Single crystal Cr3+-doped NIR phosphor: enhanced luminescence intensity and improved thermal stability†
Peng
Wang
a,
Lei
Zhong
a,
Yingyuan
Chen
 a,
Yuefei
Xiang
a,
Jing
Yan
a,
Yuefei
Xiang
a,
Jing
Yan
 a,
Chunyan
Jiang
*ab,
Lei
Zhou
a,
Chunyan
Jiang
*ab,
Lei
Zhou
 *a and
Mingmei
Wu
*a and
Mingmei
Wu
 *a
*a
aZhuhai Key Laboratory of Optoelectronic Functional Materials and Membrane Technology, School of Chemical Engineering and Technology, Sun Yat-sen University, Zhuhai 519082, China. E-mail: jiangchy92@outlook.com; zhoul8@mail.sysu.edu.cn; ceswmm@mail.sysu.edu.cn
bState Key Laboratory of Extreme Photonics and Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou 310027, China
First published on 24th April 2025
Abstract
Single crystals of Cr3+-doped guanidine-based organic–inorganic hybrid fluorides (GA3GaF6:Cr3+) with sizes up to 6 × 5 × 2 mm3 were successfully synthesized using a facile room-temperature solvent exchange crystallization method. The GA3GaF6:Cr3+ single crystal exhibits near-infrared (NIR) emission centered at 802 nm with a full width at half-maximum (FWHM) of 130 nm upon blue light excitation. Compared to polycrystalline powders, the GA3GaF6:Cr3+ single crystal demonstrates enhanced luminescence intensity and improved thermal stability. Notably, the bright green GA3GaF6:Cr3+ single crystal maintains its structural integrity at temperatures up to 540 K without degradation and retains over 75% of its initial luminescence intensity at 373 K. Furthermore, the GA3GaF6:Cr3+ single crystals exhibit excellent environmental stability, preserving 77% of their initial luminescence intensity after exposure to high temperature and high humidity (85 °C, 85% RH) for 5 days. This work represents a significant advancement in the development of organic–inorganic hybrid fluoride single-crystal materials for near-infrared emitting devices.
1. Introduction
Broadband NIR light sources are widely used in various fields, including biomedical imaging, night vision, non-destructive food testing, plant growth, and quantitative composition analysis.1–7 Conventional NIR light sources, such as tungsten halogen lamps, rely on high-temperature thermal radiation from a tungsten filament. However, these lamps require temperatures above 250 °C for the halogen to react with the tungsten on the glass shell, making them unsuitable for direct contact with humans or plants.8 Additionally, tungsten halogen lamps suffer from low luminescence efficiency, high energy consumption, slow response times, bulky packaging, and limited lifespans, which hinder their application in portable devices.9,10 An alternative NIR light source is achieved by integrating multiple NIR light-emitting diode (LED) chips to produce multi-spectral overlapping emissions. This approach improves portability due to its fast response time, high luminescence efficacy, compact size, and extended lifespan. However, the spectral output of such systems can vary over time due to differences in operating currents and the decay rates of NIR LED chips across different emission bands. Balancing the operating currents requires additional power supply modules, significantly increasing the overall cost of the NIR light source.11 To address these challenges, phosphor-converted LEDs (pc-LEDs) have emerged as a promising solution.12,13 By coating a blue InGaN chip with broadband NIR phosphor excitable by blue light, pc-LEDs enable the development of portable and intelligent NIR light sources.14 These devices offer advantages such as cost-effectiveness, compact size, high luminescence efficacy, and long operational lifespans, making them highly suitable for modern applications.15 Recently, most NIR phosphors have focused on Cr3+-doped broadband NIR luminescent materials.16 The outermost electron configuration of Cr3+ is 3d3, and its energy level structure is highly sensitive to changes in the crystal field strength. Studies have shown that the broadband NIR emission of Cr3+ can be finely tuned by modulating the crystal field strength.17 Additionally, the excitation peak of Cr3+ matches well with blue-light chips, making it a promising candidate for phosphors of NIR pc-LEDs.18A significant amount of heat is generated due to the large Stokes shift between blue light absorption and NIR light emission in NIR phosphors. To mitigate the adverse effects of heat, which limit their practical performance, two key strategies are essential: (1) the development of efficient and thermally stable broadband NIR-emitting phosphors, and (2) the advancement of NIR phosphors with other forms, such as ceramics and single crystals.19,20 Conventional powdery phosphors, which contain numerous grain boundaries in polycrystalline forms, tend to generate surface defects that lead to substantial luminescence quenching, thereby restricting their practical applications. In contrast, single crystals are considered ideal materials for optoelectronic devices due to their minimal defects, superior light absorption capacity, longevity, and stability.21 Moreover, single crystal materials can be directly integrated into LED chips to serve dual functions: phosphors and organic resins. This integration can separate the phosphor layer from the blue LED chip layer, forming a remote phosphor structure.22 Importantly, this design reduces heat generation in devices and improves the luminescence efficiency of LEDs, making them particularly suitable for high-power devices.23,24 Most single crystal preparation methods rely on high-temperature melt crystallization techniques, such as the Bridgman and Czochralski methods,25 or conventional solution crystallization techniques, including temperature cooling, solvent evaporation, and solvent exchange crystallization.26 Numerous studies have focused on the preparation of Cr3+-doped single crystals under high-temperature conditions. For example, Zhang et al. used an RF induction-heated EFG furnace to grow Cr3+:β-Ga2O3 single crystals,27 and Chen et al. employed a hydrothermal reaction at 423 K for 12 hours to synthesize Cs2AgInCl6:Cr3+ single crystals.28 However, these high-temperature methods inevitably result in substantial thermal energy consumption. Solvent exchange method, which is unaffected by temperature fluctuations, enables room-temperature crystal growth by increasing the concentration of target crystals through the selection of suitable anti-solvents (e.g., ethanol, toluene, diethyl ether).29 This method significantly reduces the complexity and cost of crystal preparation, offering a promising approach to expand the methods for growing Cr3+-doped single crystal NIR phosphors.
Among the numerous organo-inorganic hybrid materials, zero-dimensional (0D) organo-inorganic metal halides have demonstrated considerable promise in a number of applications due to their straightforward synthesis, substantial Stokes shifts, and broad emission spectra.30 0D organic–inorganic metal halides possess a distinctive spatial confinement structure. It has been demonstrated that organic cations have the capacity to separate metal halide anionic groups, enabling the organic ligands to protect the inorganic components with luminescent activity effectively, thereby forming a self-modifying structure that exhibits resistance to moisture.31 Moreover, the composition of 0D organic–inorganic metal halide comprises a variety of metal ions and halogen ligands, which exhibit diverse coordination modes, including tetrahedral, octahedral, and tetragonal. This enables the tuning of emission properties and assists in regulating crystal symmetry and photoluminescence.32 Organo-inorganic fluorides are more stable and possess lower phonon energy and weaker electron–phonon coupling strength than organo-inorganic chlorides, bromides, and iodides, making them emerging as a preferred option for transition metal or rare earth metal ion-doped phosphor matrix materials.33
In this work, a series of Cr3+-doped guanidinium-based 0D organic–inorganic hybrid fluoride (GA3GaF6:Cr3+) single crystals with broadband NIR emission were successfully synthesized using the solvent exchange method. The GA3GaF6:Cr3+ single crystal exhibits an emission peak at 802 nm with a FWHM of 130 nm. Detailed investigations were conducted on the crystal structure, including morphology, luminescence properties, and stability. The results reveal that the GA3GaF6:Cr3+ single crystal demonstrates good NIR emission performance and thermal stability. Specifically, the luminescence intensity of the optimum GA3GaF6:Cr3+ single crystal is enhanced by 51% compared to the corresponding polycrystalline powder. Furthermore, the thermal stability of the GA3GaF6:Cr3+ single crystal and its resistance to temperature and humidity were further verified. These findings highlight the advantages of single-crystal NIR phosphors over powdery counterparts, offering a promising paradigm for the application of organic–inorganic hybrid fluoride single crystals in NIR light sources and paving the way for future developments in single-crystal NIR phosphors.
2. Experimental section
2.1. Raw materials
The raw materials are CrF3 (AR), NH4HF2 (99%), Ga(NO3)3·xH2O (99.99%), HF acid (48 wt%), [C(NH2)3]2CO3 (Guanidinium carbonate, abbreviated as GA2CO3, 99%), Al(OH)C4H6O4 (99%), ethanol (AR), Acetic acid (HAc, 99.9%). All of them were used without any further purification.2.2. Preparation of (NH4)3CrF6
The Cr3+ source (NH4)3CrF6 was synthesized according to the method reported by He et al.34 For the specific steps, 20 mmol CrF3 and 240 mmol NH4HF2 were completely dissolved in 10 mL of distilled water in a plastic beaker, followed by continuously stirring for 24 hours. Afterward, the precipitates were collected and washed with ethanol and acetic acid several times. Finally, the products were dried at 65 °C for 12 hours in a vacuum oven to obtain (NH4)3CrF6.2.3. Preparation of GA3GaF6:Cr3+ and GA3AlF6:Cr3+ saturation solution
GA3GaF6:Cr3+ saturation solution was prepared by a room temperature dissolution method. Taking GA3GaF6:10%Cr3+ as an example. First, 25 mmol of GA2CO3 was dissolved in a plastic beaker containing 3 mL of HF solution under stirring. When the raw materials were fully dissolved, a transparent solution (solution A) was obtained. Second, 5 mmol of Ga(NO3)3·xH2O was dissolved in 4 mL of HF solution in another plastic beaker (solution B), and then 0.5 mmol of (NH4)3CrF6 was put into this solution and stirred continuously for 5 minutes until completely dissolved. After that, solution A and B were mixed, and then about 0.5 mL of HF solution was added slowly with vigorous stirring until a bright green transparent saturation solution formed. Subsequently, the solution was filtered. GA3AlF6:Cr3+ saturation solution was prepared with a similar procedure by using Al(OH)C4H6O4 instead of Ga(NO3)3·xH2O, and the use of an additional amount of HF acid solution was required in this procedure.2.4. Growth of GA3GaF6:Cr3+ and GA3AlF6:Cr3+ single crystal
GA3GaF6:Cr3+ and GA3AlF6:Cr3+ single crystals were prepared by a solvent exchange method.35Fig. 1a shows a flowchart illustrating the process of growing GA3GaF6:Cr3+ single crystals. The beaker containing GA3GaF6:Cr3+ or GA3AlF6:Cr3+saturation solution was sealed in a plastic box filled with ethanol, and the box was placed in a fume cupboard. After diffusion for 3 days, single crystals were precipitated in the bottom of the beaker. The remaining solution in the beaker was then poured out, and the green single crystals were washed three times with ethanol and dried at 65 °C for 2 hours. Fig. 1b exhibits the specific shape of the single crystal after growth, with the size measured at 6 × 5 × 2 mm3. When the single crystal is placed on a piece of squared paper, the intersecting lines underneath the single crystal are visible, indicating good transparency.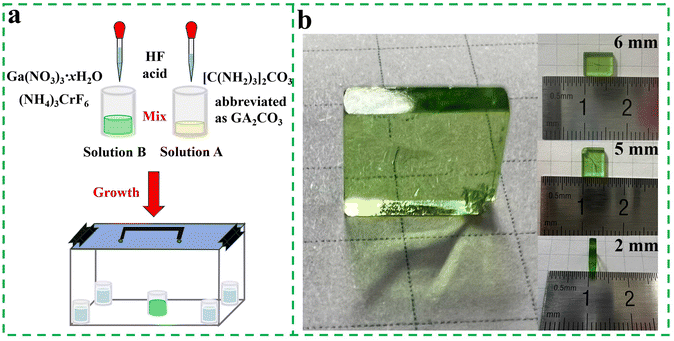 | ||
| Fig. 1 (a) Illustration of the preparation process of GA3GaF6:Cr3+ single crystal. (b) Photograph of the synthesized GA3GaF6:Cr3+ single crystal. | ||
2.5. Characterizations
Powder XRD patterns were tested by a Bruker D8 ADVANCE powder diffractometer operating at 40 kV and 40 mA with Cu-Kα radiation (λ = 1.54056 Å). The Fullprof program was used to perform Rietveld refinements. The chemical composition was measured with X-ray photoelectron spectroscopy spectra (XPS, Thermo Fisher/ESCALAB Oxi). The precise doping concentration of Cr3+ in the single crystals was measured by the inductively coupled plasma optical emission spectroscopy (OPtima8300 ICP-OES). The crystal morphology and elemental mapping were measured using a field emission scanning electron microscope (FE-SEM, Apreo 2S HiVac, Thermo Fisher Scientific) equipped with an energy-spectrometer (Escalab 250Xi, Thermo Fisher Scientific). The diffuse reflectance spectra (DRS) were collected on a UV-VIS spectrophotometer (UH4150) with BaSO4 as the reference standard. The thermogravimetric (TG) curve and derivative thermogravimetry (DTG) curve were measured using a thermal gravimetric analyzer (NETZSCH TG209F1 Libra). The photoluminescence (PL) and decay curves were measured by the Edinburgh FLS1000 spectrofluorometer equipped with detector R5509-72 photomultiplier (PMT1700), a 450 W xenon lamp and a 100 W microsecond flash lamp (μF2) were used as excitation light, respectively. The temperature-dependent luminescence was measured by the same spectrophotometer with a temperature controller (TS600V-SM, Wuhan Congtical Technology Co., Ltd).3. Results and discussion
At room temperature, Ga(NO3)3·xH2O and (NH4)3CrF6 are dissolved in HF acid solution to form isolated octahedron groups [GaF6]3− and [CrF6]3−via the chemical reaction in eqn (1) and (2). Meanwhile, guanidine carbonate is dissolved in HF acid. The nucleophilic lone pair of electrons on the nitrogen atoms coordinates with H+ ions from HF acid, forming a covalent bond between the imine nitrogen and hydrogen. Protonation converts the nitrogen centre into the [NH2]+ configuration. This process generates a large number of [C(NH2)3]+ cations with charge characteristics analogous to alkali metal cations.36,37 Subsequent introduction of [C(NH2)3]+ into the [GaF6]3− and [CrF6]3− systems yields GA3GaF6:Cr3+via the reaction described in eqn (3). Based on the above reaction mechanism, a series of different concentrations of Cr3+-doped GA3GaF6:Cr3+ single crystal samples can be synthesized by varying the mole ratios of Ga(NO3)3·xH2O and (NH4)3CrF6. The possible reaction processes for the GA3GaF6:Cr3+ single crystal are as follows:| Ga(NO3)3·xH2O(s) ↔ [GaF6]3− (aq) + 3NO3− (aq) | (1) |
| (NH4)3CrF6(s) ↔ [CrF6]3− (aq) + 3NH4+ (aq) | (2) |
| (1−x)[GaF6]3− (aq) + x[CrF6]3− (aq) + 3[GA]+ (aq) ↔ GA3Ga1−xCrxF6(s) | (3) |
Fig. 2a shows the XRD patterns of GA3GaF6:xCr3+(x = 0%–12.44%). Theoretically, the input Cr3+ doping concentrations are 0–30%. The corresponding precise Cr3+ doping concentrations determined by inductively coupled plasma emission spectroscopy (ICP-OES), are presented in Table S1 (ESI†). All the diffraction peaks in the samples match well with the standard sample, indicating that Cr3+ doping did not introduce impurities and is successfully incorporated into the GA3GaF6 host lattice. With increasing Cr3+ doping concentration, Ga3+ (r = 0.62 Å, CN = 6) is gradually replaced by a smaller ionic radius Cr3+ (r = 0.615 Å for six coordination), which causes the crystal plane spacing to decrease and the diffraction peaks gradually shifting to a higher angle (inset of Fig. 2a). In order to investigate the crystal structure of GA3GaF6, Rietveld structure refinement of measured XRD was implemented on the Fullprof package. A representative refined XRD pattern of GA3GaF6:10.55%Cr3+ is shown in Fig. 2b. The refinement results demonstrate convergence with reliable residual factors of Rp = 4.73% and Rwp = 6.77%. In addition, the structure refinement results for samples with other Cr3+ doping concentrations are shown in Table S2 (ESI†), and all the corresponding crystallographic data are provided in Tables S3–S8 (ESI†). The results indicate that the GA3GaF6 belongs to a typical cubic crystal system with a space group of Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (205). Fig. 2c shows the crystal structure view of GA3GaF6. The Ga atom is coordinated with six F atoms to form the [GaF6] octahedron. The [GaF6] octahedra are completely separated by [GA] cations, resulting in a 0D structure. Fig. 2d presents the structural differences between [Ga1F6] and [Ga2F6], highlighting the variances in bond lengths and bond angles. Due to the similar ionic radii and the same valence state, Cr3+ will preferentially occupy the lattice sites of Ga3+, which means that Cr3+ may occupy both Ga3+ lattice sites with slight differences. To further verify the successful doping of Cr3+, the presence of C, N, Ga, F, and Cr in the samples was confirmed using X-ray photoelectron spectroscopy (XPS) respectively (Fig. 2e). Furthermore, the SEM image in Fig. 2f exhibits that the typical GA3GaF6:10.55%Cr3+ single crystal has a highly regular cubic morphology and high crystallinity with a smooth and flat crystal surface, which is well-suited for integration with the blue LED chip and facilitates the application of NIR pc-LEDs. The energy dispersive spectrum (EDS) of the GA3GaF6:10.55%Cr3+ single crystal shows that the elements N, Ga, F, C, and Cr are uniformly distributed within the crystal (Fig. S1, ESI†). The elemental ratios by EDS analysis are displayed in Fig. S2 (ESI†). The atomic ratio of Cr: Ga (8.22%:10.69%) is close to the ICP precise composition, verifying the correctness of the actual Cr3+ doping concentration.
(205). Fig. 2c shows the crystal structure view of GA3GaF6. The Ga atom is coordinated with six F atoms to form the [GaF6] octahedron. The [GaF6] octahedra are completely separated by [GA] cations, resulting in a 0D structure. Fig. 2d presents the structural differences between [Ga1F6] and [Ga2F6], highlighting the variances in bond lengths and bond angles. Due to the similar ionic radii and the same valence state, Cr3+ will preferentially occupy the lattice sites of Ga3+, which means that Cr3+ may occupy both Ga3+ lattice sites with slight differences. To further verify the successful doping of Cr3+, the presence of C, N, Ga, F, and Cr in the samples was confirmed using X-ray photoelectron spectroscopy (XPS) respectively (Fig. 2e). Furthermore, the SEM image in Fig. 2f exhibits that the typical GA3GaF6:10.55%Cr3+ single crystal has a highly regular cubic morphology and high crystallinity with a smooth and flat crystal surface, which is well-suited for integration with the blue LED chip and facilitates the application of NIR pc-LEDs. The energy dispersive spectrum (EDS) of the GA3GaF6:10.55%Cr3+ single crystal shows that the elements N, Ga, F, C, and Cr are uniformly distributed within the crystal (Fig. S1, ESI†). The elemental ratios by EDS analysis are displayed in Fig. S2 (ESI†). The atomic ratio of Cr: Ga (8.22%:10.69%) is close to the ICP precise composition, verifying the correctness of the actual Cr3+ doping concentration.
Fig. 3a presents the PLE and PL spectra of GA3GaF6:Cr3+ single crystal at room temperature. The emission spectrum displays a broad band with a maximum emission wavelength of 802 nm and a FWHM of approximately 130 nm. This phenomenon can be attributed to the fact that within the weak octahedral crystal field environment of Cr3+ coordinated with F, the lowest excited state energy level of Cr3+ is the spin-triplet state 4T2. The spin-allowed 4T2 → 4A2 transition of Cr3+, accompanied by electron–phonon coupling, produces a broad emission spectrum. In the excitation spectrum, the broad band with a peak value of 440 nm belongs to the 4A2 → 4T1 (4F) transition of Cr3+. This excitation band dominates with a large FWHM, illustrating a good match with the commercial blue LED chip. Additionally, the excitation spectrum exhibits a 4A2 → 4T1 (4P) transition with a peak at 288 nm, as well as a 4A2 → 4T2 (4F) transition at 660 nm. As shown in Fig. 3b, the emission spectrum of the GA3GaF6:10.55%Cr3+ single crystal can be deconvolved well into two Voigt spectral lines with spectral peaks of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 837 (845 nm) and 12
837 (845 nm) and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631 cm−1 (792 nm), respectively.38,39 This suggests that the two distinct crystallographic allies [Ga1F6] and [Ga2F6] octahedra are substituted by Cr3+ concurrently. Fig. 3c presents the luminescence of GA3GaF6:Cr3+ single crystals with different Cr3+ doping concentrations. The luminescence intensity initially increases with the increasing Cr3+ doping concentration. Subsequently, the luminescence intensity decreases upon reaching 10.55% due to the concentration quenching effect (Fig. 3d). The emission peak position and FWHM remain relatively constant as the Cr3+ concentration increases. Fig. 3e depicts the decay curves of GA3GaF6:xCr3+ single crystals (x = 5.08–12.44%) with varying doping concentrations. The fitted decay lifetime exhibits a gradual decrease with increasing Cr3+ doping concentration because of the enhanced probability of non-radiative transitions induced by the concentration quenching effect. Fig. 3f compares the luminescence intensity of the optimum GA3GaF6:10.55%Cr3+ single crystal and corresponding polycrystalline powder. It is observed that the single crystal exhibits a notable luminescence advantage over the polycrystalline powders. The PL intensity of GA3GaF6:10.55%Cr3+ single crystal increases by 51% compared with polycrystalline powder. Fig. S3a–e (ESI†) exhibits the comparison of the luminescence intensity of single crystals and polycrystalline powders of different Cr3+ doping concentrations. All single crystals exhibit a more substantial enhancement relative to polycrystalline powders. The trend of the percentage enhancement of the integral intensity is shown in Fig. S3f (ESI†), and the average percentage enhancement of the luminescence intensity is 46%.
631 cm−1 (792 nm), respectively.38,39 This suggests that the two distinct crystallographic allies [Ga1F6] and [Ga2F6] octahedra are substituted by Cr3+ concurrently. Fig. 3c presents the luminescence of GA3GaF6:Cr3+ single crystals with different Cr3+ doping concentrations. The luminescence intensity initially increases with the increasing Cr3+ doping concentration. Subsequently, the luminescence intensity decreases upon reaching 10.55% due to the concentration quenching effect (Fig. 3d). The emission peak position and FWHM remain relatively constant as the Cr3+ concentration increases. Fig. 3e depicts the decay curves of GA3GaF6:xCr3+ single crystals (x = 5.08–12.44%) with varying doping concentrations. The fitted decay lifetime exhibits a gradual decrease with increasing Cr3+ doping concentration because of the enhanced probability of non-radiative transitions induced by the concentration quenching effect. Fig. 3f compares the luminescence intensity of the optimum GA3GaF6:10.55%Cr3+ single crystal and corresponding polycrystalline powder. It is observed that the single crystal exhibits a notable luminescence advantage over the polycrystalline powders. The PL intensity of GA3GaF6:10.55%Cr3+ single crystal increases by 51% compared with polycrystalline powder. Fig. S3a–e (ESI†) exhibits the comparison of the luminescence intensity of single crystals and polycrystalline powders of different Cr3+ doping concentrations. All single crystals exhibit a more substantial enhancement relative to polycrystalline powders. The trend of the percentage enhancement of the integral intensity is shown in Fig. S3f (ESI†), and the average percentage enhancement of the luminescence intensity is 46%.
The universality of improved luminescence intensity in single crystals was further verified by the GA3AlF6:Cr3+ sample. Fig. 4a displays the emission spectra of GA3AlF6:Cr3 single crystals with various Cr3+ doping concentrations. An enhancement in luminescence intensity with increasing Cr3+ concentration is observed until the Cr3+ doping concentration reaches 16% in GA3AlF6 single crystal. The powder XRD pattern of GA3AlF6:16%Cr3+ single crystal is shown in Fig. S4 (ESI†), which is well matched to the standard card (ICSD#81-0138). As shown in Fig. 4b, the optimum GA3AlF6:16%Cr3+ single crystal exhibits an enhancement of 37% compared with the corresponding powder sample, which is comparable to the average enhancement between single crystal and powder of GA3GaF6:Cr3+. The NIR luminescence intensity of the GA3GaF6:10.55%Cr3+ single crystal is stronger than that of the GA3AlF6:16%Cr3+ single crystal, which can be ascribed to the closer radius and more suitable doping sites of Ga3+ than Al3+ when compared with Cr3+. As illustrated in Fig. S5 (ESI†), the maximum emission peak of GA3AlF6:16%Cr3+ is located at 795 nm, which is blue-shifted compared to that of GA3GaF6:10.55%Cr3+. This shift can be attributed to the smaller ionic radius of Al3+ (r = 0.535 Å) compared to that of Cr3+ and Ga3+. With the replacement of the smaller Al3+ by Cr3+, the distance between Cr3+ and the ligand F− is reduced, thereby providing a strong crystal field environment for Cr3+. To further explore the origin of improved luminescence in single crystal, the absorptance differences between single crystal and polycrystalline powder were investigated. As shown in Fig. 4c, the diffuse reflectance spectra of GA3GaF6:xCr3+ (x = 5.08%–12.44%) single crystals exhibit high absorption at peaks of 288, 440 and 646 nm. The absorption peak at 440 nm corresponds well with the excitation spectra. In particular, when the concentration of Cr3+ increased from 5.08% to 12.44%, the absorption changed by 23% (from 63% to 86%). In comparison with the corresponding polycrystalline powders (Fig. 4d), the absorption of the single crystals is much higher, which exceeds more than 3 times. This finding suggests that the single crystal is anticipated to demonstrate superior luminescence performance due to high absorption as a result of longer optical path length.40
In order to assess the thermal stability of the hybrid single-crystal material under practical application conditions, TG and DTG analysis curves of GA3GaF6:10.55%Cr3+ single crystal were first investigated (Fig. 5a). The samples begin to lose weight and undergo decomposition when the temperature reaches 540 K. From the DTG curve, the decomposition rate peaks at approximately 590 K. This indicates that the GA3GaF6:10.55%Cr3+ single crystal demonstrates relatively high chemical stability at high temperatures and exhibits superior thermal stability when compared with other non-fluoride-based metal halides (e.g., chloride, bromide, and iodide).33 Typically, the operating temperature of pc-LEDs reaches approximately 423 K. The higher stability temperature indicates a promising application prospect for the GA3GaF6:Cr3+ single crystal. The emission spectra of GA3GaF6:10.55%Cr3+ single crystal and their corresponding polycrystalline powder within the temperature range of 298 to 423K are displayed in Fig. 5b. As the temperature increases, the non-radiative transition probability exhibits a corresponding enhancement due to the lattice vibration quenching, leading to the monotonically decreasing NIR emission intensities of the single crystal and polycrystalline powder. When the temperature increases, the peak position is gradually red-shifted from 802 to 823 nm due to the expansion of the lattice, which causes a decrease in the crystal field strength (Fig. 5c). The GA3GaF6:10.55%Cr3+ single crystal presents good luminescence stability at high temperature. When the temperature increases to 373 K, the integrated luminescence of GA3GaF6:10.55%Cr3+ single crystal maintains 76% of the initial intensity at room temperature state, while the value of GA3GaF6:10.55%Cr3+ polycrystalline powder is 58% (Fig. 5d). This is due to that the absence of grain boundaries and inter-particle gaps in the crystal lattice structure of the single crystal material ensures a better thermal conductivity under high temperature condition. In addition, polycrystalline powder has more internal defects than single crystal. The energy transfer from Cr3+ to defects is accelerated under high temperature, which further enhances the luminescence quenching and leads to a faster luminescence decreasing trend compared to single crystal.41 To further investigate the luminescence properties of the single crystal at low temperatures, the low-temperature dependent spectra of the single crystal was measured. The results are shown in Fig. S6 (ESI†), when the temperature is gradually decreased from 273 to 83 K, the emission peak is gradually blue-shifted from 800 to 780 nm, as well as the FWHM is reduced from 130 to 96 nm. Due to the weakening of the phonon-electron vibrational coupling at low temperature, the emission intensity gets stronger. When the temperature is further lowered to 183 K, there are two small spikes on the left and right sides of the emission peaks, which are most noticeable when the temperature reaches 83 K. There is no R-line emission of 2E → 4A2 during the whole temperature range. Then, the crystal field strength was calculated by Formulae S1–S4 (ESI). The results show Dq/B = 1.772 at 85 K, which is smaller than that at the intersection of 4T2 and 4T1 states (Dq/B = 2.3),42 suggesting that GA3GaF6 provides a weak octahedral crystal field for Cr3+ and enables broadband emission under blue light excitation.
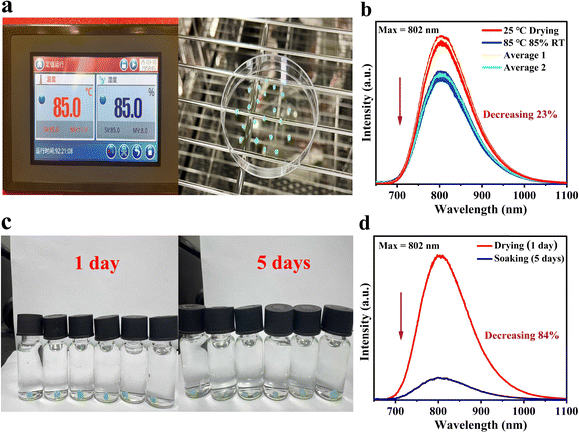
To further assess the stability, GA3GaF6:10.55%Cr3+ single crystals were kept in a constant temperature and humidity chamber with a temperature of 85 °C and a relative humidity of 85% for 5 days, according to the China Standard (GB/T 23595.4-2009) (Fig. 6a). After the procedure, the single crystals were naturally cooled to room temperature. The luminescence intensity of the single crystal samples before and after the placement is compared to reflect their temperature and humidity resistance to some degree. The luminescence intensity of various single crystal particles was demonstrated in Fig. 6b. The average luminescence intensity indicates a 77% preservation of the original intensity after 5 days, suggesting that the single crystal possesses a certain degree of temperature and humidity resistance. Furthermore, the testing condition was extended to an extreme, the additional experiment was conducted in which single crystals were directly immersed in water at room temperature for 5 days (Fig. 6c). It is observed that the single crystals experience partial fragmentation after this soaking condition. Following this observation, their luminescence was tested after the single crystals dried naturally at ambient temperature. As shown in Fig. 6d, the luminescence intensity of the single crystals after 5 days of soaking retains only 26% compared to the original single crystals, evidencing its inadequate waterproof performance. In the future, the selection of organic cations, with a better protection of the luminescent centers, may help to achieve more desirable waterproof performance and enhance the stability of single crystals, which will allow for better application in many different water environments.
4. Conclusions
In summary, a series of broadband NIR emission GA3GaF6:xCr3+ single crystals were successfully synthesized using a simple room-temperature solvent exchange method. The optimal luminescence performance of GA3GaF6:xCr3+ single crystals is achieved at x = 10.55%. The single crystals exhibit broadband emission spanning 650–1100 nm, with a peak at 802 nm and a FWHM of 130 nm. Notably, the luminescence intensity of the optimum GA3GaF6:10.55%Cr3+ single crystal exhibits a 51% increase compared to its polycrystalline powder counterpart. At 373 K, the single crystal retains 76% of its initial luminescence intensity measured at room temperature, significantly outperforming the powder sample, which retains only 58%. GA3AlF6:Cr3+ single crystals further validate the universality of the luminescence enhancement phenomenon in single crystals. Additionally, the temperature and humidity resistance of the GA3GaF6:Cr3+ single crystals were evaluated to comprehensively assess their stability. The results demonstrate that the single crystal exhibits good resistance to high temperature and humidity. This work provides valuable insights into the room-temperature preparation of Cr3+-doped organic–inorganic hybrid fluoride single crystals and highlights their potential to expand the range of available NIR light sources.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 52272174, 52002411), the Joint Funds of NSFC-Guangdong Province (No. U22A20135, No. U1801253).References
- Z. Liao, M. Sójka, J. Zhong and J. Brgoch, Chem. Mater., 2024, 36, 4654–4663 CrossRef CAS.
- X. Dai, X. Zou, M. Wei, X. Zhang, B. Dong, X. Li, Y. Cong, D. Li, J. Zhao, M. S. Molokeev and B. Lei, Adv. Opt. Mater., 2024, 12, 2401608 CrossRef CAS.
- E. Shirshin, B. Yakimov, D. Davydov, A. Baev, G. Budylin, N. Fadeev, L. Urusova, N. Pachuashvili, O. Vasyukova and N. Mokrysheva, Anal. Methods, 2024, 16, 175–178 RSC.
- Z. Chen, R. Zhou and P. Ren, RSC Adv., 2024, 14, 8053–8066 RSC.
- R. Ma, T. Zhang, B. Cao, X. Gong, C. Deng and W. Huang, Dalton Trans., 2024, 53, 17629–17641 RSC.
- X. Ma, X. Guo, B. Lin, H. Wang, Q. Dong, S. Huang, L. Li and H. Zang, Anal. Methods, 2024, 16, 537–550 RSC.
- L. Gao, D. Wang, L. Zhong, J. Yue, L. Nie, L. Li, Z. Meng, G. Cao, Y. Lai and H. Zang, New J. Chem., 2023, 47, 20920–20927 RSC.
- V. Rajendran, H. Chang and R.-S. Liu, Opt. Mater., X, 2019, 1, 100011 CAS.
- F. Zhao, Z. Song and Q. Liu, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS.
- X. Meng, Z. Wang, X. Huo, M. Zhou, Y. Wang and P. Li, Mater. Chem. Front., 2024, 8, 3608–3615 RSC.
- X. Fu, X. Wang and X. Rao, Biosys. Eng., 2017, 163, 87–93 CrossRef.
- Z. Chen, S. Zhang, Z. Li, H. Ye, H. Yan, J. Xu, L. Gao, Y. Li and S. Zhang, Inorg. Chem. Front., 2024, 11, 6898–6908 RSC.
- C. Wang, J. Lin, X. Zhang, H. Dong, M. Wen, S. Zhao, S. Yuan, D. Zhu, F. Wu and Z. Mu, J. Alloys Compd., 2023, 942, 168893 CrossRef CAS.
- Z. Jia, C. Yuan, Y. Liu, X.-J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed.
- Y. Zhu, Y. Yang, Y. Zhu, Y. Chen, Z. Liu, F. Lei, H. Yu, Q. Mao, J. Zhong and J. Wang, J. Mater. Chem. C, 2023, 11, 10694–10702 RSC.
- M.-H. Fang, P.-Y. Huang, Z. Bao, N. Majewska, T. Leśniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, C.-W. Yang, K.-M. Lu, H.-S. Sheu and R.-S. Liu, Chem. Mater., 2020, 32, 2166–2171 CrossRef CAS.
- Y. Jin, Z. Zhou, R. Ran, S. Tan, Y. Liu, J. Zheng, G. Xiang, L. Ma and X.-J. Wang, Adv. Opt. Mater., 2022, 10, 2202049 CrossRef CAS.
- D. Huang, H. Zhu, Z. Deng, H. Yang, J. Hu, S. Liang, D. Chen, E. Ma and W. Guo, J. Mater. Chem. C, 2021, 9, 164–172 RSC.
- Z. Liu, X. Qin, Q. Chen, Q. Chen, Y. Jing, Z. Zhou, Y. S. Zhao, J. Chen and X. Liu, Adv. Opt. Mater., 2022, 10, 2201254 CrossRef CAS.
- G. Liu, W. Chen, Z. Xiong, Y. Wang, S. Zhang and Z. Xia, Nat. Photonics, 2024, 18, 562–568 CrossRef CAS.
- S. Zhao, W. Cai, H. Wang, Z. Zang and J. Chen, Small Methods, 2021, 5, 2001308 CrossRef CAS.
- H.-C. Kuo, C.-W. Hung, H.-C. Chen, K.-J. Chen, C.-H. Wang, C.-W. Sher, C.-C. Yeh, C.-C. Lin, C.-H. Chen and Y.-J. Cheng, Opt. Express, 2011, 19, A930–A936 CrossRef CAS PubMed.
- Y.-H. Song, E. K. Ji, S.-H. Bak, Y. N. Kim, D. B. Lee, M. K. Jung, B. W. Jeong and D.-H. Yoon, Chem. Eng. J., 2016, 287, 511–515 CrossRef CAS.
- Y. Zhou, C. Yu, E. Song, Y. Wang, H. Ming, Z. Xia and Q. Zhang, Adv. Opt. Mater., 2020, 8, 2000976 CrossRef CAS.
- V. Vezin, T. Sugawara, R. K. Ryuichi Komatsu and S. U. Satoshi Uda, Jpn. J. Appl. Phys., 1997, 36, 5950 CrossRef CAS.
- Y. Cho, H. R. Jung and W. Jo, Nanoscale, 2022, 14, 9248–9277 RSC.
- J. Zhang, W. Mu, K. Zhang, J. Sun, J. Zhang, N. Lin, X. Zhao, Z. Jia and X. Tao, CrystEngComm, 2020, 22, 7654–7659 RSC.
- D. Chen, X. Zhang, J. Wei, L. Zhou, P. Chen, Q. Pang and J. Z. Zhang, Inorg. Chem. Front., 2022, 9, 4695–4704 RSC.
- X. Zhang, F. Li and R. Zheng, J. Mater. Chem. C, 2020, 8, 13918–13952 RSC.
- L. Zhou, J.-F. Liao and D.-B. Kuang, Adv. Opt. Mater., 2021, 9, 2100544 CrossRef CAS.
- P. Cai, S. Wang, T. Xu, Y. Tang, X. Yuan, M. Wan, Q. Ai, J. Si, X. Yao, Y. Cao, M. K. Rabchinskii, P. N. Brunkov and Z. Liu, J. Lumines., 2020, 228, 117661 CrossRef CAS.
- B. Li, J. Jin, M. Yin, X. Zhang, M. S. Molokeev, Z. Xia and Y. Xu, Angew. Chem., Int. Ed., 2022, 61, e202212741 CrossRef CAS.
- T. Xu, P. Cai, Q. Ai, Q. He, J. Si, X. Yao and Z. Liu, J. Lumin., 2022, 248, 118979 CrossRef CAS.
- F. He, E. Song, Y. Zhou, H. Ming, Z. Chen, J. Wu, P. Shao, X. Yang, Z. Xia and Q. Zhang, Adv. Funct. Mater., 2021, 31, 2103743 CrossRef CAS.
- J. Zhou, Y. Wang, Y. Chen, Y. Zhou, B. Milićević, L. Zhou, J. Yan, J. Shi, R.-S. Liu and M. Wu, Angew. Chem., Int. Ed., 2021, 60, 3940–3945 CrossRef CAS.
- H. Ming, Y. Zhao, Y. Zhou, S. Zhang, Y. Wang, E. Song, Z. Xia and Q. Zhang, ACS Appl. Electron. Mater., 2020, 2, 4134–4145 CrossRef CAS.
- K. Sadman, Q. Wang and K. R. Shull, ACS Macro Lett., 2019, 8, 117–122 CrossRef CAS PubMed.
- Y. Wang and P. D. Townsend, J. Phys.: Conf. Ser., 2012, 398, 012003 CrossRef.
- J. Mooney and P. Kambhampati, J. Phys. Chem. Lett., 2013, 4, 3316–3318 CrossRef CAS.
- Z. Wang, Z. Yang, N. Wang, Q. Zhou, J. Zhou, L. Ma, X. Wang, Y. Xu, M. G. Brik, M. D. Dramićanin and M. Wu, Adv. Opt. Mater., 2020, 8, 1901512 CrossRef CAS.
- S. Arya, P. Mahajan, R. Gupta, R. Srivastava, N. K. Tailor, S. Satapathi, R. R. Sumathi, R. Datt and V. Gupta, Prog. Solid State Chem., 2020, 60, 100286 CrossRef CAS.
- Q. Lin, Q. Wang, M. Liao, M. Xiong, X. Feng, X. Zhang, H. Dong, D. Zhu, F. Wu and Z. Mu, ACS Appl. Mater. Interfaces, 2021, 13, 18274–18282 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01433f |
| This journal is © The Royal Society of Chemistry 2025 |