Open Access Article
Open Access ArticleHealth risk assessment of polychlorinated biphenyls in a fish species (Clarias gariepinus) from southwestern rivers, Nigeria†
I. A.
Ololade
 *a,
A. O.
Apata
ab,
O. J.
Oloyede
a,
O. I.
Akindumila
c,
O. P.
Asanga
d and
F. F.
Oloye
e
*a,
A. O.
Apata
ab,
O. J.
Oloyede
a,
O. I.
Akindumila
c,
O. P.
Asanga
d and
F. F.
Oloye
e
aEnvironmental Monitoring Unit, Department of Chemical Sciences, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria. E-mail: isaac.ololade@aaua.edu.ng
bPuget Sound Naval Shipyard, Bremerton, Washington 98314, USA
cDepartment of Chemistry and Biochemistry, University of Toledo, USA
dDepartment of Chemistry, Clemson University, 211S, Palmetto Blvd, Clemson, South Carolina 29643, USA
eDepartment of Chemistry, Division of Physical and Computational Sciences, University of Pittsburgh, Bradford, Pennsylvania 16701, USA
First published on 22nd April 2025
Abstract
The catfish (Clarias gariepinus) is commonly eaten in Nigeria, especially in the southwestern region. In this study, the levels of polychlorinated biphenyls (PCBs) in the muscles of Clarias gariepinus from six major rivers in the area were measured using gas chromatography with an electron capture detector. The PCB concentration ranged from 4.63 to 21.96 mg kg−1 in the dry season and from 5.26 to 23.52 mg kg−1 in the wet season. There were significant differences in PCB concentrations between the two seasons. The ∑PCB concentrations at any location were above the Food and Drug Administration tolerance level of 2.0 mg kg−1 and other regulatory limits. The study found that chlorinated PCB congeners with a high octanol–water partition coefficient (Kow) dominated the congener profiles. The most dominant congener was #101, comprising 12.3% to 17.8% of the total PCB concentration. The study also found that the levels of non-carcinogenic hazard quotient (HQ) and hazard index (HI) were below 1, suggesting no non-carcinogenic health risks from consuming Clarias gariepinus. However, the cumulative cancer risks (∑CR) fall within the low CR classification (10−6–10−4) by USEPA for all age categories. Also, the toxic equivalent quantity range was comparatively and significantly higher than the estimated non-carcinogenic screening values, suggesting potential health concerns. The study concluded that regular and continuous consumption of Clarias gariepinus as a significant portion of the diet may expose humans to unacceptable PCB concentrations due to residual environmental concentrations rather than a recent introduction.
Environmental significanceThis study highlights critical environmental issues linked to the contamination of aquatic ecosystems with polychlorinated biphenyls (PCBs). PCBs, being highly persistent and bioaccumulative, pose a long-term threat to biodiversity and human health through bio-magnification in the food chain. The research findings emphasize the vulnerability of aquatic environments, especially in regions with poor waste management practices, to industrial, agricultural, and domestic pollutants. The elevated PCB levels in Clarias gariepinus, a staple fish species in Nigeria, underline the urgent need for stricter regulation of waste discharge into water bodies. The results serve as a baseline for identifying hotspots of PCB contamination and encourage proactive monitoring of aquatic ecosystems. Concentrating on the effects of PCBs, the prevention of indiscriminate waste disposal and the preservation of local water resources to safeguard public health and preserve ecological balance are promoted by this study. The study also highlights the possible health risks of eating contaminated fish, underscoring the significance of protecting aquatic biodiversity for future generations. |
1. Introduction
Polychlorinated biphenyls (PCBs) are synthetic organochlorine chemicals that were utilized as coolants and lubricants in transformers, generators, and electrical capacitors due to their electrical insulating qualities, low burning capacity, and chemical inertness.1 They were also utilized to make plasticizers for rubber and polyvinyl chloride polymers.2 Historically, they were made in the United States and Europe, and consequent to their highly toxic nature, the US government banned their production in 1977.3 Going by the persistence in the environment, PCBs were included in the United Nations Environmental Programme Stockholm Convention (2001) list of persistent organic pollutants (POPs) and later in 2004 by the Organization for Economic Co-operation and Development.4 However, despite the ban, PCB-containing electrical transformers and capacitors in use at that time were approved to remain in place or “grandfathered” into continued use.5 The spread continues because of slow degradation and high environmental persistence at dangerous levels. High levels of these chemicals have been discovered in non-producing regions such as Africa,6 and this has been linked to PCB atmospheric deposition. In Nigeria, the importation of used and old electrical equipment from developed nations, including electrical transformers and oils, during the 1970s and 1980s contributed to the presence of PCBs in the environment, particularly in the energy production sector. Additionally, run-offs from dumpsites where wastes from various sectors—including plasticizers, paints, adhesives, lubricants, and capacitors—are indiscriminately discarded have also been recognized as significant local sources of PCBs in Nigerian aquatic habitats and many other parts of the world.7Generally, PCBs and various organic pollutants enter the aquatic environment and become major sinks and sources in biological systems, including sediment, affecting the entire ecosystem.8,9 Most of these pollutants accumulate in organisms, reaching levels several times higher than in the water. The behavior and distribution of PCBs in aquatic systems depend significantly on the octanol–water partitioning coefficient (Kow).10,11 The Kow values are often used as a measure of the hydrophobic nature to estimate bioconcentration factors (accumulation after uptake from water), toxicity, and aquatic solubility.12,13 This is based on the assumption that the uptake of an organic substance is driven by its hydrophobicity.
Previous and most recent studies have identified fish as one suitable bioindicator of contaminants, including PCBs.14,15 Going by the feeding nature of some fish species including Clarias gariepinus, they can be exposed to such contaminated sediments, particularly during sediment remobilization. People can be indirectly exposed to this toxic chemical by consuming contaminated fish. In a recent study, the primary route of PCB absorption in man has been observed to be dietary exposure via the ingestion of aquatic animals, particularly fish.16 The accumulation of poorly metabolized compounds in fish can thus indicate the level of pollution in an aquatic system.17 The Environmental Protection Agency (EPA) rates PCBs as “probable human carcinogens” since they cause cancer in laboratory animals. In addition, the biological effects can cause cardiovascular disease, immune system suppression, neuro-behavioral changes, hypertension, hypothyroidism, infertility, and disorders of the reproductive system, among others.1
One of the most widely distributed African freshwater omnivorous fish is the African sharp-tooth catfish Clarias gariepinus. Clarias gariepinus and others like Heterobranchus dorsalis, Clarias buthupogon, Clarias anguillaris, and Clarias nigrodigitatus are the most common species found in Nigeria.18 Due to its hardiness and excellent growth rate, the gariepinus species is a common and significant cultured species, highly valued in Nigeria as a staple food for local communities and an ideal species for aquaculture. Clarias gariepinus is commonly used for pollution monitoring and is regarded as having high ecological significance. Therefore, it is a suitable organism for monitoring organic pollutants in aquatic environments.19 The relatively high-fat content of catfish meat allows fat-soluble environmental pollutants, including PCBs, to accumulate steadily.20 Several studies in Nigeria have investigated the levels of various toxicants in Clarias sp.18,21–25 Similarly, some previous studies on catfish and other fishes in Nigeria reported wide PCB concentration ranges including 0.064–4.254 mg kg−1 in Wupa river, Nigeria,24 0.00444–0.00501 mg kg−1 in Otamiri River,26 and 1.64–16.4 mg kg−1 and 0.56 mg kg−1 in Ogun and Ona rivers, respectively in Nigeria.27 In some other fishes, a range of 0.795–1.830 mg kg−1 and 0.557–1.688 mg kg−1 had been reported.28,29 These concentration ranges suggest that catfish consumption may be a significant source of PCB exposure.30,31
In several ecotoxicological studies, fish have been used to monitor several pollutants, including PCBs.19,32–34 Generally, the use of biological groups such as phytoplankton, invertebrates, and fish for pollution monitoring is a vital and rapidly growing field.17,25,35,36 In a recent report from the location investigated in this study, concentrations of PCBs were detected in sediments at levels of concern.37 In Nigeria and most African nations, information on PCB distribution across major rivers, coupled with congener-specific data, is limited. In addition, there is very scarce data on non-ortho congeners. Non-ortho PCBs (such as PCBs 77, 126, and 169) are considered particularly toxic due to their structural similarity to dioxins and are often referred to as coplanar PCBs. A better understanding of factors increasing PCB body burden in fish will help identify and provide solutions for exposure through subsistence fishing and other potential sources. Therefore, the objectives of this study are (i) to provide reliable data on PCBs in the Clarias gariepinus species with regard to tissue concentration from selected rivers within Southwestern Nigeria, (ii) to quantify the daily dietary intake of PCBs, and (iii) to evaluate the potential health risks associated with fish consumption from the study locations.
2. Materials and methods
2.1. The study area
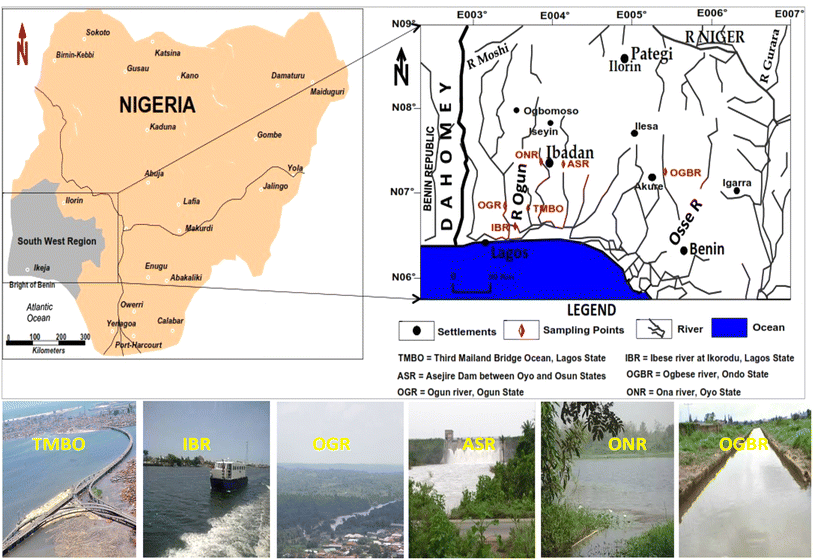
The locations and rivers examined (Fig. 1) have been detailed elsewhere.9,36–42 In summary, the six major rivers [Ibese River: IBR, Ogbese River: OGBR, Ona River: ONR, Asejire River: ASR, Ogun River: OGR, and the Ocean housing the Third Mainland Bridge (TMBO), Lagos] studied in this research are highly significant within the southwestern geopolitical zone in Nigeria (Fig. 1). Two of the rivers (TMBO and IBR) are situated in the city of Lagos State, a densely populated industrial and economic city in Nigeria. The remaining rivers are found in other states, where they serve as major water sources for agriculture, including fishing and irrigation, as well as for domestic activities.39,40 One of the rivers (ASR) has a dam on it that provides water for the waterworks in Oyo State, Nigeria, for processing potable water for the state. The areas surrounding these rivers are typically home to various industries, including food production, beverages, agriculture, and pharmaceuticals. Unfortunately, untreated effluents from these industries are discharged directly into the river through a network of canals. Additionally, drains are also connected to these rivers, leading to an indiscriminate discharge of various waste products. During the wet season, the magnitude of waste becomes huge due to run-off activities.2.2. Sample collection and preparation
Sampling was carried out during the dry and wet seasons of 2020 to 2022, as recently reported.37,42,43 Catfish (C. gariepinus) were sampled from all the investigated rivers. The ready availability at both seasons of the year was part of the reason for using this species in the current study. Fish were caught with the help of local fishermen. At each location, not less than fifteen (15) samples were obtained randomly, from which those considered of the same weight (115–122 g) and length (8.5–9.2 cm) were used for the analysis. Samples were washed with the river water, wrapped in hexane-treated aluminum foil, and placed inside an open-glass vessel within an ice box in the field. All samples were kept frozen at −20 °C on arrival in the laboratory before analysis. The head, backbone, fins, and tails were carefully removed from the partially frozen fish during preparation. Only the muscle of the fish, being the most desirable for consumption, was taken, chopped into small portions, homogenized, and separately pooled to form a composite for each river. The samples were later preserved under liquid nitrogen before analysis.2.3. Chemicals and standards
All the chemicals used, including dichloromethane and n-hexane (BDH, Poole, UK), and a standard PCB mixture (Supelco, Bellefonte, PA, USA) were of pesticide residue grade. Sulfuric acid and silica gel were from Fisher Scientific. Other solvents and chemicals, including anhydrous sodium sulfate, were obtained from Darmstadt, Germany.2.4. Extraction and analysis
The muscle tissues were sampled and processed for analysis, with three samples taken from each river, totaling eighteen samples per season. The extraction (in triplicate) follows EPA Method 8082A with a slight modification. Briefly, the fish samples (15 g each) were homogenized in excess anhydrous Na2SO4 (in a proportion of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Na2SO4: tissue) to remove excess moisture. The samples were Soxhlet extracted with 200 mL hexane: acetone (1
1, Na2SO4: tissue) to remove excess moisture. The samples were Soxhlet extracted with 200 mL hexane: acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 8 h (a mixture of hexane-acetone limits interference during extraction and improves the signal-to-noise ratio). Obtained fat extracts (after three cycles of extraction) were cleaned on sulfuric acid (certified ACS, Fisher Scientific)–silica gel (40% [vol/wt], Fisher Scientific). The final volume of the purified extract was reduced to 2 mL and transferred into a vial ready for gas chromatography-electron capture detector (GC-ECD) analysis.
1 v/v) for 8 h (a mixture of hexane-acetone limits interference during extraction and improves the signal-to-noise ratio). Obtained fat extracts (after three cycles of extraction) were cleaned on sulfuric acid (certified ACS, Fisher Scientific)–silica gel (40% [vol/wt], Fisher Scientific). The final volume of the purified extract was reduced to 2 mL and transferred into a vial ready for gas chromatography-electron capture detector (GC-ECD) analysis.
The extract was analyzed according to EPA Method 8082A with an Agilent 7820A gas chromatograph (Hewlett–Packard) coupled with an electron capture detector, as described previously.37 Briefly, the carrier gas (He) flow rate is 6 mL min; the makeup gas (argon/methane or nitrogen) flow rate is 30 mL min; injector temperature is 205 °C; detector temperature is 290 °C; initial temperature is 140 °C which will be maintained for 2 min; the temperature program is from 140 °C to 240 °C with holding for 5 min; and the final temperature is 265 °C which will be maintained for 18 min. Data acquisition was performed using Varian Star software V5.52. PCB standards were used to prepare a calibration for the measurement.
Quantification was achieved using an external standard calibration method. The method requires that a five-point calibration is obtained for linearity and to establish the response factor for a minimum of 5 peaks from each standard. The five-point calibration generated (congener peak areas versus standard concentrations) produced a regression coefficient (r2) of 0.9997.
2.5. Quality control and assurance
Apart from using pesticide residue grade reagents, this study employed matrix spike methods, procedural blanks (n = 3), and recoveries of standards (13C12-PCBs) for quality control and assurance. PCBs were not detected in the procedural blanks, indicating that there were no interference peaks present. Recoveries were determined by external standard calibration (n = 3) with five replicate samples and duplicate GC injections. The recovery from the matrix spiked sample ranged from 93.7–109%, while the surrogate PCB recoveries ranged from 88.9–96.7%. The limit of detection (LOD) and limit of quantification (LOQ, 0.2–0.6 mg kg−1) were based on signal-to-noise ratios of 3 and 10, respectively.2.6. Data analysis and risk assessment
In this study, we evaluated both carcinogenic and non-carcinogenic risks, following our recent publications.9,37–43 The assessment of the non-carcinogenic risks involved the estimation of the target hazard quotient (THQ) and hazard index (HI). However, the assessment of carcinogenic risks was based on toxic equivalency factors (TEFs): measures of the toxicity of other congeners in relation to the most toxic dioxin compound (2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-TCDD),44 through which the toxic equivalent quantity (TEQ) was estimated following the referenced guidelines.45 (See Text S1†).The exposure assessment focused on the ingestion pathway, which is a primary route of exposure through consumption among children, adolescents, and adults. The exposure dose via ingestion was estimated based on established constants from the literature (See Table S1†).46–48 In this study, the incremental lifetime cancer risk via ingestion (CRig) of C. gariepinus was estimated following the United States Environmental Protection Agency (USEPA) guidelines.49 The qualitative description of the CR as prescribed by the Risk Assessment Guidance was strictly followed in assessing the model (See Text S1†).49
Descriptive statistics were estimated using the Origin 2018 software. The seasonal trend in PCBs was evaluated with a one-way analysis of variance (ANOVA). In this study, a p-value of < 0.05 was considered statistically significant.
3. Results and discussion
3.1. PCB distribution in fish
The seasonal mean contents of PCBs in edible tissues of Clarias gariepinus from Southwestern rivers in Nigeria are summarized in Table 1 and Fig. 2. Of the 209 PCB congeners, twenty-five congeners were detected across all locations. In general, all samples are mixtures of Aroclor. That is, no sample in the data set represents a 100% contribution from a single source. Varying contributions of multiple sources impact every sample. However, based on the Nigerian environment, and the percentage of chlorine content, Aroclor 1242, which contains 42% chlorine by weight, and Aroclor 1254, which contains 54% chlorine by weight in relation to their industrial applications in electrical components, including heat transfer fluids, transformers, and capacitors within Nigeria, are suspected Aroclor formulations for the congeners detected in this study. In approximately 85% of all the samples, the individual PCB concentrations were below 1.0 mg kg−1 across all locations and seasons. The muscle tissues' total PCB concentrations (the sum of the twenty-five PCB congeners) varied across the two seasons (Fig. 2). In the wet season of 2020 and 2021, the sum of the PCBs ranged from 5.43–22.14 mg kg−1 with a median value of 11.98 mg kg−1, and from 5.26–23.52 mg kg−1 with a median value of 12.94 mg kg−1, respectively. However, in the dry season of 2019, the PCB content ranged from 4.66–21.96 mg kg−1 with a median value of 11.79 mg kg−1, while in 2020, it ranged from 4.63 mg kg−1–21.50 mg kg−1 with a median value of 11.57 mg kg−1 across all locations (Table 1). The data showed no significant differences in the median values across the various sampling seasons, which suggests (i) a small increase in PCBs across months and (ii) stable sources of this pollutant regardless of the season involved. In addition, fish, being a migratory species, may not necessarily be stable at a location to bioaccumulate, considering the rivers in question. Thus, dilution as the fish migrates across different ionic strengths within the river may be responsible for the lack of major seasonal differences. A more critical assessment showed that the ∑PCB content recorded at each of the locations is slightly higher during the wet season (Fig. 2).| PCB | K ow | ASR | ONR | OGBR | OGR | IBR | TMB0 |
|---|---|---|---|---|---|---|---|
| a ∑Co-PCB: Coplanar PCBs – PCB-77, 81, 105, 114, 118, 126, 156, 157, 167, and 169. ∑Marker-PCB: ICES indicator PCBs – PCB-28, −52, −101, −118, −138, −153, and −180. | |||||||
| PCB 8 | 5.301 | 0.039 ± 0.009 | 0.023 ± 0.004 | 0.011 ± 0.004 | 0.033 ± 0.006 | 0.170 ± 0.020 | 0.142 ± 0.020 |
| PCB 18 | 5.551 | 0.307 ± 0.020 | 0.277 ± 0.020 | 0.167 ± 0.020 | 0.325 ± 0.030 | 0.460 ± 0.040 | 0.659 ± 0.030 |
| PCB 28 | 5.691 | 0.150 ± 0.020 | 0.194 ± 0.020 | 0.129 ± 0.020 | 0.229 ± 0.020 | 0.338 ± 0.020 | 0.487 ± 0.040 |
| PCB 44 | 5.811 | 0.120 ± 0.010 | 0.228 ± 0.010 | 0.114 ± 0.010 | 0.191 ± 0.020 | 0.256 ± 0.020 | 0.331 ± 0.010 |
| PCB 52 | 6.091 | 0.160 ± 0.020 | 0.260 ± 0.030 | 0.084 ± 0.009 | 0.280 ± 0.030 | 0.139 ± 0.020 | 0.630 ± 0.040 |
| PCB 66 | 5.452 | 0.230 ± 0.040 | 0.270 ± 0.020 | 0.230 ± 0.020 | 0.310 ± 0.030 | 0.550 ± 0.030 | 0.693 ± 0.020 |
| PCB 77 | 6.523 | 0.120 ± 0.020 | 0.240 ± 0.020 | 0.230 ± 0.310 | 0.340 ± 0.030 | 0.490 ± 0.030 | 0.830 ± 0.040 |
| PCB 81 | 6.367 | 0.640 ± 0.030 | 0.870 ± 0.460 | 0.450 ± 0.020 | 0.640 ± 0.040 | 1.270 ± 0.050 | 2.080 ± 0.070 |
| PCB 101 | 7.071 | 0.880 ± 0.030 | 2.090 ± 0.080 | 0.800 ± 0.030 | 1.680 ± 0.040 | 2.070 ± 0.040 | 2.720 ± 0.060 |
| PCB 105 | 6.657 | 0.130 ± 0.020 | 0.540 ± 0.040 | 0.087 ± 0.020 | 0.540 ± 0.030 | 1.040 ± 0.050 | 1.440 ± 0.060 |
| PCB 114 | 6.657 | 0.150 ± 0.020 | 0.340 ± 0.040 | 0.120 ± 0.020 | 0.290 ± 0.040 | 0.440 ± 0.030 | 0.630 ± 0.020 |
| PCB 118 | 7.121 | 0.055 ± 0.006 | 0.010 ± 0.003 | 0.020 ± 0.006 | 0.009 ± 0.003 | 0.011 ± 0.003 | 0.022 ± 0.005 |
| PCB 126 | 6.897 | 0.280 ± 0.020 | 1.570 ± 0.040 | 0.220 ± 0.020 | 1.660 ± 0.050 | 2.080 ± 0.070 | 2.870 ± 0.060 |
| PCB 128 | 6.961 | 0.750 ± 0.030 | 1.760 ± 0.050 | 0.380 ± 0.020 | 1.450 ± 0.060 | 2.060 ± 0.080 | 2.450 ± 0.060 |
| PCB 138 | 7.441 | 0.008 ± 0.004 | 0.007 ± 0.003 | 0.009 ± 0.003 | 0.008 ± 0.003 | 0.009 ± 0.003 | 0.008 ± 0.004 |
| PCB 153 | 7.751 | 0.056 ± 0.009 | 0.010 ± 0.003 | 0.021 ± 0.003 | 0.008 ± 0.003 | 0.009 ± 0.004 | 0.030 ± 0.009 |
| PCB 156 | 7.187 | 0.009 ± 0.003 | 1.050 ± 0.030 | 0.009 ± 0.003 | 0.007 ± 0.004 | 1.610 ± 0.060 | 0.060 ± 0.010 |
| PCB 157 | 7.187 | 0.650 ± 0.020 | 0.912 ± 0.003 | 0.433 ± 0.010 | 1.700 ± 0.050 | 1.208 ± 0.004 | 2.070 ± 0.080 |
| PCB 167 | 7.277 | 0.220 ± 0.010 | 0.009 ± 0.003 | 0.190 ± 0.005 | 0.009 ± 0.003 | 0.008 ± 0.003 | 0.010 ± 0.004 |
| PCB 169 | 7.427 | 0.340 ± 0.030 | 0.490 ± 0.020 | 0.280 ± 0.010 | 0.540 ± 0.030 | 0.990 ± 0.020 | 0.018 ± 0.008 |
| PCB 170 | 7.277 | 0.009 ± 0.003 | 0.008 ± 0.003 | 0.008 ± 0.002 | 0.011 ± 0.004 | 0.010 ± 0.005 | 0.010 ± 0.003 |
| PCB 180 | 6.961 | 0.670 ± 0.030 | 0.902 ± 0.030 | 0.570 ± 0.020 | 0.960 ± 0.050 | 1.450 ± 0.040 | 2.060 ± 0.050 |
| PCB 187 | 7.177 | 0.130 ± 0.010 | 0.009 ± 0.003 | 0.064 ± 0.005 | 0.330 ± 0.020 | 0.010 ± 0.005 | 0.008 ± 0.003 |
| PCB 195 | 7.567 | 0.093 ± 0.020 | 0.530 ± 0.020 | 0.073 ± 0.005 | 0.130 ± 0.020 | 0.330 ± 0.030 | 0.440 ± 0.040 |
| PCB 206 | 9.143 | 0.234 ± 0.008 | 0.350 ± 0.020 | 0.290 ± 0.008 | 0.390 ± 0.020 | 0.690 ± 0.020 | 1.660 ± 0.060 |
| Mean∑PCB | 6.797 | 13.306 | 5.246 | 13.584 | 17.735 | 22.618 | |
| ∑Co-PCB | 2.599 ± 0.226 | 6.031 ± 0.497 | 2.046 ± 0.154 | 5.742 ± 0.627 | 9.179 ± 0.677 | 10.042 ± 1.052 | |
| ∑Marker-PCB | 1.974 ± 0.345 | 3.477 ± 0.772 | 1.636 ± 0.319 | 3.178 ± 0.638 | 4.029 ± 0.838 | 5.952 ± 1.096 | |
| % Marker-PCB | 30.73 | 26.83 | 32.74 | 26.30 | 22.78 | 26.61 | |
Fish sampled from the TMBO recorded the highest concentrations of PCBs. In general, based on the location, the PCB concentrations in the tissues decreased in the order TMBO > IBR > ONR > OGR > OGBR > ASR, with PCB-153 markedly dominant in all tissues. Thus, the study showed a statistically significant difference between the mean of all rivers as determined by one-way ANOVA [F (2.516) = 0.0046, p < 0.05]. Between ONR and OGR, there were no significant mean differences (p > 0.05). Similarly, between ASR and OGBR, no observable significant difference was recorded (P > 0.05). However, there are significant differences between IBR, TMBO, and other locations investigated in the study. The relatively lower concentrations of PCBs found at ASR and OGBR in fish indicate a relatively low rate of PCB pollution input.
3.2. Profiles of the PCB congeners in fish
Across all samples, seasons, and locations, the most dominant congener was PCB-101 (comprising 12.3–17.8% of the ∑PCB concentration), followed by PCB-128 (7.8–15.1%), PCB-126 (4.3–13.7%), PCB-180 (7.6–10.6%), PCB-157 (6.8–10.1%), and PCB-81 (5.2–10.1%). The octanol–water partitioning coefficients (Kow) of PCBs 81, 101, 126, 128, 157, and 180 are 6.367, 7.071, 6.897, 6.961, 7.187, and 6.961, respectively.50 This parameter (Kow) is very high among the respective isomeric group and PCB numbers detected in the study. These high Kow values increase PCB bioaccumulation because they are more soluble in lipids, leading to accumulation in the fatty tissues of fish. Therefore, PCBs 81, 101, 126, 128, 157, and 180 can be considered to have high lipophilicity and persist in the tissues of the fish analyzed in the study. PCBs 101, 126, and 180 were particularly present in higher concentrations than the other congeners in the tissues. The two commonly detected PCB congeners in animal samples, PCBs 153 and 138, were detected in this study.51 Interestingly, both PCBs were the least predominant in the study, even though they are highly lipophilic and have less tendency to be tightly bound to sediments, which should naturally enhance their availability for bioaccumulation.52The coplanar (dioxin-like PCBs PCB-77, 81, 105, 114, 118, 126, 156, 157, 167, and 169) PCBs ranged from 2.05–10.04 mg kg−1 across all locations (Table 1), with OGR and TMBO recording the lowest and highest load, respectively. Similarly, the summarized statistical estimates of the Marker PCBs or Indicator PCBs PCB-28, 52, 101, 118, 138, 153, and 180 are included in Table 1. These indicator PCBs represent different PCB patterns to account for non-dioxin-like PCBs.53 They are known to be persistent and highly bioaccumulative in the food chain.53 Total indicator PCB concentrations (range) of 1.97 (0.008–0.879) mg kg−1, 3.48 (0.008–2.093) mg kg−1, 1.64 (0.009–0.800) mg kg−1, 3.18 (0.008–1.682) mg kg−1, 4.03 (0.009–2.071) mg kg−1, and 5.95 (0.008–2.719) mg kg−1 were recorded at ASR, ONR, OGBR, OGR, IBR, and TMBO, respectively. Apart from PCBs-118, 138, and 153, all the other indicator PCBs exceeded the EU regulation of 0.075 mg kg−1 limit for ocean fish.54 In general, the average percentage concentration of the sum of the indicator PCBs to ∑PCB across all locations (Table 1) was 27.7% (range 22.8–32.7%). This percentage is lower than the prescribed 50% attributable to the six indicator PCBs by the European Food Safety Authority.53 The range falls within 24–42% in marine fish obtained from tsunami-stricken areas following the Great East Japan Earthquake in 2011.55
Bioaccumulation was reasonably high for PCBs 101 and 180 compared to PCBs 138 and 153, which have similar characteristics.56 This suggests the contribution of other factors in controlling the rate of bioaccumulation, metabolism, and elimination from the organism.57,58 The enhanced proportion of PCBs 101 (12.3–17.8%) and 180 (7.8–10.6%) of all PCB congeners across all locations possibly reflects a low rate of biotransformation, resistance to metabolism, and slow rate of elimination by cytochrome P-450 iso-enzymes, while reduced levels of PCBs 153 and 138 may reflect an increased metabolic and elimination rate.59–61
3.3. Influence of the physical and chemical properties of PCBs on bioaccumulation
The study shows that factors like equilibrium partition between the fish and ambient water possibly play a vital role in PCB uptake and elimination. Clarias gariepinus is a migratory species, capable of being exposed to PCBs either by dermal, inhalation, or ingestion routes. Consequently, defining levels of PCBs in fish revolves around several factors, including the physicochemical properties of the water. In this study, the order of decreasing homolog concentration follows TeCB > HeCB > PeCB > HpCB > TCB ≈ NoCB > OcCB > DCB (Fig. 3). It is not surprising that DCB is the lowest across all locations due to its ability to reach a quicker equilibrium between ambient water and C. gariepinus based on the relatively lower partition coefficient (Kow). The trend equally reveals that highly chlorinated PCB congeners with high Kow dominated the congener profiles. For instance, the high percentage levels (40–61%) of planar congeners (PCBs-77, 81, 105, 114, 118, 126, 156, 157, 167, and 169) can be attributed to their favorable accumulation via cell membranes.62 In addition, varied PCB concentrations and congeners can also be related to the rate of metabolism.63The trend as reported (proportion of the low-chlorinated PCBs) suggests the fish were not significantly contaminated with fresh PCBs because fish metabolize PCBs slowly compared to mammals.64 By implication, the level of PCBs reported in this study may not be associated with fresh contamination of high levels of PCBs, which should have enhanced the proportions of the low-chlorinated PCBs in contrast to what was obtained in this study. Previous studies have shown that the detection of PCB-28 and PCB-52 suggests recent exposure.65 However, the percentage contributions of these two PCBs (#28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.76–2.36%; #52
1.76–2.36%; #52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93–2.80%) are lower compared to those of most other congeners detected in the study. In other words, the level of PCBs obtained in tissues of C. gariepinus in this study was most likely derived majorly from residual concentrations in the environment and less from recent introduction.
0.93–2.80%) are lower compared to those of most other congeners detected in the study. In other words, the level of PCBs obtained in tissues of C. gariepinus in this study was most likely derived majorly from residual concentrations in the environment and less from recent introduction.
3.4. Comparison of PCB concentration with other studies
In comparison to previous studies (Table 2), the levels of PCBs in this study showed significant differences. The ∑25 PCB concentrations found in Clarias gariepinus from the rivers in this study were similar to the range of concentrations found in muscles of Clarias gariepinus from Ogun River, Nigeria (1.64–16.4 mg kg−1),66 and in fish from Galveston Bay, Texas (0.29–110 mg kg−1).67 However, the range of PCB concentrations in this study is much higher than those in several reports in Nigeria, including the levels found in Tilapia zilli from Lagos Lagoon (0.56–2.94 mg kg−1),68 fish from Eleyele Reservoir, Southwestern Nigeria (0.33–2.53 mg kg−1,69Clarias gariepinus from Ovia River, Southern Nigeria (0.001–0.003 mg kg−1),24 and Clarias gariepinus from Ogun River and Ona River (1.64–16.4 mg kg−1 and 0.56 mg kg−1), respectively.66| Locations | Name of species | Fish part | Concentrations | References |
|---|---|---|---|---|
| a DS: Dry season; WS: Wet season. | ||||
| Southwest rivers, Nigeria | Clarias gariepinus | Muscle | DS: 4.63–21.96 mg kg−1 | This study |
| WS: 5.26–23.53 mg kg−1 | ||||
| Wupa river, Abuja, Nigeria | Clarias anguillaris | 64–4254 ng g−1 | ||
| Ovia river, Southern Nigeria | Clarias gariepinus | Muscle | 0.001–0.003 mg kg−1 | Tongo and Ezemonye (2018) |
| Ogun river, Nigeria, Ona river, Nigeria | Clarias gariepinus | Muscle | 1.64–16.4 mg kg−1 | Adeogun et al. (2016) |
| 0.56 mg kg−1 | ||||
| Lagos lagoon | Tilapia zilli (adult) | Muscle | 00.56–2.94 mg kg−1 | Adeyemi et al. (2009) |
| Gulf of Mexico, Ionian sea | Bluefin tuna: Thunnus thynnus Yellowfin tuna: Thunnus albacares | 8.08 ± 5.08 (w/w) | Nicklisch, et al. (2017), Storelli et al. (2008) | |
| 5.3–35.0 (15.9 ± 8.0) (w/w) | ||||
| Hartbeespoort dam, South Africa | Clarias gariepinus | Muscle | 0 mg kg−1 (5 year old catfish) | Rimayi and Chimuka (2019) |
| Common carp (Cyprinus carpio) | Muscle | 1.8 mg kg−1 | Rimayi and Chimuka (2019) | |
| Laguna de Términos, Campeche | Lepisosteus tropicus | Muscle | 0.782 ng g−1 | Carvalho et al. (2009) |
| Crassostrea spp | Muscle | 0.013–4.847 ng g−1 | Carvalho et al. (2009) | |
| Coatzacoalcos river estuary, Veracruz, Mexico | Centropomus parallelus | Muscle | 0.20 ng g−1 | Espinosa-Reyes et al. (2012) |
| Coast of Florida, United States | M. cephalus | Muscle | 3.4–59.3 ng g−1 | Karouna-Renier et al. (2011) |
| Red sea coast, Saudi Arabia | C. chanos, M. cephalus | Muscle | 0.22–44.3 ng g−1 (w/w) | Batang et al. (2016) |
| 33.9–82.5 ng g−1 (w/w) | ||||
| Zemplinska Sirara water reservoir | Common carp (Cyprinus carpio) | Muscle | 10.2 mg kg−1 | Brazova et al. (2011) |
| Zemplinska Sirara water reservoir | Freshwater bream (Abramis brama) | Muscle | 128.0 mg kg−1 | Brazova et al. (2011) |
| Coast of Malacca, Malaysia | Lutjanus argentimaculatus | Muscle | 0.43–0.67 ng g−1 (w/w) | Mohamad et al. (2015) |
| Coastal lagoons, central Mexican Pacific | Mugil sp. | Muscle | Bdl–0.13 ng g−1 (w/w) | Ramírez-Ayala (2021) |
In addition, various studies have reported lower concentrations of contaminants in different parts of the world. For instance, in Hartbeespoort Dam, South Africa, Clarias gariepinus had 0 mg kg−1 and Cyprinus carpio had 1.8 mg kg−1.33 Luxembourg River fish were reported to have concentrations ranging from 0.05 to 3.50 mg kg−1.70 Marine fish from tsunami-stricken areas of Japan showed concentrations ranging from 0.00044 to 0.086 mg kg−1,55 while marine fish from the Persian Gulf exhibited concentrations from 0.0072 to 0.0902 mg kg−1.71 In the Red Sea coast (Saudi Arabia), PCB values were recorded in the range of 0.0002–0.0443 mg kg−1 for C. Chanos and 0.0339–0.0825 mg kg−1 forM. cephalus.72 Additionally, muscles of M. cephalus from 15 sites on the coast of Florida, United States, were reported to have concentrations ranging from 0.0034 to 0.0593 mg kg−1,73 while Mugil spp from Coastal lagoons, Central Mexican Pacific, showed concentrations from Bdl to 0.16 ng g−1 w/w,34 which are far below the range recorded in this study. On the other hand, a much higher concentration of 128.0 mg kg−1 was found in Abramis brama from the Zemplinska Sirara Water reservoir, surpassing the mean concentration obtained in this study (Table 2).74
3.5. Human health risks
| Minimum | Maximum | ∑EDI | ||
|---|---|---|---|---|
| ASR | CHD | 5.94 × 10−3 | 0.671 | 4.894 |
| ADL | 4.89 × 10−3 | 0.551 | 4.027 | |
| ADT | 3.56 × 10−3 | 0.402 | 2.935 | |
| ONR | CHD | 5.72 × 10−3 | 1.590 | 8.425 |
| ADL | 4.70 × 10−3 | 1.312 | 6.933 | |
| ADT | 3.43 × 10−3 | 0.956 | 5.053 | |
| OGBR | CHD | 5.91 × 10−3 | 0.609 | 3.807 |
| ADL | 4.85 × 10−3 | 0.501 | 3.133 | |
| ADT | 3.54 × 10−3 | 0.365 | 2.283 | |
| OGR | CHD | 5.72 × 10−3 | 1.295 | 9.208 |
| ADL | 4.70 × 10−3 | 1.066 | 7.577 | |
| ADT | 3.43 × 10−3 | 0.777 | 5.522 | |
| IBR | CHD | 6.27 × 10−3 | 1.581 | 11.384 |
| ADL | 5.16 × 10−3 | 1.301 | 9.367 | |
| ADT | 3.76 × 10−3 | 0.948 | 6.827 | |
| TMBO | CHD | 5.85 × 10−3 | 2.189 | 17.044 |
| ADL | 4.81 × 10−3 | 1.802 | 14.025 | |
| ADT | 3.51 × 10−3 | 1.313 | 10.222 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 children, 319
634 children, 319![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 182 adolescents, and 437
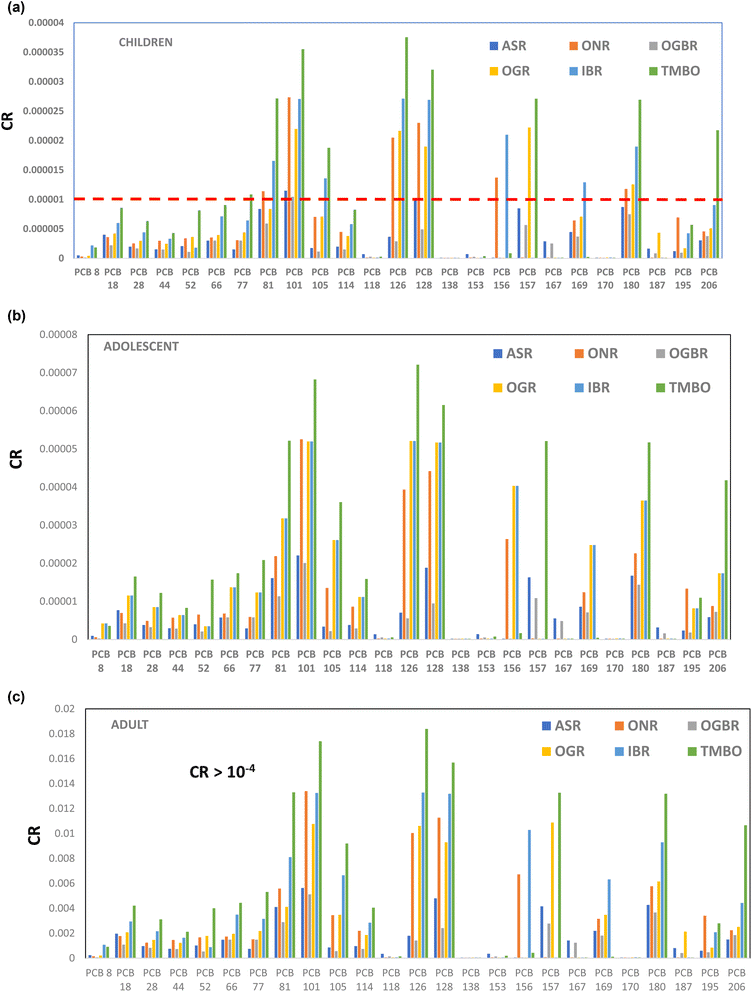
182 adolescents, and 437![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 adults may be at risk of cancer through consumption of fish from the investigated rivers (Fig. 5).
915 adults may be at risk of cancer through consumption of fish from the investigated rivers (Fig. 5).
| ASR | ONR | OGBR | OGR | IBR | TMBO | SV | ||
|---|---|---|---|---|---|---|---|---|
| a SV: Screening value: values in brackets are SV for carcinogenic risks while those outside brackets are for non-carcinogenic risks. | ||||||||
| Children | Min | 7.129 × 10−6 | 6.855 × 10−6 | 7.083 × 10−6 | 6.855 × 10−6 | 7.518 × 10−6 | 7.015 × 10−6 | 0.0260 (0.0066) |
| Max | 8.032 × 10−4 | 1.913 × 10−3 | 7.310 × 10−4 | 1.554 × 10−3 | 1.897 × 10−3 | 2.626 × 10−3 | ||
| Total | 5.871 × 10−3 | 1.011 × 10−2 | 4.567 × 10−3 | 1.104 × 102 | 1.365 × 10−2 | 2.044 × 10−2 | ||
| Adolescents | Min | 9.781 × 10−6 | 9.405 × 10−6 | 9.718 × 10−6 | 9.405 × 10−6 | 1.031 × 10−5 | 9.624 × 10−6 | 0.0425 (0.0106) |
| Max | 1.102 × 10−3 | 2.625 × 10−3 | 1.003 × 10−3 | 2.131 × 10−3 | 2.602 × 10−3 | 3.603 × 10−3 | ||
| Total | 8.054 × 10−3 | 1.386 × 10−2 | 6.266 × 10−3 | 1.515 × 10−2 | 1.873 × 10−2 | 2.805 × 10−2 | ||
| Adults | Min | 1.189 × 10−5 | 1.143 × 10−5 | 1.181 × 10−5 | 1.143 × 10−5 | 1.253 × 10−5 | 1.170 × 10−5 | 0.0437 (0.0109) |
| Max | 1.339 × 10−3 | 3.190 × 10−3 | 1.219 × 10−3 | 2.590 × 10−3 | 3.163 × 10−3 | 4.379 × 10−3 | ||
| Total | 9.788 × 10−3 | 1.685 × 10−2 | 7.615 × 10−2 | 1.842 × 10−2 | 2.277 × 10−2 | 3.409 × 10−2 | ||
To further establish the potency of PCBs to cause carcinogenic health risks, toxic equivalency factors (TEFs) were used to estimate the toxic equivalent quantity (TEQ) (Table 5). Based on the mean PCB concentrations of both seasons, the total non-ortho PCBs (77, 81, 126, and 169) ranged from 3.19–29.27, the mono-ortho PCBs (105, 114, 118, 156, 157, and 167) ranged from 0.03–0.18, and the di-ortho PCBs (170–180) ranged from 8.05 × 10−4–2.36 × 10−3. The PCB-TEQ values ranged from 3.22 to 29.45 (Table 5). The TEQ range was comparatively and significantly higher than the estimated non-carcinogenic screening values (SV) of 0.026, 0.0425, and 0.0437, and carcinogenic SV of 0.0066, 0.0106, and 0.0109 for children, adolescents, and adults, respectively (included in Table 4). The SV represents the PCB threshold concentration of potential public health concern.80 Based on the SV values, the PCB levels obtained in the current study are of serious potential health concern.
| PCBs | TEF | ASR | ONR | OGBR | OGR | IBR | TMBO | |
|---|---|---|---|---|---|---|---|---|
| a TEQ: toxic equivalent concentration = Σ [concentration of each dioxin-like congener X 2,3,7,8-TCDD TEF]. b TEF: toxic equivalent factor; $: Ding et al. (2012). | ||||||||
| Non-ortho | 77 | 0.005 | 0.058 | 0.118 | 0.116 | 0.169 | 0.246 | 0.415 |
| 81 | 0.0003 | 0.019 | 0.026 | 0.014 | 0.019 | 0.038 | 0.062 | |
| 126 | 0.1 | 2.800 | 15.684 | 2.211 | 16.575 | 20.754 | 28.735 | |
| 169 | 0.01 | 1.026 | 1.481 | 0.849 | 1.629 | 2.963 | 0.054 | |
| ∑(77–169) | 3.904 | 17.308 | 3.189 | 18.393 | 24.001 | 29.266 | ||
| Mono-ortho | 105 | 0.0001 | 4.018 × 10−3 | 0.016 | 2.624 × 10−3 | 0.016 | 0.031 | 0.043 |
| 114 | 0.0005 | 7.578 × 10−3 | 0.017 | 5.763 × 10−3 | 0.014 | 0.022 | 0.032 | |
| 118 | 0.0001 | 1.637 × 10−3 | 2.947 × 10−4 | 6.405 × 10−4 | 2.873 × 10−4 | 3.150 × 10−4 | 0.001 | |
| 156 | 0.0005 | 4.425 × 10−4 | 0.052 | 4.300 × 10−4 | 3.750 × 10−4 | 0.080 | 0.003 | |
| 157 | 0.0005 | 3.248 × 10−2 | 5.800 × 10−4 | 0.022 | 0.085 | 4.112 × 10−4 | 0.104 | |
| 167 | 0.00001 | 6.622 × 10−4 | 2.827 × 10−5 | 5.801 × 10−4 | 2.655 × 10−5 | 2.527 × 10−5 | 2.835 × 10−5 | |
| ∑(105–167) | 0.047 | 0.087 | 0.032 | 0.117 | 0.134 | 0.182 | ||
| Di-ortho | 170 | 0.0001 | 2.580 × 10−4 | 2.542 × 10−4 | 2.325 × 10−4 | 3.165 × 10−4 | 3.360 × 10−4 | 2.940 × 10−4 |
| 180 | 0.00001 | 6.672 × 10−4 | 9.015 × 10−4 | 5.728 × 10−4 | 9.617 × 10−4 | 1.453 × 10−3 | 2.061 × 10−3 | |
| ∑(170–180) | 9.252 × 10−4 | 1.156 × 10−3 | 8.053 × 10−4 | 1.278 × 10−3 | 1.789 × 10−3 | 2.355 × 10−3 | ||
| TEQ = ∑ (77–180) | 3.952 | 17.396 | 3.221 | 18.511 | 24.138 | 29.451 | ||
| % Non-ortho PCBs | 99.16 | 99.60 | 99.25 | 99.45 | 99.56 | 99.56 | ||
| % PCB-126 | 54.18 | 82.60 | 55.35 | 85.78 | 80.12 | 80.81 | ||
The non-ortho PCB congeners constituted over 99.2% of the ∑TEQ values across all the locations investigated in this study. One member, PCB-126, is the most toxic, representing between 55.4 and 85.8% of the total dioxin-like PCB contributions across all locations, in addition to the high contribution to the total PCBs in the study. In addition, the dioxin-like PCBs represented 40%, 47%, 41%, 48%, 52%, and 45% of the mean total PCBs recorded at ASR, ONR, OGBR, OGR, IBR, and TMBO, respectively.
It is evident from the study that PCBs continue to be present in fish, and the significance of intake via ingestion was also highlighted. High accumulation of PCBs in adults over several years is particularly of concern, especially in the mother's body where it is usually stored in the fat in breast milk through which breastfeeding infants are exposed.81,82 Other studies have shown prenatal transfer of PCBs by crossing the placental barrier.83,84 Therefore, relevant stakeholders at all levels of government are to take proactive measures that will prevent or at least minimize the release of these carcinogenic substances (PCBs) and other related carcinogens into the aquatic environment to safeguard human health.
4. Conclusions
This study found a seasonal increase in polychlorinated biphenyls (PCBs) in Clarias gariepinus fish from six rivers in southwestern Nigeria, with higher concentrations during the wet season. All recorded PCB levels exceeded regulatory limits, primarily featuring highly chlorinated PCBs, which made up over 86% of the total concentrations. The detection of PCB 28 and PCB 52 suggests ongoing contamination. Health risk assessments indicated no significant non-carcinogenic effects from PCB exposure through fish consumption, but low carcinogenic risks were noted, particularly for children, who are the most vulnerable. Recreational and subsistence fishers near the TMBO and IBR areas of Lagos State are especially at risk. To address these risks, public health policies should improve education on the importance of preserving aquatic ecosystems and preventing waste disposal into rivers. This study underscores the need for monitoring water and sediment quality for PCBs to reduce their environmental impact and supports a comprehensive assessment of aquatic species in southwestern Nigeria for PCBs and other persistent organic pollutants (POPs). These measures aim to enhance health risk mitigation and improve environmental safety.Data availability
All data and related information not in the ESI† are available from the corresponding author (E-mail: isaac.ololade@aaua.edu.ng).Author contributions
Isaac Ayodele Ololade: conceptualization; data curation; formal analysis; funding acquisition; investigation; mthodology; resources; validation; project administration; visualization; writing – original draft; writing – review & editing; Abiodun Oyewumi Apata: investigation; methodology; writing – review & editing; Olubunmi Jerome Oloyede, Ifeoluwa Akindumila, and Olubunmi Jerome Oloyede: methodology, software, data curation, manuscript draft preparation. Omotayo Precious Asanga and Francis Femi Oloye: validation, software and data curation. Writing of the article and approval of the final manuscript were done by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate and would like to acknowledge all the graduate students in the Environmental Monitoring Unit, Department of Chemical Sciences, Adekunle Ajasin University, Akungba-Akoko, Nigeria for their assistance during the sampling and laboratory work of this study. Special thanks are given to the staff of the Central Research Laboratory, Adekunle Ajasin University, Akungba-Akoko (AAUA), Nigeria for providing an enabling environment for the laboratory work of this study. The authors would also like to thank the reviewers for their constructive feedback.References
- H. E. Obanya, C. Ntor, C. U. Okoroafor and R. Nwanze, Occurrence of a Polychlorinated Biphenyl (PCB) Congener in Surface Water, Sediments and Blackchin Tilapia (Sarotherodonmelanotheron) from Ologe Lagoon, Nigeria, J. Appl. Sci. Environ. Manag., 2019, 23(10), 1805–1811 CAS.
- N. M. Niehoff, E. C. Zabor, J. Satagopan, A. Widell, T. R. O'Brien, M. Zhang, N. Rothman, T. K. Grimsrud, S. K. Van Den Eeden and L. S. Engel, Prediagnostic serum polychlorinated biphenyl concentrations and primary liver cancer: A case-control study nested within two prospective cohorts, Environ. Res., 2020, 187, 109690 CrossRef CAS PubMed.
- R. Gioia, A. J. Akindele, S. A. Adebusoye, K. A. Asante, S. Tanabe, A. Buekens and A. J. Sasco, Polychlorinated biphenyls (PCBs) in Africa: a review of environmental levels, Environ. Sci. Pollut. Res., 2013, 21(10), 6278–6289 CrossRef PubMed.
- United Nations Environmental Programme Stockholm Convention, The 12 initial POPs under the Stockholm Convention, 2001, [cited 2 February 2018]. Available from: http://www.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx.
- J. P. Berninger and D. E. Tillitt, Polychlorinated Biphenyl Tissue-Concentration Thresholds for Survival, Growth, and Reproduction in Fish, Environ. Toxicol. Chem., 2019, 38(4), 712–736 CrossRef CAS PubMed.
- O. E. Akinrinade, W. A. Stubbings, M. A.-E. Abdallah, O. Ayejuyo, R. Alani and S. Harrad, Atmospheric concentrations of polychlorinated biphenyls, brominated flame retardants, and novel flame retardants in Lagos, Nigeria indicate substantial local sources, Environ. Res., 2022, 204, 112091 CrossRef CAS PubMed.
- M. H. Wong, S. C. Wu., W. J. Deng, X. Z. Yu, Q. Luo, A. O. W. Leung and A. S. Wong, Export of toxic chemicals–A review of the case of uncontrolled electronic-waste recycling, Environ. Pollut., 2017, 149(2), 131–140 CrossRef.
- M. P. Okoh, Exposure to Organo-Chlorinated Compound, PolyChlorinated Biphenyl (PCB), environmental and public health Implications: A Nigeria Case study, Int. J. Chem. Stud., 2015, 2, 14–21 Search PubMed.
- I. A. Ololade, A. O. Apata, N. A. Oladoja, B. A. Alabi and O. O. Ololade, Polycyclic aromatic hydrocarbons in rivers and health risk consequences of human exposure: a Nigerian case study, Int. J. Energy Wat. Res., 2023 DOI:10.1007/s42108-023-00236-1.
- C. T. Chiou, V. H. Freed, D. W. Schmedding and R. L. Kohnert, Partition coefficient and bioaccumulation of selected organic chemicals, Environ. Sci. Technol., 1977, 11(5), 475–478 CrossRef CAS.
- G. Patil, Correlation of aqueous solubility and octanol-water partition coefficient based on molecular structure, Chemosphere, 1991, 22(8), 723–738 CrossRef CAS.
- K. B. Woodburn, W. J. Doucette and A. W. Andren, Generator column determination of octanol/water partition coefficients for selected polychlorinated biphenyl congeners, Environ. Sci. Technol., 1987, 18, 457–459 CrossRef PubMed.
- D. W. Hawker and D. W. Connell, Octanol–water partition coefficients of polychlorinated biphenylcongeners, Environ. Sci. Technol., 1988, 22, 382–387 CrossRef CAS.
- L. Bervoets and R. Blust, Metal concentrations in water, sediment, and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor, Environ. Pollut., 2003, 126(1), 9–19 CrossRef CAS PubMed.
- X. Cao, R. Lu, Q. Xu, X. Zheng, Y. Zeng and B. Mai, Distinct biomagnification of chlorinated persistent organic pollutants in adjacent aquatic and terrestrial food webs, Environ. Pollut., 2023, 317, 120841 CrossRef CAS PubMed.
- M. Pruvost-Couvreura, B. Le Bizeca, I. Margaritis, J. Volatier, C. Béchaux and G. Rivière, Impact of dietary guidelines on lifetime exposure to chemical contaminants: divergent conclusions for two bioaccumulative substances, Food Chem. Toxicol., 2020, 145, 111672 CrossRef PubMed.
- A.-W. A. Abdel-Warith, E.-S. M. I. Younis, N. A. Al-Asgah, A. M. Rady and H. Y. Allam, Bioaccumulation of lead nitrate in tissues and its effects on hematological and biochemical parameters of Clarias gariepinus, Saudi J. Biol. Sci., 2020, 27(3), 840–845 CrossRef CAS PubMed.
- I. A. Ololade and O. Ogini, Behavioural and hematological effects of zinc on African Catfish, Clarias gariepinus, Int. J. Fish. Aquac., 2009, 1(2), 022–027 CAS.
- S. Squadrone, L. Favaro, M. Prearo, B. Vivaldi, P. Brizio and M. C. Abete, NDL-PCBs in muscle of the European catfish (Silurus glanis): An alert from Italian rivers, Chemosphere, 2013, 93(3), 521–525 CrossRef CAS PubMed.
- H. Fiedler, K. S. Cooper, M. H. Bergek, C. Rappe, M. Bonner, F. Howell, K. Willett and S. Safe, PCDD, PCDF, and PCB in farm-raised catfish from Southeast United States - concentrations, sources, and CYP1A induction, Chemosphere, 1998, 37(9–12), 1645–1656 CrossRef CAS PubMed.
- J. N. Aguigwo, Studies on acute toxicity of Cassava leaf extracts on African catfish Clarias angullaris, J. Aquat. Sci., 1998, 13, 29–32 Search PubMed.
- R. Maheswaran, A. Devapanl, S. Muralidharan, B. Velmurugan and S. Ignaeimuthu, Haematological studies of fresh water fish, Clarias batradrus (L) exposed to mercuric chloride, Int. J. Integr. Biol., 2008, 2(1), 49–54 CAS.
- I. Tongo and L. Ezemonye, Human Health Risk Assessment of PAHs in Fish and Shellfish from Amariaria Community, Bonny River, Nigeria, J. Appl. Sci. Environ. Manag., 2018, 22(5), 731 CAS.
- T. A. Ayandiran, O. O. Fawole and M. A. Ogundiran, Polycyclic aromatic hydrocarbon concentrations in Clarias gariepinus from Oluwa River, Ondo State, Nigeria, Res. J. Environ. Toxicol., 2022, 16(1), 1–11 CrossRef CAS.
- I. A. Ololade, A. O. Apata, B. A. Alabi, O. I. Akindumila, O. J. Oloyede and B. A. Obasusi, Polycyclic aromatic hydrocarbons (PAHs) in fish (Clarias gariepinus) of southwestern rivers, Nigeria: Occurrence, distribution, and potential human exposure risks, Reg. Stud. Mar. Sci., 2024, 103687 Search PubMed.
- K. E. Onwuka, O. C. Atasie, S. O. E. Okereke, N. E. Enenwa, I. A. Onuabuchi and C. E. Osuigwe, Risk Assessment of Polychlorinated biphenyls (PCBs) in Fish Samples from Otamiri River, Imo State, Nigeria, Int. J. Green Herb. Chem., 2022, 8(2), 10–19 Search PubMed.
- A. O. Adeogun, A. V. Chukwuka, C. P. Okoli and A. Arukwe, Concentration of polychlorinated biphenyl (PCB) congeners in the muscle of Clarias gariepinus and sediment from inland rivers of southwestern Nigeria and estimated potential human health consequences, J. Toxicol. Environ. Health, Part A, 2016, 79(21), 969–983 CrossRef CAS PubMed.
- J. P. Unyimadu, O. Osibanjo and J. O. Babayemi, Polychlorinated Biphenyls in Brackish Water Fish in the River Niger, Nigeria, J. health pollut., 2018, 8(17), 31–42 CrossRef PubMed.
- V. O. Eyenubo, V. O. Peretomode, F. Egharevba, S. A. Osakwe and O. G. Avwioro, Polychlorinated biphenyls (PCBs) in sediments and fish from dredged tributaries and creeks of river Ethiope, South-South, Nigeria: sources, risk assessment and bioaccumulation, J. Niger. Soc. Phys. Sci., 2024, 6, 1951 CrossRef.
- M. Weintraub and L. S. Birnbaum, Catfish consumption as a contributor to elevated PCB levels in a non-Hispanic black subpopulation, Environ. Res., 2008, 107(3), 412–417 CrossRef CAS PubMed.
- D. R. Luellen, M. J. LaGuardia, T. D. Tuckey, M. C. Fabrizio, G. W. Rice and R. C. Hale, Assessment of legacy and emerging contaminants in an introduced catfish and implications for the fishery, Environ. Sci. Pollut. Res. Int., 2018, 25(28), 28355–28366 CrossRef CAS PubMed.
- K. Akutsu, K. Kuwabara, Y. Konishi, H. Matsumoto, Y. Murakami and Y. Tanaka, et al., Congener-specific analysis of PCBs in food samples by using GC/MS. ShokuhinEiseigakuZasshi, Food Hyg. Saf. Sci., 2005, 46, 99–108 CrossRef CAS PubMed.
- C. Rimayi and L. Chimuka, Organ-specific bioaccumulation of PCBs and PAHs in African sharptooth catfish (Clarias gariepinus) and common carp (Cyprinus carpio) from the Hartbeespoort Dam, South Africa, Environ. Monit. Assess., 2019, 191, 700 CrossRef CAS PubMed.
- E. Ramírez-Ayala, M. A. Arguello-Pérez, A. Tintos-Gómez, J. H. Hernández-Anguiano, R. Y. Pérez-Rodríguez, C. A. Ilizaliturri-Hernández, G. Núñez-Nogueira and C. A. Sepúlveda-Quiroz, Persistent organic pollutants (POPs) in fish from two coastal lagoons of the central Mexican Pacific, Lat. Am. J. Aquat. Res., 2015, 49(4), 663–670 CrossRef.
- C. N. Ibeto, W. C. Nkechi and N. R. Ekere, Health Risks of Polychlorinated Biphenyls (PCBs) Levels in Fish and Sediment from River Niger (Onitsha Axis), J. Aquat. Food Prod. Technol., 2019, 2, 138–149 CrossRef.
- R. Riaz, C. A. de Wit and R. N. Malik, Persistent organic pollutants (POPs) in fish species from different lakes of the lesser Himalayan region (LHR), Pakistan: The influence of proximal sources in distribution of POPs, Sci. Total Environ., 2020, 760, 143351 CrossRef PubMed.
- A. Apata, I. A. Ololade, N. A. Oladoja, B. A. Alabi and O. O. Ololade, Seasonal congener survey for polychlorinated biphenyls in sediments of south-western rivers, Nigeria: Occurrence, sources, and ecotoxicological risks, Reg. Stud. Mar. Sci., 2022, 55, 102623 Search PubMed.
- A. Apata, I. A. Ololade, N. A. Oladoja, B. A. Alabi and O. O. Ololade, Polycyclic aromatic hydrocarbons in selected rivers in southwestern Nigeria: Seasonal distribution, source apportionment and potential risk assessment, Reg. Stud. Mar. Sci., 2022, 52, 102318 Search PubMed.
- I. A. Ololade, A. Apata, N. A. Oladoja, B. A. Alabi and O. O. Ololade, Microplastic particles in river sediments and water of southwestern Nigeria: insights on the occurrence, seasonal distribution, composition, and source apportionment, Environ. Sci. Pollut. Res., 2023, 31(1), 1314–1330 CrossRef PubMed.
- I. A. Ololade, B. O. Adetiba, F. F. Oloye, O. O. Ololade, N. A. Oladoja, S. B. Obadawo, M. M. Anifowose, T. A. Akinnifesi, D. Akerele, A. B. Alabi and A. O. Adeola, Bioavailability of polycyclic aromatic hydrocarbons (PAHs) and Environmental Risk (ER) Assessment: The case of the Ogbese river, Nigeria, Reg. Stud. Mar. Sci., 2017, 9, 9–16 Search PubMed.
- I. A. Ololade, I. A. Arogunrerin, N. A. Oladoja, O. O. Ololade and A. B. Alabi, Concentrations and Toxic Equivalency of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyl (PCB) Congeners in Groundwater around Waste Dumpsites in South-West Nigeria, Arch. Environ. Contam. Toxicol., 2021, 80(1), 134–143 CrossRef CAS PubMed.
- I. A. Ololade, A. O. Apata, B. A. Alabi, O. I. Akindumila, O. J. Oloyede and B. A. Obasusi, Polycyclic aromatic hydrocarbons (PAHs) in fish (Clarias gariepinus) of southwestern rivers, Nigeria: Occurrence, distribution, and potential human exposure risks, Reg. Stud. Mar. Sci., 2024, 77, 103687 Search PubMed.
- I. A. Ololade, A. O. Apata, N. A. Oladoja, O. J. Oloyede, O. O. Ololade, O. P. Asanga and F. F. Oloye, Occurrence, seasonal distribution and probabilistic source-specific health risk assessment of dissolved trace metals in southwestern rivers, Nigeria, Sci. Total Environ., 2025, 963, 178342 CrossRef CAS PubMed.
- C. Ding, H. Ni and H. Zheng, Parent and halogenated polycyclic aromatic hydrocarbons in rice and implications for human health in China, Environ. Pollut., 2012, 168, 80–86 CrossRef CAS PubMed.
- M. Van den Berg, L. S. Birnbaum, M. Denison, M. D. Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, M. Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker and R. E. Peterson, The 2005 World Health Organization Reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds, Toxicol. Sci., 2006, 93, 223–241 CrossRef CAS PubMed.
- U. C. Ugochukwu and A. Ochonogor, Groundwater contamination by polycyclic aromatic hydrocarbon due to diesel spill from a telecom base station in a Nigerian City: assessment of human health risk exposure, Environ. Monit. Assess., 2018, 190(4), 249 CrossRef PubMed.
- USEPA, Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part A). Available online: https://rais.ornl.gov/documents/HHEMA.pdf, 2001a, (accessed on March 31st, 2017).
- USEPA, Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E). Available online: https://rais.ornl.gov/documents/HHEMA.pdf, 2001b, (accessed on 31st March 2017).
- USEPA, Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). EPA/540/1–89/002Washington, D.C.: Oice of Emergency and and Remedial Response, U.S. Environmental Protection Agency, https://www.epa.gov/oswer/riskassessment/ragsa/pdf/rags-vol-pta_complete.pdf, 1989 Search PubMed.
- R. Eisler and A. A. Belisle, Planar PCB Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review Contaminant Hazard Reviews, Biological Report 31, 1996, pp. 1–96 Search PubMed.
- World Health Organization Regional Office for Europe, Air quality guidelines, 2nd edn, Copenhagen, Denmark, Cap 5.10 PCB, 2000 Search PubMed.
- V. A. McFarland and J. U. Clarke, Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis, Environ. Health Perspect., 1989, 81, 225–239 CrossRef CAS PubMed.
- European Food Safety Authority, Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to the presence of non-dioxin-like polychlorinated biphenyls (PCB) in feed and food, EFSA J., 2005, 284, 1–137 Search PubMed.
- European Union Commission regulation, Commission regulation (EU) No 1259/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non-dioxin-like PCBs in foodstuffs, Off. J. Eur. Union, 2011, 320, 18–23 Search PubMed.
- Y. Uekusa, S. Takatsuki, T. Tsutsumi, H. Akiyama, R. Matsuda, R. Teshima, A. Hachisuka and T. Watanabe, Determination of polychlorinated biphenyls in marine fish obtained from tsunami-stricken areas of Japan, PLoS One, 2017, 12(4), e0174961 CrossRef PubMed.
- J. Xie, Z. Bian, T. Lin, L. Tao, Q. Wu and M. Chu, Global occurrence, bioaccumulation factors and toxic effects of polychlorinated biphenyls in tuna: A review, Emerging Contam., 2020, 6, 388–395 CrossRef.
- D. Jacob and E. H. Boer, 8-year study on the elimination of PCBs and other organochlorine compound from eel under nature conditions, Environ. Sci. Technol., 1994, 28, 2242–2248 CrossRef PubMed.
- L. Nie, M. L. Wise, D. M. Peterson and M. Meydani, Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production, Atherosclerosis, 2006, 186(2), 260–266 CrossRef CAS PubMed.
- M. M. Storelli, E. Casalino, G. Barone and G. O. Marcotrigiano, Persistent organic pollutants (PCBs and DDTs) in small size specimens of bluefin tuna (Thunnus thynnus) from the Mediterranean Sea (Ionian Sea), Environ. Int., 2008, 34(4), 509–513 CrossRef CAS PubMed.
- M. M. Storelli, S. Losada, G. O. Marcotrigiano, L. Roosens, G. Barone, H. Neels and A. Covaci, Polychlorinated biphenyl and organochlorine pesticide contamination signatures in deep-sea fish from the Mediterranean Sea, Environ. Res., 2009, 109(7), 851–856 CrossRef CAS PubMed.
- M. Masci, E. Orban and T. Nevigato, Organochlorine pesticide residues: An extensive monitoring of Italian fishery and aquaculture, Chemosphere, 2014, 94, 190–198 CrossRef CAS PubMed.
- A. H. Buckman, N. Veldhoen, G. Ellis, J. K. B. Ford, C. C. Helbing and P. S. Ross, PCB-Associated Changes in mRNA Expression in Killer Whales (Orcinus orca) from the NE Pacific Ocean, Environ. Sci. Technol., 2011, 45(23), 10194–10202 CrossRef CAS PubMed.
- D. Mackay, W. Y. Shiu and K. C. Ma, Illustrated Handbook of Physical-Chemical Properties of Environmental Fate for Organic Chemicals, CRC press, vol. 5, 1997 Search PubMed.
- A. A. Elskus, J. J. Stegeman, J. W. Gooch, D. E. Black and R. J. Pruell, Polychlorinated biphenyl congener distributions in winter flounder as related to gender, spawning site, and congener metabolism, Environ. Sci. Technol., 1994, 28(3), 401–407 CrossRef CAS PubMed.
- S. Janković, M. Ćurčić, T. Radičević, S. Stefanović, M. Lenhardt, K. Durgo and B. Antonijević, Non-dioxin-like PCBs in ten different fish species from the Danube river in Serbia, Environ. Monit. Assess., 2010, 181(1–4), 153–163 Search PubMed.
- A. O. Adeogun, A. V. Chukwuka, P. O. Chukwunonso and A. Arukwe, Concentration of polychlorinated biphenyl (PCB) congeners in the muscle of Clarias gariepinus and sediment from inland rivers of southwestern Nigeria and estimated potential human health consequences, PubMed, 2016, 79(21), 969–983 CAS.
- E. M. Oziolor, J. N. Apell, Z. C. Winfield, J. A. Back, S. Usenko and C. W. Matson, Polychlorinated biphenyl (PCB) contamination in Galveston Bay, Texas: Comparing concentrations and profiles in sediments, passive samplers, and fish, Environ. Pollut., 2018, 236, 609–618 CrossRef CAS PubMed.
- D. Adeyemi, G. Ukpo, C. Anyakora and J. Uyimadu, Polychlorinated biphenyl in fish samples from Lagos Lagoon, Nigeria, Afr. J. Biotechnol., 2009, 8, 2811–2815 CAS.
- Y. Yang, Q. Xie, X. Liu and J. Wang, Occurrence, distribution and risk assessment of polychlorinated biphenyls and polybrominated diphenyl ethers in nine water sources, Ecotoxicol. Environ. Saf., 2015, 115, 55–61 CrossRef CAS PubMed.
- J. L. Hugla, I. Thys and L. Hoffman, Contamination par les PCBs et les pesticides organochlorés des poissons du Grand-DuchédeLuxembourg: incidence possible surles populations de loutre (Lutralutra L.). Annales de Limnologie, Int. J. Limnol., 1998, 34, 201–209 CrossRef.
- A. Jafarabadi, A. Riyahi Bakhtiari, S. Mitra, M. Maisano, T. Cappello and C. Jadot, First polychlorinated biphenyls (PCBs) monitoring in seawater, surface sediments and marine fish communities of the Persian Gulf: Distribution, levels, congener profile and health risk assessment, Environ. Pollut., 2019, 253, 78–88 CrossRef PubMed.
- Z. B. Batang, N. Alikunhi, M. Gochfeld, J. Burger, R. AlJahdali and H. Al-Jahdali, Congenerspecific levels and patterns of polychlorinated biphenyls in edible fish tissue from the central Red Sea coast of Saudi Arabia, Sci. Total Environ., 2016, 572, 915–925 CrossRef CAS PubMed.
- N. K. Karouna-Renier, R. A. Snyder, T. Lange, S. Gibson, J. G. Allison, M. E. Wagner and K. R. Rao, Largemouth bass (Micropterus salmoides) and striped mullet (Mugil cephalus) as vectors of contaminants to human consumers in northwest Florida, Mar. Environ. Res., 2011, 72, 96–104 CrossRef CAS PubMed.
- T. Brázová, V. Hanzelová, D. Miklisová, D. Šalgovičová and Ľ. Turčeková, Biomonitoring of polychlorinated biphenyls (PCBs) in heavily polluted aquatic environment in different fish species, Environ. Monit. Assess., 2011, 184(11), 6553–6561 CrossRef PubMed.
- FDA (Food and Drug Administration), Fish and Fisheries Products Hazards and Controls Guidance, Centre for Food Safety and Applied Nutrition, US Food and Drug Administration, 3rd edn, 2001 Search PubMed.
- European Union Commission Regulation EU, Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance), Off. J. Eur. Union, 2006, 364, 264–288 Search PubMed.
- J. M. Llobet, A. Bocio, J. I. Domingo, A. Teixido, C. Casas and L. Mϋller, Levels of Polychlorinated Biphenyls in Foods from Catalonia Spain: Estimated Dietary Intake, J. Food Prot., 2003, 663, 479–484 CrossRef PubMed.
- X. Li, T. Gan, X. Yang, J. Zhou, J. Dai and M. Xu, Human health risk of organochlorine pesticides OCPs and polychlorinated biphenyls PCBs in edible fish from Huairou Reservoir and Gaobeidian Lake in Beijing, China, Food Chem., 2008, 109, 348–354 CrossRef CAS PubMed.
- USEPA, Risk Assessment Guidance for Superfund, https://www.epa.gov/risk/regional-screeningconcentrations-rsls, 2010.
- K. C. Cheung, H. M. Leung, K. Y. Kong and M. H. Wong, Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment, Chemosphere, 2007, 66, 460–468 CrossRef CAS PubMed.
- Institute of Medicine, Dioxins and Dioxin-like Compounds in the Food Supply, National Academy Press, Washington, DC, http://books.nap.edu/openbook.php?record_id=10763%26page=R1, 2003 Search PubMed.
- K. J. Hooper, M. She, J. Sharp, N. Chow, R. Jewell, R. Gephart and A. Holden, Depuration of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from California first-time mothers (primiparae), Environ. Health Perspect., 2007, 115(9), 1271–1275 CrossRef CAS PubMed.
- Agency for Toxic Substances and Disease Registry, Toxicological Profile for Polychlorinated Biphenyls (PCBs), U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=142%26tid=26, 2000 Search PubMed.
- J. B. Herbstman, A. Sjodin, B. J. Apelberg, F. R. Witter, D. G. Patterson, R. U. Halden, R. S. Jones, A. Park, Y. Zhang and J. Heidler, Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population, Environ. Health Perspect., 2007, 115(12), 1794–1800 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00399c |
| This journal is © The Royal Society of Chemistry 2025 |