetic fibers and microfibers).23,61 Meanwhile, nylon (polyamide) and rayon (semi-synthetic polymer) are known for their usage in fishing materials and ropes and have also been used in clothing industries.62 The contribution of these materials could be induced by discharge from ships, such as ghost fishing nets, fishing gear, and maritime-based operational waste, or can be from land-based sources into the marine environment.
Other types of polymers were also detected via pyrolysis-GC/MS, indicating its sensitivity in detecting smaller microplastic particles (Table 3). The widespread presence of PE, PP, PET and polyamide polymers, which typically float due to their lower densities compared to seawater, can be attributed to various factors in the aquatic ecosystem.63,64 Parameters such as hydrodynamics and flow, alongside environmental factors including salinity, temperature, pH, surface currents and upwelling events, will contribute to differences in polymer distribution in marine environments. For instance, higher temperatures can accelerate the degradation of certain polymers, altering their structural integrity, thus leading to differential fragmentation rates and types, such as microfibers, fragments and filaments.65,66 Furthermore, surface currents in the SCS waters are known to be stronger than those in the SOM, which can influence the spatial distribution of polymers, and consequently their degradation rates in both water regions.57 It is worth noting that the interconnectedness of the SOM and SCS with major regional seas (e.g., the Java Sea and Andaman Sea) could facilitate the long-range transport of different microplastics with different polymer compositions. However, due to the lack of recorded physical parameters in this study, no conclusive remark can be made pertaining to the environmental factors, highlighting the need for future monitoring and investigation. Additionally, pooling samples for analysis based on the particle sizes (i.e., large vs. small microplastics using two separate analyses of FTIR and pyrolysis-GC/MS, respectively) also limits detailed information about each polymer’s concentration relative to individual sample characteristics, including fish dynamics and feeding behaviours. Addressing this limitation in future studies is crucial for developing a more comprehensive understanding of microplastic distribution and its ecological implications.
| Region | South China Sea (ppm) | Strait of Malacca (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | Silver pomfret | Yellowtail scad | Indian mackerel | Round scad | Silver pomfret | Yellowtail scad | Indian mackerel | Round scad |
| a “<” refers to detected below the limit of quantitation, LOQ, of respective polymers; “n.d.” refers to undetected. | ||||||||
| PE | <20 | 50 | n.d. | n.d. | <20 | <20 | <20 | 60 |
| PP | <10 | <10 | n.d. | n.d. | <10 | n.d. | <10 | <10 |
| PVC | <10 | 60 | 70 | <10 | <10 | n.d. | <10 | n.d. |
| PA | <20 | n.d. | 50 | 60 | n.d. | n.d. | n.d. | 40 |
| PET | <40 | n.d. | n.d. | n.d. | n.d. | n.d. | 60 | <40 |
| PC | n.d. | n.d. | n.d. | 90 | n.d. | n.d. | n.d. | <40 |
3.5. Estimated human intake of microplastics from fish consumption
The potential ingestion of microplastics through fish consumption in Malaysia, as highlighted in this study, emphasizes significant implications for ecological and human health. Based on the calculation of the microplastic ingestion by all species, it is revealed that a Malaysian adult who consumes 122 g of fish daily may potentially ingest 1.129–1.582 microplastics per day (Table 4).24 It is also estimated that there is annual intake of 577.429 microplastics per capita (microplastics/kg/bw/year) from consuming silver pomfret, followed by round scad (561.043 microplastics/kg/bw/year), Indian mackerel (421.062 microplastics/kg/bw/year) and yellowtail scad (411.962 microplastics/kg/bw/year). These results are significantly higher than the consumption for marine fish species collected in the wet market of Kota Kinabalu, Malaysia, with an intake of 12.928 microplastic particles/kg/bw/year.61 This disparity may be influenced by the geographic characteristics of the SCS and SOM, which are among the busiest water regions globally. These waterways have high maritime activities and are influenced by industrial activities from the neighboring area, and serve as interconnection passages and transport between the China Seas, Java Seas and Andaman Seas.67 The microplastic dietary intake through fish consumption in this study also falls within the range of values reported globally, although with notable differences in magnitude, for example, Iran (174.43 particles/kg/bw/year) and Nigeria (2.2 × 10−7 particles/kg/bw/year). These differences may be attributed to varying levels of microplastic contamination and Malaysia’s substantial per capita seafood consumption.68,69| Common name | Species | Mean (microplastics/gram) | Estimated daily intake (microplastics/kg/bw) | Estimated yearly intake (microplastics/kg/bw/year) |
|---|---|---|---|---|
| Silver pomfret | P. argentus | 0.804 | 1.582 | 577.429 |
| Yellowtail scad | A. mate | 0.574 | 1.129 | 411.962 |
| Indian mackerel | R. kanagurta | 0.586 | 1.154 | 421.062 |
| Round scad | D. punctatus | 0.781 | 1.537 | 561.043 |
Other than fish, consumption of seafood including bivalves also contributes significantly to microplastic ingestion in humans. For instance, the microplastic ingestion rates through bivalves are the highest in China, with 8369 microplastics/capita/year, and France (1070 microplastics/capita/year).70 The same authors reported Thailand and Vietnam adults may potentially consume 233 and 245 microplastics/capita/year. These values fall within the broader range of estimates reported in other countries such as Malaysia (21.8–93.5 microplastics/capita/year) and Hong Kong (0–358.53 microplastics/capita/year), though significantly lower than those reported for Turkey (1584.02–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053.85 microplastics/capita/year).71–73 Nevertheless, the presence of microplastics in the edible tissues aforementioned reflects the pervasive contamination by microplastics (and potentially nanoplastics) in marine ecosystems, where the pollutants ingested by organisms may be translocated to various tissues, transferred through the food web, and thus disrupt trophic dynamics.13 This could also threaten biodiversity in critical marine biodiversity hotspots surrounding Malaysian and Southeast Asian waters. While these findings provide valuable insight and raise concerns about the cumulative effects of dietary microplastic exposure, particularly given the reliance on fish and seafood as a primary protein source, nonetheless, it is not yet possible to draw a conclusion. The environmental factors that dictate microplastic presence require a broader discussion in assessment of regional microplastic contamination of commercially significant fish species from the South China Sea and Straits of Malacca. Therefore, critical assessment of the methods for microplastic extraction and identification is necessary, to consider many factors, for instance contamination risk, limit of detection and the risk of misclassifying fibers. Importantly, further research is necessary to enhance the consistency of the data by expanding the sample sizes, especially to have larger sample volumes and sampling seasons, involve a wider variety of species and cover more sampling sites. This is needed to assess the extent of microplastic contamination and its potential implications.
053.85 microplastics/capita/year).71–73 Nevertheless, the presence of microplastics in the edible tissues aforementioned reflects the pervasive contamination by microplastics (and potentially nanoplastics) in marine ecosystems, where the pollutants ingested by organisms may be translocated to various tissues, transferred through the food web, and thus disrupt trophic dynamics.13 This could also threaten biodiversity in critical marine biodiversity hotspots surrounding Malaysian and Southeast Asian waters. While these findings provide valuable insight and raise concerns about the cumulative effects of dietary microplastic exposure, particularly given the reliance on fish and seafood as a primary protein source, nonetheless, it is not yet possible to draw a conclusion. The environmental factors that dictate microplastic presence require a broader discussion in assessment of regional microplastic contamination of commercially significant fish species from the South China Sea and Straits of Malacca. Therefore, critical assessment of the methods for microplastic extraction and identification is necessary, to consider many factors, for instance contamination risk, limit of detection and the risk of misclassifying fibers. Importantly, further research is necessary to enhance the consistency of the data by expanding the sample sizes, especially to have larger sample volumes and sampling seasons, involve a wider variety of species and cover more sampling sites. This is needed to assess the extent of microplastic contamination and its potential implications.
3.6. Translocation of microplastics to fish tissue and relationship with potential human consumption
The detection of microplastics in the tissues of commercial fish species raises concerns regarding human exposure through dietary intake. While existing studies suggest that microplastics predominantly reside in the gastrointestinal tracts of fish (i.e., parts typically removed prior to consumption), the identification of microplastics in fish flesh tissues, which are consumed by humans, reveals potential pathways for exposure. According to the Food and Agriculture Organization of the United Nations, global fish and seafood consumption reached 20.2 kg per capita in 2020, a trend that is escalating in response to sociodemographic demands and the nutritional value of fish and seafood.74,75 During the ingestion of microplastics by fish, smaller particles can accumulate in their gastrointestinal tract (GIT), potentially causing blockages and leading to malnutrition. This accumulation can impair the growth, health and survival of the fish. Microplastic may enter fish via branchial intrusion, in which particles enter through their gills and attach to filaments during respiration.76 This could reduce the surface area for oxygen exchange, leading to respiratory stress and hypoxia of the organism, while also increasing the risk of physical damage and infections.77,78 Microplastics may get translocated from the GIT and gills to other tissues, including muscle tissues, via the bloodstream or mucous layer.79The slender nature and high aspect ratio (smaller width but greater length) of fiber microplastics allows them to penetrate tissue more easily compared to other morphologies, such as fragments of film shapes.80 This elongated shape also contributes to their persistence within tissues, where they may cause localized inflammation or be transported across the mucous layer into deeper tissue structures. Once embedded in muscle, microplastics can induce oxidative stress and lipid peroxidation through the leaching of toxic additives, potentially impairing neuromuscular function and reducing swimming efficiency.81,82 Although muscle tissues generally contain lower microplastic levels than the GIT or gills, their presence raises concerns for both fish health and human exposure through seafood consumption.
Nonetheless, the direct health risks and implications of ingesting microplastics have remained unclear and under investigation; however, the associated chemical pollutants pose significant risks. These pollutants include additives, plasticizers, and environmental toxins absorbed by the plastics, such as heavy metals and persistent organic pollutants, which may leach from microplastics into fish tissues and ultimately affect humans in the food chain.83,84 When humans ingest these chemicals, they may accumulate in the body, leading to long-term health risks such as endocrine-hormone disruption, carcinogenic effects, and inflammation.85 Further, it has also been hypothesized that smaller-sized microplastics and nanoplastics can penetrate biological membranes and reach human tissues.62,86 The dietary intake of smaller microplastics and nanoplastics, which are categorized as solid pollutants, may lead to oxidative stress, immune responses, and other types of cellular harm. Moreover, it is important to note that these emerging contaminants tend to increase in concentration along the food chain, potentially exposing top consumers, particularly humans, to elevated risks of harm.87 Nonetheless, the debate on potential risks to human health needs to be greatly enhanced by contextualizing the extent of observed microplastic contamination with specific and relevant hazards, with limited overlap between these areas currently. While the direct biological consequences of consuming fish containing microplastics are still under investigation, continuous exposure to microplastics and their associated toxicants through seafood consumption represents a potential public health concern. This highlights the need for further research to assess the presence of microplastics in edible fish tissues and the long-term impacts of chronic ingestion on human health.88 Animal model studies have shown that chronic exposure to microplastics can lead to metabolic disorders, reproductive toxicity, and immune system alterations, and adverse effects related to the additives and chemicals absorbed by microplastics.89
Furthermore, reducing human exposure to microplastics depends on implementing stricter regulations and preventive measures in fisheries and plastic management. The results of the study indicate that fiber microplastics were substantially prevalent in the tissues of four commercially important fish species from the South China Sea and Straits of Malacca, with Rastrelliger kanagurta showing the highest abundance. However, ingestion rates may vary within population and sub-populations based on factors such as demographic, age, gender and dietary preferences. Additionally, it is important to better elucidate the possible sources of microplastics to contribute to the knowledge on the origin and pathways of these contaminants. For instance, riverine inputs, wastewater effluents, and maritime activities, with the assistance of polymer composition identification and oceanographic data, should be further examined to determine the primary contributors to microplastic pollution in these areas. Filling in this knowledge would lead to a more comprehensive and scientifically rigorous evaluation of commercial fish species as vectors of microplastic contamination in human health implications. Nevertheless, while much remains to be learned about the direct health risks, the findings of this study serve as a wake-up call for systematic monitoring of microplastic contamination in seafood, and thus will increase public awareness and implementation of stricter measures to prevent plastic from entering marine food chains, which are particularly crucial for Malaysia and the Southeast Asia region – one of the world’s unique and ecologically significant coastal regions.
4. Conclusions
In conclusion, this study documents the presence of microplastics in the tissues of four different commercial fish species from two distinct sampling areas in Malaysian waters: the South China Sea (Terengganu waters) and the Straits of Malacca. The SCS commercial fish contained an average of 10.28 particles per ind., while the SOM commercial fish had 7.63 particles per ind. in their flesh tissues. The highest number of microplastics detected in South China Sea fish corresponded to Indian mackerel (an epipelagic neritic fish species) ingesting 10.8 particles per ind., while the highest in the Straits of Malacca was 12.7 particles per ind. in yellowtail scad (a pelagic species). The physical characteristics of the microplastics showed that black and fiber-shape are the most dominant color and shape of microplastics found in samples in both water regions. The smallest average size of microplastics found in the edible tissue was 0.04 millimeter, which was from South China Sea fish, highlighting the ability of tiny particles to accumulate in biological tissues. The prevalence of smaller microplastics also raises concerns about their potential to translocate within organisms and their increased bioavailability to marine life.Microplastic was found in higher concentrations in pelagic fish than in benthic fish, with fish from areas with higher environmental particle counts ingesting more microplastics, likely due to their feeding habits and exposure to high microplastic concentrations in the surface waters. The amount of microplastic in fish flesh was not influenced by the species’ trophic level, but appeared related to the size and mass of the fish. Additionally, the estimated daily intake (EDI) of microplastics through fish consumption of these four species ranges from 1.129 to 1.582 microplastics per capita. Annually, the highest ingestion is observed with silver pomfret consumption, amounting to 577.429 microplastics/kg/bw/year. These results highlight the potential for human exposure to microplastics through fish consumption, particularly in regions where fish is a dietary staple, such as Southeast Asia. This current finding supports the theory that microplastics are present in fish tissues; however definitive conclusions should be approached with caution. Therefore, to confirm the low residence duration of microplastics in fish tissues, a more rigorous monitoring program should be considered, and targeted dietary laboratory experimental research is necessary.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Y. S. Ibrahim: writing – original draft, methodology, investigation, conceptualization. N. I. Abd Razak: writing – original draft, methodology, investigation. N. S. Roslan: writing – original draft, writing – review & editing. A. A. M. Ali: writing – review & editing. N. F. Omar: methodology, investigation. K. M. K. K. Yusof: methodology, investigation, writing – review & editing. C. Chinglenthoiba: writing – original draft, writing – review & editing. N. N. Mohamad: resources and funding acquisition, writing – review & editing. S. T. Anuar: conceptualization, resources and funding acquisition, writing – original draft, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Ministry of Higher Education, Malaysia (FRGS/1/2024/WAS02/UMT/02/2). This article shows a successful collaborative work between university, government research institute and private laboratory. Therefore, we would like to thank the Ministry of Higher Education, Malaysia for the FRGS award FRGS59773 (FRGS/1/2024/WAS02/UMT/02/2) and the facilities provided by FSSM and CRAFTS. We also would like to thank ALS Technichem (M) Sdn Bhd for their technical support, especially for the py-GC/MS in their facility. We are also thankful for the support from FRI, especially in supporting the micro-FTIR cost for this project. This project also would not have been able to succeed without tremendous support from MRIG Lab members and students.References
- J. C. Anderson, B. J. Park and V. P. Palace, Microplastics in aquatic environments: implications for Canadian ecosystems, Environ. Pollut., 2016, 218, 269–280 CrossRef CAS PubMed.
- Y. S. Ibrahim, R. Rathnam, S. T. Anuar and W. M. A. W. M. Khalik, Isolation and characterisation of microplastic abundance in Lates calcarifer from Setiu Wetlands, Malaysia, Malays. J. Anal. Sci., 2017, 21(5), 1054–1064 Search PubMed.
- A. Lusher, P. C. H. Hollman and J. Mendoza-Hill, Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety, FAO Fisheries and Aquaculture Technical Paper No. 615, 2017, p. 34 Search PubMed.
- E. M. Tuuri and S. C. Leterme, How plastic debris and associated chemicals impact the marine food web: A review, Environ. Pollut., 2023, 321, 121156 CrossRef CAS PubMed.
- M. Arienzo, L. Ferrara and M. Trifuoggi, Research progress in transfer, accumulation and effects of microplastics in the oceans, J. Mar. Sci. Eng., 2021, 9(4), 433 CrossRef.
- A. A. Mohd Ali, A. A. Khalid, N. I. Abd Razak, N. S. Maulana, N. S. Roslan, R. S. Razmi, W. M. Ruseli, Y. S. Ibrahim, M. Jaafar, R. Shahrudin, K. Ismail and S. T. Anuar, A review on the presence of microplastics in environmental matrices within Southeast Asia: elucidating risk information through an analysis of microplastic characteristics such as size, shape, and type, Water Emerging Contam. Nanoplast., 2024, 3, 12 Search PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband, R. Goodhead, J. Moger and T. S. Galloway, Microplastic ingestion by zooplankton, Environ. Sci. Technol., 2013, 47(12), 6646–6655 CrossRef CAS PubMed.
- C. G. Avio, S. Gorbi and F. Regoli, Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea, Mar. Environ. Res., 2015, 111, 18–26 CrossRef CAS PubMed.
- P. Makhdoumi, H. Hossini and M. Pirsaheb, A review of microplastic pollution in commercial fish for human consumption, Rev. Environ. Health, 2023, 38(1), 97–109 CrossRef CAS PubMed.
- C. Rubio-Armendáriz, S. Alejandro-Vega, S. Paz-Montelongo, Á. J. Gutiérrez-Fernández, C. J. Carrascosa-Iruzubieta and A. Hardisson-de la Torre, Microplastics as emerging food contaminants: a challenge for food safety, Int. J. Environ. Res. Public Health, 2022, 19(3), 1174 CrossRef PubMed.
- M. E. McHale and K. L. Sheehan, Bioaccumulation, transfer, and impacts of microplastics in aquatic food chains, J. Environ. Exposure Assess., 2024, 3, 15 CAS.
- A. A. Koelmans, A. Bakir, G. A. Burton and C. R. Janssen, Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies, Environ. Sci. Technol., 2016, 50(7), 3315–3326 CrossRef CAS PubMed.
- X. Dong, X. Liu, Q. Hou and Z. Wang, From natural environment to animal tissues: A review of microplastics (nanoplastics) translocation and hazards studies, Sci. Total Environ., 2023, 855, 158686 CrossRef CAS PubMed.
- A. V. Filgueiras, I. Preciado, A. Cartón and J. Gago, Microplastic ingestion by pelagic and benthic fish and diet composition: A case study in the NW Iberian shelf, Mar. Pollut. Bull., 2020, 160, 111623 CrossRef CAS PubMed.
- J. Wu, M. Lai, Y. Zhang, J. Li, H. Zhou, R. Jiang and C. Zhang, Microplastics in the digestive tracts of commercial fish from marine ranching in East China Sea, China, Case Stud. Chem. Environ. Eng., 2020, 2, 100066 CrossRef.
- M. N. Woods, M. E. Stack, D. M. Fields, S. D. Shaw and P. A. Matrai, Microplastic fiber uptake, ingestion, and egestion rates in the blue mussel (Mytilus edulis), Mar. Pollut. Bull., 2018, 137, 638–645 CrossRef CAS PubMed.
- N. I. Ahmad, W. R. Wan Mahiyuddin, T. R. Tengku Mohamad, C. Y. Ling, S. F. Daud, N. C. Hussein, N. A. Abdullah, R. Shaharudin and L. H. Sulaiman, Fish consumption pattern among adults of different ethnics in Peninsular Malaysia, Food Nutr. Res., 2016, 60(1), 32697 CrossRef PubMed.
- A. Karami, A. Golieskardi, C. K. Choo, N. Romano, Y. B. Ho and B. Salamatinia, A high-performance protocol for extraction of microplastics in fish, Sci. Total Environ., 2017, 578, 485–494 CrossRef CAS PubMed.
- Z. Feng, T. Zhang, Y. Li, X. He, R. Wang, J. Xu and G. Gao, The accumulation of microplastics in fish from an important fish farm and mariculture area, Haizhou Bay, China, Sci. Total Environ., 2019, 696, 133948 CrossRef CAS PubMed.
- A. L. Lusher, K. Munno, L. Hermabessiere and S. Carr, Isolation and extraction of microplastics from environmental samples: an evaluation of practical approaches and recommendations for further harmonization, Appl. Spectrosc., 2020, 74(9), 1049–1065 CrossRef CAS PubMed.
- B. Beckingham, A. Apintiloaiei, C. Moore and J. Brandes, Hot or not: systematic review and laboratory evaluation of the hot needle test for microplastic identification, Microplast. Nanoplast., 2023, 3(1), 8 CrossRef.
- S. T. Anuar, R. S. Altarawnah, A. A. Mohd Ali, B. Q. Lee, W. M. A. W. M. Khalik, K. M. K. K. Yusof and Y. S. Ibrahim, Utilizing pyrolysis–gas chromatography/mass spectrometry for monitoring and analytical characterization of microplastics in polychaete worms, Polymers, 2022, 14(15), 3054 CrossRef CAS PubMed.
- M. Go, A. Ybañez, A. Illano, F. Cababat and L. De La Calzada, Microplastics in beach sediments, seawater, and common fish in tourist destinations, Global J. Environ. Sci. Manage., 2024, 10(4), 1993–2008 CAS.
- E. Von Goh, S. A. Ali, S. R. Mitra and F. McCullough, Understanding the patterns of fish and seafood consumption and its nutritional roles among a Malaysian population to inform sustainable development, Asia Pac. J. Sustainable Agric. Food Energy, 2021, 9(1), 1–13 Search PubMed.
- N. H. Mohamed Nor, M. Kooi, N. J. Diepens and A. A. Koelmans, Lifetime accumulation of microplastic in children and adults, Environ. Sci. Technol., 2021, 55(8), 5084–5096 CrossRef CAS PubMed.
- C. Y. Manullang, M. P. Patria, A. Haryono, S. T. Anuar, R. D. Susanto, M. S. Abdul, M. Fadli and Z. Wei, Ingestion of microplastics in the planktonic copepod from the Indonesian Throughflow pathways, Sains Malays., 2024, 53(8), 1873–1887 CrossRef.
- N. K. Y. Susanti, A. Mardiastuti and S. Hariyadi, Microplastics in fishes as seabird preys in Jakarta Bay Area, IOP Conf. Ser.: Earth Environ. Sci., 2022, 967(1), 012033 CrossRef.
- P. T. Ningrum, A. H. S. Negoro, D. E. Indahyani, Kusnadi and Y. Nurdiansyah, Microplastic contamination in marine fish and shells in the coastal areas of Jember Regency, Indonesia, J. Ilm. Perikan. dan Kelaut., 2023, 15(1), 201 CrossRef.
- M. Najihah, M. Mohd Ali, K. M. Jansar, K. K. Ku Yaacob and N. H. Asgnari, Microplastic contamination in Indian mackerel: A study of prevalence and potential health risks for Malaysian consumers, Phys. Chem. Earth, 2025, 138, 103864 CrossRef.
- N. Jaafar, A. Azfaralariff, S. M. Musa, M. Mohamed, A. H. Yusoff and A. M. Lazim, Occurrence, distribution and characteristics of microplastics in gastrointestinal tract and gills of commercial marine fish from Malaysia, Sci. Total Environ., 2021, 799, 149457 CrossRef CAS PubMed.
- Y. H. Foo, S. Ratnam, E. V. Lim, M. Abdullah, V. J. Molenaar, A. T. Shau Hwai, S. Zhang, H. Li and N. B. Mohd Zanuri, Microplastic ingestion by commercial marine fish from the seawater of Northwest Peninsular Malaysia, PeerJ, 2022, 10, e13181 CrossRef PubMed.
- S. Sarijan, S. Azman, M. I. M. Said and M. H. Lee, Ingestion of microplastics by commercial fish in Skudai River, Malaysia, EnvironmentAsia, 2019, 12(3), 75–84 Search PubMed.
- W. Fischer and P. J. P. Whitehead, Fishery and Aquaculture Economics and Policy Division, 1974, https://openknowledge.fao.org/handle/20.500.14283/e9163e Search PubMed.
- R. Md Amin, E. S. Sohaimi, S. T. Anuar and Z. Bachok, Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea, Mar. Pollut. Bull., 2020, 150, 110616 CrossRef CAS PubMed.
- K. Mattsson, S. Jocic, I. Doverbratt and L.-A. Hansson, Nanoplastics in the Aquatic Environment, Microplastic Contamination in Aquatic Environments, Elsevier, 2018, pp. 379–399 Search PubMed.
- K. M. K. K. Yusof, S. T. Anuar, Y. Mohamad, M. Jaafar, N. Mohamad, Z. Bachok, N. Mohamad and Y. S. Ibrahim, First evidence of microplastic pollution in the surface water of Malaysian Marine Park islands, South China Sea during COVID-19, Mar. Pollut. Bull., 2023, 194, 115268 CrossRef CAS PubMed.
- P. G. Ryan, A simple technique for counting marine debris at sea reveals steep litter gradients between the Straits of Malacca and the Bay of Bengal, Mar. Pollut. Bull., 2013, 69(1–2), 128–136 CrossRef CAS PubMed.
- A. Rebelein, I. Int-Veen, U. Kammann and J. P. Scharsack, Microplastic fibers — Underestimated threat to aquatic organisms?, Sci. Total Environ., 2021, 777, 146045 CrossRef CAS PubMed.
- C. M. Rochman, E. Hoh, T. Kurobe and S. J. Teh, Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress, Sci. Rep., 2015, 3, 3263 CrossRef PubMed.
- H. Kye, J. Kim, S. Ju, J. Lee, C. Lim and Y. Yoon, Microplastics in water systems: A review of their impacts on the environment and their potential hazards, Heliyon, 2023, 9, e14359 CrossRef CAS PubMed.
- C. Y. Manullang, M. P. Patria, A. Haryono, S. T. Anuar, M. Fadli, R. D. Susanto and Z. Wei, Vertical distribution of microplastic along the main gate of Indonesian Throughflow pathways, Mar. Pollut. Bull., 2024, 199, 115954 CrossRef CAS PubMed.
- S. S. Ali, M. H. M. Alsharbaty, R. Al-Tohamy, M. Schagerl, M. Al-Zahrani, M. Kornaros and J. Sun, Microplastics as persistent and vectors of other threats in the marine environment: toxicological impacts, management and strategical roadmap to end plastic pollution, Environ. Chem. Ecotoxicol., 2025, 7, 229–251 CrossRef CAS.
- F. T. Mercy and A. K. M. Rashidul Alam, Assessment of microplastic contamination in shrimps from the Bay of Bengal and associated human health risk, Mar. Pollut. Bull., 2024, 201, 116185 CrossRef CAS PubMed.
- K. Miserli, C. Lykos, A. G. Kalampounias and I. Konstantinou, Screening of microplastics in aquaculture systems (fish, mussel, and water samples) by FTIR, scanning electron microscopy–energy dispersive spectroscopy and micro-Raman spectroscopies, Appl. Sci., 2023, 13, 9705 CrossRef CAS.
- A. Delre, M. Goudriaan, V. H. Morales, A. Vaksmaa, R. T. Ndhlovu, M. Baas, E. Keijzer, T. de Groot, E. Zeghal, M. Egger, T. Röckmann and H. Niemann, Plastic photodegradation under simulated marine conditions, Mar. Pollut. Bull., 2023, 187, 114544 CrossRef CAS PubMed.
- M. P. Born, C. Brüll and H. Schüttrumpf, Implications of a new test facility for fragmentation investigations on virgin (micro) plastics, Environ. Sci. Technol., 2023, 57(28), 10393–10403 CrossRef CAS PubMed.
- Y. Huo, Y. Niu, Z. Sun, Y. Li and J. Niu, Surface/subsurface damage mechanisms and inhibition strategies in machining of hard and brittle materials: a systematic review, Surf. Interfaces, 2024, 54, 105088 CrossRef.
- X. Yan, C. Chio, H. Li, Y. Zhu, X. Chen and W. Qin, Colonization characteristics and surface effects of microplastic biofilms: Implications for environmental behavior of typical pollutants, Sci. Total Environ., 2024, 937, 173141 CrossRef CAS PubMed.
- A. J. Pete, P. J. Brahana, M. Bello, M. G. Benton and B. Bharti, Biofilm formation influences the wettability and settling of microplastics, Environ. Sci. Technol. Lett., 2023, 10(2), 159–164 CrossRef CAS.
- I. V. Kirstein, S. Kirmizi, A. Wichels, A. Garin-Fernandez, R. Erler, M. Löder and G. Gerdts, Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles, Mar. Environ. Res., 2016, 120, 1–8 CrossRef CAS PubMed.
- H. Luo, C. Liu, D. He, J. Xu, J. Sun, J. Li and X. Pan, Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions, J. Hazard. Mater., 2022, 423, 126915 CrossRef CAS PubMed.
- P. Wardrop, J. Shimeta, D. Nugegoda, P. D. Morrison, A. Miranda, M. Tang and B. O. Clarke, Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish, Environ. Sci. Technol., 2016, 50, 4037–4044 CrossRef CAS PubMed.
- S. Liu, J. Huang, W. Zhang, L. Shi, K. Yi, H. Yu, C. Zhang, S. Li and J. Li, Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects, J. Environ. Manage., 2022, 302, 113995 CrossRef CAS PubMed.
- S. S. Ali, T. Elsamahy, R. Al-Tohamy and J. Sun, A critical review of microplastics in aquatic ecosystems: Degradation mechanisms and removing strategies, Environ. Sci. Ecotechnol., 2024, 21, 100427 CrossRef CAS PubMed.
- H. Ma, L. Chao, H. Wan and Q. Zhu, Microplastic Pollution in Water Systems: Characteristics and Control Methods, Diversity, 2024, 16(1), 70 CrossRef CAS.
- W. Liu, C. Gu, J. Li, Y. Zhang, X. Zhang, P. Zhang and X. Liu, High Salinity Alters the Adsorption Behavior of Microplastics towards Typical Pollutants and the Phytotoxicity of Microplastics to Synechococcus, Sustainability, 2024, 16(3), 1107 CrossRef CAS.
- R. H. Roseli, N. Maisarah, N. Shakila, A. Wahid and M. S. Irwan, Temperature And Salinity Characteristics In The Southern South China Sea And Strait Of Malacca, Universiti Malaysia Terengganu Journal of Undergraduate Research, 2022, 4(1), 99–116 CrossRef.
- L. Qiang, J. Cheng, S. Mirzoyan, L. J. Kerkhof and M. M. Häggblom, Characterization of microplastic-associated biofilm development along a freshwater–estuarine gradient, Environ. Sci. Technol., 2021, 55(24), 16402–16412 CrossRef CAS PubMed.
- F. Mendrik, R. Fernández, C. R. Hackney, C. Waller and D. R. Parsons, Non-buoyant microplastic settling velocity varies with biofilm growth and ambient water salinity, Commun. Earth Environ., 2023, 4, 30 CrossRef.
- Y. K. Müller, T. Wernicke, M. Pittroff, C. S. Witzig, F. R. Storck, J. Klinger and N. Zumbülte, Microplastic analysis—are we measuring the same? Results on the first global comparative study for microplastic analysis in a water sample, Anal. Bioanal. Chem., 2020, 412, 555–560 CrossRef PubMed.
- P. Gao, N. Q. I. Mohd Noor, U. H. Mohamad Razali, M. H. Mohd Yusop and S. Md Shaarani, Anthropogenic particles in the muscle, gill, and gastrointestinal tract of marine fish sold for human consumption, Heliyon, 2023, 9(10), e20835 CrossRef CAS PubMed.
- Y. S. Ibrahim, S. T. Anuar, A. A. Azmi, W. M. A. W. M. Khalik, S. Lehata, S. R. Hamzah, D. Ismail, Z. F. Ma, A. Dzulkarnaen, Z. Zakaria, N. Mustaffa, S. E. Tuan Shariff and Y. Y. Lee, Detection of microplastics in human colectomy specimens, JGH Open, 2021, 5(1), 116–121 CrossRef PubMed.
- J. Huang, H. Chen, Y. Zheng, Y. Yang, Y. Zhang and B. Gao, Microplastic pollution in soils and groundwater: Characteristics, analytical methods and impacts, Chem. Eng. J., 2021, 425, 131870 CrossRef CAS.
- F. Yu, Q. Qin, X. Zhang and J. Ma, Characteristics and adsorption behavior of typical microplastics in long-term accelerated weathering simulation, Environ. Sci.: Processes Impacts, 2024, 26, 882–890 RSC.
- A. Puthcharoen and S. Leungprasert, Determination of microplastics in soil and leachate from the landfills, Thai Environ. Eng. J., 2019, 33, 39–46 Search PubMed.
- D. T. Ha, H. D. Tong and T. T. Trinh, Insights into hydrothermal gasification process of microplastic polyethylene via reactive molecular dynamics simulations, Sci. Rep., 2024, 14, 18771 CrossRef CAS PubMed.
- P. H. Kok, S. Wijeratne, M. F. Akhir, C. Pattiaratchi, N. H. Roseli and F. S. Mohamad Ali, Interconnection between the southern South China Sea and the Java Sea through the Karimata Strait, J. Mar. Sci. Eng., 2021, 9, 1040 CrossRef.
- P. Makhdoumi, H. Hossini, Z. Nazmara, K. Mansouri and M. Pirsaheb, Occurrence and exposure analysis of microplastic in the gut and muscle tissue of riverine fish in Kermanshah province of Iran, Mar. Pollut. Bull., 2021, 173, 112915 CrossRef CAS PubMed.
- M. O. Michael and E. Oshodin, Accumulation of Microplastics in African Sharptooth Catfish Clarias gariepinus from Ikpoba Reservoir, Benin City, Nigeria, BIMA J. Sci. Technol., 2024, 8(3B), 152–162 Search PubMed.
- J. Ding, Y. Sun, C. He, J. Li and F. Li, Towards risk assessments of microplastics in bivalve mollusks globally, J. Mar. Sci. Eng., 2022, 10(2), 288 CrossRef.
- P. Mohan, F. S. Hamid, H. Furumai and K. Nishikawa, Beneath the Surface: Exploring Microplastic Intricacies in Anadara granosa, Mar. Environ. Res., 2024, 199, 106581 CrossRef CAS PubMed.
- İ. C. Tunçelli and N. Erkan, Microplastic Pollution in Wild and Aquacultured Mediterranean Mussels from the Sea of Marmara: Abundance, Characteristics, and Health Risk Estimations, Environ. Res., 2024, 242, 117787 CrossRef PubMed.
- T. W. Lam, Y. C. J. Tsui, Y. L. Cheng, A. T. H. Ma and L. Fok, Microplastic Contamination in Edible Clams from Popular Recreational Clam-Digging Sites in Hong Kong and Implications for Human Health, Sci. Total Environ., 2023, 875, 162576 CrossRef CAS PubMed.
- Food and Agriculture Organization of the United Nations, The State of World Fisheries and Aquaculture, FAO, Rome, 2022, DOI:10.4060/cc0461en.
- E. D. Maciel, J. G. Sonati, J. A. Galvão and M. Oetterer, Fish consumption and lifestyle: a cross-sectional study, Food Sci. Technol., 2019, 39, 141–145 CrossRef.
- E. Conger, M. Dziobak, E. J. Berens McCabe, T. Curtin, A. Gaur, R. S. Wells, J. E. Weinstein and L. B. Hart, An analysis of suspected microplastics in the muscle and gastrointestinal tissues of fish from Sarasota Bay, FL: exposure and implications for apex predators and seafood consumers, Environments, 2024, 11(9), 185 CrossRef PubMed.
- M. Abdel-Tawwab, M. N. Monier, S. H. Hoseinifar and C. Faggio, Fish response to hypoxia stress: growth, physiological, and immunological biomarkers, Fish Physiol. Biochem., 2019, 45, 997–1013 CrossRef CAS PubMed.
- W. Liang, B. Li, M.-C. Jong, C. Ma, C. Zuo, Q. Chen and H. Shi, Process-oriented impacts of microplastic fibers on behavior and histology of fish, J. Hazard. Mater., 2023, 448, 130856 CrossRef CAS PubMed.
- C. Ma, Q. Chen, J. Li, B. Li, W. Liang, L. Su and H. Shi, Distribution and translocation of micro- and nanoplastics in fish, Crit. Rev. Toxicol., 2021, 51(9), 740–753 CrossRef CAS PubMed.
- S. Acharya, S. S. Rumi, Y. Hu and N. Abidi, Microfibers from synthetic textiles as a major source of microplastics in the environment: A review, Text. Res. J., 2021, 91(17–18), 2136–2156 CrossRef CAS.
- A. K. M. M. Hasan, M. Hamed, J. Hasan, C. J. Martyniuk, S. Niyogi and D. P. Chivers, A review of the neurobehavioural, physiological, and reproductive toxicity of microplastics in fishes, Ecotoxicol. Environ. Saf., 2024, 282, 116712 CrossRef CAS PubMed.
- C. R. Multisanti, S. Ferrara, G. Piccione and C. Faggio, Plastics and their derivatives are impacting animal ecophysiology: A review, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2025, 291, 110149 CAS.
- B. D. Abera and M. A. Adimas, Health benefits and health risks of contaminated fish consumption: Current research outputs, research approaches, and perspectives, Heliyon, 2024, 10(13), e33905 CrossRef PubMed.
- P. N. Manavi and A. Mazumder, Potential risk of mercury to human health in three species of fish from the southern Caspian Sea, Mar. Pollut. Bull., 2018, 130, 1–5 CrossRef CAS PubMed.
- J. Domenech and R. Marcos, Pathways of human exposure to microplastics, and estimation of the total burden, Curr. Opin. Food Sci., 2021, 39, 144–151 CrossRef CAS.
- N. S. Roslan, Y. Y. Lee, Y. S. Ibrahim, S. T. Anuar, K. M. K. K. Yusof, L. A. Lai and T. Brentnall, Detection of microplastics in human tissues and organs: A scoping review, J. Global Health, 2024, 14, 04179 CrossRef PubMed.
- B. Jiang, A. E. Kauffman, L. Li, W. McFee, B. Cai, J. Weinstein, J. R. Lead, S. Chatterjee, G. I. Scott and S. Xiao, Health impacts of environmental contamination of micro-and nanoplastics: a review, Environ. Health Preventative Med., 2020, 25, 1 CrossRef PubMed.
- S. M. O’Neill and J. Lawler, Knowledge gaps on micro and nanoplastics and human health: A critical review, Case Stud. Chem. Environ. Eng., 2021, 3, 100091 CrossRef.
- E. Jeong, J.-Y. Lee and M. Redwan, Animal exposure to microplastics and health effects: A review, Emerging Contam., 2024, 10, 100369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00425f |
| This journal is © The Royal Society of Chemistry 2025 |