Flexible organic crystal-quantum dot hybrids with adjustable waveguides†
Xuesong
Yang
,
Boyang
Gao
,
Yi
Liu
 ,
Baolei
Tang
*,
Hao
Zhang
* and
Hongyu
Zhang
,
Baolei
Tang
*,
Hao
Zhang
* and
Hongyu
Zhang
 *
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Qianjin Street, Changchun, 130012, P. R. China. E-mail: tangbl15@mails.jlu.edu.cn; hao_zhang@jlu.edu.cn; hongyuzhang@jlu.edu.cn
First published on 17th February 2025
Abstract
Organic molecular crystals have shown great potential in integrated photonics due to their long-distance ordered packing structures and optical merits. However, single-component organic crystals often emit mono-color light, limiting full exploitation of their advantages. Herein, we demonstrate a modular approach to impart multicolor luminescence to organic single crystals while preserving their crystallinity. Semiconductor quantum dots (QDs) with distinguishable fluorescence are conformally coated onto flexible organic crystals via a layer-by-layer electrostatic self-assembly strategy. The resultant hybrid crystals integrate emissions from both QDs and original crystals, and can function as flexible optical waveguides with adjustable outputs. Exciting different positions of the hybrid crystals leads to multicolor output signals at the crystal end, which can be decoded into encrypted messages. This work provides a facile strategy to combine the advantages of QDs and organic crystals for diverse photonic applications.
Introduction
Organic molecular crystals have garnered tremendous research interest as emerging photonic materials due to their intrinsically low dielectric constants, diversified choices of chromophores, and amenable flexibility and processability.1–12 Their highly ordered molecular packing configurations and rich intermolecular interactions endow pronounced optical properties, enabling diverse applications such as organic lasers, field-effect transistors, and photovoltaics.13–20 In particular, their long-range energy migration, low propagation loss of light, and mechanical bendability make organic crystals intriguing platforms for integrated photonics and optoelectronics.21–26 A variety of optical components have been created by manipulating organic microcrystals, including beam splitters, interferometers and couplers.17,18,27,28 Nevertheless, single-component organic crystals often emit light of a defined wavelength, limiting full exploitation of their potential for multicolor photonics. Composite approaches by doping or mixed crystallization have been explored, but often lead to disruption of molecular arrangements and thereby degraded performance.29–33 It remains challenging yet desirable to develop new strategies that can impart multicolor fluorescence to organic crystals while preserving their optical merits and mechanical compliance. Semiconductor quantum dots (QDs) act as light-emitting materials spanning visible to infrared wavelengths, boasting high photoluminescence (PL) quantum yields, substantial absorption cross sections, and exceptional photostability.34–37 Recently, a burgeoning avenue has emerged, focusing on the integration of organic crystals with diverse functional materials like magnetic nanoparticles, conductive substances, and polymers. This integration yields hybrid materials showcasing multifunctionality for a spectrum of applications—ranging from multi-directional optical signal transmission to the realms of soft robotics and flexible electronics.38–43 However, the fusion of QDs with organic crystals remains unexplored. The amalgamation of QDs’ strengths with those of organic crystals holds immense potential, necessitating nuanced strategies to forge hybrid components that uphold their photophysical properties without compromise. Researchers have attempted to achieve modulation of multicolored optical signals through QD-polymer hybrids or doped organic crystals, and have demonstrated a wide range of potential applications.44–47 However, hybrid materials exhibit unique advantages over the above materials. Hybrid organic crystals have long-range ordered structures and low optical loss coefficients, and maintain the crystallinity of the crystals while maintaining excellent flexibility. These properties make the hybrid materials very promising for applications in optical electronic components.Here we demonstrate a modular and scalable approach to impart multicolor luminescence to flexible organic crystals by conformal QD coatings, while preserving the crystallinity, mechanical flexibility, and optical transducing capability of the original crystals. Four aqueous CdTe QDs emitting distinctive green, yellow, orange and red fluorescence were employed. Elastically bendable organic crystals were modified by alternate deposition of polycations and polyanions to form uniform coatings. Afterwards, the surface-charged crystals were immersed in QD aqueous solutions, enabling electrostatic self-assembly of multiple luminescent QD layers. The resultant hybrid crystals coupled emissions from both components and acted as flexible optical waveguides. Excitation at different positions led to multicolor output signals at the crystal ends, which were decoded into encrypted messages. The modular integration of QDs with organic crystals creates diverse possibilities for optical waveguides, light modulation, and integrated photonics.
Results and discussion
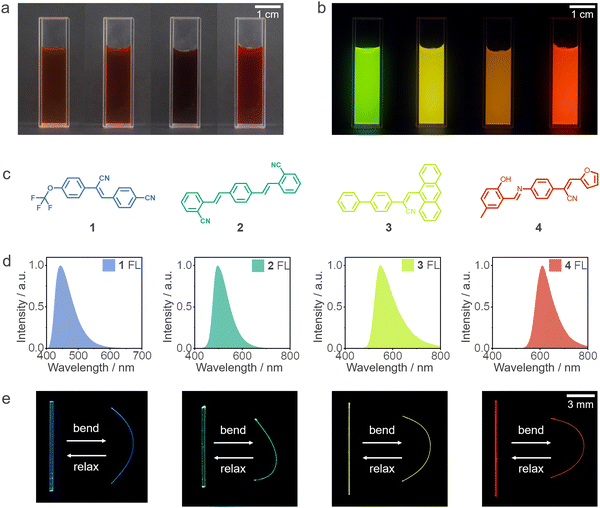
Aqueous CdTe QDs with negative charges were directly synthesized in aqueous media according to the previous method.48 As illustrated in Fig. S1 (ESI†), CdTe QDs with specific PL emission colors, spanning from green to red, were obtained by altering the reaction duration. Four CdTe QDs with distinct green, yellow, orange, and red emission were employed for further investigations (Fig. 1a and b), which correspond to the PL emission centered at 560, 583, 610, and 631 nm, respectively (Fig. S2 and S3, ESI†). As shown in Fig. 1c, four organic small-molecule compounds, namely 1–4, were synthesized to prepare hybrid materials, following established procedures outlined in prior reports.41,49 Solvent evaporation was utilized to grow needle-like crystals of these compounds. These crystals exhibited dimensions, with lengths reaching the order of centimeters, while their widths and thicknesses ranged from tens to hundreds of micrometers. The emission spectra of crystals 1–4 were characterized, as depicted in Fig. 1d. Fig. 1e demonstrates the flexibility of these crystals, as they can be bent without fracturing or breaking when subjected to an external force applied to their ends, and rebound after relaxing. This flexibility can be attributed to the extensive intermolecular interactions prevalent within their structures, notably including hydrogen bonding and π–π interactions.As shown in Fig. 2a, the fabrication of hybrid materials for multicolor fluorescence can be delineated into the ensuing two phases: primordially, modifying the crystal surface via an aqueous solution comprising positively charged poly(diallyldimethylammonium chloride) (PDDA) and negatively charged poly(sodium styrenesulfonate) (PSS), referred to as PDDA/PSS//1–4; subsequently, employing the PDDA and negatively charged CdTe to deposit upon the surface of the polymer-encased crystals, culminating in the production of the ultimate hybrid materials, referred to as PDDA/CdTe//PDDA/PSS//1–4 (henceforth denoted as Q//1–4, for convenience (Q = quantum dot)). The thickness of the hybrid materials was examined using scanning electron microscopy (SEM). As depicted in Fig. 2a, the thickness of the PDDA/PSS layer was determined to be approximately 460 nm, while the PDDA/CdTe layer exhibited a thickness of around 450 nm. As shown in Fig. 2b and c, visual records of distinct PDDA/CdTe layers alongside the corresponding spectra of the QD deposition sites, with varying numbers of deposition, were recorded. The color of the QDs situated on the surface of Q//2 progressively intensified as the number of PDDA/CdTe layers increased, and simultaneously, the spectra experienced a gradual red-shift (Fig. S4, ESI†). This led to a transformation in the fluorescence color on the side of crystal 2, transitioning from green to red. The roughness of the hybrid material was found to be minimal, with an average roughness value of 74 nm, as depicted in Fig. 2d. The surface exhibited a consistent and even dispersion of QDs, firmly affixed to the crystal surface (Fig. 2e). As shown in Fig. 2f, the surface of crystal 1 underwent individual depositions of four distinct fluorescent QDs (green, yellow, orange, and red), resulting in the transformation of the originally blue fluorescent crystal 1 into various fluorescent hybrid materials. In addition, the hybrid materials remained fluorescent after repeated bending and after a long period of placement (20 °C, RH = 31%) (Fig. S5–S7, ESI†). To test whether the mechanical properties of the crystals were significantly changed after coating with quantum dots, the mechanical properties of the 2, 3, Q//2, and Q//3 crystals were measured and compared (Fig. S8, ESI†). The results confirmed that the mechanical properties of the hybrid organic crystals were essentially unchanged compared to the pristine crystals. As shown in Fig. S9 (ESI†), to realize the high-volume preparation of hybrid materials, multiple crystals were fixed on the same glass plate, and the preparation efficiency was effectively improved by repeating the automated self-assembly process several times through the coater. These experiments demonstrated the efficacy and ease of the fluorescent hybridized material preparation method.
Accurate control over the length and placement of the QD coating was paramount for advancing the applications of the hybrid materials. Precise adjustment of the crystal length during its immersion into the CdTe aqueous solution enabled the tuning of the QD coating length for PDDA/PSS//1–4. The resulting fluorescence of Q//1–4 comprised a combination of the CdTe coating and the pristine crystals 1–4 (Fig. 3a). In Fig. S10 (ESI†), spectroscopic measurements were conducted for both the CdTe coating and the overlapping region of the pristine crystals 1–4 with the CdTe coating. The CIE coordinates revealed an evident optical coupling between the fluorescence of the QD coatings and the crystals themselves. Likewise, in the subsequent experiment, two distinct QD solutions with different fluorescence (yellow, red) were sequentially deposited onto the surface of PDDA/PSS//1–4. Initially, PDDA/PSS//1–4 was immersed in the CdTe (red) solution, followed by a reduction in the immersion time in the CdTe (yellow) solution (Fig. 3b). This process led to the emergence of Q//1–4 fluorescence, comprising a harmonious amalgamation of the CdTe (yellow and red) coatings, the CdTe (red) coatings, and the pristine crystals 1–4. The fluorescence signals emitted from the CdTe (yellow and red) coatings, as well as CdTe (red), were acquired and shown in Fig. S11 (ESI†). The CIE coordinates indicate a substantial redshift in the fluorescence of the crystals, signifying a significant alteration in their optical characteristics (Fig. S11b, ESI†). The creation of segmentally deposited QD coatings on the crystal surface involved a spin-coating process, wherein small droplets are dispersed from the tip of a syringe needle, aspirating an aqueous solution of CdTe (green, yellow, and red) onto the surface of PDDA/PSS//1, as illustrated in Fig. 3c. Through spectroscopic analysis, it was verified that the fluorescence of the QDs was coupled to the crystal emission, and the CIE coordinates further demonstrated a noticeable shift towards red gamut (Fig. 3d and Fig. S12a, ESI†). Furthermore, utilizing the same approach, CdTe (green, yellow, orange, red) can be selectively deposited at distinct locations on the same crystal surface (Fig. 3e and Fig. S12b, ESI†). The aforementioned experiments demonstrated the precise control attainable over the position and length of the QD coatings on the crystal surface.
Organic crystal structures offer numerous advantages, including less-defects and long-range ordered arrangements.50 Flexible organic crystals exhibited exceptional optical waveguide performance. Recently, the utilization of controlled hybrid organic crystals in multi-directional optical waveguides has proven highly advantageous, showcasing sensitivity and durability under external stimuli, such as magnetic fields and humidity control.38,39 The impressive performance of flexible organic optical waveguides in the visible and near-infrared spectrum has been well-documented.20,51,52 Demonstrations of various applications, such as interferometers and splitters, have showcased the manipulation of microcrystals using AFM probes, revealing the immense potential of flexible organic crystals for future technological advancements.53–55
As shown in Fig. 4a, hybrid organic crystals were achieved by depositing CdTe QDs onto the surface of PDDA/PSS//1, resulting in distinct fluorescence characteristics. To confirm that the hybrid organic crystal had the performance of the optical signal transmission, the optical waveguide performance of Q//1 was tested. The emission intensity of the tip gradually decreased as the distance from the irradiation position to the tip increases, which was due to the increased loss of emitted light as the propagation distance increases. Distance-dependent emission spectra were acquired by irradiating distinct positions of Q//1 using the identical excitation light from a 355 nm pump laser. The emission spectra corresponding to each irradiation position was collected at the uncoated CdTe end of Q//1, as depicted in Fig. 4e. As shown in Fig. 4a–d, the excitation of 355 nm light at different locations of the hybrid organic crystal (either at the original crystal or the CdTe coating) results in discernible light signals. The fluorescence of the QDs transduces through the crystal interior, reaching the tip of the crystal. The spectral signals confirmed the optical coupling between the fluorescence of the crystal and that of the QDs (Fig. 4e). This coupling phenomenon of fluorescence mainly depended on the radiative energy transfer mechanism. As shown in Fig. 1d and Fig. S2 (ESI†), the existence of the emission spectrum of the organic crystal overlapped with the absorption spectrum of the quantum dots. When the organic crystal was irradiated by UV light, the fluorescence emitted by the crystal could effectively excite the quantum dots to emit fluorescence. For example, in Fig. 4d and e, when the organic crystal at position 0 (uncoated quantum dots) was excited by UV light, the output signal spectra contained both the blue signal of the organic crystal and the red signal of the quantum dots, which further demonstrated the existence of fluorescence coupling. Fig. S13a–d (ESI†) demonstrates that the optical signals transmitted by the hybrid material exhibited a wide distribution in the CIE coordinate diagram. In a similar manner, the emission spectra corresponding to each irradiation position were collected at the CdTe-coated end of Q//1 (Fig. 4f). The discrepancy between the collected optical signals at the CdTe-coated end and those obtained at the uncoated CdTe end of Q//1 was attributed to the excitation of CdTe QDs by the blue fluorescence of crystal 1. The coupling between the fluorescence of the crystal and that of the QDs was evident, further affirming their interconnected optical behavior, and the optical signal exhibited extensive distribution in the CIE coordinate plots (Fig. S13e–h, ESI†). In addition, the hybrid material still has excellent optical signal transmission in the bending state (Fig. 4g–j). To ascertain the enhanced diversity of optical signal transmission in hybrid organic crystals compared to pristine crystals, optical signal transmission experiments were conducted on crystal 1. The emission spectra corresponding to each irradiation point position of crystal 1 were collected and subsequently fitted to the data in Fig. S14a–d (ESI†), respectively, utilizing the literature program as described.56 As shown in Fig. S14e and f (ESI†), the optical loss coefficients of crystal 1 in the straight and bent states are 0.14698 dB mm−1 and 0.15618 dB mm−1, and no optical signal coupling was observed during the experiments. As shown in Fig. S15 (ESI†), when one end of the crystal Q//1,2 was irradiated by infrared light at 650 nm, the optical signal could be transmitted to the other end, which demonstrated that the hybrid material could perform passive optical signal transmission. To investigate whether quantum dot coating affect the optical signal transmission of organic crystals, the optical loss coefficients of crystal 2 before and after coating with quantum dots were characterized. As shown in Fig. S16 (ESI†), the optical waveguide experiments were performed on crystal 2 and Q//2, respectively. The optical loss coefficient of crystal 2 was calculated to be 0.06651 dB mm−1, while the optical loss coefficients of the Q//2 crystals varied less in different samples, which were 0.09017 dB mm−1, 0.07677 dB mm−1, and 0.10437 dB mm−1, respectively. The experimental results showed that the optical loss coefficients of the hybrid materials were slightly higher than those of the original crystals, indicating that the nanoparticles had almost no effect on the optical signal transmission of the organic crystals. As shown in Fig. S17 (ESI†), the wavelength dependence of the optical signal output of the hybrid crystal Q//1 was explored by different excitation wavelengths of the light source. When excited with 365 nm UV light, the emission spectra of the crystals showed blue fluorescence and fluorescence of red quantum dots; when excited with 530 nm UV light, the emission spectra of the crystals showed fluorescence of the light source and red quantum dots, which indicated that the 530 nm light source was unable to efficiently excite the organic crystals; while under 660 nm UV excitation, the emission spectra of the crystals showed infrared light, which proved that the 660 nm light source was unable to excite the crystal materials and quantum dots, which confirmed the wavelength dependence of the hybrid materials. In order to evaluate the optical signal transmission capability of the hybrid materials, the optical loss coefficients of the hybrid organic crystals and other state-of-the-art optical waveguide materials were compared,23,24,53,57–59 and the hybrid crystals showed excellent performance in optical signal transmission capability as shown in Table S1 (ESI†). To further assess the durability of the hybrid materials, optical waveguide experiments of the hybrid organic crystals were performed under different numbers of bending cycles and under different periods of placement. As shown in Fig. S18 and S19 (ESI†), the optical loss coefficients of Q//3 after 0, 500, and 1000 times of bending were 0.13098 dB mm−1, 0.14316 dB mm−1, and 0.15738 dB mm−1, respectively; and the optical loss coefficients in the pristine state and after 7 days of placement were 0.15289 dB mm−1 and 0.16596 dB mm−1, respectively. These experimental results showed that the hybrid crystals maintained excellent optical signal transmission performance after many times of bending and long term placement.
Encryption technology boasts a vast array of applications within the sphere of information security, and its significance grows in tandem with the continuous advancement of digitalization and the Internet. In Fig. S20 (ESI†), an encrypted messaging instrument using hybrid organic crystals has been designed, where signals were encoded through different spectral signal outputs and identified through the use of ASCII binary (Table S2, ESI†). The hybrid organic crystal was situated atop the silicon wafer, with the uncoated CdTe-coated end connected to the optical signal receiver. By manipulating the position of the 355 nm excitation light (either uncoated or CdTe-coated), distinct optical signals can be captured, with the original fluorescence signal of the crystal designated as “0”, and the coupled optical signal as “1”. Fig. 5a–f illustrates that the left tip of Q//1 emitted two different fluorescence spectra, one in blue and the other in violet, each corresponding to unique emission spectra. The decryption process encompasses three essential steps: firstly, the collection of spectral data; secondly, the conversion of crystal spectra and coupling spectra into binary representations “0” or “1,” respectively; and finally, the translation of the binary code into letters according to the ASCII code. As shown in Fig. 5d–f, the binary codes corresponding to the three encrypted messages were “01001010,” “01001100,” and “01010101,” which, upon decryption, yield the corresponding letters “JLU”, signifying the esteemed abbreviation of Jilin University. Akin experiments were conducted on Q//2, as shown in Fig. 5g–l, where the three encrypted messages correspond to the binary codes “01001110,” “01011001,” and “01010101,” which, upon decryption, transform into the corresponding letters “NYU”. These outcomes convincingly demonstrated the versatile applicability of hybridized organic crystals in multifaceted encryption methodologies. As shown in Fig. S21 (ESI†), the distance of optical signal transmission was changed by adjusting the position of the quantum dot coating of the excitation light source, and the output optical signals were collected on the right side to characterize the error of the output signals of the hybrid crystals. The experimental results show that when the transmission distance is increased by 10 mm, the hybrid materials output signal still maintained a high degree of discrimination. In addition, the hybrid material was placed under different ambient light conditions (dark, low light, normal lighting, and strong light) for encrypted transmission of optical signals, and the experimental results showed that its encrypted spectral signals had a high degree of recognition (Fig. S22, ESI†).
Conclusions
In summary, we have crafted polychromatic fluorescent hybrid organic crystals through a straightforward and efficient methodology. This method enables the creation of hybrid materials regardless of the inherent structures of the crystals, allowing for the seamless optical integration of both crystals and QDs. Variably hued QD coatings, exhibiting distinct luminescent shades, have been deposited onto the crystalline surface. Furthermore, the dimensions and placement of these coatings are amenable to precise adjustments. The resultant hybridized materials retain their exceptional aptitude for optical signal propagation. By manipulating the position of the excitation light source, diverse optical signals can be adroitly transmitted, thus effectuating the capacity for information encryption. The luminescent hybrid materials exhibit pronounced advantages in the orchestration of signal conveyance within pliable optical waveguides, thereby augmenting the potential utility of flexible organic crystals in the realms of optical signal transmission and flexible optoelectronics.Author contributions
X. Y., B. T. and B. G. performed the experiments. Y. L., Hao. Z., and H. Z. supervised the experiments. B. T. Hao. Z. and H. Z. conceived the project.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (523B2032, 52173164, 52373181) and the Natural Science Foundation of Jilin Province (20230101038JC).Notes and references
- S. Hayashi, T. Koizumi and N. Kamiya, Cryst. Growth Des., 2017, 17, 6158–6162 CrossRef CAS.
- S. Hayashi and T. Koizumi, Angew. Chem., Int. Ed., 2016, 55, 2701–2704 CrossRef CAS PubMed.
- S. Hayashi, S. Yamamoto, D. Takeuchi, Y. Ie and K. Takagi, Angew. Chem., Int. Ed., 2018, 57, 17002–17008 CrossRef CAS PubMed.
- P. Commins, D. P. Karothu and P. Naumov, Angew. Chem., Int. Ed., 2019, 58, 10052–10060 CrossRef CAS PubMed.
- L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS PubMed.
- P. Gupta, D. P. Karothu, E. Ahmed, P. Naumov and N. K. Nath, Angew. Chem., Int. Ed., 2018, 57, 8498–8502 CrossRef CAS PubMed.
- M. Annadhasan, D. P. Karothu, R. Chinnasamy, L. Catalano, E. Ahmed, S. Ghosh, P. Naumov and R. Chandrasekar, Angew. Chem., Int. Ed., 2020, 59, 13821–13830 CrossRef CAS PubMed.
- N. Mitetelo, D. Venkatakrishnarao, J. Ravi, M. Popov, E. Mamonov, T. V. Murzina and R. Chandrasekar, Adv. Opt. Mater., 2019, 7, 1801775 CrossRef.
- Y. Takasaki, T. Sasaki and S. Takamizawa, Cryst. Growth Des., 2020, 20, 6211–6216 CrossRef CAS.
- T. Mutai, T. Sasaki, S. Sakamoto, I. Yoshikawa, H. Houjou and S. Takamizawa, Nat. Commun., 2020, 11, 1824 CrossRef CAS PubMed.
- B. Liu, Q. Di, W. Liu, C. Wang, Y. Wang and H. Zhang, J. Phys. Chem. Lett., 2019, 10, 1437–1442 CrossRef CAS PubMed.
- R. Huang, C. Wang, Y. Wang and H. Zhang, Adv. Mater., 2018, 30, 1800814 CrossRef PubMed.
- H. Liu, Z. Lu, Z. Zhang, Y. Wang and H. Zhang, Angew. Chem., Int. Ed., 2018, 57, 8448–8452 CrossRef CAS PubMed.
- H. Liu, Z. Lu, B. Tang, C. Qu, Z. Zhang and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 12944–12950 CrossRef CAS PubMed.
- B. Tang, B. Liu, H. Liu and H. Zhang, Adv. Funct. Mater., 2020, 30, 2004116 CrossRef CAS.
- X. Chu, Z. Lu, B. Tang, B. Liu, K. Ye and H. Zhang, J. Phys. Chem. Lett., 2020, 11, 5433–5438 CrossRef CAS PubMed.
- M. Annadhasan, A. R. Agrawal, S. Bhunia, V. V. Pradeep, S. S. Zade, C. M. Reddy and R. Chandrasekar, Angew. Chem., Int. Ed., 2020, 59, 13852–13858 CrossRef CAS PubMed.
- J. Ravi and R. Chandrasekar, Adv. Opt. Mater., 2021, 9, 2100550 CrossRef CAS.
- Q. Di, L. Li, X. Miao, L. Lan, X. Yu, B. Liu, Y. Yi, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 5280 CrossRef CAS PubMed.
- D. P. Karothu, G. Dushaq, E. Ahmed, L. Catalano, S. Polavaram, R. Ferreira, L. Li, S. Mohamed, M. Rasras and P. Naumov, Nat. Commun., 2021, 12, 1326 CrossRef CAS PubMed.
- S. Tang, K. Ye, P. Commins, L. Li, P. Naumov and H. Zhang, Adv. Opt. Mater., 2023, 11, 2200627 CrossRef CAS.
- X. Pan, A. Zheng, X. Yu, Q. Di, L. Li, P. Duan, K. Ye, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202203938 CrossRef CAS PubMed.
- Y. Wei, X. Xu, X. Yang and H. Zhang, Cryst. Growth Des., 2023, 23, 8204–8211 CrossRef CAS.
- S. Tang, K. Ye and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202210128 CrossRef CAS PubMed.
- B. Tang, X. Yu, K. Ye and H. Zhang, Adv. Opt. Mater., 2022, 10, 2101335 CrossRef CAS.
- B. Liu, H. Liu, H. Zhang, Q. Di and H. Zhang, J. Phys. Chem. Lett., 2020, 11, 9178–9183 CrossRef CAS PubMed.
- V. V. Pradeep and R. Chandrasekar, Adv. Opt. Mater., 2022, 10, 2201150 CrossRef CAS.
- A. V. Kumar, E. Mamonov, T. Murzina and R. Chandrasekar, Adv. Opt. Mater., 2023, 11, 2201507 CrossRef CAS.
- S. Cui, T. Zhu, L. Zhang, X. Ye, Q. Han, C. Ge, Q. Guo, X. Zheng, Q. Lin, C. Li, J. Jiang, W. Yuan, Y. Liu and X. Tao, Adv. Opt. Mater., 2022, 10, 2102355 CrossRef CAS.
- Z. Qin, C. Gao, W. W. H. Wong, M. K. Riede, T. Wang, H. Dong, Y. Zhen and W. Hu, J. Mater. Chem. C, 2020, 8, 14996–15008 RSC.
- J. Han, W. Feng, D. Y. Muleta, C. N. Bridgmohan, Y. Dang, G. Xie, H. Zhang, X. Zhou, W. Li, L. Wang, D. Liu, Y. Dang, T. Wang and W. Hu, Adv. Funct. Mater., 2019, 29, 1902503 CrossRef.
- R. Yoshida, T. Tachikawa and S. Ito, Chem. Commun., 2022, 58, 6781–6784 RSC.
- R. Ding, F. Dong, M. An, X. Wang, M. Wang, X. Li, J. Feng and H. Sun, Adv. Funct. Mater., 2019, 29, 1807606 CrossRef.
- V. Notot, W. Walravens, M. Berthe, N. Peric, A. Addad, X. Wallart, C. Delerue, Z. Hens, B. Grandidier and L. Biadala, ACS Nano, 2022, 16, 3081–3091 CrossRef CAS PubMed.
- Y. Shu, X. Lin, H. Qin, Z. Hu, Y. Jin and X. Peng, Angew. Chem., Int. Ed., 2020, 59, 22312–22323 CrossRef CAS PubMed.
- B. Chen, D. Li and F. Wang, Small, 2020, 16, 2002454 CrossRef CAS PubMed.
- M. J. Smith, C. H. Lin, S. Yu and V. V. Tsukruk, Adv. Opt. Mater., 2019, 7, 1801072 CrossRef.
- X. Yang, L. Lan, L. Li, X. Liu, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 2322 CrossRef CAS PubMed.
- L. Lan, X. Yang, B. Tang, X. Yu, X. Liu, L. Li, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200196 CrossRef CAS PubMed.
- L. Lan, L. Li, Q. Di, X. Yang, X. Liu, P. Naumov and H. Zhang, Adv. Mater., 2022, 34, 2200471 CrossRef CAS PubMed.
- X. Yang, L. Lan, X. Pan, X. Liu, Y. Song, X. Yang, Q. Dong, L. Li, P. Naumov and H. Zhang, Nat. Commun., 2022, 13, 7874 CrossRef CAS PubMed.
- X. Yang, L. Lan, L. Li, J. Yu, X. Liu, Y. Tao, Q. Yang, P. Naumov and H. Zhang, Nat. Commun., 2023, 14, 3627 CrossRef CAS PubMed.
- X. Yang, L. Lan, X. Pan, Q. Di, X. Liu, L. Li, P. Naumov and H. Zhang, Nat. Commun., 2023, 14, 2287 CrossRef CAS PubMed.
- R. Yoshida, T. Tachikawa and S. Ito, Chem. Commun., 2022, 58, 6781–6784 RSC.
- Y. Xia, C. Zhu, F. Cao, Y. Shen, M. Ouyang and Y. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202217547 CrossRef CAS PubMed.
- Q. Zhao, L. Ye, Z. Cheng, S. Hong, R. Penty and I. White, Opt. Laser Technol., 2020, 131, 106358 CrossRef CAS.
- X. Peng, Z. Wu and Y. Ding, Nanomaterials, 2024, 14, 1463 CrossRef CAS PubMed.
- H. Zhang, Z. Zhou, B. Yang and M. Gao, J. Phys. Chem. B, 2003, 107, 8–13 CrossRef CAS.
- L. Lan, H. Liu, X. Yu, X. Liu and H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 11283–11287 CrossRef CAS PubMed.
- L. Sun, Y. Wang, F. Yang, X. Zhang and W. Hu, Adv. Mater., 2019, 31, 1902328 CrossRef PubMed.
- B. Liu, Z. Lu, B. Tang, H. Liu, H. Liu, Z. Zhang, K. Ye and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 23117–23121 CrossRef CAS PubMed.
- J. Ravi, M. Annadhasan, A. V. Kumar and R. Chandrasekar, Adv. Funct. Mater., 2021, 31, 2100642 CrossRef CAS.
- A. V. Kumar, M. Godumala, J. Ravi and R. Chandrasekar, Adv. Opt. Mater., 2024, 12, 2302807 CrossRef CAS.
- A. V. Kumar and R. Chandrasekar, Adv. Opt. Mater., 2023, 11, 2201009 CrossRef CAS.
- M. Annadhasan, S. Basak, N. Chandrasekhar and R. Chandrasekar, Adv. Opt. Mater., 2020, 8, 2000959 CrossRef CAS.
- Y. Li, Z. Ma, A. Li, W. Xu, Y. Wang, H. Jiang, K. Wang, Y. Zhao and X. Jia, ACS Appl. Mater. Interfaces, 2017, 9, 8910–8918 CrossRef CAS PubMed.
- Q. Chen, B. Tang, K. Ye and H. Zhang, Adv. Mater., 2024, 36, 2311762 CrossRef CAS PubMed.
- J. Qi, L. Lan, Q. Chen, L. Li, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202417409 CrossRef CAS PubMed.
- R. Huang, B. Tang, K. Ye, C. Wang and H. Zhang, Adv. Opt. Mater., 2019, 7, 1900927 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc00112a |
| This journal is © The Royal Society of Chemistry 2025 |