Ultrasmall metal nanoclusters as efficient luminescent probes for bioimaging
Xinyue
Dou
*a,
Sariah
Saalah
a,
Chel-Ken
Chiam
a,
Jianping
Xie
 *b and
Coswald Stephen
Sipaut
*a
*b and
Coswald Stephen
Sipaut
*a
aChemical Engineering Programme, Faculty of Engineering, Universiti Malaysia Sabah, Kota Kinabalu 88400, Sabah, Malaysia. E-mail: jojoba2021@126.com; css@ums.edu.my
bDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore 117585, Singapore. E-mail: chexiej@nus.edu.sg
First published on 16th December 2024
Abstract
Ultrasmall metal nanoclusters (NCs, <2 nm) have emerged as a novel class of luminescent probes due to their atomically precise size and tailored physicochemical properties. The rapid advancements in the design and utilization of metal NC-based luminescent probes are facilitated by the atomic-level manipulation of metal NCs. This review article explores (i) the engineering of metal NCs’ functions for bioimaging applications, and (ii) the diverse uses of metal NCs in bioimaging. We begin by presenting an overview of the engineering functions of metal NCs as luminescent probes for bioimaging applications, highlighting key strategies for enhancing NCs’ luminescence, biocompatibility and targeting capabilities towards biological specimens. Our discussion then centers on the bioimaging applications of metal NCs in subcellular organelles, individual cells, tissues, and entire organs. Finally, we offer a perspective on the challenges and potential developments in the future use of metal NCs for bioimaging applications.
1. Introduction
Ultrasmall metal nanoclusters (NCs) with core sizes of ≤2 nm, like Au, Ag, Cu, and Pt, are novel functional nanomaterials.1–7 They have been extensively studied in domains including sensing,8,9 catalysis,10–17 environmental protection,18–20 energy conversion,2,21,22 and biomedicine.5,7,23–27 Particularly in biomedicine, metal NCs are highly valuable in bioimaging as their potent photoluminescence (PL) aids non-invasive bioimaging for biospecimen labelling and disease diagnosis.28,29 The metallic core-ligand shell structure of NCs can be precisely modulated at the atomic level,30,31 allowing customization for specific bioimaging uses.7 Even a single atom change can significantly alter the PL emission wavelength of metal NCs,32,33 offering numerous functional tailoring opportunities to metal NCs for catering to different bioimaging requirements.Engineered nanomaterials, including inorganic nanoparticles (metal,34 quantum dots,35 carbon/silicon-based36–38) and organic luminescent probes (small/large molecules),39,40 show clinical promise as PL probes. Inorganic variants offer tunable emission, large Stokes shift, and photostability, while organic ones allow structural and chemical versatility. Metal NCs combine the advantages of both inorganic and organic types, featuring molecule-like properties,41–46 ultrasmall size,47,48 diverse and customizable surface chemistry,49–52 adjustable size and composition,53–55 large Stokes shifts,56 excellent biocompatibility,5,7,26 robust photostability,20,57,58 and effective renal clearance.59,60 These characteristics present significant opportunities for various bioimaging applications.
The engineering of metal NCs has advanced from the nanoscale to the atomic scale, prompting intensified efforts to precisely manipulate their physicochemical properties for targeted applications. This has resulted in the development of diverse metal NCs as PL probes for a range of bioimaging scenarios. To regulate the physicochemical properties of metal NCs, the engineering of metal NCs has been advanced in three main areas. The first focus is on the size/structural tuning of metal NCs,30,31,47,48,61,62 where atomic precision NCs of varying sizes and architectures are synthesized to achieve unique physicochemical characteristics. For instance, due to variations in size and structure, Au22(GSH)18 NCs (here GSH denotes glutathione) exhibit PL emission near the first near-infrared window (NIR-I; 800–1000 nm),63–65 whereas Au25(GSH)18 NCs display PL emission in the second near-infrared window (NIR-II; 1000–1700 nm).66 Research in this arena not only endows metal NCs with intriguing characteristics for bioimaging but also offers new insights into the relationship between the size/structure of metal NCs and their bioimaging performance.58,67
Secondly, NC engineering emphasizes customizing their composition to achieve innovative or improved physicochemical properties.10,12,68 Heteromeric NCs with dual, triple, or even quadruple metallic compositions have been synthesized,32,69–73 to display composition-dependent characteristics (e.g., PL properties and stability) that are invaluable for applications as PL probes.65,66
Beyond the engineering of the metal core, the surface ligand control of metal NCs also presents a very interesting issue.27,74–77 This third domain of NCs engineering can be harnessed to modulate biocompatibility and biointeraction with NCs.77,78 Ligand engineering introduces additional functionalities, enabling metal NCs to surpass the limitations of conventional PL probes and achieve enhanced bioimaging efficacy.7,77 These properties make metal NCs suitable for imaging a wide range of biological targets, from subcellular organelles and individual cells to tissues and entire organs. To date, hundreds of metal NCs with diverse physicochemical properties and functions have been designed as PL probes,7,28,79 and have demonstrated the potential in providing high-resolution images with excellent contrast, facilitating advanced studies in various bioimaging applications.
Given the rapid advancements in this area, there is a need to delicately review the current state of research on luminescent metal NCs in bioimaging. This review aims to provide an overview of the engineering functions of metal NCs as PL probes, discuss various synthetic strategies employed to enhance their PL properties and targeting ability to biospecimens, and explore their diverse applications in bioimaging. By summarizing recent progress and identifying future directions, this article seeks to offer valuable insights for researchers working in the field of nanomedicine and bioimaging.
2. Engineering functions of metal NCs-based PL probes
Metal NCs must meet several criteria for bioimaging applications, including strong PL, excellent biocompatibility, and robust photostability, as well as stability under physiological conditions. They should also exhibit high specificity or selectivity towards biospecimens (e.g., subcellular organelles,80 cells,9,44,81 tissues,79,82 and organs60,65,83), which impacts PL imaging efficacy and potential side effects. For instance, tumor targeting capabilities are essential for tumor diagnosis via PL imaging.7,74,84,85 For intracranial brain tissue imaging, metal NCs must traverse the blood–brain barrier (BBB) to reach the brain tissues.86,87 This section will explore various strategies for PL regulation, enhancing biocompatibility, implementing targeting functions, and improving stability.2.1 Synthetic strategies of highly luminescent metal NCs
As mentioned above, producing sufficient PL signals is crucial for achieving high-quality imaging with metal NCs-based PL probes, so it is necessary to deploy highly luminescent metal NCs as PL probes. After years of research, several effective strategies have been developed to obtain highly luminescent metal NCs, including metal doping, ligand engineering, and surface motif modulation. In this subsection, the synthesis strategies of such highly luminescent metal NCs will be discussed, with an emphasis on the mechanisms of PL enhancement.Metal doping methods include co-reduction, galvanic reactions, anti-galvanic reactions,89 and under potential deposition.70 The primary mechanisms of metal doping for PL enhancement include: (1) the increase of the electropositivity of the metal core to facilitate ligand-to-metal charge transfer (LMCT) or ligand-to-metal–metal charge transfer (LMMCT);43,90 (2) the modification of the electronic and geometric structure of metal NCs;32,43,91 (3) the utilization of the relativistic effects of the dopants.92 For instance, Wu and colleagues synthesized an alloyed Ag2Au25(SR)18 NC by reacting Au25(SR)18 with AgNO3, which exhibited approximately 3.5 times stronger PL compared to either Au25(SR)18 or AgxAu25−x(SR)18 NCs.90 This enhancement is attributed to the anchoring of two Ag+ ions, with lower electronegativity (∼1.93), onto the Au25(SR)18 NC's surface (note: the electronegativity of Au is ∼2.54) without altering their valence states. This process increases the electropositivity of the metal core, thereby enhancing PL through facilitated LMCT. Oh et al. found Ag-doped Au NCs doubled QY and improved stability, with emission adjustable from 670–820 nm.93
Most alloy metal NCs position their heterometal atoms at the center of the metal core,12,91,94–96 but doping metal atoms on surface motifs can also significantly enhance PL through increasing the electropositivity of the metal core. For example, Wang et al. employed Ag doping to modify the PL of Au NCs by engineering Au(I)-thiolate motifs in the shell, achieving up to a 3.9-fold increase in PL intensity (Fig. 1e and f).97 By adjusting the reduction degree and shell thickness, they shifted PL from orange to red. This method synthesizes highly luminescent bi-metallic NCs with aggregation-induced emission (AIE). Advanced techniques like electrospray ionization mass spectrometry (ESI-MS) facilitate the study of the correlation between doping behavior and optical properties.72,94 For example, Zheng et al. observed Au atoms diffusing into Ag25 NCs, influencing optical absorption.94
 | ||
| Fig. 1 (a) and (b) NIR-II PL enhancement of Au25 NCs via Er, Ag, Cu, and Zn doping. Reproduced with permission from the ref. 66, Copyright 2019 WILEY-VCH. (c) and (d) PL enhancement of AIE-featured Ag29 NCs via single-atom Au doping. Reproduced with permission from the ref. 32, Copyright 2024 The Royal Society of Chemistry. (e) and (f) PL enhancement of AIE-featured Au NCs via Ag doping. Reproduced with permission from the ref. 97, Copyright 2019 Wiley-VCH. | ||
In addition to increasing electropositivity, modification of the electronic and geometric structure of the metal core via metal doping is also a common way to enhance the PL of metal NCs. For example, Liu et al. used CO to synthesize NIR-II luminescent GSH-protected Au25 NCs doped with Er, Ag, Cu, and Zn, enhancing NIR-II PL by 3–5.2 times (Fig. 1a and b).66 In particular, under 808 nm excitation, the quantum yield (QY) of the Au24Cu1 NCs obtained was as high as 4%, comparable to conventional NIR-II dyes. The authors found that the heterogeneous metal atom doping could effectively regulate the HOMO–LUMO transition, which may be the reason for the enhancement of NIR-II PL. He et al. reported Au-doped Au1Ag28(DHLA)12 had 4.7 times the PL intensity of Ag29(DHLA)12 NCs (Fig. 1c and d), highlighting PL dependence on dopant species.32
Xie et al. demonstrated the utilization of relativistic effects of dopants for PL enhancement by employing density functional theory (DFT) calculations to explore the relationship between the degree of Au doping and the PL properties of AuxAg29−x NCs where x ranges from 1 to 5.92 Their findings revealed that the first Au heteroatom invariably occupied the innermost position of the icosahedral kernel, while the second to fifth Au heteroatoms predominantly localized on the surface of the icosahedral M13 kernel. Additional DFT calculations indicated that replacing Ag with Au significantly boosted PL intensity due to the relativistic effect of the Au heteroatoms. This enhancement in PL could become increasingly pronounced in heavily Au-doped NCs, such as Au3Ag26, Au4Ag25, and Au5Ag24 NCs.92
In earlier research, Wu et al. discovered that Au25 NCs exhibit significantly increased PL when shielded by those ligands containing electron-rich atoms (e.g., nitrogen, oxygen) or groups (e.g., –COOH, NH2).99 Zhu's group compared the PL of Au25(SePh)18, Au25(SC2H4Ph)18, and Au25(NAPS)18 NCs (here NAPS represents 2-(naphthalen-2yl)ethanethiolate).104,105 They found that Au25(SePh)18's weaker PL is due to Se–Au bonds’ poor LMCT or LMMCT contribution compared to S–Au bonds. Au25(NAPS)18 NCs showed 6.5 times higher PL than Au25(SC2H4Ph)18, due to NAPS's stronger charge-donating capability. Recently, Liu et al. discovered that the NIR-II PL intensity of Au25(GSH)18 NCs is markedly superior to that of Au25(Cys)18 and Au25(HCys)18 NCs (Fig. 2a; here HCys denotes homocysteine).66 The enhancement of NIR-II PL in Au25(GSH)18 NCs can be attributed to the following reasons: (1) GSH contains more electron-rich functional groups (two carboxyl groups and one amino group) compared to Cys and HCys (each possessing only one carboxyl group and one amino group), facilitating stronger LMCT or LMMCT; (2) the greater steric hindrance of GSH, relative to Cys and HCys, effectively restricts the ligand stretching of Au25 NCs, thereby minimizing energy loss caused by non-radiative relaxation.
 | ||
| Fig. 2 (a) Comparison in the NIR-II PL intensity between Au25(GSH)18, Au25(Cys)18 and Au25(HCys)18 NCs. Reproduced with permission from the ref. 66, Copyright 2019 WILEY-VCH. (b) PL enhancement of Au22(GSH)18 NCs via surface conjugation of arginine. Reproduced with permission from the ref. 103, Copyright 2024 The Royal Society of Chemistry. | ||
Furthermore, ligand engineering can be employed to modify the bulkiness or rigidity of the ligands in metal NCs, a method proven effective in enhancing their PL. For instance, Pyo et al. markedly amplified the PL of Au22 NCs by a factor of eight through the strategic alteration of GSH ligands with aromatic molecules and folic acid.101 This modification resulted in a more rigid ligand shell, facilitating more efficient energy transfer from the ligands to the metal core. The PL of pyrene-modified Au22 NCs experienced a 37-fold enhancement, upon the introduction of electrostatic interactions with tetraoctylammonium cations. This interaction significantly enhanced the energy transfer efficiency, raising it from 14% to 80%. Recently, Dou et al. also discovered that the PL intensity of Au22(GSH)18 NCs increases by up to 3.63-fold following the surface conjugation with arginine molecules (Fig. 2b).103
Beyond enhancing PL, ligand engineering can also adjust the emission wavelength of metal NCs. For example, Shibu et al. used 3-mercapto-2-butanol (MB) molecules to replace some GSH ligands of Au25 NCs, resulting in Au25(MB)x(GSH)18−x products with blue shifts of 20–30 nm in their PL spectrum.106 Therefore, it can be concluded that ligand engineering profoundly influences the optical properties of metal NCs, facilitating the creation of highly luminescent metal NCs for bioimaging applications.
In recent years, AIE theory proposed by Tang et al. has guided the design of metal NCs-based luminescent probes through surface motif engineering,56,107,108 enhancing their design and PL mechanisms. Surface motifs strengthen intra- and inter-cluster metallophilic interactions and reduce nonradiative energy loss,109,110 significantly boosting PL.
In 2012, Xie group used AIE-featured Au(I)–GSH complexes to protect in situ generated Au(0) cores, forming highly luminescent Au(0)@Au(I)–GSH NCs (Fig. 3a).109 These complexes emitted strong orange PL at 610 nm due to enhanced Au(I)⋯Au(I) interactions. In the solid state, the PL intensity increased further, driven by the formation of extended Au(I)–GSH motifs on the NC surface. This configuration restricted ligand stretching/vibration, minimized non-radiative energy loss, enhanced Au(I)⋯Au(I) interactions and concurrently facilitated LMCT or LMMCT. Inspired by this, researchers engineered metal NCs’ surface for PL enhancement through the AIE mechanism. It should be mentioned that the AIE mechanism involves the deployment of elongated M(I)-SR motifs on the NC's surface to restrict ligand stretching/vibration, strengthen M(I)⋯M(I) interactions, and facilitate LMCT or LMMCT. For instance, Xie and his coworkers created Au22(GSH)18 NCs with red emission at 665 nm (QY of 10%), featuring an Au8 core capped with elongated motifs, i.e., two [RS(AuSR)3] + two [RS(AuSR)4] motifs.63,111 Similarly, they also connected short Au(I)–GSH motifs with Ag(I), amplifying PL based on the above-mentioned AIE mechanism.110 Recently, a succession of AIE-featured metal NCs exhibiting robust PL have been progressively developed.43,112–117
 | ||
| Fig. 3 (a) The core–shell structure (upper panel) and optical spectra (lower panel) of the luminescent the Au(0)@Au+-GSH NCs with AIE. Reproduced with permission from ref. 109, Copyright 2012 American Chemical Society. (b) The surface motif features (upper panel) and luminescent spectra (lower panel) of 600 nm- and 810 nm-emitting Au NCs with two different local Au bonding environments. Reproduced with permission from ref. 118, Copyright 2016 Wiley-VCH. (c) Schematic illustration of the synthesis of AIE-featured Cu NCs (upper panel). UV-Vis absorption, PL spectra (lower panel), and digital photos (insets) of blue-emitting Cu NCs and cyan-emitting Cu NCs. Reproduced with permission from ref. 119, Copyright 2024 Elsevier Ltd. | ||
In addition, Liu et al. adjusted the PL emission of GSH-protected Au NCs from 600 nm to 810 nm by fine-tuning the surface coverage of Au(I)–GSH motifs (Fig. 3b).118 High coverage led to strong charge transfer and 600 nm emission, while low coverage resulted in weak charge transfer and 810 nm emission. Wu et al. found that altering the length of surface M(I)-SR motifs could tune emission wavelengths and change the PL origin. Shorter Au(I)-SR motifs shifted emission to the NIR-II range and altered PL from phosphorescence to fluorescence. This effect was more pronounced with greater aggregation. Very recently, Wang et al. designed AIE-featured Cu NCs with adjustable PL by controlled aggregation of Cu(I)–GSH motifs on in situ generated Cu(0) cores, forming Cu(0)@Cu(I)-thiolate core–shell NCs at the elevated temperature (80 °C; Fig. 3c).119 Varying the reaction pH from 3 to 12 adjusted the PL color from blue to cyan by modulating the aggregation degree of Cu(I)–GSH motifs. At present, a considerable number of research studies focusing on the assembly of AIE-featured metal NCs for PL enhancement have emerged,113,116,120–124 demonstrating substantial advancements. The surge in such studies underscores the growing interest and commitment within the NCs field to explore and optimize PL properties through metal NCs assembly for relevant applications.
Overall, the surface motif engineering for augmenting NC's PL has garnered widespread recognition in the field, potentially spurring further research into the design of AIE-featured metal NCs and deeper fundamentals of NC's PL mechanisms at the molecular level.
2.2 Biocompatibility
For metal NCs to be effective as luminescent probes in bioimaging applications, they must demonstrate outstanding biocompatibility.This encompasses several critical factors, including minimal cytotoxicity, good water solubility, low metabolic toxicity, and negligible adverse effects on organs, which will be separately discussed in this subsection.
 | ||
| Fig. 4 Cytotoxicity of AIE-featured Cu NCs on L929 cells. PL microscope images of viable cells stained with Calcein AM (green color) and non-viable cells stained with ethidium homodimer 1 (red color) after 24 h treatment with 200 μM of (a) blue-emitting Cu NCs (i.e., B–Cu NCs in the figure) and (c) cyan-emitting Cu NCs (i.e., C–Cu NCs). Cell viability evaluated by MTT assay after 24 h treatment with different concentration of blue-emitting Cu NCs (b) and cyan-emitting Cu NCs (d) using CuSO4 as positive control. Reproduced with permission from ref. 119, Copyright 2024 Elsevier Ltd. | ||
 | ||
| Fig. 5 (a) Schematic illustration of the core–shell structure of GSH-Au25 NCs and BSA-Au25 NCs. Reproduced with permission from ref. 85, Copyright 2013 WILEY-VCH. (b) Size-dependent renal clearance behaviors of Au10–11, Au15, Au18 and Au25, 1.7 nm GSH-Au NCs, 2.5 nm and 6 nm GSH-Au NPs. Reproduced with permission from ref. 131, Copyright 2017 Springer Nature. | ||
In addition to renal clearance, liver clearance of metal NCs is also crucial. While renal clearance has been well-studied, liver clearance has been less explored.135 Large nanomaterials can be degraded by liver macrophages and excreted via the bile duct.40,136 Indeed, metal NCs can undergo liver detoxification to reduce toxicity, with GSH-mediated biotransformation being a key pathway. Jiang et al. used indocyanine green (ICG)-modified GSH-Au25 NCs to study this process, finding that GSH and Cys in liver cells replace Au NCs’ ligands, altering retention time and targeting of Au NCs.135 This GSH-mediated biotransformation is also applicable to large Au nanoparticles (NPs) ranging from 5 to 100 nm. Thus, the liver regulates metal NC transport, enhancing disease-targeting while minimizing off-target accumulation and health risks, aiding in bioimaging-directed disease diagnostics.
Overall, the surface chemistry, stability, and density of NCs, coupled with HD, must be meticulously considered to modulate renal clearance for luminescent bioimaging applications, while hepatic excretion also plays a role in the elimination of metal NCs from the body. However, the clearance of metal NCs entails intricate biological interactions within the body, necessitating more thorough investigations to elucidate the metabolic mechanisms of these metal NCs.
 | ||
| Fig. 6 (a) The bio-distribution of Au44MBA26-Cy7 NCs in the heart, liver, spleen, lung, and kidney 24 h after the intravenous injection of the Au44MBA26-Cy7 NCs (Cy7 denotes heptamethine dye). (b) Haematoxylin and eosin (H & E)-stained histological sections of heart, liver, spleen, lung, and kidney tissues obtained from mice in the saline, and PTT groups with the Au44MBA26 and Au44MBA26-Cy7 NCs. Reproduced with permission from ref. 75, Copyright 2023 Royal Society of Chemistry. | ||
Achieving these characteristics requires meticulous selection of both the metal cores and the surface ligands. The metal cores need to be chosen for their inherent properties that are compatible with biological environments, while the surface ligands should be selected for their ability to interact benignly with biological molecules. Research has demonstrated that Au NCs frequently meet these stringent criteria.7,75–77 These metals are typically non-toxic and exhibit favorable interactions with biological systems. Moreover, they can be functionalized with a variety of biocompatible ligands, including peptides, proteins, or biomolecules, which further enhance their compatibility and functionality in bioimaging applications. By carefully engineering these NCs, scientists can develop highly effective luminescent probes that are safe and efficient for use in medical diagnostics and research.
2.3 Targeting property of metal NCs-based luminescent probes
The effectiveness of metal NCs in bioimaging is significantly influenced by their capacity to target on specific biological structures (e.g., tumors). Achieving this precision in targeting is made possible through the functionalization of metal NCs with specialized molecules that exhibit selective binding properties to certain cell types or biomolecules. For instance, by attaching antibodies or aptamers to the surface of metal NCs,137–139 these entities can be steered towards specific cancer cells or pathogenic bacteria with high accuracy. This method of targeted delivery not only improves the clarity and accuracy of imaging results but also enhances the precision of diagnostic capabilities. Additionally, the exceptional targeting capability of metal NCs-based PL probes also serves to minimize any off-target-induced potential biotoxicity or side effects.Taking the malignant tumor targeting as an example, a variety of metal NCs-based diagnostic probes have been developed for tumor diagnosis.7,75,76,82,140 Metal NCs-based probes developed for tumor diagnosis must be highly selective, effective, and have minimal side effects. These probes exhibit passive tumor-targeting through the EPR effect. For instance, GSH-Au NCs exhibit prolonged tumor retention and expedited clearance compared to traditional dyes, owing to their ability to evade rapid renal elimination.141 The passive targeting ability of metal NCs depends on their size, density, surface chemistry, and the tumor microenvironment.128 Active tumor targeting of metal NCs is achieved by applying tumor-targeting ligands on their surface. For instance, grafting a tumor-targeting peptide, luteinizing-hormone-releasing hormone (LHRH) on 64Cu NC@BSA resulted in 4× higher tumor uptake (Fig. 7a).142 Other molecules like folic acid and epidermal growth factor receptor (EGFR) antibody have also been used.143–145 Selection depends on targeting receptors in different tumors, considering toxicity and in vivo clearance to avoid increased HD and side effects.
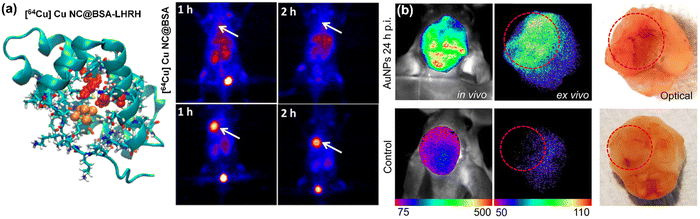 | ||
| Fig. 7 (a) Positron emission tomography (PET) images of lung-tumor-bearing mice 1 h after injection with (upper) 64Cu NC@BSA and (lower) 64Cu NC@BSA-LHRH. Adapted with permission from ref. 142 Copyright 2015 American Chemical Society. (b) Invasive in vivo, ex vivo PL imaging, and optical imaging of advanced glioma-bearing brain with (upper row, 24 h p.i.) or without (lower row) administration of Au NCs. Adapted with permission from ref. 146, Copyright 2017 Tsinghua University Press and Springer-Verlag Berlin Heidelberg. | ||
Another crucial aspect of metal NCs-based luminescent probes is their ability to target brain tissues for intracranial disease diagnosis, which is challenging due to the BBB, a semipermeable central nervous system (CNS) boundary composed of endothelial tight junctions, pericytes, and astrocyte foot processes.147 Renal-clearable metal NCs can bypass the BBB through the leaky blood–tumor barrier, as demonstrated by luminescent GSH-Au NCs targeting glioblastoma multiforme with high efficiency and specificity (Fig. 7b).146 However, more studies are needed to understand how surface chemistry, size, and composition affect extravasation and clearance of infiltrated NCs in brain tissues.
2.4 Photostability and stability of metal NCs under physiological conditions
Photostability is an essential attribute for luminescent probes used in bioimaging applications. It ensures that these probes can maintain their PL properties even when subjected to prolonged exposure to light, which is crucial for obtaining accurate and reliable imaging results but avoiding decomposition-caused toxicity issues.148 Additionally, metal NCs-based luminescent probes also must demonstrate resilience within physiological conditions. This includes enduring varying pH levels and ionic strengths that are commonly found in biological environments. To achieve this level of stability, surface modification techniques are frequently employed.44,50 These techniques involve altering the surface of the NCs to protect their core structure from environmental factors that could lead to degradation. By enhancing the stability of these probes through such modifications, researchers can ensure that the PL properties remain intact over extended periods. This is particularly important for long-term imaging sessions, as it prevents a significant loss of signal quality, thereby allowing for more effective and accurate observations. Robust photostability thus plays a pivotal role in the success of bioimaging studies, enabling continuous and consistent data collection without the interference of signal deterioration.3. Bioimaging applications of metal NCs-based luminescent probes
Metal NCs feature ultrasmall size, strong PL, diverse and customizable surface chemistry, adjustable size and composition, large Stokes shifts, excellent biocompatibility, robust photostability, and effective renal clearance. These characteristics allow metal NCs as excellent PL probes for imaging a wide range of biological targets, from subcellular organelles and individual cells to tissues and entire organs. This section discusses the applications of metal NCs in bioimaging of subcellular organelles, cells, biological tissues and even organs.3.1 Sub-cellular organelle imaging of metal NCs
Cells are the fundamental units of all living organisms and serve as the foundation of life. Each cell is a sophisticated and well-organized system. Taking animal cells as an example, animal cells have a cell membrane, cytoplasm, and nucleus. The cytoplasm includes a matrix and sub-cellular organelles like the endoplasmic reticulum, mitochondria, Golgi apparatus, ribosomes, lysosomes, and centrosomes.149 Organelles perform specific functions to ensure normal cellular functioning and overall organismal activities. For instance, mitochondria are recognized as the “powerhouses” of cells responsible for adenosine triphosphate (ATP) production through oxidative phosphorylation to provide energy for cellular processes.150 Additionally, mitochondria participate in regulating apoptosis (cell death) and calcium ion storage while possessing their own DNA capable of independent replication;150 Golgi apparatus processes, modifies, and transports proteins via vesicles, also aiding in lipid transport and lysosome formation;151 lysosomes contain hydrolases that degrade macromolecules like proteins, lipids, and polysaccharides, playing a crucial role in autophagy by removing damaged organelles and maintaining cellular health.152 Generally, the structure of cells and the functions of their organelles are crucial for life activities. Each organelle has its unique and irreplaceable role, collectively forming a precise and efficient system that ensures the normal operation of an organism from a single cell to the whole. Therefore, PL imaging of organelles is of great significance.Metal NCs have been effectively used for sub-cellular organelle imaging due to their small size and engineerable surface chemistry. They can penetrate cellular compartments such as mitochondria or nuclei, providing detailed images that aid in understanding intracellular processes.
The PL imaging of mitochondria utilizing luminescent metal NCs exhibits a pronounced size-dependent effect. For example, in 2018, Gao et al. engineered two Au NCs with identical surface chemistry yet differing molecular sizes, namely Au5Peptide3 and Au22Peptide10, which exhibited distinct mitochondrial uptake behaviors.84 The authors found that Au5Peptide3 and Au22Peptide10 could be taken byhuman nasopharyngeal cancer (CNE1) cells, but only Au5Peptide3 induced apoptosis significantly because CNE1 cells took up 11.6 times more Au5Peptide3 than Au22Peptide10 in mitochondria (Fig. 8a and b). The mitochondrial enrichment effect of Au5Peptide3 enhanced its antitumor efficacy by diminishing anti-apoptotic protein (Mcl-1) and augmenting pro-apoptotic protein (Puma) of mitochondria, thereby altering mitochondrial potential and activating the caspase pathway to induce apoptosis.
 | ||
| Fig. 8 (a) Confocal PL images of Au5Peptide3 and Au22Peptide10 NCs in CNE1 cells co-stained with LysoTracker Green or MitoTracker Green. (b) ICP-MS result of Au5Peptide3 and Au22Peptide10 in the whole cell and the cell extracted mitochondria. Reproduced with permission from ref. 84 Copyright 2018 American Chemical Society. (c) Confocal PL images of Au44MBA26-Cy7 NCs in live HeLa cells co-stained with MitoTracker Green or LysoTracker Green. Reproduced with permission from ref. 75, Copyright 2023 Royal Society of Chemistry. | ||
To enhance the targeting ability of metal NCs, Pan et al. engineered an innovative luminescent AuAg NC-based probe with AIE for targeted mitochondrial imaging through ligand engineering by conjugating (4-carboxybutyl)triphenylphosphonium bromide (TPP) on the surface.80 By integrating TPP, which facilitates mitochondrial targeting, the resultant AuAg NCs@TPP probe boasted several advantages, including an ultrasmall size (less than 3 nm), strong PL with a 610 nm emission, an extended PL lifetime (7.2 μs), amphiphilic surface chemistry, and exceptional stability. These attributes rendered it successful for targeted mitochondrial imaging.
Recently, Yang et al. devised a Au NC-based probe for cancer theranostics by conjugating NIR-II luminescent Au44MBA26 NCs with aromatic photothermal molecules (Cy7) via click chemistry.75 Through confocal PL microscopy, they investigated the uptake behavior of HeLa cells for the as-designed Au44MBA26-Cy7 probe and discovered that the green PL from the lysosome-specific dye LysoTracker Green strongly correlated with the red PL channel of the Au44MBA26-Cy7 probe (Fig. 8c), indicating predominant localization of the probe within the lysosomes.
In summary, the distinctive advantages of metal NCs have facilitated their use in PL imaging of specific sub-cellular organelles. Nevertheless, research in this domain remains nascent. Future endeavors must focus on advancing targeted imaging of additional sub-cellular organelles, which may help to delve into the diagnostic and therapeutic mechanisms of various diseases at the subcellular level.
3.2 Cell and bacteria imaging of metal NCs
Metal NCs exhibit ultrasmall size, strong PL, high photostability, large Stokes shift, diverse surface chemistry, and low toxicity, making them ideal for PL imaging of cells and bacteria. In 2011, orange-emitting D-penicillamine-Au NCs were reported for HeLa cell imaging with minimal toxicity.153 Subsequent developments led to various luminescent NCs with specific functionalities for cell imaging. For example, Yu et al. found that luminescent Au NCs, shielded by GSH and cysteamine (referred to as GC-Au NCs), exhibit pH-dependent cell imaging capabilities in the presence of serum proteins due to the NC's pH-dependent adsorption onto live cell membranes.81 Specifically, as illustrated in Fig. 9a, the GC-Au NCs fail to image HeLa cells at pH 7.4 in phosphate-buffered saline (PBS) due to minimal interaction with cells, yet they succeed when the extracellular pH is reduced to pH 6.0 and further to pH 5.3.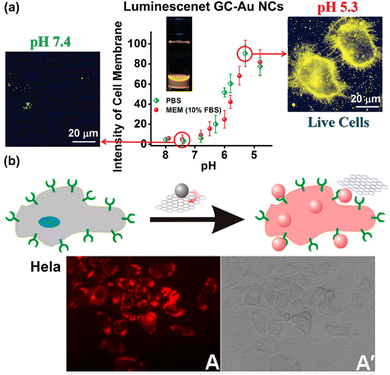 | ||
| Fig. 9 (a) pH-dependent PL images of GC-AuNPs on live HeLa cells. Adapted with permission from ref. 81, Copyright 2011 American Chemical Society. (b) Schematic depiction of the turn-on PL imaging mechanism, along with the PL imaging results of the Tf-Au NCs/GO probe, for bioimaging HeLa cells with overexpressed Tf receptors. Adapted with permission from ref. 154, Copyright 2013 American Chemical Society. | ||
To achieve targeted imaging of cells and bacteria, two primary strategies are employed for the synthesis of metal NCs-based PL probes. The first strategy involves post-surface modification of metal NCs using functional molecules with targeting capabilities, while the second relies on specific biomolecules with targeting abilities as stabilizers for the direct synthesis of metal NCs-based PL probes. As a demonstration of the initial strategy, Chahande et al. functionalized Au NCs with acyl homoserine lactones (AHLs; possessing bacterial targeting capability) of varying carbon chain lengths to create an Au NC-based PL probe for sensing Escherichia coli (E. coli) and Cronobacter sakazakii (C. sakazakii).155 The authors discovered that the developed PL probe could label E. coli irrespective of the AHL's carbon chain length variation, whereas only the probe conjugated with C8-chain AHL could selectively illuminate C. sakazakii. The meticulously designed metal NCs-based PL probes, following post-surface modification, are frequently utilized for in vivo imaging of biological tissues and organs, and will be elaborated upon in subsequent subsections.
In addition to the post-surface modification, the second strategy is also commonly used for the design of metal NCs-based PL probes for cell imaging. Some biomolecules retain their functions within metal NCs’ protecting shell, giving these stabilized metal NCs specific biological activity. For instance, transferrin (Tf) can be used to synthesize Tf-Au NCs, which target cells with Tf receptors. A turn-on PL probe that integrates Tf-Au NCs with graphene oxide (GO) has demonstrated the capability to image HeLa cells (Fig. 9b), which are known to overexpress transferrin receptors.154 The underlying mechanism is that the PL of Tf-Au NCs could be quenched by GO but restored by Tf receptors, allowing targeted cell imaging through PL (Fig. 9b). Moreover, peptides and DNAs can be employed to stabilize and functionalize metal NCs with targeting capabilities.156–158 For instance, the cyclic arginine-glycine-aspartic acid RGD peptide was used to develop Au NCs-based PL probe for selective FL imaging of melanoma A375 cells.159 Additionally, antimicrobial peptide (AMP)-protected Au NCs are utilized for both diagnosing and treating various forms of cancer as well as infections.160–162 In another application, the G-quadruplex AS1411 sequence and the MUC1 aptamer are employed to synthesize Ag NCs, which are then used for imaging purposes.163,164 These Ag NCs effectively visualize nucleolin and mucin-overexpressing cancer cells, aiding in accurate detection. Furthermore, DNA-Ag NCs that have been modified with cationic polyelectrolytes demonstrate enhanced stability, making them highly effective for rapid cell imaging.165 This modification ensures that the NCs remain stable and functional over extended periods, thus improving the efficiency and accuracy of cellular imaging techniques.
3.3 Tissue imaging of metal NCs
On a larger scale, metal NCs can be harnessed for tissue imaging. Their ability to penetrate tissue matrices allows them to provide detailed images that are essential for studying tissue architecture or diagnosing diseases at early stages. Notably, while the PL in the visible spectrum (400–800 nm) of metal NCs is readily utilized for imaging sub-cellular organelles and cells due to shallow penetration requirements, the red PL of metal NCs is also frequently used for in vivo imaging of biological tissues. For instance, Xiao et al. employed amphiphilic yet positively charged peptide to modify GSH-Au NCs aggregates (AP1P2PEG NCs), resulting in red PL that exhibited remarkable accumulation in solid liver tumors.74 This facilitated PL imaging of solid liver tumors (Fig. 10a) and induced prolonged stress in the endoplasmic reticulum, promoting the synergistic enhancement of autophagy and apoptosis in liver tumor cells.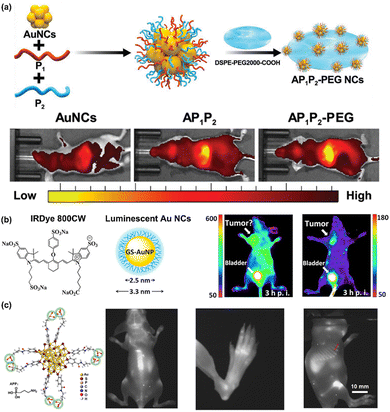 | ||
| Fig. 10 (a) Schematic illustration of AP1P2-PEG NCs synthesis and in vivo NIR-II PL images of solid tumors in the liver at 24 h after intravenous injection of NCs (Au NCs, AP1P2, and AP1P2-PEG NCs) labeled with Cy5 dye. Reproduced with permission from ref. 74, Copyright 2023 Wiley-VCH GmbH. (b) In vivo NIR-I PL images of the tumor within mice 3 h after injection with (left) IRDye 800CW and (right) ultrasmall GSH-Au NCs. Adapted with permission from ref. 141, Copyright 2013 American Chemical Society. (c) In vivo NIR-II PL images of bones of a mouse after intravenous injection of the Au44MBA26-P probe. Reproduced with permission from ref. 77, Copyright 2023 South China University of Technology, AIE Institute and John Wiley & Sons Australia, Ltd. | ||
However, it should be emphasized that in vivo PL imaging of biotissues and organs typically necessitates longer PL wavelengths such as those in the near-infrared ranges (NIR-I: 800–1000 nm; NIR-II: 1000–1700 nm).7 This is because NIR-I/II PL offers higher spatiotemporal resolution, reduced tissue damage, and deeper tissue penetration with minimal biological background interference.7,166–168 Consequently, an array of NIR-I and NIR-II emitting metal NCs-based PL probes has been developed for noninvasive imaging and theranostics of biotissues and organs in vivo. For instance, NIR-I-emitting Au NCs have been used to label tissues, serving as PL imaging contrast agents to assist in head and neck tumor surgeries.169 Recently, Zheng and colleagues developed a renal-clearable NIR-I-emitting Au NCs probe for rapid tumor detection.141 Particularly, they developed GSH-Au NCs, mimic small dye molecules like IRDye 800CW in stability and renal clearance but have longer tumor retention and faster normal tissue clearance (Fig. 10b). This PL probe shows the enhanced permeability and retention effect persists in small NCs, allowing rapid tumor detection without significant accumulation in reticuloendothelial organs, promising for cancer diagnosis and therapy.
NIR-II PL imaging, with its enhanced tissue penetration and superior spatiotemporal resolution, has emerged as a highly promising alternative to visible and NIR-I PL imaging.7 Recently, a variety of NIR-II emitting metal NCs-based probes have been developed for biotissue imaging.65,66,75–77,82 For example, Yuan et al. created a Au NCs-based probe for targeted bone imaging by modifying NIR-II emitting Au44 NCs with surface phosphorylation.77 This phosphorylation augments the probe's bone-targeting capability through densely concentrated phosphate groups, enabling in vivo bone-targeted NIR-II PL imaging (Fig. 10c). Recently, a series of NIR-II emitting Au NCs-based probes have been developed for PL imaging of tumors,75,76,82 cerebral vessels,66 gastrointestinal structures,170 and bone tissues,77,171 promoting the diagnosis and treatment of relevant diseases.
3.4 Organ imaging of metal NCs
Organ imaging represents one of the most advanced applications of luminescent metal NCs. Their enhanced penetration depth and targeting capabilities enable visualization of entire organs, providing valuable information for preclinical studies and potential clinical diagnostics. For example, Pan et al. recently developed a PL probe emitting at 610 nm by conjugating AIE-featured AuAg NCs with hydrophobic TPP molecules, demonstrating its efficacy for in vivo brain imaging in living mice (Fig. 11a).80 To minimize the biological background interference, Zheng's team recently applied renal-clearable NIR-I emitting GSH-Au NCs as contrast agents for PL imaging of kidney clearance kinetics (KCK) in normal mice.60,83,172 This approach allowed to noninvasively monitor KCK with a 50-fold increase in kidney contrast enhancement compared to conventional organic dyes. Moreover, the authors also confirmed that the in vivo PL imaging with renal-clearable NIR-I emitting GSH-Au NCs is capable of noninvasively detecting kidney dysfunction and stages (Fig. 11b), revealing adaptive function in a mouse model of unilateral obstructive nephropathy, undiagnosable by routine markers. This may aid early diagnosis and intervention for liver issues like acetaminophen-induced injuries. Later, Zhang and colleagues devised a probe based on NIR-II emitting Au21Cu1 NCs, enabling three-dimensional monitoring of cisplatin-induced renal injury through NIR-II light-sheet microscopy (Fig. 11c).65 | ||
| Fig. 11 (a) Highly luminescent AuAg NCs@TPP as PL probe for in vivo PL imaging of the brain within living mice using pristine AuAg NCs as reference. Reproduced with permission from ref. 80, Copyright 2023 the Royal Society of Chemistry and the Chinese Chemical Society. (b) The establishment of unilateral ureteral obstruction (UUO) model in mice (left), and the PL images of mice kidneys before and after intravenous injection of GSH-Au NCs (right). Reproduced with permission from ref. 83, Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Schematic illustration of the cisplatin-induced AKI disease model (left), and the real-time PL imaging of mice kidneys with intravenous injection of NIR-II emitting Au21Cu1 NCs (right). Reproduced with permission from ref. 65, Copyright 2023 American Association for the Advancement of Science. | ||
In summary, luminescent metal NCs-based probes hold vast potential in organ PL imaging. Looking ahead, with continued refinement and advancement in synthesis methods, surface modification techniques, and multimodal imaging systems, it is anticipated that more effective and precise luminescent metal NCs will be engineered, offering more robust and safer diagnostic tools for clinical applications.
4. Perspective and conclusions
Ultrasmall luminescent metal NCs have strong PL, ultrasmall size, diverse and customizable surface chemistry, adjustable size and composition, large Stokes shifts, excellent biocompatibility, robust photostability, and effective renal clearance, which made them promising as luminescent probes for bioimaging. The robust PL of metal NCs is essential for creating metal NC-based PL probes. Through ongoing research, various synthetic strategies have been devised, including metal doping, ligand engineering, surface motif modulation, and structural adjustments. These approaches have significantly advanced the synthesis of highly luminescent metal NCs, providing excellent platforms for the development of PL probes.The NCs can be tailored with excellent biocompatibility in terms of minimal cytotoxicity, good water solubility, low metabolic toxicity, and negligible adverse effects on organs. They are developed with desired functionalities, such as targeting functionality, making them suitable for selective PL imaging for a variety of biospecimens such as different sub-cellular organelles, cells, tissues, and organs. The good photostable properties of metal NCs under various conditions enable them to be employed as agents for both in vitro and in vivo PL imaging.
Recent advancements of metal NCs have greatly broadened their range of potential applications. They are now being explored for use in PL imaging at multiple scales, from the minute details of subcellular organelles and cells to the larger scope of tissues and even entire organs. This scalability makes them incredibly useful across a wide spectrum of medical and scientific research areas.
Despite significant progress in the construction and preclinical application of metal NCs-based PL bioimaging probes, several obstacles must be overcome for successful clinical translation. For instance, a systematic, long-period evaluation of the toxicity of NCs should be conducted using various animal models (e.g., from mice to nonhuman primates).7 Such efforts would help to provide a clearer horizon in terms of in vivo metabolic pathways as well as comprehensive toxicological data related to metal NCs. Moreover, diverse biological interactions of metal NCs, such as with protein corona, must be thoroughly investigated to optimize their efficacy as PL bioimaging probes because of the intricacy of in vivo environments and the diversiform surface chemistry of metal NCs.
Furthermore, the PL efficiency of metal NCs remains relatively low, particularly when considering water-soluble varieties. Most of these water-soluble metal NCs show QYs that do not exceed 15%. Although the methods outlined in Section 2.1 have demonstrated their effectiveness in boosting the PL of metal NCs (e.g., tens of times PL enhancement), metal NCs’ PL lag behind quantum dots and organic dyes, which achieve over 30% QYs. Further advancements are needed for metal NCs to compete with these phosphors.
Moreover, when examining the various types of metal NCs that function as PL probes emitting light in the visible and NIR-I spectrum, it becomes evident that significant advancements are needed for NIR-II emitting metal NCs-based probes. Currently, progress in this domain is restricted to a few water-soluble metal NCs successfully developed to emit PL in the NIR-II range. This suggests that the field is still in its early stage and highlights the need for more comprehensive and targeted research efforts to explore and expand the potential applications and capabilities of these NIR-II emitting metal NCs-based probes. Furthermore, understanding the PL mechanism of metal NCs is essential for precise customization. This knowledge allows for effective tailoring of NCs to meet specific PL imaging demands, enhancing performance and adaptability. It enables significant advancements by providing precise control over imaging characteristics like wavelength, stability, and PL lifetimes, expanding applications in scientific and medical fields.
In addition, designing metal NCs-based PL probes with tailored pharmacokinetics for in vivo bioimaging applications remains a formidable challenge. It is crucial to strike a delicate balance between blood circulation half-life and renal clearance kinetics for specific in vivo bioimaging applications. In recent years, various chiral metal NCs have been documented; however, their use in bioimaging remains relatively constrained. Chiral metal NCs may display unique bio-nano interactions compared to their achiral counterparts, impacting their potential in selective PL bioimaging applications.
In summary, we have examined the tailorable functions of metal NCs for PL bioimaging applications and explored the recent advancements in metal NCs-based luminescent probes for imaging a diverse array of biological targets, ranging from subcellular organelles and individual cells to tissues and whole organs. The extensive chemistry associated with metal NCs has resulted in their intricate designs and applications as PL imaging probes. Further incorporation of metal NCs into complex systems will pave the way for clinical breakthroughs.
Data availability
All relevant data are within the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Faculty of Engineering, Universiti Malaysia Sabah for their support and assistance in this work. The authors acknowledge Prof. Coswald's group for their guidance and support in this project. The authors gratefully acknowledge the financial support from the Natural Science Foundation of Shandong Province (ZR2019YQ07, China), and that from the Ministry of Education, Singapore, Academic Research Fund (AcRF, Grant No. A-0009186-01-00 and A-8000054-01-00).References
- S. Li, N.-N. Li, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Rev., 2024, 124, 7262–7378 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- Z. Liu, Z. Wu, Q. Yao, Y. Cao, O. J. H. Chai and J. Xie, Nano Today, 2021, 36, 101053 CrossRef CAS.
- X. Wang, B. Yin, L. Jiang, C. Yang, Y. Liu, G. Zou, S. Chen and M. Zhu, Science, 2023, 381, 784–790 CrossRef CAS PubMed.
- G. Yang, Z. Wang, F. Du, F. Jiang, X. Yuan and J. Y. Ying, J. Am. Chem. Soc., 2023, 145, 11879–11898 CrossRef CAS PubMed.
- S. Qian, Z. Wang, Z. Zuo, X. Wang, Q. Wang and X. Yuan, Coord. Chem. Rev., 2022, 451, 214268 CrossRef CAS.
- L. Shang, J. Xu and G. U. Nienhaus, Nano Today, 2019, 28, 100767 CrossRef.
- X. Cai, Y. Tian, H. Wang, S. Huang, X. Liu, G. Li, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2024, e202414030 Search PubMed.
- L. Qin, F. Sun, X. Ma, G. Ma, Y. Tang, L. Wang, Q. Tang, R. Jin and Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 26136–26141 CrossRef CAS PubMed.
- K. Kwak, W. Choi, Q. Tang, M. Kim, Y. Lee, D.-E. Jiang and D. Lee, Nat. Commun., 2017, 8, 14723 CrossRef PubMed.
- V. K. Kulkarni, B. N. Khiarak, S. Takano, S. Malola, E. L. Albright, T. I. Levchenko, M. D. Aloisio, C.-T. Dinh, T. Tsukuda, H. Häkkinen and C. M. Crudden, J. Am. Chem. Soc., 2022, 144, 9000–9006 CrossRef CAS PubMed.
- Q. You, H. Wang, Y. Zhao, W. Fan, W. Gu, H.-L. Jiang and Z. Wu, J. Am. Chem. Soc., 2024, 146, 9026–9035 CrossRef CAS PubMed.
- Q.-Y. Wang, Z.-B. Sun, M. Zhang, S.-N. Zhao, P. Luo, C.-H. Gong, W.-X. Liu and S.-Q. Zang, J. Am. Chem. Soc., 2022, 144, 21046–21055 CrossRef CAS PubMed.
- S. Biswas, A. Pal, M. K. Jena, S. Hossain, J. Sakai, S. Das, B. Sahoo, B. Pathak and Y. Negishi, J. Am. Chem. Soc., 2024, 146, 20937–20944 CrossRef CAS PubMed.
- Z. Liu, J. Chen, B. Li, D.-E. Jiang, L. Wang, Q. Yao and J. Xie, J. Am. Chem. Soc., 2024, 146, 11773–11781 CrossRef CAS PubMed.
- Y. Lin, Y. Cao, Q. Yao, O. J. H. Chai and J. Xie, Ind. Eng. Chem. Res., 2020, 59, 20561–20581 CrossRef CAS.
- F. Q. Liu, Y. B. Liu, A. F. Yao, Y. X. Wang, X. F. Fang, C. S. Shen, F. Li, M. H. Huang, Z. W. Wang, W. G. Sand and J. P. Xie, Environ. Sci. Technol., 2020, 54, 5913–5921 CrossRef CAS PubMed.
- H. Zhu, X. Yuan, Q. Yao and J. Xie, Nano Energy, 2021, 88, 106306 CrossRef CAS.
- X. Wang, L. Zhao, X. Li, Y. Liu, Y. Wang, Q. Yao, J. Xie, Q. Xue, Z. Yan, X. Yuan and W. Xing, Nat. Commun., 2022, 13, 1596 CrossRef CAS PubMed.
- X. Wang, Y. Tong, W. Feng, P. Liu, X. Li, Y. Cui, T. Cai, L. Zhao, Q. Xue, Z. Yan, X. Yuan and W. Xing, Nat. Commun., 2023, 14, 3767 CrossRef PubMed.
- X. Jiang, B. Du, Y. Huang and J. Zheng, Nano Today, 2018, 21, 106–125 CrossRef CAS PubMed.
- K. Zheng and J. Xie, Trends Chem., 2020, 2, 665–679 CrossRef CAS.
- K. Zheng and J. Xie, Acc. Mater. Res., 2021, 2, 1104–1116 CrossRef CAS.
- Z. Lin, N. Goswami, T. Xue, O. J. H. Chai, H. Xu, Y. Liu, Y. Su and J. Xie, Adv. Funct. Mater., 2021, 31, 2105662 CrossRef CAS.
- Z. Pang, N. Ren, Y. Wu, J. Qi, F. Hu, Y. Guo, Y. Xie, D. Zhou and X. Jiang, Adv. Mater., 2023, 35, 2303562 CrossRef CAS PubMed.
- S. Li, J. Wei, Q. Yao, X. Song, J. Xie and H. Yang, Chem. Soc. Rev., 2023, 52, 1672–1696 RSC.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef CAS.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS PubMed.
- Q. Yao, X. Yuan, T. Chen, D. T. Leong and J. Xie, Adv. Mater., 2018, 30, 1802751 CrossRef PubMed.
- Q. He, Z. Jiang, H. Jiang, S. Han, G. Yang, X. Yuan and H. Zhu, Nanoscale, 2024, 16, 14310–14318 RSC.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- M. I. Lucío, M.-E. Kyriazi, J. Hamilton, D. Batista, A. Sheppard, E. Sams-Dodd, M. V. Humbert, I. Hussain, M. Christodoulides and A. G. Kanaras, ACS Appl. Mater. Interfaces, 2020, 12, 27994–28003 CrossRef PubMed.
- X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS PubMed.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed.
- S. Zhou, Q. Zhong, Y. Wang, P. Hu, W. Zhong, C.-B. Huang, Z.-Q. Yu, C.-D. Ding, H. Liu and J. Fu, Coord. Chem. Rev., 2022, 452, 214309 CrossRef CAS.
- H. Ding, T. Xiao, F. Ren, Y. Qiu, Z. Shen, X. Chen, E. Mijowska and H. Chen, BMEMat, 2024, 2, e12085 CrossRef.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- P. Cheng and K. Pu, Nat. Rev. Mater., 2021, 6, 1095–1113 CrossRef CAS.
- T. Jia, Z.-J. Guan, C. Zhang, X.-Z. Zhu, Y.-X. Chen, Q. Zhang, Y. Yang and D. Sun, J. Am. Chem. Soc., 2023, 145, 10355–10363 CrossRef CAS PubMed.
- S.-S. Zhang, S. Havenridge, C. Zhang, Z. Wang, L. Feng, Z.-Y. Gao, C. M. Aikens, C.-H. Tung and D. Sun, J. Am. Chem. Soc., 2022, 144, 18305–18314 CrossRef CAS PubMed.
- X. Kang and M. Z. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- W. Zhong, K. Liang, W. Liu and L. Shang, Chem. Sci., 2023, 14, 8823–8830 RSC.
- W.-Q. Shi, L. Zeng, R.-L. He, X.-S. Han, Z.-J. Guan, M. Zhou and Q.-M. Wang, Science, 2024, 383, 326–330 CrossRef CAS PubMed.
- J.-H. Huang, Y.-J. Liu, Y. Si, Y. Cui, X.-Y. Dong and S.-Q. Zang, J. Am. Chem. Soc., 2024, 146, 16729–16736 CrossRef CAS PubMed.
- X. Yuan, L. L. Chng, J. Yang and J. Y. Ying, Adv. Mater., 2020, 32, 1906063 CrossRef CAS PubMed.
- Z. Wang, X. Pan, S. Qian, G. Yang, F. Du and X. Yuan, Coord. Chem. Rev., 2021, 438, 213900 CrossRef CAS.
- X. Yuan, B. Zhang, Z. Luo, Q. Yao, D. T. Leong, N. Yan and J. Xie, Angew. Chem., Int. Ed., 2014, 53, 4623–4627 CrossRef CAS PubMed.
- X. Yuan, N. Goswami, I. Mathews, Y. Yu and J. Xie, Nano Res., 2015, 8, 3488–3495 CrossRef CAS.
- X. Yuan, N. Goswami, W. Chen, Q. Yao and J. Xie, Chem. Commun., 2016, 52, 5234–5237 RSC.
- Q. Yao, L. Liu, S. Malola, M. Ge, H. Xu, Z. Wu, T. Chen, Y. Cao, M. F. Matus, A. Pihlajamäki, Y. Han, H. Häkkinen and J. Xie, Nat. Chem., 2023, 15, 230–239 CrossRef CAS PubMed.
- X. Zou, X. Kang and M. Zhu, Chem. Soc. Rev., 2023, 52, 5892–5967 RSC.
- Y. Zhou, L. Liao, S. Zhuang, Y. Zhao, Z. Gan, W. Gu, J. Li, H. Deng, N. Xia and Z. Wu, Angew. Chem., Int. Ed., 2021, 60, 8668–8672 CrossRef CAS PubMed.
- S. Qian, F. Liu, H. Zhu, Y. Liu, T. Feng, X. Dou and X. Yuan, Polyoxometalates, 2023, 2, 9140049 CrossRef.
- N. Goswami, Q. Yao, Z. Luo, J. Li, T. Chen and J. Xie, J. Phys. Chem. Lett., 2016, 7, 962–975 CrossRef CAS PubMed.
- T. Chen, H. Lin, Y. Cao, Q. Yao and J. Xie, Adv. Mater., 2022, 34, 2103918 CrossRef CAS PubMed.
- H. Lin, X. Song, O. J. H. Chai, Q. Yao, H. Yang and J. Xie, Adv. Mater., 2024, 36, 2401002 CrossRef CAS PubMed.
- J. Xu, C. Peng, M. Yu and J. Zheng, Wiley Interdiscip. Rev.:Nanomed. Nanobiotechnol., 2017, 9, e1453 Search PubMed.
- B. Du, M. Yu and J. Zheng, Nat. Rev. Mater., 2018, 3, 358–374 CrossRef.
- Y. Cao, T. Chen, Q. Yao and J. Xie, Acc. Chem. Res., 2021, 54, 4142–4153 CrossRef CAS PubMed.
- Q. Yao, Z. Wu, Z. Liu, Y. Lin, X. Yuan and J. Xie, Chem. Sci., 2021, 12, 99–127 RSC.
- Y. G. Srinivasulu, N. Goswami, Q. Yao and J. Xie, J. Phys. Chem. C, 2021, 125, 4066–4076 CrossRef CAS.
- J. Ding, M. Zhao, Y. Li, K. Zhang, H. Chen, X. Hu, L. Li, Y. Su, X. Yuan and Z. Lin, Chem. Eng. J., 2024, 496, 153726 CrossRef CAS.
- H. Ma, X. Zhang, L. Liu, Y. Huang, S. Sun, K. Chen, Q. Xin, P. Liu, Y. Yan, Y. Wang, Y. Li, H. Liu, R. Zhao, K. Tan, X. Chen, X. Yuan, Y. Li, Y. Liu, H. Dai, C. Liu, H. Wang and X.-D. Zhang, Sci. Adv., 2023, 9, eadh7828 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- H. Yu, B. Rao, W. Jiang, S. Yang and M. Zhu, Coord. Chem. Rev., 2019, 378, 595–617 CrossRef CAS.
- X. Cai, G. Saranya, K. Shen, M. Chen, R. Si, W. Ding and Y. Zhu, Angew. Chem., Int. Ed., 2019, 58, 9964–9968 CrossRef CAS PubMed.
- H. Xiang, H. Yan, J. Liu, R. Cheng, C.-Q. Xu, J. Li and C. Yao, J. Am. Chem. Soc., 2022, 144, 14248–14257 CrossRef CAS PubMed.
- X. Yuan, X. Dou, K. Zheng and J. Xie, Part. Part. Syst. Charact., 2015, 32, 613–629 CrossRef.
- S. Wang, Q. Li, X. Kang and M. Zhu, Acc. Chem. Res., 2018, 51, 2784–2792 CrossRef CAS PubMed.
- Q. Yao, Y. Feng, V. Fung, Y. Yu, D.-E. Jiang, J. Yang and J. Xie, Nat. Commun., 2017, 8, 1555 CrossRef PubMed.
- Y. Zhang, W. Zhang, T.-S. Zhang, C. Ge, Y. Tao, W. Fei, W. Fan, M. Zhou and M.-B. Li, J. Am. Chem. Soc., 2024, 146, 9631–9639 CrossRef CAS PubMed.
- F. Xiao, Y. Chen, J. Qi, Q. Yao, J. Xie and X. Jiang, Adv. Mater., 2023, 35, 2210412 CrossRef CAS PubMed.
- G. Yang, X. Mu, X. Pan, Y. Tang, Q.-F. Yao, Y. Wang, F. Jiang, F. Du, J. Xie, X. Zhou and X. Yuan, Chem. Sci., 2023, 14, 4308–4318 RSC.
- G. Yang, X. Pan, W. Feng, Q. Yao, F. Jiang, F. Du, X. Zhou, J. Xie and X. Yuan, ACS Nano, 2023, 17, 15605–15614 CrossRef CAS PubMed.
- G. Yang, K. Liu, Y. Wang, X. Pan, J. Ye, Y. Li, F. Du, T. Feng and X. Yuan, Aggregate, 2024, 5, e435 CrossRef CAS.
- Y. Shi, Z. Wu, M. Qi, C. Liu, W. Dong, W. Sun, X. Wang, F. Jiang, Y. Zhong, D. Nan, Y. Zhang, C. Li, L. Wang and X. Bai, Adv. Mater., 2024, 36, 2310529 CrossRef CAS PubMed.
- D. Sang, X. Luo and J. Liu, Nano-Micro Lett., 2023, 16, 44 CrossRef PubMed.
- X. Pan, Z. Zuo, Z. Wang, G. Yang, H. Zhu, Y. Li and X. Yuan, Mater. Chem. Front., 2023, 7, 1146–1152 RSC.
- M. Yu, C. Zhou, J. Liu, J. D. Hankins and J. Zheng, J. Am. Chem. Soc., 2011, 133, 11014–11017 CrossRef CAS PubMed.
- X. Song, W. Zhu, X. Ge, R. Li, S. Li, X. Chen, J. Song, J. Xie, X. Chen and H. Yang, Angew. Chem., Int. Ed., 2021, 60, 1306–1312 CrossRef CAS PubMed.
- M. Yu, J. Zhou, B. Du, X. Ning, C. Authement, L. Gandee, P. Kapur, J.-T. Hsieh and J. Zheng, Angew. Chem., Int. Ed., 2016, 55, 2787–2791 CrossRef CAS PubMed.
- J. Zhai, Y. Jia, L. Zhao, Q. Yuan, F. Gao, X. Zhang, P. Cai, L. Gao, J. Guo, S. Yi, Z. Chai, Y. Zhao and X. Gao, ACS Nano, 2018, 12, 4378–4386 CrossRef CAS PubMed.
- X.-D. Zhang, J. Chen, Z. Luo, D. Wu, X. Shen, S.-S. Song, Y.-M. Sun, P.-X. Liu, J. Zhao, S. Huo, S. Fan, F. Fan, X.-J. Liang and J. Xie, Adv. Healthcare Mater., 2014, 3, 133–141 CrossRef CAS PubMed.
- S. Zha, H. Liu, H. Li, H. Li, K.-L. Wong and A. H. All, ACS Nano, 2024, 18, 1820–1845 CrossRef CAS PubMed.
- W. Zhang, G. Gao, Z. Ma, Z. Luo, M. He and T. Sun, Natl. Sci. Rev., 2020, 7, 763–774 CrossRef CAS PubMed.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Acc. Chem. Res., 2018, 51, 3114–3124 CrossRef CAS PubMed.
- Z. Gan, N. Xia and Z. Wu, Acc. Chem. Res., 2018, 51, 2774–2783 CrossRef CAS PubMed.
- C. Yao, J. Chen, M.-B. Li, L. Liu, J. Yang and Z. Wu, Nano Lett., 2015, 15, 1281–1287 CrossRef CAS PubMed.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Jin, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- X.-Y. Xie, P. Xiao, X. Cao, W.-H. Fang, G. Cui and M. Dolg, Angew. Chem., Int. Ed., 2018, 57, 9965–9969 CrossRef CAS PubMed.
- E. Oh, J. B. Delehanty, L. D. Field, A. J. Mäkinen, R. Goswami, A. L. Huston and I. L. Medintz, Chem. Mater., 2016, 28, 8676–8688 CrossRef CAS.
- K. Zheng, V. Fung, X. Yuan, D.-E. Jiang and J. Xie, J. Am. Chem. Soc., 2019, 141, 18977–18983 CrossRef CAS PubMed.
- Y. Negishi, K. Igarashi, K. Munakata, W. Ohgake and K. Nobusada, Chem. Commun., 2012, 48, 660–662 RSC.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880–11883 CrossRef CAS PubMed.
- Z. Wang, Z. Zhu, C. Zhao, Q. Yao, X. Li, H. Liu, F. Du, X. Yuan and J. Xie, Chem. – Asian J., 2019, 14, 765–769 CrossRef CAS PubMed.
- Z. Gan, Y. Lin, L. Luo, G. Han, W. Liu, Z. Liu, C. Yao, L. Weng, L. Liao, J. Chen, X. Liu, Y. Luo, C. Wang, S. Wei and Z. Wu, Angew. Chem., Int. Ed., 2016, 55, 11567–11571 CrossRef CAS PubMed.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed.
- J. Xu, J. Li, W. Zhong, M. Wen, G. Sukhorukov and L. Shang, Chin. Chem. Lett., 2021, 32, 2390–2394 CrossRef CAS.
- K. Pyo, V. D. Thanthirige, S. Y. Yoon, G. Ramakrishna and D. Lee, Nanoscale, 2016, 8, 20008–20016 RSC.
- L. Zhu, Y. Zeng, M. Teubner, B. Grimm-Lebsanft, A. R. Ziefuß, C. Rehbock, M. A. Rübhausen, S. Barcikowski, W. J. Parak and I. Chakraborty, ACS Appl. Nano Mater., 2021, 4, 3197–3203 CrossRef CAS.
- X. Dou, S. Saalah, C.-K. Chiam, J. Xie and C. S. Sipaut, Nanoscale, 2024, 16, 20089–20099 RSC.
- Y. Song, J. Zhong, S. Yang, S. Wang, T. Cao, J. Zhang, P. Li, D. Hu, Y. Pei and M. Zhu, Nanoscale, 2014, 6, 13977–13985 RSC.
- S. Wang, X. Zhu, T. Cao and M. Zhu, Nanoscale, 2014, 6, 5777–5781 RSC.
- E. S. Shibu, M. A. H. Muhammed, T. Tsukuda and T. Pradeep, J. Phys. Chem. C, 2008, 112, 12168–12176 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen, Y. Chen, Z. Chen, D. Dang, D. Ding, S. Ding, Y. Duo, M. Gao, W. He, X. He, X. Hong, Y. Hong, J.-J. Hu, R. Hu, X. Huang, T. D. James, X. Jiang, G.-I. Konishi, R. T. K. Kwok, J. W. Y. Lam, C. Li, H. Li, K. Li, N. Li, W.-J. Li, Y. Li, X.-J. Liang, Y. Liang, B. Liu, G. Liu, X. Liu, X. Lou, X.-Y. Lou, L. Luo, P. R. McGonigal, Z.-W. Mao, G. Niu, T. C. Owyong, A. Pucci, J. Qian, A. Qin, Z. Qiu, A. L. Rogach, B. Situ, K. Tanaka, Y. Tang, B. Wang, D. Wang, J. Wang, W. Wang, W.-X. Wang, W.-J. Wang, X. Wang, Y.-F. Wang, S. Wu, Y. Wu, Y. Xiong, R. Xu, C. Yan, S. Yan, H.-B. Yang, L.-L. Yang, M. Yang, Y.-W. Yang, J. Yoon, S.-Q. Zang, J. Zhang, P. Zhang, T. Zhang, X. Zhang, X. Zhang, N. Zhao, Z. Zhao, J. Zheng, L. Zheng, Z. Zheng, M.-Q. Zhu, W.-H. Zhu, H. Zou and B. Z. Tang, ACS Nano, 2023, 17, 14347–14405 CrossRef CAS PubMed.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
- X. Dou, X. Yuan, Y. Yu, Z. Luo, Q. Yao, D. T. Leong and J. Xie, Nanoscale, 2014, 6, 157–161 RSC.
- Y. Yu, Z. Luo, D. M. Chevrier, D. T. Leong, P. Zhang, D.-E. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 1246–1249 CrossRef CAS PubMed.
- X. Kang, S. Wang and M. Zhu, Chem. Sci., 2018, 9, 3062–3068 RSC.
- Y. Liu, D. Yao and H. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 12071–12080 CrossRef CAS PubMed.
- X.-H. Wu, P. Luo, Z. Wei, Y.-Y. Li, R.-W. Huang, X.-Y. Dong, K. Li, S.-Q. Zang and B. Z. Tang, Adv. Sci., 2019, 6, 1801304 CrossRef PubMed.
- Z. Wu, Q. Yao, O. J. H. Chai, N. Ding, W. Xu, S. Zang and J. Xie, Angew. Chem., Int. Ed., 2020, 59, 9934–9939 CrossRef CAS PubMed.
- D. Bera and N. Goswami, J. Phys. Chem. Lett., 2021, 12, 9033–9046 CrossRef CAS PubMed.
- Y.-E. Shi, J. Ma, A. Feng, Z. Wang and A. L. Rogach, Aggregate, 2021, 2, e112 CrossRef CAS.
- J. Liu, P. N. Duchesne, M. Yu, X. Jiang, X. Ning, R. D. Vinluan, P. Zhang and J. Zheng, Angew. Chem., Int. Ed., 2016, 55, 8894–8898 CrossRef CAS PubMed.
- Y. Wang, Z. Zuo, Z. Wang, Y. Wu, J. Linghu, Y. Liu, H. Zhu, X. Dou, T. Feng and X. Yuan, Chem. Eng. J., 2024, 492, 152216 CrossRef CAS.
- D. Bera, M. Baruah, A. K. Dehury, A. Samanta, Y. S. Chaudhary and N. Goswami, J. Phys. Chem. Lett., 2022, 13, 9411–9421 CrossRef CAS PubMed.
- Z. Wu, Q. Yao, S.-Q. Zang and J. Xie, Natl. Sci. Rev., 2020, 8, nwaa208 CrossRef PubMed.
- Y. Zhong, J. Zhang, T. Li, W. Xu, Q. Yao, M. Lu, X. Bai, Z. Wu, J. Xie and Y. Zhang, Nat. Commun., 2023, 14, 658 CrossRef CAS PubMed.
- Y. Zhong, X. Wang, T. Li, Q. Yao, W. Dong, M. Lu, X. Bai, Z. Wu, J. Xie and Y. Zhang, Nano Lett., 2024, 24, 6997–7003 CrossRef CAS PubMed.
- Z. Yin, Z. Wang, X. Dai, N. Liu, S. Wang, G. Li, F. Du and X. Yuan, ACS Sustainable Chem. Eng., 2020, 8, 15336–15343 CrossRef CAS.
- X. Dou, X. Chen, H. Zhu, Y. Liu, D. Chen, X. Yuan, Q. Yao and J. Xie, Dalton Trans., 2019, 48, 10385–10392 RSC.
- X. Piao, H. Yin, S. Guo, H. Wang and P. Guo, Adv. Sci., 2019, 6, 1900951 CrossRef CAS PubMed.
- T. Meng, J. Zheng, C. S. Shin, N. Gao, D. Bande, H. Sudarjat, W. Chow, M. S. Halquist, F.-S. Yu, G. Acharya and Q. Xu, ACS Nano, 2024, 18, 20679–20693 CrossRef CAS PubMed.
- M. Yu, J. Xu and J. Zheng, Angew. Chem., Int. Ed., 2019, 58, 4112–4128 CrossRef CAS PubMed.
- C. Peng, Y. Huang and J. Zheng, J. Controlled Release, 2020, 322, 64–80 CrossRef CAS PubMed.
- S. Tang, Y. Huang and J. Zheng, Angew. Chem., Int. Ed., 2020, 59, 19894–19898 CrossRef CAS PubMed.
- B. Du, X. Jiang, A. Das, Q. Zhou, M. Yu, R. Jin and J. Zheng, Nat. Nanotechnol., 2017, 12, 1096–1102 CrossRef CAS PubMed.
- J. Liu, M. Yu, X. Ning, C. Zhou, S. Yang and J. Zheng, Angew. Chem., Int. Ed., 2013, 52, 12572–12576 CrossRef CAS PubMed.
- C. Zhou, M. Long, Y. Qin, X. Sun and J. Zheng, Angew. Chem., Int. Ed., 2011, 50, 3168–3172 CrossRef CAS PubMed.
- S. Tang, C. Peng, J. Xu, B. Du, Q. Wang, R. D. Vinluan III, M. Yu, M. J. Kim and J. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16039–16043 CrossRef CAS PubMed.
- X. Jiang, B. Du and J. Zheng, Nat. Nanotechnol., 2019, 14, 874–882 CrossRef CAS PubMed.
- W. Poon, B. R. Kingston, B. Ouyang, W. Ngo and W. C. W. Chan, Nat. Nanotechnol., 2020, 15, 819–829 CrossRef CAS PubMed.
- K. J. Malawska, S. Takano, K. Oisaki, H. Yanagisawa, M. Kikkawa, T. Tsukuda and M. Kanai, Bioconjugate Chem., 2023, 34, 781–788 CAS.
- B. Dhanasekaran, M. Chandran, G. Chellasamy, M. Veerapandian, S. Govindaraju and K. Yun, ACS Appl. Bio Mater., 2024, 7, 6065–6077 CrossRef CAS PubMed.
- Y. Zhang, Z. Yang, L. Song, Y. Li and Q. Lin, J. Colloid Interface Sci., 2024, 659, 1003–1014 CrossRef CAS PubMed.
- X.-D. Zhang, Z. Luo, J. Chen, X. Shen, S. Song, Y. Sun, S. Fan, F. Fan, D. T. Leong and J. Xie, Adv. Mater., 2014, 26, 4565–4568 CrossRef CAS PubMed.
- J. Liu, M. Yu, C. Zhou, S. Yang, X. Ning and J. Zheng, J. Am. Chem. Soc., 2013, 135, 4978–4981 CrossRef CAS PubMed.
- F. Gao, P. Cai, W. Yang, J. Xue, L. Gao, R. Liu, Y. Wang, Y. Zhao, X. He, L. Zhao, G. Huang, F. Wu, Y. Zhao, Z. Chai and X. Gao, ACS Nano, 2015, 9, 4976–4986 CrossRef CAS PubMed.
- F. Liu, T. Yang, X. Chang, L. Chen, C. Cheng, X. Peng, H. Liu, Y. Zhang and X. Chen, Natl. Sci. Rev., 2024, 11, nwae113 CrossRef PubMed.
- T. T. Jia, G. Yang, S. J. Mo, Z. Y. Wang, B. J. Li, W. Ma, Y. X. Guo, X. Y. Chen, X. L. Zhao, J. Q. Liu and S. Q. Zang, ACS Nano, 2019, 13, 8320–8328 CrossRef CAS PubMed.
- X.-D. Zhang, J. Chen, J. Yang, J.-Y. Wang, X. Shen, S.-S. Song, H. Wang, H. He, X. Wang, S. Fan, Y.-M. Sun and M. Guo, J. Mater. Chem. B, 2015, 3, 4735–4741 RSC.
- C. Peng, X. Gao, J. Xu, B. Du, X. Ning, S. Tang, R. M. Bachoo, M. Yu, W.-P. Ge and J. Zheng, Nano Res., 2017, 10, 1366–1376 CrossRef CAS PubMed.
- S. Ding, A. I. Khan, X. Cai, Y. Song, Z. Lyu, D. Du, P. Dutta and Y. Lin, Mater. Today, 2020, 37, 112–125 CrossRef CAS PubMed.
- H. Santhakumar, R. V. Nair, D. M. Govindachar, G. Periyasamy and R. S. Jayasree, ACS Sustainable Chem. Eng., 2023, 11, 2102–2114 CrossRef CAS.
- T. D. Pollard, W. C. Earnshaw, J. Lippincott-Schwartz and G. T. Johnson, Cell Biology, Elsevier, 3rd edn, 2016 Search PubMed.
- F. R. Palma, B. N. Gantner, M. J. Sakiyama, C. Kayzuka, S. Shukla, R. Lacchini, B. Cunniff and M. G. Bonini, Oncogene, 2024, 43, 295–303 CrossRef CAS PubMed.
- S. Xu, K.-C. Yan, Z.-H. Xu, Y. Wang and T. D. James, Chem. Soc. Rev., 2024, 53, 7590–7631 RSC.
- C. Settembre and R. M. Perera, Nat. Rev. Mol. Cell Biol., 2024, 25, 223–245 CrossRef CAS PubMed.
- L. Shang, R. M. Dorlich, S. Brandholt, R. Schneider, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Nanoscale, 2011, 3, 2009–2014 RSC.
- Y. Wang, J.-T. Chen and X.-P. Yan, Anal. Chem., 2013, 85, 2529–2535 CrossRef CAS PubMed.
- A. M. Chahande, D. Lathigara, A. A. Prabhune and R. N. Devi, Arch. Microbiol., 2021, 203, 4293–4301 CrossRef CAS PubMed.
- X. Ouyang, N. Jia, J. Luo, L. Li, J. Xue, H. Bu, G. Xie and Y. Wan, JACS Au, 2023, 3, 2566–2577 CrossRef CAS PubMed.
- Y. Chen, M. L. Phipps, J. H. Werner, S. Chakraborty and J. S. Martinez, Acc. Chem. Res., 2018, 51, 2756–2763 Search PubMed.
- M. Madhu, W.-B. Tseng, Y.-S. Chou, A. S. Krishna Kumar, C.-Y. Lu, P.-L. Chang and W.-L. Tseng, Anal. Chem., 2024, 96, 9007–9015 CrossRef CAS PubMed.
- H.-Q. Yin, F.-L. Bi and F. Gan, Bioconjugate Chem., 2015, 26, 243–249 CrossRef CAS PubMed.
- D. Pranantyo, P. Liu, W. Zhong, E.-T. Kang and M. B. Chan-Park, Biomacromolecules, 2019, 20, 2922–2933 CrossRef CAS PubMed.
- Y. Zheng, W. Liu, Y. Chen, C. Li, H. Jiang and X. Wang, J. Colloid Interface Sci., 2019, 546, 1–10 Search PubMed.
- S. Zhu, X. Wang, S. Li, L. Liu and L. Li, ACS Appl. Mater. Interfaces, 2020, 12, 11063–11071 Search PubMed.
- Y.-B. Su, X. Zhao, L.-J. Chen, H.-L. Qian and X.-P. Yan, Talanta, 2021, 233, 122567 CrossRef CAS PubMed.
- B. Feng, Y. Xing, J. Lan, Z. Su and F. Wang, Talanta, 2020, 212, 120796 CrossRef CAS PubMed.
- D. Lyu, J. Li, X. Wang, W. Guo and E. Wang, Anal. Chem., 2019, 91, 2050–2057 CrossRef CAS PubMed.
- H. Zhao, H. Xiao, Y. Liu and H. Ju, BMEMat, 2023, 1, e12032 CrossRef.
- W. Shao, F. Zhao, J. Xue and L. Huang, BMEMat, 2023, 1, e12009 CrossRef.
- H. Yin, W. Jiang, Y. Liu, D. Zhang, F. Wu, Y. Zhang, C. Li, G. Chen and Q. Wang, BMEMat, 2023, 1, e12023 CrossRef.
- C. Colombé, X. Le Guével, A. Martin-Serrano, M. Henry, E. Porret, C. Comby-Zerbino, R. Antoine, I. Atallah, B. Busser, J.-L. Coll, C. A. Righini and L. Sancey, Nanomed. Nanotechnol., 2019, 20, 102011 CrossRef PubMed.
- W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Angew. Chem., Int. Ed., 2020, 59, 22431–22435 CrossRef CAS PubMed.
- D. Li, Q. Liu, Q. Qi, H. Shi, E.-C. Hsu, W. Chen, W. Yuan, Y. Wu, S. Lin, Y. Zeng, Z. Xiao, L. Xu, Y. Zhang, T. Stoyanova, W. Jia and Z. Cheng, Small, 2020, 16, 2003851 CrossRef CAS PubMed.
- J. Xu, M. Yu, P. Carter, E. Hernandez, A. Dang, P. Kapur, J.-T. Hsieh and J. Zheng, Angew. Chem., Int. Ed., 2017, 56, 13356–13360 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
