Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lipid droplet specific BODIPY based rotors with viscosity sensitivity to distinguish normal and cancer cells: impact of molecular conformation†
Charutha
Kalarikkal
a,
Anjali‡
b,
Sarbani
Bhattacharjee‡
b,
Koyeli
Mapa
*b and
Chinna Ayya Swamy
P
 *a
*a
aMain group Organometallics Optoelectronic Materials and Catalysis lab, Department of Chemistry, National Institute of Technology, Calicut, 673601, India. E-mail: swamy@nitc.ac.in
bProtein Homeostasis Laboratory, Department of Life Sciences, School of Natural Sciences, Shiv Nadar Institution of Eminence, Delhi-NCR, Greater Noida, Gautam Buddha Nagar, Uttar Pradesh 201314, India
First published on 11th December 2024
Abstract
Lipid droplets (LDs) are dynamic, multifunctional organelles critical for regulating energy balance, cell signaling, membrane formation, and trafficking. Recent studies have highlighted LDs as emerging cancer biomarkers, with cancer cells typically exhibiting a higher number and viscosity of LDs compared to normal cells. This discovery paves the way for developing molecular probes that can monitor intracellular viscosity changes within LDs, offering a powerful tool for early cancer diagnosis, recurrence monitoring, and therapeutic interventions. In this study, we designed and synthesized two series of donor–acceptor (D–A) conjugated BODIPY-cyanostilbene based fluorophores (5a–c and 6a–c) by fine-tuning the cyanostilbene unit with three distinct substituents (OMe, H, Cl) and modulating the molecular conformation via rigidifying the indacene core. While the terminal substituents had a minimal effect on the optical properties, changes in molecular conformation significantly impacted the photophysical behavior of the fluorophores. Compounds 5a–c function as molecular rotors, with the free rotation of the meso-biphenyl rings leading to non-radiative deactivation of the excited state, resulting in weak emission. Additionally, this structural feature makes them highly responsive to changes in viscosity. As the glycerol concentration increased from 0% to 99%, the fluorescence intensity of compounds 5a, 5b, and 5c increased dramatically by 17-fold, 78-fold, and 43-fold, respectively. In contrast, compounds 6a–c, with restricted phenyl ring rotation due to tetra-methyls on the indacene unit, showed only a modest 2–3-fold increment in fluorescence intensity under similar conditions. These fluorophores possess several key advantages, including high selectivity for LDs, good photostability, sensitivity to viscosity, and responsiveness to polarity and pH. Moreover, they effectively differentiate between normal and cancer cells, making them valuable tools for cancer diagnosis and potential therapeutic applications.
1. Introduction
Lipid droplets (LDs), once considered simple fat reserves within cells, are now recognized as dynamic organelles playing pivotal roles in maintaining energy homeostasis.1 Structurally, LDs are composed of a core of neutral lipids like triglycerides and cholesterol esters, encased in a phospholipid monolayer embedded with specific proteins.2,3 This configuration helps LDs to prevent lipotoxicity by regulating cellular stress responses and assist in lipid trafficking, protein storage, and degradation.4–6 LDs also interact with other organelles including mitochondria, the endoplasmic reticulum, lysosomes, and peroxisomes, coordinating cellular metabolism.6,7 A particularly significant aspect of LD biology is the impact of cellular viscosity on their functionality. Viscosity influences cellular processes like molecular diffusion, signal transduction, and enzymatic activities, which can regulate apoptosis, metabolism, and membrane formation.8 Disruptions in LD function are linked to several diseases, including obesity, diabetes, non-alcoholic fatty liver disease, atherosclerosis, cardiovascular disease, and cancer.3,9 Recent findings have uncovered a link between LDs and cancer, with malignant cells often displaying higher numbers, larger sizes, and greater motility of LDs compared to normal cells.10 This relationship emphasizes the potential of LDs as biomarkers for cancer detection, treatment, and prognosis. Therefore, understanding their dynamics and cellular functions opens new avenues for therapeutic interventions, particularly in metabolic and cancer-related diseases.Traditional techniques, including Raman microscopy, transmission light microscopy, electron microscopy, and mass spectrometry, have been crucial for visualizing LDs.11 However, their practical applicability is limited by several factors, including intricate sample preparation, difficulty in real-time tracking of sub-cellular changes, low cell permeability, and the need for cell fixation.11,12 Fluorescence imaging has revolutionized this field by offering non-invasive, real-time imaging of living cells including LDs, with high spatial and temporal resolution using specially designed fluorescent probes.13–15 To date, many small molecular probes have been developed that target LDs including commercially used Nile Red and BODIPY 493/503.16–19 However, these probes still face significant challenges, including non-specific binding to other intracellular components, poor photostability, and high background fluorescence. Additionally, the spectroscopic limitations reduce their effectiveness in multimodal imaging techniques.20 As a result, the development of more efficient and selective probes remains essential to improve the accuracy of LD visualization and to better understand their dynamic behavior in live cells.
In the realm of small organic molecular probes, 4,4′-difluoro-4-bora-3a,4a-diaza-s-indacene also known as boron dipyrromethene (BODIPY) and their derivatives stand out due to the structural versatility, excellent photostability, high quantum yields, low cytotoxicity, high biocompatibility, and strong lipophilicity making them ideal for LD staining.21,22 These properties have made them invaluable across diverse fields, including biological sensing, optoelectronics, and as photosensitizers.23 A notable advancement in BODIPY research is the use of meso-substituted BODIPY derivatives as molecular rotors to study intracellular viscosity at the single-cell level. Kuimova et al. pioneered this approach in 2008 by modifying the meso-position of the BODIPY core to measure viscosity using fluorescence lifetime imaging microscopy (FLIM).24 In low viscosity environments, these probes exhibit weak fluorescence due to non-radiative deactivation, while high viscosity restricts rotation, enhancing fluorescence.25,26 Similarly, cyanostilbene-based organic fluorophores have gained significant attention due to their rigid and twisted structure, strong solid-state luminescence, and twisted intramolecular charge transfer (TICT) characteristics. They exhibit a wide range of applications including organic solar cells, organic light-emitting devices, liquid crystal displays, bioimaging, sensing, molecular switches and so on.27 However, there is still scope for enhancement in the field of molecular rotors such as developing organelle-specific rotors to provide disease related insights, more photostable probes suited for multi modal imaging and super resolution microscopy, and minimal sensitivity to solvent polarity and temperature which are key attributes for achieving high spatial resolution and rapid data acquisition in live-cell imaging.
Our research centres on the design, synthesis, and characterization of a new class of donor–acceptor (D–A) conjugated BODIPY-cyanostilbene-based fluorophores (5a–c and 6a–c), which specifically target LDs and distinguish normal cells from cancer cells by detecting intracellular viscosity variations. A standout feature of these fluorophores is their high selectivity towards viscosity, showing minimal interference from other environmental factors such as solvent polarity, pH changes, or the presence of competing species, making them highly suitable for the accurate tracking of dynamic physicochemical processes within LDs. We optimized their photophysical properties through two strategies: introducing different terminal groups (OMe, H, Cl) on the cyanostilbene moiety and incorporating four methyl groups to the BODIPY core to modify molecular conformation. Although modifications at the meso-position had minimal impact on optical properties, changes in conformation significantly affected the electronic interaction between the BODIPY and cyanostilbene units, thereby influencing their photophysical behavior. Compounds 5a–c showed low fluorescence in low-viscosity environments due to free rotation causing non-radiative deactivation, but exhibited enhanced fluorescence in high-viscosity environments, making them highly sensitive molecular rotors. In contrast, 6a–c adopted a rigid conformation due to steric effects of the methyl groups, exhibiting bright fluorescence even in low-viscosity environments but reduced sensitivity to viscosity changes. Our study shows that compounds 5a–c, in particular, are well-suited for applications where sensitivity to viscosity is critical, such as the real-time imaging of intracellular environments or distinguishing between normal and cancerous cells. This research paves the way for the development of next-generation fluorophores with enhanced selectivity, photostability, and tunability, which could have broad implications in both bioimaging and therapeutic diagnostics.
2. Results and discussion
2.1 Synthesis and spectroscopic analysis
In the present work, we aimed to develop and synthesize two distinct series of D–A conjugated BODIPY-based fluorophores (5a–c and 6a–c), carefully designed by fine-tuning the cyanostilbene derivatives and varying the molecular conformation to enhance their optical properties (Chart 1). The design strategy focused on two key aspects: (1) introducing three different terminal substituents (OMe, H, Cl) at the cyanostilbene core to modulate electronic effects, and (2) incorporating four methyl groups at the indacene unit of the BODIPY core to control the molecular conformation. The synthetic routes leading to the target molecules 5a–c and 6a–c are detailed in Scheme 1, and the full synthetic procedures are provided in the ESI.† To summarize, the synthetic strategy employed a stepwise approach, starting with the synthesis of intermediates, followed by cross-coupling reactions and achieved the desired products. The first step involved the Knoevenagel condensation reaction between 4-bromo benzaldehyde and the corresponding benzyl cyanides. This reaction proceeded efficiently, producing the desired intermediates 1a–c in excellent yields. Next, the intermediates 1a–c were subjected to palladium-catalyzed Suzuki–Miyaura cross-coupling reaction yielding compounds 2a–c. Furthermore, the synthesis of compounds 3 and 4, which form the core BODIPY structures, were synthesized through a two-step process. First, a trifluoroacetic acid (TFA)-catalyzed condensation of 4-bromo benzaldehyde with the corresponding pyrroles was performed. This reaction produced the dipyrromethane intermediates, which were subjected to oxidation reaction by using DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) to form the dipyrromethene scaffold. In the final step, the dipyrromethenes were complexed with BF3·Et2O in dichloromethane (CH2Cl2), resulting in the formation of the stable BODIPY cores (compounds 3 and 4). In the final synthetic step, compounds 3 and 4 underwent palladium-catalyzed Suzuki–Miyaura cross-coupling reactions with the intermediates 2a–c. This reaction allowed for the attachment of the cyanostilbene moiety to the BODIPY core, which leads to the formation of target molecules 5a–c and 6a–c. All newly synthesized compounds were thoroughly characterized using a range of advanced analytical techniques such as 1H NMR, 13C NMR, 11B NMR, and 19F NMR spectroscopy, as well as high-resolution mass spectrometry (HRMS) to confirm their structure and purity.2.2 Photophysical properties
The preliminary photophysical properties of our target molecules (5a–c and 6a–c) were investigated in dichloromethane (DCM) solvent, and the corresponding UV-vis absorption and fluorescence spectra are shown in Fig. 1 and Fig. S40 (ESI†). A comprehensive summary of the collected data is provided in Table 1. The UV-vis absorption spectra of the synthesized compounds fall within the range of 300–550 nm. For compound 5a, three prominent absorption bands were observed as a strong absorption band around 500 nm, corresponding to the BODIPY π → π* (S0 → S1) transition, the cyanostilbene π → π* transition (∼360 nm), and a broad peak around ∼410–480 nm which might be due to the transitions resulting from the electronic interactions between the BODIPY core and the cyanostilbene unit. Compounds 5b and 5c displayed similar absorption patterns, with a peak around 500 nm, also characteristic of the BODIPY π → π* transition and higher-energy transitions near 340 nm, corresponding to the cyanostilbene π → π* transition, along with a shoulder peak around 385 nm that may be indicative of weak electronic communication between the BODIPY and cyanostilbene chromophores. Similarly, compounds 6a–c displayed similar absorption profiles, featuring two absorption bands. The lower energy transition (∼501 nm) originates from the π → π* (S0 → S1) transition of the BODIPY core, while the higher energy transition (∼350 nm) is associated with the π → π* transition of the cyanostilbene unit. Interestingly, the comparable absorption behavior across all synthesized compounds, regardless of the terminal substituents on the cyanostilbene unit, suggested only weak electronic coupling between the BODIPY core and cyanostilbene unit.28,29 The absorption spectra of compounds 6a–c appeared to be the sum of individual contributions from both chromophores, indicating minimal electronic conjugation between them.30 To investigate the effect of terminal substituents on the cyanostilbene unit and their influence on the emission spectra, we conducted fluorescence studies in DCM using an excitation wavelength of 350 nm, specifically targeting the cyanostilbene moiety. This approach allowed us to directly assess how the different substituents modulate the photophysical behavior of the compounds. For example, for compounds 5a–c, excitation at 350 nm led to a strong emission band between 520–525 nm, which corresponds to the S1 → S0 transition of the BODIPY core. Additionally, a weak and broad emission band was observed in the 380–480 nm region, characteristic of the cyanostilbene moiety. This dual emission pattern indicates partial excitation energy transfer from the cyanostilbene donor to the BODIPY acceptor. Such energy transfer is dependent on several factors, including spectral overlap between the emission of the cyanostilbene donor and the absorption of the BODIPY acceptor, favorable orientation of the two chromophores, and their close proximity.31,32 The weak residual cyanostilbene emission suggests that energy transfer in compounds 5a–c is partial, likely due to the free rotation of the phenyl rings attached to the BODIPY core, which disrupts efficient energy transfer. In contrast, compounds 6a–c exhibited a complete energy transfer from donor (cyanostilbene) to acceptor (BODIPY). Upon excitation at 350 nm, these compounds displayed a single, intense emission band around 515 nm, corresponding to the S1 → S0 transition of the BODIPY core, with no observable emission from the cyanostilbene unit. This suggests that complete excitation energy transfer occurs from the cyanostilbene donor to the BODIPY acceptor in compounds 6a–c. The higher efficiency of energy transfer in these compounds can be attributed to the restricted rotation of the phenyl rings, likely caused by the steric hindrance introduced by the methyl groups on the indacene unit.31,32 This structural rigidity allows for better electronic coupling between the donor and acceptor, resulting in complete energy transfer. A slight bathochromic shift was noted in the emission spectra of compounds 5a–c compared to compounds 6a–c. This shift is attributed to the electronic communication between the BODIPY core and cyanostilbene units, which influence the emission wavelength. Furthermore, the fluorescence quantum yields of compounds 5a–c were much lower than that of compounds 6a–c. This reduced intensity is likely a consequence of the free rotation of the biphenyl rings in compounds 5a–c, leading to non-radiative deactivation of the excited state, as described in Table 1.22,28 Moreover, we can conclude that varying the terminal substitutions on the cyanostilbene unit had minimal impact on the optical properties of our target molecules. | ||
| Fig. 1 (A) Normalized absorption (10 μM) and (B) normalized emission spectra of compounds 5a–c and 6a–c in DCM (2 μM, λex = 350 nm). | ||
| Compounds | λ abs (nm)/ε (×104) | λ em (nm) | Φ f |
|---|---|---|---|
| a Quantum yields are calculated using quinine sulfate (0.1 M in H2SO4, ΦF = 57.7%) solution as a reference and using the following formula ϕ = ϕF × I/IR × AR/A × η2/ηR2 where ϕ = quantum yield, I = integral area of the emission peak, A = absorbance at λex, and η = refractive index of the solvent. | |||
| 5a | 361 (2.9), 428 (1.3), 499 (0.8) | 438, 522 | 06 |
| 5b | 339 (3.1), 500 (3.3) | 524 | 07 |
| 5c | 343 (3.0), 500 (3.5) | 525 | 10 |
| 6a | 352 (2.9), 500 (3.2) | 515 | 50 |
| 6b | 344 (2.5), 501 (4.3) | 514 | 50 |
| 6c | 347 (1.6), 501 (2.6) | 515 | 54 |
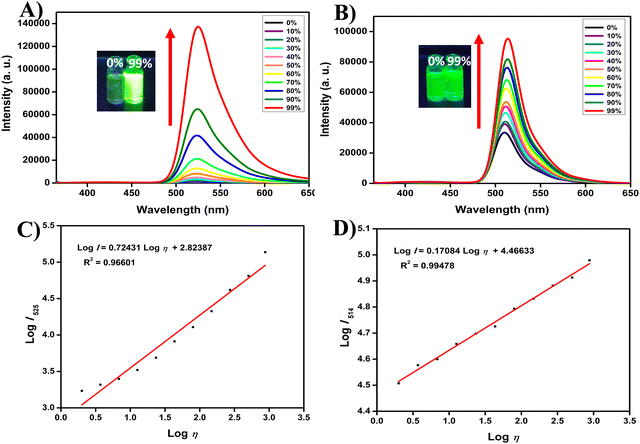
2.3 Spectral response to viscosity
After thoroughly analyzing the fundamental optical properties of our target molecules, we shifted our focus to examining their molecular rotor characteristics by precisely studying the relationship between fluorescence intensity and viscosity. To achieve this, we controlled the viscosity of the environment by varying the ratios of DMSO and glycerol in a miscible system, creating a viscosity range from 2 cP (DMSO) to 878.32 cP (glycerol). This setup allowed us to assess how changes in viscosity impacted the photophysical behavior of the target compounds. Our results, depicted in Fig. 2, and Fig. S41, S42 (ESI†), demonstrate that compound 5b exhibited a very weak fluorescence emission at 525 nm when excited at 350 nm in pure DMSO (2 cP), a low-viscosity solvent. This weak emission is primarily attributed to the intramolecular free rotation of the meso bi-phenyl rings in 5b, which facilitates non-radiative deactivation of the excited state. As the viscosity increased with the addition of glycerol, the photophysical behavior of 5b changed dramatically. With increasing glycerol concentration (0% to 99%), the maximum fluorescence intensity of 5b increased by a remarkable 78-fold. This sharp increase in fluorescence intensity can be attributed to the restriction of intramolecular rotation in the higher-viscous environment. When the intramolecular motion is hindered, the molecule is forced to undergo radiative decay rather than non-radiative processes, leading to a significant enhancement in fluorescence emission.33,34 The behavior of compounds 5a and 5c followed a similar trend to 5b, with fluorescence intensity enhancements of 17-fold and 43-fold, respectively. This suggests that for all the compounds in the 5a–c series, the intramolecular free rotation of the meso bi-phenyl rings is the dominant factor influencing fluorescence intensity in low-viscous environments, and restricting this rotation via increasing viscosity leads to a notable fluorescence enhancement. Furthermore, the incorporation of an electron-donating methoxy (OMe) group at the cyanostilbene unit in 5a promotes free rotation of the rotatable single bonds, causing non-radiative deactivation of the excited state. This is evidenced by the notably lower quantum yield of 5a compared to 5b and 5c, along with the different electronic distributions observed in DFT studies (discussed in detail in Section 2.5 DFT Studies). Additionally, the free rotation of the methoxy group might have increased the energy barrier, even in highly viscous environments, resulting in reduced sensitivity to viscosity. In the case of 5b and 5c, DFT studies indicate similar electronic distributions; however, 5b exhibits a slightly lower quantum yield than 5c, suggesting faster non-radiative decay. The terminal substitution (–Cl) in 5c likely increases the energy barrier slightly compared to 5b, resulting in lower viscosity sensitivity for 5c. These results are consistent with previous reports,35 which emphasize the critical role of the energy barrier in determining viscosity sensitivity. For 5b, the faster non-radiative decay and lower energy barrier could explain its enhanced sensitivity to viscosity relative to the other compounds. In contrast to the 5a–c series, 6a–c displayed only modest increment in the fluorescence intensity (2 to 3-fold) as the viscosity was increased. This limited response is attributed to the structural rigidity introduced by the four methyl groups on the indacene unit of the 6a–c series. The presence of these methyl groups significantly reduces the free rotation of the meso bi-phenyl rings, even in low-viscous environments like DMSO. As a result, compounds 6a–c already exhibit relatively strong fluorescence emission in low-viscosity conditions, and their fluorescence increases only marginally with higher viscosity. Furthermore, the orthogonal orientation effectively decouples the two chromophores resulting in a minimal effect of terminal substitutions on their optical properties, particularly viscosity sensitivity. In addition to the qualitative observations, we also analyzed the relationship between fluorescence intensity and viscosity quantitatively. Across all the target molecules, we found an excellent linear correlation between the logarithm of fluorescence intensity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I) and the logarithm of viscosity (log
I) and the logarithm of viscosity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η) within the viscosity range of 2 cP to 878.32 cP, as illustrated by the Förster–Hoffmann equation:36,37
η) within the viscosity range of 2 cP to 878.32 cP, as illustrated by the Förster–Hoffmann equation:36,37log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = C + x I = C + x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η η | (1) |
2.4 Selectivity and competitive studies
To further investigate the influence of solvent polarity on both the ground and excited states of our target molecules, we conducted a series of absorption and emission studies across solvents with varying dielectric constants. The results, outlined in Table S1 and Fig. S43 (ESI†), reveal that the absorption spectra of the target molecules (5a–c and 6a–c) remained consistent with all solvents, implying that the ground-state electronic structure is not affected by the solvent environment.30 This indicates that the electronic transitions responsible for absorption are not significantly altered by the polarity of the medium. On the other hand, the fluorescence spectra showed only very slight changes in intensity with the solvent polarity, which strongly suggested the minimal effect of the solvent environment on the excited-state properties of the compounds (Fig. 3A, B and Fig. S44, ESI†). However, a stark contrast emerged when we examined the fluorescence behavior of the compounds in viscous media, which we have discussed detailed in the previous section.Next, to evaluate the selectivity of these compounds towards other competing factors, we conducted fluorescence studies in the presence of various ions (Zn2+, Fe3+, Cu2+, Na+, K+, Ca2+, CO32−, HCO3−, and NO2−), amino acids (Ser, Asn, Thr, and Tyr), reactive oxygen species (ClO− and H2O2) and reactive sulphur species (GSH, Cys, and Hcy), which are common in biological systems (Fig. 3C, D and Fig. S45, ESI†). The results showed no significant change in fluorescence intensity in response to the presence of these ions, confirming that the fluorescence response of the compounds is highly specific to viscosity variations. This specificity is significant for biological applications, as it ensures that the compounds can reliably detect changes in viscosity without interference from fluctuations in ion concentration. We also investigated the effect of pH on the fluorescence behavior of the compounds, given that pH levels vary widely within different cellular compartments and between healthy and diseased tissues. For this purpose, we tested compounds 5b and 6b in two viscosity environments: DMSO (low-viscosity) and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of glycerol/DMSO (high-viscosity) (Fig. 3E, F and Fig. S46, ESI†). The results revealed that the fluorescence intensity of all compounds remained constant across a wide pH range, confirming that pH changes have minimal impact on their optical properties. However, a significant increase in fluorescence intensity was observed when the viscosity increased, independent of pH.36 Overall, our findings demonstrate that the target molecules exhibit robust photophysical properties with a high degree of sensitivity to viscosity changes while maintaining stability against solvent polarity, pH variations, and interference from other biological species. These characteristics make them ideal candidates for probing the microenvironment of LDs and other intracellular structures where viscosity plays a key role in cellular function and disease progression. The excellent selectivity and responsiveness of these compounds lay a strong foundation for their further development as fluorescent probes in bioimaging and diagnostic applications.
1 mixture of glycerol/DMSO (high-viscosity) (Fig. 3E, F and Fig. S46, ESI†). The results revealed that the fluorescence intensity of all compounds remained constant across a wide pH range, confirming that pH changes have minimal impact on their optical properties. However, a significant increase in fluorescence intensity was observed when the viscosity increased, independent of pH.36 Overall, our findings demonstrate that the target molecules exhibit robust photophysical properties with a high degree of sensitivity to viscosity changes while maintaining stability against solvent polarity, pH variations, and interference from other biological species. These characteristics make them ideal candidates for probing the microenvironment of LDs and other intracellular structures where viscosity plays a key role in cellular function and disease progression. The excellent selectivity and responsiveness of these compounds lay a strong foundation for their further development as fluorescent probes in bioimaging and diagnostic applications.
2.5 DFT studies
To further understand the structural and electronic characteristics of the target molecules, density functional theory (DFT) calculations were carried out for compounds 5a–c and 6a–c. These calculations were aimed at exploring how molecular conformation and terminal substituents affect their electronic properties. The computations were conducted using Gaussian 09W, with the B3LYP/6-31G(d,p) level of theory in the gas phase, without enforcing symmetry constraints.38 One key focus of the study was to evaluate the dihedral angles between the meso-phenyl ring and the BODIPY core, as these angles significantly influence the degree of conjugation between the two chromophores (Fig. S47, ESI†). For compounds 5a–c, the dihedral angle was calculated to be approximately 53°, suggesting that the meso-phenyl ring and the BODIPY core are not completely planar but still allow for partial electronic interaction. This partial conjugation promotes a moderate degree of electronic communication between the two chromophores, influencing the optical behavior of the molecules, particularly with respect to energy transfer and fluorescence properties. In contrast, compounds 6a–c exhibited a much larger dihedral angle, around 90°, indicating that the meso-phenyl ring is orthogonal to the BODIPY core. This orthogonal orientation, caused by steric hindrance from the methyl groups on the indacene unit, decouples the two chromophores, preventing significant electronic interaction.31 The DFT calculations also provided insights into the electronic distribution within the molecules (Fig. 4 and Table S2, Fig. S48, ESI†). In compounds 5b and 5c, the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) are more confined to the BODIPY core, while the HOMO−1 and LUMO+1 are situated on the cyanostilbene units. Specifically, in compound 5a, the presence of an electron-donating OMe group shifts the localization of the HOMO and LUMO+1 to the cyanostilbene unit, while the HOMO−1 and LUMO remain on the BODIPY core. The non-orthogonal orientation of the meso phenyl ring in compounds 5a–c, leads to moderate mixing of the molecular orbitals (MOs) between the two chromophores, particularly the LUMO and LUMO+1 orbitals, while the HOMO−1 and HOMO orbitals remain unaffected. Conversely, for compounds 6a–c, the orbital distribution shows a distinct separation between the chromophores. In compounds 6a and 6b, the electronic distribution is confined to the indacene unit for HOMO and LUMO orbitals, while the HOMO−1 and LUMO+1 orbitals are localized on the cyanostilbene units. In the case of compound 6c, the HOMO and LUMO+1 orbitals are concentrated on the indacene unit, while the HOMO−1 and LUMO are localized on the cyanostilbene derivative. This complete localization of the electronic distribution to each chromophore implies that the absorption spectra of 6a–c represent a simple summation of the individual chromophore contributions, with minimal inter-chromophore interaction. Additionally, the introduction of various terminal substituents on the cyanostilbene unit-OMe (electron-donating), H (neutral), and Cl (electron-withdrawing) has very little effect on the absorption spectra of compounds 6a–c. This is due to the orthogonal orientation of the meso-phenyl ring, which inhibits significant electronic communication between the chromophores. In contrast, compounds 5a–c, with their non-orthogonal meso-phenyl rings, allow for moderate orbital overlap between the cyanostilbene and BODIPY units. This partial orbital mixing enhances electronic communication in compounds 5a–c, leading to more pronounced optical effects and sensitivity to environmental changes. However, the emission spectra may still vary based on energy transfer efficiency between the cyanostilbene and BODIPY units. This structural and orbital distinction underlies the different properties observed between the two series of compounds. | ||
| Fig. 4 Important frontier molecular orbitals (MOs) of compounds 5b (A), and 6b (B). Calculations were based on the optimized ground state geometry at the B3LYP/6-31G(d,p) level with Gaussian 09. | ||
To further understand the photophysical behavior of the target compounds, time-dependent density functional theory (TD-DFT) calculations were performed to examine their UV-vis absorption properties in the gas phase. These calculations were based on the ground-state optimized geometries and employed the Franck–Condon principle to simulate vertical excitations. The predicted electronic transitions, their oscillator strengths, and the molecular orbitals involved are detailed in both Fig. S49 and Table S3 (ESI†). For compounds 5b (λmax calc. = 354 nm, λmax exp. = 339 nm), 5c (λmax calc. = 360 nm, λmax exp. = 345 nm), 6a (λmax calc. = 360 nm, λmax exp. = 350 nm), and 6b (λmax calc. = 383 nm, λmax exp. = 343 nm), the predominant transition originates from HOMO−1 to LUMO+1, corresponding to the π → π* transition of the cyanostilbene unit. In contrast, for compound 5a (λmax calc. = 377 nm, λmax exp. = 360 nm), the major excitation involves a transition from HOMO to LUMO+1, whereas compound 6c (λmax calc. = 367 nm, λmax exp. = 348 nm) shows a similar transition, originating from HOMO−1 to LUMO, involving the cyanostilbene unit. The lower energy transition (λmax exp. = 500 nm) for compounds 5b (λmax calc.= 415 nm), 5c (λmax calc. = 419 nm), 6a (λmax calc. = 409 nm), and 6b (λmax calc. = 409 nm), is from HOMO to LUMO, corresponding to the π → π* (S0 → S1) transition of the BODIPY core. For compound 5a (λmax calc. = 412 nm), the lower energy transition occurs between HOMO−1 to LUMO, while in compound 6c (λmax calc. = 410 nm), it involves HOMO to LUMO+1 transitions. Additionally, the broad peak in compound 5a may stem from overlapping electronic transitions between the BODIPY core and cyanostilbene unit, reflecting their partial conjugation. Overall, these calculated results align well with the experimental UV-vis absorption data, validating the presence of distinct electronic transitions in both chromophore systems.
2.6 Lipid droplet specificity
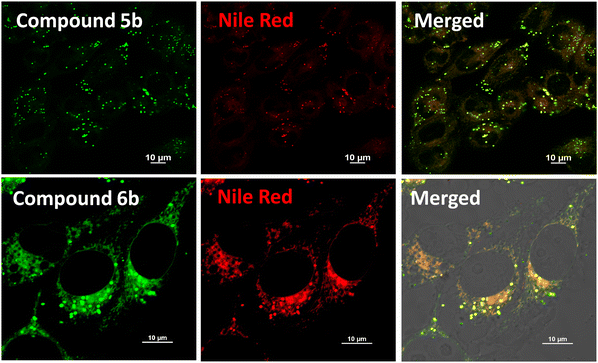
To further assess the potential of our target compounds for bioimaging applications, we conducted an in-depth evaluation of their performance in live-cell imaging. First, the cytotoxicity of the compounds was tested on HeLa cells using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, which measures cell viability following compound exposure. Cells were treated with increasing concentrations of the compounds for 24 hours. As illustrated in Fig. S50 (ESI†), the compounds displayed low cytotoxicity, with cell viability remaining above 90% at concentrations as high as 1 μM. Although a decrease in cell viability was noted at higher concentrations, it is important to emphasize that the concentrations necessary for effective cellular imaging are much lower, approximately 500 nM, thereby reducing any concerns about potential toxicity during imaging studies. Next, we examined the cellular localization of the compounds using confocal fluorescence microscopy. Upon treatment with the compounds, HeLa cells exhibited distinct fluorescence localized in small, punctate structures within the cytoplasm. These bright fluorescent spots suggested a potential accumulation in organelles, and their distribution and appearance led us to hypothesize that the compounds were likely localizing in LDs, as seen with similar fluorescent probes.24,39 To confirm this hypothesis, co-localization studies were performed using Nile Red, a well-known LD marker, as shown in Fig. 5 and Fig. S51 (ESI†). The green fluorescence of the compounds and the red fluorescence of Nile Red showed complete overlap, confirming that the compounds selectively target LDs within the cells and the Pearson's correlation coefficient was determined to be 0.83 for compound 5b and 0.85 for compound 6b (Fig. S51, ESI†). The LD specificity of our target fluorophores is likely due to their highly conjugated aromatic structure, which imparts lipophilic properties and exhibits a strong tendency to accumulate within the hydrophobic core of LDs.26 In addition to the Nile Red experiments, further co-localization studies were carried out using other organelle-specific dyes, including Mito-Tracker (for mitochondria), Lyso-Tracker (for lysosomes), and Hoechst (for nuclei), as illustrated in Fig. S52 (ESI†). No significant overlap was observed between these markers and the fluorescence of the compounds, further reinforcing their selective localization to LDs. Interestingly, we observed a marked difference in fluorescence intensity between the two series of compounds. Compounds 6a–c consistently exhibited stronger fluorescence signals compared to 5a–c. This difference may be attributed to the molecular rotor behavior of the 5a–c series, which is more prone to intramolecular motions that can quench fluorescence, whereas the 6a–c series has a more rigid molecular structure, preventing such quenching and resulting in higher fluorescence intensity. This enhanced fluorescence makes the 6a–c series particularly promising for high-sensitivity imaging. Furthermore, we have also evaluated the photostability of our target fluorophores. As shown in Fig. S53 and S54 (ESI†), the fluorescence intensity remained nearly unchanged over 50 minutes, indicating that the probes exhibit good photostability over an extended period. Taken together, these findings highlight the strong potential of both series of compounds for selective LD imaging in living cells, with excellent biocompatibility and minimal cytotoxicity, and good photostability. The superior brightness of the 6a–c series suggests that they could be especially useful in applications requiring high-contrast imaging, while the unique properties of the 5a–c series may offer additional advantages in dynamic studies of LD behavior. These results pave the way for further exploration of these compounds in advanced bioimaging techniques.2.7 Discriminating normal cells and cancer cells
Cancer remains one of the most critical global health challenges, with its high mortality rate continuing to drive the search for more effective diagnostic and therapeutic approaches. Among the emerging biomarkers, LDs have gained attention due to their increased number and altered properties in cancer cells.10,40 LDs play a crucial role in vital processes such as energy metabolism, oxidative stress regulation, and autophagy all of which are closely tied to cancer progression.6 Notably, cancer cells tend to have more LDs with higher viscosity compared to their normal cells.10,41 Leveraging this information, we conducted an experiment to test whether our compounds could differentiate cancer cells from normal cells by targeting these unique LD characteristics. In this study, HEK293T cells were selected as the model for non-cancerous cells, while HeLa cells served as the cancer cell model. Both cell types were exposed to identical experimental conditions, followed by fluorescence imaging. As shown in Fig. 6 and Fig. S55 and S56 (ESI†), the fluorescence images revealed a clear distinction between the two cell types. HeLa cells showed a marked increase in the number of LDs, as well as significantly stronger fluorescence intensity compared to HEK293T cells, which exhibited only faint fluorescence when treated with the same compounds. This clear contrast in fluorescence response between cancer and normal cells is likely due to the dual effect of a higher quantity of LDs and increased viscosity in cancer cells. Quantitative analysis of the fluorescence intensity confirmed that compounds 5a, 5b, and 5c produced 8.7-fold, 2.8-fold, and 7.4-fold enhanced fluorescence in HeLa cells compared to HEK293T cells, respectively. Similarly, compounds 6a, 6b, and 6c demonstrated 12.7-fold, 6.8-fold, and 3.3-fold enhanced fluorescence in HeLa cells than in HEK293T cells. These findings suggest that the unique characteristics of LDs in cancer cells can be exploited for selective targeting. By utilizing the differences in LD number and viscosity, our compounds demonstrate a strong potential for distinguishing cancer cells from normal cells. This approach could provide a valuable tool for cancer detection and may also be applicable in developing new strategies for targeted cancer therapies.3. Conclusions
In this study, we introduced two novel series of D–A conjugated BODIPY-cyanostilbene fluorophores (5a–c and 6a–c), designed by varying the terminal groups on the cyanostilbene unit (OMe, H, Cl) and manipulating molecular conformation through the addition of methyl groups on the indacene core. Our findings revealed that altering the meso-position of the BODIPY core had minimal influence on the optical properties of the fluorophores, while controlling the conformation of the molecules played a crucial role in fine-tuning the electronic communication between the BODIPY and cyanostilbene chromophores, significantly impacting their photophysical behavior. Compounds 5a–c demonstrated weak fluorescence in low-viscosity media due to free rotation around the meso-phenyl rings, which enabled non-radiative decay of the excited state. However, in high-viscosity environments like glycerol, this rotation was restricted, resulting in a remarkable increase in emission. Specifically, the fluorescence intensity of compounds 5a, 5b, and 5c increased by 17-fold, 78-fold, and 43-fold, respectively, with increasing glycerol concentration (0% to 99%). In contrast, compounds 6a–c, with methyl groups on the indacene unit that limit free rotation, displayed strong fluorescence even in low-viscosity media, and only a modest 2–3-fold increase was observed in high-viscosity conditions. These experimental results were further correlated by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations, which highlighted the role of molecular conformation in influencing the optical properties. Beyond their sensitivity to viscosity, the fluorophores exhibited excellent selectivity to polarity and pH, along with minimal interference from other biological molecules, making them versatile probes for biological systems. A key highlight of these fluorophores is their selective localization in LDs, which are known to be involved in various metabolic processes and are often upregulated in cancer cells. The compounds displayed a clear ability to differentiate cancer cells from normal cells, leveraging the increased number and viscosity of LDs in cancer cells. This property suggests that these fluorophores could serve as useful tools for cancer diagnosis, enabling early detection through the monitoring of LD behavior. Additionally, their strong response to environmental changes and good photostability makes them valuable candidates for a wide range of biological applications, particularly in imaging and diagnostic fields.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. A. S. P. sincerely acknowledges the Science and Engineering Research Board (SERB) for the EEQ grant (EEQ/2021/000180). C. K. gratefully thanks the Department of Science and Technology, New Delhi, for awarding the DST-Inspire Fellowship (IF210349). C. K. and C. A. S. P. also thank NIT Calicut for the NMR (CMC), HRMS (DST-FIST) and HPC (CCMS) facility. K. M. acknowledges the funding support from the Science and Engineering Research Board (SERB), Government of India, for Core Research Grant (SERB/CRG/2022/006517) and SNIoE core funding. A. N. and S. B. acknowledge the SNIoE PhD fellowship. A. N., S. B. and K. M. acknowledge the SNU DST-FIST grant [SR/FST/LS-1/2017/59(c)] for the confocal microscopy facility. We thank Rajan Singh for his help at the confocal microscopy facility at SNIoE.References
- R. V. J. Farese and T. C. Walther, Lipid droplets finally get a little respect, Cell, 2009, 139, 855–860 CrossRef CAS PubMed.
- J. A. Olzmann and P. Carvalho, Dynamics and functions of lipid droplets, Nat. Rev. Mol. Cell Biol., 2019, 20, 137–155 CrossRef CAS PubMed.
- A. Zadoorian, X. Du and H. Yang, Lipid droplet biogenesis and functions in health and disease, Nat. Rev. Endocrinol., 2023, 19, 443–459 CrossRef CAS PubMed.
- F. Geltinger, L. Schartel, M. Wiederstein, J. Tevini, E. Aigner, T. K. Felder and M. Rinnerthaler, Friend or foe: lipid droplets as organelles for protein and lipid storage in cellular stress response, aging and disease, Molecules, 2020, 25, 5053–5083 CrossRef CAS PubMed.
- E. Jarc and T. Petan, Lipid droplets and the management of cellular stress, Yale J. Biol. Med., 2019, 92, 435–452 CAS.
- (a) M. Gao, X. Huang, B.-L. Song and H. Yang, The biogenesis of lipid droplets: lipids take center stage, Prog. Lipid Res., 2019, 75, 100989–100999 CrossRef CAS PubMed; (b) M. F. Renne and H. Hariri, Lipid droplet-organelle contact sites as hubs for fatty acid metabolism, trafficking, and metabolic channeling, front, Cell Dev. Biol., 2021, 9, 726261–726271 Search PubMed; (c) Y. Jin, Y. Tan, J. Wu and Z. Ren, Lipid droplets: a cellular organelle vital in cancer cells, Cell Death Discovery, 2023, 9, 254–262 CrossRef CAS PubMed; (d) J. Z. Hsia, D. Liu, L. Haynes, R. Cruz-Cosme and Q. Tang, Lipid droplets: formation, degradation, and their role in cellular responses to flavivirus infections, Microorganisms, 2024, 12, 647–670 CrossRef CAS PubMed.
- Y.-X. Zhou, S.-Y. Wu, X. Zhang and F.-G. Wu, Lipid droplet-targeting optical biosensors: design strategies and applications, Anal. Chem., 2024, 175, 117703–117729 CAS.
- (a) D. Su, C. L. Teoh, L. Wang, X. Liu and Y.-T. Chang, Motion-induced change in emission (MICE) for developing fluorescent probes, Chem. Soc. Rev., 2017, 46, 4833–4844 RSC; (b) J. Yin, L. Huang, L. Wu, J. Li, T. D. James and W. Lin, Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions, Chem. Soc. Rev., 2021, 50, 12098–12150 RSC.
- B. C. Farmer, A. E. Walsh, J. C. Kluemper and L. A. Johnson, Lipid droplets in neurodegenerative disorders, Front. Neurosci., 2020, 14, 742–754 CrossRef PubMed.
- A. L. S. Cruz, E. A. Barreto, N. P. B. Fazolini, J. P. B. Viola and P. T. Bozza, Lipid droplets: platforms with multiple functions in cancer hallmarks, Cell Death Dis., 2020, 11, 105–120 CrossRef PubMed.
- T. K. Fam, A. S. Klymchenko and M. Collot, Recent advances in fluorescent probes for lipid droplets, Materials, 2018, 11, 1768–1786 CrossRef PubMed.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, New strategies for fluorescent probe design in medical diagnostic imaging, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- H. Zhu, J. Fan, J. Du and X. Peng, Fluorescent probes for sensing and imaging within specific cellular organelles, Acc. Chem. Res., 2016, 49, 2115–2126 CrossRef CAS PubMed.
- H. Tian, A. C. Sedwick, H.-H. Han, S. Sen, G.-R. Chen, Y. Zang, J. L. Sessler, T. D. James, J. Li and X.-P. He, Fluorescent probes for the imaging of lipid droplets in live cells, Coord. Chem. Rev., 2021, 427, 213577–213590 CrossRef CAS.
- Y. Zhao, W. Shi, X. Li and H. Ma, Recent advances in fluorescent probes for lipid droplets, Chem. Commun., 2022, 58, 1495–1509 RSC.
- L. Wang, X. Chen, X. Ran, H. Tang and D. Cao, Recent advance of lipid droplets fluorescence imaging with aggregation-induced emission luminogens (AIEgens), Dyes Pigm., 2022, 203, 110332–110345 CrossRef CAS.
- P. Greenspan, E. P. Mayer and S. D. Fowler, Nile red: a selective fluorescent stain for intracellular lipid droplets, J. Cell Biol., 1985, 100, 965–973 CrossRef CAS PubMed.
- B. Qiu and M. C. Simon, BODIPY 493/503 staining of neutral lipid droplets for microscopy and quantification by flow cytometry, Bio-Protoc., 2016, 6, e1912 Search PubMed.
- E. E. Spangenburg, S. J. P. Pratt, L. M. Wohlers and R. M. Lovering, Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle, J. Biomed. Biotechnol., 2011, 598358 Search PubMed.
- M. Collot, T. K. Fam, P. Ashokkumar, O. Faklaris, T. Galli, L. Danglot and A. S. Klymchenko, Ultrabright and fluorogenic probes for multicolor imaging and tracking of lipid droplets in cells and tissues, J. Am. Chem. Soc., 2018, 140, 5401–5411 CrossRef CAS PubMed.
- (a) J. Karolin, L. B.-A. Johansson, L. Strandberg and T. Ny, Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (BODIPY) derivatives in liquids, lipid membranes, and proteins, J. Am. Chem. Soc., 1994, 116, 7801–7806 CrossRef CAS; (b) R. Ziessel, G. Ulrich and A. Harriman, The chemistry of Bodipy: a new el dorado for fluorescence tools, New J. Chem., 2007, 31, 496–501 RSC; (c) L. D. Lavis and R. T. Raines, Bright ideas for chemical biology, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed; (d) N. Boens, V. Leen and W. Dehaen, Fluorescent indicators based on BODIPY, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (e) H. Lu, J. Mack, Y. Yang and Z. Shen, Structural modification strategies for the rational design of red/NIR region BODIPYs, Chem. Soc. Rev., 2014, 43, 4778–4823 RSC.
- (a) A. Loudet and K. Burgess, BODIPY dyes and their derivatives; syntheses and spectroscopic properties, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Harriman, The chemistry of fluorescent Bodipy dyes: versatility unsurpassed, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- (a) P. Kaur and K. Singh, Recent advances in the application of BODIPY in bioimaging and chemosensing, J. Mater. Chem. C, 2019, 7, 11361–11405 RSC; (b) M. Poddar and R. Misra, Recent advances of BODIPY based derivatives for optoelectronic applications, Coord. Chem. Rev., 2020, 421, 213462–213683 CrossRef CAS; (c) B. M. Squeo, L. Ganzer, T. Virgili and M. Pasini, Molecules, 2021, 26, 153–182 CrossRef CAS PubMed; (d) F.-Z. Li, Z. Wu, C. Lin, Q. Wang and G.-C. Kuang, Results Chem., 2022, 4, 100384–100398 Search PubMed; (e) I. S. Yadav and R. Misra, Design, synthesis and functionalization of BODIPY dyes: applications in dye-sensitized solar cells (DSSCs) and photodynamic therapy (PDT), J. Mater. Chem. C, 2023, 11, 8688–8723 Search PubMed; (f) D. Pinjari, Y. Patil and P. Misra, Near-infrared absorbing aza-BODIPY dyes for optoelectronic applications, Chem. – Asian J., 2024, 19, e202400167 CrossRef CAS PubMed.
- M. K. Kuimova, G. Yahioglu, J. A. Levitt and K. Suhling, Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging, J. Am. Chem. Soc., 2008, 130, 6672–6673 CrossRef CAS PubMed.
- B. M. Uzhinov, V. L. Ivanov and M. Y. Melnikov, Molecular rotors as luminescence sensors of local viscosity and viscous flow in solutions and organized systems, Russ. Chem. Rev., 2011, 80, 1179–1190 Search PubMed.
- (a) K. Li, Y. Wang, Y. Li, W. Shi and J. Yan, Development of BODIPY-based fluorescent probes for imaging Aβ aggregates and lipid droplet viscosity, Talanta, 2024, 227, 126362–126371 CrossRef PubMed; (b) G. Li, J. Li, Y. Otsuka, S. Zhang, M. Takahashi and K. Yamada, A BODIPY-based fluorogenic probe for specific imaging of lipid droplets, Materials, 2020, 13, 677–686 CrossRef CAS PubMed; (c) R. Žvirblis, K. Maleckaitė, J. Dodonova-Vaitkūnienė, D. Jurgutis, R. Žilėnaitė, V. Karabanovas, S. Tumkevičius and A. Vyšniauskas, A red-emitting thiophene-modified BODIPY probe for fluorescence lifetime-based polarity imaging of lipid droplets in living cells, J. Mater. Chem. B, 2023, 11, 3919–3928 RSC; (d) M. Olšinová, P. Jurkiewicz, M. Pozník, R. Šachl, T. Prausová, M. Hof, V. Kozmík, F. Teplý, J. Svoboda and M. Cebecauer, Di- and tri-oxalkyl derivatives of a boron dipyrromethene (BODIPY) rotor dye in lipid bilayers, Phys. Chem. Chem. Phys., 2014, 16, 10688–10697 RSC; (e) J. Zhu, N. K. Tan, K. Kikuchi, A. Kaur and E. J. New, BODIPY-based fluorescent indicators for lipid droplets, Anal. Sens., 2024, 4, e202300049 CAS; (f) J. E. Chambers, M. Kubánková, R. G. Huber, I. López-Duarte, E. Avezov, P. J. Bond, S. J. Marciniak and M. K. Kuimova, An optical technique for mapping microviscosity dynamics in cellular organelles, ACS Nano, 2018, 12, 4398–4407 CrossRef CAS PubMed.
- (a) L. Zhu and Y. Zhao, Cyanostilbene-based intelligent organic optoelectronic materials, J. Mater. Chem. C, 2013, 1, 1059–1065 RSC; (b) B. Kim, H. R. Yeom, W.-Y. Choi, J. Y. Kim and C. Yang, Synthesis and characterization of a bis-methanofullerene-4-nitro-α-cyanostilbene dyad as a potential acceptor for high-performance polymer solar cells, Tetrahedron, 2012, 68, 6696–6700 CrossRef CAS; (c) P. Mahalingavelar and S. Kanvah, α-cyanostilbene: a multifunctional spectral engineering motif, Phys. Chem. Chem. Phys., 2022, 24, 23049–23075 RSC; (d) A. Gao, Q. Wang, H. Wu, J.-W. Zhao and X. Cao, Research progress on AIE cyanostilbene-based self-assembly gels: design, regulation and applications, Coord. Chem. Rev., 2022, 471, 214753–214775 CrossRef CAS; (e) B.-K. An, J. Gierschner and S. Y. Park, π-conjugated cyanostilbene derivatives: a unique self-assembly motif for molecular nanostructures with enhanced emission and transport, Acc. Chem. Res., 2012, 45, 544–554 CrossRef CAS PubMed; (f) S. Sasaki, G. P. C. Drummen and G.-I. Konishi, Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry, J. Mater. Chem. C, 2016, 4, 2731–2743 RSC; (g) M. Martínez-Abadía, R. Giménez and M. B. Ros, Self-assembled α-cyanostilbenes for advanced functional materials, Adv. Mater., 2018, 30, 1704161–1704199 Search PubMed; (h) A. Afrin and P. C. A. Swamy, Symphony of light: AIE and MFC in carbazole-based cyanostilbenes, J. Mater. Chem. C, 2024, 12, 1923–1944 Search PubMed; (i) A. Afrin and P. C. A. Swamy, Tailoring emission color shifts in mechanofluorochromic-active AIE systems of carbazole-based d–π–a conjugates: impact of π spacer unit variants, J. Org. Chem., 2024, 89, 7946–7961 CrossRef CAS PubMed; (j) A. Afrin and P. C. A. Swamy, Aggregation induced emission and reversible mechanofluorochromism active carbazole-anthracene conjugated cyanostilbenes with different terminal substitutions, New J. Chem., 2023, 47, 18919–18932 RSC.
- T. Gayathri, A. K. Barui, S. Prashanthi, C. R. Patra and S. P. Singh, meso-Substituted BODIPY fluorescent probes for cellular bio-imaging and anticancer activity, RSC Adv., 2014, 4, 47409–47413 RSC.
- R. P. Nandi, P. C. A. Swamy, P. Dhanalakshmi, S. K. Behera and P. Thilagar, Effect of the molecular conformation on excitation energy transferrin conformationally constrained boryl-BODIPY dyads, Inorg. Chem., 2021, 60, 5452–5462 CrossRef CAS PubMed.
- P. C. A. Swamy, S. Mukherjee and P. Thilagar, Multichannel-emissive V-shaped boryl-BODIPY dyads: synthesis, structure, and remarkably diverse response toward fluoride, Inorg. Chem., 2014, 53, 4813–4823 CrossRef PubMed.
- P. C. A. Swamy, S. Mukherjee and P. Thilagar, Dual emissive borane-BODIPY dyads: molecular conformation control over electronic properties and fluorescence response towards fluoride ions, Chem. Commun., 2013, 49, 993–995 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, Singapore, 2006 Search PubMed.
- Y. Zhang, Z. Li, W. Hu and Z. Liu, A mitochondrial-targeting near-infrared fluorescent probe for visualizing and monitoring viscosity in live cells and tissues, Anal. Chem., 2019, 91, 10302–10309 CrossRef CAS PubMed.
- L. Zhang, G. Li, H. Zheng and W. Lin, A mitochondria-targeted fluorescent probe with viscosity sensitivity to distinguish normal and cancer cells, New J. Chem., 2024, 48, 4565–4569 RSC.
- (a) X. Liu, W. Chi, Q. Qiao, S. V. Kokate, E. P. Cabrera, Z. Xu, X. Liu and Y.-T. Chang, Molecular mechanism of viscosity sensitivity in BODIPY rotors and application to motion-based fluorescent sensors, ACS Sens., 2020, 5, 731–739 CrossRef CAS PubMed; (b) S. Toliautas, J. Dodonova, A. Žvirblis, I. Čiplys, A. Polita, A. Devižis, S. Tumkevičius, J. Šulskus and A. Vyšniauskas, Enhancing the viscosity-sensitive range of a BODIPY molecular rotor by two orders of magnitude, Chem. – Eur. J., 2019, 25, 10342–10349 CrossRef CAS PubMed.
- Y. Wu, W. Shu, C. Zeng, B. Guo, J. Shi, J. Jing and X. Zhang, A mitochondria targetable and viscosity sensitive fluorescent probe and its applications for distinguishing cancerous cells, Dyes Pigm., 2019, 168, 134–139 CrossRef CAS.
- M. Liu, J. Weng, S. Huang, W. Yin, H. Zhang, Y. Jiang, L. Yang and H. Sun, Water-soluble fluorescent probes for differentiating cancer cells and normal cells by tracking lysosomal viscosity, Chem. Commun., 2023, 59, 3570–3573 RSC.
- N. Gupta, S. I. Reja, V. Bhalla, M. Gupta, G. Kaur and M. Kumar, A bodipy based fluorescent probe for evaluating and identifying cancer, normal and apoptotic C6 cells on the basis of changes in intracellular viscosity, J. Mater. Chem. B, 2016, 4, 1968–1977 RSC.
- (a) D. Jurgutis, G. Jarockyte, V. Poderys, J. Dodonova-Vaitkuniene, S. Tumkevicius, A. Vysniauskas, R. Rotomskis and V. Karabanovas, Exploring BODIPY-based sensor for imaging of intracellular microviscosity in human breast cancer cells, Int. J. Mol. Sci., 2022, 23, 5687–5703 CrossRef CAS PubMed; (b) C. Caltagirone, M. Arca, A. M. Falchi, V. Lippolis, V. Meli, M. Monduzzi, T. Nylander, A. Rosa, J. Schmidt, Y. Talmon and S. Murgia, Solvatochromic fluorescent BODIPY derivatives as imaging agent in camptothecin loaded hexasomes for possible theranostic applications, RSC Adv., 2015, 5, 23443–23449 RSC.
- D. Delmas, A. K. Cotte, J.-L. Connat, F. Hermetet, F. Bouyer and V. Aires, Emergence of lipid droplets in the mechanisms of carcinogenesis and therapeutic responses, Cancers, 2023, 15, 4100–4116 CrossRef CAS PubMed.
- R. Munir, J. Lisec, J. V. Swinnen and N. Zaidi, Lipid metabolism in cancer cells under metabolic stress, Br. J. Cancer, 2019, 120, 1090–1098 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb02405b |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |