Open Access Article
Open Access ArticleIodide substituted halide-rich lithium argyrodite solid electrolytes with improved performance for all solid-state batteries†
Adwitiya
Rao
 a,
Jacob
Rempel
a,
Jacob
Rempel
 a,
Ming
Jiang
a,
Ming
Jiang
 b,
Parvin
Adeli
c,
Chae-Ho
Yim
b,
Parvin
Adeli
c,
Chae-Ho
Yim
 d,
Mohamed
Houache
d,
Yaser
Abu-Lebdeh
d and
Chandra Veer
Singh
d,
Mohamed
Houache
d,
Yaser
Abu-Lebdeh
d and
Chandra Veer
Singh
 *ae
*ae
aDepartment of Materials Science and Engineering, University of Toronto, Toronto, Ontario M5S 3E4, Canada. E-mail: chandraveer.singh@utoronto.ca
bInstitute of Physical Science and Information Technology, Anhui University Hefei, Hefei 230601, China
cDepartment of Chemistry, Department of Chemical Engineering and the Waterloo Institute for Nanotechnology, University of Waterloo, Waterloo, Ontario N2L 3G1, Canada
dEnergy, Mining, and Environment Research Centre, National Research Council of Canada, 1200 Montreal Road, Ottawa, Ontario K1A 0R6, Canada
eDepartment of Mechanical and Industrial Engineering, University of Toronto, 5 King's College Road, Toronto, Ontario M5S 3G8, Canada
First published on 29th April 2025
Abstract
Halogen substitution has been a widely accepted strategy to boost ionic conductivity of lithium argyrodites. Mixed halide argyrodites containing Cl and Br have been shown to be promising candidates as solid electrolytes, featuring high room temperature ionic conductivities >10 mS cm−1. This study focuses on the less explored halide-rich Cl–I mixed halide argyrodites as solid-state electrolytes, comparing them to their Cl–Br analogues. DFT calculations reveal that Cl–I argyrodites possess enhanced phase stability and electrode compatibility. Despite differences in the type of halogen used, Cl–I and Cl–Br argyrodites exhibit similar ionic conductivities at equivalent Cl/X (X = Br, I) ratios. AIMD simulations of Li5.5PS4.5Cl1.5−xIx systems identify an optimal I and Cl content of 0.75 each, yielding a maximum conductivity of 23.5 mS cm−1, attributed to enlarged Li+ migration channels.
1. Introduction
Over the past decade, there has been a growing interest in electric vehicles powered by lithium-ion batteries, due to their immense potential in reduction of “well-to-wheel” greenhouse emissions. Typically, liquid electrolytes have been a popular choice in commercial lithium-ion batteries as they exhibit fast Li+ ion conduction (10−2–10−1 S cm−1 at room temperature). However, utilization of liquid electrolytes poses safety concerns due to their thermal instability, volatility, flammability, and Li dendrite formation. Solid state electrolyte (SSE) batteries, besides their promise to enable the use of Li metal and increase energy density, can provide a noticeable advantage over conventional liquid batteries with regards to safety and in recent years have begun to outperform conventional liquid batteries.1–6One of the most notable compound groups for applications in SSEs are sulfide-based lithium argyrodites which have shown great progress in recent years through the discovery of compounds featuring very high ionic conductivities. By making use of various cationic and anionic substitution strategies, several compounds have been discovered with ionic conductivities in the range of 10−4–10−2 S cm−1.7 Amongst all other strategies, halogen substituted lithium argyrodites, i.e. Li7−yPS6−yXy (X = Cl, Br, I) have been more popular. This is due to the resulting lithium vacancies generated when halogens are added and the S−2/X− anionic site disorder, which occurs in argyrodites substituted with Cl and Br anions, where the halogens, to some degree, swap their 4a crystallographic positions with 4d sites of S−2 ions, due to being similar in size. This shift results in a modification of the energy landscape for Li+ ions, which results in better transport kinetics. Such a phenomenon is absent in the case of I− anions as they only occupy 4a crystallographic positions in the structure.8 Previous experimental studies have reported an ionic conductivity value of 1.9 mS cm−1 for Li6PS5Cl and 0.7 mS cm−1 for Li6PS5Br. However, depending on the synthetic method used, the ionic conductivity could be increased to ∼3.1 mS cm−1 for both Li6PS5Cl and Li6PS5Br.9,10
Based on the halogen substitution strategy to generate lithium vacancies, excess halogen substituted or halide-rich argyrodites of the type Li6−xPS5−xX1+x (0 < x < 0.5) have also been studied. Out of all the compounds, Li5.5PS4.5Cl1.5 exhibited the best performance, reaching an experimental ionic conductivity value of 12 mS cm−1. Synthesizing a pure crystalline phase of Li6−xPS5−xX1+x (X = Br, I) proved to be difficult due to the large anionic size and the formation of LiX (X = Br, I) phase which negatively affected ionic conductivity.11 Yu et al. were able to create a stable Li5.5PS4.5Br1.5 phase, which exhibited an ionic conductivity of 4.35 mS cm−1.12 Zhang et al. were able to synthesize a stable Li5.5PS4.5I1.5, which had an ionic conductivity of 0.31 mS cm−1.13 Other literature studies on similar single halogen substitution strategies involve an addition of other elements such as Si, Ge, Sb, or Se for boosting conductivity via vacancy generation, change in anionic polarizability etc.14–16
Mixed halide rich argyrodites have gained significant popularity in recent years, with argyrodites containing 2 or more halogens substituted in the structure which have yielded ionic conductivity values greater than 10 mS cm−1. Experimental and computational studies done on Li6−xPS5−xClBrx showed that Li5.3PS4.3ClBr0.7 was the best choice amongst other candidates in the family, with an ionic conductivity of 24 mS cm−1 at room temperature with a low lithium ion migration barrier.17 Li et al. conducted a thorough exploration of the Li5.5PS4.5Cl1.5−xBrx chemical space, establishing the role of configurational entropy in boosting ionic conductivity of these systems, and discovering a novel Li5.5PS4.5Cl0.8Br0.7 phase exhibiting a bulk ionic conductivity of 22.7 mS cm−1.18 There have been limited experimental studies on Cl–I mixed halide rich compositions. Most notably, Li5.4PS4.4Cl1.4I0.2 and Li5.6PS4.6Cl1.3I0.1 with an ionic conductivity of 16.43 and 10.45 mS cm−1 respectively.19,20
Although mixed halide substitution strategies show a lot of promise, there exist a few unanswered questions which demand exploration. In this work, we focus on the less explored Cl–I mixed halide argyrodite chemical space, to evaluate and compare their performance with the widely explored Cl–Br chemical space. In the first section, ground state calculations revealed that Cl–I mixed argyrodites exhibited a better overall stability than their Cl–Br analogues due to their low energy phase equilibrium compounds and lower charge per unit volume leading to lower electrostatic repulsions in the structure. Furthermore, interface reaction energy calculations show that Cl–I mixed compounds have a slight edge in terms of compatibility with commercial electrodes. AIMD simulations showed that despite having different halogens altogether, both Cl–I and Cl–Br mixed argyrodites exhibited similar ionic conductivity, if the composition ratios of Cl/Br and Cl/I are the same. Lastly, AIMD simulations were conducted to understand the effect of varying I/Cl ratios on ionic conductivity for Li5.5PS4.5Cl1.5−xIx systems. The maximum ionic conductivity of 23.5 mS cm−1 was achieved for x = 0.75, due to this composition having the maximum average Li Voronoi polyhedron volume, indicating wide migration channels. To this end, these results may be used to complement experimental work, to provide a sense of direction to researchers working on screening different formulations of these mixed halide rich Cl–I argyrodites.
2. Computational details
2.1 Density functional theory (DFT) calculations
All the calculations were carried out within the DFT framework, implemented in Vienna Ab Initio Simulation Package (VASP).21 The projector augmented-wave pseudopotentials were used to describe the interaction between ions and electrons, and the exchange–correlation effects were treated using the Perdew–Burke–Ernzerhof (PBE) functional under the generalized gradient approximation (GGA).22 The initial structure for Li6PS5Cl, used as the basis of this work was sourced from the ICSD (collection code 418490).23 Stable structure for excess Li6PS5ClXy (X = Cl, Br, I; 0 < y < 0.5) was determined by halogen substitution of 4d sulfur sites, and rigorous enumeration using pymatgen.24 Structural optimization for all the systems was performed using Monkhorst–Pack grid k-points of 4 × 4 × 4 and an energy cut-off of 520 eV. All the optimized 1 × 1 × 1 argyrodite structures had a lattice parameter ∼10 Å, which is a moderate system size for AIMD calculations.252.2 Ab initio molecular dynamics (AIMD) simulations
AIMD calculations were then performed with a time-step of 2 fs. For each species, the structure underwent five different AIMD calculations, where the diffusivity would be determined at four different temperatures, in intervals of 100, from 600 K to 1000 K. The simulations were run for a total of 110 ps using NVT ensemble with a single Γ-center k-point grid, with the initial. The lithium-ion diffusivity in the structures was determined by computing the total mean squared displacement of lithium ion during AIMD simulations over 100 ps, after initially equilibrating the system for 10 ps. The mean square displacement (MSD) over time was used to calculate the diffusion coefficient (D) as shown in eqn (1). | (1) |
 | (2) |
2.3 Electrode interface compatibility
To understand possible degradation causing side-reactions at the interface with common cathode materials, a pseudo binary model was employed. To assess the phase equilibria, the calculation of the energy minimum was performed using the reaction energy (ΔED). The determination of ΔED involved comparing the energy of relevant phases within their compositional space. By utilizing the grand potential phase diagram, the phase equilibria Ceq (C, μM) of a specific phase with composition C in equilibrium with the chemical potential μM of element M can be identified. The reaction energy is determined using eqn (3). | (3) |
3. Results and discussion
3.1 Comparison of Br and I substituted structures
The lithium atoms in the initial structure referenced from ICSD, populated the 48 h sites. Stoichiometric Li6PS5Cl was obtained by iteratively removing Li atoms from the reference structure via rigorous enumeration and coulomb energy calculations using pymatgen.24 The resulting structure with an occupancy of 0.5 for 48 h sites is shown in Fig. 1(a).To keep the computational costs reasonable, 4a/4d antisite site disorder between Cl− and S2− ions have been avoided. Halogen substitutions were carried out on 4d sites containing S2− ions, with Cl, Br and I atoms with rigorous enumeration. These structures were optimized using DFT and the structures with minimum energy was considered for further analysis and AIMD simulations. It was found that both Br and I atoms preferred 4a sites over 4d sites, with the difference in energy being 0.14 eV per atom and 0.35 eV per atom for Br and I respectively. This site selectivity is due to the higher ionic radius of Br− and I− ions, as compared to S−2 and Cl− ions.
The variation in Energy above convex hull (Ehull) and formation energy with respect to halogen composition in shown in Fig. 1(b). Although perfectly stable compounds are on the convex hull, with energy above hull equal to 0, we consider a threshold value of 40 meV per atom for determining stable argyrodite configurations. This is because most of the compounds in ICSD were determined to have Ehull < 36, which means that some of these compounds could be stabilized via entropic effects.28,29 The obtained energy above hull (Ehull) value of 24 meV for Li6PS5Cl is in good agreement with the literature value of 21 meV.25 We observe that for pure Cl substitution case the Ehull increases to 28 meV for Li5.5PS4.5Cl1.5. However, we observe that addition of Br leads to a slight decrease in Ehull and addition of I results in a decrease in Ehull resulting in the most phase stable configurations, with Li5.5PS4.5ClI0.5 being the most phase stable compounds out of all with Ehull value of 18 meV. This implies that the addition of small amounts of larger halogens such as Br and especially I result in the formation of stable configurations. The phase decomposition products for all argyrodites were Li3PS4, Li2S and LiX (X = Cl, Br, I). Therefore, in this case Ehull = Ef(argyrodite) − (aEf(Li3PS4) + bEf(Li2S) + cEf(LiX)), where Ef denotes formation energy and a, b, c are constants. As documented in the Materials Project,30 the formation energy for LiX follows the order of LiCl > LiBr > LiI (ELiCl = −2.03 eV per atom, ELiBr = −1.83 eV per atom, ELiI = −1.39 eV per atom). Despite Li–Cl and Li–Br interactions being energetically more favorable for the argyrodites than Li–I interactions, as evidenced by slightly lower formation energies for I-substituted systems, the resulting Ehull value was still lower than their Cl and Br-substituted counterparts. To better understand this phenomenon, Bader charge calculations were conducted for all halides with a total halogen content of 1.5, to determine the average Bader charge and Bader charge per unit volume as documented in Table 1.
| Material | Q Cl (|e|) | Q Cl (|e| Å−3) | Q Br (|e|) | Q Br(|e| Å−3) | Q I (|e|) | Q I (|e| Å−3) |
|---|---|---|---|---|---|---|
| Li5.5PS4.5Cl1.5 | −0.898 | −0.0232 | — | — | — | — |
| Li5.5PS4.5ClBr0.5 | −0.897 | −0.0231 | −0.889 | −0.0202 | — | — |
| Li5.5PS4.5ClI0.5 | −0.897 | −0.0231 | — | — | −0.857 | −0.0163 |
An increase in halogen content would lead to an increase in electrostatic repulsion in the anionic sublattice which could affect the stability of the system. A lower Bader charge for Li5.5PS4.5ClI0.5 as compared to Li5.5PS4.5Cl1.5 and Li5.5PS4.5ClBr0.5 indicated a lower charge polarization in this system. This resulted in a lower charge per unit volume for Li5.5PS4.5ClI0.5 systems which caused the decrease of overall electrostatic repulsion in the anionic sublattice of these systems. As a result of this phenomenon, despite Li–Cl and Li–Br interactions being much stronger than Li–I interactions, the formation energy for I-substituted systems did not decrease significantly. This phenomenon combined with lower energy phase decomposition products, resulted in a higher phase stability of I-substituted systems as compared to Cl and Br-substituted systems.
Band structures were determined as shown in Fig. 1(c) and (d) for Li5.5PS4.5ClBr0.5 and Li5.5PS4.5ClI0.5, respectively. Band structures for all other compounds of study are shown in Fig. S1 in the ESI.† The bandgap for Li5.5PS4.5ClBr0.5 was 2.19 eV, which was lower than the value of 2.32 eV for Li5.5PS4.5ClI0.5. A larger bandgap is desirable for application in solid-state batteries, as it prevents leakage of electrons and increases dendrite formation resistance, resulting in an increased stability. Overall, all compounds exhibited poor electronic conductivity and were sufficiently insulating for application as solid-state electrolytes, with Li5.5PS4.5ClI0.5 being the best candidate amongst all of them.
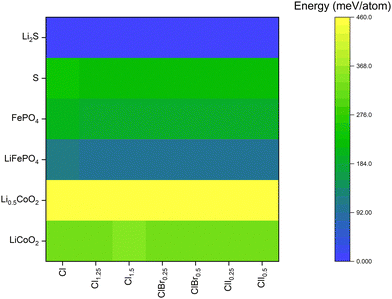
The electrode compatibility of all compounds with common cathodes like LiCoO2, LiFePO4 or sulfur (S) with their lithiated/delithiated configurations were studied to determine the practical applicability of these materials as solid electrolytes. All detailed results for have been provided in Tables S2–S4 (ESI†).
The reaction energy for each argyrodite composition with respective cathode materials has been shown in Fig. 2. In the case of LiCoO2 and Li0.5CoO2, the interfacial reaction energy is quite high, which makes argyrodites an unsuitable class of materials for this type of cathode. Furthermore, the delithiated variant Li0.5CoO2, showed much higher reaction energies, which implied that the cathode would become even more susceptible to degradation during the beginning of discharge and end of charge cycles. The general trend that was observed was that reaction energy increased with an increase in halogen content in the system. However, for all systems, I-substituted compounds performed slightly better as the reaction products included LiX (X = Cl, Br, I), and as discussed before, due to LiI having the lowest formation energy, the energy of reaction for I-substituted compounds was slightly lower.
In the case of LiFePO4/FePO4, the reaction energy was much lower, making LiFePO4 a better cathode material than LiCoO2. Similar trend of delithiated FePO4 having higher reaction energy than LiFePO4 was observed, but the overall reaction energies were still much lower than LiCoO2. In these systems, however, an increased halogen content showed a lower reaction energy. This could be due to the lower concentration of sulfur in these systems, due to Fe having a high affinity for S atoms. Amongst Cl, Br and I rich halogen compounds, I-substituted compounds had a better edge due to LiI formation with LiFePO4 and I2 formation with FePO4, both having lower formation energies than LiCl/LiBr.
For S cathodes, the reaction energies were higher than LiFePO4 but were in the range of FePO4. However, the lithiated variant Li2S showed no interfacial reaction with any argyrodite, thereby making argyrodites an excellent material of choice for batteries with S cathodes. In this case however, Cl-rich argyrodites performed slightly better than Br and I-substituted argyrodites due to the formation of low energy side products such as SCl, SBr and I2. However, both Br and I-substituted compounds exhibited similar performance with the S electrode.
Overall, the ground state calculations highlighted an increased phase stability and slightly better electrode compatibility for Cl–I mixed halide systems as compared to their Cl–Br analogues, for the same I/Cl and Br/Cl ratios. Furthermore, for a deeper comparison of both material classes, AIMD simulations were conducted to ascertain the Li+ ion transport characteristics in these systems.
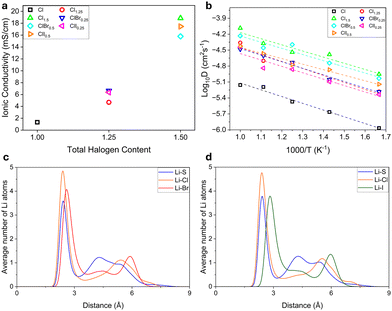
The log plot of diffusivity vs. temperature and ionic conductivities vs. total halogen mole fraction is shown in Fig. 3a and b, respectively. The AIMD simulations showed that the ionic conductivity of Li6PS5Cl, Li5.75PS4.75Cl1.25 and Li5.5PS4.5Cl1.5 was 1.33 mS cm−1, 4.69 mS cm−1, and 18.86 mS cm−1, respectively. We obtained a good fit for the experimental conductivity for Li6PS6Cl and Li5.75PS4.75Cl1.25 obtained by Adeli et al., which was 2.5 mS cm−1 and 4.2 mS cm−1 respectively.11 The predicted ionic conductivity for Li5.5PS4.5Cl1.5 was slightly higher than the literature value of 12 mS cm−1, however, both were in the order of 10 mS cm−1. There are multiple factors that could have resulted in this difference. Firstly, Li+ ionic conductivity was determined from diffusivity at high temperatures using Arrhenius law, which could cause the resulting variation. Secondly, in the experimental structure, Cl− ions have shown to occupy both 4a and 4c sites, whereas, we have only considered 4a sites, based on the reference ICSD structure. Moreover, 4a/4d site disorder has not been considered in this work due to simplicity. These slight structural variations could also result in discrepancies in ionic conductivity. Lastly, synthesis methods, macroscopic conditions and different time scales of measurements could also result in the resulting difference. For the mixed halide system, we compare the AIMD results with the available data for Li5.5PS4.5ClBr0.5. The calculated conductivity of 15.79 mS cm−1 was in good agreement with the experimental conductivity of 17 mS cm−1.
To further understand the effect of change in Li transport behavior by the addition of larger halogens in the structure, the radial distribution of Li atoms around anions at 600 K, was plotted for Li5.5PS4.5ClBr0.5 and Li5.5PS4.5ClI0.5, as shown in Fig. 3(c) and (d) respectively. Although average Li clustering was highest around Cl− ions, the peaks for Br− and I− showed significant clustering of Li around these ions showing high Li–Br and Li–I interactions. An increase in concentration of Br and I would further increase these interactions, resulting in sluggish diffusion kinetics.31 Li5.5PS4.5ClBr0.5 showed a slightly lower ionic conductivity than Li5.5PS4.5ClI0.5 as both systems had polarizable ions and the latter had a smaller RDF peak for I− ions which implied lesser Li–I interactions as compared to Li–Br interactions in Li5.5PS4.5ClBr0.5. However, it is to be noted that irrespective of the type of halogen, all argyrodites with total halogen content = 1.5 exhibited ionic conductivities in the order of 10 mS cm−1 which makes them perfectly suitable for application as solid electrolytes. Despite the small difference in ionic conductivities of Li5.5PS4.5ClBr0.5 and Li5.5PS4.5ClI0.5, it was not significant enough to claim that Cl–I mixed argyrodites outperformed Cl–Br systems in terms of ionic transport. However, due to Cl–Br and Cl–I systems showing very similar ionic conductivities for similar Br/Cl and I/Cl ratios, we can expect these systems to exhibit similar ionic conductivities despite having different halogens, if their element ratios with respect to Cl in the system and total halogen content were the same.
3.2 Effect of I/Cl ratios on ionic transport in Li5.5PS4.5Cl1.5−xIx
AIMD simulations were performed to gain a deeper understanding of ionic transport in Cl–I mixed argyrodites, while keeping the total halogen content constant, at 1.5. Fig. 4(a) and (c) show the variation of ionic conductivity and activation energy with x in Li5.5PS4.5Cl1.5−xIx and the calculated diffusivities for temperatures in the range of 600–1000 K. The ionic conductivity shows an initial increasing trend, with the ionic conductivity maximizing at 23.5 mS cm−1 for the I/Cl ratio (x = 0.75), followed by a decreasing trend. In the previous section, we showed that Cl–Br and Cl–I mixed halide argyrodites would show very similar transport properties for the same atomic ratios. Comparing the ionic conductivity trend obtained for these systems with the experimental results of Li et al. for the Li5.5PS4.5Cl1.5−xBrx system, show a similar increasing–decreasing trend, with the maximum ionic conductivity obtained at a near equiatomic composition (x = 0.7).18 Furthermore, the range of ionic conductivity values obtained through our AIMD simulations for these Cl–I systems is also in the range of bulk ionic conductivity measurements through PFG NMR for Cl–Br systems, with the maximum value being >20 mS cm−1.18 Although there is no literature on the exact I/Cl ratios chosen in this work, our AIMD results are in good agreement with the experimental results for Li5.4PS4.4Cl1.4I0.2 exhibiting an ionic conductivity of 16.43 mS cm−1 for a pellet-sintered sample and Li5.6PS4.6Cl1.3I0.1 with an ionic conductivity of 10.45 mS cm−1, by accurately capturing the range of ionic conductivities which is >10 mS cm−1. Increasing I content in the structure also resulted in a decrease followed by an increase in activation barrier for ionic transport, minimizing at 0.18 eV for the equiatomic composition.Voronoi method was used to determine the Li+ coordination polyhedron, to understand the structure–property relationship governing ionic conductivity in these systems. A larger polyhedron volume would indicate wider migration channels for the Li+ ion to move through the lattice, resulting in a lower activation barrier, boosting ionic conductivity. Fig. 4(b) shows the variation of the average volume of Li+ ion coordination polyhedron and ionic conductivity with varying I/Cl ratios. The average volume shows an increasing–decreasing trend, like ionic conductivity, maximizing for the equiatomic Cl–I composition at 9.39 Å3. The structures undergo lattice expansion due to the addition of I atoms into the structure, which is the main cause of this volume increase, which explains the increasing trend and widening of the migration channels. However, for x > 0.75, the addition of I into the structure resulted in a decrease in average polyhedron volume which is unexpected, as substitution of smaller Cl atoms with larger I atoms would result in lattice expansion. To understand this phenomenon, each Li+ coordination polyhedron was analyzed separately to highlight the effect of local disorder due to structural mismatch caused by mixed halide substitution. Fig. 4(d) shows a schematic of a Li+ coordination polyhedron in Li5.5PS4.5Cl1.25I0.25 and Fig. 4(e)–(g) show individual histograms capturing the overall polyhedron volume distribution for Li5.5PS4.5Cl1.5, Li5.5PS4.5Cl0.75I0.75 and Li5.5PS4.5I1.5. Histograms for other compositions are available in the Fig. S2 of ESI.† Due to an increased concentration of Li vacancies for this composition, instead of forming a standard 4-atom coordinated tetrahedral polyhedron, certain Li sites form a larger distorted 5-atom coordinated polyhedron. These polyhedra contribute the most to ionic conductivity due to having much larger volumes and skewed the results shown in Fig. 4b, causing an increasing-decreasing trend. Till x = 0.75, addition of I resulted in overall increase in polyhedron volumes, including the 5-atom coordinated one, as marked in Fig. 4d, however a further increase in I concentration resulted in reversion of some of the 5-atom coordinated polyhedra back to a 4-atom coordinated configuration, significantly reducing the width of migration channel, thereby lowering the average Li+ polyhedron volume. For x = 1.5, all the 5-atom coordinated polyhedra reverted back to 4 atom coordination, resulting in the lowest ionic conductivity out of all the systems, at 4.3 mS cm−1.
Overall, for Cl–I systems, these results indicate a strong dependency of ionic conductivity on local coordination environments of Li+ ions, where a faster conduction is facilitated by wider migration channels and the presence of 5-atom coordinated Li+ ions. Li et al. discussed the role of site configurational entropy for the increasing-decreasing trend observed in the case of Li5.5PS4.5Cl1.5−xBrx systems, as entropy maximizes for a near equiatomic composition. However, that discussion is beyond the scope of this work, as anti-site disorder was avoided for simplification of systems in this study, which contributes significantly to site configurational entropy.
Conclusions
In this study, we investigated the transport properties of mixed halide argyrodites, with a focus on Cl–I halide rich compositions through first-principles calculations. The results revealed that Cl–I systems offer better phase stability, and have a slightly better compatibility with commercial electrodes, than Cl–Br systems. Additionally, Cl–I and Cl–Br systems exhibited similar ionic conductivities at room temperature, if the I/Cl and Br/Cl ratios for the compositions were the same. AIMD simulations for different I/Cl ratios revealed a novel composition Li5.5PS4.5Cl0.75I0.75 which exhibited an ionic conductivity of 23.5 mS cm−1 at 300 K with a low activation barrier of 0.18 eV. Overall, the ionic conductivity for the Li5.5PS4.5Cl1.5−xIx (x = [0–1.5]) systems showed an increasing–decreasing trend. The major reason behind this was the widening of migration channels due to substitution of smaller Cl atoms with larger I atoms and reversion of 5-atom coordinated Li+ coordination polyhedra to 4-atom coordinated configurations.Author contributions
Adwitiya Rao performed DFT and AIMD calculations, conducted the analysis, and prepared the manuscript. Jacob Rempel contributed to DFT and AIMD calculations. Ming Jiang, Parvin Adeli, Chae-Ho Yim, and Mohamed Houache contributed to the discussion and development of this work. Yaser Abu-Lebdeh and Chandra Veer Singh initiated this research project and contributed to the ideation, discussion, and funding of this work.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research project has been funded by the University of Toronto and the National Research Council of Canada (RGPIN-2018-04642). The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Innovation, and the Ontario Research Fund. This work was supported in part by a contribution from the National Research Council of Canada (NRC) through the NRC/University of Toronto Collaboration Centre in Green Energy Materials (CC-GEM).References
- J. Speirs, M. Contestabile, Y. Houari and R. Gross, Renewable Sustainable Energy Rev., 2014, 35, 183–193 CrossRef.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- J. C. Bachman, S. Muy, A. Grimaud, H.-H. Chang, N. Pour, S. F. Lux, O. Paschos, F. Maglia, S. Lupart, P. Lamp, L. Giordano and Y. Shao-Horn, Chem. Rev., 2016, 116, 140–162 CrossRef CAS PubMed.
- X. Yang, X. Gao, C. Zhao, Q. Sun, Y. Zhao, K. Adair, J. Luo, X. Lin, J. Liang, H. Huang, L. Zhang, S. Lu, R. Li and X. Sun, Energy Storage Mater., 2020, 27, 198–204 CrossRef.
- J. Wu, S. Liu, F. Han, X. Yao and C. Wang, Adv. Mater., 2021, 33(6), 2000751 CrossRef CAS PubMed.
- Q. Jia, Z. Yao, J. Xiang, J. Shi, Y. Zhou, J. Huang, H. Zhang, X. Zhang, Y. Yang and J. Tu, Adv. Funct. Mater., 2025, 2426053 CrossRef.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- C. Yu, F. Zhao, J. Luo, L. Zhang and X. Sun, Nano Energy, 2021, 83, 105858 CrossRef CAS.
- R. P. Rao and S. Adams, Phys. Status Solidi A, 2011, 208, 1804–1807 CrossRef CAS.
- P. R. Rayavarapu, N. Sharma, V. K. Peterson and S. Adams, J. Solid State Electrochem., 2012, 16, 1807–1813 CrossRef CAS.
- P. Adeli, J. D. Bazak, K. H. Park, I. Kochetkov, A. Huq, G. R. Goward and L. F. Nazar, Angew. Chem., Int. Ed., 2019, 58, 8681–8686 CrossRef CAS PubMed.
- C. Yu, Y. Li, W. Li, K. R. Adair, F. Zhao, M. Willans, J. Liang, Y. Zhao, C. Wang, S. Deng, R. Li, H. Huang, S. Lu, T.-K. Sham, Y. Huang and X. Sun, Energy Storage Mater., 2020, 30, 238–249 CrossRef.
- Z. Zhang, C. Yu, R. Xu, L. Peng, H. Ren, J. Zhang, L. Zhang, S. Cheng and J. Xie, Scr. Mater., 2022, 210, 114475 CrossRef CAS.
- M. Jiang, Z.-W. Chen, A. Rao, L.-X. Chen, X.-T. Zu and C. V. Singh, J. Mater. Chem. C, 2022, 10, 18294–18302 RSC.
- L. Zhou, N. Minafra, W. G. Zeier and L. F. Nazar, Acc. Chem. Res., 2021, 54, 2717–2728 CrossRef CAS PubMed.
- H. Schneider, H. Du, T. Kelley, K. Leitner, J. ter Maat, C. Scordilis-Kelley, R. Sanchez-Carrera, I. Kovalev, A. Mudalige, J. Kulisch, M. M. Safont-Sempere, P. Hartmann, T. Weiβ, L. Schneider and B. Hinrichsen, J. Power Sources, 2017, 366, 151–160 CrossRef CAS.
- S. V. Patel, S. Banerjee, H. Liu, P. Wang, P.-H. Chien, X. Feng, J. Liu, S. P. Ong and Y.-Y. Hu, Chem. Mater., 2021, 33, 1435–1443 CrossRef CAS.
- S. Li, J. Lin, M. Schaller, S. Indris, X. Zhang, T. Brezesinski, C. Nan, S. Wang and F. Strauss, Angew. Chem., Int. Ed., 2023, 62(50), e202314155 CrossRef CAS PubMed.
- H. Yan, R. Song, R. Xu, S. Li, Q. Lin, X. Yan, Z. Wang, C. Yu and L. Zhang, J. Energy Chem., 2023, 86, 499–509 CrossRef CAS.
- G. Liu, J. Zhang, J. Yang, J. Chen, X. Xiao and X. Yao, J. Energy Chem., 2025, 100, 50–58 CrossRef CAS.
- J. Hafner, J. Comput. Chem., 2008, 29, 2044–2078 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- D. Zagorac, H. Müller, S. Ruehl, J. Zagorac and S. Rehme, J. Appl. Crystallogr., 2019, 52, 918–925 CrossRef CAS PubMed.
- S. P. Ong, W. D. Richards, A. Jain, G. Hautier, M. Kocher, S. Cholia, D. Gunter, V. L. Chevrier, K. A. Persson and G. Ceder, Comput. Mater. Sci., 2013, 68, 314–319 CrossRef CAS.
- Z. Deng, Z. Zhu, I.-H. Chu and S. P. Ong, Chem. Mater., 2017, 29, 281–288 CrossRef CAS.
- Y. Mo, S. P. Ong and G. Ceder, Chem. Mater., 2012, 24, 15–17 CrossRef CAS.
- S. P. Ong, L. Wang, B. Kang and G. Ceder, Chem. Mater., 2008, 20, 1798–1807 CrossRef CAS.
- W. Li, R. Jacobs and D. Morgan, Comput. Mater. Sci., 2018, 150, 454–463 CrossRef CAS.
- O. I. Malyi, K. V. Sopiha and C. Persson, ACS Appl. Mater. Interfaces, 2019, 11, 24876–24884 CrossRef CAS PubMed.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- M. A. Kraft, S. P. Culver, M. Calderon, F. Böcher, T. Krauskopf, A. Senyshyn, C. Dietrich, A. Zevalkink, J. Janek and W. G. Zeier, J. Am. Chem. Soc., 2017, 139, 10909–10918 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc00529a |
| This journal is © The Royal Society of Chemistry 2025 |