 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Growth of MnWO4 nanowires on W(110) by high-temperature oxygen-assisted molecular beam epitaxy
Kalina
Fornal
a,
Clara
Gutiérrez-Cuesta
 b,
Adolfo
del Campo
b,
Adolfo
del Campo
 c,
Anna
Mandziak
c,
Anna
Mandziak
 d,
Pawel
Nita
d,
Pawel
Nita
 de,
José Emilio
Prieto
de,
José Emilio
Prieto
 b,
José F.
Marco
b,
José F.
Marco
 b and
Juan
de la Figuera
b and
Juan
de la Figuera
 *b
*b
aUniversity of Aberdeen, Aberdeen, UK
bInstituto de Química Física Blas Cabrera, CSIC, Madrid 28006, Spain. E-mail: juan.delafiguera@csic.es
cInstituto de Cerámica y Vidrio, CSIC, Madrid 28049, Spain
dSolaris National Synchrotron Radiation Centre, Kraków 30-392, Poland
eFaculty of Physics, Astronomy and Applied Computer Science, Jagiellonian University, Kraków 30-348, Poland
First published on 5th September 2025
Abstract
We describe the growth of synthetic hübnerite (MnWO4) by high-temperature oxygen-assisted molecular beam epitaxy on W(110). The hübnerite nanowires have widths of hundreds of nanometers, heights of tens of nanometers and lengths in the range of millimeters. The growth was followed in real time by low-energy electron microscopy (LEEM). The nanowires were characterized in situ by low-energy electron microscopy, X-ray absorption and X-ray photoelectron spectroscopy in photoemission microscopy, as well as ex situ by atomic force microscopy, optical microscopy and Raman spectroscopy. Hübnerite can be grown on W(110) by dosing only manganese in a molecular oxygen environment, likely due to the formation of highly mobile WOx species with diffusion lengths of the order of hundreds of micrometers. These species can react with the deposited Mn and be efficiently incorporated into the wolframite structure of hübnerite. The strongly anisotropic growth observed may stem from the inherent anisotropy of the wolframite lattice. We propose that this method may be applicable to the growth of other tungstates as well.
1 Introduction
Synthetic hübnerite, MnWO4, has been suggested as a multifunctional material1 in fields such as photocatalysis,2–4 or for applications in supercapacitors.5 It is insulating with a band gap higher than 2.6 eV, and thus transparent to visible light. Below 13 K it is a single phase multiferroic material6–9 combining a spiral magnetic ordering with ferroelectricity.Hübnerite belongs to the family of the tungstates of the 3d transition metals such as FeWO4, CoWO4 or NiWO4, which all share the same wolframite structure. Wolframite, the iron–manganese tungstate, is itself one of the most common commercial tungsten ores.10 The wolframite structure belongs to the monoclinic P2/c space group. It is composed of edge-sharing oxygen distorted octahedra surrounding W6+ and Mn2+ cations, forming zig-zag chains.
Nanowires are of interest from an applied point of view, often presenting a high surface-to-volume ratio. From a fundamental perspective they are useful for studying confinement in the perpendicular directions while keeping bulk-like properties in the axial direction. They possess morphologies which can benefit from the application of novel growth methods.11–13 In the particular case of hübnerite nanowires, their growth has been performed by the solvothermal method,14 and by incorporation of Mn onto W18O49 nanowires.15 Instead, in the present work, we describe the growth of hübnerite nanowires on W(110) single crystals by oxygen-assisted high-temperature molecular beam epitaxy (MBE) depositing only the manganese component and taking advantage of the special characteristics of the oxygen-tungsten interaction to provide the tungsten component.
The growth of oxide ultrathin films and nanostructures on metal substrates has advantages for their characterization, as well as interest for applications in catalysis.16 Our method consists of depositing the required cations from a metallic source in a background of oxidizing gas like molecular oxygen at a temperature high enough to activate surface diffusion, coupled to real time observations by low-energy electron microscopy (LEEM).17 One prerequisite for such approach to be successful is that the substrate is less easily oxidized than the deposited metal. Using this procedure, we have grown spinel and rock-salt oxides on Ru(0001) single crystals as well as on Ru(0001) thin films on sapphire. Complication factors are that often three-dimensional growth is achieved and that different oxide phases might be present on the surface. Even taking into account these problems, high-temperature oxygen-assisted MBE growth allows obtaining, for example, ferrimagnetic micrometer-wide crystals of iron-containing oxides such as magnetite,18 maghemite,19 cobalt ferrite20 and nickel ferrite,21 with magnetic domains which are orders of magnitude larger than those usually found in typical thin films. Cobalt22 and nickel oxide23 antiferromagnetic crystals have also been grown in this way, as well as ceria.24 In all those cases, ruthenium acts as an inert substrate which plays only a minor role in the growth process by breaking oxygen molecules into atomic oxygen and providing an epitaxial template with a Ru–Ru distance which is close to the oxygen–oxygen distance in the oxides.
However, when attempting the same approach to grow oxides on W(110), the role of the substrate is very different. This is related to the different oxide formation mechanism and reactivity of ruthenium and tungsten. For low oxygen exposures, both the Ru(0001)25,26 and W(110)27 surfaces are covered by an atomic layer of oxygen chemisorbed on the surface, which upon cooling to room temperature gives rise to coverage-dependent ordered structures. Increasing the oxygen dose results in the formation of genuine oxide crystals. In the case of Ru(0001), the growth takes place initially in the form of RuO2 islands having the rutile structure. A higher oxygen pressure or a stronger oxydizing agent such as NO228–30 is required to further grow the oxide islands on this substrate. But RuO2 is very stable in UHV.29 Tungsten, instead, gives rise to WO3 upon oxidizing.31 Many studies determined that WO3 has a much higher vapour pressure than RuO2, and that it sublimates into several tungsten oxides in UHV conditions at temperatures around 1400 K32–34 explaining the propensity of tungsten to be etched by oxygen, as initially studied by Langmuir.35,36 Even before sublimation, it is likely that WOx units are highly mobile on the W surface, presumably by a “sky-hook” effect in which an adsorbate enhances surface diffusion by weakening the bonding of the adatoms to the substrate as observed in S/Cu(111)37,38 and other systems.39,40 These oxide units would provide a source of W6+ for the growth of ternary oxides upon deposition of an additional cation. This effect has been observed in the growth of cerium tungstate during the initial stages of the deposition of cerium on W(110) in a molecular oxygen background pressure41,42 at 700 K. With continued deposition, a pure cerium oxide was obtained, as the transport of W6+ through the tungstate is kinetically hindered. If however, the growth mode is purely three dimensional, the availability of W6+ is still possible through the uncovered areas.
2 Experimental
The growth was performed at the low-energy electron-microscopy (LEEM) facility of the Instituto de Química Física Blas Cabrera in Madrid and at the DEMETER beamline of the Solaris synchrotron in Kraków. Both sites are equipped with Elmitec LEEM III instruments; in the latter case, it is integrated with a hemispherical energy spectrometer. Using an electron beam as the illumination source, both microscopes can acquire LEEM images of the surface during growth by MBE, as well as low-energy electron diffraction patterns. At Solaris, the DEMETER-PEEM instrument uses a soft X-ray beam to illuminate the sample, causing it to emit electrons through X-ray absorption and photoemission. These emitted electrons are used to create images that reveal the chemical composition of the sample. By tuning the energy of the X-ray beam, the instrument can produce spatially resolved X-ray absorption spectra. Additionally, the built-in energy spectrometer allows to perform X-ray photoelectron spectroscopy (XPS) from specific regions of the sample.The W(110) crystal used as a substrate was cleaned by multiple steps of flashing at 1500 °C in a molecular oxygen atmosphere (1 × 10−6 mbar). Manganese was deposited from a 5 mm thick manganese rod inside a home-made electron bombardment doser. The dose rate was calibrated by depositing manganese on the clean W(110) surface. Ex situ characterization of the nanostructures was performed by optical microscopy, atomic force microscopy and Raman spectroscopy. The Raman spectra were acquired with a commercial Witec Alpha 300RA confocal Raman spectrometer, using a 100× objective with a numerical aperture of 0.95. The light source was a 532 nm laser operated at 1 mW power, selected in order to avoid modification of the samples. The spectra presented are the average of 5 scans, each acquired with a 30 s integration time.
3 Results and discussion
In order to grow an oxide by oxygen-assisted MBE, crucial parameters are the oxygen pressure and the substrate temperature. As discussed in detail in ref. 43, a pressure of 10−6 mbar, compatible with direct observation by LEEM, should be able to oxidize Mn to Mn2+ at any growth temperature. The growth temperature of 800 °C was selected based on the previous experience with the growth by oxygen-assisted MBE of rock-salt and spinel oxides on Ru(0001).18,20,22 On the one hand, the temperature should be as high as possible to promote long-range diffusion of the deposited species and to promote good crystallinity. On the other hand, the temperature should be low enough so that the desired oxide does not decompose. Given that W-oxides decompose at temperatures above 1000 °C, we selected 800 °C in this work. We have later confirmed that the nanostructures grown decompose at a temperature of 850 °C.A sequence of images from a movie acquired during the deposition of manganese on the oxygen-coveredW(110) surface at 800 °C is shown in Fig. 1. The starting surface, which presents atomic steps imaged as faint dark lines in the first frame (Fig. 1a), was already exposed for several minutes to a pressure of 1 × 10−6 mbar of molecular oxygen. With these conditions, if the sample is cooled down to room temperature, it presents the so-called 337 reconstruction.44,45 From etching experiments of oxygen on tungsten31,34 it is clear that at elevated temperatures, it involves mobile WOx units. The manganese doser shutter was opened just before the acquisition of Fig. 1b, where islands initially nucleate at the steps, and then grow into larger sizes (Fig. 1c–e). The initial surface is nearly completely covered in Fig. 1f. In Fig. 1g the complete surface is already covered by a mixed Mn–W oxide. Continuing the deposition, eventually some nanostructures were detected on the surface; their growth could be followed in LEEM. However, the nucleation rate is very small, with just a few structures found in millimeter-sized areas. The sample was then cooled down in oxygen for further characterization. The grown structures decompose at a temperature of 850 °C.
An alternative way of obtaining the nanostructures, still with a very low density, was to stop the growth after the surface is completely covered by a mixed Mn–W layer and to cool down to room temperature. The sample was then transferred (in air) from the Madrid LEEM instrument into the Solaris one for further processing and characterization. In the Solaris station, the sample was initially degassed for several hours at 250 °C. At such stage, the surface still looked the same in LEEM as observed in the Madrid instrument. After a brief anneal to 800 °C in vacuum for a few minutes, again a few nanostructures could be located on the surface of the crystal. Fig. 1h shows a low-energy electron microscopy (LEEM) image of the central region of a nanostructure with a distinctly elongated shape. This structure is composed of several nanowires that appear to branch out from a common central point, where a zig-zag pattern is visible. The same region is shown in photoemission microscopy (Fig. 2a) and with a wider field of view in Fig. 2b using ex situ optical microscopy, revealing that the nanowires extend to lengths of several hundred micrometers. Fig. 2c presents an atomic force microscopy (AFM) image of the same area, acquired in contact mode. As illustrated by the height profile in Fig. 2d, the nanowires range in width from several hundred nanometers to nearly one micrometer, with heights reaching up to 100 nanometers. The zig-zag feature is clearly resolved in the AFM image and is seen to increase in height along its length.
In order to determine the composition and structure of the nanowires, we resorted to the use of several selected-area spectroscopies. We first present in Fig. 3 the X-ray absorption spectra at the Mn L2,3 edges and at the O K-edge. In the first case, the two main structures appear well separated, by about 10 eV, due to the spin–orbit splitting between the  and
and 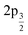 core levels. The L3 structure appears in the range 637–645 eV, while the L2 appears in the 650–655 eV range. The manganese spectrum has been reported for both manganese oxide and manganese tungstate.46 The shape of each absorption edge, specially in the case of the L3 one, depends strongly on the multiplet structure, the effects of the local crystal fields and hybridization with the O 2p ligands and on the Mn 3d–3d and 2p–3d Coulomb and exchange interactions. We note that the Mn XAS signal from the areas outside the nanowires presented roughly the same shape (not shown) although the signal was quite noisy with very low intensity. For the oxygen edge, the signal outside the nanowires was negative, indicating that it was dominated by the absorption by oxygen in the mirrors of the X-ray optics prior to the PEEM instrument. We used it to normalize the spectra from the nanowire, shown in Fig. 3b.
core levels. The L3 structure appears in the range 637–645 eV, while the L2 appears in the 650–655 eV range. The manganese spectrum has been reported for both manganese oxide and manganese tungstate.46 The shape of each absorption edge, specially in the case of the L3 one, depends strongly on the multiplet structure, the effects of the local crystal fields and hybridization with the O 2p ligands and on the Mn 3d–3d and 2p–3d Coulomb and exchange interactions. We note that the Mn XAS signal from the areas outside the nanowires presented roughly the same shape (not shown) although the signal was quite noisy with very low intensity. For the oxygen edge, the signal outside the nanowires was negative, indicating that it was dominated by the absorption by oxygen in the mirrors of the X-ray optics prior to the PEEM instrument. We used it to normalize the spectra from the nanowire, shown in Fig. 3b.
 | ||
| Fig. 3 XAS spectrum (black) acquired from the central part of the wire shown in Fig. 2a. (a) XAS spectra at the Mn L2 and L3 absorption edges. Shifted up, in orange, a multiplet calculation is shown (details in the main text). (b) XAS spectrum at the oxygen K-edge. | ||
To model the Mn experimental spectra, we calculated the X-ray absorption spectra using the Quanty47 code via the Crispy interface.48 The spectrum (Fig. 3a, shown in orange) has been calculated for octahedrally coordinated Mn2+ ions. We have used a 10Dq crystal field of 0.9 eV (as reported by variable pressure studies on MnWO449) and a spin–orbit coupling of 7.0 eV was chosen to best fit the experimental data. The values for intra-atomic 3d–3d and 2p–3d Coulomb interactions were taken from ref. 46 as 7.0 eV and 8.0 eV, respectively and the Slater integrals were reduced to 83% of their Hartree–Fock values. The simulated spectrum reproduces well the experimental peak positions and general shapes of the absorption edges, indicating the presence of high-spin, octahedrally coordinated Mn2+ ions in the structure.
For the O K-edge, the multiplet effects and the core-hole spin–orbit coupling do not need to be taken into account, since the core-hole resides in a spherically symmetrical 1s orbital localized on the oxygen atom. In fact, the shape of the O K-edge spectra shows a more direct correspondence with the empty p-states on the oxygen as they are affected by hybridization with the metal cations.50,51 The O K-edge X-ray absorption spectrum (Fig. 3b) can be divided into three regions. The first one consists of the intense peak at 532 eV. The second comprises the peak at 536 eV and the third the broader feature at 540–548 eV. The first region corresponds to electronic transitions to the unoccupied oxygen 2p states50,52 hybridized with the empty W6+ t2g orbitals, with some minor additional contribution from the manganese d levels (which are just less than 1 eV apart). The second peak is likely associated to hybridized oxygen 2p orbitals with the W6+ eg levels, which are located approximately 5 eV above the t2g levels in WO3.52 This interpretation is supported by the fact that WO3, like MnWO4, exhibits octahedral coordination around the tungsten atoms. In our case, the separation between them, which is directly the crystal field 10Dq parameter, would then be 4.0 eV. Finally, the third broader feature is attributed to the hybridization of oxygen states with the more delocalized s and p orbitals of the cations conduction band.52
Spatially resolved W 4f X-ray photoelectron spectra were acquired from the wire and the surrounding area with a photon energy of 200 eV; they are shown in Fig. 4. The W 4f spectral region is quite complex showing three different spin–orbit doublets which suggests that tungsten is present in three different chemical states at the surface both at the nanowire and the outside region. The peaks near 33.5 eV and 31.3 eV can be assigned to metallic tungsten, while those at about 38.5 eV and 36.4 eV correspond to a W6+ chemical species.53 W6+ peaks are expected to appear at energies about 6 eV higher than those corresponding to metallic tungsten due to the increased charge on the ions. The difference observed in our spectra amounts to 5 eV in the case of the wire, and to 4.2 eV in the case of wetting layer, where the W6+ are shifted to slighly lower binding energies (by 0.5–0.7 eV with respect to the wire). As it has been noted in the discussion of the XAS spectra, the ligand–metal charge transfer between oxygen and tungsten lowers the formal charge of the tungsten cation, resulting in a smaller energy difference between peaks. In any case, the chemical shifts noted in our spectra remain in agreement with values published for W6+ compounds. Finally, the doublet appearing at binding energies slightly higher than those of the doublet corresponding to metallic tungsten has been associated with the occurrence of regions in the sample where oxygen is adsorbed on metallic tungsten. This brings about a slight decrease in electron density on tungsten and, hence, a small increase in the corresponding binding energies of the W 4f core levels. The main difference between both regions is the relative weight of the different components: while the nanowire is composed mostly of W6+, the metallic W and O/W components dominate in the outside region.
 | ||
| Fig. 4 W 4f XPS spectra acquired from the MnWO4 nanowire and from the outside region (black: experimental data, gray: Shirley background). The fit is discussed in the text. | ||
Fig. 5 shows a characteristic Raman spectrum acquired from the central part of the wire (no Raman peaks were detected outside the nanowires). The wolframite monoclinic structure has a large number (36) of phonon modes as obtained from group-theory analysis (8Ag + 10Bg + 8Au + 10Bu).54 Of those, there should be 18 Raman-active modes (8Ag + 10Bg). While there are studies performed on hübnerite minerals,55–58 a more complete characterization has been performed by polarized Raman on single crystals of MnWO4,59 detecting all the Raman active phonon modes and identifying their atomic vibrations by means of density functional calculations. In our case, we readily detect (Fig. 5) nearly all the 18 modes in our nanowire (marked in the inset by red dots). The remaining modes, Bg(2,4) and Ag(3) have intensities close to the noise level. We have fitted the spectrum to a sum of Lorentzian peaks and a third-order polynomial for the background (shown by an orange dashed line). The fitted Lorentzian peak positions, widths and amplitudes are shown in Table 1, together with the values reported for a single crystal.59 The difference between the single-crystal results and the nanowire grown on W(110) is at most 3 cm−1, suggesting that our MnWO4 is also a single crystal. Thus the nanowire is thick enough to relax to the bulk structure of hübnerite. A promising venue to research is to study ultrathin wires to find possible structural modifications or phonon confinement effects for nanostructures like those obtained before the last annealing stage.
| Mode | Ref. 59 | Position | Width | Amplitude |
|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | arb. units | |
| Bg(1) | 89 | 90 | 6 | 56 |
| Ag(1) | 129 | 129 | 4 | 158 |
| Bg(2) | 160 | — | — | — |
| Bg(3) | 166 | 170 | 10 | 10 |
| Bg(4) | 177 | — | — | — |
| Ag(2) | 206 | 207 | 6 | 48 |
| Ag(3) | 258 | — | — | |
| Bg(5) | 272 | 269 | 20 | 10 |
| Bg(6) | 294 | 295 | 3 | 16 |
| Ag(4) | 327 | 328 | 8 | 62 |
| Bg(7) | 356 | 363 | 9 | 13 |
| Ag(5) | 397 | 400 | 5 | 62 |
| Bg(8) | 512 | 512 | 6 | 15 |
| Ag(6) | 545 | 548 | 8 | 72 |
| Bg(9) | 674 | 675 | 9 | 25 |
| Ag(7) | 698 | 700 | 8 | 37 |
| Bg(10) | 774 | 778 | 17 | 27 |
| Ag(8) | 885 | 888 | 6 | 1000 |
4 Conclusions
We have grown nanostructured synthetic hübnerite (MnWO4) by molecular beam epitaxy of manganese on a W(110) substrate in a molecular oxygen background. The nanostructures have been characterized by both in situ (X-ray absorption and X-ray photoelectron spectroscopies as well as low-energy electron microscopy) and ex situ (Raman spectroscopy and atomic force microscopy) techniques. The formation of a highly diffusive tungsten oxide allows for the growth of the tungstate by depositing only manganese. The nanostructures are tens of nanometers thick, hundreds of nanometers wide and with lengths in the range of millimetres. From current experiments which we will submit shortly to the scientific literature, we propose that this simple growth method based on the deposition of a single metal on W(110) by high-temperature oxygen-assisted molecular beam epitaxy can be successfully extended to the growth of other transition metal or alkaline-earth tungstates, although the particular morphology of the grown nanostructures will likely depend on whether more isotropic (e.g. tetragonal scheelite) or more anisotropic (e.g. monoclinic wolframite) structures are formed.Author contributions
The work was planned by JdlF and JEP, the samples were grown by KF and CG-C, and the synchrotron characterization was performed by AM, PN, CG-C, JdlF. JFM contributed to the XPS analysis and AdC performed the Raman, AFM and optical microscopy. The manuscript was written by JdlF and CG-C with input and revisions from all the other authors.Conflicts of interest
There are no conflicts to declare.Data availability
Data are available at the DIGITAL.CSIC open repository under DOI https://doi.org/10.20350/digitalCSIC/17530.Acknowledgements
This work was supported by Grants PID2021-124585NB-C31 and TED2021-130957B-C54 funded by MCIN/AEI/10.13039/501100011033, by “ERDF A way of making Europe” and by the “European Union NextGenerationEU/PRTR”, and by the BEETHOVEN project funded by the European Commission under grant agreement 101129912. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Education and Culture Executive Agency (EACEA). Neither the European Union nor EACEA can be held responsible for them. This publication was partially developed under the provision of the Polish Ministry of Science and Higher Education project “Support for research and development with the use of research infrastructure of the National Synchrotron Radiation Centre Solaris” under contract no. 1/SOL/2021/2.References
- M. Assis, A. C. M. Tello, F. S. A. Abud, P. Negre, L. K. Ribeiro, R. A. P. Ribeiro, S. H. Masunaga, A. E. B. Lima, G. E. Luz Jr, R. F. Jardim, A. B. F. Silva, J. Andrés and E. Longo, Appl. Surf. Sci., 2022, 600, 154081 CrossRef CAS.
- X. Li, D. Teschner, V. Streibel, T. Lunkenbein, L. Masliuk, T. Fu, Y. Wang, T. Jones, F. Seitz, F. Girgsdies, F. Rosowski, R. Schlögl and A. Trunschke, Chem. Sci., 2019, 10, 2429–2443 RSC.
- K. S. Kumar, K. Vaishnavi, P. Venkataswamy, G. Ravi, K. Ramaswamy and M. Vithal, J. Indian Chem. Soc., 2021, 98, 100140 CrossRef CAS.
- S. Han, Y. Wang, D. Zhang and H. Cong, Chem. Commun., 2023, 59, 6857–6860 RSC.
- A. M. Sorouri, A. Sobhani-Nasab, M. R. Ganjali, S. Manani, H. Ehrlich, Y. Joseph and M. Rahimi-Nasrabadi, Appl. Mater. Today, 2023, 32, 101819 CrossRef.
- O. Heyer, N. Hollmann, I. Klassen, S. Jodlauk, L. Bohaty, P. Becker, J. A. Mydosh, T. Lorenz and D. Khomskii, J. Phys.: Condens. Matter, 2006, 18, L471 CrossRef CAS.
- A. H. Arkenbout, T. T. M. Palstra, T. Siegrist and T. Kimura, Phys. Rev. B:Condens. Matter Mater. Phys., 2006, 74, 184431 CrossRef.
- K. Taniguchi, N. Abe, T. Takenobu, Y. Iwasa and T. Arima, Phys. Rev. Lett., 2006, 97, 097203 CrossRef CAS.
- K. Taniguchi, N. Abe, H. Umetsu, H. A. Katori and T. Arima, Phys. Rev. Lett., 2008, 101, 207205 CrossRef CAS PubMed.
- E. Lassner and W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, Springer, US, Boston, MA, 1999 Search PubMed.
- B. Mandl, J. Stangl, E. Hilner, A. A. Zakharov, K. Hillerich, A. W. Dey, L. Samuelson, G. Bauer, K. Deppert and A. Mikkelsen, Nano Lett., 2010, 10, 4443–4449 CrossRef CAS.
- M. Fickenscher, T. Shi, H. E. Jackson, L. M. Smith, J. M. Yarrison-Rice, C. Zheng, P. Miller, J. Etheridge, B. M. Wong, Q. Gao, S. Deshpande, H. H. Tan and C. Jagadish, Nano Lett., 2013, 13, 1016–1022 CrossRef CAS PubMed.
- K. Pemasiri, H. E. Jackson, L. M. Smith, B. M. Wong, S. Paiman, Q. Gao, H. H. Tan and C. Jagadish, J. Appl. Phys., 2015, 117, 194306 CrossRef.
- S.-J. Chen, X.-T. Chen, Z. Xue, J.-H. Zhou, J. Li, J.-M. Hong and X.-Z. You, J. Mater. Chem., 2003, 13, 1132–1135 RSC.
- W. B. Hu, X. L. Nie and Y. Z. Mi, Mater. Charact., 2010, 61, 85–89 CrossRef CAS.
- H.-J. Freund, J. Am. Chem. Soc., 2016, 138, 8985–8996 CrossRef CAS.
- J. I. Flege and D. C. Grinter, Prog. Surf. Sci., 2018, 93, 21–45 CrossRef CAS.
- M. Monti, B. Santos, A. Mascaraque, O. Rodríguez de la Fuente, M. A. Niño, T. O. Menteş, A. Locatelli, K. F. McCarty, J. F. Marco and J. de la Figuera, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 85, 020404 CrossRef.
- M. Monti, B. Santos, A. Mascaraque, O. Rodríguez de la Fuente, M. A. Nino, T. O. Menteş, A. Locatelli, K. F. McCarty, J. F. Marco and J. de la Figuera, J. Phys. Chem. C, 2012, 116, 11539–11547 CrossRef CAS.
- L. Martín-García, A. Quesada, C. Munuera, J. F. Fernandez, M. Garca-Hernandez, M. Foerster, L. Aballe and J. de la Figuera, Adv. Mater., 2015, 27, 5955–5960 CrossRef.
- A. Mandziak, J. D. L. Figuera, S. Ruiz-Gómez, G. D. Soria, L. Perez, P. Prieto, A. Quesada, M. Foerster and L. Aballe, Sci. Rep., 2018, 8, 17980 CrossRef PubMed.
- A. Mandziak, J. de la Figuera, A. Quesada, A. Berja, C. Granados-Miralles, J. E. Prieto, L. Aballe, M. Foerster, M. A. Nino and P. Nita, Ultramicroscopy, 2023, 253, 113795 CrossRef CAS.
- A. Mandziak, G. D. Sori, J. E. Prieto, P. Prieto, C. Granados-Miralles, A. Quesada, M. Foerster, L. Aballe and J. de la Figuera, Sci. Rep., 2019, 9, 13584 CrossRef PubMed.
- B. Kaemena, S. D. Senanayake, A. Meyer, J. T. Sadowski, J. Falta and J. I. Flege, J. Phys. Chem. C, 2013, 117, 221–232 CrossRef CAS.
- P. Piercy, Phys. Rev. B:Condens. Matter Mater. Phys., 1992, 45, 1869–1877 CrossRef CAS PubMed.
- S. Lizzit, A. Baraldi, A. Groso, K. Reuter, M. V. Ganduglia-Pirovano, C. Stampfl, M. Scheffler, M. Stichler, C. Keller, W. Wurth and D. Menzel, Phys. Rev. B:Condens. Matter Mater. Phys., 2001, 63, 205419 CrossRef.
- P. K. Wu, M. C. Tringides and M. G. Lagally, Phys. Rev. B:Condens. Matter Mater. Phys., 1989, 39, 7595–7610 CrossRef CAS.
- J. I. Flege, J. Hrbek and P. Sutter, Phys. Rev. B:Condens. Matter Mater. Phys., 2008, 78, 165407 CrossRef.
- H. Over, Chem. Rev., 2012, 112, 3356–3426 CrossRef CAS.
- J. I. Flege, J. Lachnitt, D. Mazur, P. Sutter and J. Falta, Phys. Chem. Chem. Phys., 2015, 18, 213–219 RSC.
- A. E. Lee, K. E. Singer and D. Tabor, Proc. R. Soc. London, Ser. A, 1971, 323, 523–539 CAS.
- J. C. Batty and R. E. Stickney, J. Chem. Phys., 1969, 51, 4475–4484 CrossRef CAS.
- J. C. Batty and R. E. Stickney, J. Chem. Phys., 1969, 51, 4485–4492 CrossRef CAS.
- J. C. Batty and R. E. Stickney, Oxid. Met., 1971, 3, 331–355 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1913, 35, 105–127 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1915, 37, 1139–1167 CrossRef CAS.
- P. J. Feibelman, Phys. Rev. Lett., 2000, 85, 606–609 CrossRef CAS PubMed.
- W. Ling, N. Bartelt, K. Pohl, J. de la Figuera, R. Hwang and K. McCarty, Phys. Rev. Lett., 2004, 93, 166101 CrossRef CAS.
- M. Kalff, G. Comsa and T. Michely, Phys. Rev. Lett., 1998, 81, 1255–1258 CrossRef CAS.
- S. Horch, H. Q. Lorensen, S. Helveg, E. Læsgaard, I. Stensgaard, K. W. Jacobsen, J. K. Norskov and F. Besenbacher, Nature, 1999, 398, 134 CrossRef CAS.
- T. Skala, N. Tsud, M. N. Orti, T. O. Menteş, A. Locatelli, K. C. Prince and V. Matolin, Phys. Chem. Chem. Phys., 2011, 13, 7083–7089 RSC.
- T. Skala and V. Matolin, Surf. Interface Anal., 2016, 48, 111–114 CrossRef CAS.
- F. V. E. Hensling, W. Braun, D. Y. Kim, L. N. Majer, S. Smink, B. D. Faeth and J. Mannhart, APL Mater., 2024, 12, 040902 CrossRef CAS.
- K. Radican, S. I. Bozhko, S.-R. Vadapoo, S. Ulucan, H.-C. Wu, A. McCoy and I. V. Shvets, Surf. Sci., 2010, 604, 1548–1551 CrossRef CAS.
- D. Wilgocka-Slezak, T. Giela, K. Freindl, N. Spiridis and J. Korecki, Appl. Surf. Sci., 2020, 528, 146712 CrossRef CAS.
- N. Hollmann, Z. Hu, T. Willers, L. Bohaty, P. Becker, A. Tanaka, H. H. Hsieh, H.-J. Lin, C. T. Chen and L. H. Tjeng, Phys. Rev. B:Condens. Matter Mater. Phys., 2010, 82, 184429 CrossRef.
- M. W. Haverkort, M. Zwierzycki and O. K. Andersen, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 85, 165113 CrossRef.
- M. Retegan, Crispy: v0.8.0, 2024 DOI:10.5281/zenodo.1008184.
- J. Ruiz-Fuertes, S. López-Moreno, J. López-Solano, D. Errandonea, A. Segura, R. Lacomba-Perales, A. Munoz, S. Radescu, P. Rodríguez-Hernandez, M. Gospodinov, L. L. Nagornaya and C. Y. Tu, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 86, 125202 CrossRef.
- F. M. F. de Groot, M. Grioni, J. C. Fuggle, J. Ghijsen, G. A. Sawatzky and H. Petersen, Phys. Rev. B:Condens. Matter Mater. Phys., 1989, 40, 5715–5723 CrossRef CAS PubMed.
- F. Frati, M. O. J. Y. Hunault and F. M. F. de Groot, Chem. Rev., 2020, 120, 4056–4110 CrossRef CAS PubMed.
- B. Chen, J. Laverock, L. F. J. Piper, A. R. H. Preston, S. W. Cho, A. DeMasi, K. E. Smith, D. O. Scanlon, G. W. Watson, R. G. Egdell, P.-A. Glans and J.-H. Guo, J. Phys.: Condens. Matter, 2013, 25, 165501 CrossRef CAS PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of xps data, 1995 Search PubMed.
- D. Diaz-Anichtchenko, J. E. Aviles-Coronado, S. López-Moreno, R. Turnbull, F. J. Manjón, C. Popescu and D. Errandonea, Inorg. Chem., 2024, 63, 6898–6908 CrossRef CAS PubMed.
- W. P. Griffith, J. Chem. Soc. A, 1970, 286–291 RSC.
- V. V. Fomichev and O. I. Kondratov, Spectrochim. Acta, Part A, 1994, 50, 1113–1120 CrossRef.
- J. T. Kloprogge, M. L. Weier, L. V. Duong and R. L. Frost, Mater. Chem. Phys., 2004, 88, 438–443 CrossRef CAS.
- R. L. Frost, L. Duong and M. Weier, Spectrochim. Acta, Part A, 2004, 60, 1853–1859 CrossRef PubMed.
- M. N. Iliev, M. M. Gospodinov and A. P. Litvinchuk, Phys. Rev. B:Condens. Matter Mater. Phys., 2009, 80, 212302 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |



