Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deep-red to NIR solution-processed OLEDs of donor-decorated quinoxaline-based TADF aggregates†
Abhishek Kumar
Gupta
 *ab,
David B.
Cordes
*ab,
David B.
Cordes
 a,
Joydip
De
a,
Joydip
De
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
Stuart
Warriner
c,
Ifor D. W.
Samuel
a,
Stuart
Warriner
c,
Ifor D. W.
Samuel
 *b and
Eli
Zysman-Colman
*b and
Eli
Zysman-Colman
 *a
*a
aOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, Fife KY16 9ST, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk
bOrganic Semiconductor Centre, SUPA. School of Physics and Astronomy, University of St Andrews, North Haugh, St Andrews, Fife KY16 9SS, UK
cSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds, UK
First published on 22nd January 2025
Abstract
We report the use of an extended π-conjugated electron-deficient “pyrazino[2,3-g]quinoxaline (PQ)” as a strong, planar acceptor in a donor–acceptor thermally activated delayed fluorescence (TADF) emitter design to achieve deep-red to NIR emission. A pair of multi-donor–acceptor (multi-D–A) emitters, 4DMAC-TPPQ and 4PXZ-TPPQ, using PQ as the strong acceptor and dimethylacridine (DMAC) and phenoxazine (PXZ) as strong donors, respectively, emit at 650 and 762 nm in toluene, which is significantly red-shifted in comparison to the parent compound TPPQ (λPL = 456 nm). Both compounds show aggregation-induced emission enhancement in THF:water mixtures. Both compounds exhibit TADF as doped films in 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP) and as neat films. The emission of the neat film is similar to that in toluene with λPL of 654 and 770 nm for 4DMAC-TPPQ and 4PXZ-TPPQ, respectively. Solution-processed organic light-emitting diodes (SP-OLEDs) of neat 4DMAC-TPPQ showed electroluminescence (λEL) at 685 nm and an EQEmax of 0.3%, while the device with 4PXZ-TPPQ emitted at an λEL of 780 nm and showed an EQEmax of 0.04%. The OLEDs with 4PXZ-TPPQ showed the most red-shifted emission amongst those employing TADF emitters containing a quinoxaline-type acceptor.
Introduction
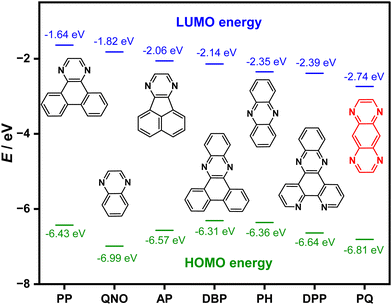
Materials that emit in the deep-red (650–700 nm) to near-infrared (NIR) (700–1400 nm) wavelength range are used in numerous applications, including biomedical imaging,1–3 forensic science,4,5 chemical sensors,6,7 dyes in photovoltaic cells,8 fibre optic telecommunications,9–11 and electroluminescent devices.12 Both transition metal complexes11–13 and conjugated polymers1,14 can be considered rather mature classes of red emitters; however, the development of purely organic emitters in the deep-red to NIR region is challenging because of three main issues. Firstly, red emitters are not as emissive as higher energy analogues due to the energy gap law, which states that radiative decay decreases and nonradiative decay accelerates as the emission wavelength shifts to the red. This is due to vibrational coupling between the excited state and the ground state.15,16 Secondly, the use of large π-systems, particularly in terms of the electron acceptor in donor–acceptor emitter design, leads to aggregation-caused quenching (ACQ) of the emission.16,17 Thirdly, most organic compounds are fluorescent and so only the electrogenerated singlet excitons are available to produce light in organic light emitting diodes (OLEDs), which limits the efficiency of these devices.15,16One strategy to overcome these design limitations is to use thermally activated delayed fluorescence (TADF) emitters as these can harvest both singlet and dark triplet excitons to produce light in OLEDs.18,19 To design an efficient NIR TADF emitter, the compound needs to possess a small singlet–triplet energy gap (ΔEST), which will enable a fast reverse intersystem (RISC) that should outcompete nonradiative decay pathways involving triplet excitons. This requires the use of rigid, planar and strong donor and acceptor motifs that are reasonably weakly coupled to each other to extend π-conjugation.15,16 A second plausible strategy is to develop an emitter showing simultaneous TADF and aggregation-induced emission (AIE).20,21 Our emitter design adheres to this latter paradigm. We envisioned having a strong acceptor motif (i.e., ELUMO < −2.50 eV); however, the choice of acceptor moieties is somewhat limited, and most NIR TADF emitters (λPL > 650 nm) contain one of benzothiadiazole (BT),22,23 pyridal[2,1,3]thiadiazole (PT),24 naphtho[2,3-c][1,2,5]thiadiazole (NZP)25 and pyrazine derivatives.7,26,27 Out of these acceptor motifs, the most common pyrazine derivatives are phenanthropyrazine (PP), quinoxaline (QNO), acenaphthopyrazine (AP), dibenzophenazine (DBP), phenazine (PH), and dipyridophenazine (DPP), and their acceptor strengths are shown in Fig. 1.
With the goal of pushing the emission further into the NIR, our acceptor design contains a dipyrazine embedded within an extended π-conjugative framework in the form of a pyrazine-quinoxaline (PQ). This motif can also be thought of as a fused tetraazaanthracene. Consequently, the presence of four nitrogen atoms makes the system quite electron deficient, reflected in a LUMO energy of −2.74 eV (Fig. 1). Because of their strong accepting nature, PQ-based materials have been explored as fluorescent sensors,28 as motifs in organic liquid-crystalline semiconductors,29–31 and as components in OLEDs23 and organic photovoltaics (OPVs).29 Chen et al. reported an AIE active fluorescent donor–acceptor system based on PQ derivatives that exhibited green and red emission from their aggregates, and utilized these in bio-imaging applications.32 Vishwakarma et al. reported a PQ based columnar liquid crystalline material (PQ10) and its use as a fluorescent emitter for green OLEDs.30 Due to the impressive hole transporting mobility of PQ10, it was also used as a hole transporting material for photovoltaic applications.29
In our bid to design NIR emitters, we decorated the PQ moiety with four electron-donating groups, either dimethylacridane (DMAC) or phenoxazine (PXZ),33–35 each separated by a phenylene bridge to the central PQ acceptor. We cross-compare the optoelectronic properties of 4DMAC-TPPQ, and 4PXZ-TPPQ with that of the electron-acceptor TPPQ (Scheme 1), including assessing whether each of these compounds shows aggregation-induced emission enhancement (AIE). TPPQ emits in the blue (λPL = 456 nm) in toluene, while 4DMAC-TPPQ and 4PXZ-TPPQ emit at 650 and 762 nm, respectively (Fig. 4). Their corresponding ΔEST values, obtained in glassy 2-MeTHF at 77 K, are 0.97, 0.41 and 0.24 eV. All these compounds show AIE in THF:H2O mixtures; indeed, upon increasing the water fraction in THF solution, the blue PL band peaking at 490 nm intensified, which is associated with locally-excited emissions from TPPQ. Additionally, for 4DMAC-TPPQ, there is the appearance of a deep-red band at 650 nm and for 4PXZ-TPPQ a band at 790 nm emerges, both of which are associated with aggregate formation (Fig. 5). 4DMAC-TPPQ shows emission from 627 to 654 nm and 4PXZ-TPPQ shows emission from 682 to 770 nm by varying the doping concentration from 5 to 50 wt% in CBP and in neat film with lowering the ΔEST to 0.29 and 0.04 eV, respectively (Fig. S34, ESI† and Table 2). Finally, we fabricated doped (in CBP) and neat film solution-processed OLEDs (SP-OLEDs) of 4DMAC-TPPQ and 4PXZ-TPPQ. The neat film SP-OLEDs with 4DMAC-TPPQ emitted with an electroluminescence maximum, λEL, of 685 nm, while neat film SP-OLEDs with 4PXZ-TPPQ emitted at an λEL of 780 nm. Notably, the PL spectrum of 4PXZ-TPPQ and the EL spectrum of the OLED with this emitter were the most red-shifted among those of the 19 other TADF emitters containing a quinoxaline-type acceptor (Fig. S43 and Table S11, ESI†). Remarkably, we also enabled the TADF phenomena in TPPQ containing emitters, which was absent in previously reported compounds containing the TPPQ moiety.30,32
Results and discussion
The α-diketone derivatives of benzil-containing 9,9-dimethyl-9,10-dihydroacridine (DMAC-α-DK) and 10H-phenoxazine (PXZ-α-DK) moieties were synthesized through a palladium-catalysed Buchwald–Hartwig cross-coupling between 4,4′-dibromobenzil and DMAC or PXZ according to our previously reported procedure.34 Benzil, DMAC-α-DK and PXZ-α-DK were condensed with 1,2,4,5-tetraaminobenzene tetrahydrochloride to afford pyrazinyl[2,3-g]quinoxaline derivatives TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ, respectively, in good yield (Scheme 1). Their identity and purity were determined by a combination of 1H and 13C NMR spectroscopy, high-pressure liquid chromatography (HPLC), high-resolution mass spectrometry (HRMS), elemental analysis, and single crystal X-ray diffraction (SC XRD) (Fig. 1 and Fig. S1–S15, ESI†). The decomposition temperature (Td), defined as the 5% weight loss of the material, is 356 °C for TPPQ, 445 °C for 4DMAC-TPPQ and 495 °C for 4PXZ-TPPQ (Fig. S16, ESI†). Thus, all three compounds show high thermal stability. DSC measurements reveal very high glass transition temperatures (Tg) of 292 °C, 432 °C, and 450 °C for TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ, respectively.Single crystals of TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ suitable for X-ray diffraction were grown by slow evaporation from either a mixture of dichloromethane and hexane, or chloroform and THF. The structures of TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ are found in the centrosymmetric space groups P21/c (TPPQ) and P21/n (both other structures). For TPPQ and 4DMAC-TPPQ the asymmetric unit contains one half of an emitter molecule, as well as one or two molecules of dichloromethane, respectively, while the asymmetric unit of the structure of 4PXZ-TPPQ contains a complete emitter molecule. The four phenyl rings attached to the central core of PQ in TPPQ are somewhat twisted, with dihedral angles between phenyl rings and the planes of PQ of 34.32(14) and 57.55(14)° (Fig. 2). The corresponding dihedral angles are 34.74(16) and 49.47(6)° in 4DMAC-TPPQ, and 47.81(12), 47.84(12), 48.60(5) and 58.02(15)° in 4PXZ-TPPQ, while the dihedral angles between the bridging phenylene rings and the mean plane of the central six-membered heterocycle of the donor moieties show more twist at 69.95(6) and 78.23(7)° for 4DMAC-TPPQ, and 59.27(13), 62.24(13), 68.40(6) and 73.17(18)° for 4PXZ-TPPQ (Fig. 2a). This leads to the donor and acceptor groups being closer to co-planar in 4PXZ-TPPQ than in 4DMAC-TPPQ, the angles between the PQ plane and the central ring of the DMAC are 29.54(6) and 33.37(6)° in 4DMAC-TPPQ, while in 4PXZ-TPPQ the equivalent angles are decreased to 12.12(14), 15.25(13), 16.26(16) and 20.8(4)°. The DMAC groups show bowed conformations, with angles between phenyl ring planes of 23.23(8) and 33.19(8)°, while in 4PXZ-TPPQ three of the PXZ donor groups show slightly bowed conformations (angle between phenyl ring planes 14.1(6), 14.68(16), 15.87(15)°) while one is closer to planar (angle between phenyl ring planes 5.8(2)°). The structure of TPPQ is a DCM solvate form of the previously reported structure, showing a very similar molecular conformation, but with molecules packed in a herringbone fashion (Fig. S17a, ESI†), rather than the previously encountered parallel sheet structure.32 There are two sets of C–H⋯π intermolecular interactions between adjacent TPPQ molecules. One of these arises between a peripheral phenyl hydrogen and the central ring of the PQ group (H⋯centroid distance of 2.81 Å, C⋯centroid distance of 3.472(4) Å), which leads to chains of TPPQ molecules along the [0 1 0] axis. These are linked into two-dimensional sheets in the (1 0 0) plane (Fig. 2b) by the second set of C–H⋯π interactions, arising between symmetry-related phenyl rings (H⋯centroid distance of 2.94 Å, C⋯centroid distance of 3.608(5) Å). The crystal structure of 4DMAC-TPPQ shows a combination of weak π⋯π and C–H⋯π intermolecular interactions, leading to a brickwork packing (Fig. S17b, ESI†). There are two sets of C–H⋯π intermolecular interactions between adjacent molecules. One of these arises between a bridging phenyl hydrogen and a phenyl of a DMAC group (H⋯centroid distance of 3.00 Å, C⋯centroid distance of 3.729(2) Å), the second between methyl hydrogen and a pyrazine ring of the PQ group (H⋯centroid distance of 2.99 Å, C⋯centroid distance of 3.537(3) Å). The CH⋯π interactions result in the formation of sheets in the (−1 0 1) plane (Fig. 2b), which are further supported by the π⋯π interactions between one pyrazine ring and another phenyl of a DMAC (centroid⋯centroid distance 3.8477(11)). In the structure of 4PXZ-TPPQ, molecules show undulating layered packing, with channels for solvent (Fig. S17c, ESI†). This complex shows a more significant and more complex arrangement of supramolecular interactions. This comprises two-dimensional sheets in the (0 0 1) plane, built up of a combination of nine sets of interactions (Fig. 2b). These include two sets of π⋯π interactions from the central benzene ring of the PQ group to two peripheral phenyls, forming a three-layered π-stack (centroid⋯centroid distances of 3.570(2) and 3.616(2) Å), four sets of weak hydrogen bonds between four different aromatic CH groups of the bridging phenyls and two pyrazine nitrogen and two oxygen atoms of the PXZ groups (H⋯N distances of 2.47 and 2.60 Å, corresponding C⋯N distances of 3.302(4) and 3.439(4) Å; H⋯O distances of 2.59 Å, with C⋯O distances of 3.414(5) and 3.448(6) Å), and three sets of CH⋯π interactions between three different aromatic CH groups of the bridging phenyls and one other bridging phenyl and two proximal phenyl groups of PXZ (H⋯centroid distances of 2.93 to 3.00 Å, C⋯centroid distances of 3.753(4) to 3.901(3) Å). These sheets are in turn weakly connected into a three-dimensional supramolecular structure via two further sets of dimer-forming π⋯π interactions between two phenyl groups of PXZ and their symmetry-related neighbours (centroid⋯centroid distances of 3.726(10) and 3.947(3) Å) at the close points of approach of the undulations (Fig. S17c, ESI†).
Theoretical studies
Density functional theory (DFT) and time-dependent DFT calculations within the Tamm–Dancoff approximation (TDA-DFT) were performed to provide insight into the geometries and energies of the ground and the excited states of TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ. The ground-state geometries were optimized at the PBE0/6-31G(d,p) level of theory in the gas phase starting from the geometries obtained from the single crystal structures (Fig. 3). The central PQ moiety is almost planar in all three molecules, with dihedral angles between the planes of both pyrazine nitrogen in symmetrical pyrazine rings of 3.88° in TPPQ, 3.74° in 4DMAC-TPPQ and 3.79° in 4PXZ-TPPQ. In TPPQ, all four phenyl rings are equally twisted to the PQ with an angle of 39.68°, whereas the dihedral angles between the phenylene rings and PQ in 4DMAC-TPPQ are 41.97 and 45.88° and in 4PXZ-TPPQ are 43.31 and 43.49°. In 4DMAC-TPPQ, the DMAC moieties are strongly twisted with respect to the phenylene bridge (76.56 and 76.24°), while in 4PXZ-TPPQ the PXZ moieties are slightly less twisted (70.64 and 71.37°, Fig. S18, ESI†). Moreover, the DMAC units retained their puckered/bowed conformations, with dihedral angles between the phenyl ring planes of 31.72° and 28.96°. Their conformation is well matched with that in the corresponding crystal structure; however, the PXZ units exhibited more planar conformations than seen in the crystal structure, with dihedral angles between their phenyl ring planes of 2.07° and 2.32°. The electron density of the highly occupied molecular orbital (HOMO) is delocalized over the whole TPPQ molecule, while the lowest unoccupied molecular orbital (LUMO) is somewhat more concentrated on the pyrazino[2,3-g]quinoxaline. The HOMO/LUMO levels are −6.23/−2.56 eV. A similar electron density distribution pattern of the orbitals of TPPQ has been previously reported, though those calculations diverge from those reported here as they included a THF environment and were carried out at the lower B3LYP/6-31G(d) level.32 However, the literature calculated HOMO level was slightly shallower at −6.15 eV, whilst the LUMO level was deeper at −2.80 eV. Both 4DMAC-TPPQ and 4PXZ-TPPQ exhibit a similar pattern in the distribution of the electron densities to TPPQ, with the HOMO distributed across the four donors and the LUMO localized mainly on the PQ acceptor (Fig. 3). Consequently, the HOMO levels are shallower compared to TPPQ at −5.29 eV for 4DMAC-TPPQ and −4.79 eV for 4PXZ-TPPQ, in line with their increasing donor strength, and the LUMO levels are stabilized compared to TPPQ, at −2.87 eV for 4DMAC-TPPQ and −2.99 eV for 4PXZ-TPPQ. The HOMO–LUMO gap, ΔEHOMO–LUMO, thus decreases from 3.67 eV for TPPQ to 2.42 eV for 4DMAC-TPPQ and 1.80 eV for 4PXZ-TPPQ.The excited-state properties were calculated using time-dependent density functional theory (TD-DFT) within the Tamm–Dancoff approximation (TDA-DFT) based on the optimized ground-state geometries. The oscillator strength, f, of the S0 → S1 transition in TPPQ is high at 0.808, indicating a significant overlap of the electron density between the HOMO and LUMO (Fig. S19 and Table S3, ESI†). However, the f for the S0 → S1 transition of 4DMAC-TPPQ and 4PXZ-TPPQ is much lower at 0.027 and 0.091, respectively, reflecting the charge-transfer (CT) nature of this transition in these two compounds (Fig. S19 and Tables S4, S5, ESI†). The S1 energy of TPPQ is 3.11 eV, while those of 4DMAC-TPPQ (2.03 eV) and 4PXZ-TPPQ (1.59 eV) are significantly stabilized (Fig. 3 and Tables S2–S5, ESI†). This trend in S1 energies mirrors the trend in the HOMO–LUMO gaps. The T1 state of TPPQ, mainly located on the pyrazine rings, is 2.07 eV and, like the S1 state, also has a locally-excited (LE) character. The T1 state of 4DMAC-TPPQ at 2.01 eV is slightly lower in energy, while the T1 state of 4PXZ-TPPQ at 1.57 eV is significantly stabilized, reflecting their CT character (Fig. 3 and Table S6, ESI†). The corresponding calculated singlet–triplet energy gaps (ΔEST) of 4DMAC-TPPQ (0.01 eV) and 4PXZ-TPPQ (0.02 eV) are much smaller than that of TPPQ (1.03 eV) (Fig. 3). There are multiple high-lying triplet states in 4DMAC-TPPQ and 4PXZ-TPPQ, some of which have a different orbital type to S1, and may participate in the TADF process in these two compounds (Fig. 3 and Table S7, ESI†). Additionally, at an excited T1 geometry, there is a decrease in the S1–T1 spin–orbit coupling matrix element (SOCME) from 3.99 cm−1 in TPPQ to 0.01 cm−1 in 4DMAC-TPPQ and 0.02 cm−1 in 4PXZ-TPPQ (Table S6, ESI†). It should be noted that the SOCME of S1–Tn (n > 1) in 4DMAC-TPPQ (〈S1|HSO|T3〉 0.03 cm−1) and in 4PXZ-TPPQ (〈S1|HSO|T3〉 is 0.06 cm−1) is larger because of the large changes in orbital character between these two states (Table S6, ESI†). All these studies support a RISC process could proceed via the T3 to S1 in 4DMAC-TPPQ and the T3 to S1 in 4PXZ-TPPQ as T3 lies just 0.06 eV above S1 for 4DMAC-TPPQ and 0.05 eV above S1 for 4PXZ-TPPQ, so is readily accessible because of their relatively large SOCME between these states.
Electrochemistry
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were used to infer the HOMO/LUMO levels of TPPQ, 4DMAC-TPPQ, and 4PXZ-TPPQ in DCM. The voltammograms are shown in Fig. 4a and the data are summarized in Table 1. As expected, there is no observable oxidation wave for TPPQ, while there are two 1-electron reversible reduction waves at Ered1 = −1.00 V and Ered2 = −1.49 V vs. SCE in DCM, in line with the literature-reported electrochemistry.32 The corresponding orbital energies are LUMO = −3.34 eV and LUMO+1 = −2.85 eV. 4DMAC-TPPQ possesses a quasi-reversible oxidation wave at Eox at 1.00 V vs. SCE, which is assigned to the oxidation of the DMAC donor moieties as this value aligns with the previously reported Eox of 1.04 V for the DMAC-containing compound DMAC-α-DK.34 The reversible oxidation wave of 4PXZ-TPPQ is shifted cathodically at 0.79 V vs. SCE and is attributed to the oxidation of the PXZ donor moieties; indeed, the Eox of 4PXZ-TPPQ is similar to that of a related PXZ-containing compound, PXZPDO (Eox = 0.83 V).33 The HOMO levels thus are −5.34 and −5.13 eV for 4DMAC-TPPQ and 4PXZ-TPPQ, respectively, which are in line with the values and trends calculated using DFT (vide supra). The reduction waves are assigned to the reduction of the TPPQ acceptor, at around −1.00 V vs. SCE for both emitters. The corresponding LUMO energy levels are very similar, at −3.45 and −3.44 eV for 4DMAC-TPPQ and 4PXZ-TPPQ, respectively; the LUMO+1 for 4DMAC-TPPQ is −2.94 eV and for PXZ-TPPQ is −2.93 eV, quite close to the LUMO+1 energy of TPPQ. The estimated HOMO level of TPPQ is inferred to be −6.12 eV from the difference between the LUMO level and the optical gap (ΔEg = 2.78 eV) of TPPQ (Table 1). The HOMO–LUMO energy gap of TPPQ (2.78 eV) is much larger than that of 4DMAC-TPPQ (1.89 eV) and 4PXZ-TPPQ (1.69 eV).| Emitters | E ox /V | E red /V | HOMOb/eV | LUMOb/eV | E opt/eV |
|---|---|---|---|---|---|
| a E ox and Ered are anodic and cathodic peak potentials, respectively, obtained from DPV using Fc/Fc+ as the internal reference and referenced versus SCE (0.46 V vs. SCE) in DCM with 0.1 M [nBu4N]PF6 as the supporting electrolyte.36 b E HOMO/LUMO = −(Eox/Ered + 4.8) eV,37 where Eox is the anodic peak potential and Ered is the cathodic peak potential calculated from DPV relative to Fc/Fc+. c E HOMO = |ELUMO − Eopt| where Eopt is the optical band gap obtained from the intersection of the normalized absorption and emission spectra in toluene (Fig. S25, ESI). | |||||
| TPPQ | — | −1.00 | −6.12c | −3.34 | 2.78 |
| 4DMAC-TPPQ | 1.00 | −0.89 | −5.34 | −3.45 | 2.32 |
| 4PXZ-TPPQ | 0.79 | −0.90 | −5.13 | −3.44 | 2.08 |
Solution-state photophysical studies
The photophysical properties of all three molecules were first studied in toluene solution (Fig. 4b and Table S8, ESI†). There are three distinct absorption bands in the UV-vis absorption spectra of 4DMAC-TPPQ and 4PXZ-TPPQ, while there are only two bands in the spectrum of TPPQ. The strong absorption band at 314 nm present in all three spectra corresponds to a LE π–π* transition of the TPPQ moiety (ε = 69.9 × 103 M−1 cm−1 for TPPQ, ε = 70.5 × 103 M−1 cm−1 for 4DMAC-TPPQ and ε = 86.9 × 103 M−1 cm−1 for 4PXZ-TPPQ); the intensity of this band is influenced by the decoration about the acceptor (Fig. 4b). The absorption band located at 413 nm (ε = 42.9 × 103 M−1 cm−1 for TPPQ, ε = 28.2 × 103 M−1 cm−1 for 4DMAC-TPPQ and ε = 32.9 × 103 M−1 cm−1 for 4PXZ-TPPQ) is of mixed LE/CT character, based on an analysis of the TD-DFT calculations, where the LE transition is centred on the acceptor and the CT transition occurs from the phenylene groups to the PQ (Fig. S19, ESI†). The weakly absorptive CT band of 4PXZ-TPPQ (ε = 7.57 × 103 M−1 cm−1 at λabs = 506 nm) and 4DMAC-TPPQ (ε = 8.75 × 103 M−1 cm−1 at λabs = 475 nm) reflects the poor orbital overlap between the donor and the PQ acceptor (Fig. 4); notably, the red-shifted band in 4PXZ-TPPQ is consistent with its smaller HOMO–LUMO gap compared to that of 4DMAC-TPPQ, as observed in the electrochemistry study. The photoluminescence (PL) of TPPQ does not change as a function of solvent, with a peak maximum, λPL, of around 456 nm, and a shoulder band at around 476 nm (Fig. 4b). This, coupled with the reasonably narrow FWHM (56 nm), indicates that the emission emanates from a LE state. In contrast, 4DMAC-TPPQ and 4PXZ-TPPQ show unstructured and broad emission that is sensitive to solvent polarity and thus is assigned to originating from a CT state. In toluene, 4DMAC-TPPQ emits at a λPL of 650 nm, while 4PXZ-TPPQ emits at a λPL of 762 nm (Fig. 4b and Table S8, ESI†).Unexpectedly, we observed that TPPQ showed photochromic behaviour in ethereal solvents. On exposure to UV light (365 nm) at room temperature in THF solution, over 6 minutes the blue band at 456 nm decreased in intensity while a broad, green band at 556 nm appeared, with a corresponding iso-emissive point at 504 nm (Fig. S21, ESI†). At 77 K in glassy 2-MeTHF, there is no appearance of the green emission band and only the narrow blue emission band at 448 nm is observed (Fig. S22, ESI†). There were no changes in the 1H NMR spectrum of TPPQ in THF-d8 before and after exposure to UV light (Fig. S23, ESI†), which confirms that that there is no photodegradation of TPPQ. From these experiments, the appearance of the new green emission band at 556 nm in TPPQ is attributed to excimer formation. To corroborate these conclusions, we modelled a TPPQ dimer, which has a more stable S1 energy of 2.86 eV compared to the S1 energy of the TPPQ monomer of 3.11 eV (Fig. S20, ESI†). Both 4DMAC-TPPQ and 4PXZ-TPPQ also showed a similar but weak photochromic behaviour in ethereal solvents (Fig. S24, ESI†).
The photoluminescence quantum yield (ΦPL) in degassed toluene of 4DMAC-TPPQ is 13.7%, higher than those of TPPQ (2.1%) and 4PXZ-TPPQ (0.8%). Upon exposure to air, the ΦPL for TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ decreases to 1.9, 7.3, and 0.6%, respectively. The PL decays of the three emitters in toluene under degassed and aerated conditions are shown in Fig. S26 (ESI†). There is only a single decay component observed for all three compounds, with lifetimes, τPL, of 7.9 ns for TPPQ, 20.2 ns for 4DMAC-TPPQ and 1.98 ns for 4PXZ-TPPQ.
The steady-state PL and phosphorescence spectra of 4DMAC-TPPQ and 4PXZ-TPPQ in 2-MeTHF glass at 77 K were measured to ascertain the S1 and T1 levels from their respective onsets; the S1 and T1 levels of TPPQ were measured in methylcyclohexane (MCH) at 77 K to mitigate against possible photochromism in 2-MeTHF (Fig. S27, ESI†). In glassy MCH, the λPL of TPPQ is around 456 nm, while the delayed emission spectrum of TPPQ, attributed to phosphorescence, peaks at 766 nm. Notably, there is a weak residual prompt emission at around 460 nm. The corresponding S1 and T1 energies are 2.82 and 1.85 eV, and thus the ΔEST is 0.97 eV. The S1 and T1 energies of 4DMAC-TPPQ are 2.26 and 1.85 eV, while for 4PXZ-TPPQ they are 2.08 and 1.84 eV (Fig. S27, ESI†). Thus, in these two compounds the S1 energies follow the trend calculated by DFT and the observed HOMO–LUMO gaps determined by electrochemistry, while the T1 energies, which are effectively the same as that of TPPQ, reflect the same 3LE state. The corresponding ΔEST values of 4DMAC-TPPQ and 4PXZ-TPPQ are 0.41 and 0.24 eV, respectively.
Aggregation-induced emission (AIE)
TPPQ has previously been reported as an AIE-active moiety.32 Aware of this, we assessed whether 4DMAC-TPPQ and 4PXZ-TPPQ would also show AIE and thus explored their PL spectra in THF/H2O mixtures (Fig. 5a–c). Firstly, we re-confirmed the AIE behaviour of TPPQ in THF/H2O mixtures. The emission peaking at 456 and 476 nm of TPPQ in THF slightly red-shifts and increases in intensity by 1.8-fold upon increasing the water fraction (fw) to 60% (Fig. 5c and Fig. S28a, ESI†). On further increasing the fw beyond 80%, the emission intensity then decreases and red-shifts further to 492 nm. We confirmed the formation of crystalline aggregates by powder XRD (Fig. S29, ESI†). Comparison of the powder XRD patterns to those simulated from the SC XRD data indicates structural differences, particularly in the cases of TPPQ and 4DMAC-TPPQ, likely arising from the influence of solvent and its ordering on the structures. However, in the case of 4PXZ-TPPQ, which lacks ordered solvent in its structure, a stronger similarity is seen between calculated and determined PXRD patterns. In comparison to TPPQ, 4DMAC-TPPQ shows very weak emission at λPL of 568 nm, while 4PXZ-TPPQ does not show any emission in THF, which points to non-radiative decay being significant in THF. Upon increasing fw to 80%, the λPL of 4DMAC-TPPQ red-shifts to 656 nm and becomes as much as 20-fold more intense (Fig. 5b and Fig. S28b, ESI†). Upon increasing the fw from 80 to 95%, the emission intensity slightly decreases and further red-shifts to 690 nm. In the case of 4PXZ-TPPQ, upon increasing the fw to 40%, a new emission band at λPL of 790 nm appears, the intensity of which increases 23-fold at fw = 80% (Fig. 5c and Fig. S28c, ESI†). When the fw > 80%, the PL intensity decreases and slightly blue-shifts to 770 nm. In both molecules, the observed AIE is attributed to the formation of aggregates, as found in their crystal structures. The red-shifted emission observed from the aggregates formed in the 80% H2O/THF mixture in both molecules likely occurs because of stronger intermolecular interactions between adjacent donor and acceptor moieties that modulate the energy of intermolecular CT states. To understand the emissive behaviour of the crystalline aggregates in THF/H2O solution (fw = 80%), we measured the PL spectra of the dried aggregates isolated from the THF/H2O solution. These spectra closely resemble those of the neat films of TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ (Fig. S30, ESI†), indicating that the excited-state species are likely the same for both types of samples.Photophysical studies in thin films
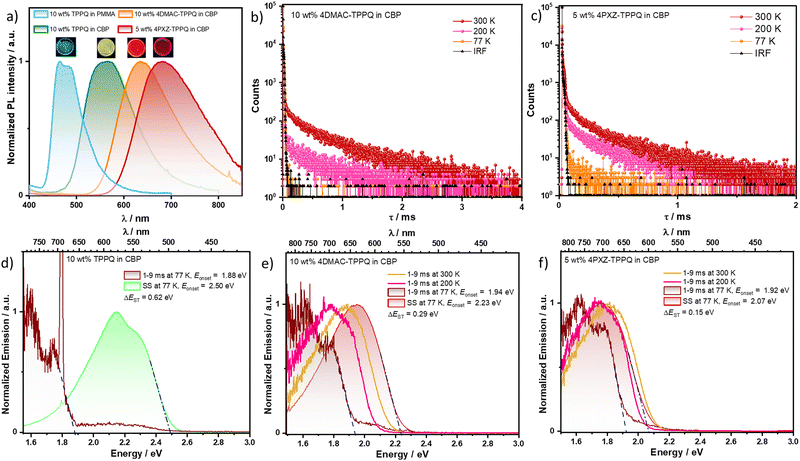
We next focused our attention on the photophysical properties of TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ doped in poly(methylmethacrylate) (PMMA) and in 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP) and in neat thin films. Initially, we measured the photophysical properties of the three emitters as 10 wt% doped films in PMMA as the polarity of this host closely mimics that of toluene. The absorption bands of all three compounds are of similar energy to those in toluene, with the weak CT band observed in the range of 451–650 nm for 4DMAC-TPPQ while it is more red-shifted for 4PXZ-TPPQ (Fig. S31a, ESI†). Upon photoexcitation at 315 nm, TPPQ shows a structured emission profile at 466 nm (ΦPL = 4.7%), similar to that measured in toluene, while the CT-based unstructured emission is at 635 nm for 4DMAC-TPPQ (ΦPL = 14.3%) and at 681 nm for 4PXZ-TPPQ (ΦPL = 3.0%), emission that is blue-shifted by 15 and 81 nm, respectively, compared to the λPL in toluene (Fig. S31b, ESI†). The emission of TPPQ decays with bi-exponential kinetics, with an average lifetime, τPL, of 2.1 ns. The time-resolved PL of 4DMAC-TPPQ and 4PXZ-TPPQ shows multiexponential decay kinetics, with average prompt lifetimes, τp, of 25.2 and 15.8 ns, respectively (Fig. S32, ESI†), and average delayed lifetimes, τd, of 390.9 and 342.5 μs, respectively (Fig. S33 and Table S8, ESI†). The contribution from the delayed emission increases with increasing temperature, indicating that its origin is TADF.To best evaluate whether these compounds could act as promising emitters in red-to-NIR OLEDs, we next turned our attention to assessing the photophysics of these three compounds in an OLED-relevant host, CBP, as this host matrix has a sufficiently high triplet energy (T1 = 2.6 eV) to confine excitons onto the emitters.38 The λPL of the 10 wt% doped film of TPPQ in CBP is 548 nm and the PL spectrum is much broader (FWHM = 100 nm) and around 80 nm bathochromically shifted compared to that in PMMA (λPL = 466 nm) and in solutions (λPL = 456 nm) (Fig. 6). There is only a small red-shift (only 10 nm) of the λPL and moderate decrease in the ΦPL upon increasing the doping concentration of TPPQ from 5 to 30 wt% in CBP (Fig. S34a and Table S10, ESI†). To understand the large red-shift in the emission band of TPPQ in CBP in comparison to both in solution and in PMMA, we checked the PL spectrum of TPPQ in another host, 2,2′,2′′-(1,3,5-benzinetriyl)-tris(1-phenyl-1H-benzimidazole) (TPBi), Fig. S34a (ESI†). The 5 wt% doped films of TPPQ in TBPi emits at λPL of 470 nm and has a ΦPL of 4.8%, which is very similar to the properties in toluene and in the 10 wt% doped film in PMMA. Notably, the remarkably red-shifted emission of the 10 wt% doped film of TPPQ in CBP is reminiscent of the emission profile after UV exposure in ethereal solvents (vide supra). This may imply that there is an exciplex between CBP and TPPQ that emits at a similar energy to the TPPQ excimer. The photophysical properties of the doped films (5–50 wt%) of 4DMAC-TPPQ and 4PXZ-TPPQ in CBP and the neat films are shown in Fig. S34b, c and S35–S40 (ESI†) and Table 2. Of these films, the highest ΦPL was found to be 21.0% for 4DMAC-TPPQ and 10.0% for 4PXZ-TPPQ as 10 and 5 wt% doped films in CBP, respectively. The λPL of the 10 wt% doped film of 4DMAC-TPPQ in CBP is 635 nm, which is very similar to that of the 10 wt% doped film in PMMA (Fig. 6 and Fig. S31, ESI†). The emission for this compound does not significantly change across a doping range from 5–50 wt% and even as the neat film (λPL ranging from 627 to 654 nm, Fig. S34b, ESI† and Table 2). The λPL of 4PXZ-TPPQ is significantly shifted to the NIR region compared to that of 4DMAC-TPPQ, at 682 nm for the 5 wt% doped film, at 713 nm for the 50 wt% doped film, and 770 nm for the neat film (Fig. S34c, ESI†). The substantially red-shifted emission as a function of doping concentration of 4PXZ-TPPQ in comparison to 4DMAC-TPPQ may be attributed to the formation of aggregates in the former resulting in strong interactions between adjacent chromophores. The emission profile of the neat films of 4DMAC-TPPQ and 4PXZ-TPPQ match well those in THF:H2O (fw = 80%), indicating that the species responsible for the emission in each is the excimer.
| Doping/wt% | λ PL /nm | Φ PL /% | E S1 /eV | E T1 /eV | ΔESTf/eV | τ p /ns | τ d /μs | k RISC /s−1 (×103) | k ISC /s−1 (×107) | k s,nr /s−1 (×107) | k s,r i /s−1 (×106) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Spin-coated film and annealed at 55 °C for 1 minute. b Drop-cast film and annealed at 70 °C for 1 minute. c PL measured under vacuum, λexc = 340 nm for all films except 100 wt% 4PXZ-TPPQ, which has λexc = 465 nm. d Photoluminescence quantum yield (ΦPL) of thin films determined using an integrating sphere (λexc = 305 nm or 340 nm) under a N2 atmosphere at 298 K. The values in parentheses are in the presence of O2 at 298 K. e E S1 and ET1 were obtained from the onset of the steady-state and phosphorescence spectra (time window: 1–9 ms), respectively, of doped films in CBP at 77 K, λexc = 340 nm, and for 100 wt% 4PXZ-TPPQ, λexc = 465 nm. f ΔEST = ES1 − ET1. g Prompt lifetime was measured by TCSPC, λexc = 375 nm, the average lifetimes were calculated by using the equation τavg = ΣAiτi2/ΣAiτi, (where Ai is the pre-exponential for lifetime τi). h Delayed emission was measured by MCS, using a microsecond flash lamp as an excitation source, λexc = 340 nm. i k s,nr, ks,r, kRISC and kISC were calculated according to the method given in ref. 39 and 40. | |||||||||||
| TPPQ | |||||||||||
| 10a | 548 | 8.8 (8.5) | 2.50 | 1.88 | 0.62 | 42.8 | — | — | — | — | — |
| 4DMAC-TPPQ | |||||||||||
| 10a | 635 | 21.0 (17.2) | 2.23 | 1.94 | 0.29 | 25.2 | 564.5 | 1.32 ± 0.8 | 2.00 ± 1.3 | 2.6 | 6.8 |
| 100a | 654 | 4.3 (4.1) | 2.12 | 1.83 | 0.29 | 27.2 | 109.4 | 5.03 ± 4.5 | 1.85 ± 1.7 | 3.3 | 1.5 |
| 4PXZ-TPPQ | |||||||||||
| 5a | 682 | 10.2 (8.2) | 2.09 | 1.94 | 0.15 | 17.6 | 303.9 | 2.48 ± 1.6 | 3.17 ± 2.1 | 4.1 | 4.7 |
| 100b | 770 | 1.5 (1.0) | 1.85 | 1.81 | 0.04 | 3.7 | 2.05g | 489 ± 243 | 17.7 ± 8.8 | 17.6 | 2.7 |
At 77 K, the λPL of the steady-state PL spectrum of the 10 wt% doped film of TPPQ in CBP is 578 nm, while the delayed emission spectrum peaks at a λPh of 775 nm (Fig. 6). The corresponding S1 and T1 energies are 2.50 and 1.88 eV, thus the ΔEST is 0.62 eV. The phosphorescence of TPPQ in CBP closely resembles that in glassy MCH, indicating that it occurs from the same 3LE state. The S1 and T1 energies of the 10 wt% doped film of 4DMAC-TPPQ in CBP are 2.23 and 1.94 eV, while those of the 5 wt% doped film of 4PXZ-TPPQ in CBP are 2.07 and 1.92 eV (Fig. 6). The corresponding ΔEST for 4DMAC-TPPQ is 0.29 eV, and 0.15 eV for 4PXZ-TPPQ. Given that the T1 state energy remains effectively the same for the same LE state, the smaller ΔEST values for the three emitters in CBP are due to the stabilization of the S1 state. In comparison to the doped film (5 wt%), the S1 energy of the neat film of 4PXZ-TPPQ is significantly red shifted at 1.85 eV (ΔS1 = 0.25 eV), while the neat film of 4DMAC-TPPQ showed a small red-shift at 2.12 eV (ΔS1 = 0.11 eV) in comparison to the doped film of 10 wt% 4DMAC-TPPQ in CBP, however, the T1 energies of the neat films of both emitters are similarly red-shifted. The ΔEST of neat 4PXZ-TPPQ is significantly smaller at 0.04 eV, while ΔEST of neat 4DMAC-TPPQ is the same as the doped film, at 0.29 eV (Fig. S38, S40, ESI† and Table 2). These large declines in S1 energy and ΔEST of the neat film 4PXZ-TPPQ in comparison to doped films could be explained by the synergistic effect of the transformation of exciton states due to the formation of aggregates and shortening intermolecular distance because of planar phenoxazine motifs, which induce π–π interactions between adjacent molecules in neat film 4PXZ-TPPQ compared with the doped film.21
As expected, no delayed emission was observed in the 10 wt% doped film of TPPQ in CBP and the emission decays with biexponential kinetics, with an average lifetime, τPL of 42.8 ns at 300 K. In contrast, the 10 wt% doped film of 4DMAC-TPPQ and 5 wt% doped film of 4PXZ-TPPQ in CBP show multiexponential decay kinetics, with an average prompt lifetime, τp of 25.2 and 17.6 ns, while the average delayed lifetime (τd) is 564.5 and 303.9 μs at a λPL of 635 and 680 nm, respectively at 300 K (Fig. 6b and c). The corresponding estimated kRISC and kISC of the 10 wt% 4DMAC-TPPQ in CBP is 1.32 × 103 and 2.00 × 107 s−1 which slightly increase to 2.48 × 103 and 3.17 × 107 s−1 for the 5 wt% doped 4PXZ-TPPQ in CBP, respectively (Table 2). On the other hand, the τp of the neat film of 4DMAC-TPPQ is 27.2 ns and the τd is 109.4 μs, while the τp and τd for the neat film of 4PXZ-TPPQ are much shorter at 3.7 ns and 2.05 μs, respectively. Therefore, the kRISC of the neat film of 4PXZ-TPPQ significantly increased (4.9 × 105 s−1) in comparison to the neat film of 4DMAC-TPPQ (5.03 × 103 s−1) which is strongly aligned with the reduced ΔEST of neat 4PXZ-TPPQ. The temperature-dependent time-resolved PL decay studies of both emitters indicate that these compounds are TADF active not only in doped films but also as their aggregates (Fig. S38 and S40, ESI†).
We next measured the delayed emission spectrum as a function of temperature of the respective 10 and 5 wt% films of 4DMAC-TPPQ and 4PXZ-TPPQ in CBP (Fig. 6e, f and Table S10, ESI†). There is an apparent shifting of the delayed emission, attributed to phosphorescence, as a function of temperature. The T1 state of 4DMAC-TPPQ red-shifts from 2.15 eV at 300 K to 2.06 eV at 200 K to 1.94 eV at 77 K (Fig. 6e and Table S10, ESI†). Similarly, the T1 state of 4PXZ-TPPQ red-shifts from 2.10 eV at 300 K to 2.02 eV at 200 K and 1.92 eV at 77 K (Fig. 6f and Table S10, ESI†). The apparent blue-shift of triplet states from 77 to 300 K in both molecules could be due to the fact that at 300 K the delayed emission originates from the monomer while at 77 K the delayed emission comes from the aggregate. This is supported by the TDA-DFT calculations (vide supra).
OLED characterization
Having identified CBP as a viable host, we next proceeded to fabricate solution-processed OLEDs (SP-OLEDs) with 4DMAC-TPPQ and 4PXZ-TPPQ; furthermore, recognizing the AIE properties of these emitters, we also explored SP-OLEDs using neat films of both emitters. We employed a bottom-emitting OLED device architecture consisting of: indium tin oxide (ITO)/poly(3,4-ethylenedioxy-thiophene):poly(styrenesulfonate) (PEDOT:PSS) (40 nm)/F8-TFB (25 nm)/EML (25 nm for doped and 30 nm for neat film EMLs)/2,3,5-tri[(3-pyridyl)-phen-3-yl]benzene (TmPyPB) (70 nm)/LiF (1 nm)/Al (100 nm) (Fig. S41, ESI†). ITO and Al serve as the anode and cathode, respectively, PEDOT:PSS acts as the hole-transporting layer, F8-TFB acts as the electron-blocking layer (EBL) and hole-transporting layer (HTL), TmPyPB acts as the electron-transporting layer (ETL), and LiF acts as the electron-injection layer by modifying the work function of the aluminum cathode (Fig. 7b). The EQE–luminance curves and electroluminescence spectra (EL) are shown in Fig. 7 and the current density and luminance versus voltage are shown in Fig. S42 (ESI†) and the performance of the OLEDs is summarized in Table 3. The EL spectra mirror the corresponding PL spectra. The λEL of the SP-OLEDs with 4DMAC-TPPQ ranged from 640 to 670 nm for the 10 and 50 wt% doped film in CBP EMLs, while the neat film SP-OLEDs emitted at an λEL of 683 nm. As expected, the SP-OLEDs with 4PXZ-TPPQ emitted further into the NIR, with an λEL of 686 nm (5 wt% in CBP), 735 nm (50 wt% in CBP), and 780 nm (for neat film devices) (Table 3). Of the devices fabricated with 4DMAC-TPPQ, the highest EQEmax was achieved with 10 wt% doping at 3.9%; the efficiency decreased to 1.9% at 100 cd m−2. The 50 wt% and neat film devices generally exhibited relatively low EQE, with EQEmax of 0.8 and 0.3%, respectively (Table 3). The EQEmax for the device with 5 wt% 4PXZ-TPPQ in CBP was 1.7%; the EQEmax was 0.3% for the 50 wt% doped device and 0.04% for the neat film OLEDs, values that are consistent with their lower ΦPL. For comparison, we fabricated vacuum-deposited devices (VD-OLEDs) with 5 wt% 4PXZ-TPPQ in CBP, using the device structure shown in Fig. S41 (ESI†) and Table 3. Notably, the VD-OLEDs performance improved, reflected in the higher EQEmax of 3.2% at λEL at 681 nm, a similar EL spectrum to that observed for the 5 wt% SP-OLEDs. The fabrication of VD-OLEDs was not possible for 4DMAC-TPPQ because its molecular weight was too high. The turn-on voltages for the SP-OLEDs range from 4.0 to 5.2 V, while that for the VD-OLEDs is decreased at 3.8 V. Furthermore, the exciton utilization efficiency (EUE) of the SP-OLEDs with 4DMAC-TPPQ and 4PXZ-TPPQ is high as 74 and 67%, respectively, indicating that there is significant triplet exciton harvesting in these devices. The EL spectrum of the neat film SP-OLED with 4PXZ-TPPQ mirrors the PL spectrum (λPL = 770 nm). These devices are, to the best of our knowledge, the reddest of those based on emitters containing quinoxaline acceptors (Fig. S43 and Table S11, ESI†).| Doping/wt% | V on /V | λ EL /nm | EQEc/% | EUEe/% |
|---|---|---|---|---|
| a The turn-on voltage at lowest luminance and 10 cd m−2 in parenthesis. b The electroluminescence maximum recorded at 7 or 8 V. c EQEmax/EQE10/EQE100. d Device was fabricated by vacuum deposition. e EUE = exciton utilization efficiency, calculated according to the equation: EQE = ηext × ΦPL of film × EUE × γ, in which ηext = optical outcoupling efficiency, assumed to be 25% for an isotropic film, and γ = charge-balance factor, assumed to be 100%. | ||||
| 4DMAC-TPPQ | ||||
| 10 | 4.2 (5.6) | 640 | 3.9/3.2/1.9 | 74.2 |
| 50 | 4.4 (6.1) | 670 | 0.8/0.7/0/8 | 30.2 |
| 100 | 4.0 (5.6) | 685 | 0.3/0.3/— | 27.9 |
| 4PXZ-TPPQ | ||||
| 5 | 5.2 (8.8) | 686 | 1.7/1.2/0.6 | 66.7 |
| 50 | 4.4 (8.2) | 735 | 0.3/0.3/0.2 | 34.3 |
| 100 | 4.6 (7.3) | 780 | 0.04/0.04/— | 10.7 |
| 5d | 3.8 (4.8) | 681 | 3.2/2.3/1.4 | — |
Conclusion
We have developed deep-red and NIR TADF emitters containing an extended π-conjugated pyrazine-quinoxaline (PQ) acceptor with a particularly deep LUMO at −2.74 eV. The compounds 4DMAC-TPPQ and 4PXZ-TPPQ emit at 650 and 762 nm in toluene, which are significantly red-shifted in comparison to the donor-free parent compound TPPQ (λPL = 456 nm). AIE behavior was observed in THF:water mixtures of all compounds. While TPPQ is fluorescent, both 4DMAC-TPPQ and 4PXZ-TPPQ exhibit TADF as both doped films in CBP and as neat films. The 10 wt% doped film of 4DMAC-TPPQ in CBP emits at 635 nm and the ΔEST is 0.29 eV, while the neat film emits at 654 nm yet has the same ΔEST. On the other hand, the 5 wt% doped film of 4PXZ-TPPQ in CBP emits at 682 nm and the ΔEST is 0.15 eV, while the neat film emits at 770 nm and the ΔEST is much smaller at 0.04 eV. The undoped solution-processed OLEDs (SP-OLEDs) with 4DMAC-TPPQ and 4PXZ-TPPQ are particularly remarkable, emitting at 685 and 780 nm and exhibiting EQEmax of 0.3 and 0.04%, respectively.Data availability
The research data supporting this publication can be accessed at https://doi.org/10.17630/63338f36-6958-437d-acb2-447a2ff03f96.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AKG is grateful to the Royal Society for a Newton International Fellowship NF171163. We acknowledge support from the Engineering and Physical Sciences Research Council of the UK (grants EP/W007517/1, EP/P010482/1, and EP/L017008/1). We are also grateful for financial support from the University of St Andrews Restarting Research and Restarting Interdisciplinary Research Funding Schemes (SARRF and SARIRF) which are funded through the Scottish Funding Council grant reference SFC/AN/08/020. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 101025143 (TADF-DLC-OLEDs). The authors thank Dr Tomas Matulaitis for helpful discussions and Dr Gavin Peters for TGA/DSC measurements.References
- S. Wang, J. Liu, G. Feng, L. G. Ng and B. Liu, NIR-II Excitable Conjugated Polymer Dots with Bright NIR-I Emission for Deep In Vivo Two-Photon Brain Imaging Through Intact Skull, Adv. Funct. Mater., 2019, 29, 1808365, DOI:10.1002/adfm.201808365.
- A. T. Eggebrecht, S. L. Ferradal, A. Robichaux-Viehoever, M. S. Hassanpour, H. Dehghani, A. Z. Snyder, T. Hershey and J. P. Culver, Mapping distributed brain function and networks with diffuse optical tomography, Nat. Photonics, 2014, 8, 448–454, DOI:10.1038/nphoton.2014.107.
- Q. He, M. Wang, L. Zhao, B. Xu, W. Tian and L. Zhang, Near-infrared aggregation-induced emission materials: Bibliometric analysis and their application in biomedical field, Aggregate, 2024, 5, e505, DOI:10.1002/agt2.505.
- R. Singh, A. K. Gupta and C. P. Pradeep, Synthesis of a New Series of Organic Solid-State Near-Infrared Emitters: The Role of Crystal Packing and Weak Intermolecular Interactions and Application in Latent Fingerprint Detection, Cryst. Growth Des., 2021, 21, 1062–1076, DOI:10.1021/acs.cgd.0c01392.
- M. Kono, H. Ueki and S.-I. Umemura, Near-infrared finger vein patterns for personal identification, Appl. Opt., 2002, 41, 7429–7436, DOI:10.1364/AO.41.007429.
- Z. Lan, Y. Lei, W. K. E. Chan, S. Chen, D. Luo and F. Zhu, Near-infrared and visible light dual-mode organic photodetectors, Sci. Adv., 2020, 6, eaaw8065, DOI:10.1126/sciadv.aaw8065.
- C. Si, A. K. Gupta, B. Basumatary, A. P. McKay, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Multi-Responsive Thermally Activated Delayed Fluorescence Materials: Optical ZnCl2 Sensors and Efficient Green to Deep-Red OLEDs, Adv. Funct. Mater., 2024, 34, 2315935, DOI:10.1002/adfm.202315935.
- X. Zhao, C. Yao, T. Liu, J. C. Hamill Jr., G. O. Ngongang Ndjawa, G. Cheng, N. Yao, H. Meng and Y.-L. Loo, Extending the Photovoltaic Response of Perovskite Solar Cells into the Near-Infrared with a Narrow-Bandgap Organic Semiconductor, Adv. Mater., 2019, 31, 1904494, DOI:10.1002/adma.201904494.
- B. Xu, C. Jin, J.-S. Park, H. Liu, X. Lin, J. Cui, D. Chen and J. Qiu, Emerging near-infrared luminescent materials for next-generation broadband optical communications, InfoMat, 2024, 6, e12550, DOI:10.1002/inf2.12550.
- J.-C. G. Bünzli and S. V. Eliseeva, Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion, J. Rare Earths, 2010, 28, 824–842, DOI:10.1016/S1002-0721(09)60208-8.
- H. Q. Ye, Z. Li, Y. Peng, C. C. Wang, T. Y. Li, Y. X. Zheng, A. Sapelkin, G. Adamopoulos, I. Hernández, P. B. Wyatt and W. P. Gillin, Organo-erbium systems for optical amplification at telecommunications wavelengths, Nat. Mater., 2014, 13, 382–386, DOI:10.1038/nmat3910.
- K. Tuong Ly, R.-W. Chen-Cheng, H.-W. Lin, Y.-J. Shiau, S.-H. Liu, P.-T. Chou, C.-S. Tsao, Y.-C. Huang and Y. Chi, Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance, Nat. Photonics, 2017, 11, 63–68, DOI:10.1038/nphoton.2016.230.
- R. Nagata, H. Nakanotani and C. Adachi, Near-Infrared Electrophosphorescence up to 1.1 μm using a Thermally Activated Delayed Fluorescence Molecule as Triplet Sensitizer, Adv. Mater., 2017, 29, 1604265, DOI:10.1002/adma.201604265.
- A. Zampetti, A. Minotto, B. M. Squeo, V. G. Gregoriou, S. Allard, U. Scherf, C. L. Chochos and F. Cacialli, Highly Efficient Solid-State Near-infrared Organic Light-Emitting Diodes incorporating A–D–A Dyes based on α,β-unsubstituted “BODIPY” Moieties, Sci. Rep., 2017, 7, 1611, DOI:10.1038/s41598-017-01785-2.
- Y. Xiao, H. Wang, Z. Xie, M. Shen, R. Huang, Y. Miao, G. Liu, T. Yu and W. Huang, NIR TADF emitters and OLEDs: challenges, progress, and perspectives, Chem. Sci., 2022, 13, 8906–8923, 10.1039/d2sc02201j.
- H. U. Kim, T. Kim, C. Kim, M. Kim and T. Park, Recent Advances in Structural Design of Efficient Near-Infrared Light-Emitting Organic Small Molecules, Adv. Funct. Mater., 2023, 33, 2208082, DOI:10.1002/adfm.202208082.
- A. K. Gupta, A. Kumar, R. Singh, M. Devi, A. Dhir and C. P. Pradeep, Facile Synthesis of an Organic Solid State Near-Infrared-Emitter with Large Stokes Shift via Excited-State Intramolecular Proton Transfer, ACS Omega, 2018, 3, 14341–14348, DOI:10.1021/acsomega.8b02116.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238, DOI:10.1038/nature11687.
- J. M. Dos Santos, D. Hall, B. Basumatary, M. Bryden, D. Chen, P. Choudhary, T. Comerford, E. Crovini, A. Danos, J. De, S. Diesing, M. Fatahi, M. Griffin, A. K. Gupta, H. Hafeez, L. Hämmerling, E. Hanover, J. Haug, T. Heil, D. Karthik, S. Kumar, O. Lee, H. Li, F. Lucas, C. F. R. Mackenzie, A. Mariko, T. Matulaitis, F. Millward, Y. Olivier, Q. Qi, I. D. W. Samuel, N. Sharma, C. Si, L. Spierling, P. Sudhakar, D. Sun, E. Tankelevičiūtė, M. Duarte Tonet, J. Wang, T. Wang, S. Wu, Y. Xu, L. Zhang and E. Zysman-Colman, The Golden Age of Thermally Activated Delayed Fluorescence Materials: Design and Exploitation, Chem. Rev., 2024, 124, 13736–14110, DOI:10.1021/acs.chemrev.3c00755.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: New Vistas at the Aggregate Level, Angew. Chem., Int. Ed., 2020, 59, 9888–9907, DOI:10.1002/anie.201916729.
- J. Xue, Q. Liang, R. Wang, J. Hou, W. Li, Q. Peng, Z. Shuai and J. Qiao, Highly Efficient Thermally Activated Delayed Fluorescence via J-Aggregates with Strong Intermolecular Charge Transfer, Adv. Mater., 2019, 31, 1808242, DOI:10.1002/adma.201808242.
- Z. Hu, L. Feng and P. Yang, 2,1,3-Benzothiadiazole Deriva tive Small Molecule Fluorophores for NIR-II Bioimaging, Adv. Funct. Mater., 2024, 34, 2310818, DOI:10.1002/adfm.202310818.
- Y. Liu, Z. Deng, J. Li, J. Xie, X. Feng, Z. Qiu, G. Xie, Z. Zhao and B. Z. Tang, Deep-red/NIR AIEgens based on electron-withdrawing dithiafulvalene-fused benzothiadiazole for solution-processed non-doped OLEDs, Mater. Chem. Front., 2023, 7, 5431–5438, 10.1039/d3qm00598d.
- J. Jiang, Z. Xu, J. Zhou, M. Hanif, Q. Jiang, D. Hu, R. Zhao, C. Wang, L. Liu, D. Ma, Y. Ma and Y. Cao, Enhanced Pi Conjugation and Donor/Acceptor Interactions in D–A–D Type Emitter for Highly Efficient Near-Infrared Organic Light-Emitting Diodes with an Emission Peak at 840 nm, Chem. Mater., 2019, 31, 6499–6505, DOI:10.1021/acs.chemmater.8b04894.
- T. Liu, L. Zhu, C. Zhong, G. Xie, S. Gong, J. Fang, D. Ma and C. Yang, Naphthothiadiazole-Based Near-Infrared Emitter with a Photoluminescence Quantum Yield of 60% in Neat Film and External Quantum Efficiencies of up to 3.9% in Nondoped OLEDs, Adv. Funct. Mater., 2017, 27, 1606384, DOI:10.1002/adfm.201606384.
- G. Hong, C. Si, A. K. Gupta, C. Bizzarri, M. Nieger, I. D. W. Samuel, E. Zysman-Colman and S. Bräse, Fluorinated dibenzo[a,c]-phenazine-based green to red thermally activated delayed fluorescent OLED emitters, J. Mater. Chem. C, 2022, 10, 4757–4766, 10.1039/d1tc04918f.
- T. Huang, D. Liu, J. Jiang and W. Jiang, Quinoxaline and Pyrido[x,y-b]pyrazine-Based Emitters: Tuning Normal Fluorescence to Thermally Activated Delayed Fluorescence and Emitting Color over the Entire Visible-Light Range, Chem. – Eur. J., 2019, 25, 10926–10937, DOI:10.1002/chem.201902116.
- V. K. Vishwakarma, V. Adupa, K. Anki Reddy and A. Ammathnadu Sudhakar, Experimental and theoretical investigations of acid sensing properties of pyrazino[2,3-g]quinoxaline derivatives, J. Mol. Struct., 2021, 1225, 129120, DOI:10.1016/j.molstruc.2020.129120.
- A. Shah, V. K. Vishwakarma, N. Lhouvum, A. A. Sudhakar, P. Kumar, A. K. Srivastava, F. Dubois, T. Chomchok, N. Chattham and D. P. Singh, Pyrazino[2,3-g]quinoxaline core-based organic liquid crystalline semiconductor: Proficient hole transporting material for optoelectronic devices, J. Mol. Liq., 2024, 393, 123535, DOI:10.1016/j.molliq.2023.123535.
- V. K. Vishwakarma, S. Nath, M. Gupta, D. K. Dubey, S. S. Swayamprabha, J.-H. Jou, S. K. Pal and A. A. Sudhakar, Room-Temperature Columnar Liquid Crystalline Materials Based on Pyrazino[2,3-g]quinoxaline for Bright Green Organic Light-Emitting Diodes, ACS Appl. Electron. Mater., 2019, 1, 1959–1969, DOI:10.1021/acsaelm.9b00477.
- X. Wang, Y. Zhou, T. Lei, N. Hu, E.-Q. Chen and J. Pei, Structural–Property Relationship in Pyrazino[2,3-g]quinoxaline Derivatives: Morphology, Photophysical, and Waveguide Properties, Chem. Mater., 2010, 22, 3735–3745, DOI:10.1021/cm100798q.
- M. Chen, X. Hu, J. Liu, B. Li, N. L. C. Leung, L. Viglianti, T. S. Cheung, H. H. Y. Sung, R. T. K. Kwok, I. D. Williams, A. Qin, J. W. Y. Lam and B. Z. Tang, Rational design of red AIEgens with a new core structure from non-emissive heteroaromatics, Chem. Sci., 2018, 9, 7829–7834, 10.1039/c8sc02810a.
- A. K. Gupta, W. Li, A. Ruseckas, C. Lian, C. L. Carpenter-Warren, D. B. Cordes, A. M. Z. Slawin, D. Jacquemin, I. D. W. Samuel and E. Zysman-Colman, Thermally Activated Delayed Fluorescence Emitters with Intramolecular Proton Transfer for High Luminance Solution-Processed Organic Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2021, 13, 15459–15474, DOI:10.1021/acsami.1c02248.
- A. K. Gupta, T. Matulaitis, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Highly twisted α-diketone-based thermally activated delayed fluorescence emitters and their use in organic light-emitting diodes, Can. J. Chem., 2022, 100, 224–233, DOI:10.1139/cjc-2021-0208.
- S. Li, T. Cheng, C. Yin, S. Zhou, Q. Fan, W. Wu and X. Jiang, Phenothiazine versus Phenoxazine: Structural Effects on the Photophysical Properties of NIR-II AIE Fluorophores, ACS Appl. Mater. Interfaces, 2020, 12, 43466–43473, DOI:10.1021/acsami.0c12773.
- N. G. Connelly and W. E. Geiger, Chemical Redox Agents for Organometallic Chemistry, Chem. Rev., 1996, 96, 877–910, DOI:10.1021/cr940053x.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications, Adv. Mater., 2011, 23, 2367–2371, DOI:10.1002/adma.201004554.
- M. A. Baldo and S. R. Forrest, Transient analysis of organic electrophosphorescence: I. Transient analysis of triplet energy transfer, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 10958–10966, DOI:10.1103/PhysRevB.62.10958.
- F. B. Dias, T. J. Penfold and A. P. Monkman, Photophysics of thermally activated delayed fluorescence molecules, Methods Appl. Fluoresc., 2017, 5, 012001, DOI:10.1088/2050-6120/aa537e.
- Y. Tsuchiya, S. Diesing, F. Bencheikh, Y. Wada, P. L. dos Santos, H. Kaji, E. Zysman-Colman, I. D. W. Samuel and C. Adachi, Exact Solution of Kinetic Analysis for Thermally Activated Delayed Fluorescence Materials, J. Phys. Chem. A, 2021, 125, 8074–8089, DOI:10.1021/acs.jpca.1c04056.
Footnote |
| † Electronic supplementary information (ESI) available: Compound characterization (1H NMR, 13C NMR, elemental analysis, and HRMS), SCXRD, photophysical studies, OLED data, and DFT calculations. Crystallographic information for TPPQ, 4DMAC-TPPQ and 4PXZ-TPPQ. CCDC 2406567–2406569. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc05238b |
| This journal is © The Royal Society of Chemistry 2025 |