Solid additive-enhanced performance in near-infrared organic photodetectors for broadband–narrowband dual-mode detection
Abstract
Organic photodetectors (OPDs) are essential for applications in environmental monitoring, medical diagnostics, and telecommunications, particularly for detecting infrared to near-infrared (NIR) light. However, improving the detectivity of OPDs in these regions remains challenging due to the presence of high dark current and limited responsivity. In this study, we present the first use of a solid additive, ferrocene (Fc), in the active layer of PTB7-Th:BT-CIC to enhance its performance. This approach significantly reduced the dark current density to 6.81 × 10−9 A cm−2 and improved the responsivity to 0.45 A W−1 at 800 nm, with an external quantum efficiency (EQE) of 70%. Compared with 1-chloronaphthalene (CN), a commonly used liquid additive, Fc provided superior improvements in both electrical and optical properties as well as enhanced the molecular arrangement and carrier transport. Additionally, Fc optimization reduced the root-mean-square white noise (In,rms) to a minimum of 1.92 × 10−14 A with a specific detectivity 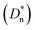 of 2.54 × 1012 Jones. Furthermore, we integrated an input optical filtering (IOF) perovskite filter into the OPD system, enabling narrowband NIR detection with an EQE of 43.9%, a responsivity of 0.294 A W−1 at 830 nm, and a full width at half maximum (FWHM) of 84.6. We also realized broadband–narrowband dual-mode NIR OPDs by introducing a transparent electrode under bidirectional illumination. Our study highlights the pioneering use of solid additives to optimize the performance of NIR OPDs while showcasing advanced detection capabilities by integrating perovskite filters and transparent electrodes.
of 2.54 × 1012 Jones. Furthermore, we integrated an input optical filtering (IOF) perovskite filter into the OPD system, enabling narrowband NIR detection with an EQE of 43.9%, a responsivity of 0.294 A W−1 at 830 nm, and a full width at half maximum (FWHM) of 84.6. We also realized broadband–narrowband dual-mode NIR OPDs by introducing a transparent electrode under bidirectional illumination. Our study highlights the pioneering use of solid additives to optimize the performance of NIR OPDs while showcasing advanced detection capabilities by integrating perovskite filters and transparent electrodes.



 Please wait while we load your content...
Please wait while we load your content...