ACQ/AIE transition of rofecoxib-based analogues via combined ring expansion and amino dimethylation strategies and their multiple stimuli-responsive fluorescent behaviors†
Jingming
Zhou‡
a,
Yongbo
Wei‡
a,
Yu
Cheng‡
b,
Xia
Wang
a,
Tong
Wu
a,
Weiwei
Zhang
a,
Yinyin
Yao
c,
Yusheng
Lu
*c,
Hongqiang
Qiu
 *b and
Lijun
Xie
*b and
Lijun
Xie
 *a
*a
aFujian Provincial Key Laboratory of Screening for Novel Microbial Products, Fujian Institute of Microbiology, Fuzhou, Fujian 350007, P. R. China. E-mail: lijunxie8224@outlook.com
bDepartment of Pharmacy, Fujian Medical University Union Hospital, 29 Xin Quan Rd, Gulou, Fuzhou, 350001, Fujian, P. R. China. E-mail: hongqiangqiu@fjmu.edu.cn
cFujian-Taiwan-Hongkong-Macao Science and Technology Cooperation Base of Intelligent Pharmaceutics, College of Material and Chemical Engineering, Minjiang University, Fuzhou, Fujian 350108, P. R. China. E-mail: lu_yu_sheng@126.com
First published on 2nd April 2025
Abstract
In this work, two innovative molecular design philosophies have been successfully developed to achieve the fluorescence conversion of 4 targets from aggregation-caused quenching (ACQ) to aggregation-induced emission (AIE). First, this conversion was achieved by employing a ring expansion strategy, where the pyrrolidinyl group of F0 was replaced with a piperidyl group, resulting in the target molecule FA. Next, by dimethylating the amino group of F0, the resulting compound FB also exhibited AIE properties. Moreover, when these two strategies were combined, the corresponding compound FAB exhibited the most obvious AIE properties with the highest quantity yield in the solid state (ΦPL,solid = 17.72%). The fluorescent behaviors of these three compounds in different states were elucidated clearly by analyzing their single X-ray crystal and theoretical calculation data. In addition, their potential multi-functional applications were investigated in mechano-sensors, trace water sensors and lipid droplet (LD) imaging. Conclusively, this work not only presents a better understanding of structure–fluorescence property relationship, but also offers two new strategies for developing new promising AIEgens.
Introduction
Over the past two decades, the development of new luminogens has attracted increasing attention due to their potential applications in mechano-sensors, optical storage, memory chips, security systems, etc.1–5 However, most conventional luminescent materials exhibit weakened or even totally quenched emission in the aggregated state or solid state, which is defined as the aggregation-caused quenching (ACQ) effect.6–10 In contrast to this phenomenon, Tang and his coworkers11,12 discovered aggregation-induced emission (AIE), describing the enhanced fluorescence of materials in the aggregated states. Thereafter, scientists have been developing a large number of AIEgens via the construction of new AIE scaffolds and their derivatization. In recent years, due to the rich source of ACQ fluorophores, the ACQ to AIE transformation has been a hot research topic for enriching the family of AIEgens.Several strategies are employed for the ACQ to AIE transformation. One of the strategies is the combination of ACQ and AIE through the changes of the original π structure for the twisted, suppressed π–π stacking but prolonged π system.13 Another way is to migrate substituent groups including the migration of CN,14 pyrrolidine,15 piperidine,16 and so on.17,18 The introduction of bulky alkyl groups into the ACQ unit is also feasible.19 These strategies are effective due to the inhibition of strong π–π stacking by increasing the steric hindrance without disturbing its π system to a large degree. However, almost all these reported cases were investigated and reported from only one aspect. Therefore, it would be meaningful to systematically explore the relationship between the structures and fluorescent properties (RSF) of targets, which has been rarely reported.20,21 Obviously, the development of new RSF will provide multiple ACQ-to-AIE strategies to obtain molecules with stronger AIE properties.
Herein, we report the ACQ to AIE transformation by two subtle structural modification strategies: ring expansion and amino dimethylation. Based on the two strategies, we transformed F0, an ACQ molecule, into AIEgens FA, FB, and FAB (Scheme 1). Specifically, by replacing F0's pyrrole ring with a piperidine ring, the synthesized FA exhibited AIE properties. Meanwhile, by introducing the dimethyl unit into the amino group of F0, the FB compound also showed obvious AIE properties. Excitingly, by combining these two strategies together, the compound FAB displayed further enhanced AIE properties. The influences of ring expansion and amino dimethylation on ACQ-to-AIE conversion were analyzed via single X-ray data. The slight ring expansion or amino dimethylation could significantly affect the molecular conformation and intermolecular packing mode, motivating their opposite photophysical properties. Two structural modifications combined can more significantly enhance the AIE effect of compounds. This work could be a potential guidance for other luminogens to achieve these similar ACQ-to-AIE conversion without the disturbance of its original π system.
Results and discussion
Molecular design, synthesis and characterization
The non-π-conjugated region of F0 consists mainly of an amino group and a pyrrole ring (Scheme 1). Since ACQ properties are often caused by strong π–π packing and other reasons,6,14 it is possible to achieve the ACQ-to-AIE conversion by changing molecular stacking modes and weakening π–π packing through increasing the steric hindrance of non-conjugated groups without disturbing the original π system. Therefore, two subtle modification strategies on the structure of ACQ molecule F0 were applied to synthesize AIE-active molecules FA and FB in this study, respectively. One is ring expansion by substitution of pyrrole ring for piperidine ring while the other is the introduction of dimethylamino group into the amino group in F0 (Scheme 1). FAB was obtained via the combination of the two strategies. The synthesis route is shown in Scheme 1. The products were characterized via1H and 13C NMR spectroscopy and high-resolution mass spectrometry (Fig. S1–S18, ESI†).Photophysical properties and theoretical calculations
The UV-vis absorption and emission spectra of F0, FA, FB and FAB were measured in a 50 μM solution of dimethyl sulfoxide (DMSO). The results reveal that F0, FA, FB, and FAB have maximum absorption peaks with wavelengths (λabs) at 446 nm, 438 nm, 458 nm, and 450 nm, respectively, as shown in Fig. 1A–D. Regarding emission characteristics, the maximum fluorescence of F0 is at 623 nm, while compounds FA, FB, and FAB exhibit red shifts, peaking at 632 nm, 642 nm, and 662 nm, respectively, as shown in Fig. 1A–D. To provide more insight into the effect of different structural variations on the photophysical properties of compounds, the main transitions for F0, FA, FB, and FAB were modeled using Multiwfn22 and VMD software23 from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). The HOMO–LUMO energy gaps calculated by density functional theory (DFT) at the B3LYP/6-311G*(d,p) level are also displayed in Fig. 1E–H. The HOMOs of these compounds are distributed less in the benzene ring connected to the lactone nucleus, while their LUMOs are distributed in the benzene ring connected to the lactone nucleus. This may be due to the N-atoms at both ends providing abundant electron density that participates in π-conjugation. The spatial separation of HOMO and LUMO orbitals facilitates charge transfer from the electron donor (D) to the electron acceptor (A) upon excitation, forming a pronounced intramolecular charge transfer (ICT) effect. The spatial separation of the HOMO/LUMO in F0 is the most pronounced, thus it exhibits the highest quantum yield in solution (Table 1). The energy gaps between HOMO and LUMO were determined to be 2.85, 3.22, 2.71 and 2.78 eV for F0, FA, FB and FAB, respectively (Fig. 1E–H), which was consistent with their λabs absorption spectra (Fig. 1A–D).| Compounds | Φ PL,solution (%) | Φ PL,agg. (%) | Φ PL,solid (%) |
|---|---|---|---|
| a Quantum yield measurement of four compounds in DMSO and their aggregates in DMSO/H2O mixtures has been carried out in comparison to an accepted standard (relative method of quantum yield measurement) viz. quinine sulphate in 0.1 M H2SO4 (λex = 350 nm, 25 °C), using the equation of Φ = ΦR (I/IR)(AR/A)(n2/nR2).24 Here, Φ is the quantum yield, ΦR = quantum yield of quinine sulphate under the stated conditions (0.577), I is integrated emission intensity of sample, A is the optical density of the sample, and n is the refractive index.25 | |||
| F0 | 1.27 | −0.05 (fw = 60%) | −0.06 |
| FA | 0.49 | 1.12 (fw = 80%) | 0.45 |
| FB | 0.72 | 4.19 (fw = 100%) | 1.44 |
| FAB | 0.72 | 12.91 (fw = 70%) | 17.72 |
Solvatochromism
The UV-vis absorption (Fig. S19A–D, ESI†) and photo-luminescence (PL) (Fig. 2A–D) spectra of F0, FA, FB and FAB were measured in different solvents, with emission maximum and Stokes shifts (Δν) listed in Table 2. As shown in Fig. S19A–D (ESI†) and Table 2, with the increase of solvent polarity, the absorption spectra of the four compounds showed small red shifts and similar absorption intensities. However, their emission spectra showed a relatively bright emission in non-polar solvents such as THF, CHCl3 and DCM, while they exhibited pronounced red shifts with significant decrease in the PL intensity in high-polarity solvents. The above-mentioned phenomena may be due to the occurrence of the typical TICT effect.26 In polar solvents, molecules with donor–acceptor (D–A) architectures undergo a conformational twist, creating a twisted intramolecular charge transfer (TICT) state. The TICT state exhibits a longer emission wavelength compared to its initial state due to its lower energy gap.| Compounds | Solvents | λ abs (nm) | λ em (nm) | Stokes shiftc (nm) | Δνd (cm−1) |
|---|---|---|---|---|---|
| a λ abs of F0, FA, FB and FAB in various solvents. b λ em of F0, FA, FB and FAB in various solvents. c The Stokes shift values. d Δν was calculated using the equation Δν [cm−1] = (1/λabs − 1/λem) × 107. | |||||
| F0 | DMSO | 446 | 623 | 177 | 6370 |
| DMF | 440 | 615 | 175 | 6467 | |
| EtOH | 430 | 601 | 171 | 6616 | |
| CHCl3 | 412 | 583 | 171 | 7119 | |
| THF | 426 | 571 | 145 | 5961 | |
| DCM | 414 | 593 | 179 | 7291 | |
| FA | DMSO | 438 | 632 | 194 | 7008 |
| DMF | 434 | 604 | 170 | 6485 | |
| EtOH | 426 | 600 | 174 | 6807 | |
| CHCl3 | 416 | 574 | 158 | 6617 | |
| THF | 426 | 564 | 138 | 5744 | |
| DCM | 416 | 590 | 174 | 7089 | |
| FB | DMSO | 458 | 642 | 184 | 6258 |
| DMF | 452 | 626 | 174 | 6149 | |
| EtOH | 446 | 612 | 166 | 6082 | |
| CHCl3 | 446 | 592 | 146 | 5530 | |
| THF | 442 | 584 | 142 | 5501 | |
| DCM | 444 | 598 | 154 | 5800 | |
| FAB | DMSO | 450 | 662 | 212 | 7116 |
| DMF | 446 | 636 | 190 | 6698 | |
| EtOH | 440 | 612 | 172 | 6387 | |
| CHCl3 | 444 | 594 | 150 | 5688 | |
| THF | 440 | 594 | 154 | 5892 | |
| DCM | 442 | 604 | 162 | 6068 | |
ACQ and AIE properties of F0, FA, FB and FAB
The ACQ and AIE properties were investigated through absorption (Fig. S20, ESI†) and emission spectra (Fig. 3A–D) for each compound in a 10 μM DMSO solution, upon slow addition of water fraction [fw (v/v%)]. Due to the TICT effect caused by the increased solvent polarity, the PL intensities of F0, FA, FB and FAB decreased when the fw was below 30% (Fig. 3E–H). However, as the water content further increased above 30%, their emission peaks experienced slight blue shifts. Specifically, F0, FA, FB and FAB blue-shifted by 45 nm (623 nm → 578 nm), 52 nm (632 nm → 580 nm), 76 nm (642 nm → 566 nm), and 96 nm (662 nm → 566 nm), respectively. This shift may be attributed to a reduction in molecular coplanarity as they aggregated under higher water fractions. Meanwhile, as an ACQ molecule, the fluorescence intensity of F0 in the aggregated state remained low (Fig. 3E), while the fluorescence intensities of FA, FB and FAB were significantly enhanced, demonstrating AIE properties (Fig. 3F–H). In detail, the aggregation-induced planarization of AIEgens restricts intramolecular rotation (RIR), thereby enhancing the conjugation of excited electrons and leading to intensified emission.27,28 Interestingly, the emission wavelengths (λem) of FB and FAB gradually red-shifted back to 608 nm and 604 nm, respectively, as the water fraction continued to increase, which may result from the combined effects of the TICT and AIE phenomena.29 To better study the emission in aggregate state, αAIE is defined as the ratio of maximum PL intensity to the intensity at fw = 0%. FAB displayed an αAIE of 17.1 (fw = 70%, ΦPL,agg. = 12.91%) that is higher than FA (αAIE = 2.5, fw = 80%, ΦPL,agg. = 1.12%) and FB (αAIE = 6.1, fw = 100%, ΦPL,agg. = 4.19%), suggesting that these three compounds exhibit a much stronger emission in the aggregated states (listed in Table 1).Single crystal X-ray analysis
The red FA crystals (CCDC 2284607†), the red FB crystals (CCDC 2284616†) and orange FAB crystals (CCDC 2284543†) were obtained by slow evaporation of acetone, acetone and ethyl acetate solutions, respectively (Fig. 4A–C). Suitable single crystals were selected for single-crystal X-ray diffraction analysis, and the crystallographic data are summarized in Table S1 (ESI†). FA and FAB crystallize in the monoclinic system with the P21/c space group, while FB crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. We analyzed the impact of ring expansion on the π–π stacking of the molecules, by comparing the structures of F0 and FA, as well as FB and FAB. It was speculated that the lower steric resistance of the pyrrole ring in F0 led to more pronounced π–π stacking contributing to the pronounced ACQ property of F0 (Fig. 3E). Unlike F0, the significant steric hindrance was caused by the piperidine ring in FA, which resulted in weak π–π packing (Fig. 4D). Similarly, the stacking mode of FAB differed significantly from that of FB. While FB and FAB had very similar molecular structures, the larger dihedral angle (50.2°) between rings D–E of the piperidine ring in FAB, compared to that (38.8°) of the pyrrole ring in FB (Fig. 4B, C, E and F), leading to greater distance between FAB layers. The vertical height of ring E is 3.061 Å in FAB, which is greater than the vertical height of 2.230 Å in FB. This weakens the π–π stacking. Specifically, FB exhibits a simple tiling pattern with layer-by-layer slip stacking (Fig. S22, ESI†), whereas FAB displays a more complex mode (Fig. S23, ESI†). Due to the large steric hindrance of the piperidine rings, the dimeric units in FAB arrange in a W-shape (white molecules) to optimize space. The distances between the centers of rings C and D in the adjacent molecular layers of FB are 3.693 Å (Fig. 4K), while those in FAB are 3.842 Å (Fig. 4L). This weakness of π–π stacking led to more AIE properties of FAB.
space group. We analyzed the impact of ring expansion on the π–π stacking of the molecules, by comparing the structures of F0 and FA, as well as FB and FAB. It was speculated that the lower steric resistance of the pyrrole ring in F0 led to more pronounced π–π stacking contributing to the pronounced ACQ property of F0 (Fig. 3E). Unlike F0, the significant steric hindrance was caused by the piperidine ring in FA, which resulted in weak π–π packing (Fig. 4D). Similarly, the stacking mode of FAB differed significantly from that of FB. While FB and FAB had very similar molecular structures, the larger dihedral angle (50.2°) between rings D–E of the piperidine ring in FAB, compared to that (38.8°) of the pyrrole ring in FB (Fig. 4B, C, E and F), leading to greater distance between FAB layers. The vertical height of ring E is 3.061 Å in FAB, which is greater than the vertical height of 2.230 Å in FB. This weakens the π–π stacking. Specifically, FB exhibits a simple tiling pattern with layer-by-layer slip stacking (Fig. S22, ESI†), whereas FAB displays a more complex mode (Fig. S23, ESI†). Due to the large steric hindrance of the piperidine rings, the dimeric units in FAB arrange in a W-shape (white molecules) to optimize space. The distances between the centers of rings C and D in the adjacent molecular layers of FB are 3.693 Å (Fig. 4K), while those in FAB are 3.842 Å (Fig. 4L). This weakness of π–π stacking led to more AIE properties of FAB.
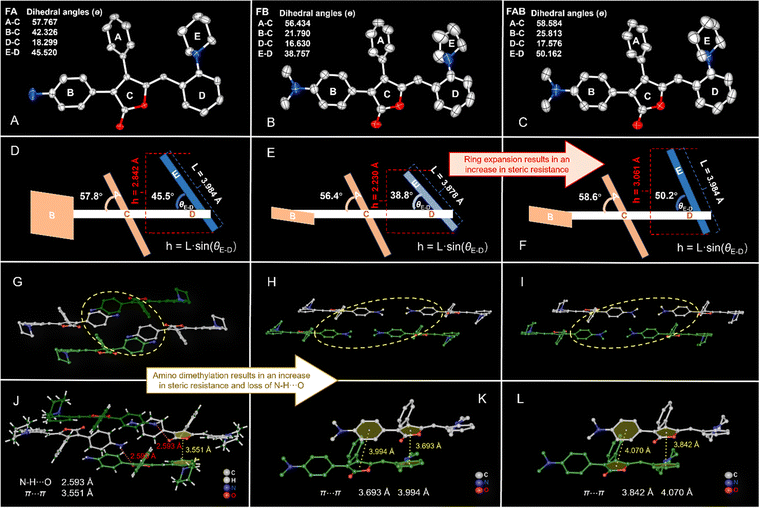 | ||
| Fig. 4 Single crystal structure, steric hindrance diagram and stacking structure of FA (A), (D), and (G), FB (B), (E), and (H) and FAB (C), (F), and (I), the rings A–E in Fig. 4A–C are corresponding to the rings A–E in Fig. 4D–F, respectively. Various intermolecular interactions were observed in the crystals of FA (J), FB (K), FAB (L). Dihedral angles were calculated from the crystal structures. Hydrogen atoms in the crystal structures were omitted for clarity except in Fig. 4J. | ||
Next, we further analyzed the impact of the dimethylamino substitution for the amino group on the molecular properties by comparing the structures of FA and FAB. Each pair of FA molecules forms dimeric units in an end-to-end arrangement. The crystal packing of FA displays a typical layer-by-layer stacking pattern (Fig. 4G and Fig. S21, ESI†). Molecules within the same layer are arranged in a glide pattern, with the dimeric units represented by the white molecules in Fig. 4G. The adjacent molecules are head-to-head with different orientations (yellow rings in Fig. 4G and light green and dark green molecules in Fig. 4G and Fig. S21, ESI†). Notably, the B ring, due to the small steric hindrance of the amino group, can twist more freely to achieve a more favourable angle, resulting in molecules being more closely packed within the same layer compared to FAB. On the other hand, intermolecular N–H⋯O hydrogen bonding (2.593 Å) occurs within adjacent molecular layers, which brings the adjacent molecular layers closer together, resulting in π–π stacking interactions (3.551 Å) between rings C and D also being observed between neighboring layers (Fig. 4J). But unlike FA, the N–H⋯O hydrogen bond disappears in FAB due to the demethylation of the amino group. Additionally, the steric hindrance of the dimethylamino group is larger than that of the amino group, which likely resulted in a smaller distortion angle between rings B and C compared to FA. These two factors led to the dimeric units in FAB being stacked in a relatively regular slip-stacked arrangement between layers. Overall, the steric resistance increased by decreasing in π–π stacking between the molecular layers is likely the main factor contributing to the enhanced AIE properties in FAB.
Mechanochromism and rewritable application
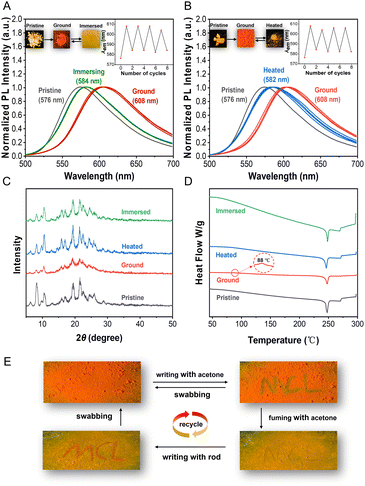
Strongly twisted conformations often result in a loose arrangement of molecules that are sensitive to pressure stimuli. Therefore, compounds with AIE properties are considered to be good candidates for mechanical fluorescence materials.30 Since FAB exhibited the best AIE performance among the targets, it was chosen for further investigation into its mechanochromic luminescence (MCL) behavior. Under UV irradiation at 365 nm, the pristine FAB powder emitted orange-yellow fluorescence with an emission peak at 576 nm. After grinding the powder with a pestle in an agate mortar, the ground FAB exhibited dark orange-red fluorescence, showing a 32 nm redshift to 608 nm (Fig. 5A). The weaker fluorescence emission and the redshift phenomena are likely due to the disruption of the original ordered structure, which transformed the packing mode into a tighter configuration and enhanced the conjugation effect between the molecules. Next, the reversibility of the MCL was investigated. The ground sample returned to its initial orange-yellow emission upon immersion in acetone or heating at 90 °C for 20 min. Furthermore, grinding–immersing and grinding–heating processes could be performed reversibly many times without fatigue (Fig. 5A and B). These results demonstrate that FAB possesses distinct mechanochromic properties.To further investigate the mechanochromic mechanism, powder X-ray diffraction (PXRD) and differential scanning calorimetry (DSC) were performed on solid FAB in its pristine, ground, fumed, and heated states. The diffractograms of the pristine sample displayed multiple sharp peaks (Fig. 5C), corresponding to crystalloid FAB. After grinding, the sharp peaks almost turned into weak, broad, and diffuse patterns, which suggested a transition from a crystalline state to an amorphous state. When the ground sample was immersed in acetone or heated at 90 °C, similar sharp and strong peaks reappeared in their diffraction patterns (Fig. 5C), indicating their pristine crystalline state had been restored. This observation shows that the amorphous molecules could be rearranged into an ordered crystalline state using these two treatments, confirming that the mechanochromic behavior of FAB is driven by changes in its crystal structure. DSC studies further supported this phase transition, showing a weak exothermic peak at 88 °C (Fig. 5D), which corresponds to the phase change from the crystalline state (emitting orange-yellow fluorescence) to the amorphous state (emitting orange-red fluorescence).
The reversible MCL behavior exhibited by compound FAB, in contrast to traditional irreversible systems, positions it as a viable alternative for rewritable optical devices.31 An example of stimulus-response write and erase for compound FAB is shown in Fig. 5E. First, ground FAB powder was spread on filter paper, displaying a typical orange-red fluorescence under UV light. Then, acetone was used as ink to write “MCL” on the FAB paper, as shown in Fig. 5E. In the presence of acetone, FAB transforms into a crystalline state. The method for erasing “MCL” involved exposing the FAB paper to acetone vapor for a few seconds, causing the background's orange-red fluorescence turn yellow and the “MCL” characters to become unclear. Subsequently, under mechanical force, such as using a rod, “MCL” was written on the FAB paper, revealing the orange-red fluorescence from the amorphous FAB. The original orange-red background was recovered by wiping the FAB paper with cotton. The write–erase procedure was demonstrated to be reversible and could be cycled several times. This reversible switching mechanism offers a cost-effective and eco-friendly alternative to conventional inks,32 validated through multiple fatigue-resistant cycles. The combination of stimulus-responsive transitions and robust reversibility highlights FAB's potential for anti-counterfeiting applications and sustainable rewritable data storage systems.
Detection of trace water in organic solvents
Fluorescence spectrometry has the advantage of high sensitivity and fast response in the detection of trace substances. With AIEgen's fluorescence characteristics, there have been many reports on the use of fluorescence spectroscopy for trace matter monitoring.27,28,33–35 Organic solvents often contain trace amounts of water, which can affect certain reactions that require strict control over water content.36 The fluorescence of FAB is strongly influenced by solvent polarity, motivating us to explore its potential applications as a trace water detector. We used THF as the model solvent to measure the emission spectra at different water contents (Fig. 6A). As the water fraction gradually increased, the fluorescence of FAB showed a rapid decrease in emission intensity. When the water fraction (fw) reached 2.0% (v/v), a significant quenching effect was observed, with a quenching efficiency of 76% (Fig. 6A). A clear linear relationship between water content and fluorescence intensity was observed in the range of 0 to 0.3%, with a correlation coefficient of 0.9902 (Fig. 6B). According to the 3-sigma rule, the limit of detection (LoD) for this system was as low as 0.032%. This not only surpasses the 1 ppm limit of the classic Karl Fischer titrator (volumetric method), but also offers the advantages of faster detection. These results demonstrate that FAB can be employed as a promising new fluorescent probe for sensitively detecting water content in organic solvents.Lipid droplet imaging of FAB
Lipid droplets (LDs) are essential cellular organelles involved in lipid metabolism, energy storage, and signal transduction. Molecular tools capable of specifically labeling LDs enable researchers to monitor dynamic changes in their quantity, size, polarity, composition, and distribution during metabolic processes, thereby revealing critical biological information about cellular or tissue-level metabolic states. FAB is expected to exhibit LDs-targeted imaging characteristics due to its pronounced AIE behavior and lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFAB = 6.4, calculated by using ALOGPS 2.1 software). First, FAB shows low cytotoxicity (Fig. S24, ESI†), with no apparent apoptosis observed in cells even at concentrations exceeding 10 μM. Then, its potential for LD imaging was tested and displayed in Fig. 6C–F, where it was co-stained with the commercially available LD-staining dye BODIPY503. FAB-stained MCF-7 cells exhibited a clear red channel signal (Fig. 6C), and a significant overlap with the green light from the LD dye was observed (Fig. 6D and F), indicating their co-localization. The Pearson's correlation coefficient for their co-localization was 0.89 (Fig. 6G). Furthermore, plot analysis of regions of interest (ROI) across the cells revealed a close correlation between the green and red channels (Fig. 6H). These demonstrate FAB's localization specificity for LDs. Combined with its low cytotoxicity, FAB demonstrates high potential as a tool for lipid droplet imaging in live cells.
PFAB = 6.4, calculated by using ALOGPS 2.1 software). First, FAB shows low cytotoxicity (Fig. S24, ESI†), with no apparent apoptosis observed in cells even at concentrations exceeding 10 μM. Then, its potential for LD imaging was tested and displayed in Fig. 6C–F, where it was co-stained with the commercially available LD-staining dye BODIPY503. FAB-stained MCF-7 cells exhibited a clear red channel signal (Fig. 6C), and a significant overlap with the green light from the LD dye was observed (Fig. 6D and F), indicating their co-localization. The Pearson's correlation coefficient for their co-localization was 0.89 (Fig. 6G). Furthermore, plot analysis of regions of interest (ROI) across the cells revealed a close correlation between the green and red channels (Fig. 6H). These demonstrate FAB's localization specificity for LDs. Combined with its low cytotoxicity, FAB demonstrates high potential as a tool for lipid droplet imaging in live cells.
Conclusions
In summary, two systematic molecular strategies were developed to facilitate the transformation from ACQ to AIE molecules based on a typical ACQ molecule, F0. One strategy involved ring extension, while the other focused on amino dimethylation without altering the conjugated backbone. Using these two approaches, two innovative rofecoxib-based fluorescent probes, FA and FB, as well as FAB, were developed. Remarkably, by combining these two strategies, the obtained FAB exhibited further improved AIE properties due to significant synergistic effects of them. X-ray single crystal structure analysis confirmed the AIE characteristics of FA, FB and FAB, revealing that slight modifications to non-conjugated substituents had a significant impact on their π–π stacking modes, which in turn induced contrasting fluorescence behaviors. These findings not only provide a novel series of insights into structural modification strategies for converting ACQ to AIE, but also highlight the broad potential for application of FAB. For example, FAB may find promising uses in rewritable data storage, trace water detection, and LD imaging. These experimental results bolster our confidence that the establishment of novel RSF will guide the design of highly promising AIEgens in the future.Data availability
All data included in this study will be made available upon request from the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Department of Science and Technology of Fujian Province (Grant Number 2024R1043), the National Natural Science Foundation of China (NSFC, Grant Number 81703555) and the Key Research and Industrialization Project for Technological Innovation of Fujian Province (No. 2022G003).Notes and references
- S. Takahashi, S. Nagai, M. Asami and S. Ito, Mater. Adv., 2020, 1, 708–719 CAS.
- F. Zhao, Z. Chen, C. Fan, G. Liu and S. Pu, Dyes Pigm., 2019, 164, 390–397 CAS.
- K. Zheng, H. Yang, F. Ni, Z. Chen, S. Gong, Z. Lu and C. Yang, Adv. Opt. Mater., 2019, 7, 1900727 CAS.
- Z. Mao, Z. Yang, Y. Mu, Y. Zhang, Y. F. Wang, Z. Chi, C. C. Lo, S. Liu, A. Lien and J. Xu, Angew. Chem., Int. Ed., 2015, 54, 6270–6273 CAS.
- Z. Ma, Z. Wang, X. Meng, Z. Ma, Z. Xu, Y. Ma and X. Jia, Angew. Chem., Int. Ed., 2016, 55, 519–522 CrossRef CAS PubMed.
- Y. Q. Dong, J. W. Y. Lam and B. Z. Tang, J. Phys. Chem. Lett., 2015, 6, 3429–3436 CrossRef CAS.
- L. Yan, T. Qing, R. Li, Z. Wang and Z. Qi, RSC Adv., 2016, 6, 63874–63879 RSC.
- M. Chen, X. Hu, J. Liu, B. Li, N. L. C. Leung, L. Viglianti, T. S. Cheung, H. H. Y. Sung, R. T. K. Kwok, I. D. Williams, A. Qin, J. W. Y. Lam and B. Z. Tang, Chem. Sci., 2018, 9, 7829–7834 RSC.
- R. Isci, E. Tekin, K. Kaya, S. P. Mucur, S. F. Gorkem and T. Ozturk, J. Mater. Chem. C, 2020, 8, 7908–7915 CAS.
- Q. Qi, Y. Liu, X. Fang, Y. Zhang, P. Chen, Y. Wang, B. Yang, B. Xu, W. Tian and S. X.-A. Zhang, RSC Adv., 2013, 3, 7996–8002 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 Search PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 CAS.
- Monika, A. Verma, M. K. Tiwari, N. Subba and S. Saha, J. Photochem. Photobiol., A, 2022, 433, 114130 CAS.
- S. Pratihar, A. Bhattacharyya and E. Prasad, J. Photochem. Photobiol., A, 2020, 396, 112458 Search PubMed.
- X. Wang, X. Lin, R. Li, Z. Wang, W. Liu, L. Chen, N. Chen, T. Dai, S. Sun, Z. Li, J. Hao, B. Lin and L. Xie, Mol., 2021, 27, 193 Search PubMed.
- X. Wang, W. Liu, X. Lin, L. Chen, Z. Wang, Z. Xie, L. Wang, M. Hu, H. Jiang and L. Xie, Dyes Pigm., 2022, 198, 109992 CAS.
- H. Wang, H. Xing, J. Gong, H. Zhang, J. Zhang, P. Wei, G. Yang, J. W. Y. Lam, R. Lu and B. Z. Tang, Mater. Horiz., 2020, 7, 1566–1572 RSC.
- J. Li, T. Shan, M. Yao, Y. Gao, X. Han, B. Yang and P. Lu, J. Mater. Chem. C, 2017, 5, 2552–2558 RSC.
- L. Wang, Y. Zhang, X. Huang, Y. Liu, Y. Cheng, W. Fan, L. Zheng and Q. Cao, J. Mater. Chem. C, 2023, 11, 9308–9315 RSC.
- M.-L. Hebestreit, M. Schneider, H. Lartian, V. Betz, M. Heinrich, M. Lindic, M. Y. Choi and M. Schmitt, Phys. Chem. Chem. Phys., 2019, 21, 14766–14774 RSC.
- S. Sasaki, S. Suzuki, W. M. C. Sameera, K. Igawa, K. Morokuma and G.-i Konishi, J. Am. Chem. Soc., 2016, 138, 8194–8206 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- H. Yan, X. Meng, B. Li, S. Ge and Y. Lu, J. Mater. Chem. C, 2017, 5, 10589–10599 RSC.
- Y. Zhang, Y. Peng, J. Hou and Z. Jiang, High Power Laser Part. Beams, 2013, 25, 500–504 CAS.
- F. Lin, Y. Liu, Z. Zhang, Y. Feng, Z.-Q. Yu and L. Wang, Dyes Pigm., 2021, 186, 108977 CAS.
- K. Khurshid, S. A. Shahzad, M. A. Assiri, A. Shabbir, T. Javida and H. Irshada, RSC Adv., 2024, 14, 21682 CAS.
- A. Shabbir, S. A. Shahzad, A. Y. A. Alzahrani, Z. A. Khan, M. Yar and W. Rauf, Spectrochim. Acta, Part A, 2025, 327, 125414 CAS.
- Y. Li, Z. Cai, S. Liu, H. Zhang, S. T. H. Wong, J. W. Y. Lam, R. T. K. Kwok, J. Qian and B. Z. Tang, Nat. Commun., 2020, 11, 1255 CAS.
- X. Huang, Y. Zhou, L. Qian, M. Liu, Y. Cheng and H. Wu, J. Mater. Chem. C, 2018, 6, 5075–5096 CAS.
- H. Sun, W.-H. Sun, Y. Jiang, J.-H. Wei, Y. Zhao, R. Zhang and Z.-H. Ni, Dyes Pigm., 2020, 173, 107938 CrossRef CAS.
- W. Yang, Y. Yang, L. Zhan, K. Zheng, Z. Chen, X. Zeng, S. Gong and C. Yang, Chem. Eng. J., 2020, 390, 124626 CrossRef CAS.
- A. Majeed, S. A. Shahzad, M. A. Assiri, K. O. Khan, H. Rabale and A. Shabbir, Spectrochim. Acta, Part A, 2025, 331, 125802 CrossRef CAS.
- P. Chen, H. Zhu, L. Kong, X. Xu, Y. Tian and J. Yang, Dyes Pigm., 2020, 172, 107832 CrossRef CAS.
- K. Zhang, T.-T. Chen, Y.-J. Shen, L.-F. Zhang, S. Ma and Y. Huang, Sens. Actuators, B, 2020, 311, 127887 CrossRef CAS.
- H. S. Jung, P. Verwilst, W. Y. Kim and J. S. Kim, Chem. Soc. Rev., 2016, 45, 1242–1256 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: The detailed experimental procedure and related spectroscopic data. CCDC 2284607, 2284616 and 2284543. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5tc00289c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |