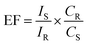Open Access Article
Open Access ArticleFabrication of large-area nanostructures of pine needles with a dewdrop array for surface-enhanced Raman spectroscopy†
Jing
Du
a,
Kuanguo
Li
 *b,
Yonghua
Lu
*b,
Yonghua
Lu
 *ac and
Pei
Wang
ac
*ac and
Pei
Wang
ac
aDepartment of Optics and Optical Engineering, University of Science and Technology of China, Hefei, 230026, China. E-mail: yhlu@ustc.edu.cn
bSchool of Physics and electronic information, Anhui Normal University, Wuhu, 241002, China. E-mail: likguo@ahnu.edu.cn
cHefei Comprehensive National Science Center, Hefei, China
First published on 6th March 2025
Abstract
Surface-enhanced Raman spectroscopy (SERS) depends on the development of a nanostructured substrate on which the excitation of a localized surface plasmon enhances the Raman scattering signals. Herein, we proposed a large-area three-dimensional (3D) pine needle with a dewdrop array (PNDA) nanostructure that can be easily fabricated via a film deposition technique with the help of a self-assembled polystyrene microsphere template and ultra-thin anodized aluminum oxide mask. Electromagnetic hotspots generated at the cracks or gaps between adjacent island structures of the PNDA are responsible for the SERS enhancement factor of 6.7 × 106 when the structural parameters of the PNDA substrate are optimized. Experiments demonstrated that the rhodamine 6G (R6G) molecule can be probed with the PNDA substrate at the lowest concentration of 10−9 M using SERS. The homogeneity of the substrate was confirmed by verifying the relative standard deviation (RSD) of Raman spectra at different sites (6.5% at 611 cm−1 and 8.3% at 1652 cm−1). Moreover, crystal violet (CV) molecules were probed using our SERS experiment at the lowest detection concentration of 10−8 M. The results confirm that the PNDA structure is a reliable and sensitive SERS substrate to detect trace amounts of pollutants in an aquatic environment.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) is a sensitive vibrational spectroscopy technique that can detect a molecular fingerprint at extremely low concentration. SERS technology has been broadly applied in various fields of biomedicine,1–4 environment monitoring,5,6 food safety7–10 and photocatalysis.11–13 The high sensitivity of SERS detection is attributed to the electromagnetic “hotspots” at the sharp tip14,15 or confined gap16–18 of the metallic nanostructures because of their local surface plasmon resonance. For the development of a sensitive SERS technique, the fabrication of metallic nanostructures that allow dense and uniform “hotspots” is particularly important.Classical top-down techniques of electron beam lithography (EBL),19,20 nanoimprint lithography (NIL)21,22 and focused ion beam (FIB) lithography23,24 have been utilized to fabricate ordered nanostructures as SERS substrates owing to their accurate controllability and remarkable uniformity. However, these techniques suffer from the limitations of being expensive, having a low throughput and being time-consuming. To circumvent the above limitations, certain low-cost fabrication methods, including displacement Talbot lithography (DTL),25 electrodeposition,26,27 microsphere lithography28–30 and anodized aluminum oxide (AAO),31–33 were developed to fabricate large-area ordered SERS substrates. Among these low-cost methods, fabrication with a monolayer of polystyrene (PS) microsphere template34,35 or ultra-thin AAO (UTAAO) mask36,37 is more attractive owing to its high throughput and low cost. Many two-dimensional (2D)18,34,36 and three-dimensional (3D)11,13,38,39 ordered arrays of nanostructures have been fabricated using the self-assembled PS microsphere template or UTAAO mask. Compared with 2D nanostructures, 3D plasmonic nanostructures can yield additional electromagnetic “hotspots,” which are helpful for enhancing SERS sensitivity.13,29,40 Therefore, the development of large-area 3D-ordered SERS substrates using cheap and facile approaches are worth exploring.
Herein, we proposed a large-area 3D pine needle with a dewdrop array (PNDA) substrate fabricated via the simple deposition of silica and silver film with a self-assembled PS microsphere template and UTAAO mask. Because of the prominent enhancement of the electric field at the cracks and gaps between adjacent island structures, SERS with a PNDA substrate was used to probe rhodamine 6G (R6G) molecules with high sensitivity and good uniformity at different measurement sites. Using the PNDA substrate, crystal violet (CV) molecules, known as a hazardous pollutant in water, were sensitively detected using SERS with a linear correlation between the Raman signal and concentration. Results indicate that the PNDA structure is a reliable and sensitive SERS substrate to probe the trace amount of pollutants in an aquatic environment.
2. Experimental
2.1. Materials
Rhodamine 6G (R6G), crystal violet (CV), silver wire (99.9%), acetone (AR, GC ≥ 99.5%), ethanol (AR, GC ≥ 99.7%), sulfuric acid (H2SO4, AR, GC ≥ 95%) and hydrogen peroxide (H2O2, AR, GC ≥ 30%) were purchased from Sinopharm Chemical Reagent Co., Ltd. PS microspheres (diameters of 500, 1000 and 2000 nm) were obtained from Huge Biotechnology (Shanghai). The UTAAO mask (125 nm period, 100 nm pore diameter, 400 nm thickness) was obtained from TopMembranes Technology (Shenzhen). The 18.0 MΩ deionized water utilized for the experiments was supplied by our laboratory.2.2. Fabrication of PNDA
Glass slides were sequentially subjected to ultrasonic cleaning in acetone, ethanol, piranha solution (98% H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% H2O2 = 3
30% H2O2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
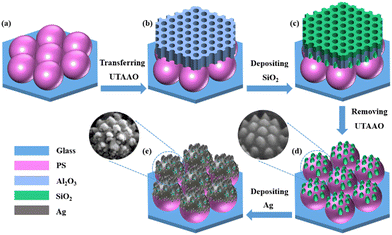
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and deionized water. As can be seen in Fig. 1, the PNDA substrate was fabricated by depositing silica and silver through the UTAAO mask and self-assembled PS microsphere template. First, a centimeter-sized monolayer of PS microsphere array was self-assembled at the gas–liquid interface34,35 and then transferred to a cleaned glass slide (Fig. 1(a)). After drying naturally, the PS microsphere array on the glass slide was placed on a hot plate and baked at 108 °C for 10 min. Second, a UTAAO mask was transferred to the surface of the PS microspheres array (Fig. 1(b)). The commercially bought UTAAO was prudently shifted onto the hydrophilic and smooth Si substrate, and then, the UTAAO held by a Si substrate was gently inserted in the deionized water. The UTAAO mask floated on the water surface because of surface tension, and then, the UTAAO was laid on the PS microsphere array by inserting the substrate prepared in the first step. Third, silicon oxide (SiO2) was deposited on the surface of PS microspheres through the holes of the UTAAO mask (Fig. 1(c)) using electron beam (E-beam) evaporation at the rate of 1 Å s−1. SiO2 nanopillars were maintained on the surface of PS microspheres after blowing off the UTAAO mask with nitrogen gas (Fig. 1(d)). Finally, Ag was deposited through high-vacuum thermal evaporation at a rate of 1 Å s−1 (Fig. 1(e)), and then, a centimeter-sized PNDA substrate was obtained.
1) and deionized water. As can be seen in Fig. 1, the PNDA substrate was fabricated by depositing silica and silver through the UTAAO mask and self-assembled PS microsphere template. First, a centimeter-sized monolayer of PS microsphere array was self-assembled at the gas–liquid interface34,35 and then transferred to a cleaned glass slide (Fig. 1(a)). After drying naturally, the PS microsphere array on the glass slide was placed on a hot plate and baked at 108 °C for 10 min. Second, a UTAAO mask was transferred to the surface of the PS microspheres array (Fig. 1(b)). The commercially bought UTAAO was prudently shifted onto the hydrophilic and smooth Si substrate, and then, the UTAAO held by a Si substrate was gently inserted in the deionized water. The UTAAO mask floated on the water surface because of surface tension, and then, the UTAAO was laid on the PS microsphere array by inserting the substrate prepared in the first step. Third, silicon oxide (SiO2) was deposited on the surface of PS microspheres through the holes of the UTAAO mask (Fig. 1(c)) using electron beam (E-beam) evaporation at the rate of 1 Å s−1. SiO2 nanopillars were maintained on the surface of PS microspheres after blowing off the UTAAO mask with nitrogen gas (Fig. 1(d)). Finally, Ag was deposited through high-vacuum thermal evaporation at a rate of 1 Å s−1 (Fig. 1(e)), and then, a centimeter-sized PNDA substrate was obtained.
Surface morphology of all samples was characterized using scanning electron microscopy (SEM) (Hitachi, SU8220) at 3 kV. Moreover, the typical SEM images of the samples in different fabrication stage are shown in Fig. S1 (ESI†), and the low-magnification SEM images of the large area of SiO2 nanopillars on the PS array and the PNDA structure are shown in Fig. S2 (ESI†).
2.3. Raman spectrum measurement
R6G molecules were used as probing analytes to estimate the Raman performance of the PNDA substrate. The sample was excited using a 532 nm laser with an objective lens (40×, NA = 0.6), the Raman scattering light was collected after a long pass filter and the Raman spectrum was recorded using the HORIBA iHR550 spectrometer. The excitation laser power of 0.05 mW was used for the R6G probe and 0.1 mW for the CV molecules. The accumulation time was set as 1 s to obtain the Raman spectra of R6G and 5 s to obtain the Raman spectra of CV. After eliminating the baseline and fluorescence background using Origin, clean Raman spectra were obtained.2.4. Electromagnetic field simulation
Distribution of the electromagnetic field in the nanostructure was numerically simulated using the 3D finite-difference time-domain (FDTD) method. The morphology and geometric parameters of nanostructures was obtained from the corresponding SEM images in the simulation. During the FDTD simulation, periodic boundary conditions are imposed along the x- and y-axes, while a perfectly matched layer (PML) is employed at the boundary of the z-axis. A 532 nm plane wave, linearly polarized along the x-direction, is incident on the sample along the z-axis. The y-normal monitor was used to retrieve the near-field electric field distribution within the x–z plane for the nanostructure. A grid size of 1 nm was set to ensure simulation accuracy.3. Results and discussion
3.1. Parameter optimization for PNDA
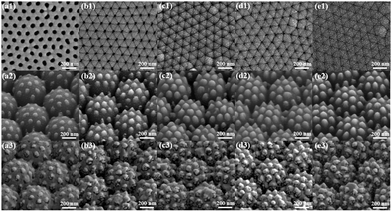
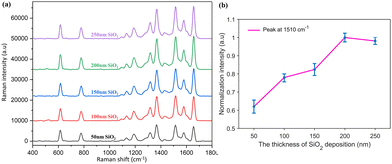
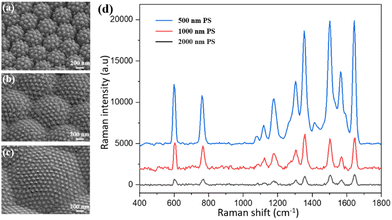
As shown in Fig. 1, the PNDA substrate was fabricated when proper fabrication parameters were chosen. To promote the SERS performance, many fabrication parameters, including the deposition thickness of Ag and SiO2 and the size of PS microspheres, were optimized. We fabricated substrates with different parameters and then characterized the surface morphology through SEM and the SERS of R6G probes on substrates. The detailed influences of every parameter are presented in the following sections.As shown in Fig. 3(a1–e1), the nanopores of AAO gradually shrink because of the deposition of SiO2 and are almost blocked at the SiO2 deposition thickness of 250 nm. The size of the SiO2 nanopillars (Fig. 3(a2–e2)) also increases as the SiO2 deposition thickness increases before the nanopores of AAO are blocked. The SEM images of the different PNDA substrates after Ag deposition are displayed in Fig. 3(a3–e3). The Ag film breaks into additional nanograins as the size of SiO2 nanopillars grows, and therefore, many gaps and cracks are introduced in the Ag film. PNDA substrates with different sizes of SiO2 nanopillars were soaked in 10−5 M R6G for 2 h and then dried using nitrogen gas. Raman spectra of R6G probes for the different substrates were obtained (Fig. 4(a)), and the relative intensities for the typical Raman peak at 1510 cm−1 of R6G molecules are illustrated in Fig. 4(b). As revealed by the Raman spectra, a more pronounced enhancement of the Raman signal is observed for substrates fabricated with larger SiO₂ nanopillars. Note that Raman intensity is slightly weakened for the 250-nm-sized SiO2 deposition thickness possibly because the SiO2 nanopillar size fluctuates when the nanopores of AAO are almost blocked. Therefore, we adopted the SiO2 deposition thickness of 200 nm as the optimal choice for subsequent analyses.
3.2. SERS performance of the PNDA substrate
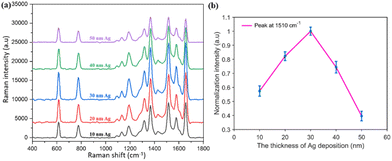
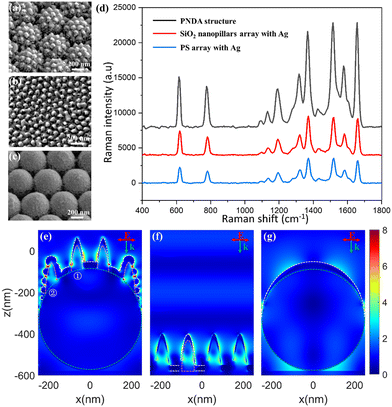
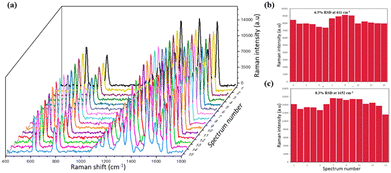
The PNDA substrate was fabricated with the following optimal parameters: an Ag deposition thickness of 30 nm, SiO2 deposition thickness of 200 nm and PS microsphere diameter of 500 nm. The enhancement of Raman scattering in the optimized PNDA substrate was clarified by comparing the Raman spectra of R6G molecules on the PNDA substrate and the substrates that comprise PS microspheres covered in Ag or SiO2 nanopillars covered in Ag. Moreover, the SERS performance of the PNDA substrate, including sensitivity, uniformity and versatility, was investigated.All the samples were soaked in 10−5 M R6G solutions for 2 h, and then, the Raman spectrum was obtained. As can be seen in Fig. 6(d), the Raman spectrum is distinctly enhanced more by the PNDA substrate than by the other two substrates. Quantitatively, the SERS of the PNDA substrate at 1510 cm−1 is approximately threefold stronger than that of the SiO2 nanopillars array substrate covered with 30 nm Ag and fourfold stronger than that of 500 nm PS microsphere array substrate covered with 30 nm Ag. The different Raman intensities can be attributed to the different electromagnetic enhancement of substrates, which can be clarified using FDTD simulations. The simulated electromagnetic field distributions in the cross-section of the three substrates are shown in Fig. 6(e–g). The boundaries of the different materials are indicated by dashed lines: the white dashed line indicates the boundary of silver, the green dashed line indicates the boundary of the PS microsphere, and the pink dashed line indicates the boundary between silica and silver. As can be seen in Fig. 6(e), the electromagnetic field is enhanced by not only the propagating surface plasmon along the surface of nanopillars but also the localized surface plasmon caused by the gaps, cracks and silver protrusions.
Moreover, enhanced electromagnetic field from the localized surface plasmon can be seen as a bright “hotspot” in simulations. As can be seen in Fig. 6(f), cracks such as that on the left bottom of the no. 1 SiO2 nanopillar is wider than those in Fig. 6(e), and localized surface plasmon cannot be excited in such wide cracks with only surface plasmons propagating along the surface nanopillars. For the PS microsphere covered with a silver film, the simulation indicates that only the propagating surface plasmon is excited (Fig. 6(g)). Simulations demonstrate that the local electromagnetic field of the PNDA substrate is enhanced more than the other two substrates, which explains why the SERS signal is highest for the PNDA substrate and weakest for the substrate of the PS array with a Ag film (Fig. 6(d)).
With the aim of estimating the SERS activity, we determined the enhancement factor (EF) of the PNDA substrate according to the following equation:37
3.3. Versatility of the PNDA substrate
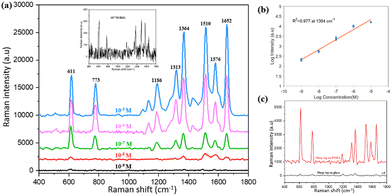
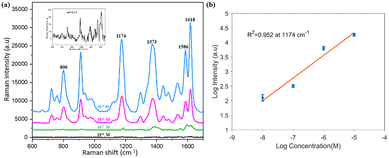
We demonstrated that PNDA can work not only as a SERS substrate for R6G molecules but also for versatile probe molecules. CV is used in fish farming because it is bactericidal and antiparasitic as well as toxic and teratogenetic43 and thus harmful to human health; therefore, it is meaningful to detect the presence of CV. Fig. 9(a) shows the Raman spectra corresponding to Raman intensities with a variety of CV solution concentrations (from 10−5 to 10−8 M) tested using PNDA substrates, which were soaked in these CV solutions for 2 h and dried using nitrogen gas. When CV concentrations slowly decrease, the Raman intensities gradually decrease as well. The inset of Fig. 9(a) is the Raman spectrum with 10−8 M CV, which indicates that the spectrum remains distinguishable. Furthermore, Fig. 9(b) demonstrates the Raman intensities spectra with diverse concentrations of CV solutions, which ranges from 10−5 to 10−8 M at 1174 cm−1, and shows splendid linear correlation, and the correlation coefficient is 0.952 in the logarithmic coordinate. The results demonstrate that the PNDA structure can be used for the quantitative detection of contaminants in water.4. Conclusions
We demonstrated a facile construction procedure based on the self-assembled PS microsphere template and UTAAO mask with a thin film deposition technique to fabricate a large-area PNDA structure. Owing to the prominent enhancement of electric field at the cracks and gaps between adjacent island structures, the optimized PNDA substrate exhibited prominent SERS property with high sensitivity (EF = 6.7 × 106) and uniformity (RSD = 6.5% and 8.3%) for measuring R6G analytes. Moreover, the PNDA substrate was demonstrated to effectively detect crystal violet (CV) molecules, known as a hazardous pollutant in water, exhibiting robust SERS sensitivity and a strong linear correlation between the Raman signal and concentration levels. Results indicate that the PNDA substrate can be potentially applied for the development of high-property SERS technique aimed at accurately identifying trace pollutants in an aquatic environment.Data availability
All data supporting the findings of this study are presented in the article and ESI.† Additional data are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2023YFF0715501; 2023YFF0715502), National Natural Science Foundation of China (U20A20216), and Anhui Provincial Key Research and Development Project (202304a05020009). Nanofabrication and SEM characterizations were carried out at the USTC Center for Micro and Nanoscale Research and Fabrication.References
- F. Lu, L. M. Li, K. Shen, Y. Y. Qian, P. F. Zhang, Y. Yang, Q. S. Zhu, Y. Huang, C. X. Yan and W. Wei, Microchim. Acta, 2024, 191, 653 CrossRef CAS PubMed.
- C. Y. Song, Y. Liu, X. Y. Jiang, J. J. Zhang, C. Dong, J. X. Li and L. H. Wang, Talanta, 2019, 205, 120137 CAS.
- M. Zhu, J. Y. Gao, Z. Y. Chen, X. Sun, Y. Duan, X. C. Tian, J. Gu, Q. Q. Shi and M. Sun, Microchim. Acta, 2024, 191, 40 CrossRef CAS PubMed.
- Y. B. Wang, H. J. Ni, H. Li, J. Chen, D. Q. Zhang and L. L. Fu, Chem. Eng. J., 2022, 442, 136140 CrossRef CAS.
- Z. H. Li, K. H. Han, A. X. Zhang, T. Wang, Z. L. Yan, Z. Ding, Y. H. Shen, M. F. Zhang and W. Zhang, Talanta, 2024, 266, 125070 CrossRef CAS PubMed.
- T. T. Zhang, L. Zhang, S. Y. Wu, G. Y. Wang, X. H. Huang, W. H. Li, C. Liu, Z. Kong, J. S. Li and R. Lu, J. Agric. Food Chem., 2024, 72, 865–873 CrossRef CAS PubMed.
- J. Meng, S. H. Qin, L. Zhang and L. B. Yang, Appl. Surf. Sci., 2016, 366, 181–186 CrossRef CAS.
- X. J. Li, L. J. Li, Y. Z. Wang, X. H. Hao, C. Z. Wang, Z. S. Yang and H. F. Li, Spectrochim. Acta, Part A, 2023, 299, 122877 CrossRef CAS PubMed.
- X. Y. Shao, Q. Zhao, J. Y. Xia, M. X. Xie, Q. Z. Li, Y. Q. Tang, X. F. Gu, X. F. Ning, S. S. Geng, J. Fu and S. Tian, Talanta, 2024, 274, 125989 CrossRef CAS PubMed.
- Q. Z. Wang, Y. J. Zhao, T. Bu, X. Wang, Z. H. Xu, H. Zhangsun and L. Wang, Sens. Actuators, B, 2022, 352, 131025 CrossRef CAS.
- Q. Chu, W. Wang, S. Guo, E. Park, S. Jin, Y. Park, L. Chen, Y. C. Liu and Y. M. Jung, ACS Appl. Mater. Interfaces, 2023, 15, 11304–11313 CrossRef CAS PubMed.
- J. Guo, M. Zhang, Z. Z. Yin, C. S. Ding, P. Chen, W. Gan, H. Yu and Z. Q. Sun, Appl. Surf. Sci., 2022, 592, 153265 CrossRef CAS.
- Y. Jiao, Y. Y. Pan, M. R. Yang, Z. Li, J. Yu, R. Fu, B. Y. Man, C. Zhang and X. F. Zhao, Nanophotonics, 2024, 13(3), 307–318 CrossRef CAS PubMed.
- M. J. Mulvihill, X. Y. Ling, J. Henzie and P. D. Yang, J. Am. Chem. Soc., 2010, 132, 268–274 CrossRef CAS PubMed.
- X. X. Li, X. Lin, X. L. Zhao, H. Y. Wang, Y. Y. Liu, S. Lin, L. Wang and S. L. Cong, Appl. Surf. Sci., 2020, 518, 146217 CrossRef CAS.
- Z. D. Yan, W. Du, L. L. Tu, P. Gu, Z. Huang, P. Zhan, F. X. Liu and Z. L. Wang, J. Raman Spectrosc., 2015, 46, 795–801 CrossRef CAS.
- Y. Q. Liu, L. S. Zhang, X. Liu, Y. Z. Zhang, Y. Z. Yan and Y. Zhao, Spectrochim. Acta, Part A, 2022, 270, 120803 CrossRef CAS PubMed.
- S. S. Yang, G. Q. Liu, L. P. Meng, X. Wang, Y. Xiong, Q. P. Luo and S. J. Feng, Opt. Mater., 2021, 112, 110788 CrossRef CAS.
- W. S. Yue, Z. H. Wang, Y. Yang, L. Q. Chen, A. Syed, K. Wong and X. B. Wang, J. Micromech. Microeng., 2012, 22, 125007 CrossRef.
- N. Chamuah, G. P. Vaidya, A. M. Joseph and P. Nath, Plasmonics, 2017, 12, 1353–1358 CrossRef CAS.
- Y. L. Wang, Z. S. Wang, C. N. Chen, J. Q. Liu, J. X. Lu and N. Lu, Anal. Chem., 2023, 95, 14184–14191 CrossRef CAS PubMed.
- V. Suresh, L. Ding, A. B. Chew and F. L. Yap, ACS Appl. Nano Mater., 2018, 1, 886–893 CrossRef CAS.
- Y. Y. Lin, J. D. Liao, Y. H. Ju, C. W. Chang and A. L. Shiau, Nanotechnology, 2011, 22, 185308 CrossRef PubMed.
- X. Q. Zhang, W. J. Salcedo, M. M. Rahman and A. G. Brolo, Surf. Sci., 2018, 676, 39–45 CrossRef CAS.
- Y. Wang, L. T. Dong, M. N. Liu, M. Z. Ouyang, J. Yang and Z. B. Zhang, IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, Chengdu, China, 2023, pp. 290–294.
- C. Liu, J. Wu, S. Wang and J. H. Fang, J. Electroanal. Chem., 2022, 909, 116120 CrossRef CAS.
- T. T. Fan, Y. Ke, L. J. Zhang, L. Cai and Z. B. Li, CrystEngComm, 2022, 24, 4764–4771 RSC.
- J. R. Liang, L. Z. Yu, Y. X. Wang, T. Xue, D. Y. Lei, Z. Y. Wang and X. Z. Li, Adv. Opt. Mater., 2022, 10, 2102615 CrossRef CAS.
- S. H. Luo, A. Mancini, E. K. Lian, W. Q. Xu, R. Berte and Y. Li, Nanomaterials, 2022, 12, 3842 CrossRef CAS PubMed.
- Y. J. Xia, M. N. Sun, R. J. Huang, S. Y. Qi, L. Zhang, Y. R. Jia, Z. H. Li, H. L. Xu, M. K. Wang, W. Huang, J. T. Zhang, A. G. Wu and B. Chen, New J. Chem., 2024, 48, 10104 RSC.
- S. S. Yan, C. H. Zhu, A. Y. Wang, J. C. Sun, Y. J. Hang, B. Chen, X. J. Wang, N. Q. Wu, H. B. Tang, L. Y. Wen and G. W. Meng, Adv. Opt. Mater., 2023, 11, 2300508 CrossRef CAS.
- Y. Q. Liu, M. H. Li, L. L. Liang, C. Feng, Y. Z. Zhang, X. Liu and Y. Zhao, Opt. Mater., 2022, 128, 112381 CrossRef CAS.
- J. Ma, W. Liu, Z. Ma, P. S. Song, Y. Q. Zhao, F. H. Yang and X. D. Wang, Nanoscale, 2019, 11, 20194–20198 RSC.
- S. S. Yan, H. B. Tang, J. C. Sun, C. H. Zhu, Q. J. Pan, B. Chen and G. W. Meng, Adv. Opt. Mater., 2024, 12, 2302010 CrossRef CAS.
- M. Kong, Y. Y. Wu, D. D. Men, Q. Q. Ding and H. H. Zhang, RSC Adv., 2023, 13, 36181–36187 RSC.
- K. G. Li, X. Y. Tang, H. Y. Wang, M. H. Huang, G. J. Liu, Y. Zhou, W. X. Huang, Z. W. Zuo and Y. H. Lu, Appl. Surf. Sci., 2023, 613, 156117 CrossRef CAS.
- K. G. Li, X. Y. Tang, H. Y. Wang, M. H. Huang, G. J. Liu, Y. Zhou, W. X. Huang and Z. W. Zuo, Appl. Surf. Sci., 2024, 669, 160544 CrossRef CAS.
- D. Wang, G. C. Xu, X. S. Zhang, H. Y. Gong, L. Jiang, G. L. Sun, Y. Li, G. R. Liu, Y. Li, S. K. Yang and X. Liang, Sens. Actuators, B, 2022, 359, 131512 CrossRef CAS.
- Y. Wang, B. Ai, Z. Y. Wang, Y. D. Guan, G. Xiao and G. Zhang, Appl. Surf. Sci., 2023, 615, 156271 CrossRef CAS.
- K. G. Li, X. Y. Tang, G. J. Liu, J. J. Mi, J. Du, W. X. Huang, Z. W. Zuo and Y. H. Lu, Appl. Surf. Sci., 2021, 570, 151069 CrossRef CAS.
- L. W. Ma, Y. Huang, M. J. Hou, Z. Xie and Z. J. Zhang, Sci. Rep., 2015, 5, 15442 CrossRef CAS PubMed.
- H. Y. Wei and H. Eilers, J. Phys. Chem. Solids, 2009, 70, 459–465 CrossRef CAS.
- X. J. Chen, Q. L. Wang, L. X. Qin, X. X. Liu, S. Z. Kang, T. Y. Zhang and X. Q. Li, Mater. Adv., 2022, 3, 2583 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed SEM pictures and diagram for film deposition. See DOI: https://doi.org/10.1039/d5tc00211g |
| This journal is © The Royal Society of Chemistry 2025 |