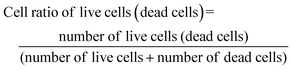Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quaternary ammonium-functionalized carbon nanotubes/alginate nanocomposite hydrogels support myoblast growth and differentiation†
Ludovica
Ceroni‡
a,
Tianqi
Feng‡
b,
Laura
Calvillo
 a,
Stefano
Casalini
a,
Stefano
Casalini
 a,
Patrick
Van Rijn
a,
Patrick
Van Rijn
 *b and
Enzo
Menna
*b and
Enzo
Menna
 *a
*a
aDepartment of Chemical Sciences, University of Padova & INSTM, Via Marzolo 1, 35131, Padova, Italy. E-mail: enzo.menna@unipd.it
bDepartment of Biomaterials & Biomedical Technology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands. E-mail: p.van.rijn@umcg.nl
First published on 26th May 2025
Abstract
Carbon nanotube (CNT) composite hydrogels are promising materials for tissue engineering due to the biocompatibility of the matrix and the electrical conductivity of the filler, which is crucial for promoting the growth and functions in electroactive tissues. While pristine CNTs are insoluble, we synthesized and fully characterized a water-soluble CNT derivative (fCNT) bearing quaternary ammonium groups, and we homogeneously dispersed it within alginate-based hydrogels. Through external and internal gelation we obtained two plain and two fCNT-filled hydrogels (HG1 and HG2 and HG1-fCNT and HG2-fCNT, respectively), and we compared the physical properties of the four different materials. A measurement setup and an approach were specifically designed for the electrical characterization of our hydrogel samples, showing that the addition of a low amount (0.1 mg mL−1) of fCNT enhanced the conductivity of the hydrogel from internal gelation (HG2-fCNT) by more than one order of magnitude, from 5.7 × 10−10 to 2.8 × 10−8 S cm−1. Even more interestingly, HG2-fCNT featured a faster transmission of low frequency signals (with time scales from 1 ms to 100 ms, typical of electroactive biological tissues) than the other samples. Finally, the behavior of the four hydrogels as scaffolds for muscle tissue engineering was compared through studies of myoblast viability, proliferation, and differentiation. A relevant improvement in differentiation (more than doubling the number and area of myotubes and the fusion index) was obtained by adding the fCNT in the case of HG2-fCNT, in line of its superior electrical properties. These outcomes hint at the feasibility of using the fCNT combined with the alginate hydrogel in order to support the myoblast growth and proliferation.
1. Introduction
In the field of tissue engineering, there is particular interest in creating a stimulating environment for cell growth and differentiation of specific tissues and organs with the intention of repairing or replacing them.1 Scaffolds for tissue engineering are biomaterials designed to exhibit customizable properties with the purpose of sustaining cell functions and enhancing cell responses.2 These materials should exhibit a controllable microstructure and adequate porosity, thus allowing for the transport of nutrients, cell migration within the construct, and the diffusion of waste or the exit of degradation products.3 Moreover, the mechanical properties of the scaffolds are of crucial importance in ensuring adequate support for the growing tissue. Ideally, the stiffness of the scaffold should be consistent with the anatomical site being treated.2 Certain tissues could additionally benefit from the specific electric properties of the environment. Indeed, electroactive tissues rely on signal transduction based on electrochemical potential for their development and functions. For instance, ventricular muscle, nerve, lung, cardiac, and skeletal muscle display electrical conductivity ranging between 0.03 and 0.6 S m−1,4 and their cellular functions, including growth, migration, adhesion, proliferation and differentiation, can be modulated by electrical signals. In this case, biomaterials with electroactive components represent a new generation of “smart” biomaterials, which enable the direct transfer of electrical and electromechanical stimuli to cells with or without external electrical stimulation.5 For this reason, carbon-based materials are promising candidates as building blocks in scaffolds for tissue engineering. Specifically, carbon nanotubes (CNTs) have attracted much attention thanks to their unique combination of chemical and physical properties.6 Due to their extended C-sp2 structure, they exhibit high electrical conductivity and modulation of electrostatic potential that can be exploited in the formation of electroactive composite materials, which have already been extensively studied especially for the regeneration of cardiac and neuronal tissues.7–11 CNTs did not demonstrate only support of cell growth but also enhancement of cell adhesion to the substrate, proliferation, and differentiation, in particular osteogenic, neuronal and myogenic.12–15 Furthermore, CNTs demonstrated to function as a reinforcing agent within polymer matrices, thereby modulating the stiffness of the composite material to meet specific requirements and introducing micro- and nano-scale morphologies.16In the specific area of muscle tissue engineering, myogenic cells are isolated from the tissue and made to proliferate and fuse into myotubes in 2D cultures that can then rearrange in parallel and organize in 3D to obtain new engineered muscle tissue.17 The most frequently employed scaffolds for this purpose are hydrogels, thanks to their structural and compositional resemblance to the natural extracellular matrix (ECM), as well as their capacity to facilitate cell proliferation and survival.18 These materials possess a soft and flexible texture, similar to that of living tissues, and a high level of hydrophilicity due to the presence of hydrophilic groups (e.g. –NH2, –COOH, –OH, and –CONH2) which ensure the bidirectional flow of water or biological fluids.19 Alginate, in particular, is a well-known hydrogel based on polysaccharides from natural sources that differ in the relative amount of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues and is typically crosslinked by Ca2+ ions between the G units.20 This material is biocompatible, biodegradable, biologically inert, and it is characterized by a high-water content.18,21 Alginate exhibits an adjustable shape and porosity, fast gelling behaviour and the ability to adapt the mechanical properties to mimic those of natural tissues depending on the type, amount and crosslinking density of the polymer in the hydrogel.22–24 Thanks to these properties, it was already used for organ repair, for example in bone24,25 and cartilage tissue engineering.26,27
As skeletal muscle tissues possess electrical excitable properties, the incorporation of CNTs as fillers within hydrogel matrices results in the formation of electroactive composite materials which facilitate the local transmission of electrical stimuli, thereby enhancing cell responses and tissue regeneration. CNT-based materials have been successfully shown to contribute to myogenesis and muscle tissue recovery due to their electrical and mechanical properties.15,28 The problem associated with the use of carbon nanostructures is that they are highly hydrophobic and tend to aggregate in any solvent, and in particular in water, through π–π stacking and van der Waals interactions.29 Chemical modification of CNTs is an effective strategy to limit aggregation and promote homogeneous dispersion of the nanostructure in matrices and to obtain hybrid scaffolds with uniform properties.30 Moreover, several studies suggest that the functionalization of CNTs could enhance their biocompatibility and biodegradability, thus making them safer for a biomedical use.31 Indeed, through covalent functionalization, we previously obtained CNT derivatives soluble in organic solvents and we used them as fillers in polymer-based scaffolds for the proliferation of the human neuronal precursor cell line SH-SY5Y and neurite extension.9,32 We also reported on the synthesis of water-soluble CNT derivatives that could be effectively dispersed in oxidized polyvinylalcohol matrices. The resulting composites showed promising features as conductive nerve conduits.10 Functionalisation can also be considered a strategy for modulating the interactions of the nanostructure with the matrix. For example, when charges are introduced onto the surface of CNTs, electrostatic interactions can be initiated with charged polymers, thus enabling the grafting of CNTs into the matrix and preventing their uncontrolled release towards the cell environment. A similar strategy, based on charge interactions between a positively charged polyelectrolyte and alginate, was successfully employed in the past.33
In the present study, we focus on the functionalization of multi-walled CNTs with positively charged quaternary ammonium groups to obtain a water-dispersible CNT derivative (fCNT), which specifically promotes potential interactions with carboxylate units of the alginate chains. The hybrid scaffolds were prepared with the aim of improving electrical response, weight retention and mechanical stability. Moreover, two distinct alginate gelation methods were employed and subsequently compared on the basis of their divergent physical properties. Finally, the response of myoblasts to these scaffolds was studied, with the ultimate purpose of obtaining well-suited materials that resemble the structure and mechanical properties of muscle tissues.
2. Experimental
Multi-walled CNTs (outer diameter: 8–15 nm and length: 0.5–2 μm), purity >95%, were purchased from ACS Material (Pasadena, CA, USA). Sodium alginate with a low viscosity between 5.0 and 40.0 cps (Product Number: W201502) and solvents and reagents used in the synthesis procedures were all purchased from Sigma-Aldrich (Milan, Italy). Concerning the cell experiments, DMEM-HG, fetal bovine serum, pen/streptomycin, insulin–transferrin–selenium were purchased from Gibco, and dexamethasone, paraformaldehyde and live/dead staining were purchased from Sigma-Aldrich.2.1. Synthesis of CNT-PhN(CH3)3+ (fCNT)
Multi-walled CNTs (70.0 mg, 5.82 mmol of carbon) were mixed with a solution of 4-amino-N,N,N-trimethylbenzene ammonium iodide (810.51 mg, 2.91 mmol) in 17 mL of Milli-Q water. The reaction mixture was heated to 80 °C under nitrogen flux and magnetic stirring; then, isopentyl nitrite (0.824 mL, 5.82 mmol) was added. After 4 hours, the reaction mixture was allowed to cool to room temperature. The dispersion was filtered on a Millipore PC 0.1 μm membrane (VCTP), and the product was washed on the filter with 50 mL of distilled water and then removed from the filter through sonication in 50 mL of distilled water. The filtration/washing procedure was repeated four times using water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1
methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and one time using methanol. The methanol dispersion was finally dried under nitrogen flux to afford the multi-walled CNT derivative (fCNT) as a solid black powder.
1) and one time using methanol. The methanol dispersion was finally dried under nitrogen flux to afford the multi-walled CNT derivative (fCNT) as a solid black powder.
2.2. Characterization of fCNT
The fCNT standard dispersion (100 μL) and a solution of 1 mg mL−1 of NaCl in water (160 μL) were added to Milli-Q water (700 μL) and placed in a folded capillary cell for zeta potential analysis. The ZP measurement was carried out in NaCl 30 mM, equilibrating at 25 °C for 120 s. The Smoluchowski equation was used as a theoretical model for the measurement and the resulting value was an average of 3 measurements of 10–100 runs each.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RCF for 10 minutes. The supernatant was removed and the precipitate was further washed by repeating the same procedure five more times. Then, an alginate@fCNT dispersion in 1 mL of Milli-Q water was bath sonicated for 10 minutes and diluted 10 times for the measurements. For comparison, a control sample was also prepared according to the same procedure starting from the fCNT stock dispersion (without alginate). The DLS size and ZP analysis were carried out using a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK) using a clear disposable zeta cell, setting the scattering angle at 173°, equilibrating at 25 °C for 120 s. The reported value of the hydrodynamic mean diameter was an average of 3 measurements of 11 runs each, with a run duration of 10 s. For data evaluation, the Smoluchowski equation was used as a theoretical model.
000 RCF for 10 minutes. The supernatant was removed and the precipitate was further washed by repeating the same procedure five more times. Then, an alginate@fCNT dispersion in 1 mL of Milli-Q water was bath sonicated for 10 minutes and diluted 10 times for the measurements. For comparison, a control sample was also prepared according to the same procedure starting from the fCNT stock dispersion (without alginate). The DLS size and ZP analysis were carried out using a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK) using a clear disposable zeta cell, setting the scattering angle at 173°, equilibrating at 25 °C for 120 s. The reported value of the hydrodynamic mean diameter was an average of 3 measurements of 11 runs each, with a run duration of 10 s. For data evaluation, the Smoluchowski equation was used as a theoretical model.
2.3. Preparation of the scaffolds HG1, HG1-fCNT, HG2, and HG2-fCNT
Four alginate hydrogels, without and with fCNT as a filler, were prepared following two different gelation techniques, namely the external gelation (hereafter HG1 and HG1-fCNT) and the internal gelation (hereafter HG2 and HG2-fCNT). Table 1 summarizes reagents and quantities for both approaches. Samples were prepared in the form of discs using a circular polydimethylsiloxane (PDMS) mold previously fabricated. All solutions were prepared with Milli-Q water. The external gelation was carried out by pouring 1 mL of alginate solution 2 w/v% in the circular PDMS mold; then, a dialysis membrane was positioned on top of the alginate solution followed by a transwell insert. Next, 3 mL of a 50 mM CaCl2 solution were poured into the transwell and the system was left to equilibrate in the fridge overnight to allow Ca2+ ions to pass through the membrane and crosslink the alginate chains. Instead, for the internal gelation protocol, 0.5 mL of alginate solution 4 w/v%, 0.1 mL of Milli-Q water and 0.2 mL of a 300 mM dispersion of CaCO3 were vortexed and mixed in an ultrasonic bath. Once CaCO3 was homogeneously dispersed in the alginate solution, 0.2 mL of a 600 mM solution of glucono-delta-lactone (GDL) freshly prepared was added. The mixture was finally poured into a circular PDMS mold, left at room temperature for 5 hours to make sure that the hydrogel is formed and then stored in the fridge until use.| Reagents | External gelation | Internal gelation | ||
|---|---|---|---|---|
| HG1 | HG1-fCNT | HG2 | HG2-fCNT | |
| fCNT (w/v%) | — | 0.01 (0.1 mg mL−1) | — | 0.01 (0.1 mg mL−1) |
| Alginate (w/v%) | 2 | 2 | ||
| CaCO3 (mM) | — | 60 | ||
| GDL (mM) | — | 120 | ||
| CaCl2 (mM) | 50, incubate 23 h 4 °C | — | ||
For the preparation of hybrid hydrogels, a fCNT stock dispersion of 1 mg mL−1 was prepared through tip sonication for 3 minutes. Then, before gelation, the stock dispersion was added to the alginate solution to achieve the final fCNT concentration of 0.1 mg mL−1.
2.4. Characterization of the scaffolds
 where I is the current, dV is the scanned potential, m is the mass of the hydrogel (viz. mHG1 = 22.1 mg; mHG1-fCNT = 22.9 mg; mHG2 = 27.1 mg; mHG2-fCNT = 27.7 mg), ΔV is the potential range (i.e. 400 mV), and v is the scan rate (i.e. from 50 mV s−1 to 500 mV s−1). Regarding CA, we applied a step potential of 200 mV for 5 seconds, and the acquisition time was 10 ms. Finally, EIS was performed by applying a series of sinusoidal waves featuring an amplitude of 10 mV and a set point potential of 0 V (versus OCP). We selected a range of frequencies from 105 to 10−1 Hz.
where I is the current, dV is the scanned potential, m is the mass of the hydrogel (viz. mHG1 = 22.1 mg; mHG1-fCNT = 22.9 mg; mHG2 = 27.1 mg; mHG2-fCNT = 27.7 mg), ΔV is the potential range (i.e. 400 mV), and v is the scan rate (i.e. from 50 mV s−1 to 500 mV s−1). Regarding CA, we applied a step potential of 200 mV for 5 seconds, and the acquisition time was 10 ms. Finally, EIS was performed by applying a series of sinusoidal waves featuring an amplitude of 10 mV and a set point potential of 0 V (versus OCP). We selected a range of frequencies from 105 to 10−1 Hz.
The whole batch of electrochemical measurements was performed by sealing the sample holder thereby keeping wet the surrounding environment from water vapor. This technical aspect preserved the overall hydration of the hydrogel, and it allowed us to avoid keeping a droplet of solution on top of it.
2.5. Cell experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) in PBS with 1% FBS for 3 hours at 4 °C and then incubated with goat anti-mouse IgG H&L (Alexa Fluor® 488, 1
200) in PBS with 1% FBS for 3 hours at 4 °C and then incubated with goat anti-mouse IgG H&L (Alexa Fluor® 488, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
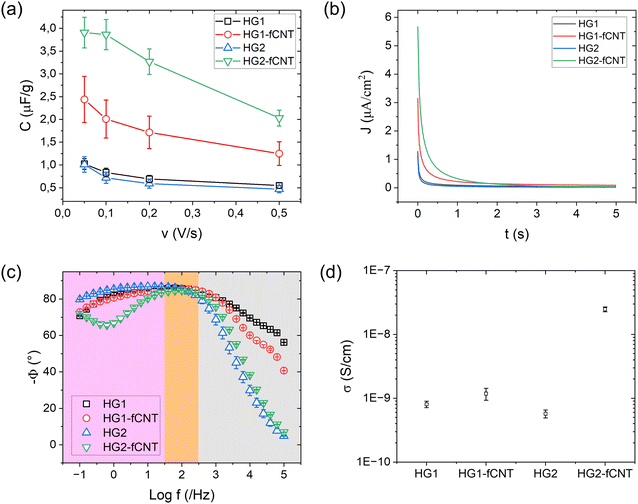
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) in PBS with 1% FBS and DAPI for 1 hour, following with PBS washing in 3 times. The imaging was done using a fluorescence microscope (Microscope DM4000b). The Myotube number, area, diameter and length were evaluated using Fiji software. CellProfiler software evaluated the fusion index and the percentage of total nuclei inside the myotubes. Three independent samples were analyzed (n ≥ 5).
500) in PBS with 1% FBS and DAPI for 1 hour, following with PBS washing in 3 times. The imaging was done using a fluorescence microscope (Microscope DM4000b). The Myotube number, area, diameter and length were evaluated using Fiji software. CellProfiler software evaluated the fusion index and the percentage of total nuclei inside the myotubes. Three independent samples were analyzed (n ≥ 5).
3. Results and discussion
3.1. fCNT synthesis and characterization
CNTs were decorated with trimethyl ammonium benzene groups through the diazotization reaction to obtain fCNT, as shown in Fig. 1. The diazotization reaction was performed in water at 80 °C (see Experimental details in section 2.1).37 The diazonium precursor 4-amino-N,N,N-trimethylbenzene ammonium iodide was obtained through methylation of N,N-dimethylbenzene-1,4-diamine38 and activated in situ with isopentyl nitrite. The method herein employed for CNT functionalization relies on a synthetic strategy readapted from our previous works10,37,39 and based on the Tour diazotization reaction.40 This type of reaction allows the covalent attachment of substituted benzene rings to carbon nanostructures and leads to a good degree of functionalization. The reaction is fast, effective, well-reproducible, free from hazardous reagents or soluble toxic contaminants and, being carried out in water, it does not leave residual organic solvents adsorbed on the nanostructures. Moreover, a proper choice of reaction conditions allows for limited damage to the structure of the basal plane and thus retains the properties of the nanotube, unlike functionalization based on harsh oxidative treatments. | ||
| Fig. 1 Reaction scheme of CNT functionalization with 4 amino-N,N,N-trimethylbenzene ammonium iodide through the diazotization reaction. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– moieties.43 Indeed, azobenzene bonds may form during the functionalization, through diazo coupling of diazonium salts with N,N,N-trimethylbenzene ammonium groups already anchored on the CNT surface, or from the radical splitting of a diazoanhydride formed throughout the radical propagation mechanism proposed for the Tour reaction.44,45 This result is in agreement with similar evidence already reported for this kind of CNT functionalization.39 We hypothesized that azo coupling may compete with the reduction of the diazonium salt thus increasing the amount of nitrogen species on the surface and leading to the formation of multiaryl layers on CNTs. On the other hand, it cannot be excluded that a small fraction of the aniline reagent remains adsorbed on CNTs, since the –NH2 component would overlap with the –N
N– moieties.43 Indeed, azobenzene bonds may form during the functionalization, through diazo coupling of diazonium salts with N,N,N-trimethylbenzene ammonium groups already anchored on the CNT surface, or from the radical splitting of a diazoanhydride formed throughout the radical propagation mechanism proposed for the Tour reaction.44,45 This result is in agreement with similar evidence already reported for this kind of CNT functionalization.39 We hypothesized that azo coupling may compete with the reduction of the diazonium salt thus increasing the amount of nitrogen species on the surface and leading to the formation of multiaryl layers on CNTs. On the other hand, it cannot be excluded that a small fraction of the aniline reagent remains adsorbed on CNTs, since the –NH2 component would overlap with the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– signal (Fig. 2b). However, the difference between the ratios of the two N components for fCNT and for the aniline reagent excludes the hypothesis that all aniline molecules are adsorbed on the CNT surface without reacting. The surface composition of fCNT was obtained from the C 1s, N 1s and O 1s peak regions, taking into account the corresponding sensitivity factors. Accordingly, a nitrogen component of 3.0 at% is introduced by the functionalization process.
N– signal (Fig. 2b). However, the difference between the ratios of the two N components for fCNT and for the aniline reagent excludes the hypothesis that all aniline molecules are adsorbed on the CNT surface without reacting. The surface composition of fCNT was obtained from the C 1s, N 1s and O 1s peak regions, taking into account the corresponding sensitivity factors. Accordingly, a nitrogen component of 3.0 at% is introduced by the functionalization process.
| Sample | C sp2 | C sp3 | C–OH/C–N | Carbonyl groups | Carboxylic groups |
|---|---|---|---|---|---|
| CNT | 284.1 eV | 285.0 eV | 286.0 | 287.5 eV | 288.9 eV |
| 79.1% | 8.3% | 5.7% | 4.3% | 2.6% | |
| Fcnt | 284.1 eV | 285.0 eV | 286.0 | 287.5 eV | 288.9 eV |
| 74.6% | 10.2% | 11.1% | 4.1% | 0% |
A hydrodynamic mean diameter of 291.0 ± 3.6 nm was measured for alginate@fCNT, which falls in the same size range obtained for the fCNT control sample (313.0 ± 9.1 nm), also considering the above-mentioned approximation about the shape of the nanoparticles. This evidence rules out the presence of macroaggregates of alginate chains and confirms the effectiveness of the washing procedure. Once the removal of excess alginate was verified, the interaction between fCNT and alginate was investigated through ZP analysis. The negative ZP value (−36.7 ± 0.4 mV) obtained for alginate@fCNT, compared with the positive potential of fCNT (33.4 ± 0.2 mV), can be ascribed to the alginate carboxylate groups.51 This indicates that the negatively charged polymer is still wrapping the nanotubes, even after repeated washing steps, thanks to strong interactions with the positively charged fCNT surface. This evidence supports the hypothesis that ammonium groups help to stabilize the dispersion of fCNT in the hydrogel scaffold and prevent migration into the cell environment.
3.2. Scaffold preparation, characterization and cell studies
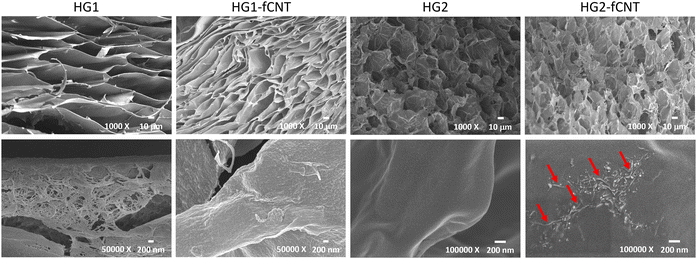
Since the physical properties of an alginate hydrogel are affected by its specific preparation procedure,52 we considered two different gelation techniques based on ionic crosslinking with Ca2+ ions, in order to test if they give rise to different responses to the inclusion of the fCNT filler, and in turn different cell behaviors (details on scaffold preparation are provided in Section 2.3). Specific preparation conditions were chosen to obtain values of stiffness in the range of 25–90 kPa, which is typically measured for skeletal muscle tissues in murine53–55 and rabbit.56The external gelation method is based on an external crosslink source and gelation is achieved by the established equilibrium between alginate and CaCl2 solutions, which are physically separated by a dialysis membrane. Usually, this technique leads to the formation of a stiff but not uniform material. Instead, the internal gelation occurs from inside the hydrogel after homogeneously mixing the alginate solution with a CaCO3 crosslinker solution and an acid component.57 This method is known to lead to the formation of a soft and highly porous hydrogel due to the evolution of CO2 from the CaCO3 source during the crosslinking52 and allows tuning of the mechanical and chemical properties of hydrogels by changing the amount of Ca2+ and alginate used.22,58 Plain alginate hydrogels HG1 and HG2 were obtained through external gelation and internal gelation, respectively. By adding a fCNT dispersion to the alginate solution before gelation, composite hydrogels HG1-fCNT and HG2-fCNT were prepared, with a 0.01 w/v% fCNT loading. This value, much lower than what can be found in the literature,16,30 was chosen to minimize toxicity. A thorough characterization of the alginate discs was carried out to study the different properties provided by the two gelation methods and by the presence of fCNT. The scaffolds were tested in terms of mechanical, electrical, and cell adhesion properties, promoting myoblast growth and differentiation.
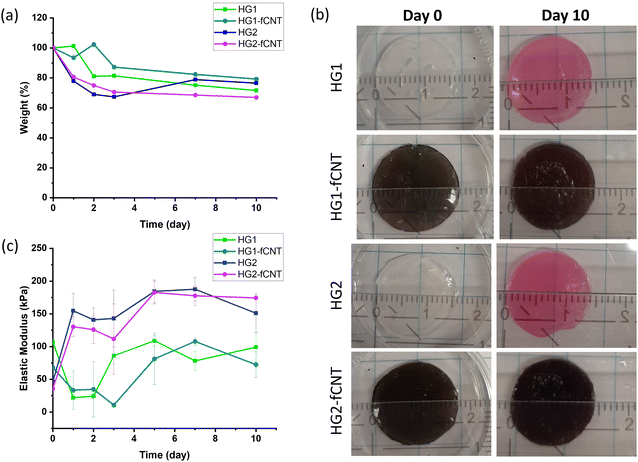 | ||
| Fig. 5 (a) Weight changes, (b) gross appearance and (c) elastic modulus of hydrogel samples in contact with cell culture medium for ten days. | ||
In this instance, we demonstrated that the simple addition of calcium ions into the cell medium can stabilize the crosslinking network, thereby preventing the degradation of the material, which is often associated with the uncontrolled exchange of divalent ions with the surrounding medium and subsequent dissolution.19 As reported in the literature, this degradation tendency can be reduced by using different crosslinkers, such as Al3+ and Fe3+ cations, which bind to alginate more efficiently,61 polyelectrolyte as linear polyethyleneimine (LPEI),33 or covalent crosslinkers through carbodiimide chemistry.62,63 In addition, to overcome these issues, alginate can be coupled with other biomaterials such as cellulose, collagen or hyaluronic acid.60,64
The differences between bare HG2 and HG2-fCNT observed in cell adhesion and differentiation could be explained, as already mentioned, by the exposure of fCNT particles on the hydrogel surface, acting as adhesion sites, but also by the difference in electrical behaviour detectable precisely at low frequencies, in the range of bioelectrical signalling. We already demonstrated that the dispersion of CNT derivatives in nonconductive biomaterials can improve the interaction with cells, helping the differentiation in electroactive tissues.10,32 Specifically, the literature reported several examples supporting the hypothesis that electrical conductivity at low frequencies could be beneficial for cell growth and differentiation.65,67,70 Moreover, the presence of CNTs in alginate hydrogels probably enhances the very first cell adhesion onto these substrates, consequently affecting cell fate.16,30 On the other hand, HG1 was not affected by the presence of fCNT; this could be because carbon nanotubes were not exposed on the surface of the material; therefore, they were not directly in contact with the cells, and the surface electrical conductivity of HG1 and HG1-fCNT was the same at low frequencies. Another explanation for this peculiar behavior could be that both HG1 and HG1-fCNT showed a mechanical stiffness of about 100 kPa with little fluctuation over a period of ten days. This stiffness value is very close to that of the skeletal muscle tissue of 25–90 kPa, indicating that the samples probably had the best mechanical properties for myoblast growth. In fact, stiffness is one of the most important factors for cell growth and differentiation. If modulated properly, it can adapt to the mechanical properties of the tissue of interest and help cells to attach and grow well on the scaffold.81 Indeed, both HG1-type hydrogels displayed better cell adhesion than HG2 already in the first 24 hour, and this may have influenced cell fate also in terms of differentiation.
4. Conclusions
CNTs were successfully functionalized with positively charged trimethyl ammonium benzene groups affording fCNT derivatives with good dispersibility in water. The soluble CNT derivatives were homogeneously dispersed in alginate-based hydrogels obtained through two different gelation methods (external gelation for the HG1 series, by addition of CaCl2, and internal gelation for the HG2 series, based on CaCO3).The physical properties of the resulting materials, either with or without CNT fillers, were compared and correlated with the effects on myoblast growth and differentiation. It was shown that the gelation routes significantly influenced relevant features such as pore morphology and stiffness, while the addition of fCNT lowered the elastic modulus in both matrices. A thorough electrical characterization was carried out through a methodology and a setup that we have specifically designed for our hydrogel scaffolds. This allowed us to highlight that, while the native electrical properties of the two kinds of hydrogels are very similar, the inclusion of fCNT has a prominent effect on HG2. Indeed, HG2-fCNT, besides showing a higher conductivity, transmitted low frequency signals (typical of electroactive biological tissues) at a higher rate compared to the other examined scaffolds.
The proposed functionalization of CNTs allowed us to obtain composite scaffolds fulfilling cell viability and cytocompatibility with both gelation methods. Interestingly, the addition of fCNT had a more relevant effect on the scaffold obtained through internal gelation. Precisely, in HG2-fCNT, where the nanotubes provide a significant improvement in electrical properties, there is also an enhancement in cell differentiation. We can therefore conclude that HG2-fCNT is the most promising scaffold for tissue engineering applications, considering not only the observed effect on cells but also its potential advantage in active electrical stimulation. Noteworthy, the observed effects were obtained with a very low loading of fCNT, thanks to the specific functionalization strategy.
Further steps towards the use of such hybrid scaffolds may explore strategies to achieve an aligned topography on the surface to further promote myoblast differentiation into myotubes. The addition of proteins and growth factors within the hydrogel is also an option to stimulate cells and recreate an improved biomimetic environment.82,83 Moreover, testing our scaffold also on different cell lines responding to electric stimuli, such as neurons, may lead to similar or even more interesting results.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Patrick van Rijn reports a relationship with BiomACS BV that includes equity or stocks, being the co-founder, scientific advisor, and share-holder of BiomACS BV, a biomedical oriented screening company. The authors declare no other competing interests.Acknowledgements
Financial support from the University of Padova is acknowledged by EM, LCa for grant P-DiSC#05BIRD2021-UNIPD (CORDER) and by SC for grant P-DiSC#11NexuS_BIRD2020-UNIPD (CARBON-FET). The PhD fellowship of L.Ce. was financed by PON 2014-2020 (National Operative Program ‘Research and Innovation’) D.M. n. 1061, August 10th 2021, Action IV.5. The PhD fellowship of T. F. was financed by the Chinese Scholarship Council (No. 202106170045).References
- A. S. Mao and D. J. Mooney, Regenerative Medicine: Current Therapies and Future Directions, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(47), 14452–14459, DOI:10.1073/pnas.1508520112.
- M. I. Echeverria Molina, K. G. Malollari and K. Komvopoulos, Design Challenges in Polymeric Scaffolds for Tissue Engineering, Front. Bioeng. Biotechnol., 2021, 9, 617141, DOI:10.3389/fbioe.2021.617141.
- C. M. Murphy and F. J. O’Brien, Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds, Cell Adhes. Migr., 2010, 4(3), 377–381, DOI:10.4161/cam.4.3.11747.
- P. Zarrintaj, S. Manouchehri, Z. Ahmadi, M. R. Saeb, A. M. Urbanska, D. L. Kaplan and M. Mozafari, Agarose-Based Biomaterials for Tissue Engineering, Carbohydr. Polym., 2018, 187, 66–84, DOI:10.1016/j.carbpol.2018.01.060.
- X. Lu, W. Zhang, C. Wang, T.-C. Wen and Y. Wei, One-Dimensional Conducting Polymer Nanocomposites: Synthesis, Properties and Applications, Prog. Polym. Sci., 2011, 36(5), 671–712, DOI:10.1016/j.progpolymsci.2010.07.010.
- C. Cha, S. R. Shin, N. Annabi, M. R. Dokmeci and A. Khademhosseini, Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering, ACS Nano, 2013, 7(4), 2891–2897, DOI:10.1021/nn401196a.
- M. Barrejón, S. Marchesan, N. Alegret and M. Prato, Carbon Nanotubes for Cardiac Tissue Regeneration: State of the Art and Perspectives, Carbon, 2021, 184, 641–650 CrossRef.
- E. L. Hopley, S. Salmasi, D. M. Kalaskar and A. M. Seifalian, Carbon Nanotubes Leading the Way Forward in New Generation 3D Tissue Engineering, Biotechnol. Adv., 2014, 32(5), 1000–1014, DOI:10.1016/j.biotechadv.2014.05.003.
- N. Vicentini, T. Gatti, M. Salerno, Y. S. H. Gomez, M. Bellon, S. Gallio, C. Marega, F. Filippini and E. Menna, Effect of Different Functionalized Carbon Nanostructures as Fillers on the Physical Properties of Biocompatible Poly (l-Lactic Acid) Composites, Mater. Chem. Phys., 2018, 214, 265–276 CrossRef CAS.
- E. Stocco, S. Barbon, L. Ceroni, M. Confalonieri, G. Pulzato, S. Pressi, A. D’Osualdo, M. Contran, R. Boscolo-Berto, C. Tiengo, S. Todros, P. G. Pavan, V. Macchi, R. De Caro, L. Calvillo, E. Menna and A. Porzionato, Partially Oxidized Polyvinyl Alcohol + Functionalized Water Soluble Multiwalled Carbon Nanotubes: A New Conductive Nanocomposite Material with Promising Implications for Neuroregeneration, J. Sci. Adv. Mater. Devices, 2024, 9(3), 100762, DOI:10.1016/j.jsamd.2024.100762.
- G. Suarato, S. Pressi, E. Menna, M. Ruben, E. M. Petrini, A. Barberis, D. Miele, G. Sandri, M. Salerno, A. Schirato, A. Alabastri, A. Athanassiou, R. Proietti Zaccaria and E. L. Papadopoulou, Modified Carbon Nanotubes Favor Fibroblast Growth by Tuning the Cell Membrane Potential, ACS Appl. Mater. Interfaces, 2024, 16(3), 3093–3105, DOI:10.1021/acsami.3c14527.
- L. P. Zanello, B. Zhao, H. Hu and R. C. Haddon, Bone Cell Proliferation on Carbon Nanotubes, Nano Lett., 2006, 6(3), 562–567, DOI:10.1021/nl051861e.
- G. Scapin, P. Salice, S. Tescari, E. Menna, V. De Filippis and F. Filippini, Enhanced Neuronal Cell Differentiation Combining Biomimetic Peptides and a Carbon Nanotube-Polymer Scaffold, Nanomedicine, 2015, 11(3), 621–632 CrossRef CAS PubMed.
- A. Fabbro, M. Prato and L. Ballerini, Carbon Nanotubes in Neuroregeneration and Repair, Adv. Drug Delivery Rev., 2013, 65(15), 2034–2044, DOI:10.1016/j.addr.2013.07.002.
- S. Ahadian, J. Ramón-Azcón, M. Estili, X. Liang, S. Ostrovidov, H. Shiku, M. Ramalingam, K. Nakajima, Y. Sakka, H. Bae, T. Matsue and A. Khademhosseini, Hybrid Hydrogels Containing Vertically Aligned Carbon Nanotubes with Anisotropic Electrical Conductivity for Muscle Myofiber Fabrication, Sci. Rep., 2014, 4(1), 4271, DOI:10.1038/srep04271.
- E. D. Yildirim, X. Yin, K. Nair and W. Sun, Fabrication, Characterization, and Biocompatibility of Single-walled Carbon Nanotube-reinforced Alginate Composite Scaffolds Manufactured Using Freeform Fabrication Technique, J. Biomed. Mater. Res., Part B, 2008, 87B(2), 406–414, DOI:10.1002/jbm.b.31118.
- P. E. Kosnik; R. G. Dennis and H. H. Vandenburgh, Tissue Engineering Skeletal Muscle, Functional tissue engineering, Springer, 2003, pp. 377–392 Search PubMed.
- I. M. El-Sherbiny and M. H. Yacoub, Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges, Glob. Cardiol. Sci. Pract., 2013, 3, 38, DOI:10.5339/gcsp.2013.38.
- K. Y. Lee and D. J. Mooney, Hydrogels for Tissue Engineering, Chem. Rev., 2001, 101(7), 1869–1880, DOI:10.1021/cr000108x.
- A. D. Augst, H. J. Kong and D. J. Mooney, Alginate Hydrogels as Biomaterials, Macromol. Biosci., 2006, 6(8), 623–633 CrossRef CAS PubMed.
- K. Joyce, G. T. Fabra, Y. Bozkurt and A. Pandit, Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties, Signal Transduct. Target. Ther., 2021, 6(1), 122, DOI:10.1038/s41392-021-00512-8.
- E. A. Growney Kalaf, R. Flores, J. G. Bledsoe and S. A. Sell, Characterization of Slow-Gelling Alginate Hydrogels for Intervertebral Disc Tissue-Engineering Applications, Mater. Sci. Eng., C, 2016, 63, 198–210, DOI:10.1016/j.msec.2016.02.067.
- H.-J. Kong, K. Y. Lee and D. J. Mooney, Decoupling the Dependence of Rheological/Mechanical Properties of Hydrogels from Solids Concentration, Polymer, 2002, 43(23), 6239–6246 CrossRef CAS.
- C. K. Kuo and P. X. Ma, Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties, Biomaterials, 2001, 22(6), 511–521 CrossRef CAS PubMed.
- M. M. Stevens, R. P. Marini, D. Schaefer, J. Aronson, R. Langer and V. P. Shastri, In Vivo Engineering of Organs: The Bone Bioreactor, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(32), 11450–11455, DOI:10.1073/pnas.0504705102.
- S. C. N. Chang, J. A. Rowley, G. Tobias, N. G. Genes, A. K. Roy, D. J. Mooney, C. A. Vacanti and L. J. Bonassar, Injection Molding of Chondrocyte/Alginate Constructs in the Shape of Facial Implants, J. Biomed. Mater. Res., 2001, 55((4)), 503–511 CrossRef CAS PubMed.
- M. Stevens, A Rapid-Curing Alginate Gel System: Utility in Periosteum-Derived Cartilage Tissue Engineering, Biomaterials, 2004, 25(5), 887–894, DOI:10.1016/j.biomaterials.2003.07.002.
- A. Patel, S. Mukundan, W. Wang, A. Karumuri, V. Sant, S. M. Mukhopadhyay and S. Sant, Carbon-Based Hierarchical Scaffolds for Myoblast Differentiation: Synergy between Nano-Functionalization and Alignment, Acta Biomater., 2016, 32, 77–88, DOI:10.1016/j.actbio.2016.01.004.
- N. Karousis, N. Tagmatarchis and D. Tasis, Current Progress on the Chemical Modification of Carbon Nanotubes, Chem. Rev., 2010, 110(9), 5366–5397, DOI:10.1021/cr100018g.
- B. Joddar, E. Garcia, A. Casas and C. M. Stewart, Development of Functionalized Multi-Walled Carbon-Nanotube-Based Alginate Hydrogels for Enabling Biomimetic Technologies, Sci. Rep., 2016, 6(1), 32456, DOI:10.1038/srep32456.
- H. J. Johnston, G. R. Hutchison, F. M. Christensen, S. Peters, S. Hankin, K. Aschberger and V. Stone, A Critical Review of the Biological Mechanisms Underlying the in Vivo and in Vitro Toxicity of Carbon Nanotubes: The Contribution of Physico-Chemical Characteristics, Nanotoxicology, 2010, 4(2), 207–246 CrossRef CAS PubMed.
- N. Vicentini, T. Gatti, P. Salice, G. Scapin, C. Marega, F. Filippini and E. Menna, Covalent Functionalization Enables Good Dispersion and Anisotropic Orientation of Multi-Walled Carbon Nanotubes in a Poly(l-Lactic Acid) Electrospun Nanofibrous Matrix Boosting Neuronal Differentiation, Carbon, 2015, 95, 725–730, DOI:10.1016/j.carbon.2015.08.094.
- P. T. Kühn, T. L. Meijer, I. Schiavon, M. Van Poll, J. Van Aken, S. Groen, R. Kuijer, T. G. Van Kooten and P. Van Rijn, Non-Covalently Stabilized Alginate Hydrogels as Functional Cell Scaffold Material, Macromol. Biosci., 2016, 16(11), 1693–1702, DOI:10.1002/mabi.201600214.
- P. Sharma, H. Busscher, T. Terwee, S. Koopmans and T. van Kooten, A Comparative Study on the Viscoelastic Properties of Human and Animal Lenses, Exp. Eye Res., 2011, 93(5), 681–688 CrossRef CAS PubMed.
- A. M. Almonacid Suarez, M. G. L. Brinker, L. A. Brouwer, I. Van Der Ham, M. C. Harmsen and P. Van Rijn, Topography-Mediated Myotube and Endothelial Alignment, Differentiation, and Extracellular Matrix Organization for Skeletal Muscle Engineering, Polymers, 2020, 12(9), 1948, DOI:10.3390/polym12091948.
- A. M. Almonacid Suarez, Q. Zhou, P. van Rijn and M. C. Harmsen, Directional Topography Gradients Drive Optimum Alignment and Differentiation of Human Myoblasts, J. Tissue Eng. Regen. Med., 2019, 13(12), 2234–2245 CrossRef CAS PubMed.
- L. Ceroni, S. Benazzato, S. Pressi, L. Calvillo, E. Marotta and E. Menna, Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes, Nanomaterials, 2024, 14(6), 522, DOI:10.3390/nano14060522.
- C. Hadad, X. Ke, M. Carraro, A. Sartorel, C. Bittencourt, G. Van Tendeloo, M. Bonchio, M. Quintana and M. Prato, Positive Graphene by Chemical Design: Tuning Supramolecular Strategies for Functional Surfaces, Chem. Commun., 2014, 50(7), 885–887, 10.1039/C3CC47056C.
- P. Salice, E. Fabris, C. Sartorio, D. Fenaroli, V. Figà, M. P. Casaletto, S. Cataldo, B. Pignataro and E. Menna, An Insight into the Functionalisation of Carbon Nanotubes by Diazonium Chemistry: Towards a Controlled Decoration, Carbon, 2014, 74, 73–82, DOI:10.1016/j.carbon.2014.02.084.
- J. L. Bahr, J. Yang, D. V. Kosynkin, M. J. Bronikowski, R. E. Smalley and J. M. Tour, Functionalization of Carbon Nanotubes by Electrochemical Reduction of Aryl Diazonium Salts: A Bucky Paper Electrode, J. Am. Chem. Soc., 2001, 123(27), 6536–6542, DOI:10.1021/ja010462s.
- S. Osswald, M. Havel and Y. Gogotsi, Monitoring Oxidation of Multiwalled Carbon Nanotubes by Raman Spectroscopy, J. Raman Spectrosc., 2007, 38(6), 728–736, DOI:10.1002/jrs.1686.
- W. Cao, Z. Wang, Q. Zeng and C. Shen, 13C NMR and XPS Characterization of Anion Adsorbent with Quaternary Ammonium Groups Prepared from Rice Straw, Corn Stalk and Sugarcane Bagasse, Appl. Surf. Sci., 2016, 389, 404–410, DOI:10.1016/j.apsusc.2016.07.095.
- P. Brant and R. D. Feltham, X-Ray Photoelectron Spectra of Aryldiazo Derivatives of Transition Metals, J. Organomet. Chem., 1976, 120(3), C53–C57 CrossRef CAS.
- M. E. Lipińska, S. L. H. Rebelo, M. F. R. Pereira, J. A. N. F. Gomes, C. Freire and J. L. Figueiredo, New Insights into the Functionalization of Multi-Walled Carbon Nanotubes with Aniline Derivatives, Carbon, 2012, 50(9), 3280–3294, DOI:10.1016/j.carbon.2011.12.018.
- Z. Salmi, S. Gam-Derouich, S. Mahouche-Chergui, M. Turmine and M. Chehimi, On the Interfacial Chemistry of Aryl Diazonium Compounds in Polymer Science, Chem. Pap., 2012, 66(5), 369–391 CAS.
- P. Salice, P. Maity, E. Rossi, T. Carofiglio, E. Menna and M. Maggini, The Continuous-Flow Cycloaddition of Azomethine Ylides to Carbon Nanotubes, Chem. Commun., 2011, 47(32), 9092, 10.1039/c1cc13155a.
- S. Bhattacharjee, DLS and Zeta Potential – What They Are and What They Are Not?, J. Controlled Release, 2016, 235, 337–351, DOI:10.1016/j.jconrel.2016.06.017.
- Y. Wei, X. Ling, L. Zou, D. Lai, H. Lu and Y. Xu, A Facile Approach toward Preparation of Sulfonated Multi-Walled Carbon Nanotubes and Their Dispersibility in Various Solvents, Colloids Surf. Physicochem. Eng. Asp., 2015, 482, 507–513, DOI:10.1016/j.colsurfa.2015.07.005.
- H. Liu, J. Wang, J. Wang and S. Cui, Sulfonitric Treatment of Multiwalled Carbon Nanotubes and Their Dispersibility in Water, Materials, 2018, 11(12), 2442, DOI:10.3390/ma11122442.
- R. Li, X. Wang, Z. Ji, B. Sun, H. Zhang, C. H. Chang, S. Lin, H. Meng, Y.-P. Liao, M. Wang, Z. Li, A. A. Hwang, T.-B. Song, R. Xu, Y. Yang, J. I. Zink, A. E. Nel and T. Xia, Surface Charge and Cellular Processing of Covalently Functionalized Multiwall Carbon Nanotubes Determine Pulmonary Toxicity, ACS Nano, 2013, 7(3), 2352–2368, DOI:10.1021/nn305567s.
- G. Khachatryan, K. Khachatryan, J. Szczepankowska, M. Krzan and M. Krystyjan, Design of Carbon Nanocomposites Based on Sodium Alginate/Chitosan Reinforced with Graphene Oxide and Carbon Nanotubes, Polymers, 2023, 15(4), 925, DOI:10.3390/polym15040925.
- X. Liu, W. Yu, Y. Zhang, W. Xue, W. Yu, Y. Xiong, X. Ma, Y. Chen and Q. Yuan, Characterization of Structure and Diffusion Behaviour of Ca-Alginate Beads Prepared with External or Internal Calcium Sources, J. Microencapsulation, 2002, 19(6), 775–782, DOI:10.1080/0265204021000022743.
- A. B. Mathur, A. M. Collinsworth, W. M. Reichert, W. E. Kraus and G. A. Truskey, Endothelial, Cardiac Muscle and Skeletal Muscle Exhibit Different Viscous and Elastic Properties as Determined by Atomic Force Microscopy, J. Biomech., 2001, 34(12), 1545–1553 CrossRef CAS PubMed.
- I. V. Ogneva, D. V. Lebedev and B. S. Shenkman, Transversal Stiffness and Young's Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy, Biophys. J., 2010, 98(3), 418–424 CrossRef CAS PubMed.
- E. Defranchi, E. Bonaccurso, M. Tedesco, M. Canato, E. Pavan, R. Raiteri and C. Reggiani, Imaging and Elasticity Measurements of the Sarcolemma of Fully Differentiated Skeletal Muscle Fibres, Microsc. Res. Tech., 2005, 67(1), 27–35, DOI:10.1002/jemt.20177.
- Y. Yoshikawa, T. Yasuike, A. Yagi and T. Yamada, Transverse Elasticity of Myofibrils of Rabbit Skeletal Muscle Studied by Atomic Force Microscopy, Biochem. Biophys. Res. Commun., 1999, 256(1), 13–19, DOI:10.1006/bbrc.1999.0279.
- C. K. Kuo and P. X. Ma, Ionically Crosslinked Alginate Hydrogels as Sca!Olds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties, Biomaterials, 2001, 22, 511–521, DOI:10.1016/S0142-9612(00)00201-5.
- A. S. Sergeeva, D. A. Gorin and D. V. Volodkin, In-Situ Assembly of Ca–Alginate Gels with Controlled Pore Loading/Release Capability, Langmuir, 2015, 31(39), 10813–10821, DOI:10.1021/acs.langmuir.5b01529.
- A. Sergeeva, A. S. Vikulina and D. Volodkin, Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals, Micromachines, 2019, 10(6), 357, DOI:10.3390/mi10060357.
- H. Yan, D. Huang, X. Chen, H. Liu, Y. Feng, Z. Zhao, Z. Dai, X. Zhang and Q. Lin, A Novel and Homogeneous Scaffold Material: Preparation and Evaluation of Alginate/Bacterial Cellulose Nanocrystals/Collagen Composite Hydrogel for Tissue Engineering, Polym. Bull., 2018, 75(3), 985–1000, DOI:10.1007/s00289-017-2077-0.
- C. H. Yang, M. X. Wang, H. Haider, J. H. Yang, J.-Y. Sun, Y. M. Chen, J. Zhou and Z. Suo, Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations, ACS Appl. Mater. Interfaces, 2013, 5(21), 10418–10422, DOI:10.1021/am403966x.
- Z. Salmi, S. Gam-Derouich, S. Mahouche-Chergui, M. Turmine and M. Chehimi, On the Interfacial Chemistry of Aryl Diazonium Compounds in Polymer Science, Chem. Pap., 2012, 66(5), 369–391, DOI:10.2478/s11696-012-0135-5.
- P. Eiselt, K. Y. Lee and D. J. Mooney, Rigidity of Two-Component Hydrogels Prepared from Alginate and Poly(Ethylene Glycol)−Diamines, Macromolecules, 1999, 32(17), 5561–5566, DOI:10.1021/ma990514m.
- A. Magnani, R. Rappuoli, S. Lamponi and R. Barbucci, Novel Polysaccharide Hydrogels: Characterization and Properties, Polym. Adv. Technol., 2000, 11(8–12), 488–495 CrossRef CAS.
- M. Mehrali, A. Thakur, C. P. Pennisi, S. Talebian, A. Arpanaei, M. Nikkhah and A. Dolatshahi-Pirouz, Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials That Are Compatible with Load-Bearing and Electroactive Tissues, Adv. Mater., 2017, 29(8), 1603612 CrossRef PubMed.
- S. Marchesan, S. Bosi, A. Alshatwi and M. Prato, Carbon Nanotubes for Organ Regeneration: An Electrifying Performance, Nano Today, 2016, 11(4), 398–401 CrossRef CAS.
- J. Ramón-Azcón, S. Ahadian, M. Estili, X. Liang, S. Ostrovidov, H. Kaji, H. Shiku, M. Ramalingam, K. Nakajima and Y. Sakka, et al., Dielectrophoretically Aligned Carbon Nanotubes to Control Electrical and Mechanical Properties of Hydrogels to Fabricate Contractile Muscle Myofibers, Adv. Mater., 2013, 25(29), 4028–4034 CrossRef PubMed.
- S. Seifi, A. Shamloo, A. K. Barzoki, M. A. Bakhtiari, S. Zare, F. Cheraghi and A. Peyrovan, Engineering Biomimetic Scaffolds for Bone Regeneration: Chitosan/Alginate/Polyvinyl Alcohol-Based Double-Network Hydrogels with Carbon Nanomaterials, Carbohydr. Polym., 2024, 339, 122232 CrossRef CAS PubMed.
- A. Raslan, J. Ciriza, A. Ochoa de Retana, M. Sanjuán, M. Toprak, P. Galvez-Martin, L. Saenz-del-Burgo and J. Pedraz, Modulation of Conductivity of Alginate Hydrogels Containing Reduced Graphene Oxide through the Addition of Proteins, Pharmaceutics, 2021, 13, 1473 CrossRef CAS PubMed.
- R. Tutar, Preparation and Characterization of Conductive and Multi-Network Nanocomposite Hydrogels as Potential Scaffolds for Electroactive Tissues, New J. Chem., 2024, 48, 14736–14745, 10.1039/D4NJ01930J.
- J. R. Macdonald, W. B. Johnson, I. Raistrick, D. Franceschetti, N. Wagner, M. McKubre, D. Macdonald, B. Sayers, N. Bonanos and B. Steele,et al., Impedance Spectroscopy: Theory, Experiment, and Applications, John Wiley & Sons, 2018 Search PubMed.
- L. Li, S. Qin, J. Peng, A. Chen, Y. Nie, T. Liu and K. Song, Engineering Gelatin-Based Alginate/Carbon Nanotubes Blend Bioink for Direct 3D Printing of Vessel Constructs, Int. J. Biol. Macromol., 2020, 145, 262–271, DOI:10.1016/j.ijbiomac.2019.12.174.
- M. A. Saleemi, M. Hosseini Fouladi, P. V. C. Yong, K. Chinna, N. K. Palanisamy and E. H. Wong, Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications, Chem. Res. Toxicol., 2021, 34(1), 24–46, DOI:10.1021/acs.chemrestox.0c00172.
- K. Smetanajr, Cell Biology of Hydrogels, Biomaterials, 1993, 14(14), 1046–1050, DOI:10.1016/0142-9612(93)90203-E.
- J. A. Rowley, G. Madlambayan and D. J. Mooney, Alginate Hydrogels as Synthetic Extracellular Matrix Materials, Biomaterials, 1999, 20(1), 45–53 CrossRef CAS PubMed.
- P. Prang, R. Müller, A. Eljaouhari, K. Heckmann, W. Kunz, T. Weber, C. Faber, M. Vroemen, U. Bogdahn and N. Weidner, The Promotion of Oriented Axonal Regrowth in the Injured Spinal Cord by Alginate-Based Anisotropic Capillary Hydrogels, Biomaterials, 2006, 27(19), 3560–3569 CAS.
- J. A. Rowley and D. J. Mooney, Alginate Type and RGD Density Control Myoblast Phenotype, J. Biomed. Mater. Res., 2002, 60, 217–223, DOI:10.1002/jbm.1287.
- F. Brandl, F. Sommer and A. Goepferich, Rational Design of Hydrogels for Tissue Engineering: Impact of Physical Factors on Cell Behavior, Biomaterials, 2007, 28(2), 134–146, DOI:10.1016/j.biomaterials.2006.09.017.
- J. Peng, L. Li, Y. Nie, T. Liu and K. Song, 3D Bio-Printing Fabrication and Properties of Graphene Dispersion-Based Hybrid Scaffolds, J. Phys. Conf. Ser., 2020, 1622(1), 012062, DOI:10.1088/1742-6596/1622/1/012062.
- T. A. Partridge, Tissue Culture of Skeletal Muscle, Basic Cell Culture Protocols, 1997, pp. 131–144 DOI:10.1385/0-89603-441-0:131.
- Q.-Z. Chen, S. E. Harding, N. N. Ali, A. R. Lyon and A. R. Boccaccini, Biomaterials in Cardiac Tissue Engineering: Ten Years of Research Survey, Mater. Sci. Eng., R, 2008, 59(1–6), 1–37, DOI:10.1016/j.mser.2007.08.001.
- K. Y. Lee and D. J. Mooney, Alginate: Properties and Biomedical Applications, Prog. Polym. Sci., 2012, 37(1), 106–126, DOI:10.1016/j.progpolymsci.2011.06.003.
- A. Sergeeva, A. S. Vikulina and D. Volodkin, Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals, Micromachines, 2019, 10(6), 357 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb00601e |
| ‡ These authors contributed equally to this work as first authors. |
| This journal is © The Royal Society of Chemistry 2025 |