Open Access Article
Open Access ArticleAdvancing thermoplasmonic sensing: gold nanobipyramids for enhanced light-to-heat conversion†
Andreea Campuab,
Ioana Andreea Brezesteancd,
Septimiu-Cassian Triponef,
Simion Astilean ag and
Monica Focsan
ag and
Monica Focsan *ag
*ag
aNanobiophotonics and Laser Microspectroscopy Center, Interdisciplinary Research Institute on Bio-Nano-Sciences, Babes-Bolyai University, Treboniu Laurian No. 42, Cluj-Napoca 400271, Romania. E-mail: andreea.campu@ubbcluj.ro; monica.iosin@ubbcluj.ro
bResearch Center for Complex Physical Systems, Faculty of Sciences, Lucian Blaga University, Doctor Ion Raţiu No. 5-7, Sibiu 550012, Romania
cDepartment of Molecular and Biomolecular Physics, National Institute for Research and Development of Isotopic and Molecular Technologies, Donat No. 67-103, Cluj-Napoca 400293, Romania. E-mail: ioana.brezestean@itim-cj.ro
dRDI Laboratory of Applied Raman Spectroscopy, RDI Institute of Applied Natural Sciences (IRDI-ANS), Babes-Bolyai University, Fântânele No. 42, Cluj-Napoca 400293, Romania
eElectron Microscopy Center “Prof. C. Craciun”, Babes-Bolyai University, Clinicilor No. 5-7, Cluj-Napoca 400006, Romania. E-mail: septimiu.tripon@itim-cj.ro
fElectron Microscopy Integrated Laboratory, National Institute for Research and Development of Isotopic and Molecular Technologies, Donat No. 67-103, Cluj-Napoca 400293, Romania
gBiomolecular Physics Department, Faculty of Physics, Babes-Bolyai University, Mihail Kogalniceanu No. 1, Cluj-Napoca 400084, Romania. E-mail: simion.astilean@ubbcluj.ro
First published on 8th July 2025
Abstract
Thermoplasmonic detection is a newly emerging application in the rapidly growing and promising field of thermoplasmonics. Accordingly, herein, an in-depth evaluation of thermoplasmonic performances of gold nanobipyramids (AuBPs) dispersed in colloidal solutions and immobilized on a filter paper substrate was provided, which revealed their ability for efficient and sensitive thermoplasmonic detection of simple and complex molecules. Concretely, AuBPs in aqueous solution with optical responses in and out of resonance with the 808 nm laser line were synthesized and their intrinsic light-to-heat conversion performances were assessed, revealing photothermal efficiencies (η) up to 74%. Subsequently, colloidal AuBPs were functionalized with 4-mercaptobenzoic acid (4-MBA), which is a simple and small molecule. Consequently, η decreased by up to 4%. Furthermore, their immobilization on Whatman no. 1 filter paper through immersion resulted in the preservation of their optical properties and intrinsic thermoplasmonic activity. Thermoplasmonic detection capabilities of the plasmonic paper were tested using 4-MBA and thiol-polyethylene glycol-amine (a thermo-sensitive complex polymer). Following the functionalization of the plasmonic paper, its photothermal activity significantly decreased, causing an increase in the cooling time constant; thus, both 4-MBA and thiolated PEG were detected via thermoplasmonic detection. Moreover, a LOD of 0.19 nM and a LOQ of 0.58 nM were determined for 4-MBA, proving the high biosensing efficiency of the plasmonic paper. Hence, these results contribute to the consolidation of the versatile thermoplasmonic detection of both simple and complex interactions, being a stepping stone in the development of simple and efficient thermoplasmonic nanosensors.
1. Introduction
In the early 2000s, the first approaches to exploit the heat generated by gold nanoparticles for biomedical applications emerged,1,2 becoming the foundation of the rapidly growing and promising field called thermoplasmonics.3 Recent developments in thermoplasmonics have impacted a broad range of scientific activities and communities, extending its applications beyond its initial implementation for photothermal therapy,4 drug and gene delivery5 and photoacoustic imaging6 in nanomedicine,7 cell biology, photothermal and hot-electron chemistry,8 solar light harvesting,9 soft matter10 and nanofluidics.11 Considerable progress has been made by the scientific community in providing a better understanding of the thermoplasmonic mechanism; however certain challenges still remain when it comes to the influence of nanoscale photothermal processes on the photothermal effect.12Thermoplasmonic effect emerges as a result of the resonant excitation of the surface plasmons, which induces a series of waterfall phenomena because of the energy transfer from the photons to the localized surface electrons and ultimately to the surrounding environment in the form of heat.13 Upon the plasmon-induced resonance energy transfer, radiative and non-radiative damping processes take place, followed by the generation of hot carriers that transfer the energy to the material lattice upon their relaxation. The material lattice ultimately dissipates this energy to the surrounding environment as heat, through conduction, convection or radiation, either by localized or collective heating mechanisms. Consequently, thermal-induced processes such as mass transport, phase transitions, or chemical reactions can be induced.3 The thermoplasmonic effect is highly dependent on the light absorption capability of plasmonic materials, which is considered a detrimental factor in the photothermal performances.14 In this context, metallic nanoparticles are remarkably attractive owing to their increased energy absorption capacity at plasmon wavelengths.9 In particular, gold nanoparticles have shown great potentials to operate as localized heat nanosources owing to their localized surface plasmon resonance (LSPR); the oscillations of the localized surface conduction electrons, resulting from the interactions with incoming light, produce a considerable temperature increase. Consequently, gold nanoparticles have been extensively implemented as thermoplasmonic generators in the development of alternative cancer treatments – photothermal therapy,15–17 antibacterial activity18,19 as well as, more recently, detection of viral sequences.20 In particular, gold bipyramids (AuBPs) are good candidates for developing nanosystems as therapeutic agents for photothermal therapy.21,22 Recently, their use as thermoplasmonic nanogenerators has contributed significantly to the development of the emerging application of thermoplasmonic detection. AuBPs-based photothermal sensors have been successful in evaluating antioxidant capacity by detecting ascorbic acid up to 0.08 μM,23 developing photothermal immunoassays capable of detecting toxins up to 0.20 ng mL−1,24 realizing thermoplasmonic detection of cardiac biomarkers up to 4.2 pg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and as nanoreactors for bioassays with dynamic detection range from 5 ng μL−1 to 1 pg μL−1.26 However, in these studies, the measured signals originated from photothermally induced reactions or modifications in the environment of the nanoparticles.
25 and as nanoreactors for bioassays with dynamic detection range from 5 ng μL−1 to 1 pg μL−1.26 However, in these studies, the measured signals originated from photothermally induced reactions or modifications in the environment of the nanoparticles.
Therefore, in this work, in order to gain better insights on the photothermal mechanism, we exploited the intrinsic capabilities of AuBPs to efficiently convert light to heat for realizing thermoplasmonic detection, both in solution and when immobilized on a flexible filter paper, consolidating the detection method and developing sensitive thermoplasmonic sensors. We evaluated the photothermal conversion performances of colloidal nanoparticles by determining their photothermal efficiencies. By functionalizing the AuBPs with a small molecule (4-mercaptobenzoic acid (4-MBA)), variations in their light-to-heat conversion were monitored. AuBPs with their longitudinal LSPR at 811 nm exhibited the best photothermal features and thermoplasmonic detection abilities; therefore, they were further immobilized on a Whatman paper. The photothermal performance of the plasmonic paper was evaluated and optimized by increasing the immobilized AuBPs concentration. The thermoplasmonic detection capabilities of the optimized, flexible plasmonic-paper-based nanoplatform were verified using two target analytes–4-MBA as a small, simple molecule and thiol-polyethylene glycol-amine as a thermo-sensitive complex polymer. The mass effect and thermally induced phase transition led to modifications in the photothermal conversion performances and, implicitly, cooling time constants of the plasmonic paper. Thus, AuBPs, both in solution and immobilized on a paper substrate, were demonstrated to operate as efficient thermoplasmonic sensors that enable the detection of simple and complex molecules, contributing to a comprehensive understanding of the versatile thermoplasmonic detection technique.
2. Experimental section
2.1. Materials
Tetrachloroauric acid (HAuCl4·4H2O, 99.99%), cetyltrimethylammonium bromide (CTAB, 96%), nitric acid (HNO3, 65%), citric acid (C6H8O7), cetyltrimethylammonium chloride (CTAC), hydroxyquinoline (HQL, 99%), sodium borohydride (NaBH4, 99%), silver nitrate (AgNO3, 99%), 4-mercaptobenzoic acid (4-MBA), thiol-polyethylene glycol-amine (SH-PEG-NH2, average Mw of 3500 Da) and Whatman qualitative filter paper grade 1 were purchased from Sigma-Aldrich. All chemicals were used as received. Ultrapure water with a resistivity of ∼18.2 MΩ was used as the solvent throughout the experiments.2.2. Functionalization of the colloidal AuBPs and flexible plasmonic-paper-based nanoplatform
To evaluate the potential of the colloidal AuBPs to operate as transducers for thermoplasmonic detection, 4-mercaptobenzoic acid – as a small simple molecule, was selected. An ethanoic solution of 10−3 M of 4-MBA was prepared and mixed with AuBPs in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, thus obtaining a final 4-MBA concentration of 10−4 M.
9, thus obtaining a final 4-MBA concentration of 10−4 M.
For evaluating the thermoplasmonic detection ability of the plasmonic paper, both 4-MBA and thiol-polyethylene glycol-amine – a thermo-sensitive, complex polymer with a molecular weight of 3500 Da, were tested. Specifically, 10 μL of the 4-MBA ethanoic 10−4 M solution was added dropwise onto the plasmonic paper and allowed to dry. SH-PEG-NH2 was prepared in ultra-pure water at a concentration of 0.25 mM, and 10 μL of the thiolated PEG was added dropwise onto the plasmonic paper and allowed to dry under room temperature conditions. To determine the detection limit, 10 μL of ethanoic 4-MBA solutions with concentrations ranging from 10−4 to 10−10 M were dropped onto the plasmonic paper and allowed to dry at room temperature.
2.3. Thermographic determinations
Photothermal properties of the colloidal AuBPs aqueous solutions and plasmonic paper were determined based on thermographic determinations. To achieve this, the samples were irradiated from above using a Therapy Laser DTL–BCD–01 with an NIR excitation wavelength of 808 nm. Using an Optris PI 450 infrared camera, thermographic images were recorded during and after laser exposure at a 45° angle (Fig. S5). Colloidal AuBPs (500 μL) were exposed to laser excitation for 10 minutes, followed by the monitoring of the cooling process for another 10 minutes; thermal images were recorded in real time every 30 seconds. As for the plasmonic paper samples, the exposure and cooling times were decreased to 5 minutes with the laser on and 5 minutes with the laser off, respectively, while thermal images were recorded in real time every 5 seconds. To study the dependency of the photothermal performance on the laser power, the plasmonic paper obtained after 5 successive immersions was exposed to the 808 nm laser line with varied laser powers, specifically 184, 232, 290 and 320 mW. For the determination of the detection limit, the exposure time was optimized as 3 minutes with the laser on and 3 minutes with the laser off. All the thermal experiments were realized in a dark room to avoid external contributions and conducted in triplicate to ensure reproducibility. The presented thermal curves were obtained as the average of the triplicates, and the standard deviation (SD) and standard error (SE) were calculated.2.4. Characterization methods
The extinction spectra of the colloidal AuBPs in aqueous solution were recorded using a Jasco V-670 UV-Vis-NIR spectrophotometer with a 2 nm bandwidth and 1 nm spectral resolution. To record the LSPR spectra of the plasmonic paper samples, a portable Ocean Optics USB 4000 optical UV-Vis spectrophotometer was employed coupled with an inverted optical microscope ZEISS Axio Observer Z1 system equipped with a halogen lamp (HAL 100). The extinction spectra were recorded using a 20× ZEISS objective and an optical fibre with a 600 μm core diameter.Thermoplasmonic determinations were realized in a dark room using a Therapy Laser DTL-BCD-01 equipped with a laser probe SL1 having an 808 nm diode. The measurements were realized with a laser power of 0.29 W. To record the thermographic images, an Optris PI 450 infrared camera with an O38 standard lens was used. The as-recorded thermal maps were analysed using the software of the camera, Optris PI Connect.
3. Results and discussion
3.1. Thermoplasmonic detection capabilities of colloidal AuBPs
Gold nanoparticles of different shapes and sizes have been previously studied as photothermal agents considering their application in photothermal therapy.27–29 With the advancements in the emerging field of thermoplasmonic detection, their applicability has widened owing to their unique optical properties and intrinsic photothermal features. Accordingly, colloidal AuBPs in aqueous solutions were chemically synthesized to exhibit longitudinal LSPR responses in and out of resonance using an 808 nm laser line excitation, with the longitudinal LSPR specifically located at 693, 774 and 811 nm. The seed-mediated synthesis approach used to grow AuBPs along with their optical and morphological characterisations are described in the ESI.†To assess the thermoplasmonic detection capabilities of the colloidal AuBPs in aqueous solutions, the light-to-heat conversion performances of the bipyramidal nanoparticles were first evaluated. In particular, AuBPs samples were exposed to the 808 nm laser excitation for 10 minutes, and then, the cooling process was monitored for another 10 minutes. During the heating and cooling of the nanoparticles, thermal images were recorded every 30 seconds. Fig. 1A presents representative thermographic maps recorded at 0, 2, 5, 7 and 10 minutes for the solvent (as control) and the three AuBPs samples. Notably, all the AuBPs samples exhibited photothermal conversion performances, reaching a maximum temperature of up to 49 °C for the AuBPs with longitudinal LSPR under resonance conditions with the 808 nm laser line excitation. The solvent (ultra-pure water) used as control did not show any light-to-heat conversion abilities.
To assess the light-to-heat conversion performances, the experimentally obtained thermographic images were analysed by extracting the temperatures (T) at every 30 seconds during the heating and cooling processes. Next, the temperature difference (ΔT) at respective times was calculated as the difference between the time-corresponding T and environmental temperature (Tenv) as follows:
| ΔT = T − Tenv | (1) |
Moreover, the photothermal conversion efficiencies (η) were calculated using a previously reported analytical method relying on microscale thermal dynamics in solution, specifically, on the derivation of the energy balance that is used to fit the thermal data.30 Thus, η was defined as:
 | (2) |
As predicted, the calculated η values increased as the position of the longitudinal LSPR band approached resonance condition with the excitation laser wavelength (Table 1). Thus, η values of up to 74% were obtained for AuBPs with an LSPR of 811 nm.
| Sample | ΔTmax (°C) | τS (s) | η (%) |
|---|---|---|---|
| AuBPs 693 nm | 8 ± 1 | 376.88 | 14 |
| AuBPs 693 nm-MBA | 7 ± 1 | 304.51 | 13 |
| AuBPs 774 nm | 18 ± 1 | 260.45 | 61 |
| AuBPs 774 nm-MBA | 20 ± 1 | 269.30 | 59 |
| AuBPs 811 nm | 21 ± 1 | 252.35 | 74 |
| AuBPs 811 nm-MBA | 22 ± 1 | 259.33 | 71 |
Furthermore, the thermoplasmonic detection capabilities of AuBPs were evaluated. For this purpose, 4-mercaptobenzoic acid (4-MBA) molecule was chosen owing to its thiol active group, which is known to bind to the surface of gold.31 First, we functionalized AuBPs with 4-MBA. To verify the success of functionalization, extinction spectra of the AuBPs were recorded after their exposure to 4-MBA, and the optical response were compared with those of the as-synthesized nanoparticles. The successful functionalization with 4-MBA was confirmed by the red-shifts of 1, 3 and 4 nm recorded for the longitudinal LSPR at 693, 774 and 811 nm, respectively (Fig. 2). LSPR is known to be highly sensitive to changes in the micro-environment in the close vicinity of the nanoparticles; hence, when 4-MBA was grafted on the surface of the nanoparticles, the refractive index at their surface got modified, resulting in a red-shift in the optical response. As the electromagnetic field is highly enhanced at the tips of the AuBPs,32 their sensitivity to environmental changes is high at the tips, and therefore, only the longitudinal LSPR band was red-shifted.
 | ||
| Fig. 2 UV-Vis-NIR extinction spectra recorded before (full lines) and after (dashed lines) the functionalization of AuBPs with 4-MBA. | ||
Subsequent to the functionalization of AuBPs with 4-MBA, the samples were exposed to the 808 nm laser under the same conditions used for the as-synthesized nanoparticles. Based on the thermographic images, the thermal curves were plotted, and the photothermal efficiencies were calculated considering the mass addition of the 4-MBA. As shown in Table 1, the grafting of 4-MBA did not considerably affect the maximum temperature compared with the as-synthesized nanoparticles, indicating that the target analyte binding may have affected the intrinsic heat transfer rate of the surface oscillating electrons. However, the effect of target analyte binding was observed in photothermal conversion efficiencies: functionalization of AuBPs with 4-MBA resulted in decreased photothermal conversion efficiencies. The addition of the target analyte induced a change in the cooling process of AuBPs via a change in the cooling time constant. Thus, η was reduced by 7%, 3% and 4% (Table 1) for the AuBPs with longitudinal LSPR responses of 693, 774 and 811 nm, respectively.
Therefore, all AuBPs samples could operate as thermoplasmonic nanogenerators for thermoplasmonic detection applications with different performances, which are related to their absorbance characteristics. AuBPs with a longitudinal LSPR at 811 nm exhibited the best photothermal features and thermoplasmonic detection abilities; therefore, they were selected to subsequently develop flexible plasmonic-paper-based nanoplatforms.
3.2. Light-to-heat conversion performances of the plasmonic paper samples
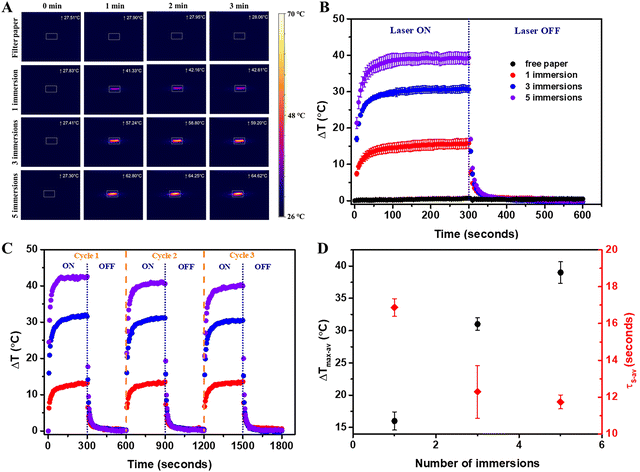
The plasmonic paper was exposed to the 808 nm laser excitation in a dark room to avoid external contributions, similar to the solution-based systems. To determine the influence of the concentration of immobilized AuBPs, filter paper immersed once, thrice and five times, respectively, were investigated. Free filter paper was tested as the control. A thermographic image was captured every 5 seconds throughout the process involving 5 minutes of exposure and 5 minutes of cooling. The characteristic thermographic images recorded at 0, 1, 2 and 3 minutes are presented in Fig. 3A. All the samples exhibited photothermal activity, excluding the free filter paper, and thermal equilibrium was reached in the first 50 seconds following laser exposure. The temperatures were extracted from the thermographic images, and an average ΔTmax of 1 °C (SD 0.41, SE 0.24) was determined for the free filter paper, while the plasmonic papers obtained after 1, 3, and 5 immersions of the filter paper reached an average ΔTmax of 16 °C (SD 2.45, SE 1.41), 31 °C (SD 1.70, SE 0.98) and 39 °C (SD 2.89, SE 1.67), respectively. As expected, the thermal curves (Fig. 3B) confirmed that a higher immobilized AuBPs concentration led to improved photothermal conversion performances. Therefore, it was demonstrated that after the immobilization of the AuBPs onto the cellulose fibres, both the optical features and the intrinsic light-to-heat conversion properties of the colloidal AuBPs were preserved. Furthermore, the reusability of the plasmonic paper was tested by exposing it to a series of three consecutive laser ON/OFF cycles. As shown in Fig. 3C, all plasmonic papers showed negligible variations in terms of temperature increase, thus exhibiting excellent thermal stability and indicating the possibility of performing multiple measurements on the same platform without altering its photothermal conversion capability. Moreover, the photothermal performances of the plasmonic paper samples were analyzed as a function of the cooling time constant (τS), which was extracted as the slope of the linear regression of the cooling profile obtained by plotting the cooling time against the negative normal logarithm of the temperature driving force (θ), which is calculated as:
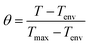 | (3) |
Furthermore, the plasmonic paper with the best performance was exposed to different laser powers (184, 232, 290 and 320 mW), and thermographic images were recorded every 5 seconds for 5 minutes with the laser on and 5 minutes with the laser off. Fig. 4A shows the average thermal curves obtained from the extraction of the temperatures from the triplicate measurements and the determination of the average ΔT. At a laser power of 184 mW, an average ΔTmax of 23 °C was obtained with an SD of 2.19 and an SE of 1.26; at a 232 mW laser power, an average ΔTmax of 36 °C was obtained with an SD of 2.25 and an SE of 1.30; at a 290 mW laser power, an average ΔTmax of 39 °C was obtained with an SD of 2.99 and an SE of 1.73; and at a 320 mW laser power, an average ΔTmax of 47 °C was obtained with an SD of 2.5 and an SE of 1.44. These results demonstrated that increasing the laser power improved the light-to-heat conversion performance. The plot of the average ΔTmax against the laser power (Fig. 4B) showed a linear dependency between the photothermal performance and the power of the irradiation source.
 | ||
| Fig. 4 (A) Average thermal curves obtained for the tested laser powers (184, 232, 290 and 320 mW). (B) Plot of the maximum temperature reached as a function of the employed laser power. | ||
3.3. Thermoplasmonic detection abilities of the flexible plasmonic-paper-based nanoplatform
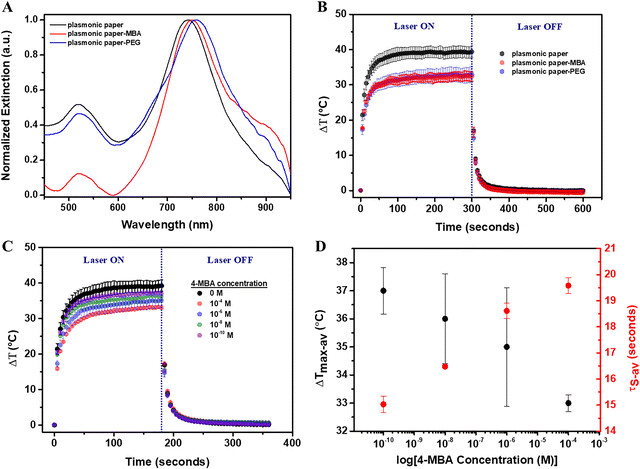
In order to evaluate the thermoplasmonic detection capabilities of the flexible plasmonic-paper-based nanoplatform, two molecules were tested: 4-MBA – a small molecule and SH-PEG-NH2 – a linear, heterobifunctional thermo-sensitive polymer with a molecular weight of 3500 Da, as a complex molecule. 4-MBA was used to verify if the thermoplasmonic performance relied on the same mechanism as in solution, specifically the modification of the intrinsic heat transfer rate of the surface oscillating electrons or it relied on the mass effect. Both molecules bound to the AuBPs through their thiol active groups. Extinction spectra were recorded before and after their functionalization (Fig. 5A). After the functionalization with 4-MBA 10−4 M, the longitudinal LSPR of the immobilized AuBPs was red-shifted by 7 nm (Fig. 5A-red spectrum), while a 16 nm red-shift was induced by the attachment of the SH-PEG-NH2 (Fig. 5A-blue spectrum), confirming the successful functionalization of the plasmonic paper with both 4-MBA (denoted as plasmonic paper-MBA) and SH-PEG-NH2 (denoted as plasmonic paper-PEG).Subsequently, the plasmonic paper-MBA and plasmonic paper-PEG were exposed to the 808 nm laser excitation for 5 minutes, and thermographic monitoring was maintained for another 5 minutes to allow the samples to cool to the environmental temperature. Fig. 5B shows the thermal curves obtained from the analysis of the thermographic images. Initially, the functionalization with 4-MBA 10−4 M resulted in a substantial decrease in the photothermal activity, and ΔT was 5 °C lower than that of the unfunctionalized plasmonic paper. This decrease in the light-to-heat conversion performance was confirmed by the increase of τS from 11.74 to 19.58 s (SD 0.54, SE 0.31). In this case, the decrease in photothermal efficiency resulted from the effect of mass addition. Furthermore, compared with the plasmonic paper, the heating process was rather slower as the plasmonic paper reached thermal equilibrium at around 60 seconds of laser exposure, while the plasmonic paper-MBA required several more seconds to equilibrate. The thermal curve of the plasmonic paper-PEG showed a similar behavior as the recorded temperature reached 33 °C. Experimentally, the temperature recorded using the thermographic camera represented the maximum temperature recorded on a specific, user-defined area. Therefore, it is assumed that the local temperature reached at the nanoparticle level is much higher. In the case of the plasmonic paper-PEG, the polymer suffered a phase change leading to a τS of 11.79 s (SD 2.48, SE 1.43) after 60 seconds of laser exposure, which is comparable with the cooling time constant of the plasmonic paper. It has been reported in literature that PEG is highly stable as a bulk material at freezing temperatures, while it gradually degrades in air or as a solute. Depending on its molecular weight, its melting point varies from 5 to 62 °C;33 therefore, relatively low temperatures can result in a phase transfer. Furthermore, the limits of detection (LOD) and quantification (LOQ) of the plasmonic paper were evaluated. Notably, the plasmonic paper was functionalized with 4-MBA ethanoic solutions of concentrations ranging between 10−4 to 10−10 M. The functionalized plasmonic papers were then exposed to an 808 nm laser line for 3 minutes followed by 3 minutes of cooling. Based on the thermographic images recorded in triplicate, average thermal curves were obtained (Fig. 5C), demonstrating that the mass addition decreased the light-to-heat conversion performance. As the 4-MBA concentration decreased, the average ΔTmax increased towards the ΔTmax of the unfunctionalized plasmonic paper. As shown in Fig. 5D, the SE in for ΔTmax was rather high and did not permit an accurate detection. Therefore, τS, which is highly sensitive to the addition of mass, was extracted from the cooling process. Fig. 5D presents the dependency of the determined average τS to the logarithmic value of the 4-MBA concentration. A high concentration of 4-MBA increased the average τS to 19.58 s (SD 0.52, SE 0.31) for 4-MBA 10−4 M. With decreasing concentration, the average τS decreased, resulting in the values of 18.61 s (SD 0.5, SE 0.29) for 4-MBA 10−6 M, 16.48 s (SD 0.11, SE 0.06) for 4-MBA 10−8 M, and 15.03 s (SD 0.54, SE 0.31) for 4-MBA 10−10 M, respectively. Moreover, an LOD of 0.19 nM and LOQ of 0.58 nM were calculated using the following equations:
 | (4) |
 | (5) |
The proposed flexible plasmonic-paper-based nanoplatform exhibited enhanced thermoplasmonic detection capabilities for 4-MBA molecule compared with the performances of the corresponding colloidal nanoparticles. Although the colloidal AuBPs showed a 6% increase in the cooling time constant after the functionalization with 4-MBA, the plasmonic paper-MBA exhibited a 67% increase in τS. Furthermore, the nanomolar LOD and LOQ were determined, which demonstrated the ability of the plasmonic paper to be efficiently implemented in thermoplasmonic detection applications.
4. Conclusions
In summary, this work reported the intrinsic thermoplasmonic properties of colloidal gold nanobipyramids (AuBPs) and AuBPs-based paper nanoplatforms in order to provide an in-depth evaluation of their thermoplasmonic performances, which revealed their ability for the efficient and sensitive thermoplasmonic detection of simple and complex molecules. AuBPs with longitudinal LSPR responses located at 693, 774 and 811 nm were synthesized in an aqueous solution, and their intrinsic light-to-heat conversion performances were assessed upon their exposure to an 808 nm laser line, which realized photothermal efficiencies (η) of up to 64%. Their functionalization with 4-mercaptobenzoic acid (4-MBA) induced a decrease in η from 5% to 8%, suggesting a change in the intrinsic heat transfer rate of the oscillating electrons. Additionally, after their immobilization on Whatman no. 1 filter paper, their optical properties and intrinsic thermoplasmonic activity were preserved. The photothermal conversion performances were significantly decreased after the functionalization of the plasmonic paper with 4-MBA, which resulted in an increased cooling-time constant. The functionalization with thiol-polyethylene glycol-amine thermo-sensitive polymer also highlighted the light-to-heat conversion performances; however, the cooling time constant revealed no change, suggesting a phase transition of the polymer owing to high local temperatures. An LOD of 0.19 nM and an LOQ of 0.58 nM were determined for 4-MBA, proving the high biosensing efficiency of the plasmonic paper. Hence, these results contribute to the consolidation of the versatile thermoplasmonic detection of both simple and complex interactions, being a stepping stone in the development of simple and efficient thermoplasmonic nanosensors.Author contributions
Andreea CAMPU: conceptualization, methodology, investigation, formal analysis, writing – original draft, review and editing, project administration, funding acquisition; Ioana Andreea Brezestean: investigation; Septimiu-Cassian Tripon: investigation; Simion ASTILEAN: writing – review and editing, Monica FOCSAN: writing – review and editing, project administration, funding acquisition.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was supported by the Babes-Bolyai University through the Starting Research Grant (SRG-UBB), contract no. 32984/23.06.2023 and by the project “Plasmon mediated biology: Exploitation of plasmonics to investigate and enhance biological processes and application to biomedical issues (acronym: BioPlasmonics)” funded by European Union – NextgenerationEU and Romanian Government, under National Recovery and Resilience Plan for Romania, contract no 760037/23.05.2023, cod PNRR-C9-I8-CF-199/28.11.2022 through the Romanian Ministry of Research, Innovation and Digitalization, within Component 9, Investment I8. I. A. B. acknowledges the financial support from the Romanian Ministry of Research, Innovation and Digitalization through the “Nucleu” Programme within the National Plan for Research, Development and Innovation 2022–2027, project PN 23 24 01 02.References
- G. Huttmann and R. Birngruber, IEEE J. Sel. Top. Quantum Electron., 1999, 5, 954–962 CrossRef CAS.
- D. Boyer, P. Tamarat, A. Maali, B. Lounis and M. Orrit, Science, 2002, 297, 1160–1163 CrossRef CAS.
- G. Baffou, Thermoplasmonics: Heating Metal Nanoparticles Using Light, Cambridge University Press, Cambridge, 2017 Search PubMed.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS.
- P. Ghosh, G. Han, M. De, C. Kim and V. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS.
- W. Li and X. Chen, Nanomedical, 2015, 10, 299–320 CrossRef CAS.
- A. Guglielmelli, F. Pierini, N. Tabiryan, C. Umeton, T. J. Bunning and L. De Sio, Adv. Photonics Res., 2021, 2, 2000198 CrossRef.
- Y. Zhao, L. Sang and Z. Ren, Sol. Energy Mater. Sol. Cells, 2024, 267, 112728 CrossRef CAS.
- G. Liu, J. Xu, T. Chen and K. Wang, Phys. Rep., 2022, 981, 1–50 CrossRef.
- A. Sepúlveda and D. Boudreau, ACS Appl. Polym. Mater., 2024, 6, 2359–2370 CrossRef.
- S. Ezendam, L. Nan, I. L. Violi, S. A. Maier, E. Cortés, G. Baffou and J. Gargiulo, Adv. Opt. Mater., 2024, 12, 2301496 CrossRef CAS.
- G. Baffou, F. Cichos and R. Quidant, Nat. Mater., 2020, 19, 946–958 CrossRef CAS PubMed.
- B. Yang, C. Li, Z. Wang and Q. Dai, Adv. Mater., 2022, 34, 2107351 CrossRef CAS.
- Y. Li, W. Li, T. Han, X. Zheng, J. Li, B. Li, S. Fan and C.-W. Qiu, Nat. Rev. Mater., 2021, 6, 488–507 CrossRef CAS.
- N. S. Abadeer and C. J. Murphy, J. Phys. Chem. C, 2016, 120, 4691–4716 CrossRef CAS.
- D. Khurana, A. K. Shaw, G. Sharma, M. Ahmed, S. K. Shukla and S. Soni, Int. Commun. Heat Mass Transf., 2024, 156, 107597 CrossRef CAS.
- M. Ghaffarlou, H. Rashidzadeh, A. Mohammadi, N. Mousazadeh, M. Barsbay, A. Sharafi, M. Gharbavi, H. Danafar and S. Javani, Sci. Rep., 2024, 14, 13299 CrossRef CAS PubMed.
- Y. Feng, Q. Sun, P. Liu, W. Fan and B. Fan, Int. J. Nanomed., 2024, 19, 6981–6997 CrossRef.
- J. Lv, Y. Qiu, L. Pan, X. Zhang, M. Li and X. Yin, Nano TransMed, 2024, 3, 100034 CrossRef.
- G. Qiu, Z. Gai, Y. Tao, J. Schmitt, G. A. Kullak-Ublick and J. Wang, ACS Nano, 2020, 14, 5268–5277 CrossRef CAS PubMed.
- X. Wu, L. Mu, M. Chen, S. Liang, Y. Wang, G. She and W. Shi, ACS Appl. Bio Mater., 2019, 2, 2668–2675 CrossRef CAS.
- M. Mohammadzadeh, S. Labbaf and A. Kermanpur, Adv. Theory Simul., 2024, 2400005 CrossRef CAS.
- J. Huang, F. Jiang, Z. Zhao, Z. Li, X. Deng, R. P. S. Han, S. Xu, Y. Tao and Z. Lin, ACS Appl. Nano Mater., 2024, 7, 14621–14628 CrossRef CAS.
- Y. Wang, L. Ma, L. Xie, Q. Wu, Y. Liu, Q. Zhao, Y. Zhang, B. Jiao and Y. He, ACS Appl. Nano Mater., 2023, 6, 17858–17868 CrossRef CAS.
- A. Campu, I. Muresan, M. Potara, D. R. Lazar, F.-L. Lazar, S. Cainap, D. M. Olinic, D. Maniu, S. Astilean and M. Focsan, J. Mater. Chem. B, 2024, 12, 962–972 RSC.
- J.-H. Lee, Z. Cheglakov, J. Yi, T. M. Cronin, K. J. Gibson, B. Tian and Y. Weizmann, J. Am. Chem. Soc., 2017, 139, 8054–8057 CrossRef CAS PubMed.
- A. Campu, M. Focsan, F. Lerouge, R. Borlan, L. Tie, D. Rugina and S. Astilean, Colloids Surf., B, 2020, 194, 111213 CrossRef CAS PubMed.
- B. J. Delgado-Corrales, V. Chopra and G. Chauhan, J. Mater. Chem. B, 2025, 13, 399–428 RSC.
- X. Zeng, L. Tang, W. Zhang, X. Hong and Y. Xiao, Small, 2025, 2412296 CrossRef CAS PubMed.
- A. Campu, A.-M. Craciun, M. Focsan and S. Astilean, Nanotechnology, 2019, 30, 405701 CrossRef CAS PubMed.
- F. M. M. Aldosari, Molecules, 2022, 27, 892 CrossRef CAS PubMed.
- A. Campu, F. Lerouge, D. Chateau, F. Chaput, P. Baldeck, S. Parola, D. Maniu, A. M. Craciun, A. Vulpoi, S. Astilean and M. Focsan, Anal. Chem., 2018, 90, 8567–8575 CrossRef CAS PubMed.
- R. Paberit, E. Rilby, J. Göhl, J. Swenson, Z. Refaa, P. Johansson and H. Jansson, ACS Appl. Energy Mater., 2020, 3, 10578–10589 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01502b |
| This journal is © The Royal Society of Chemistry 2025 |