Nb2CTx MXene integrated DyMn2O5 composites: tailored particle size and enhanced capacitance for high performance pseudocapacitors†
Komal Ali
Rao
 *a,
Muhammad Ehsan
Mazhar
a,
Javed
Ahmad
a,
Muhammad
Bilal
*a,
Muhammad Ehsan
Mazhar
a,
Javed
Ahmad
a,
Muhammad
Bilal
 b,
Muhammad Imran
Khan
b,
Muhammad Imran
Khan
 c,
Muhammad Suleman
Ahmad
a,
Muhammad
Aziz
a and
Hafeez ur
Rehman
d
c,
Muhammad Suleman
Ahmad
a,
Muhammad
Aziz
a and
Hafeez ur
Rehman
d
aInstitute of Physics Bahauddin Zakariya University, Multan-60800, Pakistan. E-mail: komalrao49@gmail.com
bSchool of Engineering and Materials Science, Queen Marry University of London-E1 4NS, UK
cResearch Institute of Sciences and Engineering (RISE), University of Sharjah, Sharjah 27272, United Arab Emirates
dCenter for Green Innovation, School of Mathematics and Physics, University of Science and Technology Beijing, Beijing, 100083, China
First published on 6th June 2025
Abstract
Nb2CTx, 2D niobium carbide, belongs to the MXene family obtained from Nb2AlCTx through selective etching of the Al layer using a non-toxic aqueous KOH solution as an alternative to the hazardous HF etching process. MXene is an emerging material for supercapacitor electrodes due to its distinctive layered structure, large surface area, superior electrical conductivity, and exceptional chemical stability. Owing to the significant energy of the MXene layers, they have a tendency to restack, which limits the effective utilization of the interlayer space for energy storage. Consequently, expanding the interlayer spacing of MXene has become a key research focus to improve its electrochemical performance. Currently, the interlayer spacing of MXene is typically expanded by incorporating nanoparticles. Although numerous bi-metallic transition metal oxides have been explored for this application, only a limited number of rare earth-based bi-metallic oxides have been investigated. Nowadays, the interlayer spacing of MXene is enhanced through the incorporation of nanoparticles. Different bi-metallic transition oxides have been used for this purpose, but very few rare earth-based bi-metallic oxides have been investigated. So in this study, we have synthesized a bi-metallic DyMn2O5/Dy2O3/MXene nanocomposite using a hydrothermal method. X-ray diffraction (XRD) analysis was conducted to determine the crystalline structure and phase purity of the materials. In addition, Fourier-transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) were performed to investigate the functional groups, chemical bonding and multiple oxidation states in the composites, respectively, providing further confirmation of successful synthesis. Scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) was employed to analyze the surface morphology and confirm the cubic-shaped pellet-like nanoparticles on the MXene layers, as well as to verify the elemental composition and uniform distribution of constituent elements. The optimized electrode material demonstrates a marvelous specific capacity of 362.92 C g−1 at a current density of 1 A g−1, along with excellent cycling stability. An asymmetric supercapacitor is fabricated using the DyMn2O5/Dy2O3/MXene nanocomposite as the anode and activated carbon as the cathode. The assembled device achieves an energy density of 46.25 W h kg−1 at a power density of 705 W kg−1, retaining 70.56% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge discharge cycles at 2 A g−1. This study introduces an efficient hydrothermal method for the synthesis of an innovative rare earth and MXene-based nanocomposite with controlled particle size for the electrode of supercapacitors.
000 charge discharge cycles at 2 A g−1. This study introduces an efficient hydrothermal method for the synthesis of an innovative rare earth and MXene-based nanocomposite with controlled particle size for the electrode of supercapacitors.
1. Introduction
Overpopulation, excessive consumption of renewable energy sources, industrial expansion, and the rapid depletion of fossil fuels have raised significant concerns regarding the global energy crisis. Energy sources that are effective, green, clean, affordable, sustainable and renewable are urgently needed to replace traditional energy sources in order to address these issues. Although there are plenty of natural resources, including wind, solar power, thermal, tidal, and hydroelectric, the scientific community still has a difficult time in storing and utilizing them.1–4 As a result, the need for advanced energy storage and conversion technologies has significantly increased. Many advanced energy storage devices, capacitors, batteries, fuel cells, sensors and super capacitors that store energy electrically and chemically are the major solutions to mitigate energy challenges.5–9 Batteries and supercapacitors10 are the most reliable and practical methods of electrochemical energy storage at the interface between a conducting electrode and an electrolyte.11–15 Supercapacitors have attracted the interest of battery makers due to their ability to reduce the gap between power density and energy density. High energy density while maintaining higher power density are both essential in the field of energy storage and transmission. On the basis of the types of electrode materials and electrochemical process, supercapacitors are commonly divided into two types, electric double layer capacitors (EDLC) and pseudocapacitors.16 Carbon or activated carbon-based materials are used as electrode materials in the first type and metal oxides/conducting polymers in the second type of supercapacitor.17,18 However, a significant effort is being made by researchers to find suitable pseudocapacitive materials and methods of production in order to build such storage units. The optimal electrode material for pseudocapacitors (PCs) with superior power and energy densities must satisfy the following stringent criteria: (i) exceptional electrical conductivity, an extensive specific surface area, and enhanced ion transport pathways to facilitate efficient charge storage and rapid electrochemical response; (ii) abundant redox-active sites coupled with ultrafast charge-transfer kinetics to enable high-rate electrochemical reactions and maximize energy storage efficiency.19–21 Transition metal oxides (TMOs) exhibit exceptional electrochemical properties, characterized by high theoretical capacities and versatile redox activity, making them highly suitable for energy storage applications. However, their practical deployment is constrained by inherent drawbacks such as limited electrical conduction, slow ion diffusion, and structural degradation during cycling. To mitigate these limitations, integrating TMOs with carbon-based materials such as graphene, carbon nanotubes, or activated carbon or novel MXene carbides—has proven to be a highly effective strategy. Sometimes, one metal in binary TMOs can be replaced by a rare earth metal because rare earth metals such as La, Dy, Er, and Ce have multiple valence states, and a high value of conductivity. These conductive matrices enhance charge transport, reduce internal resistance, and provide mechanical stability, thereby counteracting volume fluctuations during charge–discharge processes. This synergistic combination significantly improves electrochemical performance by enhancing specific capacity, rate capability, and cycling stability.22,23 Composites based on rare earth metal oxides are an example that satisfy those requirements and may be utilized to develop electrode materials with a high energy and power density, where the various valence states of the rare earth metals may be fully used for increased charge storage capacity together with a maximum operating voltage.24 The two primary chemical processes that rare earth compounds undergo are chemical redox reactions and acid base reactions. Rare earth-based metal oxides are thought to be possible pseudocapacitors because of their redox characteristics and preferred conductivity. In addition, they possess maximum bulk density, which is thought to be particularly beneficial in producing outstanding volumetric capacitance.25–30 There are a few reports of rare earth metal-based oxides functioning as the electrode of supercapacitors. The specific gravimetric capacitance of rare earth compounds is quite low, ranging from 200 to 300 F g−1, compared to other transition metal oxides including nickel oxides, cobalt oxides, and manganese oxides. However, due to their significantly higher density, rare earth compounds have a very high volumetric capacitance.31–33 As examples, LaMnO3,34 SrMnO3,35 Y2NiMnO6,36 and LaCrO337 electrodes exhibit pseudocapacitance behavior with efficient specific capacitance and remarkable cycling stability. Among these, dysprosium-based manganese oxides (DyMn2O5) offer intriguing properties due to the synergistic combination of manganese's rich redox chemistry and dysprosium's ability to stabilize the crystal structure and enhance electrical conductivity. While DyMn2O5 has been studied for its magnetic and structural properties, its electrochemical behavior remains largely unexplored. Pseudocapacitance is produced by the oxygen-containing ions in the electrolyte by manganese oxidation/reduction and direct absorption into the voids of the perovskite structure. Manganese-based metal oxides were shown to be a good substitute because to their favourable electrochemical properties, natural availability, affordability, strong redox activity, eco-friendliness, & substantial potential capacitance.38–40 Also, to further enhance the conductivity and cycling performance of DyMn2O5, MXene and other carbon-based materials can be added. MXenes have emerged as highly promising materials in energy storage research due to their advanced two-dimensional framework, rich surface chemistry, excellent mechanical properties, and exceptional electrical conductivity. Their general chemical composition is represented as Mn+1XnTx where n denotes integer values (e.g., 1, 2, 3), and T corresponds to surface functional groups such as –OH, –O, and –F. Extensive investigations have led to the discovery of more than 20 MXene variants, including VC4, Ti2C, Nb2C, and Nb4C3, among others.41–43 These different types of MXene composite with metal oxides are used in energy storage devices and electrochemical sensors also. N. Prabhakar et al. studied the Nb4C3Tx @WO composite for a high performance asymmetric supercapacitor, delivering a specific capacitance of 1045 F g−1 at 1 A g1.44 Furthermore, P. Varatharjan et al. fabricated a sensor system with remarkable selectivity, stability and excellent reproducibility for the sensing of p-nitrotoluene using MnCo2O4@Ti3C2Tx.45 U. Rajaji et al. used MoS2/S-Ti3C2/LGE as an electrochemical sensing medium for the detection of poisonous roxarsone and aristolochic acid.46 Similarly, MXene base composites with metal oxides e.g. Dy-WO3/PCNFs, δ-MnO2@Ti3C2T, MnO2/MXene, CoCr2O4@MXene, ZrO2-doped V2CTx MXene, cobalt ion-doped V2CTx MXene, Ni–Mn(OH)2@V2CTx, Mn3O4@MXene, and Ti3C4@Mn3O4@carbonized iron, are used as electrode materials of supercapacitors.47–54 Structurally, MXenes comprise alternating layers of transition metals and carbon, imparting high electrical conductivity. Their characteristic of an accordion-like morphology facilitates the reversible intercalation of ions and molecules, effectively increasing the interlayer spacing and specific surface area, making them ideal candidates for electrochemical energy storage systems. Among various MXene compositions, Nb2CTx has been extensively studied due to its layered architecture, enabling its application as an electrode material with outstanding electrochemical properties.55 However, strong interlayer van der Waals forces lead to significant stacking, reducing the accessible surface area and limiting charge storage efficiency. Consequently, expanding the interlayer spacing and preventing restacking are critical research priorities to enhance MXene's performance in energy storage applications.56Multiple literature have been reported on the preparation of DyMn2O5 that have looked at their magnetic characteristics and crystal structure.57 Despite extensive studies on individual pseudocapacitive materials, there is a critical gap in the research regarding the integration of rare earth metal oxides with MXenes for electrochemical energy storage. Based on the available literature, there are no reported studies on the synthesis and electrochemical investigation of DyMn2O5/MXene (Nb2CTx) composites. The rational design of these composites leverages the high redox activity of DyMn2O5 and the exceptional electrical conductivity of Nb2CTx MXene, addressing the limitations of both materials. The synergistic effect of these components is expected to enhance the charge transfer kinetics, improve structural stability, and increase overall electrochemical performance.
The selection of DyMn2O5 and Dy2O3 among rare earth oxides was based on their synergistic electrochemical and structural characteristics, which are particularly advantageous for energy storage systems. DyMn2O5, a mixed metal oxide containing transition and rare earth metals, provides multiple accessible oxidation states of manganese, enabling fast and reversible faradaic redox reactions. Dysprosium within this compound also contributes to improved electronic conductivity and stability through its 4f orbitals.58 Moreover, they possess high bulk density, which significantly contributes to achieving superior specific capacitance. To optimize their combined performance, various compositions were systematically investigated by adjusting the weight or molar ratios.
In this study, DyMn2O5/MXene (Nb2CTx) composites were synthesized using a hydrothermal method and evaluated as electrode materials for supercapacitor applications. The incorporation of Nb2CTx MXene is anticipated to enhance the conductivity and electrochemical stability of DyMn2O5, leading to improved power and energy density. This work not only introduces a novel electrode material, but also contributes to the broader field of MXene-based hybrid materials for advanced energy storage technologies.
2. Experimental procedure
2.1. Materials
Dysprosium(III) nitrate (Dy(NO3)3, 99% purity), manganese(II) nitrate (Mn(NO3)2, 99% purity), potassium hydroxide (KOH, 98% purity) and MAX (Nb2AlC, 99% purity) all were obtained from Sigma Aldrich. Deionized water and ethanol (99.9% purity) and no other procedures were required to clean the materials, because all materials used in the experiment were analytical quality.2.2. Synthesis of MXene (Nb2CTx)
Initially, 0.2 g of Nb2AlCTx (MAX phase powder) was dispersed in 1 mL of distilled water in a beaker. In a separate beaker, 2 g of potassium hydroxide (KOH) was dissolved in 2 mL of distilled water under stirring. The prepared KOH solution was then gradually added to the Nb2AlCTx dispersion while maintaining continuous stirring. The resulting mixture was transferred to a mortar and pestle and finely ground for 1 hour until a thick paste was formed. This paste was then placed into a stainless steel autoclave and subjected to thermal treatment at 150 °C for 24 hours. After the heating process, the autoclave was removed, and the obtained precipitates were collected and thoroughly washed with ethanol and distilled water multiple times to eliminate any residual impurities. The final product was dried in a vacuum oven at 80 °C for 24 hours, yielding a black colored Nb2CTx MXene powder, as shown in Fig. 1.2.3. Preparation of DyMn2O5 Dy2O3/MXene (Nb2CTx) and DyMn2O5/Dy2O3 composites
DyMn2O5/Dy2O3/MXene (Nb2CTx) was synthesized via a hydrothermal method. Initially, 1 mmol of dysprosium nitrate (Dy(NO3)3) and 2 mmol of manganese nitrate (Mn(NO3)2) were dissolved in 30 mL of distilled water under magnetic stirring for 1 hour. Here, 0.1 g of synthesized MXene (Nb2CTx) in 5 mL distilled water was added dropwise into the precursor solution under constant stirring. Separately, 2 g of potassium hydroxide (KOH) was dissolved in 20 mL of distilled water, and the resulting solution was gradually added dropwise to the stirred precursor solution until the pH was adjusted to 7–8. The obtained solution was then transferred into a Teflon-lined stainless steel autoclave and subjected to thermal treatment at 180 °C for 12 hours to facilitate solvent evaporation. When the solution in the autoclave was cooled down at room temperature, it was washed multiple times with ethanol and distilled water, and subsequently subjected to centrifugation five times at 4000 rpm for 20 minutes to obtained pure precipitates. The collected precipitates were dried in an oven at 80 °C for 12 h. Finally, a light yellowish powder was obtained and annealed at 750 °C for 6 hours in a furnace to enhance its structural and functional properties. A schematic diagram of the synthesis technique for the preparation of the DyMn2O5/Dy2O3/MXene (DMO/DO/MX) composite is shown in Fig. 2.For comparison, the DyMn2O5/Dy2O3 (DMO/DO) composite was synthesized by following the same amount of precursors, and the same synthesis protocols as mentioned above without adding MXene (Nb2CTx) in solution.
2.4. Preparation of the electrode
A three-electrode configuration was employed for electrochemical measurements. The system consisted of a reference electrode (Ag/AgCl), a counter electrode (platinum wire), and a working electrode composed of nickel foam coated with DyMn2O5/Dy2O3/MXene (Nb2CTx). The fabrication of the working electrode involved grinding of 0.1 g of DyMn2O5/Dy2O3/MXene (DMO/DO/MX) powder in a mortar and pestle for approximately one hour to achieve a fine particle dispersion. A few drops of water were then added to form a homogeneous aqueous slurry. The prepared slurry was uniformly applied onto a 1 cm × 1 cm nickel foam substrate, followed by drying in an oven at 75 °C for 12 hours. Using the same procedure, a DyMn2O5/Dy2O3 (DMO/DO) electrode was fabricated. The active loaded mass of DyMn2O5/Dy2O3/MXene and DyMn2O5/Dy2O3 was 2.5 mg and 2.8 mg, respectively.3. Results and discussion
3.1. Structural analysis
Fig. 3(d–f) illustrates the morphological characteristics of Nb2AlCTx and Nb2CTx. As shown in Fig. 3(d), Nb2AlCTx exhibits a well-ordered ternary layered structure with no visible delamination, and its surface remains clean and intact. In contrast, the SEM images of Nb2CTx MXene in Fig. 3(e and f) captured at resolutions of 500 nm and 1 μm respectively, reveal a distinct stacked structure with well separated layers. This transformation indicates the effective removal of the Al atomic layer from Nb2AlCTx through KOH etching, resulting in the characteristic layered morphology of Nb2CTx MXene. These observations are consistent with the XRD results, further validating the successful synthesis of Nb2CTx MXene.
Furthermore, Fig. 3(b) exhibits the XRD pattern of both synthesized samples. The characteristic peaks in the prepared sample DyMn2O5/Dy2O3 observed at 2θ = 20.6°, 29.1°, 33.7°, 35.9°, 43.0°, 53.1°, 57.3°, 58.9°, 70.9°, 78.4°, and 80.7° correspond to the (020), (201), (130), (221), (231), (042), (340), (223), (124), (451), and (601) crystal planes of orthorhombic DyMn2O5 (JCPDS # 01-07-1696). The refined lattice parameters, a = 7.290 Å, b = 8.633 Å, and c = 5.626 Å, exhibit strong agreement with standard values (7.2940, 8.5551, and 5.6875 Å, respectively). Additionally, a distinct peak at 2θ = 48.44° (•) corresponds to the (440) plane of cubic Dy2O3 (JCPDS 00-022-0612), confirming the presence of Dy2O3 as a secondary phase. The XRD pattern of the synthesized DyMn2O5/Dy2O3/MXene composite, shown in Fig. 3(b), was recorded over a broad 2θ range (10°–90°). The peaks at 2θ = 29.1°, 57.8°, 60.7°, and 80.7° correspond to the (201), (340), (430), and (601) planes of orthorhombic DyMn2O5 (JCPDS 01-07-1696). Additionally, the peaks observed at 2θ = 33.9° and 72.7° (★) correspond to the (100) and (201) planes of hexagonal niobium carbide (Nb2C), indexed to JCPDS # 00-015-0127, confirming the incorporation of the MXene phase. The distinct peak at 2θ = 48.44° (•) is also present in the composite sample, further verifying the coexistence of Dy2O3. The XRD pattern of the DyMn2O5 composite with MXene exhibits characteristic peaks corresponding to DyMn2O5, Dy2O3, and Nb2CTx, confirming the successful synthesis of the DyMn2O5/Dy2O3/MXene composite. The Debye–Scherrer formula was employed to assess the crystallite size:
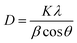 | (1) |
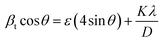 | (2) |
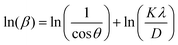 | (3) |
Both equations are the equations of a straight line, where 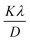 and
and 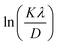 respectively, are the y-intercepts of both equations from which crystallite size is measured by W–H plots and modified W–H plots as in Fig. S1(a–d) (ESI†). Also, it is very helpful to find strain by extracting the slope and y-intercept of the linear fit as demonstrated in Fig. S1(a and b) (ESI†). The estimated values of strain, crystallite size, dislocation density and crystallinity for both samples DyMn2O5/Dy2O3 and the DyMn2O5/Dy2O3/MXene composite are given in Table 1.
respectively, are the y-intercepts of both equations from which crystallite size is measured by W–H plots and modified W–H plots as in Fig. S1(a–d) (ESI†). Also, it is very helpful to find strain by extracting the slope and y-intercept of the linear fit as demonstrated in Fig. S1(a and b) (ESI†). The estimated values of strain, crystallite size, dislocation density and crystallinity for both samples DyMn2O5/Dy2O3 and the DyMn2O5/Dy2O3/MXene composite are given in Table 1.
| Sample | Crystallite size by Scherrer (nm) | Crystallite size by W–H model (nm) | Crystallite size by modified Scherrer's equation (nm) | Micro strain ε × 10−3 | Dislocation density δ × 10−3 (nm)−2 | Crystallinity (%) |
|---|---|---|---|---|---|---|
| DyMn2O5/Dy2O3 | 61.9 | 50.6 | 58.6 | 1.5 | 0.44 | 62.5 |
| DyMn2O5/Dy2O3/Nb2CTx | 39.4 | 29.8 | 24.6 | 2.5 | 0.96 | 73.4 |
The crystallite size of DyMn2O5/Dy2O3/Nb2CTx is smaller than that of DyMn2O5/Dy2O3, indicating a higher surface area, which can contribute to enhanced specific capacitance. Furthermore, the crystallite size estimated using the Williamson–Hall (W–H) plot method is observed to be smaller than that determined by Scherrer's equation. This discrepancy arises because the W–H model accounts for microstrain and instrumental broadening effects, making it a more accurate approach for crystallite size determination.59 Dislocation density can provide details about the material's mechanical characteristics and deformation processes. The dislocation density of DyMn2O5/Dy2O3/MXene is more than that of DyMn2O5/Dy2O3, which informs us that the mechanical properties of the composite, especially its hardness and strength, have been improved.60 Furthermore, the DyMn2O5/Dy2O3/MXene composite exhibits higher crystallinity compared to DyMn2O5/Dy2O3, indicating an increase in structural order. Crystallinity is a fundamental parameter that has a significantly impact in determining the overall electrochemical functionality of materials. Highly crystalline structures typically facilitate enhanced charge/discharge kinetics, improved electrical conductivity, and greater structural stability. Therefore, precise control over crystallinity through optimized synthesis and processing techniques is essential for tailoring the electrochemical properties of materials in energy storage and conversion applications.61
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. A characteristic C–N stretching vibration has been detected at 1125 cm−1 in the MXene structure.65 Also, the vibration frequency at 1507 cm−1 is characteristic of the Nb–O stretching mode.66 The FT-IR spectrum of the composite material may offers critical insights into its electrochemical characteristics. The observed slight shift in peak position relative to the pristine DyMn2O5/Dy2O3 spectrum suggests modifications in the electronic structure arising from the incorporation of Nb2CTx. These structural alterations likely influence the material's electrochemical characteristics and efficiency by enhancing its redox activity by introducing additional redox-active sites.
O. A characteristic C–N stretching vibration has been detected at 1125 cm−1 in the MXene structure.65 Also, the vibration frequency at 1507 cm−1 is characteristic of the Nb–O stretching mode.66 The FT-IR spectrum of the composite material may offers critical insights into its electrochemical characteristics. The observed slight shift in peak position relative to the pristine DyMn2O5/Dy2O3 spectrum suggests modifications in the electronic structure arising from the incorporation of Nb2CTx. These structural alterations likely influence the material's electrochemical characteristics and efficiency by enhancing its redox activity by introducing additional redox-active sites.
 | ||
| Fig. 4 (a) XPS wide spectrum graph of DyMn2O5/Dy2O3/MXene, and (b) high resolution spectra of Dy 4d, (c) of Mn 2P, (d) of Nb 3d, (e) of C 1s, and (f) of O 1s. | ||
The high-resolution XPS spectrum of Mn 2p (Fig. 4(c)) exhibits distinct peaks at 642.9 and 654.03 eV characteristic of Mn 2p3/2 and Mn 2p1/2 spin–orbit components, respectively, while the peak at 647.6 eV represents a satellite peak. The measured spin–orbit splitting of 11.15 eV between these states is characteristic of Mn3+ based materials, further confirming the oxidation state of manganese in the synthesized sample.69Fig. 4(d) presents the high-resolution XPS spectrum of Nb 3d, where the deconvolution of the Nb4+ 3d5/2 and Nb4+ 3d3/2 peaks reveals Nb4+ oxidation states. The characteristic peak at 206.1 eV is attributed to Nb–C, while the characteristic peak at 208.9 eV corresponds to Nb–O bonding.70,71Fig. 4(e) presents the high resolution XPS spectrum of C 1s, where the deconvolution reveals distinct spectral components. The peak observed at 288.2 eV corresponds to O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating modifications in the chemical state of carbon bonded to oxygen. Additionally, the peak at 291.2 eV is identified as a satellite peak, attributed to the presence of C–O functional groups, reflecting the electronic interactions within the material.72Fig. 4(f) depicts the high resolution XPS spectrum of O 1s, where one peak at 532.8 eV is due to chemisorbed oxygen and is also indicative of carbonyl (C
O, indicating modifications in the chemical state of carbon bonded to oxygen. Additionally, the peak at 291.2 eV is identified as a satellite peak, attributed to the presence of C–O functional groups, reflecting the electronic interactions within the material.72Fig. 4(f) depicts the high resolution XPS spectrum of O 1s, where one peak at 532.8 eV is due to chemisorbed oxygen and is also indicative of carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functional groups. Meanwhile, the peak at 536.4 eV is associated with adsorbed molecular oxygen (O2) or surface-bound water (H2O), suggesting the presence of physisorbed species on the material's surface.73
O) functional groups. Meanwhile, the peak at 536.4 eV is associated with adsorbed molecular oxygen (O2) or surface-bound water (H2O), suggesting the presence of physisorbed species on the material's surface.73
3.2. Morphological and compositional analysis
The surface morphology of the DyMn2O5/Dy2O3 and DyMn2O5/Dy2O3/MXene composites was systematically analyzed using scanning electron microscopy (SEM), and the corresponding micrographs, captured at varying magnifications, are depicted in Fig. 5 and 6. As observed in Fig. 5(a–c), the DyMn2O5/Dy2O3 sample exhibits a distinct assembly of peanut-shaped microparticles that are interconnected, forming a compact structure. Fig. 5(d) demonstrates the energy-dispersive X-ray (EDX) spectrum of the as-synthesized DyMn2O5, where characteristic peaks confirm the presence of Dy (dysprosium), Mn (manganese), and O (oxygen), with their respective atomic and weight percentages detailed in the inset. The EDX analysis unequivocally verifies the compositional integrity of the synthesized material, further corroborated by X-ray diffraction (XRD) analysis. Moreover, statistical evaluation using ImageJ software, as depicted in the histogram of Fig. 5(e), indicates that the average length of the peanut-shaped DyMn2O5/Dy2O3 particles is approximately 0.34 μm.Fig. 6(a and b) presents the SEM micrographs of the DyMn2O5/Dy2O3/MXene composite at magnifications of 500 nm and 2 μm. The images reveal the presence of small, variably sized cubic-shaped platelets, which are uniformly distributed across the two-dimensional Nb2CTx (MXene) layer, with multiple interstitial voids between them. Fig. 6(d) displays the energy-dispersive X-ray (EDX) spectrum of the composite, confirming the presence of Dy (dysprosium), Mn (manganese), O (oxygen), Nb (niobium), and C (carbon), thereby substantiating the elemental composition of the as-synthesized material. Furthermore, statistical analysis performed using Image J software, as represented in the histogram of Fig. 6(e), indicates that the average particle size of the DyMn2O5/Dy2O3/MXene composite is approximately 78 nm. The extensive surface area of the MXene layers enhances heterogeneous nucleation, thereby promoting the formation of small nanoparticles. Additionally, the hydrophilic nature of MXene and the abundance of surface-anchored functional groups (e.g., –OH, –F, –O) facilitates strong interactions with precursor ions, effectively regulating their diffusion dynamics and constraining particle growth, ultimately leading to a reduction in overall nanoparticle size. The surface morphology and small particle size of DyMn2O5/Dy2O3/MXene as compared to DyMn2O5/Dy2O3 gives active sites and facilitate diffusion of ions, which may ultimately improve electrochemical properties.
3.3. Electrochemical analysis
The electrochemical properties of the synthesized materials (DMO/DO and DMO/DO/MX) were systematically investigated using cyclic voltammetry (CV), galvanostatic charge discharge (GCD), and electrochemical impedance spectroscopy (EIS). Initially, the intrinsic electrochemical behavior of both samples was assessed through CV measurements employing a three-electrode system. In this setup, an Ag/AgCl electrode served as the reference, a platinum (Pt) wire functioned as the counter electrode, and the working electrode was composed of the synthesized material coated onto a nickel (Ni) foam substrate. Cyclic voltammetry (CV) measurements were conducted for all synthesized materials MXene (Nb2CTx), DyMn2O5/Dy2O3 and DyMn2O5/Dy2O3/MXene in a 3 M KOH electrolyte across a range of scan rates of 10, 20, 30, 40, 50 and 100 mV s−1, within a potential window of 0.1–0.7 V. The corresponding CV profiles are presented in Fig. 7(a–c), illustrating the well-defined sharp redox peaks, which indicates pseudo capacitive behavior of all prepared samples. MXene CV curves, in this positive potential window (0.1–0.7 V), display pronounced redox peaks, indicating pseudocapacitive behavior. This is attributed to reversible faradaic reactions between the surface functional groups of the Nb2C MXene (e.g., –OH,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –F) and the electrolyte ions. Nb-based MXenes, like other transition-metal carbides, can undergo surface redox reactions due to multiple oxidation states of Nb (e.g., Nb5+/Nb4+), contributing to charge storage via pseudocapacitance. Meanwhile, DMO/DO and DMO/DO/MX also depict sharp redox peaks, confirming pseudocapacitive behavior. With increasing scan rates, redox peaks exhibit a shift towards higher potentials, indicating an enhancement in ion transport resistance, while the same shape of the CV curves even at higher scan rates suggests efficient charge transfer dynamics and stable electrochemical behavior of the electrode material.74,75 DMO/DO/MX demonstrates a large integrated area under the CV curve at 10 mV s−1, indicating a significantly enhanced specific capacitance relative to DMO/DO as shown in Fig. 7(d). The specific capacitance of all electrodes, MXene, DMO/DO and DMO/DO/MX is determined through the following equations.76
O, –F) and the electrolyte ions. Nb-based MXenes, like other transition-metal carbides, can undergo surface redox reactions due to multiple oxidation states of Nb (e.g., Nb5+/Nb4+), contributing to charge storage via pseudocapacitance. Meanwhile, DMO/DO and DMO/DO/MX also depict sharp redox peaks, confirming pseudocapacitive behavior. With increasing scan rates, redox peaks exhibit a shift towards higher potentials, indicating an enhancement in ion transport resistance, while the same shape of the CV curves even at higher scan rates suggests efficient charge transfer dynamics and stable electrochemical behavior of the electrode material.74,75 DMO/DO/MX demonstrates a large integrated area under the CV curve at 10 mV s−1, indicating a significantly enhanced specific capacitance relative to DMO/DO as shown in Fig. 7(d). The specific capacitance of all electrodes, MXene, DMO/DO and DMO/DO/MX is determined through the following equations.76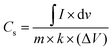 | (4) |
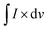 is the integral sweep area of the curve, m is the active mass of the electrode, (ΔV) is potential window and k denotes scan rate. The calculated specific capacitances of MXene are 105.9 F g−1, 61.13 F g−1, 50.24 F g−1, 44.30 F g−1, 39.10 F g−1, and 23.92 F g−1, for DMO/DO and 703.9 F g−1, 394.5 F g−1, 285.9 F g−1, 241.2 F g−1, 208.6 F g−1 and 130.6 F g−1, and for DMO/DO/MX are 863.1 F g−1, 629.36 F g−1, 530.2 F g−1, 413.9 F g−1, 368.3 F g−1 and 213.9 F g−1 at scan rates of 10 mV s−1, 20 mV s−1, 30 mV s−1, 40 mV s−1, 50 mV s−1 and 100 mV s−1, respectively (Table S1, ESI†). DMO/DO/MX has significantly high specific capacitance as compared to DMO/DO, which may be governed by the presence of Nb2CTx MXene. DyMn2O5 undergoes redox reactions involving Mn3+/Mn2+ and Mn4+/Mn3+ transitions, while Dy2O3 dissolves in an alkaline medium, forming hydroxylated species and niobium carbide undergoes surface hydroxylation and redox reactions contributing to charge storage.
is the integral sweep area of the curve, m is the active mass of the electrode, (ΔV) is potential window and k denotes scan rate. The calculated specific capacitances of MXene are 105.9 F g−1, 61.13 F g−1, 50.24 F g−1, 44.30 F g−1, 39.10 F g−1, and 23.92 F g−1, for DMO/DO and 703.9 F g−1, 394.5 F g−1, 285.9 F g−1, 241.2 F g−1, 208.6 F g−1 and 130.6 F g−1, and for DMO/DO/MX are 863.1 F g−1, 629.36 F g−1, 530.2 F g−1, 413.9 F g−1, 368.3 F g−1 and 213.9 F g−1 at scan rates of 10 mV s−1, 20 mV s−1, 30 mV s−1, 40 mV s−1, 50 mV s−1 and 100 mV s−1, respectively (Table S1, ESI†). DMO/DO/MX has significantly high specific capacitance as compared to DMO/DO, which may be governed by the presence of Nb2CTx MXene. DyMn2O5 undergoes redox reactions involving Mn3+/Mn2+ and Mn4+/Mn3+ transitions, while Dy2O3 dissolves in an alkaline medium, forming hydroxylated species and niobium carbide undergoes surface hydroxylation and redox reactions contributing to charge storage.| Mn2O5 + 2e− + H2O ↔ 2MnO2 + 2OH− | (5) |
| MnO2 + e− + OH− ↔ MnOOH | (6) |
| Dy3+ + 3OH− ↔ Dy(OH)3 | (7) |
| Dy2O3 + 6OH− + 3H2O ↔ 2Dy(OH)6−3 | (8) |
| Dy (OH)3 + e− ↔ Dy(OH)2 + OH− | (9) |
| Nb2C + 2OH− ↔ Nb2C (OH)2 + 2e− | (10) |
| Nb2C+ 4OH− ↔ 2NbO (OH) + 2H2O + 4e− | (11) |
| NbO (OH) + e− + OH− ↔ NbO2 + H2O | (12) |
The electrochemical performance of the DMO/DO/MX system is predominantly governed by faradaic redox transitions involving Mn, Dy, and Nb species, which contribute to charge storage. Additionally, the surface hydroxylation of Nb2C enhances its pseudocapacitive behavior, while the 3 M KOH alkaline electrolyte supplies hydroxyl ions, facilitating ion transport and interfacial redox reactions. Various methodologies have been proposed for distinguishing between diffusion-controlled and capacitive (non-diffusion-limited) processes. Dunn (2007) introduced a normalization formula for cyclic voltammetry (CV) kinetics analysis, as represented in eqn (13). Using this approach, the contributions of capacitive and diffusion-controlled processes in the DMO/DO/MX electrode were quantified based on the given equation.77
| i(v) = k1(V) + k2(V)1/2 = i (capacitive) + i (diffusion) | (13) |
In this context, i(v) represents the current at a given potential V, while k1(V) and k2(V)1/2 correspond to the contributions from diffusion limiting (surface capacitive effects) and diffusion-controlled processes, respectively. The graphical representation highlights the dual influence of capacitive effects and ion diffusion on the total capacitance. Furthermore, Fig. 7(e) depicts the total capacitance contribution of the DMO/DO/MX electrode at a scan rate of 20 mV s−1. At scan rates of 10, 20, 30, 40, 50, and 100 mV s−1, the electrode DMO/DO/MX exhibits diffusion controlled contributions of 72.61%, 63.41%, 60.28%, 54.59%, 51.01%, and 45.26%, respectively. Notably, the diffusion controlled contribution of the DMO/DO/MX electrode dominates, which confirms the pseudo capacitive behavior. At higher scan rates, the time available for ion diffusion decreases, thus enhancing the capacitive controlled contribution.
Fig. 8(a) shows the galvanostatic charge discharge (GCD) curves of Nb2CTx MXene at 1, 2, 3 and 5 A g−1. Fig. 8(b) presents the comparison of the GCD curves of DMO/DO and DMO/DO/MX electrodes recorded across a current density of 1 A g−1 within the potential window (0–0.5 V), providing insights into their electrochemical performance under charge discharge conditions. The observed reduction in the potential window during GCD measurements as compared to CV curves can be attributed to internal resistance, kinetic constraints of redox reactions, and the need to avoid electrolyte decomposition at higher potentials. All electrodes demonstrate a nonlinear charge–discharge curve, confirming the characteristic of purely faradaic behavior of both materials, and also supported the CV results by suggesting a diffusion-controlled redox process. Notably, the DMO/DO/MX electrode exhibits the longest discharge duration as compared to DMO/DO, indicating its superior specific capacity. MXene, DMO/DO and DMO/DO/MX exhibit specific capacity of 70.23 C g−1, 285.41 C g−1 and 362.92 C g−1 respectively at 1 A g−1 within a potential window of 0–0.5 V.
Fig. 8(c) exhibits GCD curves of DMO/DO/MX at different current densities 1, 2, 3, 4, 5, 10 and 20 A g−1 within a potential window of 0–0.5 V. The specific capacity (Cs) from GCD curves of DMO/DO/MX at different current densities is calculated using the following formula78,79
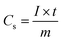 | (14) |
| S. no. | Material | Specific capacitance (F g−1) | Morphology | Synthesis method | Ref. |
|---|---|---|---|---|---|
| 1 | SrMnO3 | 446 F g−1 at 1 A g−1 | Nano fibers | Sol gel | 85 |
| 2 | CeO2/LaMnO3 | 262 F g−1 at 1 A g−1 | Spherical nanoparticles | Co-precipitation | 86 |
| 3 | DyMnO3 | 531 F g−1 at 1 A g−1 | Microwave-assisted synthesis | 87 | |
| 4 | Dy2NiMnO6 | 395.2 F g−1 at 0.5 A g−1 | Mesoporous sphere | Hydrothermal | 88 |
| 5 | La2CuMnO6 | 206 F g−1 at 1 A g−1 | Nanostructures | Hydrothermal | 89 |
| 6 | Y2NiMnO6 | 78 F g−1 at 1 A g−1 | Nanowires | Hydrothermal | 90 |
| 7 | α-MnO2 | 111 F g−1 at 5 mV s−1 | Nanorods | Hydrothermal | 91 |
| 8 | DyMn2O5/Dy2O3/MXene | 725.8 F g−1 at 1 A g−1 | Cubic pallets | Hydrothermal | This work |
Electrochemical impedance spectroscopy was used to study the impedance behavior of both electrode materials in the frequency range of 0.1 to 105 Hz with a small amplitude of 5 mV to ensure system linearity and maintain data quality during measurement. Fig. 8(e) exhibits Nyquist plots of both electrodes. In general, a Nyquist plot consists of two parts, the first part consists of a semicircle in the high frequency range and the other part represents an inclined line in the low frequency range.81 The resistance of the electrode material, electrolyte and interface of the electrolyte and electrode material can be determined by the x-intercept at a high frequency range, termed as solution resistance “Rs”. The charge transfer resistance “Rct” at the electrode material is determined by the diameter of the semicircle.73 The DMO/DO/MX electrode exhibits a relatively low charge transfer resistance (Rct = 10.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ω) as compared to DMO/DO (13.9
Ω) as compared to DMO/DO (13.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ω) indicating the enhanced capacitive performance of the DMO/DO/MX electrode. Furthermore, the Nyquist plot for DMO/DO/MX exhibits a steeper slope in the low-frequency region, with a greater inclination toward the imaginary axis.
Ω) indicating the enhanced capacitive performance of the DMO/DO/MX electrode. Furthermore, the Nyquist plot for DMO/DO/MX exhibits a steeper slope in the low-frequency region, with a greater inclination toward the imaginary axis.
This characteristic suggests a lower diffusion resistance compared to the other electrodes, indicating improved ion transport dynamics.82 These results indicate that the DMO/DO/MX electrode possesses inherently lower resistance, facilitating more efficient ion transport and electron transfer. This enhanced conductivity contributes to its high specific capacitance and stable cycling performance. The cycling lifespan of a supercapacitor is a critical parameter that significantly influences its practical applicability and long-term performance. Fig. 8(f) exhibits that the DMO/DO/MX electrode maintained 77.2% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at a current density of 5 Ag−1. These findings demonstrate that the DyMn2O5/Dy2O3 composite with MXene significantly gives a high specific capacitance with long-life stability, making it a promising candidate for pseudo capacitor applications.
000 charge–discharge cycles at a current density of 5 Ag−1. These findings demonstrate that the DyMn2O5/Dy2O3 composite with MXene significantly gives a high specific capacitance with long-life stability, making it a promising candidate for pseudo capacitor applications.
The enhanced specific capacitance and cycling stability of the DyMn2O5/Dy2O3 decorated MXene composite can be attributed to several inter-related mechanisms. Firstly, the presence of dysprosium (Dy) ions plays a crucial role in improving capacitive behavior through reversible redox reactions involving Dy3+/Dy2+ and Mn4+/Mn3+ couples. These redox transitions contribute additional faradaic charge storage, boosting the overall capacitance. Secondly, DyMn2O5 and Dy2O3 nanoparticles provide multiple active sites for electrochemical reactions, while their stable crystal structures enhance cycling durability. When integrated with MXene, a highly conductive and layered 2D material, a strong interfacial synergy emerges. MXene acts as a conductive backbone that accelerates electron transport and minimizes internal resistance, while also serving as a mechanically robust support that accommodates volume changes during charge–discharge cycles. This synergistic interaction between the redox active rare earth oxides and the conductive MXene matrix leads to improved charge storage kinetics, higher specific capacity, and excellent long term cycling stability.58,83,84
3.4. Assembly of an asymmetric super capacitor (ASC) using DyMn2O5/Dy2O3/MXene‖AC
The remarkable electrochemical performance of the DyMn2O5/Dy2O3/MXene composite necessitated a comprehensive evaluation of its energy storage potential for practical applications. To assess its practical viability, an asymmetric supercapacitor (ASC) was fabricated, integrating DMO/DO/MX as the positive electrode and activated carbon (AC) as the negative electrode (designated as DMO/DO/MX‖AC ASC). The 2 M KOH electrolyte was utilized to enhance ionic conductivity and facilitate efficient charge transfer between the electrodes. The active loaded mass of DMO/DO/MX was approximately 3.2 mg. Fig. 9(a) presents the cyclic voltammetry (CV) curves of the DMO/DO/MX and Activated carbon (AC) at different potential windows and at the same scan rate of 10 mV s−1. CV experiments were performed over progressively wider potential windows ranging from 1.1 to 1.5 V at 20 mV s−1 as depicted in Fig. 9(b). As the potential window increased, the enclosed area of each CV curve expanded, reflecting the greater energy stored at the enlarged operating potential window of 1.5 V. Above 1.5 V, oxygen evolution occurs, limiting the stability of the device. Consequently, 1.5 V was adopted as the maximum operating potential window for all subsequent electrochemical measurements of the ASC device. As anticipated, the fabricated asymmetric device achieves an extended operating voltage of 1.5 V, attributed to the synergistic integration of the electrochemically stable DMO/DO/MX positive electrode and the robust AC negative electrode.92 CV curves of DMO/DO/MX//AC were recorded at scan rates ranging from 3 to 80 mV s−1 within an operational voltage window of 0–1.5 V, as illustrated in Fig. 9(c). The presence of distinct redox peaks at 1.27 V and 0.80 V signifies a predominantly faradaic charge storage mechanism indicating pseudocapacitive behavior. A progressive increase in peak current was recorded with no significant distortion in the shape of the CV curves, even at higher scan rates, indicating the fabricated ASC's excellent rate capability and superior electrochemical reversibility. The GCD curves presented in Fig. 9(d) exhibit a non-linear quasi triangle shaped curve, reflecting efficient charge storage dynamics and favorable pseudocapacitive characteristics. The specific capacitance of the fabricated device, determined from the GCD curves was measured as 148.2, 84.5, 76.38, 72.4, 70.02, 68.1 and 60.2 F g−1 at current densities of 1, 2, 3, 4, 5, 6 and 7 A g−1, respectively. The energy density (Ed) and the critical performance metric, power density (Pd), serve as fundamental parameters for assessing the energy efficiency of the fabricated asymmetric device and they are calculated from the GCD curves using the following equations.93| Ed = (Cs × ΔV2)/(2 × 3.6) | (15) |
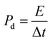 | (16) |
Fig. 9(e) illustrates the Ragone plot of the DyMn2O5/Dy2O3/MXene//AC ASC, demonstrating that the fabricated device achieves a high energy density of 46.25 W h kg−1 at a power density of 750 W kg−1. Furthermore, it retains an energy density of 18.96 W h kg−1 even at a maximum power density of 5250.20 W kg−1. This performance is comparable to, and in certain aspects surpasses, previously reported transition and rare earth-based metal oxides and MXene-based supercapacitors such as MnO2@CNT//AC (13.4 W h kg−1 at 602 W kg−1),94 Ti3C2Tx–δ-MnO2//AC (8.2 W h kg−1 at 400 W kg−1),95 λ-MnO2//Ti3C2Tx (15.5 W h kg−1 at 100 W kg−1),96 Ni dope CeO2@ d-Ti3CN (4.4 W h kg−1 at 495 W kg−1),97 Dy doped CoFe2O4 (15.23 W h kg−1 at 450 W kg−1)98 and CeO2/LaMnO3//AC (17.2 W h kg−1 at 1015 W kg−1).86 The cycling stability of the DMO/DO/MX‖AC ASC was evaluated at a current density of 2 A g−1 over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles (Fig. 9(f)), demonstrating a capacitance retention of 70.56%. This high retention exhibits the device's excellent stability and highlights its potential for high performance asymmetric supercapacitor (ASC) applications.
000 charge–discharge cycles (Fig. 9(f)), demonstrating a capacitance retention of 70.56%. This high retention exhibits the device's excellent stability and highlights its potential for high performance asymmetric supercapacitor (ASC) applications.
In this study, the fabricated system introduces a straightforward approach for synthesizing Nb2CTx-based MXene and rare earth metal-based oxide composites with outstanding cycling stability and high specific capacitance. This research offers innovative approaches for the development of flexible electrode materials with superior electrochemical performance, paving the way for next-generation portable and wearable electronic devices.
Conclusions
In this study, a novel rare earth-based bi-metallic oxide DyMn2O5/Dy2O3/MXene nanocomposite was successfully synthesized using a hydrothermal method to enhance the interlayer spacing of MXene for improved electrochemical performance. The incorporation of DyMn2O5/Dy2O3 nanoparticles effectively mitigated the restacking issue of MXene, leading to an optimized electrode material with a high specific capacitance of 362.92 C g−1 at 1 A g−1 with 77% capacitance retention over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 discharge cycles. Furthermore, an asymmetric supercapacitor assembled with the DyMn2O5/Dy2O3/MXene electrode and an activated carbon cathode exhibited a commendable energy density of 46.825 W h kg−1 at a power density of 750 W kg−1, retaining 70.56% of its initial capacitance after 10
000 discharge cycles. Furthermore, an asymmetric supercapacitor assembled with the DyMn2O5/Dy2O3/MXene electrode and an activated carbon cathode exhibited a commendable energy density of 46.825 W h kg−1 at a power density of 750 W kg−1, retaining 70.56% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 2 A g−1. These findings highlight the potential of rare earth-based MXene nanocomposites as high-performance electrode materials for next-generation supercapacitors, demonstrating the effectiveness of the hydrothermal approach in tailoring nanostructured materials for energy storage applications.
000 cycles at 2 A g−1. These findings highlight the potential of rare earth-based MXene nanocomposites as high-performance electrode materials for next-generation supercapacitors, demonstrating the effectiveness of the hydrothermal approach in tailoring nanostructured materials for energy storage applications.
Author contributions
Komal Ali Rao: the conceptualization, formal analysis, investigation, methodology, data curation, software, writing original draft, editing and review, Javed Ahmad, Muhammad Bilal: resources and review Muhammad Ehsan Mazhar, Muhammad Imran Khan, Muhammad Suleman Ahmad, Muhammad Aziz and Hafeez ur rehman: review.Data availability
All data generated or analyzed during this study are included in this article. Furthermore all the related data are available from the authors upon request.Conflicts of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research article is part of the PhD research work of Komal Ali Rao performed at the Institute of Physics, Bahauddin Zakariya University Multan, Pakistan.References
- L. Bird, M. Milligan and D. Lew, Integrating variable renewable energy: Challenges and solutions, National Renewable Energy Lab.(NREL), Golden, CO, United States, 2013 Search PubMed.
- F. Ahmad, et al., A highly active, low-cost CoZn ferrite electrocatalyst in oxygen reduction reactions, Results Chem., 2025, 102095 Search PubMed.
- X. Chen, et al., Lithium insertion/extraction mechanism in Mg2Sn anode for lithium-ion batteries, Intermetallics, 2024, 169, 108306 CrossRef CAS.
- Y. Zhan, et al., Enhancing prediction of electron affinity and ionization energy in liquid organic electrolytes for lithium-ion batteries using machine learning, J. Power Sources, 2025, 629, 235992 CrossRef CAS.
- J. Liu, et al., Advanced energy storage devices: basic principles, analytical methods, and rational materials design, Adv. Sci., 2018, 5(1), 1700322 CrossRef PubMed.
- W. Abbas, et al., Study of the electrical properties and electrochemical sensing efficiency of hydrothermally synthesized Sr doped Nickel oxide nanomaterials, Phys. Scr., 2022, 97(7), 075004 CrossRef CAS.
- M. Khalil, et al., A critical review of biofuel cell cathodes, Biofuels, Bioprod. Biorefin., 2025 DOI:10.1002/bbb.2754.
- F. Ahmad, et al., Direct electron transfer chemistry of redox-active enzymes: applications in biosensor development, Biofuels, Bioprod. Biorefin., 2025 DOI:10.1002/bbb.2742.
- X. Guo, et al., Non-coordinating charge transfer enables ultrafast desolvation of hydrated zinc ions in the outer Helmholtz layer for stable aqueous Zn metal batteries, Natl. Sci. Rev., 2025, 12(4), nwaf070 CrossRef PubMed.
- J. R. Miller and P. Simon, Electrochemical capacitors for energy management, Science, 2008, 321(5889), 651–652 CrossRef CAS PubMed.
- X. Jian, et al., Carbon-based electrode materials for supercapacitor: progress, challenges and prospective solutions, J. Electr. Eng, 2016, 4(2), 75–87 Search PubMed.
- Z. Wang, et al., Enhancing battery performance under motor overload drive with a battery–supercapacitor hybrid energy storage system, J. Power Sources, 2025, 642, 236680 CrossRef CAS.
- L. Gao, et al., Nitrogen-doped carbon trapped MnMoO4 microrods toward high performance aqueous zinc-ion battery, J. Alloys Compd., 2023, 968, 172008 CrossRef CAS.
- D. Cai, et al., Binder-Free MOF-Based and MOF-Derived Nanoarrays for Flexible Electrochemical Energy Storage: Progress and Perspectives, Small, 2024, 20(12), 2305778 CrossRef CAS PubMed.
- L. Gao, et al., Zinc selenide/cobalt selenide in nitrogen-doped carbon frameworks as anode materials for high-performance sodium-ion hybrid capacitors. Advanced Composites and Hybrid, Materials, 2024, 7(5), 144 CAS.
- B. E. Conway, Electrochemical supercapacitors: scientific fundamentals and technological applications, Springer Science & Business Media, 2013 Search PubMed.
- P. Sinha and K. K. Kar, Introduction to supercapacitors, in Handbook of Nanocomposite Supercapacitor Materials II: Performance, Springer, 2020, pp. 1–28 Search PubMed.
- P. Biesheuvel, Y. Fu and M. Z. Bazant, Diffuse charge and faradaic reactions in porous electrodes, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 83(6), 061507 CrossRef CAS PubMed.
- X. Zhu, Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies, J. Energy Storage, 2022, 49, 104148 CrossRef.
- P. Bhojane, Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding, J. Energy Storage, 2022, 45, 103654 CrossRef.
- K. K. Patel, et al., Evolution and recent developments of high performance electrode material for supercapacitors: A review, J. Energy Storage, 2021, 44, 103366 CrossRef.
- Z. Zhu, H. Peelaers and C. G. Van de Walle, Electronic and protonic conduction in LaFeO3, J. Mater. Chem. A, 2017, 5, 15367–15379 RSC.
- Q. Hu, et al., Facile syntheses of cerium-based CeMO3 (M= Co, Ni, Cu) perovskite nanomaterials for high-performance supercapacitor electrodes, J. Mater. Sci., 2020, 55, 8421–8434 CrossRef CAS.
- B. Akinwolemiwa, C. Peng and G. Z. Chen, Redox electrolytes in supercapacitors, J. Electrochem. Soc., 2015, 162(5), A5054 CrossRef CAS.
- M. Majumder, et al., Rare earth metal oxide (RE 2 O 3; RE= Nd, Gd, and Yb) incorporated polyindole composites: gravimetric and volumetric capacitive performance for supercapacitor applications, New J. Chem., 2018, 42(7), 5295–5308 RSC.
- J. Lu, et al., Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach, Nat. Commun., 2014, 5(1), 5693 CrossRef CAS PubMed.
- S. Yang, et al., Studies of structure and cycleability of LiMn2O4 and LiNd0.01Mn1.99O4 as cathode for Li-ion batteries, Electrochim. Acta, 2003, 48(5), 569–573 CrossRef CAS.
- R. Singhal, et al., Synthesis and characterization of Nd doped LiMn2O4 cathode for Li-ion rechargeable batteries, J. Power Sources, 2007, 164(2), 857–861 CrossRef CAS.
- P. Ram, et al., Improved performance of rare earth doped LiMn2O4 cathodes for lithium-ion battery applications, New J. Chem., 2016, 40(7), 6244–6252 RSC.
- Y. Xie, et al., Synthesis and electrochemical properties of Sc-doped Li1.05ScxMn2−xO4 spinel as cathodic material for rechargeable Li-battery, Solid State Ionics, 2005, 176(35–36), 2563–2569 CrossRef CAS.
- H. Zhao, et al., Rare earth incorporated electrode materials for advanced energy storage, Coord. Chem. Rev., 2019, 390, 32–49 CrossRef CAS.
- S. Liang, et al., Rare-earth based nanomaterials and their composites as electrode materials for high performance supercapacitors: a review, Sustainable Energy Fuels, 2020, 4(8), 3825–3847 RSC.
- H. Huang and J.-J. Zhu, The electrochemical applications of rare earth-based nanomaterials, Analyst, 2019, 144(23), 6789–6811 RSC.
- J. T. Mefford, et al., Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes, Nat. Mater., 2014, 13(7), 726–732 CrossRef CAS PubMed.
- S. Demirel, et al., Structural, magnetic, electrical and electrochemical properties of SrCoO2.5, Sr9Co2Mn5O21 and SrMnO3 compounds, Ceram. Int., 2017, 43(17), 14818–14826 CrossRef CAS.
- T. Sharmili, et al., Investigation on Y2NiMnO6 nanostructures for energy storage applications, Appl. Phys. A: Mater. Sci. Process., 2023, 129(1), 49 CrossRef CAS.
- S. Hussain, et al., Unique hierarchical mesoporous LaCrO3 perovskite oxides for highly efficient electrochemical energy storage applications, Ceram. Int., 2019, 45(12), 15164–15170 CrossRef.
- H. Li, et al., Facile synthesis of mesoporous MnO2 microspheres for high performance AC//MnO2 aqueous hybrid supercapacitors, Electrochim. Acta, 2013, 108, 497–505 CrossRef CAS.
- K. Shimamoto, K. Tadanaga and M. Tatsumisago, All-solid-state electrochemical capacitors using MnO2/carbon nanotube composite electrode, Electrochim. Acta, 2013, 109, 651–655 CrossRef CAS.
- H. Wang, et al., Design, synthesis and the electrochemical performance of MnO2/C@ CNT as supercapacitor material, Mater. Res. Bull., 2013, 48(9), 3389–3393 CrossRef CAS.
- S. Zhao, et al., Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene, Energy Storage Mater., 2017, 8, 42–48 CrossRef.
- G. Deysher, et al., Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals, ACS Nano, 2019, 14(1), 204–217 CrossRef PubMed.
- G. Li, et al., Highly efficient Nb2C MXene cathode catalyst with uniform O-terminated surface for lithium–oxygen batteries, Adv. Energy Mater., 2021, 11(1), 2002721 CrossRef CAS.
- N. Prabhakar, et al., Effect on delamination of Nb4C3Tx MXene supported tungsten oxide for high-performance supercapacitor, J. Energy Storage, 2024, 98, 112830 CrossRef.
- P. Varatharajan, et al., A wide linear range and highly sensitive electrochemical reduction of environmental hazard (p-Nitrotoluene) using carbon-based hybrid composite (Ti3C2TX@MnCo2O4), J. Environ. Chem. Eng., 2024, 12(6), 114301 CrossRef CAS.
- U. Rajaji, et al., MoS2 sphere/2D S-Ti3C2 MXene nanocatalysts on laser-induced graphene electrodes for hazardous aristolochic acid and roxarsone electrochemical detection, ACS Appl. Nano Mater., 2022, 5(3), 3252–3264 CrossRef CAS.
- K. Shi, Z. Chen and W. Sun, Controlling of Crystal Facets by Dysprosium-Modified WO3/Carbon Nanofibers Enhance the Flexible Supercapacitor Performance, Small, 2024, 20(50), 2405769 CrossRef CAS PubMed.
- X. Li, et al., Manganese dioxide nanosheets decorated on MXene (Ti3C2Tx) with enhanced performance for asymmetric supercapacitors, Ceram. Int., 2021, 47(9), 12211–12220 CrossRef CAS.
- R. Shafique, et al., Investigations of 2D Ti3C2 (MXene)-CoCr2O4 nanocomposite as an efficient electrode material for electrochemical supercapacitors, Int. J. Energy Res., 2022, 46(5), 6689–6701 CrossRef.
- Y. Wei, et al., All pseudocapacitive MXene-MnO2 flexible asymmetric supercapacitor, J. Energy Storage, 2022, 45, 103715 CrossRef.
- S. A. Zahra, E. Ceesay and S. Rizwan, Zirconia-decorated V2CTx MXene electrodes for supercapacitors, J. Energy Storage, 2022, 55, 105721 CrossRef.
- E. Ceesay, et al., Improved pseudocapacitor and water splitting in cobalt-anchored vanadium carbide MXene nanocomposite, Int. J. Hydrogen Energy, 2024, 56, 140–146 CrossRef CAS.
- W. Zheng, et al., MXene—Manganese oxides aqueous asymmetric supercapacitors with high mass loadings, high cell voltages and slow self-discharge, Energy Storage Mater., 2021, 38, 438–446 CrossRef.
- K. O. Oyedotun, et al., Electrochemical performance of two-dimensional Ti3C2-Mn3O4 nanocomposites and carbonized iron cations for hybrid supercapacitor electrodes, Electrochim. Acta, 2019, 301, 487–499 Search PubMed.
- D. Ponnalagar, et al., Recent progress in two-dimensional Nb2C MXene for applications in energy storage and conversion, Mater. Des., 2023, 231, 112046 CrossRef CAS.
- K. Wasnik, et al., MXenes: Advances in the synthesis and application in supercapacitors and batteries, J. Mater. Res., 2022, 37(22), 3865–3889 CrossRef CAS.
- T. Tajiri, et al., Magnetic properties and crystal structure of DyMn2O5 nanoparticles embedded in mesoporous silica, Phys. Proc., 2015, 75, 1181–1186 CrossRef CAS.
- S. Vadivel and P. Sujita, Construction of ternary structured Dy2O3 nanorods/carbon spheres/few layered Dy2WO6 nanohybrids for electrochemical supercapacitors, J. Rare Earths, 2024 DOI:10.1016/j.jre.2024.09.005.
- H. P. Klug and L. E. Alexander, X-ray diffraction procedures: for polycrystalline and amorphous materials. 1974 Search PubMed.
- D. Hull and D. Bacon, Introduction to Dislocations, 5th edn, Butterworth, Heinemann, 2011 Search PubMed.
- M. C. Roco, C. A. Mirkin and M. C. Hersam, Nanotechnology research directions for societal needs in 2020: retrospective and outlook. 2011 Search PubMed.
- N. Arif, et al., Synthesis and characterization of layered Nb2C MXene/ZnS nanocomposites for highly selective electrochemical sensing of dopamine, Ceram. Int., 2021, 47(2), 2388–2396 CrossRef CAS.
- L. Xue, Study on Spectroscopy of Rare Earth Oxides and Mineral. 2003 Search PubMed.
- G. V. Chertihin and L. Andrews, Reactions of laser-ablated manganese atoms with dioxygen. Infrared spectra of MnO, OMnO, Mn (O2),(MnO) 2, and higher oxide complexes in solid argon. The, J. Phys. Chem. A, 1997, 101(45), 8547–8553 CrossRef CAS.
- B. J. Janani, et al., Robust advantage of MXene/g-C3N4 loaded on Fe2WO6/BiIO4 nano-platform for chemo-peroxidase colorimetric detection of uranyl ions, antifungal properties, photocatalytic degradation of p-chlorophenol, and eco-toxicity studies, Diamond Relat. Mater., 2024, 145, 111129 CrossRef CAS.
- Fd. O. Cantão, et al., Utilization of Sn/Nb2O5 composite for the removal of methylene blue, Quim. Nova, 2010, 33, 528–531 CrossRef.
- K. Gopinath, et al., One-pot synthesis of dysprosium oxide nano-sheets: antimicrobial potential and cyotoxicity on A549 lung cancer cells, J. Cluster Sci., 2017, 28, 621–635 Search PubMed.
- Y. Cao, et al., High-performance acetone gas sensor based on ferrite–DyFeO3, J. Mater. Sci., 2020, 55, 16300–16310 CrossRef CAS.
- Jd. A. Pereira, et al., Tuning the morphology of manganese oxide nanostructures for obtaining both high gravimetric and volumetric capacitance, Mater. Adv., 2020, 1(7), 2433–2442 RSC.
- Z. Yang, et al., XPS studies of nitrogen doping niobium used for accelerator applications, Appl. Surf. Sci., 2018, 439, 1119–1126 Search PubMed.
- M. A. Melo, et al., Niobium-doped Hematite Photoanodes Prepared through Low-Cost Facile Methods for Photoelectrochemical Water Splitting, ChemCatChem, 2023, 15(14), e202300387 CrossRef CAS.
- A. Kirakosyan, et al., Poly (styrene sulfonic acid)-grafted carbon black synthesized by surface-initiated atom transfer radical polymerization, Molecules, 2023, 28(10), 4168 Search PubMed.
- K. A. Rao, M. E. Mazhar and J. Ahmad, Facile hydrothermal synthesis of a tri-metallic Cu–Mn–Ni oxide-based electrochemical pseudo capacitor, Dalton Trans., 2024, 53(31), 13012–13021 RSC.
- S. Kaipannan and S. Marappan, Fabrication of 9.6 V high-performance asymmetric supercapacitors stack based on nickel hexacyanoferrate-derived Ni (OH) 2 nanosheets and bio-derived activated carbon, Sci. Rep., 2019, 9(1), 1104 CrossRef PubMed.
- A. Sami, et al., Enhanced supercapacitor performance of rGO-Supported BaVO3 nanostructures as advanced electrode materials, Ceram. Int., 2025, 51(7), 8480–8491 CrossRef CAS.
- B. Saravanakumar, et al., Electrochemical properties of rice-like copper manganese oxide (CuMn2O4) nanoparticles for pseudocapacitor applications, J. Alloys Compd., 2017, 723, 115–122 CrossRef CAS.
- J. Wang, et al., Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles, J. Phys. Chem. C, 2007, 111(40), 14925–14931 CrossRef CAS.
- K. Kannadasan, et al., Sea Urchin-Like NiO-CoO Heterostructure as High-Energy Supercapattery Electrode: Laboratory Prototype to Field Application of Pouch-type Device, ACS Appl. Electron. Mater., 2024, 6(7), 5269–5282 CrossRef CAS.
- N. K. Sakthivel, M. Govindasamy and P.-Y. Chen, Sonochemical Synthesis of Perovskite Embedded with Carbon Nanofibers as an Electrode Material for Energy Storage Application, J. Electrochem. Soc., 2025, 172(4), 040519 CrossRef CAS.
- R. Packiaraj, et al., Electrochemical performances of ZnO–NiO–CuO mixed metal oxides as smart electrode material for solid-state asymmetric device fabrication, Energy Fuels, 2021, 36(1), 603–617 CrossRef.
- X. Zhang, et al., Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons, J. Power Sources, 2012, 216, 290–296 CrossRef CAS.
- J. Sun, et al., Bundlelike CuCo2O4 microstructures assembled with ultrathin nanosheets as battery-type electrode materials for high-performance hybrid supercapacitors, ACS Appl. Energy Mater., 2020, 3(8), 8026–8037 CrossRef CAS.
- B. Subramanian, et al., Synthesis and Characterization of Dy2O3@TiO2 Nanocomposites for Enhanced Photocatalytic and Electrocatalytic Applications, ACS Eng. Au, 2024, 4(5), 474–490 CrossRef CAS.
- R. Fang, et al., 2 D MXene-based energy storage materials: interfacial structure design and functionalization, ChemSusChem, 2020, 13(6), 1409–1419 CrossRef CAS PubMed.
- G. George, et al., Effect of doping on the performance of high-crystalline SrMnO3 perovskite nanofibers as a supercapacitor electrode, Ceram. Int., 2018, 44(17), 21982–21992 CrossRef CAS.
- S. Nagamuthu, S. Vijayakumar and K.-S. Ryu, Cerium oxide mixed LaMnO3 nanoparticles as the negative electrode for aqueous asymmetric supercapacitor devices, Mater. Chem. Phys., 2017, 199, 543–551 CrossRef CAS.
- P. Amalthi, et al., Microwave-aided fabrication of calcium-substituted DyMnO3 nanocomposites as prospective battery-type electrode material for supercapacitors, Mater. Sci. Eng. B, 2023, 298, 116845 CrossRef CAS.
- J. Singh, et al., Mesoporous spheres of Dy 2 NiMnO 6 synthesized via hydrothermal route for structural, morphological, and electrochemical investigation, Ionics, 2020, 26, 5143–5153 CrossRef CAS.
- J. Singh, U. K. Goutam and A. Kumar, Hydrothermal synthesis and electrochemical performance of nanostructured cobalt free La2CuMnO6, Solid State Sci., 2019, 95, 105927 CrossRef CAS.
- M. Alam, et al., Electrochemical supercapacitor based on double perovskite Y2NiMnO6 nanowires, RSC Adv., 2016, 6(115), 114722 RSC.
- D. Gangwar, et al., Effect of Dy on electrochemical supercapacitive behaviour of α-MnO2 nanorods, Electrochim. Acta, 2019, 328, 135027 CrossRef CAS.
- T. Brousse, et al., Long-term cycling behavior of asymmetric activated carbon/MnO2 aqueous electrochemical supercapacitor, J. Power Sources, 2007, 173(1), 633–641 CrossRef CAS.
- B. Safdar, et al., Self-standing star-shaped tri-metallic oxides for pseudocapacitive energy storage electrode materials, Appl. Surf. Sci., 2020, 530, 147251 CrossRef CAS.
- L. Li, et al., Facile synthesis of MnO2/CNTs composite for supercapacitor electrodes with long cycle stability. The, J. Phys. Chem. C, 2014, 118(40), 22865–22872 CrossRef CAS.
- C. K. Kamaja, et al., Effect of Aqueous Electrolytes on the Performance of a Ti3C2T x (MXene)− δ-MnO2 Asymmetric Supercapacitor, Energy Fuels, 2021, 36(1), 703–709 CrossRef.
- B. T. Vetrikarasan, et al., Co-precipitation synthesis of pseudocapacitive λ-MnO2 for 2D MXene (Ti3C2Tx) based asymmetric flexible supercapacitor, J. Energy Storage, 2023, 72, 108403 CrossRef.
- I. Ashraf, et al., Ni-doped CeO2 decorated delaminated layered titanium carbonitride (d-Ti3CN) MXene for potential applications in symmetric supercapacitors, J. Alloys Compd., 2023, 952, 170043 CrossRef CAS.
- M. Sharma, et al., Combustion synthesis, structural and electrochemical studies on the dysprosium-doped cobalt ferrite nanoparticles to investigate its performance as a supercapacitor electrode, Mater. Chem. Phys., 2024, 324, 129676 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01337b |
| This journal is © The Royal Society of Chemistry 2025 |








