Open Access Article
Open Access ArticleA low-power filamentary memristor crossbar array enabled via cubic α-phase stabilized mixed-cation lead halide perovskites†
In Hyuk
Im‡
a,
Ji Hyun
Baek‡
a,
Do Yeon
Heo‡
bc,
Sung Hyuk
Park
a,
Sohyeon
Park
a,
Seung Ju
Kim
ad,
Jae Young
Kim
a,
Youngmin
Kim
a,
Yoon Jung
Lee
ae,
Kyung Ju
Kwak
a,
Hyeon Ji
Lee
a,
Soo Young
Kim
 *b and
Ho Won
Jang
*b and
Ho Won
Jang
 *af
*af
aDepartment of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul, 08826, Republic of Korea. E-mail: hwjang@snu.ac.kr
bDepartment of Materials Science and Engineering, Korea University, Seoul, 02841, Republic of Korea. E-mail: sooyoungkim@korea.ac.kr
cHydrogen Ion Materials Group, National Institute for Materials Science, Tsukuba, 305-0044, Japan
dDepartment of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA 90089, USA
eSchool of Electrical Engineering, Kookmin University, Seoul 02707, South Korea
fAdvanced Institute of Convergence Technology, Seoul National University, Suwon 16229, Republic of Korea
First published on 10th April 2025
Abstract
Halide perovskite (HP)-based resistive switching memory has demonstrated significant advantages, particularly in terms of rapid switching speed and low power consumption. To address the thermal instability associated with CH3NH3+ ions, which have been mainly focused on in related fields, we developed resistive switching memory utilizing distorted HPs (FA0.8Cs0.2PbI3), incorporating thermally stable A-site cations. Moreover, we improved nonvolatility by introducing SCN anions to stabilize the cubic α-phase. Unlike the pristine FACsPbI3 (FCPI) device untreated by the SCN−-based additive, which features an α-phase/δ-phase heterostructure and exhibits unstable switching characteristics, the FCPI-SCN device demonstrates a stable cubic α-phase and extended retention behavior. After α-phase stabilization, trap-controlled emission becomes dominant in the FCPI-SCN device, confirming the stable filament formation through the HP layer. This strategic approach effectively suppresses the formation of heterostructures, reducing planar defects that serve as preferential sites for the multiple thin filament formation, thereby promoting stable filaments within the matrix. While pristine FCPI exhibits an unstable retention time of 40 s, FCPI-SCN demonstrates significantly improved performance including low operating voltages of 0.248 V/−0.116 V, a prolonged retention time of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, and endurance over 1200 cycles. Additionally, we fabricated a 3 × 3 crossbar array with FCPI-SCN filamentary memristor devices. The crossbar array efficiently encodes and preserves letter-based bitmaps, thus showcasing its practical utility for reliable nonvolatile memory applications.
000 s, and endurance over 1200 cycles. Additionally, we fabricated a 3 × 3 crossbar array with FCPI-SCN filamentary memristor devices. The crossbar array efficiently encodes and preserves letter-based bitmaps, thus showcasing its practical utility for reliable nonvolatile memory applications.
Introduction
Conventional memory technologies have confronted a significant challenge known as the ‘memory wall,’ an inherent limitation in the von Neumann structure.1,2 This bottleneck is exacerbated by the exponential growth of data, which leads to increased latency across the entire von Neumann-based computing system. Consequently, it becomes imperative to establish a bridge between these hierarchical components.3–7 In the last decade, there has been a growing demand for novel emerging memory technologies, commonly referred to as memristors. This is due to their advantages, including ease of cell integration, enhanced energy efficiency, and outstanding performance in both memory and neuromorphic applications.8–10 While the exploration of resistive switching behaviors has predominantly revolved around various oxide-based materials,11–14 there are still limitations related to mechanical flexibility and reduced binding energy, which are critical for biomimetics and low-power consumption. These underscore the pressing need for the discovery of more advanced materials.Inorganic–organic halide perovskites (HPs) have garnered significant attention owing to their notable advantages, encompassing a tunable bandgap, controllable majority carriers, rapid ion migration, and remarkable flexibility.15–18 Of particular significance, HPs have demonstrated substantial potential in resistive random-access memory applications, demonstrating ultralow operating voltages and high on–off ratios which are based on their soft and flexible bonding matrix. Our previous works have reported on HP-based electrochemical metallization memory and artificial synaptic devices operating at ultralow voltage.19–23 This characteristic stems from the low activation energy barrier for the movement of vacancies and ions in the halide matrix. Besides, previous studies have demonstrated that HPs composed of Ag exhibit resistive switching behavior by preformed conductive filaments, with low operating voltages and high on/off ratio.19 Ag atoms can readily move within the HP matrix under an electric field, contributing to the efficient performance of the devices.
Among the various HPs, CH3NH3PbI3 (MAPbI3) has been the subject of extensive research.24,25 However, the thermal instability of the methylammonium (CH3NH3+, MA+) cation, which tends to decompose into CH3I and NH3+, poses a significant challenge.26,27 To address this issue, a mixed-cation approach involving formamidinium (HC(NH2)2+, FA+) and Cs+ cations has been pursued.28,29 The FA cation has demonstrated promise due to its enhanced thermal stability,30,31 remaining intact within the temperature range of 100 to 175 °C.32 Furthermore, the inorganic Cs cation provides enhanced long-term stability compared to its organic counterparts.26 By integrating a mixed-cation composition (FA1−xCsxPbI3), as illustrated in Fig. 1(a), endeavors have been made to engineer a distorted perovskite structure, which has tilted octahedra, thereby optimizing the Goldschmidt tolerance factor.33 Despite these advancements, FA-based HPs have faced challenges concerning the susceptibility of the black α-phase perovskites and their transition to the yellow δ-phase non-perovskites, primarily located at the grain boundary and characterized by a high density of defects.34,35 Notably, it has been established that the formation of the conductive filament is intrinsically associated with planar defective sites, such as grain boundaries and interfaces, and their modulation is crucial for achieving reliable switching operations.36,37 Interface engineering plays a critical role in controlling the resistive switching behaviour of halide perovskite-based memristors.38–40 Previous studies have highlighted the significant influence of the interface on the filamentary switching mechanism. In this respect, the heterostructure induced by phase instability may lead to irreversible switching behavior. Furthermore, the α-phase exhibits a one-dimensional (1D)-like channel, where corner-shared PbI6 octahedra align linearly, facilitating the migration of constituent ions.41–43 This structural attribute is essential for generating the I–V hysteresis behavior in HP-based resistive switching devices.
This study delves into the stabilization of the cubic α-phase in HP-based filamentary memristor devices through the introduction of thiocyanate (SCN) anions. The SCN anion, a pseudohalide ion, possesses an ionic radius (2.15–2.20 Å) similar to that of the iodine (I−) ion. Incorporation of SCN anions into HPs has shown an enhancement in both crystallinity and electronic properties.44–48 Our focus was on the FA0.8Cs0.2PbI3 distorted perovskites, known for their stable composition conducive to the cubic phase.49 In this endeavor, we strategically employed SCN treatment during the film deposition process. In the absence of SCN−-based additive treatment, the pristine FACsPbI3 (FCPI) film exhibited a mixture of α and δ-phases, resulting in α/δ-phase heterostructures. The electrons injected mainly into these α/δ-phase heterostructures may lead to the electrochemical reduction of migrating Ag cations within the HPs. Multiple thin filaments are mainly generated along the heterostructures, possessing enlarged effective surface curvature for each filament. The tendency to minimize surface energy drives the surface atom diffusion of the filaments. Therefore, the geometry of the filaments is critical in surface diffusion circumstances. The increased surface curvature of the thin filaments renders them vulnerable to surface atom diffusion. Consequently, fast surface diffusion of thin filaments leads to an unfavorable nonvolatile characteristic. Conversely, the incorporation of SCN anions contributed to enhanced stability of the black α-phase perovskite within the FCPI system, thereby promoting the formation of filaments through the halide matrix. In the SCN−-mediated FACsPbI3 (FCPI-SCN), space-charge limited conduction (SCLC) was observed in the high-resistance state (HRS), unlike the pristine case. This bulk-limited conduction suggests that the HRS current is dependent on trap-controlled emission by the charged ions that are distributed in cubic α-phase HPs. Subsequently, electrochemically dissolved Ag cations interact with carriers transferred between charged ions to recrystallize, thereby enabling the transition to the low-resistance state (LRS). This indicates that stable Ag filament formation occurs through the α-phase stabilized FCPI-SCN layer, resulting in the reduction of filament surface curvature and slow surface atom diffusion. The FCPI-SCN device showcased enhanced device performances, including low operating voltages, fast switching speed, extended retention time, improved endurance, and a heightened multilevel capability compared to its pristine counterpart. Additionally, a 3 × 3 crossbar array utilizing FCPI-SCN was constructed and evaluated for its capability to store and retrieve bitmap images representing the acronym of our laboratory: ‘O,’ ‘N,’ ‘N,’ and ‘L.’ The crossbar array reliably retained all bitmaps. This work elucidates the correlation between filamentary switching mechanisms and the matrix environment. Furthermore, the enhancement of nonvolatile switching characteristics in the FCPI-based low-powered switching device underscores the potential of halide perovskite-based conductive bridge random access memory (CBRAM) for storage-class memory applications.
Experimental
Materials preparation
Formamidinium iodide ((HC(NH2)2I, 99.99%) was purchased from GreatCellSolar Materials. Cesium iodide (CsI, 99.9%), ammonium thiocyanate (NH4SCN, 99.99%), N,N-dimethyl-formamide (DMF, 99.8%, anhydrous), and dimethyl sulfoxide (DMSO, anhydrous, ≥99.9%) were purchased from Sigma-Aldrich. Lead iodide (PbI2, 99.99%) and PMMA were purchased from Tokyo Chemical Industry (TCI).Preparation of FCPI HPs
The FCPI precursor solution was prepared by 0.8 M FAI, 0.2 M CsI, and 1 M PbI2 powders in 1 mL mixed solvent of DMSO and DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). To prepare the FCPI-SCN film, ammonium thiocyanate (NH4SCN) was added to the FCPI precursor solution at a concentration of 0.2 M, corresponding to 0.0002 mol of NH4SCN per 1 mL of precursor solution.
3 v/v). To prepare the FCPI-SCN film, ammonium thiocyanate (NH4SCN) was added to the FCPI precursor solution at a concentration of 0.2 M, corresponding to 0.0002 mol of NH4SCN per 1 mL of precursor solution.
Device fabrication
Pt (100 nm)/Ti (20 nm) was deposited on SiO2//Si substrates for bottom electrodes (BEs) via an electron beam (E-beam) evaporation method. The Pt/Ti/SiO2/Si substrates were cleaned with acetone, isopropanol, and deionized water. Then, they were treated with UV ozone. The FCPI precursors were spin-coated onto the Pt/Ti/SiO2/Si substrates at 4000 rpm for 60 s, and toluene was dropped after 8 s while spin coating. The films were heated at 180 °C for 10 min. Then, poly(methyl methacrylate) (PMMA) in chlorobenzene with the concentration of 5 mg ml−1 was spin-coated at 4000 rpm for 30 s. The films were heated at 180 °C for 3 min. Finally, 50 × 50 μm2 Ag top electrodes (TEs) were deposited onto the pristine FCPI and FCPI-SCN films via E-beam evaporation with a shadow mask.For comparison, Bi film of 100 nm thickness was deposited on an electropolished Cu foil by an E-beam evaporator at the deposition rate of 0.3 Å s−1. The base pressure of the vacuum chamber was 1.5 × 10−6 Torr.
Fabrication of a crossbar array
Three Pt bottom line electrodes were patterned via photolithography (NanoSystem Solutions Inc. DL-1000 HP system). A Pt layer was deposited via E-beam evaporation on a patterned resist and a lift-off process was done. FCPI precursors were spin-coated onto the substrate with Pt bottom line electrodes. Then, a PMMA layer was spin-coated. Finally, three Ag line electrodes were deposited by E-beam evaporation with a line-patterned shadow mask of 100 μm width.Material characterization
The top-view and cross-sectional view images of the FCPI films were obtained using a field-emission scanning electron microscope (SEM) (ZEISS, MERLIN Compact) with an in-lens secondary electron detector which was operated at an accelerating voltage of 3 kV. XRD patterns were obtained with an X-ray diffractometer (BRUKER MILLER Co., D8-Advance) at room temperature with Cu-Kα radiation (λ = 1.54056 Å). X-ray diffraction (XRD) measurements were conducted with a step size of 0.02° and a scanning speed of 5° min−1. Topological images and current mapping of the FCPI films were obtained using atomic force microscopy (AFM) (Park System, NX-10). A fluorescence spectrometer (FlouTime 300; PicoQuant) with an excitation laser of 398![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm was used to measure photoluminescence (PL) and time-resolved photoluminescence (TR-PL) spectra in the range from 600 to 900 nm.
nm was used to measure photoluminescence (PL) and time-resolved photoluminescence (TR-PL) spectra in the range from 600 to 900 nm.
Electrochemical characterization
Agilent 4156C and 4200A-SCS semiconductor analyzers and a Tektronix AFG 3021C were used to measure the electrical properties.Statistical analysis
All data were processed using Microsoft Excel (Microsoft), OriginPro 9.0 (OriginLab Co., Northampton, MA, USA), and MATLAB R2021b (MathWorks). All C-AFM images were analyzed using XEI software (Park System).Results and discussion
FACsPbI3-based resistive switching devices with thiocyanate treatment
The FCPI thin film was synthesized through a low-temperature solution process. Three precursors (FAI, CsI, and PbI2) were dissolved in a mixed solvent of DMF and DMSO. For FCPI-SCN, the precursors were dissolved alongside the SCN−-based additive. Subsequently, the resulting solution was spin-coated onto the Pt BE substrate. The Goldschmidt tolerance factor (t), defined by eqn (1), where rA, rB, and rX are the ionic radius of A, B, and X ions respectively, is a conventional parameter used to assess the ABX3 perovskite structures. | (1) |
Cubic perovskite structures are typically stable when 0.9 < t < 1.0. Given the ionic radii of FA, Pb, and I as 253 pm, 119 pm, and 220 pm respectively,50 FAPbI3 has an ideal t value of 0.99. However, the flat shape of the FA cation leads to a larger effective ionic radius, resulting in a higher effective t value.33 Therefore, the introduction of smaller Cs cations (167 pm) promotes the t value to reach the cubic perovskites range. Consequently, FAPbI3 exhibits an imperfect cubic structure, as shown in Fig. S1 (ESI†). By mixing FA with the smaller Cs cation at a molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
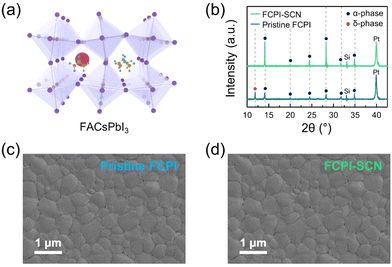
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, FA0.8Cs0.2PbI3 achieves a more stable cubic structure and high crystallinity, attributed to the decrease in the t value. However, as the Cs content increases, the orthorhombic γ-phase becomes more prevalent due to the decrease in t value. Fig. S2 (ESI†) represents the XRD measurements of FCPI HPs that included various SCN−-based additives. Recent research has demonstrated that alkali ions can impede ion migration within HPs and eliminate I–V hysteresis behavior.51,52 Thus, additives containing alkali ions were not considered. Instead, the NH4SCN was chosen due to its capability to stabilize the α-phase crystal structure and its facile removal during deposition process.53 The XRD patterns of the pristine FCPI and FCPI-SCN films are depicted in Fig. 1(b). The diffraction peaks observed at 2θ = 14.1°, 20.0°, 24.5°, 28.3°, 31.7°, 34.9°, and 39.9° are identified as the (100), (110), (111), (200), (210), (211), and (220) planes of the α-phase crystal structure, respectively. Additionally, a peak at 2θ = 11.9° corresponds to the (100) plane of the δ-phase crystal. These results indicate the successful stabilization of the cubic α-phase in HPs through the introduction of SCN anions. The increased peak intensity is attributed to the improved crystallinity observed in the FCPI-SCN film. SEM images of the FCPI and FCPI-SCN films are presented in Fig. 1(c) and (d), respectively. Furthermore, Fig. S3 (ESI†) shows the AFM images for both films. The root mean square (RMS) roughness improved from 27.7 nm in the pristine FCPI film to 23.8 nm in the FCPI-SCN film. This improvement in roughness, in conjunction with the XRD results, signifies enhanced crystallinity and film morphology.
2, FA0.8Cs0.2PbI3 achieves a more stable cubic structure and high crystallinity, attributed to the decrease in the t value. However, as the Cs content increases, the orthorhombic γ-phase becomes more prevalent due to the decrease in t value. Fig. S2 (ESI†) represents the XRD measurements of FCPI HPs that included various SCN−-based additives. Recent research has demonstrated that alkali ions can impede ion migration within HPs and eliminate I–V hysteresis behavior.51,52 Thus, additives containing alkali ions were not considered. Instead, the NH4SCN was chosen due to its capability to stabilize the α-phase crystal structure and its facile removal during deposition process.53 The XRD patterns of the pristine FCPI and FCPI-SCN films are depicted in Fig. 1(b). The diffraction peaks observed at 2θ = 14.1°, 20.0°, 24.5°, 28.3°, 31.7°, 34.9°, and 39.9° are identified as the (100), (110), (111), (200), (210), (211), and (220) planes of the α-phase crystal structure, respectively. Additionally, a peak at 2θ = 11.9° corresponds to the (100) plane of the δ-phase crystal. These results indicate the successful stabilization of the cubic α-phase in HPs through the introduction of SCN anions. The increased peak intensity is attributed to the improved crystallinity observed in the FCPI-SCN film. SEM images of the FCPI and FCPI-SCN films are presented in Fig. 1(c) and (d), respectively. Furthermore, Fig. S3 (ESI†) shows the AFM images for both films. The root mean square (RMS) roughness improved from 27.7 nm in the pristine FCPI film to 23.8 nm in the FCPI-SCN film. This improvement in roughness, in conjunction with the XRD results, signifies enhanced crystallinity and film morphology.
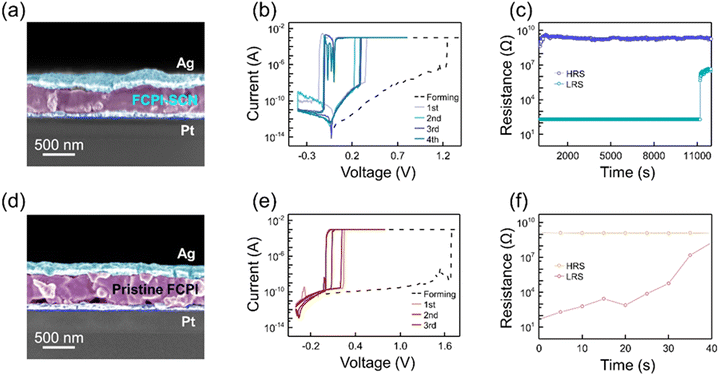
To assess its electronic characteristics, the FCPI films were synthesized on a prefabricated Pt/Ti/SiO2/Si substrate. Subsequently, a PMMA layer was spin-coated, and Ag TEs were deposited through a 50 μm × 50 μm square patterned metal mask via E-beam evaporation, resulting in the Ag TE/PMMA/HPs/Pt BE vertical-stack structure. Fig. S4 (ESI†) shows the optical image of devices fabricated in a sandwich structure with varying electrode sizes of 400 μm2, 200 μm2, 100 μm2, and 50 μm2. The SEM images in Fig. 2(a) provide the vertical structure of the FCPI-SCN device, with a FCPI thickness of 400 nm. Fig. 2(b) presents the I–V behavior of the FCPI-SCN device, with the compliance current (Icc) set to 10−3 A. A formation process was required at +1.24 V to initially switch from a high resistance state (HRS) to a low resistance state (LRS). The resistance was then switched back to HRS by applying an electric field with negative polarity. Subsequently, DC sweeps were conducted in the sequence 0 V → 0.8 V → −0.8 V → 0 V. During these sweeps, the SET voltage (VSET), where HRS is converted to LRS, was observed around +0.3 V. The RESET voltage (VRESET), where the resistance returns back to HRS, was exhibited around −0.1 V. As shown in Fig. 2(c), the HRS and LRS retention properties of both devices were evaluated by applying a pulse train of read voltage (+0.02 V). The FCPI-SCN device exhibited an impressive retention time of up to 1.1 × 104 s. The resistive switching mechanism of our devices is based on electrochemical metallization, typically observed in devices with electrochemically active Ag electrodes.54 The electrochemical metallization mechanism involves the following reaction steps. (i) Anodic dissolution: Ag → Ag+ + e−, (ii) migration of Ag cations across the solid–electrolyte, and (iii) reduction and crystallization: Ag+ + e− → Ag. Fig. S5a and b (ESI†) showcase the I–V results of Pt/PMMA/FCPI-SCN/Pt and Pt/PMMA/pristine FCPI/Pt devices, respectively, indicating that Ag metal electrodes show the important resistive switching phenomenon in the FCPI-SCN devices, as no resistive switching response was observed when an inert metal TE was used instead of the Ag TE. Fig. S6 (ESI†) illustrates the electrode size dependence of the VSET, VRESET, HRS current, and LRS current in the FCPI-SCN devices with varying electrode sizes, ranging from (50 μm)2 to (400 μm)2. VSET and VRESET remained consistent regardless of the electrode size. These findings indicate that the resistance change in our devices is driven by filamentary switching, which is independent of the electrode area.55–57 The vertical stack of Ag TE/PMMA/pristine FCPI/Pt is shown in Fig. 2(d). In contrast to the prior case, it displayed different aspects of VSET around +0.25 V and VRESET near 0 V (Fig. 2(e)). As displayed in Fig. 2(f), the pristine FCPI device demonstrated an unstable retention time of 40 s. The pristine FCPI device exhibited unstable switching performance similar to volatile threshold switching devices, which are known for their temporary filament characteristics. In contrast, the FCPI-SCN device demonstrated stable nonvolatile switching performance. These results underscore the significantly enhanced nonvolatile characteristics of the SCN−-mediated case compared to the pristine case.
Detailed observation of the switching behavior of FACsPbI3 devices
Fig. 3(a) presents the replotted I–V characteristics of the FCPI-SCN device on a double-logarithm scale. Prior to the SET process, current conduction in HRS is governed by classical trap-controlled SCLC, which comprises three distinct mechanisms:58–60 the ohmic conduction region (I ∝ V), the trap-unfilled SCLC region adhering to the Mott–Gurney law (I ∝ V2), and the trap-filled SCLC region characterized by a steep current increase. After the SET process, ohmic conduction, which is identified by a linear slope with a gradient of approximately 1 on a double-logarithmic scale, becomes predominant. Analysis of three portions in the HRS is depicted in Fig. 3(b)–(d). Initially, upon the application of the electric field, ohmic conduction, indicated by a linear slope with a gradient of approximately 1 on a double-logarithmic scale, prevails in the Ag/PMMA/FCPI-SCN/Pt device (Fig. 3(b)). Subsequently, the current conduction transitions to the trap-unfilled SCLC mechanism, demonstrated by a slope of 2 on a double-logarithmic scale (Fig. 3(c)). This shift is attributed to the low activation energy for the electrically induced generation of halide vacancies in cubic α-phase halide perovskites.33 As shown in Fig. 3(d), at higher electric fields, the current is governed by the trap-filled space-charge limited conduction (SCLC), characterized by a steep slope of 3.4 on a double-logarithmic scale. The trapping centers for electron conduction are likely attributed to migrated Ag cations. Upon reaching VSET, the reduction of Ag and electrocrystallization of the filament occur, making the overall resistance dependent on the contact between the electrode and the metallic filaments.These results suggest that in the FCPI-SCN device, the initial stage of current conduction is influenced by the ohmic interface between the electrode and cubic α-phase FCPI-SCN. As the external field increases, halide vacancies are released due to the low formation energy of Frenkel defects of iodine (V+I: positive iodine vacancy/I−I: negative interstitial iodine) in the cubic α-phase HPs.43,61,62 Consequently, these charged sites induce SCLC conduction in the FCPI-SCN device.63 At the higher field region, traps are filled by more injected carriers, leading to trap-filled SCLC. When the field reaches VSET, the electrochemically dissolved Ag cations react with carriers transferred through cubic α-phase HPs by SCLC. Consequently, the crystallization of Ag occurs and metal filaments are formed through the cubic α-phase HP film.
In contrast, the pristine FCPI device exhibits a different current conduction mechanism. Fig. S7a (ESI†) shows replotted I–V graphs of the pristine FCPI device in a double-logarithm scale. Fig. S7b and c (ESI†) represent replotted graphs for HRS and LRS of the pristine FCPI device, respectively. As shown in Fig. S7b (ESI†), the intrinsic conduction in the HRS is primarily governed by Schottky emission (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ∝ V1/2) and is observed prior to the SET process. It is attributed to the formation of the hexagonal δ-phase that possesses a higher bandgap compared to the cubic α-phase in the pristine FCPI device.64 The hexagonal δ-phase is mainly observed at the grain boundaries and interfaces cubic α-phase HPs.65,66 Before the filament is generated, the current is primarily influenced by the interfaces between the hexagonal δ-phase and electrodes. At the VSET value, the conduction mechanism in LRS is dominated by the ohmic conduction due to the formation of Ag filaments, as represented in Fig. S7c (ESI†).
I ∝ V1/2) and is observed prior to the SET process. It is attributed to the formation of the hexagonal δ-phase that possesses a higher bandgap compared to the cubic α-phase in the pristine FCPI device.64 The hexagonal δ-phase is mainly observed at the grain boundaries and interfaces cubic α-phase HPs.65,66 Before the filament is generated, the current is primarily influenced by the interfaces between the hexagonal δ-phase and electrodes. At the VSET value, the conduction mechanism in LRS is dominated by the ohmic conduction due to the formation of Ag filaments, as represented in Fig. S7c (ESI†).
Unlike the FCPI-SCN case, current conduction across the entire HRS range of the pristine FCPI device is governed by the Schottky emission, which arises from the interfaces between the hexagonal δ-phase and the electrodes. The electrochemical reaction of dissolved Ag is likely predominantly influenced by the carriers transferred through the hexagonal δ-phase. The TR-PL spectra of the pristine FCPI and FCPI-SCN films are shown in Fig. S8 (ESI†). The FCPI-SCN film exhibits longer carrier lifetime values (τ2) of 26.2 ns, compared to the pristine FCPI film displaying 14.1 ns. The values are fitted by a bi-exponential function for the PL decay kinetics: A1·exp(–t/τ1) + A2·exp(–t/τ2).67 Decreased carrier lifetime arises from coexistence with the hexagonal δ-phases in the FCPI film, which are known for being photo-inactive and defective non-perovskite phases.45,68,69 The planar defects indeed play a pivotal role as preferential pathways for generating conductive filaments due to variations in local electrical field strength.36,37,70 In the pristine FCPI device, electrons injected mainly into these α/δ-phase heterostructures participate in the electrochemical reduction of dissolved Ag cations. Hence, the heterointerfaces in the pristine FCPI facilitate the formation of numerous dendritic thin filaments within the HP layer.
Stable filament formation in SCN−-mediated FACsPbI3
The filamentary switching behavior of both the FCPI-SCN and pristine devices was visualized using conductive atomic force microscopy (C-AFM). Thin films were deposited on an Ag/Ti/SiO2/Si substrate and scanned with a biased tip while grounding the Ag BE. Fig. 4(a) and (b) illustrate the current mapping of the FCPI-SCN device during the initial 1 V scan and a subsequent 5 V scan, respectively. A distinct and localized current change is observed due to filamentary switching during the subsequent scan. The pristine FCPI device was scanned under identical conditions, as shown in Fig. 4(c) and (d). The filamentary regions in the pristine FCPI device were more dispersed, indicating that the conductive channels in the pristine FCPI were generated in a more distributed manner.Metal filaments in the solid–electrolyte are significantly influenced by the interfacial energy-related Gibbs–Thomson effect, which describes the structural evolution to minimize system energy, analogous to how a dropping cylinder of fluid splits into several spherical droplets.71,72 The driving force to minimize the surface energy triggers surface diffusion of the filaments, which can be described using eqn (2):
 | (2) |
As illustrated in Fig. 5(a), the distributed events of the filaments in the pristine FCPI device leads to the formation of multiple Ag thin filaments along heterointerfaces. It is hypothesized that filament formation in pristine FCPI primarily occurs at the heterointerfaces between the α- and δ-phases. Consequently, the large effective surface curvature (1/r) of the thin filaments accelerates their electrochemical corrosion due to a fast surface diffusion, ultimately resulting in detrimental nonvolatile characteristics. However, in the SCN-treated case, strong interaction between SCN− and Pb2+ ions is attributed to its lone pairs of electrons due to the linear molecular shape of the SCN anion, in contrast to the spherical I anion.47 This interaction facilitates the formation of Pb(SCN)42− octahedra, which possess a higher formation constant (K4) compared to PbI42− octahedra.74 As a result of these interactions, the heterostructure is prevented, thereby stabilizing the α-phase within the FCPI-SCN film. As aforementioned, the metallization of Ag occurs via electrochemical reaction by carriers transferred through the HP matrix. This leads to the formation of stable filaments through the FCPI-SCN film, thereby curtailing the effective surface curvature of the filament, as depicted in Fig. 5(b). Consequently, the FCPI-SCN device manifests improved nonvolatile characteristics.
Stable filament formation in SCN−-mediated FACsPbI3
In Fig. 6(a), the resistance distribution of LRS and HRS across 50 distinct cells in the FCPI-SCN device is depicted. It demonstrates a remarkable on/off ratio, with an average value of 4.46 × 108. Additionally, the statistical analysis illustrated in Fig. 6(b) presents the statistical distributions of VSET and VRESET across 15 individual FCPI-SCN devices. The average values of VSET and VRESET are 0.248 ± 0.025 V and −0.116 ± 0.011 V, respectively. Notably, the VSET and VRESET distributions exhibit minimal variations. As depicted in Fig. S9, the cumulative percentiles of VSET and VRESET reveal that the average cycle-to-cycle values of the FCPI-SCN devices are 0.242 ± 0.053 V and −0.11 ± 0.017 V, respectively. Furthermore, the FCPI-SCN device remained operable for up to 1200 cycles of ±1 V (50 μs/100 μs pulse durations), followed by a read voltage of +0.02 V, as displayed in Fig. 6(c). Fig. S10 (ESI†) demonstrates that the FCPI-SCN device remained operational for up to 3500 SET/RESET cycles under low-amplitude pulse conditions. This result suggests that reducing the electrical stimulus can improve the cyclability of the device. The observed fluctuation in resistance states is attributed to the relaxation behavior of the Ag filament. During the reset process, residual Ag ions require time to diffuse, and the extent of diffusion can vary from cycle to cycle.73,75 Therefore, correction techniques such as closed-loop pulse switching (CLPS)76,77 may be effective in narrowing the distribution of resistance states and enhancing device endurance. Nonvolatile switching devices intrinsically suffer from the “voltage–time dilemma” where the high operation voltage is usually needed for fast switching speed.78–80 Because this ion mass transport induces the voltage–time dilemma, we optimized the amplitude to promote reading a clear RESET states distribution and provide a large window between LRS and HRS (Fig. 6(c)). Fig. 6(d) showcases the superior range of multilevel resistance states achieved by the FCPI-SCN device. It was subjected to a DC bias sweeping sequence: 0 V → +0.8 V → 0 V → −0.4 V → 0 V with varying Iccs of 10−3, 10−4, 10−5, 10−6, 10−7, and 10−8 A. It attained a total of six distinct LRSs. The multilevel retention times were evaluated using a pulse train of the read voltage (+0.02 V), as displayed in Fig. 6(e). Each resistance state persisted for durations exceeding 103 s. Despite prior studies indicating that most electrochemical metallization memories exhibit Icc-dependent behavior, characterized by volatile switching at low Icc and nonvolatile switching at high Icc values,81,82 the FCPI-SCN device upholds its nonvolatile characteristic even at the extremely low Icc value of 10−8 A. In addition, the FCPI-SCN device demonstrates a rapid switching speed of under 50 ns, as illustrated in Fig. 6(f). Table 1 tabulates the general properties of several studies on electrometallization memories. This comparison suggests that HP-based memristors possess low operating voltages, fast switching speed, and suitable performances. Fig. S11a (ESI†) displays the XRD patterns, confirming the maintenance of the cubic α-phase after 15 days in an ambient atmosphere. Fig. S11b (ESI†) depicts the stable nonvolatile switching characteristics of the treated devices over the same period. Long-term stability was assessed by evaluating device performance after one month of storage under ambient air conditions. The switching performance including retention time and endurance cycles remained stable and reliable, as demonstrated in Fig. S12 (ESI†). These support the effectiveness of SCN treatment in enhancing the reliability of filamentary switching in HP-based devices.| Memristive materials | Device structure | Set/reset voltage | On/off ratio | Retention time | Endurance | Switching speed | Thickness (nm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Oxides | Ag/ZrO2/WS2/Pt | 0.2 V/−0.1 V | 104 | 4 × 104 s | 109 cycles | 10 ns | 100 | 83 |
| Ag/VOx/Pt | 0.23 V/−0.07 V | 103 | 4 × 104 s | 103 cycles | — | 35 | 84 | |
| Ag/SiO2/ITO | 3 V/gradual reset | 102 | 2 × 103 s | 5 × 102 cycles | 100 ns | 60 | 85 | |
| Ag/VO2/SiOx/n++Si | 2.2 V/−2.1 V | 60 | — | 30 cycles | — | — | 86 | |
| Ag/CoFe2O4/Pt | 2.5 V/−2.6 V | 103 | 103 | 5 × 102 cycles | — | 100 | 87 | |
| Ag/STO/p++-Si | 3 V/−1.8 V | 103 | 104 s | 103 cycles | 70 | 88 | ||
| 2D materials | Ag/graphdiyne oxide/Au | 0.25 V/−0.4 V | 102 | 3 × 103 s | 1.5 × 102 cycles | — | 5 | 89 |
| Ag/MoS2/Ag | 0.18 V/−0.1 V | 107 | 105 s | — | 400 ns | 100 | 90 | |
| Organic materials | Ag/polyvinyl alcohol–graphene oxide/Ag | 0.4 V/−0.8 V | 7 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × × ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 s 103 s |
2.5 × 103 cycles | — | — | 91 |
| Cu/P3HT:PCBM/ITO | 4.5 V/−2 V | 104 | 106 s | — | — | 85 | 92 | |
| Halide perovskites | Ag/BA2PbI4/Pt | 0.5 V/−0.6 V | 107 | 103 s | 2.5 × 102 cycles | 10 ms | 470 | 93 |
| Ag/PMMA/FCPI-SCN/Pt | 0.25 V/−0.12 V | 108 | 1.1 × 104 s | 1.2 × 103 cycles | 50 ns | 400 | This work | |
A 3 × 3 crossbar array incorporating the FCPI-SCN device was fabricated to assess its functionality in storing and retrieving bitmap images. The schematic of the crossbar array, constructed on the SiO2/Si substrate, is illustrated in Fig. 7(a). Fig. 7(b) and Fig. S13 (ESI†) presents an optical image of the 3 × 3 crossbar array with 100 μm-width top and bottom electrode lines. The crossbar array adopted the floating Vr biasing scheme, outlined in Fig. 7(c), whereby all neighboring cells are left floating while the target cell is biased. To validate the storage capability of the FCPI-SCN crossbar array, a 3 × 3-pixel voltage input in the shape of the letter ‘X’ (depicted in Fig. S14a, ESI†) was programmed into the array. The resulting output current, as illustrated in Fig. S14b (ESI†), successfully converted the programmed data into binary image data. Although the sneak path issue is inevitable (manifested by relatively lower detected current in the SET cell located at the center of the bitmap compared to neighboring SET cells), the readout margin within the 3 × 3 crossbar array remains acceptable. This margin was calculated using the Kirchhoff equation and meets the criterion of a 10% readout margin (Fig. S15, ESI†). However, scaling up the array size would necessitate the incorporation of selection devices to mitigate sneak path issues.94 Furthermore, input images comprising the acronym of our laboratory ‘O,’ ‘N,’ ‘N,’ and ‘L’ in a 3 × 3-pixel format were encoded (as shown in Fig. 7(d)). The resulting output current of these letters is presented in Fig. 7(e). The letter bitmap images were effectively stored and retrieved by the crossbar array. This successful demonstration of the 3 × 3 crossbar array highlights the practical applicability of the FCPI-SCN device, thereby showcasing its promising potential in advanced memory technologies. Despite the significant challenges associated with device scaling, including process compatibility and precise thickness control of the halide layer, many efforts are currently underway. First of all, halide perovskite films exhibit vulnerabilities when exposed to polar solvents such as acetone and water, which are commonly used in semiconductor manufacturing processes. Addressing this limitation is imperative for the practical deployment of halide perovskite-based resistive switching memory. Recent studies suggest that exploiting solvent orthogonality can be an effective strategy for adapting lithography techniques to halide perovskites.15,95 Notably, halide perovskite films remain undamaged when exposed to nonpolar and weakly polar solvents, including chlorobenzene, hexane, and isopropanol. Additionally, the thermal evaporation of halide-based sources can facilitate precise thickness control of halide perovskite films, enabling the fabrication of ultra-thin layers in large-scale wafer production.96,97 In the context of large-scale array fabrication, the challenge of leakage current remains unresolved. Recent studies have suggested that threshold switching memristors are suitable as selector devices in 1-selector-1-resistor (1S-1R) memristor arrays.98 Halide perovskite-based threshold switching devices have demonstrated low operating voltage and high selectivity.99,100 Employing identical material systems for both the selector and the resistor could enhance process efficiency. The next step should involve investigating the integration of these films to achieve 1S-1R operation. The progress made in halide perovskite-based memory technologies demonstrates their substantial potential for future electronics.
Conclusions
In summary, our objective was to develop a low-powered filamentary memristor device based on mixed-cation HPs, specifically focusing on FA0.8Cs0.2PbI3. By incorporating thiocyanate treatment, we successfully stabilized the cubic α-phase, leading to notable improvements in nonvolatile characteristics, such as enhanced retention and endurance. The pristine FCPI exhibits a mixed state of α and δ-phases, with heterostructures containing a high density of defects. These defects act as electron traps at the defective boundaries, facilitating the formation of multiple dendritic Ag thin filaments along the heterostructures and contributing to the fast corrosion of the filaments and unstable nonvolatile performance observed in the pristine FCPI. The reduction in the formation of the hexagonal δ-phase by-product and stabilization of cubic α-phase within FCPI-SCN substantially enhanced device performances. The Ag/PMMA/FCPI-SCN/Pt device exhibited a prolonged retention time of 1.1 × 104 s and improved endurance up to 1200 cycles. Additionally, the device could be programmed into six distinct multilevel resistance states. We also implemented a 3 × 3 crossbar array utilizing FCPI-SCN, which successfully encoded 3 × 3-pixel bitmaps representing various letters, such as ‘O,’ ‘N,’ ‘N,’ and ‘L.’ These bitmaps were reliably retained, showcasing their practical applicability for dependable resistive switching memory. In addition to elucidating the filamentary switching mechanisms, this work represents a significant step forward in the development of mixed-cation HP-based filamentary memristor devices, demonstrating the potential of novel functional materials for next-generation memory technologies. Our research opens new avenues for exploring and utilizing HPs in the realm of CBRAM.Data availability
The data supporting the findings of this study are available within this article and its ESI,† or from the corresponding authors upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National R&D Program (RS-2024-00405016 and RS-2024-00404254) through NRF (National Research Foundation of Korea), funded by the Ministry of Science and ICT. The Research Institute of Advanced Materials, Inter-University Semiconductor Research Center, SOFT Foundry, and Institute of Engineering Research at Seoul National University provided research facilities for this work.References
- Z. Wang, H. Wu, G. W. Burr, C. S. Hwang, K. L. Wang, Q. Xia and J. J. Yang, Nat. Rev. Mater., 2020, 5, 173–195 CrossRef CAS.
- J. J. Yang and R. S. Williams, ACM J. Emerg. Technol. Comput. Syst., 2013, 9, 1–20 Search PubMed.
- M. Di Ventra and Y. V. Pershin, Nat. Phys., 2013, 9, 200–202 Search PubMed.
- C. Sun, S. Okamoto, S. Hachiya, T. Yamada and K. Takeuchi, IEEE Trans. Consum. Electron., 2016, 62, 267–274 Search PubMed.
- D. B. Strukov, G. S. Snider, D. R. Stewart and R. S. Williams, Nature, 2008, 453, 80–83 CrossRef CAS PubMed.
- I. Vourkas and G. C. Sirakoulis, Memristor-Based Nanoelectronic Computing Circuits and Architectures, 2015 Search PubMed.
- I. H. Im, S. J. Kim and H. W. Jang, Adv. Intell. Syst., 2020, 2, 2000105 CrossRef.
- S. Pi, C. Li, H. Jiang, W. Xia, H. Xin, J. J. Yang and Q. Xia, Nat. Nanotechnol., 2019, 14, 35–39 CrossRef CAS PubMed.
- Z. Wang, S. Joshi, S. Savel’Ev, W. Song, R. Midya, Y. Li, M. Rao, P. Yan, S. Asapu, Y. Zhuo, H. Jiang, P. Lin, C. Li, J. H. Yoon, N. K. Upadhyay, J. Zhang, M. Hu, J. P. Strachan, M. Barnell, Q. Wu, H. Wu, R. S. Williams, Q. Xia and J. J. Yang, Nat. Electron., 2018, 1, 137–145 CrossRef.
- Y.-W. Song, J. Lee, S. Lee, W. Ham, J. H. Yoon, J.-M. Park, T. Sung and J.-Y. Kwon, Electron. Mater. Lett., 2024, 20, 725–732 CrossRef CAS.
- B. K. You, J. M. Kim, D. J. Joe, K. Yang, Y. Shin, Y. S. Jung and K. J. Lee, ACS Nano, 2016, 10, 9478–9488 CrossRef CAS PubMed.
- H. Gim, M. Lee, W. Hong and K. Hong, The Korean Sensors Society, 2024, preprint DOI:10.46670/JSST.2024.33.5.265.
- L. Wang and J. Choi, Micro Nano Syst. Lett., 2023, 11, 5 CrossRef.
- B. G. Lee, J.-Y. Lee, J. H. Choi, J. M. Seo and S.-J. Kim, Electron. Mater. Lett., 2025 DOI:10.1007/s13391-025-00555-x.
- J. Choi, J. S. Han, K. Hong, S. Y. Kim and H. W. Jang, Adv. Mater., 2018, 30, 1704002 CrossRef PubMed.
- H. M. Cho and H. W. Jang, Electron. Mater. Lett., 2025 DOI:10.1007/s13391-025-00560-0.
- M. Kerara, A. Naas, A. Gueddim and O. Meglali, Trans. Electr. Electron. Mater., 2024, 25, 665–673 CrossRef.
- A. Jabar, S. Benyoussef and L. Bahmad, Trans. Electr. Electron. Mater., 2024, 25, 519–528 CrossRef.
- J. S. Han, Q. Van Le, J. Choi, K. Hong, C. W. Moon, T. L. Kim, H. Kim, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2018, 28, 1705783 CrossRef.
- J. S. Han, Q. Van Le, H. Kim, Y. J. Lee, D. E. Lee, I. H. Im, M. K. Lee, S. J. Kim, J. Kim, K. J. Kwak, M. J. Choi, S. A. Lee, K. Hong, S. Y. Kim and H. W. Jang, Small, 2020, 16, 2003225 CrossRef CAS PubMed.
- S. J. Kim, T. H. Lee, J. M. Yang, J. W. Yang, Y. J. Lee, M. J. Choi, S. A. Lee, J. M. Suh, K. J. Kwak, J. H. Baek, I. H. Im, D. E. Lee, J. Y. Kim, J. Kim, J. S. Han, S. Y. Kim, D. Lee, N. G. Park and H. W. Jang, Mater. Today, 2022, 52, 19–30 CrossRef CAS.
- J. Choi, S. Park, J. Lee, K. Hong, D. H. Kim, C. W. Moon, G. Do Park, J. Suh, J. Hwang, S. Y. Kim, H. S. Jung, N. G. Park, S. Han, K. T. Nam and H. W. Jang, Adv. Mater., 2016, 28, 6562–6567 CrossRef CAS PubMed.
- J. Choi, Q. Van Le, K. Hong, C. W. Moon, J. S. Han, K. C. Kwon, P. R. Cha, Y. Kwon, S. Y. Kim and H. W. Jang, ACS Appl. Mater. Interfaces, 2017, 9, 30764–30771 CrossRef CAS PubMed.
- S. Poddar, Y. Zhang, L. Gu, D. Zhang, Q. Zhang, S. Yan, M. Kam, S. Zhang, Z. Song, W. Hu, L. Liao and Z. Fan, Nano Lett., 2021, 21, 5036–5044 CrossRef CAS PubMed.
- K. C. Kwon, K. Hong, Q. Van Le, S. Y. Lee, J. Choi, K. B. Kim, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2016, 26, 4213–4222 CrossRef CAS.
- S. H. Turren-Cruz, A. Hagfeldt and M. Saliba, Science, 1979, 2018(362), 449–453 Search PubMed.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D’Haen, L. D’Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. De Angelis and H. G. Boyen, Adv. Energy Mater., 2015, 5, 1500477 CrossRef.
- J. W. Lee, D. H. Kim, H. S. Kim, S. W. Seo, S. M. Cho and N. G. Park, Adv. Energy Mater., 2015, 5, 1501310 CrossRef.
- D. Ghosh, A. R. Smith, A. B. Walker and M. S. Islam, Chem. Mater., 2018, 30, 5194–5204 CrossRef CAS.
- S. S. W. S. Yang, J. Noh, Y. C. Kim, S. Ryu and J. Seo, Science, 2015, 348, 1234–1237 CrossRef PubMed.
- A. Binek, F. C. Hanusch, P. Docampo and T. Bein, J. Phys. Chem. Lett., 2015, 6, 1249–1253 CrossRef CAS PubMed.
- J. W. Lee, D. J. Seol, A. N. Cho and N. G. Park, Adv. Mater., 2014, 26, 4991–4998 CrossRef CAS PubMed.
- Z. Li, M. Yang, J. S. Park, S. H. Wei, J. J. Berry and K. Zhu, Chem. Mater., 2016, 28, 284–292 CrossRef CAS.
- J. S. Yun, J. Kim, T. Young, R. J. Patterson, D. Kim, J. Seidel, S. Lim, M. A. Green, S. Huang and A. Ho-Baillie, Adv. Funct. Mater., 2018, 28, 1705363 CrossRef.
- S. Tan, I. Yavuz, M. H. Weber, T. Huang, C. H. Chen, R. Wang, H. C. Wang, J. H. Ko, S. Nuryyeva, J. Xue, Y. Zhao, K. H. Wei, J. W. Lee and Y. Yang, Joule, 2020, 4, 2426–2442 CrossRef CAS.
- X. Zhao, J. Ma, X. Xiao, Q. Liu, L. Shao, D. Chen, S. Liu, J. Niu, X. Zhang, Y. Wang, R. Cao, W. Wang, Z. Di, H. Lv, S. Long and M. Liu, Adv. Mater., 2018, 30, 1705193 CrossRef PubMed.
- M. J. Lee, S. Han, S. H. Jeon, B. H. Park, B. S. Kang, S. E. Ahn, K. H. Kim, C. B. Lee, C. J. Kim, I. K. Yoo, D. H. Seo, X. S. Li, J. B. Park, J. H. Lee and Y. Park, Nano Lett., 2009, 9, 1476–1481 CrossRef CAS PubMed.
- H. Kim, J. S. Kim, J. Choi, Y. H. Kim, J. M. Suh, M. J. Choi, Y. S. Shim, S. Y. Kim, T. W. Lee and H. W. Jang, ACS Appl. Mater. Interfaces, 2024, 16, 2457–2466 CrossRef CAS PubMed.
- C. Muthu, A. N. Resmi, A. Ajayakumar, N. E. A. Ravindran, G. Dayal, K. B. Jinesh, K. Szaciłowski and C. Vijayakumar, Small, 2024, 20, 1–12 CrossRef PubMed.
- S. M. Lee, H. Kim, D. H. Kim, W. Bin Kim, J. M. Lee, J. Choi, H. Shin, G. S. Han, H. W. Jang and H. S. Jung, ACS Appl. Mater. Interfaces, 2020, 12, 17039–17045 CrossRef CAS PubMed.
- C. Eames, J. M. Frost, P. R. F. Barnes, B. C. O’Regan, A. Walsh and M. S. Islam, Nat. Commun., 2015, 6, 7497 CrossRef CAS PubMed.
- J. S. Han, Q. Van Le, J. Choi, K. Hong, C. W. Moon, T. L. Kim, H. Kim, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2018, 28, 1705783 CrossRef.
- M. L. Holekevi Chandrappa, Z. Zhu, D. P. Fenning and S. P. Ong, Chem. Mater., 2021, 33, 4672–4678 CrossRef CAS.
- D. Y. Heo, T. H. Lee, A. Iwan, L. Kavan, M. Omatova, E. Majkova, K. Kamarás, H. W. Jang and S. Y. Kim, J. Power Sources, 2020, 458, 228067 CrossRef CAS.
- Y. Chen, B. Li, W. Huang, D. Gao and Z. Liang, Chem. Commun., 2015, 51, 11997–11999 RSC.
- A. Halder, R. Chulliyil, A. S. Subbiah, T. Khan, S. Chattoraj, A. Chowdhury and S. K. Sarkar, J. Phys. Chem. Lett., 2015, 6, 3483–3489 CrossRef CAS PubMed.
- Q. Jiang, D. Rebollar, J. Gong, E. L. Piacentino, C. Zheng and T. Xu, Angew. Chem., Int. Ed., 2015, 54, 7617–7620 CrossRef CAS PubMed.
- Y. Yu, C. Wang, C. R. Grice, N. Shrestha, J. Chen, D. Zhao, W. Liao, A. J. Cimaroli, P. J. Roland, R. J. Ellingson and Y. Yan, ChemSusChem, 2016, 9, 3288–3297 CrossRef CAS PubMed.
- L. T. Schelhas, Z. Li, J. A. Christians, A. Goyal, P. Kairys, S. P. Harvey, D. H. Kim, K. H. Stone, J. M. Luther, K. Zhu, V. Stevanovic and J. J. Berry, Energy Environ. Sci., 2019, 12, 1341–1348 RSC.
- G. Kieslich, S. Sun and A. K. Cheetham, Chem. Sci., 2015, 6, 3430–3433 RSC.
- M. Abdi-Jalebi, Z. Andaji-Garmaroudi, S. Cacovich, C. Stavrakas, B. Philippe, J. M. Richter, M. Alsari, E. P. Booker, E. M. Hutter, A. J. Pearson, S. Lilliu, T. J. Savenije, H. Rensmo, G. Divitini, C. Ducati, R. H. Friend and S. D. Stranks, Nature, 2018, 555, 497–501 CrossRef CAS PubMed.
- D. Y. Son, S. G. Kim, J. Y. Seo, S. H. Lee, H. Shin, D. Lee and N. G. Park, J. Am. Chem. Soc., 2018, 140, 1358–1364 CrossRef CAS PubMed.
- S. Yang, W. Liu, L. Zuo, X. Zhang, T. Ye, J. Chen, C. Z. Li, G. Wu and H. Chen, J. Mater. Chem. A, 2016, 4, 9430–9436 RSC.
- R. Waser, R. Dittmann, C. Staikov and K. Szot, Adv. Mater., 2009, 21, 2632–2663 CrossRef CAS PubMed.
- S. Aussen, F. Cüppers, C. Funck, J. Jo, S. Werner, C. Pratsch, S. Menzel, R. Dittmann, R. Dunin-Borkowski, R. Waser and S. Hoffmann-Eifert, Adv. Electron. Mater., 2023, 9, 2300520 CrossRef CAS.
- W. Banerjee, W. F. Cai, X. Zhao, Q. Liu, H. Lv, S. Long and M. Liu, Nanoscale, 2017, 9, 18908–18917 RSC.
- C. C. Hsieh, A. Roy, A. Rai, Y. F. Chang and S. K. Banerjee, Appl. Phys. Lett., 2015, 106, 173108 CrossRef.
- H. F. Ling, M. D. Yi, M. Nagai, L. H. Xie, L. Y. Wang, B. Hu and W. Huang, Adv. Mater., 2017, 29, 1–9 CrossRef PubMed.
- M. A. Lampert and R. B. Schilling, Chapter 1 Current Injection in Solids: The Regional Approximation Method, 1970, pp. 1–96 Search PubMed.
- Y. C. Yang, F. Pan, Q. Liu, M. Liu and F. Zeng, Nano Lett., 2009, 9, 1636–1643 CrossRef CAS PubMed.
- D. Meggiolaro, E. Mosconi and F. De Angelis, ACS Energy Lett., 2019, 4, 779–785 CrossRef CAS.
- P. Calado, A. M. Telford, D. Bryant, X. Li, J. Nelson, B. C. O’Regan and P. R. F. Barnes, Nat. Commun., 2016, 7, 1–10 Search PubMed.
- Z. Fang, W. Chen, Y. Shi, J. Zhao, S. Chu, J. Zhang and Z. Xiao, Adv. Funct. Mater., 2020, 30, 1–9 Search PubMed.
- A. S. Thind, X. Huang, J. Sun and R. Mishra, Chem. Mater., 2017, 29, 6003–6011 CrossRef CAS.
- J. S. Yun, J. Kim, T. Young, R. J. Patterson, D. Kim, J. Seidel, S. Lim, M. A. Green, S. Huang and A. Ho-Baillie, Adv. Funct. Mater., 2018, 28, 1705363 CrossRef.
- S. Tan, I. Yavuz, M. H. Weber, T. Huang, C. H. Chen, R. Wang, H. C. Wang, J. H. Ko, S. Nuryyeva, J. Xue, Y. Zhao, K. H. Wei, J. W. Lee and Y. Yang, Joule, 2020, 4, 2426–2442 CrossRef CAS.
- W. Yang, S. Lee, H. C. Kwon, J. Tan, H. Lee, J. Park, Y. Oh, H. Choi and J. Moon, ACS Nano, 2018, 12, 11088–11097 CrossRef CAS PubMed.
- X. Song, W. Wang, P. Sun, W. Ma and Z. K. Chen, Appl. Phys. Lett., 2015, 106, 033901 CrossRef.
- A. Dehingia, U. Das and A. Roy, Mater. Today: Proc., 2022, 65, 29–34 CAS.
- H. Du, C. L. Jia, A. Koehl, J. Barthel, R. Dittmann, R. Waser and J. Mayer, Chem. Mater., 2017, 29, 3164–3173 CrossRef CAS.
- X. Xu, H. Lv, H. Liu, T. Gong, G. Wang, M. Zhang, Y. Li, Q. Liu, S. Long and M. Liu, IEEE Electron Device Lett., 2015, 36, 129–131 Search PubMed.
- W. Wang, M. Wang, E. Ambrosi, A. Bricalli, M. Laudato, Z. Sun, X. Chen and D. Ielmini, Nat. Commun., 2019, 10, 81 CrossRef CAS PubMed.
- S. A. Chekol, S. Menzel, R. W. Ahmad, R. Waser and S. Hoffmann-Eifert, Adv. Funct. Mater., 2022, 32, 2111242 CrossRef CAS.
- Q. Jiang, D. Rebollar, J. Gong, E. L. Piacentino, C. Zheng and T. Xu, Angew. Chem., Int. Ed., 2015, 54, 7617–7620 CrossRef CAS PubMed.
- I. H. Im, J. H. Baek, S. J. Kim, J. Kim, S. H. Park, J. Y. Kim, J. J. Yang and H. W. Jang, Adv. Mater., 2024, 36, 1–13 CrossRef PubMed.
- S. Paramanik and A. J. Pal, Adv. Electron. Mater., 2022, 8, 2200211 CrossRef CAS.
- T. H. Park, H. J. Kim, W. Y. Park, S. G. Kim, B. J. Choi and C. S. Hwang, Nanoscale, 2017, 9, 6010–6019 RSC.
- H. Schroeder, V. V. Zhirnov, R. K. Cavin and R. Waser, J. Appl. Phys., 2010, 107, 054517 CrossRef.
- A. Gubicza, M. Csontos, A. Halbritter and G. Mihály, Nanoscale, 2015, 7, 4394–4399 RSC.
- P. Huang, Y. Wang, H. Li, B. Gao, B. Chen, F. Zhang, L. Zeng, G. Du, J. Kang and X. Liu, IEEE Trans. Nanotechnol., 2014, 13, 1127–1132 Search PubMed.
- B. G. Chae, J. B. Seol, J. H. Song, K. Baek, S. H. Oh, H. Hwang and C. G. Park, Adv. Mater., 2017, 29, 1701752 CrossRef PubMed.
- R. A. John, Y. Demirağ, Y. Shynkarenko, Y. Berezovska, N. Ohannessian, M. Payvand, P. Zeng, M. I. Bodnarchuk, F. Krumeich, G. Kara, I. Shorubalko, M. V. Nair, G. A. Cooke, T. Lippert, G. Indiveri and M. V. Kovalenko, Nat. Commun., 2022, 13, 2074 CrossRef CAS PubMed.
- X. Yan, C. Qin, C. Lu, J. Zhao, R. Zhao, D. Ren, Z. Zhou, H. Wang, J. Wang, L. Zhang, X. Li, Y. Pei, G. Wang, Q. Zhao, K. Wang, Z. Xiao and H. Li, ACS Appl. Mater. Interfaces, 2019, 11, 48029–48038 CrossRef CAS PubMed.
- J. Ryu, K. Park, D. P. Sahu and T. S. Yoon, ACS Appl. Mater. Interfaces, 2024, 16, 26450–26459 CrossRef CAS PubMed.
- S. Gao, C. Chen, Z. Zhai, H. Y. Liu, Y. S. Lin, S. Z. Li, S. H. Lu, G. Y. Wang, C. Song, F. Zeng and F. Pan, Appl. Phys. Lett., 2014, 105, 063504 CrossRef.
- J. Li, X. Zhou, L. Xu, J. Wang, B. Wu and C. Wang, Mater. Res. Express, 2022, 9, 035003 CrossRef CAS.
- S. Munjal and N. Khare, Nanotechnology, 2021, 32, 185204 CrossRef CAS PubMed.
- N. Ilyas, J. Wang, C. Li, H. Fu, D. Li, X. Jiang, D. Gu, Y. Jiang and W. Li, J. Mater. Sci. Technol., 2022, 97, 254–263 CrossRef CAS.
- D. P. Do, V. Q. Bui, M. C. Nguyen, S. Seo, V. D. Do, J. Kim, J. Choi, H. Ko, W. J. Yu, Y. Kawazoe and H. Lee, Nano Lett., 2024, 24, 7999–8007 CrossRef CAS PubMed.
- X. Feng, Y. Li, L. Wang, S. Chen, Z. G. Yu, W. C. Tan, N. Macadam, G. Hu, L. Huang, L. Chen, X. Gong, D. Chi, T. Hasan, A. V. Y. Thean, Y. W. Zhang and K. W. Ang, Adv. Electron. Mater., 2019, 5, 1–9 CAS.
- M. K. Yadav, S. S. Kundale, S. S. Sutar, T. D. Dongale, P. Kumar and N. Panwar, J. Appl. Phys., 2023, 134, 105101 CrossRef CAS.
- S. Gao, C. Song, C. Chen, F. Zeng and F. Pan, J. Phys. Chem. C, 2012, 116, 17955–17959 CrossRef CAS.
- J. Y. Seo, J. Choi, H. S. Kim, J. Kim, J. M. Yang, C. Cuhadar, J. S. Han, S. J. Kim, D. Lee, H. W. Jang and N. G. Park, Nanoscale, 2017, 9, 15278–15285 RSC.
- I. H. Im, S. J. Kim, J. H. Baek, K. J. Kwak, T. H. Lee, J. W. Yang, D. E. Lee, J. Y. Kim, H. R. Kwon, D. Y. Heo, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2022, 33, 2211358 CrossRef.
- C. H. Lin, B. Cheng, T. Y. Li, J. R. D. Retamal, T. C. Wei, H. C. Fu, X. Fang and J. H. He, ACS Nano, 2019, 13, 1168–1176 CAS.
- S. Y. Hsiao, H. L. Lin, W. H. Lee, W. L. Tsai, K. M. Chiang, W. Y. Liao, C. Z. Ren-Wu, C. Y. Chen and H. W. Lin, Adv. Mater., 2016, 28, 7013–7019 CrossRef CAS PubMed.
- L. Gil-Escrig, C. Momblona, M. G. La-Placa, P. P. Boix, M. Sessolo and H. J. Bolink, Adv. Energy Mater., 2018, 8, 1–6 Search PubMed.
- H. J. Kim, D. S. Woo, S. M. Jin, H. J. Kwon, K. H. Kwon, D. W. Kim, D. H. Park, D. E. Kim, H. U. Jin, H. Do Choi, T. H. Shim and J. G. Park, Adv. Mater., 2022, 34, 2203643 CrossRef CAS PubMed.
- L. Tang, J. Wang, Y. Huang, H. Wang, C. Wang and Y. Yang, J. Mater. Chem. C, 2024, 12, 3622–3631 RSC.
- I. H. Im, S. J. Kim, J. H. Baek, K. J. Kwak, T. H. Lee, J. W. Yang, D. E. Lee, J. Y. Kim, H. R. Kwon, D. Y. Heo, S. Y. Kim and H. W. Jang, Adv. Funct. Mater., 2022, 33, 2211358 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc00303b |
| ‡ I. H. Im, J. H. Baek and D. Y. Heo contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |