Open Access Article
Open Access ArticleBPA and its analogues in thermal papers: an assessment of presence and dermal exposure†
Merve
Ozkaleli Akcetin
a,
Hatice Kubra
Gul
a,
Ismail Ethem
Goren
b,
Nebile
Daglioglu
b and
Perihan Binnur
Kurt-Karakus
 *a
*a
aDepartment of Environmental Engineering, Faculty of Engineering and Natural Sciences, Bursa Technical University, 16310, Bursa, Turkey. E-mail: perihan.kurt@btu.edu.tr
bInstitute of Forensic Sciences, Department of Forensic Toxicology, Ankara University, Ankara, Turkey
First published on 3rd January 2025
Abstract
Thermal papers are a significant source of exposure to bisphenol A (BPA) and other phenolic compounds (PCs), absorbed through the skin via dermal contact. This study analyzed thermal paper receipts from various commercial settings in Türkiye to assess BPA and its structural analogs. For both deterministic and probabilistic risk assessments, the estimated daily intake (EDI), hazard quotient (HQ), and hazard index (HI) were calculated for the general population and workers exposed via dermal contact from handling thermal receipts. The results showed that BPA and bisphenol S (BPS) were the most frequently detected chemicals (detected in 99% and 100% of samples, respectively) with concentrations ranging from 1.98–1061 μg per g paper and 0.070–210 μg per g paper in thermal paper receipts in Türkiye, respectively. The EDI of PCs based on the mean concentration determined in the samples for the general population ranged between 0.00000184 μg per kg per day and 0.000445 μg per kg per day, whereas it ranged between 0.0000919 μg per kg per day and 0.022 μg per kg per day for occupational exposure of workers. The EDI value based on the mean concentration detected in samples was 0.000445 μg per kg per day and 0.00223 μg per kg per day for the general population and occupational exposure, respectively. Exposure to BPS was lower, resulting in exposure values of 0.000039 μg per kg per day and 0.002 μg per kg per day for the general population and occupational exposure, respectively. Although these mean concentration based exposure levels are below the U.S. EPA reference dose (50 μg per kg per day for BPA), they exceed the more stringent European Food Safety Authority (EFSA) total daily intake (TDI) limits set for BPA (0.0002 μg per kg per day) in some cases, indicating potential health risks. The HQ and HI analyses further underscore the risks, particularly for workers, with HI values surpassing safe thresholds. The study calls for stricter regulations on BPA and its analogs in thermal papers due to the significant risks, even from BPA-free products that use BPS as a substitute.
Environmental significanceBP-A and BP-S are widely used to manufacture various consumer products such as thermal papers and sales receipts. BP-S containing products are being marketed as “BPA-free” products since the use of BPS has not been regulated. Considering the increased direct contact of handling thermal receipt papers in daily life, recent discoveries have indicated direct dermal exposure to these chemicals, resulting in their observation in urine due to absorption into skin. Moreover, significantly higher levels of BP-A and BP-S in urine samples due to occupational exposure were reported compared to general exposure. Therefore, the investigation of the transfer of these compounds into the human body is essential. |
1 Introduction
Bisphenol A (BPA, 2,2-bis(4-hydroxyphenyl) propane) is a synthetic chemical and is used in polycarbonate plastic and epoxy resin production. It has a wide application area in various consumer products and industrial applications including plastic bottles, thermal papers, dental resins, sports equipment, and compact disks (CDs)/digital versatile disks (DVDs).1–3In 2009, it was estimated that 2.2 million tons of BPA were produced globally and it is expected that the production will reach 7.3 million tons by the end of 2023.4 In thermal receipt papers, BPA has been widely used as a color developer. Due to the global restriction and regulations on BPA as a result of recognition of its negative effects on human and environmental health, it has been substituted with alternative chemicals such as bisphenol S (BPS) in thermal receipt papers. However, despite the restrictions, BPA is still in use in various products and applications. Additionally, there is limited research on the negative effects of BPS on environmental compartments and human health. A ban has entered into force in the European Union (EU) on January 2, 2020 on the use of BPA in thermal paper stating that the substance cannot legally be placed on the market in thermal paper in a concentration equal to or greater than 0.02% by weight.5 Hence, it is forecasted that 61% of all thermal paper in the EU is to be based on BPS by 2022. Outside of the EU, Switzerland is the first country to ban BPA and BPS initiating a ban on these chemicals in thermal paper. Several jurisdictions in the US introduced bills to regulate BPA in consumer products, i.e. the governor of Illinois signed the HB 2076 bill6 into law to regulate BPA in thermal paper. Use of BPA is banned in Türkiye for certain applications such as production of polycarbonate materials used in baby feeding;7,8 however, to the best knowledge of the authors, there are no regulations on BPA or BPS in thermal receipt papers in Türkiye.
The new regulation on BPA has been published by the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) under the Water Framework Directive (WFD) regulation. According to the new regulation, BPA has been added to the priority pollutant list under the WFD;9 hence, it is the first bisphenol that is listed on the priority pollutant list of the WFD.
The occurrence and levels of BPA and other phenolic compounds (PCs) have been reported in different environmental media: up to 3900 μg kg−1 in indoor dust,10 up to 9730 μg kg−1 in dust,11 up to 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg kg−1 in thermal receipts,12 up to 898.7 ng L−1 in drinking water,13 up to 46.31 μg kg−1 in sediment, up to 81.39 μg kg−1 in sediment organic matter,14 and up to 2.79 μg L−1 in human urine samples.15
000 μg kg−1 in thermal receipts,12 up to 898.7 ng L−1 in drinking water,13 up to 46.31 μg kg−1 in sediment, up to 81.39 μg kg−1 in sediment organic matter,14 and up to 2.79 μg L−1 in human urine samples.15
Due to the widespread use of BPA and its alternatives such as BPS, bisphenol Z (BPZ), bisphenol F (BPF), bisphenol C (BPC), bisphenol AP (BPAP), 2,4-bisphenol S (2,4-BPS), bisphenol E (BPE), bisphenol B (BPB), bisphenol AF (BPAF), and bisphenol P (BPP) in consumer products, the release of PCs into the environment and their subsequent transfer to humans are inevitable. The topic has gained significant public attention in recent years due to these chemicals' adverse health effects,16 especially significant negative impacts on reproductive abilities as endocrine disruptors even at low concentrations.3,12,17–20 It has been suggested that BPA may affect human reproductive and other systems by acting like human hormones.21 In response, BPS has been one of the most widely used BPA replacements introduced by manufacturers to make “BPA-free” products. However, two recent studies reported that BPS is as hormonally active as BPA,22 and, like BPA, it interferes with the endocrine (hormone) system23 in ways that may produce harmful effects, such as obesity, cancer and neurological disorders. The study by Qui et al.24 reported that both BPA and BPS alter the normal development of the reproductive system. If these chemicals are ever released into the environment, they eventually enter the human body via many pathways like oral ingestion,25 dermal contact,26 and inhalation.25,27
The main goal of the present study was to determine the levels of PCs including BPA, BPB, BPS, BPZ, BPAF, BPF and BPP in thermal papers, which were randomly collected from supermarkets, general and fast-food restaurants, gas stations, credit cards and bank machines in Türkiye. Secondly, the current study aims to estimate the daily intake of selected PCs via dermal absorption from the handling of thermal papers.
2 Materials and methods
2.1 Reagents
All solvents were of liquid chromatography grade. PCs including BPA, BPB, BPS, BPZ, BPAF, BPF and BPP were purchased from Accustandard (New Haven, CT, USA). BPA-d16 was from Dr Ehrenstorfer GmbH (Augsburg, Germany), while 13C12-BPA was from Cambridge Isotope Laboratories, Inc (Tewksbury, MA, USA).2.2 Sample collection
A total of 152 thermal receipt papers were collected randomly from commercial settings including banks, supermarkets, cafes, restaurants, gas stations, fast food stores, and health centers, from 8 different cities in Türkiye in 2020. Additionally, a thermal paper certified to be free of BPA was purchased from a supplier and replicate analysis (n = 10) was conducted for this sample. The samples were wrapped in pre-baked (at 450 °C) aluminum foil bags and were stored at −20 °C until analysis.2.3 Sample preparation
Extraction of targeted chemicals from the thermal paper samples was carried out according to the method described by Babu et al.28 with a minor revision. Briefly, the thermal paper samples were cut into small pieces using a stainless steel clipper. The clipper was cleaned using acetone before it was used for each sample. The cut pieces were blended, and a sub-sample of approximately 20–30 mg thermal paper was transferred into a 15 ml screw cap glass centrifuge tube. After addition of 522 ng of recovery compound (in acetonitrile) (BPA-d16), the cap of the tube was closed tightly and samples were kept in the dark for about an hour. 5 ml of acetonitrile was used as the extraction solvent. The sample was vortexed for 60 seconds, the cap of the tube was closed tightly and then it was kept in the dark overnight. The next day, ultrasonic extraction (for 15 minutes) was applied to the sample to extract the targeted chemicals. After extraction, the sample was centrifuged at 4000 rpm for 10 minutes and the aliquot was filtered through a 0.45 μm PTFE syringe filter. A 1 ml aliquot was transferred into an amber GC-vial and spiked with 100 ng of BPA-13C12 immediately prior to analysis as an internal standard.2.4 Instrumental analysis
A Shimadzu 8040 triple quadrupole LC-MS/MS system was used for analysis of PCs. Chromatographic separation was achieved on a Shim-pack FC-ODS (150 × 2 mm, Shimadzu, Kyoto/Japan) column. The injection volume, flow rate in the column and oven temperature were 10 μl, 0.3 ml min−1 and 40 °C, respectively. The mobile phase is composed of 10 mM ammonium acetate in water (A) and acetonitrile (B). The gradient program was set as follows: 0.0–1.0 min; 80% of solvent B, and 1.0–2 min; a linear gradient to 95% solvent A; 2.0–4.0 min, 95% solvent B; 4.0–4.01 min gradient to 50% solvent B; 5.0 min stop.The capillary voltage was kept at 4.0 kV and the vaporizer temperature was 350 °C. Detailed information on BPA and other PC precursor ions (m/z), product ions (m/z) and retention time is given in ESI Table S1.†
2.5 Quality assurance and quality control
All glassware was soaked in soapy water overnight prepared using Alconox detergent and then was rinsed first with tap water and then with Milli-Q water. All glassware and metal tools were baked at 450 °C for at least 4 hours before use. All materials were cleaned with acetone/hexane before use to prevent any contamination.Initially, it was aimed to use a BPA-free certified paper as a material to prepare blanks. However, a noticeable amount of BPA was present in the certified thermal paper; therefore, only solvent blanks were used in the current study. A solvent blank was processed for every 12 samples. None of the targeted PCs were present in blank samples; therefore, the method detection limit (MDL) was accepted to be equal to the instrument detection limit (IDL) for all targeted chemicals. For statistical analysis, if a targeted chemical was <MDL, half of the MDL was used in calculations. In the current study, the variability of the mean value of data is expressed as the standard error (SE).
The IDL values for BPA, -B, -S, -Z, -AF, -F and P were 0.050, 2.93, 0.004, 0.008, 0.003, 1.51, and 0.383 μg g−1, respectively. The recovery ratio of BPA-d16 was between 71 and 125% with an average value of 90 ± 1.16%.
2.6 Exposure assessment and risk characterization
Therefore, in the assessment of exposure to PCs via thermal paper receipts, dermal contact is considered the sole human exposure pathway due to the handling behavior of consumers as well as the high levels of PCs in such paper.11,30,31
The estimated daily intake (EDI; μg per day per kg bw) of PCs was calculated using eqn (1) modified from the equation given by Liao and Kannan:30
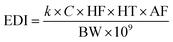 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 ng s−1 for all PCs), C is the concentration of PC determined in thermal paper (μg per g paper), and HF is the handling frequency (times per day). HT is the handling duration of receipts (5 s).1 Studies conducted in other parts of the world have taken into account different rates of HF for occupational exposure and public exposure pathways. In the current study, a HF value of 150 times per day for occupational exposure12 and a HF value of 3 times per day for public exposure12 were used in calculations. A detailed list of parameters and values used in calculations is given in ESI Table S2.†
522 ng s−1 for all PCs), C is the concentration of PC determined in thermal paper (μg per g paper), and HF is the handling frequency (times per day). HT is the handling duration of receipts (5 s).1 Studies conducted in other parts of the world have taken into account different rates of HF for occupational exposure and public exposure pathways. In the current study, a HF value of 150 times per day for occupational exposure12 and a HF value of 3 times per day for public exposure12 were used in calculations. A detailed list of parameters and values used in calculations is given in ESI Table S2.†
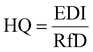 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, resulting in a TDI of 0.2 ng per kg bw per day.34 Little information is available to calculate the risk assessment of PCs through dermal absorption and it is mainly based on the calculation of EDI values.12,30 Therefore, in the current study, the oral reference dose (RfDoral) and temporary tolerable reference dose from the EFSA34 were used to calculate the RfDdermal values of PCs. In this manner, eqn (3) proposed in ref. 35 was used with a small modification to make the best assumption that could be made to estimate the RfDdermal values for the dermal absorption of the investigated PCs.
000, resulting in a TDI of 0.2 ng per kg bw per day.34 Little information is available to calculate the risk assessment of PCs through dermal absorption and it is mainly based on the calculation of EDI values.12,30 Therefore, in the current study, the oral reference dose (RfDoral) and temporary tolerable reference dose from the EFSA34 were used to calculate the RfDdermal values of PCs. In this manner, eqn (3) proposed in ref. 35 was used with a small modification to make the best assumption that could be made to estimate the RfDdermal values for the dermal absorption of the investigated PCs.| RfDdermal = RfDoral × ABSGI | (3) |
Health risks associated with exposure to multiple PCs are estimated by using the hazard index (HI) (the summation of hazard quotients (HQk) of individual PCs (k)), which can be calculated using eqn (4).42,43
| HI = ∑HQk | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 trails. Sensitivity analysis is an important tool in risk assessment as part of Monte Carlo simulation. This analysis is a technique aimed at determining the impact of specific variables on the results. The variables used in the model were based on previous studies and summarized in ESI Tables S2 and S3.†
000 trails. Sensitivity analysis is an important tool in risk assessment as part of Monte Carlo simulation. This analysis is a technique aimed at determining the impact of specific variables on the results. The variables used in the model were based on previous studies and summarized in ESI Tables S2 and S3.†
3 Results and discussion
3.1 PC concentration in thermal paper receipts
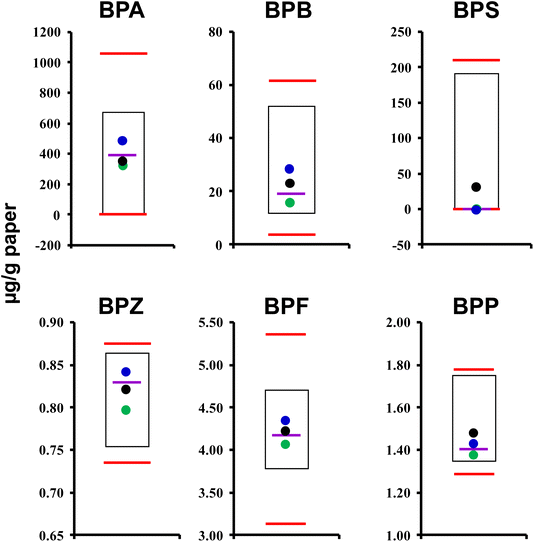
Fig. 1 shows a comparison of PC concentrations in thermal paper receipt samples analysed in the current study, while the concentration values of each targeted PC in individual samples are given in ESI Table S4.†Among the targeted bisphenols, it was found that BPA and BPS were the dominant color developers used in the thermal receipts collected from the Turkish market (n = 152) and detected in 99% (1.98–1061 μg per g paper) and 100% (0.007–210 μg per g paper) of the analysed samples, respectively. In addition to these two species, BPB, BPZ, BP-F and BPP were also determined but at a lower frequency. Generally, BPA and BPS showed higher concentration levels compared to other PCs detected in the samples (Fig. 1). BPP showed the lowest detection frequency (2.64%, n = 4) with a concentration value between 0.011 and 0.018 μg per g paper. BPAF was not present in any of the analysed thermal paper samples. The detection frequency values of PCs are ranked as follows: BPS (100%) > BPA (99.0%) > BPB (80%) > BP-F (57%) > BPZ (43%).
A comparison of detected concentrations in samples was made by grouping the samples based on a designated categorization. The first approach for such categorization was to group the samples based on the manufacturers' brand names. The collected 152 thermal paper receipts were from 10 different brands. The main grouping criterion was based on the presence of three or more samples from the same paper brand. The no-name brand papers or a sample size of 2 or less were grouped together and the results are given under “Brand f” in ESI Table S5.† The concentration ranges and mean concentration values of detected PCs in samples from different brands studied in the current research are given in ESI Table S5.† The percent contribution of each targeted PC compound to the total PC concentrations detected in each sample was calculated and is shown in ESI Fig. S1.† In most of the samples, BPA was the PC that contributed the most to the total concentration in the brands a, b, c, d, e, f, g and h (% contribution range between 1.7% and 99.7%), followed by BPS (0.1–97.9%) and BPB (1.4–38%). However, in brands i and j, BPS (0.02–98.33%) was the dominant chemical that contributed the most to the total concentration.
The second approach to categorization was grouping the samples based on the manufacturer's notifications of the “presence of BPA” in the thermal receipts. The first group was BPA-free certified paper (hereafter referred to as CTP, n = 15), the second group was BPA-free stated thermal paper (not certified by the manufacturer but stated on the back of the paper to be BPA free; hereafter referred to as STP, n = 10) and the third group was regular thermal receipt paper (hereafter referred to as RTP, n = 142). It is important to note that CTP paper was not included in the total number (n = 152) of the analysed samples and it was used to assess the presence of such chemicals in certified material. The average concentration (±SE) values of targeted chemicals in CTP, STP and RTP groups are given in Table 1.
| CTPb (n = 15) (μg per g paper) | STPc (n = 10) (μg per g paper) | RTPd (n = 142) (μg per g paper) | |
|---|---|---|---|
| a nd: not detected in the sample. b CTP: certified thermal paper. c STP: BPA-free stated thermal paper. d RTP: regular thermal receipt paper. | |||
| BPA | |||
| Min. | 37.41 | 3.34 | 0.0250 |
| Max. | 35.18 | 431.5 | 1061.1 |
| Avg ± SE | 34.06 ± 0.94 | 81.5 ± 52 | 374.4 ± 17.5 |
| Median | 35.1 | 4.11 | 397.4 |
| DF (%) | 100 | 100 | 99 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| BPS | |||
| Min. | nd | 0.09 | 0.0705 |
| Max. | nd | 207.6 | 210.3 |
| Avg ± SE | nd | 154.1 ± 25.8 | 22.4 ± 5.03 |
| Median | nd | 191.10 | 0.21 |
| DF (%) | 0 | 100 | 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| BPB | |||
| Min. | nd | 1.46 | 1.46 |
| Max. | nd | 15.5 | 61.7 |
| Avg ± SE | nd | 4.23 ± 1.85 | 19.8 ± 1.16 |
| Median | nd | 1.46 | 17.5 |
| DF (%) | 0 | 20 | 84 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| BP-F | |||
| Min. | nd | 0.75 | 0.75 |
| Max. | nd | 4.44 | 5.74 |
| Avg ± SE | nd | 2.45 ± 0.569 | 2.80 ± 0.146 |
| Median | nd | 2.27 | 4 |
| DF (%) | 0 | 50 | 58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| BPZ | |||
| Min. | nd | 0.003 | 0.003 |
| Max. | nd | 0.86 | 0.876 |
| Avg ± SE | nd | 0.09 ± 0.085 | 0.37 ± 0.034 |
| Median | nd | 0.003 | 0.003 |
| DF (%) | 0 | 10 | 45 |
Although CTP paper is expected to be completely free of BPA, this chemical was still present in CTP paper samples at a mean concentration of 34.06 ± 0.904 μg per g paper. The mean concentration of BPA in STP and RTP groups was 81.5 ± 52 μg per g paper (DF = 100%) and 374.35 ± 17.5 μg per g paper (DF = 99%), respectively. Although the mean BPA level in the CTP group was approx. 2.4–11 times lower compared to the mean levels detected in STP and RTP groups, the presence of BPA in CTP paper is still an indication that people may still be exposed to BPA even though vendors are aware of its negative effects and have a high level of awareness to use thermal paper certified as BPA free.
As some published data and government documents46,47 report that BPS is a safer alternative to BPA, it seems that BPA is still the preferred PC compound in thermal receipt papers in Türkiye. Moreover, surprisingly, a sample from the STP group showed the highest BPA concentration (431.5 μg per g paper) and showed lower levels of BPS (0.09–207.6 μg per g paper). In addition to BPA and BPS, BPB, BP-F and BPZ were also present in STP and RTP group papers.
The third approach was to group the samples based on service categories and results are shown in ESI Table S6.† Cinema, gas station and fast food receipts showed similar concentrations (398.4 ± 13.5 μg per g paper, 397.4 ± 36.6 μg per g paper, and 394 ± 35.8 μg per g paper, respectively), yet these were the highest BPA concentration level compared to other service group samples. Samples from café-restaurant settings showed the lowest mean BPA concentration (157.5 ± 100 μg per g paper). Fuel bills showed the highest mean BPB concentrations (42.1 ± 4.3 μg per g paper) but the average BPB levels in other service groups were similar (from 15.1 ± 2 μg per g paper to 29.4 ± 5.8 μg per g paper). Health center and supermarket receipts showed similar BPS concentrations (67 ± 66.74 μg per g paper and 62.2 ± 16.4 μg per g paper, respectively), yet had the highest BPS concentration level compared to other service groups, while samples from gas stations showed the lowest BPS concentration (0.22 ± 0.04 μg per g paper) level.
A comparison of BPA and BPS levels in thermal receipt papers in Türkiye to the levels reported in other countries is given in Table 2. The results of the current study for BPA and BPS are in the lower end of the reported concentrations worldwide (Table 2). Furthermore, the maximum concentration of BPA determined in the current study is approx. 20 times lower compared to the maximum concentration of BPA reported in a study conducted in Türkiye in 2016 by Yalcin et al.55 However, it is worth mentioning that Yalcin et al.55 analysed only 12 samples, yet the concentration levels reported for such a small sample size would probably not be representative of typical levels in a broader Turkish market.
| Country | Range (μg per g paper) | Reference | |
|---|---|---|---|
| BPA | BPS | ||
| a Range of median values in samples collected in 2015, 2016 and 2017; LOQ: limit of quantitation. b Concentration detected in paper currency. | |||
| Türkiye | 1.98–1061 | 0.007–210.28 | This study |
| Korea | 6165.8–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353 |
0.0138–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
49 |
| Nigeria | 1500–3160 | — | 1 |
| Brazil | <170–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
<30–8933 | 3 |
| China | 2770–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
<1–3 | 50 |
| France | <170–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
<30–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
3 |
| Spain | <170–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
<30–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
3 |
| UK | 60–63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 51 |
| Germany | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15 000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900a 900a |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–14 100–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700a 700a |
52 |
| Italy | <4–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
<0.012–3530 | 53 |
| USA | 7000–36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–26 900–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
54 |
| USA | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–26 500–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200–30 200–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
55 |
| Türkiye | 110–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
— | 48 |
| Brazil | <LOQ–42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–22 000–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12 |
| Switzerland | 560–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
8300–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
56 |
| China | 160–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
— | 57 |
| China | 2580–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
— | 31 |
| Belgium | <LOQ–2090 | — | 11 |
| USA (Albany) | — | 0.0138–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
58 |
| Other USA cities | — | 0.998–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
58 |
| Japan | — | 0.546–6130 | 58 |
| Korea | — | 0.09–11 | 58 |
| Vietnam | — | 0.1–0.55 | 58 |
| Switzerland | 8000–17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 29 |
3.2 Risk assessment of BPA and its alternatives in shopping receipts
Occupational exposure to PCs can occur through handling receipts. A study in the United States found that pregnant women employed as cashiers had the highest levels of urinary BPA concentrations, measuring 2.8 μg g−1 (equivalent to 0.0028 mg g−1), compared to pregnant women in other professions, such as teachers and industrial workers, who had concentrations of 1.8 μg g−1 and 1.2 μg g−1, respectively.59 This outcome is attributed to the frequent handling of thermal paper found in cash register receipts by women working as cashiers. The Washington Toxics Coalition estimated that an average shopper could transfer around 30 μg of BPA to their skin by handling a receipt (rubbing it five times between two fingers and a thumb), based on BPA concentrations detected in thermal paper.60 The mean daily intake (MDI) of BPS was estimated to be 12.3 ng per day for the general population through handling of thermal paper.61 Additionally, these researchers estimated the MDI of BPA from handling paper currency to be 0.102 ng per day for the general population in the United States.30 To the best of our knowledge, there is one study that has estimated the mean daily intake (MDI) of BPS from handling paper products and currency, as reported in ref. 62. That study determined that the MDI of BPS via dermal absorption in adults handling paper products and paper currency was 12.0 ng per day for the public. These findings suggest that alternatives to BPA, such as BPS, which share similar physical and chemical properties, are likely to transfer from the surface of thermal paper onto the skin and may potentially be absorbed through the skin or ingested.Thermal paper can release free PCs upon contact, which may then be absorbed into the skin, leading to exposure during handling and use. Several studies have investigated the release of BPA from POS receipts, showing that BPA can be extracted from the receipts and has the potential to be absorbed into the skin upon contact.29. It has been reported that approximately 0.17 μg of BPA migrated into the skin two hours after contact, and this amount could not be recovered by washing with water. Experimental conditions have also demonstrated that BPA can penetrate the skin under certain conditions.63
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 ng s−1 for all PCs.29 The handling frequency (HF) was determined to be 3 and 150 times per day for public and occupational exposure, respectively.12 The handling duration of receipts (HT) is 5 s,31,64 and the absorption fraction of bisphenol compounds by skin (AF) was taken as 27%.29 Based on these parameters, the daily mean occupational exposures (EDIoccupational) to BPA, BPB, BPS, BPZ, BPF and BPP from the thermal paper were 2.23 × 10−2 μg per kg per day, 1.44 × 10−3 μg per kg per day, 1.95 × 10−3 μg per kg per day, 5.11 × 10−5 μg per kg per day, 2.63 × 10−4 μg per kg per day and 9.19 × 10−5 μg per kg per day, and the daily mean public exposures (EDIpublic) were 4.45 × 10−4 μg per kg per day, 2.89 × 10−5 μg per kg per day, 3.90 × 10−5 μg per kg per day, 1.02 × 10−6 μg per kg per day, 5.26 × 10−6 μg per kg per day and 1.84 × 10−6 μg per kg per day, respectively (Table 3). According to Table 3, the EDI values were compared with the reference dose established by the USEPA (50 μg per kg per day for BPA)32 and the TDI set by the EFSA (0.0002 μg per kg per day for other BPs).34 The results indicate that the calculated EDI values for all bisphenols are well below the reference dose set by the USEPA.32 However, these values significantly exceed the EFSA's much stricter TDI limit.34 For instance, the highest mean EDI value for BPA (2.23 × 10–2 μg per kg per day) exceeded the TDI approximately 100 times.34 However, the EDI values calculated for some bisphenol analogs, such as BPZ and BPP, do not exceed TDI, suggesting a lower risk of exposure to these substances. These findings underscore the need to reassess the potential risks of bisphenol exposure, especially considering the EFSA's more rigorous safety standards. Furthermore, they highlight the importance of not overlooking health risks, even at low levels of exposure.
522 ng s−1 for all PCs.29 The handling frequency (HF) was determined to be 3 and 150 times per day for public and occupational exposure, respectively.12 The handling duration of receipts (HT) is 5 s,31,64 and the absorption fraction of bisphenol compounds by skin (AF) was taken as 27%.29 Based on these parameters, the daily mean occupational exposures (EDIoccupational) to BPA, BPB, BPS, BPZ, BPF and BPP from the thermal paper were 2.23 × 10−2 μg per kg per day, 1.44 × 10−3 μg per kg per day, 1.95 × 10−3 μg per kg per day, 5.11 × 10−5 μg per kg per day, 2.63 × 10−4 μg per kg per day and 9.19 × 10−5 μg per kg per day, and the daily mean public exposures (EDIpublic) were 4.45 × 10−4 μg per kg per day, 2.89 × 10−5 μg per kg per day, 3.90 × 10−5 μg per kg per day, 1.02 × 10−6 μg per kg per day, 5.26 × 10−6 μg per kg per day and 1.84 × 10−6 μg per kg per day, respectively (Table 3). According to Table 3, the EDI values were compared with the reference dose established by the USEPA (50 μg per kg per day for BPA)32 and the TDI set by the EFSA (0.0002 μg per kg per day for other BPs).34 The results indicate that the calculated EDI values for all bisphenols are well below the reference dose set by the USEPA.32 However, these values significantly exceed the EFSA's much stricter TDI limit.34 For instance, the highest mean EDI value for BPA (2.23 × 10–2 μg per kg per day) exceeded the TDI approximately 100 times.34 However, the EDI values calculated for some bisphenol analogs, such as BPZ and BPP, do not exceed TDI, suggesting a lower risk of exposure to these substances. These findings underscore the need to reassess the potential risks of bisphenol exposure, especially considering the EFSA's more rigorous safety standards. Furthermore, they highlight the importance of not overlooking health risks, even at low levels of exposure.
| BPA | BPB | BPS | BPZ | BPF | BPP | |
|---|---|---|---|---|---|---|
| EDI occupational | ||||||
| Minimum | 1.23 × 10−4 | 2.27 × 10−4 | 4.62 × 10−6 | 4.58 × 10−5 | 2.20 × 10−4 | 8.36 × 10−5 |
| Maximum | 6.61 × 10−2 | 3.84 × 10−3 | 1.31 × 10−2 | 5.45 × 10−5 | 3.58 × 10−4 | 1.14 × 10−4 |
| Mean | 2.23 × 10−2 | 1.44 × 10−3 | 1.95 × 10−3 | 5.11 × 10−5 | 2.63 × 10−4 | 9.19 × 10−5 |
| Median | 2.43 × 10−2 | 1.19 × 10−3 | 1.31 × 10−5 | 5.16 × 10−5 | 2.60 × 10−4 | 8.75 × 10−5 |
| 5th percentiles | 2.25 × 10−4 | 7.49 × 10−4 | 5.05 × 10−6 | 4.70 × 10−5 | 2.35 × 10−4 | 8.40 × 10−5 |
| 25th percentiles | 1.98 × 10−2 | 9.49 × 10−4 | 8.06 × 10−6 | 4.96 × 10−5 | 2.53 × 10−4 | 8.54 × 10−5 |
| 75th percentiles | 2.94 × 10−2 | 1.70 × 10−3 | 2.63 × 10−5 | 5.24 × 10−5 | 2.70 × 10−4 | 8.90 × 10−5 |
| 95th percentiles | 4.21 × 10−2 | 3.24 × 10−3 | 1.19 × 10−2 | 5.38 × 10−5 | 2.94 × 10−4 | 1.09 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| EDI public | ||||||
| Minimum | 2.47 × 10−6 | 4.55 × 10−6 | 9.25 × 10−8 | 9.16 × 10−7 | 4.39 × 10−6 | 1.67 × 10−6 |
| Maximum | 1.32 × 10−3 | 7.68 × 10−5 | 2.62 × 10−4 | 1.09 × 10−6 | 7.16 × 10−6 | 2.28 × 10−6 |
| Mean | 4.45 × 10−4 | 2.89 × 10−5 | 3.90 × 10−5 | 1.02 × 10−6 | 5.26 × 10−6 | 1.84 × 10−6 |
| Median | 4.85 × 10−4 | 2.39 × 10−5 | 2.61 × 10−7 | 1.03 × 10−6 | 5.20 × 10−6 | 1.75 × 10−6 |
| 5th percentiles | 4.49 × 10−6 | 1.50 × 10−5 | 1.01 × 10−7 | 9.39 × 10−7 | 4.71 × 10−6 | 1.68 × 10−6 |
| 25th percentiles | 3.97 × 10−4 | 1.90 × 10−5 | 1.61 × 10−7 | 9.92 × 10−7 | 5.06 × 10−6 | 1.71 × 10−6 |
| 75th percentiles | 5.87 × 10−4 | 3.41 × 10−5 | 5.26 × 10−7 | 1.05 × 10−6 | 5.39 × 10−6 | 1.78 × 10−6 |
| 95th percentiles | 8.42 × 10−4 | 6.48 × 10−5 | 2.39 × 10−4 | 1.08 × 10−6 | 5.87 × 10−6 | 2.18 × 10−6 |
EDI values (μg per kg per day) calculated in the current study were compared to EDI values detected in previous research (Table 4). To maintain consistency in EDI calculation and comparison of results with each other, BPA and BPS concentrations reported in the previously published literature were employed in eqn (1) along with the same HT, HF and AF parameters used in the calculations of the current study. As seen in Table 4, occupational or public exposure EDI values in Türkiye were lower than EDI values calculated for Nigeria, Brazil, France, Spain, China, Germany, the USA and Switzerland except for the EDI values calculated for some countries including Korea, Japan and Vietnam.61 In this study, the results obtained for BPA in the case of public exposure were 7.5 times lower than the EDI values determined in Nigeria1 and 42.5 times lower than a value calculated in another study in Türkiye55 and Switzerland.56 The EDI value for public exposure determined for BPS in this study was 325 times lower than that in Switzerland.56 In the case of occupational exposure, the results obtained for BPA were 6.4 times lower than the EDI values determined in Nigeria,1 38.2 times lower than that in Switzerland56 and 39.1 times lower than that in another study conducted in Türkiye.55 The EDI value for occupational exposure determined for BPS in this study was 318 times lower than that in Switzerland.56 The European Food Safety Authority (EFSA) and the U.S. Food and Drug Administration and European Commission Scientific Committee suggested the estimated tolerable intakes of BPA to be 0.0002 μg per kg bw per day, 50 μg per kg bw per day, and 10 μg per kg bw per day, respectively.34 In this manner, considering an adult with a body weight of 70 kg, it can be concluded that EDI values for public and occupational exposure determined based even on maximum values (EDIoccupational: 6.61 × 10−2 μg per kg per day for BPA and 1.31 × 10−2 μg per kg per day for BPS; EDIpublic: 1.32 × 10−3 μg per kg per day for BPA and 2.62 × 10−4 μg per kg per day for BPS) in the current study for BPA and BPS were far below the limit values set by the U.S. Food and Drug Administration and European Commission Scientific Committee (Table 3). However, the majority of EDI values for BPA and BPS exceed the tolerable daily intake levels set by the EFSA, indicating that these compounds could pose a significant public health risk for both occupational and public exposures. On the other hand, the EDI values for BPZ and BPP remain well within EFSA limits, suggesting a comparatively lower risk associated with these compounds. Overall, these findings highlight the need for closer monitoring of BPA and BPS exposures and a thorough assessment of their potential health risks. Similarly, none of the daily EDI values determined in the studies conducted in the countries listed in Table 4 exceed the daily limit values set by the U.S. Food and Drug Administration and the European Commission Scientific Committee based on a 70 kg adult body weight, although they do exceed the value set by the EFSA.
| Country | n | C (μg per g paper) | EDI (μg per day per kg BW) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| BPA | BPS | |||||||
| BPA | BPS | Public | Occupational | Public | Occupational | |||
| a *Median value, **mean value, ***geometric mean value, and EDI: Estimated Daily Intake (μg per kg per day). b Based on the 5th percentile concentration value. c Based on the 95th percentile concentration value. d United States, Canada, Czech Republic, Russia, Turkey, Australia, Brazil, Egypt, South Africa, China, India, Japan, Korea, Kuwait, Malaysia, the Philippines, Singapore, Thailand, Vietnam, and United Arab Emirates. e Min. value of reported mean concentrations. f Max. value of reported mean concentrations. | ||||||||
| Türkiye | 152 | 357** | 31.3** | 0.000445 | 0.022 | 0.000039 | 0.002 | This study |
| Nigeria | 80 | 2270* | — | 0.003 | 0.141 | — | — | 1 |
| Brazil | 22 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100* 100* |
— | 0.016 | 0.816 | — | — | 3 |
| France | 47 | 8440* | — | 0.011 | 0.525 | — | — | 3 |
| Spain | 43 | 9080* | — | 0.011 | 0.565 | — | — | 3 |
| China | 201 | 3610** | — | 0.004 | 0.225 | — | — | 50 |
| Germany | 311 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900** 900** |
147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000** 000** |
0.020 | 0.990 | 0.018 | 0.915 | 52 |
| USA | 33 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300** 300** |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600** 600** |
0.024 | 1.202 | 0.018 | 0.909 | 54 |
| Türkiye | 12 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830** 830** |
— | 0.017 | 0.861 | — | — | 48 |
| Brazil | 190 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300*** 300*** |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500*** 500*** |
0.020 | 1.015 | 0.021 | 1.027 | 12 |
| Switzerland | 100 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500** 500** |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200** 200** |
0.017 | 0.841 | 0.013 | 0.635 | 56 |
| China | 42 | 9380** | — | 0.012 | 0.584 | — | — | 31 |
| Switzerland | 13 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300** 300** |
— | 0.017 | 0.828 | — | — | 29 |
| US, Korea, Japan and Vietnam | 103 | 4.8–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
— | 5.98 × 10−6b to 0.017 c | 3.90 × 10−5b to 0.865 c | — | — | 61 |
| Various Countriesd | 156 | 0.001e–77.1f | — | 1.25 × 10−9 to 9.60 × 10−5 | 6.23 × 10−8 to 4.80 × 10−3 | — | — | 30 |
Hazard Quotient (HQ) and Hazard Index (HI) values calculated in the current study for PCs detected in samples for general public and occupational exposure of adults via dermal contact with thermal receipt papers are given in Table 5 and hazard index (HI) values for BPs in thermal receipts are shown in Fig. 2.
| HQoccupational | HIoccupational | ||||||
|---|---|---|---|---|---|---|---|
| BPA | BPB | BPS | BPZ | BPF | BPP | ||
| Minimum | 0.00002 | 11.4 | 0.040 | 2.29 | 11.0 | 4.18 | 28.9 |
| Maximum | 0.013 | 192 | 114 | 2.72 | 17.9 | 5.71 | 332 |
| Mean | 0.004 | 72.2 | 17.0 | 2.56 | 13.1 | 4.60 | 109 |
| Median | 0.005 | 59.7 | 0.114 | 2.58 | 13.0 | 4.37 | 79.8 |
| 5th percentiles | 0.00004 | 37.4 | 0.044 | 2.35 | 11.8 | 4.20 | 55.8 |
| 25th percentiles | 0.004 | 47.5 | 0.070 | 2.48 | 12.6 | 4.27 | 66.9 |
| 75th percentiles | 0.006 | 85.2 | 0.229 | 2.62 | 13.5 | 4.45 | 106 |
| 95th percentiles | 0.008 | 162 | 104 | 2.69 | 14.7 | 5.46 | 289 |
| HQpublic | HIpublic | ||||||
|---|---|---|---|---|---|---|---|
| BPA | BPB | BPS | BPZ | BPF | BPP | ||
| Minimum | 0.0000005 | 0.23 | 0.001 | 0.046 | 0.220 | 0.084 | 0.577 |
| Maximum | 0.0003 | 3.84 | 2.28 | 0.054 | 0.358 | 0.114 | 6.65 |
| Mean | 0.0001 | 1.44 | 0.340 | 0.051 | 0.263 | 0.092 | 2.19 |
| Median | 0.0001 | 1.19 | 0.002 | 0.052 | 0.260 | 0.087 | 1.60 |
| 5th percentiles | 0.000001 | 0.75 | 0.001 | 0.047 | 0.235 | 0.084 | 1.12 |
| 25th percentiles | 0.0001 | 0.95 | 0.001 | 0.050 | 0.253 | 0.085 | 1.34 |
| 75th percentiles | 0.0001 | 1.70 | 0.005 | 0.052 | 0.270 | 0.089 | 2.12 |
| 95th percentiles | 0.0002 | 3.24 | 2.08 | 0.054 | 0.294 | 0.109 | 5.78 |
HQ values calculated as the ratio of daily exposure to RfD for BPs ranged from 2.47 × 10−5 to 192 and 4.93 × 10−7 to 3.84 for occupational and public exposure, respectively. As seen in Table 5, the HQ values for BPA and BPS are generally well below 1, whereas the HQ values for other bisphenols are consistently above 1, indicating that there is a potential health risk derived from bisphenols other than BPA, as these HQ values exceed 1. In addition, the cumulative risk assessment (HI) for all BPs was found to be in the range of 28.9–332 and 0.557–6.65 for occupational and public exposure, respectively. For occupational exposure, it was determined that there is significant concern for health risk as the HI exceeds 1, even when considering the minimum values, whereas for public exposure, the HI exceeds 1 only for values other than the minimum (Fig. 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 ng s−1 for all PCs.29,54 The handling frequency (HF) was determined to be 3 and ranged from 96 to 150 times per day for public and occupational exposure, respectively.12,65 The handling duration of receipts (HT) ranged from 5 to 10 s (ref. 31, 57 and 65) and the absorption fraction of bisphenol compounds by skin (AF) ranged from 2.3 to 46%.29,39,63 Based on these parameters, the EDI from the thermal paper for occupational exposure to BPA, BPB, BPS, BPZ, BPF and BPP ranged from 0.004 to 16.0, 0.001 to 0.84, 0.0003 to 3.29, 2.75 × 10−5 to 0.0165, 0.0001 to 0.099 and 5.97 × 10−5 to 0.033 for BPA, BPB, BPS, BPB, BPF, and BPP, respectively (ESI Fig. S2†). For public exposure to BPA, BPB, BPS, BPB, BPF, and BPP, EDI values ranged from 0.0002 to 0.573, 3.52 × 10−5 to 0.036, 1.25 × 10−5 to 0.117, 1.04 × 10−6 to 0.0006, 6.58 × 10−6 to 0.004 and 2.37 × 10−6 to 0.001, respectively (ESI Fig. S3†). The hazard quotient (HQ) values for BPs in receipts are shown in ESI Fig. S4 and S5,† while hazard index (HI) values are shown in Fig. 3. For occupational exposure, HQoccupational ranged from 0.0006 to 3.55, 27.3 to 47
522 ng s−1 for all PCs.29,54 The handling frequency (HF) was determined to be 3 and ranged from 96 to 150 times per day for public and occupational exposure, respectively.12,65 The handling duration of receipts (HT) ranged from 5 to 10 s (ref. 31, 57 and 65) and the absorption fraction of bisphenol compounds by skin (AF) ranged from 2.3 to 46%.29,39,63 Based on these parameters, the EDI from the thermal paper for occupational exposure to BPA, BPB, BPS, BPZ, BPF and BPP ranged from 0.004 to 16.0, 0.001 to 0.84, 0.0003 to 3.29, 2.75 × 10−5 to 0.0165, 0.0001 to 0.099 and 5.97 × 10−5 to 0.033 for BPA, BPB, BPS, BPB, BPF, and BPP, respectively (ESI Fig. S2†). For public exposure to BPA, BPB, BPS, BPB, BPF, and BPP, EDI values ranged from 0.0002 to 0.573, 3.52 × 10−5 to 0.036, 1.25 × 10−5 to 0.117, 1.04 × 10−6 to 0.0006, 6.58 × 10−6 to 0.004 and 2.37 × 10−6 to 0.001, respectively (ESI Fig. S3†). The hazard quotient (HQ) values for BPs in receipts are shown in ESI Fig. S4 and S5,† while hazard index (HI) values are shown in Fig. 3. For occupational exposure, HQoccupational ranged from 0.0006 to 3.55, 27.3 to 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878, 5.14 to 25
878, 5.14 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258, 1.19 to 843, 6.70 to 4775 and 2.41 to 1599 for BPA, BPB, BPS, BPZ, BPF and BPP, respectively (ESI Fig. S4†). In contrast, for public exposure, HQpublic ranged from 2.47 × 10−5 to 0.123, 1.01 to 2018, 0.231 to 937, 0.057 to 29.8, 0.312 to 175 and 0.099 to 57.6 for BPA, BPB, BPS, BPZ, BPF and BPP, respectively (ESI Fig. S5†). These findings suggest that certain chemicals, particularly BPB, BPS, and BPF, exhibit high HQ values under both occupational and public exposure scenarios, indicating potential health risks. The wide range of HQ values observed for BPB (occupational: 27.3 to 47
258, 1.19 to 843, 6.70 to 4775 and 2.41 to 1599 for BPA, BPB, BPS, BPZ, BPF and BPP, respectively (ESI Fig. S4†). In contrast, for public exposure, HQpublic ranged from 2.47 × 10−5 to 0.123, 1.01 to 2018, 0.231 to 937, 0.057 to 29.8, 0.312 to 175 and 0.099 to 57.6 for BPA, BPB, BPS, BPZ, BPF and BPP, respectively (ESI Fig. S5†). These findings suggest that certain chemicals, particularly BPB, BPS, and BPF, exhibit high HQ values under both occupational and public exposure scenarios, indicating potential health risks. The wide range of HQ values observed for BPB (occupational: 27.3 to 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878; public: 1.01 to 2018), BPS (occupational: 5.14 to 25
878; public: 1.01 to 2018), BPS (occupational: 5.14 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258; public: 0.231 to 937), and BPF (occupational: 6.70 to 4775; public: 0.312 to 175) points to significant health concerns in both exposure scenarios. Although BPA and BPZ generally show lower HQ values, in some cases, they exceed acceptable levels (HQ < 1), which is notable and requires further investigation. The fact that many HI values exceed 1 in both occupational and public exposure highlights a substantial health risk, emphasizing the necessity for stringent monitoring and the implementation of protective measures across both exposure scenarios. The cumulative risk assessment (HI) for all BPs was found to be in the range of 572 to 50
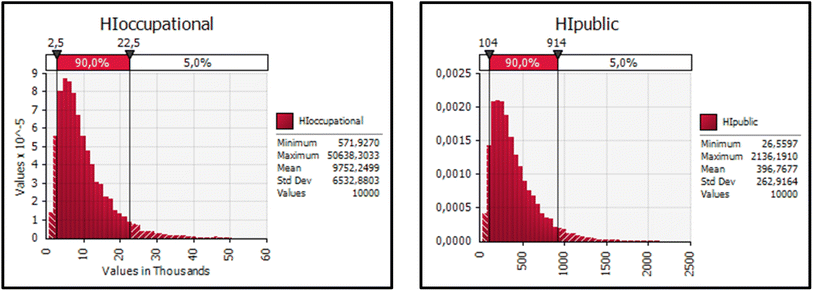
258; public: 0.231 to 937), and BPF (occupational: 6.70 to 4775; public: 0.312 to 175) points to significant health concerns in both exposure scenarios. Although BPA and BPZ generally show lower HQ values, in some cases, they exceed acceptable levels (HQ < 1), which is notable and requires further investigation. The fact that many HI values exceed 1 in both occupational and public exposure highlights a substantial health risk, emphasizing the necessity for stringent monitoring and the implementation of protective measures across both exposure scenarios. The cumulative risk assessment (HI) for all BPs was found to be in the range of 572 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 and 26.6 to 2136 for occupational and public exposure, respectively. As a result of probabilistic calculations, 90% of the HI data for occupational exposure were found to be between 2500 and 22
638 and 26.6 to 2136 for occupational and public exposure, respectively. As a result of probabilistic calculations, 90% of the HI data for occupational exposure were found to be between 2500 and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and 90% of the HI data for public exposure were found to be between 104 and 914 (Fig. 3). These data suggest that occupational exposure poses a significant health risk, while public exposure levels are also considerable, with both scenarios showing instances where the HI > 1 limit is exceeded. This highlights the need for close monitoring of BP exposure and the implementation of risk management strategies both in the workplace and among the general population to address the potential health risks associated with these exceedances.
500, and 90% of the HI data for public exposure were found to be between 104 and 914 (Fig. 3). These data suggest that occupational exposure poses a significant health risk, while public exposure levels are also considerable, with both scenarios showing instances where the HI > 1 limit is exceeded. This highlights the need for close monitoring of BP exposure and the implementation of risk management strategies both in the workplace and among the general population to address the potential health risks associated with these exceedances.
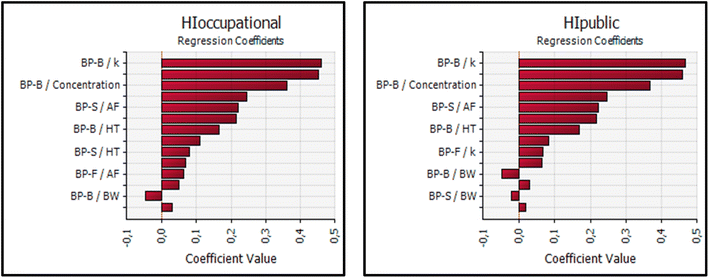 | ||
| Fig. 4 Sensitivity analysis of the hazard index (HI) for BPs in occupational and public exposure groups. | ||
These findings underscore the critical role of parameters like AF and k in the risk assessment of bisphenol exposure. Reducing these factors could effectively lower the associated health risks. The consistent influence of these parameters across both EDI and HQ calculations highlights their importance in developing strategies to manage and mitigate potential health risks in both occupational and public environments.
4 Conclusion
To the best of our knowledge, the current research is the first comprehensive study reporting PC concentrations in thermal paper receipts in Türkiye. BPA and BPS were the most frequently used bisphenol varieties in all thermal paper receipts, but BPB, BPZ, BP-F and BPP were also observed in the samples. BPS seems to be the main technical alternative to BPA in Türkiye. The model developed by the European Food Safety Agency (EFSA) estimates a typical daily dermal exposure rate of 0.059 μg per kg bw (ref. 52) for BPA. In the current study, the public and occupational daily exposure rates of BPA (based on the mean concentration value detected in the analysed samples) for a 70 kg body weight adult correspond to 4.45 × 10−4 μg per kg bw and 0.022 μg per kg bw, respectively. Hence, when comparing the results of the current study to the EFSA model, it is observed that public and occupational exposure rates are lower. Nevertheless, further studies are needed to better characterize the occurrence of bisphenols in thermal papers used in Türkiye and to assess the contribution of thermal papers to human exposure to BPA and to its proposed alternatives. Additionally, the presence of higher BPA levels in thermal papers may act as a contamination agent for “BPA” free waste paper during the recycling/reprocessing of paper and paper products.As in studies conducted in other parts of the world, BPA is still the most common color developer reported in thermal papers in Türkiye, followed by BPS, although its potential toxic effects are already widely known. Data on the occurrence and levels of BPA alternatives other than BPS in the abiotic environment are still scarce; however, such data are essential to understand the environmental fate of these chemicals. While the environmental persistence of BPA is considered very low, on the other hand, the environmental persistence of BPS is considered moderate. The ubiquitous occurrence of BPA and its less toxic analogues such as BPS, BPF and BPAF in the environment poses threats to both humans and wildlife. Several regulatory organizations across the world have established reference doses/safe limits for daily human BPA exposure because of its public health implications. However, different cells and organs may be impacted differently by the same amount of BPA depending on their level of sensitivity. Therefore, the best approach to avoid the toxicity of these chemicals is minimizing exposure to them in daily life.
Consent to publish
All the authors have given their consent for publishing the manuscript.Data availability
All data and materials reported in the article are included in the manuscript and can be found in the ESI file.†Author contributions
Conceptualization: Perihan Binnur Kurt-Karakus. Data curation: Perihan Binnur Kurt-Karakus, Merve Özkaleli Akcetin, and Nebile Daglioğlu. Formal analysis: Merve Özkaleli Akçetin and Perihan Binnur Kurt-Karakus. Investigation: Merve Özkaleli Akcetin, Hatice Kübra Gül, and İsmail Ethem Gören. Methodology: Merve Özkaleli Akçetin, Hatice Kübra Gül, İsmail Ethem Gören, and Nebile Daglioğlu. Supervision: Perihan Binnur Kurt-Karakus and Nebile Daglioglu. Visualization: Merve Özkaleli Akcetin and Perihan Binnur Kurt-Karakus. Writing – original draft: Merve Özkaleli Akçetin and Perihan Binnur Kurt-Karakus. Writing – review & editing: Perihan Binnur Kurt-Karakus, Nebile Daglioğlu, and Hatice Kübra Gül.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank volunteers who helped us collect the samples.References
- J. A. Adeyemi, M. Gallimberti, C. C. Olise, B. A. Rocha, C. O. Adedire and F. Barbosa, Evaluation of bisphenol A levels in Nigerian thermal receipts and estimation of daily dermal exposure, Environ. Sci. Pollut. Res., 2020, 27, 37645–37649 CrossRef CAS PubMed.
- I. Lee, S. Kim, K. T. Kim, S. Kim, S. Park, H. Lee, Y. Jeong, J. E. Lim, H. B. Moon and K. Choi, Bisphenol A exposure through receipt handling and its association with insulin resistance among female cashiers, Environ. Int., 2018, 117, 268–275 CrossRef CAS PubMed.
- J. M. Molina-Molina, I. Jimenez-Diaz, M. F. Fernandez, A. Rodriguez-Carrillo, F. M. Peinado, V. Mustieles, R. Barouki, C. Piccoli, N. Olea and C. Freire, Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain, Environ. Res., 2019, 170, 406–415 CrossRef CAS PubMed.
- RC, (Research Cosmos), Global bisphenol A market report 2018:Analysis 2013–2017 Report, Dublin, 2018.
- EU, Commission Regulation (EU) 2016/2235 of 12 December 2016 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bisphenol A, Report, 2016.
- ILGA, Illinois General Assembly, Public Act Report, 2019.
- O. Gazette, Turkish Food Codex-Communiqué on the restriction of the use of certain Epoxy Derivatives in substances and materials in contact with food, 2012.
- O. Gazette, Turkish Food Codex-Communiqué on Plastic substances and materials in Contact with food, Official Gazette Number, 2019.
- SCHEER, Final Opinion on Draft Environmental Quality Standards for Priority Substances under the Water Framework Directive - bisphenol-A, Europeand Comission, 2022.
- W. Wang, K. O. Abualnaja, A. G. Asimakopoulos, A. Covaci, B. Gevao, B. Johnson-Restrepo, T. A. Kumosani, G. Malarvannan, T. B. Minh, H. B. Moon, H. Nakata, R. K. Sinha and K. Kannan, A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries, Environ. Int., 2015, 83, 183–191 CrossRef CAS PubMed.
- T. Geens, L. Goeyens, K. Kannan, H. Neels and A. Covaci, Levels of bisphenol-A in thermal paper receipts from Belgium and estimation of human exposure, Sci. Total Environ., 2012, 435–436, 30–33 CrossRef CAS PubMed.
- B. A. Rocha, L. F. Azevedo, M. Gallimberti, A. D. Campiglia and F. Barbosa, High levels of bisphenol a and bisphenol s in brazilian thermal paper receipts and estimation of daily exposure, J. Toxicol. Environ. Health, Part A, 2015, 78, 1181–1188 CrossRef CAS PubMed.
- P. P. Hao, Determination of bisphenol A in barreled drinking water by a SPE-LC-MS method, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2020, 55, 697–703 CrossRef CAS PubMed.
- I. Anđelić, R. Roje-Busatto, I. Ujević, N. Vuletić and S. Matijević, Distribution of Bisphenol A in Sediment and Suspended Matter and Its Possible Impact on Marine Life in Kaštela Bay, Adriatic Sea, Croatia, J. Mar. Sci. Eng., 2020, 8, 480 CrossRef.
- L. López-Carrillo, A. Mérida-Ortega, H. Gómez-Ruiz, L. Hernández-Garciadiego and B. Gamboa-Loira, Exposure to bisphenol A and breast cancer risk in northern Mexican women, Int. Arch. Occup. Environ. Health, 2021, 94, 699–706 CrossRef PubMed.
- D. Chen, K. Kannan, H. L. Tan, Z. G. Zheng, Y. L. Feng, Y. Wu and M. Widelka, Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review, Environ. Sci. Technol., 2016, 50, 5438–5453 CrossRef CAS PubMed.
- L. Heras-Gonzalez, J. A. Latorre, M. Martinez-Bebia, D. Espino, F. Olea-Serrano and M. Mariscal-Arcas, The relationship of obesity with lifestyle and dietary exposure to endocrine-disrupting chemicals, Food Chem. Toxicol., 2020, 136, 110983 CrossRef CAS PubMed.
- J. Michałowicz, Bisphenol A – Sources, toxicity and biotransformation, Environ. Toxicol. Pharmacol., 2014, 37, 738–758 CrossRef.
- J. M. Molina-Molina, E. Amaya, M. Grimaldi, J. M. Sáenz, M. Real, M. F. Fernández, P. Balaguer and N. Olea, In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors, Toxicol. Appl. Pharmacol., 2013, 272, 127–136 CrossRef CAS.
- B. S. Rubin, Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects, J. Steroid Biochem. Mol. Biol., 2011, 127, 27–34 CrossRef CAS.
- T. Takeuchi and O. Tsutsumi, Serum bisphenol A concentrations showed gender differences, possibly linked to androgen levels, Biochem. Biophys. Res. Commun., 2002, 291, 76–78 CrossRef CAS.
- C. Liao, F. Liu and K. Kannan, Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues, Environ. Sci. Technol., 2012, 46, 6515–6522 CrossRef CAS.
- C. D. Kinch, K. Ibhazehiebo, J. H. Jeong, H. R. Habibi and D. M. Kurrasch, Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 1475–1480 CrossRef CAS PubMed.
- W. H. Qiu, Y. L. Zhao, M. Yang, M. Farajzadeh, C. Y. Pan and N. L. Wayne, Actions of Bisphenol A and Bisphenol S on the Reproductive Neuroendocrine System During Early Development in Zebrafish, Endocrinology, 2016, 157, 636–647 CrossRef CAS PubMed.
- I. Notardonato, M. V. Russo and P. Avino, Phthalates and bisphenol-A residues in water samples: an innovative analytical approach, Rendiconti Lincei. Sci. Fis. Nat., 2018, 29, 831–840 CrossRef.
- S. Lu, Y. Yu, L. Ren, X. Zhang, G. Liu and Y. Yu, Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact, Sci. Total Environ., 2018, 621, 1389–1396 CrossRef CAS PubMed.
- D. X. Liu, P. F. Wu, N. Zhao, S. S. Nie, J. S. Cui, M. R. Zhao and H. B. A. Jin, Differences of bisphenol analogue concentrations in indoor dust between rural and urban areas, Chemosphere, 2021, 276, 130016 CrossRef CAS PubMed.
- S. Babu, S. N. Uppu, B. Martin, O. A. Agu and R. M. Uppu, Unusually high levels of bisphenol A (BPA) in thermal paper cash register receipts (CRs): development and application of a robust LC-UV method to quantify BPA in CRs, Toxicol. Mech. Methods, 2015, 25, 410–416 CrossRef CAS PubMed.
- S. Biedermann, P. Tschudin and K. Grob, Transfer of bisphenol A from thermal printer paper to the skin, Anal. Bioanal. Chem., 2010, 398, 571–576 CrossRef CAS PubMed.
- C. Y. Liao and K. Kannan, High Levels of Bisphenol A in Paper Currencies from Several Countries, and Implications for Dermal Exposure, Environ. Sci. Technol., 2011, 45, 6761–6768 CrossRef CAS PubMed.
- S. Y. Lu, W. J. Chang, S. O. Sojinu and H. G. Ni, Bisphenol A in supermarket receipts and its exposure to human in Shenzhen, China, Chemosphere, 2013, 92, 1190–1194 CrossRef CAS PubMed.
- USEPA, USEPA Integrated Risk Information System (IRIS) database, 2024.
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), Scientific Opinion on the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs, Report, European Food Safety Authority, Online Version, 2015 Search PubMed.
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs, EFSA J., 2023, 21(4), 1–392 Search PubMed.
- K. Jurowski and M. Krośniak, The Toxicological Risk Assessment of Dermal Exposure of Patients Exposed to Lead and Cadmium Due to Application of Ointments with Marjoram Herb Extract (Majoranae herbae extractum), Int. J. Environ. Res. Public Health, 2023, 20(3), 1965–1971 CrossRef PubMed.
- USEPA, USEPA Integrated Risk Information System (IRIS) database, 2024.
- V. Gayrard, M. Z. Lacroix, S. H. Collet, C. Viguié, A. Bousquet-Melou, P. L. Toutain and N. Picard-Hagen, High bioavailability of bisphenol A from sublingual exposure, Environ. Health Perspect., 2013, 121, 951–956 CrossRef PubMed.
- T. A. Patterson, N. C. Twaddle, C. S. Roegge, R. J. Callicott, J. W. Fisher and D. R. Doerge, Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys, Toxicol. Appl. Pharmacol., 2013, 267, 41–48 CrossRef CAS.
- A. L. Demierre, R. Peter, A. Oberli and M. Bourqui-Pittet, Dermal penetration of bisphenol A in human skin contributes marginally to total exposure, Toxicol. Lett., 2012, 213, 305–308 CrossRef CAS.
- EC, European Comission, OPINION on the Safety of Presence of Bisphenol A in Clothing Articles, SCCS/1620/20, Scientific Committee on Consumer Safety, 2020 Search PubMed.
- V. Gavrard, M. Z. Lacroix, F. C. Grandin, S. H. Collet, H. Mila, C. Viguié, C. A. Gély, B. Rabozzi, M. Bouchard, R. Léandri, P. L. Toutain and N. Picard-Hagen, Oral Systemic Bioavailability of Bisphenol A and Bisphenol S in Pigs, Environ. Health Perspect., 2019, 127(7), 077005 CrossRef PubMed.
- S. A. Khan, S. Muhammad, S. Nazir and F. A. Shah, Heavy metals bounded to particulate matter in the residential and industrial sites of Islamabad, Pakistan: Implications for non-cancer and cancer risks, Environ. Technol. Innovation, 2020, 19, 100822 CrossRef.
- USEPA, Risk Assessment Guidance for Superfund: Volume III - Part a, Process for Conducting Probabilistic Risk Assessment, US Environmental Protection Agency, Washington, D.C, 2001 Search PubMed.
- R. A. Fallahzadeh, R. Khosravi, B. Dehdashti, E. Ghahramani, F. Omidi, A. Adli and M. Miri, Spatial distribution variation and probabilistic risk assessment of exposure to chromium in ground water supplies; a case study in the east of Iran, Food Chem. Toxicol., 2018, 115, 260–266 CrossRef CAS PubMed.
- B. Wu, Y. Zhang, X. X. Zhang and S. P. Cheng, Health risk assessment of polycyclic aromatic hydrocarbons in the source water and drinking water of China: Quantitative analysis based on published monitoring data, Sci. Total Environ., 2011, 410, 112–118 CrossRef PubMed.
- M. A. Sogorb, J. Estevez and E. Vilanova, Case study: Is bisphenol S safer than bisphenol A in thermal papers?, Arch. Toxicol., 2019, 93, 1835–1852 CrossRef CAS PubMed.
- R. Mesnage, A. Phedonos, M. Arno, S. Balu, J. C. Corton and M. N. Antoniou, Transcriptome Profiling Reveals Bisphenol A Alternatives Activate Estrogen Receptor Alpha in Human Breast Cancer Cells, Toxicol. Sci., 2017, 158, 431–443 CrossRef CAS PubMed.
- J. Lee, S. Park, J. Byun, M. Lee, Y. S. Do, Y. Kim and M. Kwon, Distribution and Potential Transdermal Human Intake of Bisphenol A and Bisphenol S from Thermal Receipt Papers in Korea Market, Exposure Health, 2021, 13, 477–485 CrossRef CAS.
- Y. J. Yang, Y. Yang, J. Zhang, B. Shao and J. Yin, Assessment of bisphenol A alternatives in paper products from the Chinese market and their dermal exposure in the general population, Environ. Pollut., 2019, 244, 238–246 CrossRef CAS PubMed.
- G. Castro, I. Rodriguez, M. Ramil and R. Cela, Direct analysis in real time accurate mass spectrometry determination of bisphenol A in thermal printing paper, Talanta, 2019, 205, 120086 CrossRef CAS PubMed.
- M. Eckardt and T. J. Simat, Bisphenol A and alternatives in thermal paper receipts - a German market analysis from 2015 to 2017, Chemosphere, 2017, 186, 1016–1025 CrossRef CAS PubMed.
- G. Russo, F. Barbato and L. Grumetto, Monitoring of bisphenol A and bisphenol S in thermal paper receipts from the Italian market and estimated transdermal human intake: A pilot study, Sci. Total Environ., 2017, 599, 68–75 CrossRef PubMed.
- K. A. Thayer, K. W. Taylor, S. Garantziotis, S. H. Schurman, G. E. Kissling, D. Hunt, B. Herbert, R. Church, R. Jankowich, M. I. Churchwell, R. C. Scheri, L. S. Birnbaum and J. R. Bucher, Bisphenol A, Bisphenol S, and 4-Hydro xyphenyl 4-Isopro oxyphenyl sulfone (BPSIP) in Urine and Blood of Cashiers, Environ. Health Perspect., 2016, 124, 437–444 CrossRef CAS PubMed.
- A. M. Hormann, F. S. vom Saal, S. C. Nagel, R. W. Stahlhut, C. L. Moyer, M. R. Ellersieck, W. V. Welshons, P. L. Toutain and J. A. Taylor, Holding Thermal Receipt Paper and Eating Food after Using Hand Sanitizer Results in High Serum Bioactive and Urine Total Levels of Bisphenol A (BPA), PLoS One, 2014, 9(10), e110509 CrossRef PubMed.
- M. S. Yalcin, C. Gecgel and D. Battal, Determination of Bisphenol A in Thermal Paper Receipts, J. Turk. Chem. Soc., Sect. A, 2016, 3, 167–174 CAS.
- D. M. Goldinger, A.-L. Demierre, O. Zoller, H. Rupp, H. Reinhard, R. Magnin, T. W. Becker and M. Bourqui-Pittet, Endocrine activity of alternatives to BPA found in thermal paper in Switzerland, Regul. Toxicol. Pharmacol., 2015, 71, 453–462 CrossRef CAS.
- R. Fan, B. Zeng, X. Liu, C. Chen, Q. Zhuang, Y. Wang, M. Hu, Y. Lv, J. Li, Y. Zhou and Z. Y. W. Lin, Levels of bisphenol-A in different paper products in Guangzhou, China, and assessment of human exposure via dermal contact, Environ. Sci.: Processes Impacts, 2015, 17, 667–673 RSC.
- C. Y. Liao, F. Liu and K. Kannan, Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues, Environ. Sci. Technol., 2012, 46, 6515–6522 CrossRef CAS.
- J. M. Braun, A. E. Kalkbrenner, A. M. Calafat, J. T. Bernert, X. Ye, M. J. Silva, D. B. Barr, S. Sathyanarayana and B. P. Lanphear, Variability and predictors of urinary bisphenol A concentrations during pregnancy, Environ. Health Perspect., 2011, 119, 131–137 CrossRef CAS PubMed.
- E. Schreder, On the Money BPA on Dollar Bills and Receipts, Washington Toxic Coalition, 2010.
- C. Liao and K. Kannan, Widespread Occurrence of Bisphenol A in Paper and Paper Products: Implications for Human Exposure, Environ. Sci. Technol., 2011, 45, 9372–9379 CrossRef CAS PubMed.
- C. Liao, F. Liu, H. Alomirah, V. D. Loi, M. A. Mohd, H. B. Moon, H. Nakata and K. Kannan, Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures, Environ. Sci. Technol., 2012, 46, 6860–6866 CrossRef CAS PubMed.
- D. Zalko, C. Jacques, H. Duplan, S. Bruel and E. Perdu, Viable skin efficiently absorbs and metabolizes bisphenol A, Chemosphere, 2011, 82, 424–430 CrossRef CAS PubMed.
- R. F. Fan, B. Y. Zeng, X. S. Liu, C. Chen, Q. W. Zhuang, Y. J. Wang, M. L. Hu, Y. S. Lv, J. N. Li, Y. X. Zhou and Z. Y. W. Lin, Levels of bisphenol-A in different paper products in Guangzhou, China, and assessment of human exposure via dermal contact, Environ. Sci.: Processes Impacts, 2015, 17, 667–673 RSC.
- L. Semerjian, N. Alawadhi and K. Nazer, Detection of bisphenol A in thermal paper receipts and assessment of human exposure: A case study from Sharjah, United Arab Emirates, PLoS One, 2023, 18(3), e0283675 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00132j |
| This journal is © The Royal Society of Chemistry 2025 |