A porphyrin metal–organic framework enhances photodynamic therapy through lymphatic circulation†
Qiuhong
Ouyang‡
a,
Xin
Li‡
a,
Yuechen
He
a,
Yuxin
Bai
a,
Xunhuan
Song
a,
Xinglv
Chen
a,
Yuzhou
Xiao
a,
Lili
Mao
b,
Min
Chen
a,
Xiaodan
Pan
a,
Weihong
Kuang
a,
Feng
Qin
*a,
Meng
Qin
 *a and
Xiaoai
Wu
*a
*a and
Xiaoai
Wu
*a
aDepartment of Nuclear Medicine, Mental Health Center and Center for Preclinical Safety Evaluation of Drugs, State Key Laboratory of Biotherapy/Collaborative Innovation Center for Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China. E-mail: qinfeng@scu.edu.cn; qinmeng212@scu.edu.cn; xiaoai.wu@scu.edu.cn
bKey laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Melanoma and Sarcoma, Peking University Cancer Hospital and Institute, Beijing 100142, China
First published on 16th June 2025
Abstract
Photodynamic therapy (PDT) has emerged as a promising strategy for cancer treatment as a local and precise therapeutic technique. However, lymph node metastasis of tumors poses a therapeutic challenge. To increase PDT's in vivo cancer effectiveness, direct targeting of lymph nodes (LNs) is the most promising approach. Herein, based on the targeting of hyaluronic acid (HA) to lymphatic endothelial cells, surface modification of porphyrin metal–organic frameworks (MOFs) was conducted to improve the therapeutic efficacy of PDT on lymphatic metastatic tumors. In this system, the prepared HA-MOFs produced equivalent reactive oxygen species (ROS) under the same light exposure as in the case of MOFs, producing consistent in vitro toxicity to tumor cells. After subcutaneous injection in mice, HA-MOFs preferentially accumulated in nearby inguinal LNs and circulated to the tumor site via the lymphatic system. In vivo experiments demonstrated that HA-MOFs circulated to the tumor site through the lymph, generating a more effective PDT therapeutic effect and significantly prolonging the median survival of hormonal mice. In conclusion, studies have demonstrated that HA-MOFs act as a powerful therapeutic platform to deliver drugs to LNs through lymphatic circulation to enhance tumor therapy.
1. Introduction
Cancer remains a major cause of death worldwide, accounting for an estimated 10 million fatalities in 2020.1 Cancer may occur from almost any organ or tissue in the body, invade neighbouring parts of the body and/or spread to other organs, and metastasis is the main cause of cancer-related deaths.2–4 First, the tumor cells invade nearby normal tissues and move along the walls of lymph nodes (LNs) or blood vessels to travel to other body parts through the lymphatic system or bloodstream. Then, staying in small distant blood vessels that invade the vessel wall, they enter the surrounding tissue and continue to grow to form small tumors.5,6 In this process, LN metastasis represents a critical stage of tumor dissemination. For numerous types of both mouse and human tumors, LNs serve as the primary pathway for distant metastasis.7 Abundant studies have shown that the lymphatic system is more likely to provide metastatic channels for tumor cells than the blood system due to the special structure of lymphatic vessels, slow flow of lymphatic fluid, serum-free lymphatic environment, and chemokine receptors.8,9 The lymphatic system acts as a major route for the dissemination of various solid tumors, such as melanoma, lung, and breast cancers, complicating treatment and subsequently decreasing patient survival rates.10–13 Therefore, the lymphatic system has become one of the research priorities in the field of tumor therapy.Despite the promising treatment, conventional chemotherapeutic agents failed to enrich the lymphatic system, with low concentrations in LNs and unsatisfactory therapeutic effects. Compared to oral and intravenous administration, interstitial drug delivery as a local mode of administration includes intramuscular, subcutaneous, and intradermal administration, with more drug enrichment in the interstitium of tissues after drug administration.14,15 Due to properties such as large gaps between lymphatic endothelial cells (LECs) and lack of a basement membrane, agents are allowed to enter the lymphatic vessels more readily.16,17 After interstitial administration the drug is more likely to penetrate the lymphatic circulatory system to reach the tumor site and accumulate, facilitating the achievement of effective therapeutic concentrations. Relevant drug carriers have been developed as lymphatic-targeted drug delivery systems to assist drug entry into lymphatic vessels and enrichment of LNs, increasing drug concentration and efficacy in the lymphatic system.18–21 Developing an efficient lymphatic circulation platform is critical for targeted LN, effective tumor enrichment, and stable elimination. In recent years, the natural polysaccharide hyaluronic acid (HA) has emerged as a medical biomaterial in multiple fields due to its biocompatibility and functionality.22–26
HA has been found to interact with the CD44 receptor, which is widely overexpressed on the surface of many malignant tumor cells. Consequently, HA-based materials have been the focus of extensive research as innovative drug carriers for targeted cancer therapies.27–29 Lymphatic endothelial hyaluronan receptor-1 (LYVE-1), a structural homologue of CD44, exhibits 43% sequence similarity and is expressed by LECs. LYVE-1 acts as a specific receptor for HA and can bind to both its soluble and immobilized forms.30–32 Unlike CD44, which is broadly expressed, LYVE-1 is specifically localized on the luminal surface of lymphatic vessels, where it coexists with HA, and is entirely absent in blood vessels.33,34 Some studies have used pure HA molecules as targeting substrates for the lymphatic system, showing a high degree of targeting to the lymphatic system in vitro and in vivo.35 In addition, it is very convenient to attach imaging or therapeutic molecules to HA due to the presence of sufficient reactive groups, such as the carboxylic acid or the hydroxyl group on the HA main chain.36 Thus, HA with lymphatic targeting and modifiable properties has great potential as a lymphatic targeting carrier in cancer therapy. More importantly, the interaction of HA with the LYVE-1 receptor promotes the targeting of tumor sites by carrying drug molecules into the lymphatic circulatory system. Delivery of the drug via subcutaneous administration is more favourable for the particles to enter the lymphatic vessels, thus reducing the drug dose to improve safety.
PDT has been extensively studied as a non-invasive approach for cancer treatment.37,38 It mainly involves irradiation of photosensitizers at the cancerous site with specific wavelengths of light, which induces the generation of reactive oxygen species (ROS) that cause cancerous cells to die, with low invasiveness and high specificity.39,40 However, for photosensitizers with phototoxicity, there needs to be selective accumulation at the tumor site to reduce damage to the surrounding normal tissue. Compared to other administration modalities, subcutaneous injection has a natural physiological advantage in enhancing drug entry into the lymphatic system. Based on the differences between capillary lymphatic vessels and capillaries in the arrangement of cellular gaps in the walls of the vessels, granular substances with a particle size of 20–150 nm are efficiently absorbed by the lymphatic system after local injection.41 Although local injections effectively improve lymphatic system accumulation, efficacy against tumor cells metastasizing to the lymphatic system remains limited. Therefore, the development of therapeutic agents that specifically target the lymphatic system by subcutaneous injection, combined with the PDT function of photosensitizers, is a promising approach to overcome this challenge.
On the basis of recent studies, the HA molecule not only specifically anchors the CD44 receptor on the tumor surface, but also can be recognized by the LYVE-1 receptor, which is abundantly distributed in LECs. Thus, HA may not only act as a targeting molecule for tumor cells, but also as a key ligand in the lymphatic system-tumor delivery pathway. By exploiting this, in this work, we designed a universal multifunctional PDT platform based on HA with tetra (4-carboxyphenyl)porphine (TCPP) as the photosensitizer, ZrOCl2·8H2O as the metal-ion chelator, and HA as the lymphatic system-targeting ligand (Scheme 1). Due to its proprietary size and HA surface modification, HA-MOFs are expected to exhibit effective lymphatic targeting and satisfactory tumor accumulation after subcutaneous injection. With the benefit of HA binding to the LYVE-1 receptor on the surface of LECs, HA-MOFs preferentially accumulate in nearby lymphatic vessels after subcutaneous injection and then circulate to the tumor site. In addition, HA also acts as a tumor-targeting molecule, generating sufficient ROS by laser irradiation after uptake via CD44-mediated endocytosis to provide excellent PDT therapeutic efficacy. In contrast to conventional PDT treatment strategies, HA is clinically approved by the FDA. This is expected to endow HA-MOFs with good biocompatibility and clinical application potential, facilitating further development and clinical translation of the PDT platform.
2. Results and discussion
2.1. Preparation and characterisation of HA-MOFs
Zr-based porphyrin MOFs were first prepared as cores according to the reported method.37 HA-MOFs were obtained by further surface modification of MOFs with HA via taking advantage of the strong coordination bonds between Zr6 clusters in the MOFs and the carboxyl groups on the molecular backbone of HA. In Fig. S1 (ESI†), the MOFs under scanning electron microscopy (SEM) show a densely distributed spherical structure. XRD patterns demonstrate the successful synthesis of MOFs, which have similar properties to PCN-224 NMOFs (Fig. S2, ESI†).37 As shown in Fig. 1a, transmission electron microscopy (TEM) verified the successful formation of HA-MOFs, revealing an average particle size of 119.11 nm as well as an increase of 28.08 nm relative to the size of the MOFs. It was also observed that the shape of the edge changed from smooth to irregular and the particles were more uniform, which should be a modified HA coating. In Fig. 1b, the average hydrodynamic diameters of MOFs and HA-MOFs were 168.62 nm and 193.22 nm with a narrow size distribution. The zeta potential of HA-MOFs was −13.25 ± 1.61 mV, and the change from positive to negative potential relative to the positive potential of MOFs demonstrated the successful modification of HA (Fig. 1c). The appearance of a characteristic peak at 3423 cm−1 in the FTIR spectra of HA-MOFs was attributed to the stretching vibration of –OH, verifying the successful modification of HA (Fig. 1d). In Fig. S3 (ESI†), the weight loss of the solvent in the pores of the MOFs and HA-MOFs is calculated by thermogravimetric (TG) curves to be 13.85% and 11.59% (30–200 °C) for the initial weight loss phase. For the subsequent weight loss phase, the structural decomposition of the MOFs and HA-MOFs is expected to result in weight losses of 28.26% and 50.10% (∼550 °C). Based on the compositional calculations, the HA content in the HA-MOFs is about 17.71 wt%. As shown in Fig. 1e and Fig. S4 (ESI†), UV-vis absorption spectra indicated that the distribution of TCPP-bound Zr ions after preparation of MOFs and HA carboxylate surface modification induced an overall red-shift of the absorption peaks at 416 nm, suggesting the presence of intermolecular conjugation. In Fig. S5 (ESI†), the experimental BET surface area of MOFs is 55.45 m2 g−1, which is lower than the theoretical value (3530.5 m2 g−1), suggesting a significant quantity of faulty sites inside the MOFs, due to localized deletions/mislocations of ligands and metal ions. The preparation route, storage conditions, and blood environment were simulated with water, saline and FBS, respectively, to determine the in vitro release profiles of HA-MOFs. The UV-Vis spectra and corresponding concentration–absorbance calibration curves showed less than 18.00% TCPP release from HA-MOFs in water, saline, and FBS within 7 days, suggesting a high in vitro stability of HA-MOFs (Fig. S6, ESI† and Fig. 1f). To visualize the in vitro release process of HA-MOFs, TEM images were captured on days 3 and 5. In Fig. S7 (ESI†), it was observed that the structure of nanoparticles disintegrated with time in different media and the particle size increased, which could be caused by the dissociation of the HA shell.2.2. Cytocompatibility and photodynamic effects of HA-MOFs
The storage stability of nanoparticles is crucial for its clinical application. Therefore, we evaluated the stability of HA-MOFs in various physiological solutions separately. HA-MOFs showed clarified in four solutions, including water, saline, DMEM, and FBS, indicating excellent water solubility (Fig. S8, ESI†). Over 7 days, HA-MOFs maintained stable particle size in various physiological solutions with PDI values below 0.2 (Fig. S9, ESI†). These results demonstrated that HA-MOFs exhibited compliant in vitro stability and dispersion, which was chemically stable possibly due to the strong interactions between Zr6 clusters and carboxylates. Consequently, to visualize the distribution of particles in tumor cells, fluorescent nanoparticles, HA5-AF-MOFs, were synthesized. The uptake of HA-MOFs by two different tumor cell types was evaluated through fluorescence-based localization of the detected particles. Over time, the green fluorescence signals in both B16F10 and H1975 cells progressively intensified (Fig. 2a and Fig. S10 and S12, ESI†). The localization of nanoparticles in tumor cells was further observed by staining the cytoskeleton and nucleus. The green fluorescence of the particles could be observed to be widely distributed in both the cytoskeleton and nucleus of the tumor cells, indicating that the cellular uptake and distribution of the nanoparticles were nonspecific (Fig. S11 and S13, ESI†). Intracellular fluorescence intensity increased significantly over 2 h and leveled off at 24 h, indicating that HA-MOFs were available for uptake by tumor cells in a short time (Fig. S14, ESI†). Furthermore, the cell counting kit (CCK-8) evaluated the toxicity of MOFs and HA-MOFs on B16F10 and H1975 cells, exhibiting no inhibitory impact on tumor cell proliferation (Fig. 2b and c). The cytotoxic effect of the nanoparticles on tumor cells was primarily due to the PDT activity of the photosensitizer TCPP. Therefore, both the concentration of TCPP and the duration of 650 nm laser irradiation were critical factors influencing the overall therapeutic efficacy. Next, the most optimal photosensitizer concentration and laser irradiation time for in vitro PDT treatment were determined by the CCK-8 method. As shown in Fig. 2d and e, MOFs and HA-MOFs containing different concentrations of TCPP were incubated with B16F10 and H1975 cells, followed by laser irradiation (650 nm, 1 W cm−2) for 10 min, which demonstrated a concentration-dependent photodynamic effect. When treated with HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1), about 94.46 ± 0.72% of B16F10 cells and 90.68 ± 0.74% of H1975 cells in the HA-MOFs group were eradicated, indicating a comparable therapeutic efficacy to that of the MOFs. In this case, the viability of B16F10 and H1975 cells decreased significantly with increasing laser irradiation time in a significant dependence (Fig. 2f and g).Additionally, the capacity of HA-MOFs to produce ROS in vitro was evaluated. In Fig. 2h, the fluorescence intensity of ROS generated by HA-MOFs under the same irradiation conditions was comparable to that observed in the MOFs group, indicating an equally robust ROS-generating capacity. To further explore the ROS-generating potential of HA-MOFs in tumor cells under laser irradiation, DCFH-DA was employed as a probe. This compound reacts with ROS to emit green fluorescence, which was subsequently quantified using both fluorescence microscopy and flow cytometry to evaluate the fluorescence intensity. In Fig. S15 and S16 (ESI†), the green fluorescence observed in both the HA-MOFs group and the light-only group was comparable to the control group and was negligible. In contrast, cells treated with HA-MOFs followed by light exposure (HA-MOFs + light group) exhibited strong green fluorescence with the highest intensity, confirming the effective light-induced ROS generation ability of HA-MOFs in vitro. In comparison to the control group, the ROS fluorescence intensity in the HA-MOFs + light group was noticeably higher, a difference further validated through statistical analysis (Fig. 2i and Fig. S17, ESI†). To visually evaluate the antiproliferative effect of HA-MOFs, propidium iodide (PI) was utilized to mark dead cells and calcein-AM was used to stain living cells. In Fig. 2j and Fig. S18, S19 (ESI†), the confocal laser scanning microscope (CLSM) images of the HA-MOFs and light group showed only green fluorescence, suggesting tumor cell survival. In contrast, the MOFs + light and HA-MOFs + light group showed distinct red fluorescence with the cell killing effect, aligning with the research results of the CCK-8 assays. The modification of HA preserved the PDT of MOFs and showed excellent in vitro tumor therapeutic effects. Furthermore, the antitumor efficacy of HA-MOFs was quantitatively evaluated using flow cytometry. In the HA-MOFs + light group, the percentage of apoptotic and necrotic B16F10 cells reached 2.82% and 6.35%. For H1975 cells, the corresponding percentages of apoptotic and necrotic cells were up to 31.0% and 20.3% (Fig. 2k). Therefore, HA-MOFs possessed significant photo-induced ROS generation ability causing cytotoxicity, showing potential application as a PDT agent.
2.3. In vivo biocompatibility of HA-MOFs
Following the in vivo therapeutic evaluation, the potential toxicity of HA-MOFs was assessed through body weight monitoring, hematological analysis, and H&E staining. As shown in Fig. 3a, healthy mice were subcutaneously injected with either PBS or HA-MOFs (TCPP concentrations equivalent to 5 mg kg−1) for 14 days. Over the 21 days, the body weight of the mice receiving HA-MOFs and PBS increased to 25.68 ± 1.84 g and 25.94 ± 1.27 g, with no significant differences between the two groups (Fig. 3b). Blood samples were collected from the mice's ocular veins on days 1, 7, and 14 for hematological and biochemical assessments. In Fig. 3c, most measurements of mice in the HA-MOFs group were close to the normal range of healthy mice, indicating no significant effects of HA-MOFs on haematological indices, hepatic and renal functions in mice at therapeutic doses. Subsequently, the mice's major organs were removed in order to stain with H&E and perform histopathological analysis to further assess any potential tissue damage or toxicity. In Fig. 3d, no significant organ damage or inflammatory lesions were detected, indicating an absence of systemic side effects in the mice. These satisfactory in vivo safety features may be attributed to the biocompatibility and targeted delivery of HA. Although further long-term biosafety studies are necessary, these in vivo results together demonstrate the favorable biosafety of HA-MOFs.2.4. In vivo distribution of HA-MOFs
The modification of HA increased the lymphatic targeting of MOFs and contributed to accumulation in LNs. Therefore, we further investigated the in vivo biodistribution, LN targeting and tumor prevention efficacy of HA-MOFs. Fluorescent nanoparticles HACy5.5-MOFs were prepared by chemically modifying the carboxyl group of HA onto the amino group and further labeling the amino group with Cy5.5. In Fig. S20 (ESI†), the 1H-NMR characteristic peaks b and c of the methylene group on the ethylenediamine moiety appeared at 3.0 ppm and 2.8 ppm, indicating the successful modification of the amino group. Approximately 20% of the carboxyl groups were substituted by ethylenediamine on each HA molecule by calculation, which corresponds to approximately 4 ethylenediamines on each HA molecule. As shown in Fig. 4a, HACy5.5-MOFs were injected subcutaneously into B16F10-tumor bearing C57BL/6J mice and the nearest inguinal LNs to the injection site were collected after 0, 12, 24, and 48 h. With the aid of an in vivo spectrum imaging system (IVIS), fluorescence images were obtained. In Fig. 4b and c, the fluorescence intensity of HACy5.5-MOFs in the LNs increased progressively, reaching its peak at 24 h, and then declined to 3.36% of the peak fluorescence after 48 h. To further investigate the localisation of HACy5.5-MOFs in LNs, mouse LNs injected for 24 h were collected and sectioned, and the specific antibody to LYVE-1 was stained to label LECs. As shown in Fig. 4d, intense red fluorescence was detected within the LNs, potentially due to the osmotic effect of the nanoparticles. In addition, the red fluorescence signal from Cy5.5 was found to co-localize extensively with the green fluorescence signal of the LYVE-1 antibody, indicating that HA-MOFs demonstrated effective lymphatic targeting by binding to HA and LYVE-1 receptors on the surface of LECs. Subsequently, we examined the aggregation effect of HA-MOFs at the tumor site. As shown in Fig. 4e and f, following the subcutaneous injection of HACy5.5-MOFs into B16F10 tumor-bearing mice, fluorescence signals were detected at the tumor site as well as in major organs after 12 h, indicating strong tumor accumulation. The fluorescence intensity of tissue homogenates from the tumor and liver peaked at 24 h, while no fluorescence signals were observed in the organs at 48 h. Based on the blood concentration–time curve, the circulatory half-life of HA-MOFs was calculated as 7.82 ± 0.22 h. Subcutaneous injection prolonged the circulation time of the blood compared to most intravenous formulations (Fig. S21, ESI†). Therefore, after subcutaneous injection of HA-MOFs, the nanoparticles enter the LNs due to the osmotic effect and targeted binding of the LYVE-1 receptor. Through lymphatic circulation, nanoparticles reach the tumor site and prolong the accumulation time. HA-MOFs possessed significant tumor-targeting ability, which ensures therapeutic efficacy in vivo.2.5. In vivo PDT efficacy
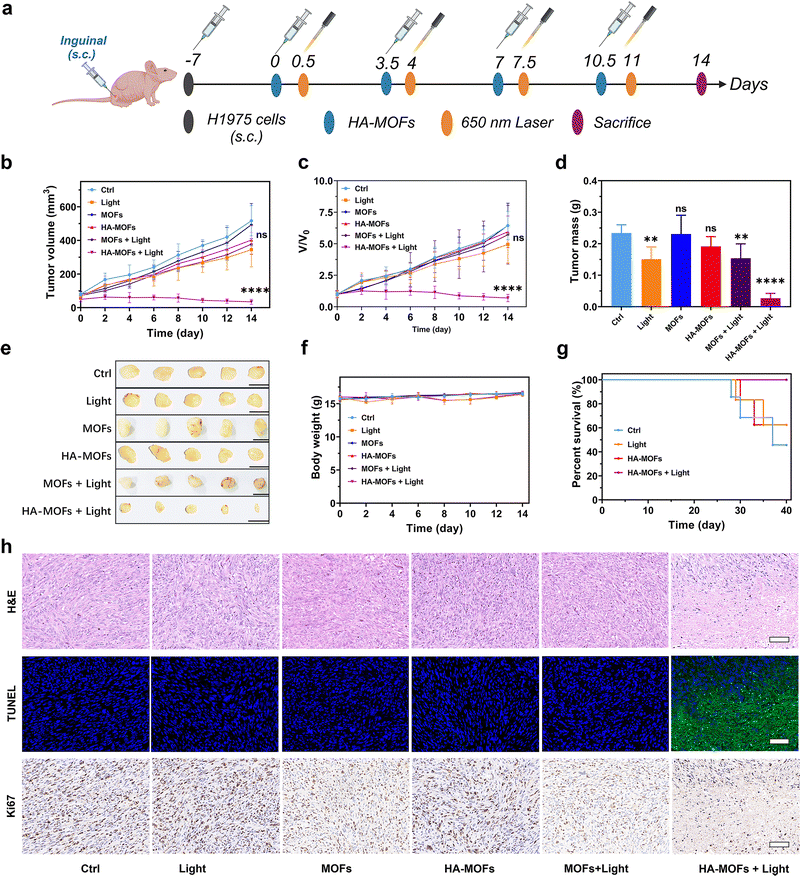
To further investigate the therapeutic effects of HA-MOFs on tumors in vivo, two subcutaneous tumor mouse models were constructed. First, forty male C57BL/6J mice were subcutaneously injected with B16F10 cells to construct a mouse model of melanoma (Fig. S22a, ESI†). After 7 days, the mice were divided into four groups: control, light, HA-MOFs, and HA-MOFs + light. Either PBS or HA-MOFs were administered subcutaneously to the respective groups. After 12 h, mice in the light group and the HA-MOFs + light group underwent laser irradiation for 10 min (650 nm, 1 W cm−2). During the treatment period, no noticeable differences in body weight, diet, or physical activity were observed between the different treatment groups and the control group (Fig. S22b, ESI†). H&E tissue staining, TUNEL immunofluorescence staining, and Ki67 immunohistochemistry of tumor sections indicated an increased level of tumor cell death in the HA-MOFs + light group (Fig. S22c, ESI†). As shown in Fig. S23 (ESI†), H&E staining of major organs after treatment revealed no notable differences compared to the control group, further confirming the biosafety of PDT both during and after treatment. Additionally, the survival rate of mice treated with HA-MOFs + light significantly improved, with 100% surviving for over 40 days (Fig. S24, ESI†). These findings suggest that HA-MOFs effectively inhibit the growth of B16F10 cells during synergistic phototherapy, leading to highly efficient melanoma treatment.On this basis, a subcutaneous tumor model was established by inoculating H1975 cells subcutaneously in mice. By adding MOFs group and MOFs + light group as experimental groups to compare and evaluate the in vivo therapeutic effects of HA-MOFs on lung cancer (Fig. 5a). As shown in Fig. 5b and c, under 650 nm laser irradiation, HA-MOFs showed some inhibitory effects on tumor growth. Nevertheless, MOFs showed no difference in PDT efficacy with the control group under laser irradiation. This is attributed to the poor permeability and lack of tumor targeting of MOFs, which resulted in the difficulty of particle aggregation in tumor tissues. Surface modification of MOFs with HA enhanced the tissue penetration and tumor targeting properties of the particles. The HA-MOFs + light group further enhanced the anti-tumor effect attributed to the effective photodynamic properties of the photosensitizer, which mainly consisted of the Fenton reaction triggered by ROS generation. The improved tumor therapeutic effect of HA-MOFs under laser treatment was further demonstrated by the tumor weight and tumor photographs taken after 14 days of treatment (Fig. 5d and e). This synergistic photodynamic therapeutic effect is likely attributed to the HA-mediated targeting of LNs and enhanced circulation to the tumor site. As shown in Fig. 5f and Fig. S25 (ESI†), no noticeable changes in body weight or evidence of tissue damage were detected, further confirming that HA-MOFs exhibit excellent biosafety. Additionally, the long-term treatment outcomes, presented in Fig. 5g, revealed a 100% survival rate in the HA-MOFs + light group, whereas the survival rates in the other groups were less satisfactory. The therapeutic impact of HA-MOF-induced PDT was further validated using H&E, TUNEL, and Ki67 staining assays. As shown in Fig. 5h, H&E staining demonstrated the most significant tissue damage in the HA-MOFs + light group, indicating a robust therapeutic effect. TUNEL staining revealed that mice in the HA-MOFs + light group exhibited the strongest green fluorescence apoptotic signal, indicating the highest level of cell death. Immunohistochemical staining showed that the HA-MOFs + light treatment group exhibited the lowest Ki67 levels, indicating effective inhibition of the proliferation process of tumor cells. In conclusion, the modification of HA enhanced the in vivo penetration effect and improved the tumor distribution of the particles. HA-MOFs synergistically eliminated tumors in vivo effectively during PDT, which provided a good therapeutic effect for such tumors prone to LN metastasis, such as melanoma and lung cancer.
3. Conclusions
In summary, HA-based PDT therapeutic agents, HA-MOFs, were successfully constructed with good lymphatic targeting and tumor ablation ability. HA-MOFs exhibited a high accumulation effect in LNs after subcutaneous injection and accumulated at the tumor site through lymphatic circulation. After a single 650 nm laser irradiation, HA-MOFs induced the generation of intracellular ROS at the same irradiation time, showing significant in vitro toxicity. The modification of HA has not decreased the PDT effect of the MOFs, and tumors were significantly ablated when treated with both HA-MOFs and a laser. Furthermore, subcutaneous injection of HA-MOFs in mice increased tumor site distribution and reduced off-target effects cause biosafety issues for phototoxic PDT drugs. In conclusion, our study reveals the behaviour of HA actively targeting LNs and demonstrates a simple and efficient synthetic method to enhance the in vivo antitumor efficacy of PDT. Since HA is clinically available and acts as a potential molecule for targeting the lymphatic system, it has great potential in the future development of lymphatic system-targeted delivery-based drugs for tumor therapy.4. Materials and methods
4.1. Materials
Tetrakis (4-carboxyphenyl) porphyrin (TCPP) was obtained from TCI. Zirconium oxychloride octahydrate (ZrOCl2·8H2O) was bought from Sigma-Aldrich. Hyaluronic acid (HA, MW: 8000 Da) was acquired from Guanglong Biological Company. 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-Methyl morpholinium chloride (DMTMM), 5-aminofluorescein (5-AF) and Cy5.5-NHS ester were purchased from Aladdin. Ethylenediamine, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were purchased from Yuanye. All cell staining kits were bought from Beyotime, including DAPI, the CCK-8 assay kit, the ROS detection kit, the Calcein-AM/PI live-dead cell staining kit, and the Annexin V-FITC apoptosis detection kit. Phalloidin conjugated Alexa Fluor 568 was purchased from Invitrogen. Mouse skin melanoma cells (B16F10) and human lung cancer cells (H1975) were bought from American Type Culture Collection. The other cell culture reagents, including RPMI 1640 medium, FBS, and PS, were obtained from Gibco. Male C57BL/6J mice (6–8 weeks, 20 g) and male BALB/c nude mice (6–8 weeks, 15 g) were provided by Beijing Huafukang Biological Science and Technology Company Limited. Every animal experiment was carried out in compliance with guidelines that were authorized by Sichuan University's West China Hospital's Institutional Animal Care and Use Committee (20240726004).4.2. Synthesis of MOFs
The preparation of MOFs followed the procedure described in ref. 37. Specifically, TCPP (100 mg), ZrOCl2·8H2O (300 mg), and BA (2.8 g) were dissolved in 100 mL of DMF. The mixture was then stirred at 90 °C for 6 h. The MOFs were isolated by centrifugation at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. Subsequently, the MOFs were washed three times with DMF, freeze-dried, and stored at 4 °C.
000 rpm for 30 min. Subsequently, the MOFs were washed three times with DMF, freeze-dried, and stored at 4 °C.
4.3. Synthesis of HA-MOFs
The MOF was mixed with HA in ultrapure water at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the mixture was agitated at room temperature for 1 h. Following the reaction, the HA-MOFs were obtained through centrifugation and freeze-dried.
4, and the mixture was agitated at room temperature for 1 h. Following the reaction, the HA-MOFs were obtained through centrifugation and freeze-dried.
4.4. Synthesis of HA5-AF-MOFs
HA (100 mg) was dissolved in aqueous K2HPO4 solution (5 mL, pH 9.0), after which DMTMM (4.15 mg, 0.5 mL) was added. For 30 min, the resultant mixture was mixed. Subsequently, 5-AF (4.34 mg, 0.5 mL) was added, while maintaining stirring at 25 °C for 2 h. The solution was centrifuged in centrifugal filtration filters (Mw: 3000 Da) to remove the unreacted 5-AF molecules. The solution of HA5-AF was stored at 4 °C for further use. HA5-AF-MOFs were subsequently synthesized using the same procedure as previously described.4.5. Synthesis of HACy5.5-MOFs
The labelling of HA was followed by the method previously reported in the paper.42 Briefly, to activate the carboxyl groups, HA (100 mg, 527 μmol carboxyl groups) was dissolved in PB (5 mL, pH 8.0) and then reacted with EDC (101 mg, 527 μmol) and NHS (60.6 mg, 527 μmol) for 1 h. Subsequently, the solution was mixed with ethylenediamine (158.3 mg) in DMSO with stirring for 24 h at 25 °C. Unreacted molecules were removed by using centrifugal filtration filters (Mw: 3000 Da) and freeze-dried. The rate of ethylenediamine substitution in HA was evaluated by 1H-NMR (400 MHz, D2O). For the imaging purposes, HA-NH2 was fluorescently labeled using Cy5.5-NHS ester. Cy5.5-NHS was dissolved in DMSO, followed by the addition of HA-NH2 at a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Cy5.5-NHS to HA-NH2). The mixture was agitated in the dark for 2 h at 25 °C. Unreacted Cy5.5-NHS was removed using a centrifugal filtration filter (Mw: 3000 Da). HACy5.5-MOFs were prepared according to the above procedure.
1 (Cy5.5-NHS to HA-NH2). The mixture was agitated in the dark for 2 h at 25 °C. Unreacted Cy5.5-NHS was removed using a centrifugal filtration filter (Mw: 3000 Da). HACy5.5-MOFs were prepared according to the above procedure.
4.6. Characterization
SEM images were captured using a Thermo Scientific Helios 5 CX. TEM images were captured using an HT78200 microscope that was run at 120 kV of acceleration. XRD patterns of the samples were measured on a Rigaku Ultima IV diffractometer. UV-vis absorbance and fluorescence intensity were measured on a Varioskan LUX multifunctional enzyme labeller. In order to determine the hydrodynamic particle size and zeta potential, Malvern Instruments' Zetasizer Nano-ZS dynamic light scattering device was utilized. A NICOLET iS50 FTIR spectrometer was used to record infrared (IR) spectra. TG curves were determined using a simultaneous thermal analyser (TG-DSC) STA 449 F3. Nitrogen adsorption–desorption isotherms were carried out on a Mack 2020 fully automated physical adsorption tester (BET) using high purity nitrogen at a set temperature of 77 K. A PE Avio 200 plasma mass spectrometer was used for analysis of Zr content.4.7. In vitro release curve of HA-MOFs
The TCPP standard solution was prepared in a solvent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to DMF. The UV absorption spectrum and absorbance–concentration standard curve (λ = 416 nm) were determined for the TCPP standard curve at room temperature. 2 mL of HA-MOFs was added (TCPP concentrations equivalent to 25 μg mL−1) into a dialysis bag (Mw: 3000 Da), and then the bag was immersed in 10 mL of a solution containing FBS, saline, and ultrapure water. The mixture was stirred at 37 °C. The release of TCPP at different times was evaluated using a Varioskan LUX multifunctional enzyme marker.
1 volume ratio of water to DMF. The UV absorption spectrum and absorbance–concentration standard curve (λ = 416 nm) were determined for the TCPP standard curve at room temperature. 2 mL of HA-MOFs was added (TCPP concentrations equivalent to 25 μg mL−1) into a dialysis bag (Mw: 3000 Da), and then the bag was immersed in 10 mL of a solution containing FBS, saline, and ultrapure water. The mixture was stirred at 37 °C. The release of TCPP at different times was evaluated using a Varioskan LUX multifunctional enzyme marker.
4.8. Storage stability of HA-MOFs
HA-MOFs (TCPP concentrations equivalent to 20 μg mL−1) were dissolved in PBS and stored at 4 °C for 7 days. The hydrodynamic particle size and PDI values were recorded to evaluate in vitro stability.4.9. Intracellular uptake of HA-MOFs by tumor cells
B16F10 and H1975 cells were inoculated in 24-well plates with cell crawls at a density of 2 × 105 cells per well and incubated for 24 h. 1 mL of serum-free medium-configured HA5-AF-MOFs (TCPP concentrations equivalent to 25 μg mL−1) was added to each well. After treatment of cells for 2 h, 6 h, 12 h, and 24 h, the cells were fixed in paraformaldehyde, stained with phalloidin and DAPI, and fluorescence images were captured on a CLSM.4.10. In vitro ROS generation curve of HA-MOFs
The ROS generation capacity of HA-MOFs was determined using a ROS detection kit. DCFH-DA (10 μM) was mixed with MOFs and HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1) in a well plate. Following exposure to a 650 nm laser (1 W cm−2) for varied durations, the intensity of fluorescence at 525 nm (λex = 488 nm) was determined.4.11. CCK-8 assay
After being seeded into 96-well plates, B16F10 and H1975 cells were grown for 24 h. After adding various MOFs and HA-MOFs concentrations to the wells, the culture was incubated for 24 h. After adding 10 μL of CCK-8 solution to each well, the cells were treated for 2 h at 37 °C. Using an enzyme marker, the well plates’ absorbance at 450 nm was measured.4.12. Cytotoxicity assay
The most applicable TCPP concentration and laser irradiation time for PDT treatment were screened by measuring cell survival at different combinations of HA-MOFs concentrations and laser irradiation times. Briefly, after being planted into 96-well plates, B16F10 and H1975 cells were cultured overnight. Then, different MOFs and HA-MOFs concentrations were introduced. The cells were incubated for an additional 24 h after being subjected to a 650 nm laser (1 W cm−2) for 10 min. The vitality of the cells in each well was evaluated using the CCK-8 kit. Additionally, MOFs and HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1) were selected and cocultured with cells for 4 h. Following various periods of exposure to laser light, the cells’ viability was assessed.4.13. Evaluation of intracellular ROS generation
B16F10 and H1975 cells were seeded in 6-well plates and cultured for a period of 24 h. HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1) were added to the experimental group. The cells were incubated for 4 h before being subjected for 10 min to a 650 nm laser (1 W cm−2). The intracellular ROS generation induced by HA-MOFs was assessed using a ROS assay kit. Intracellular ROS fluorescence intensity was observed and quantified by means of fluorescence microscopy and flow cytometry.4.14. Live/dead cell staining
Following overnight incubation, B16F10 and H1975 cells were planted into 6-well plates. Subsequently, MOFs and HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1) were added, and the culture was incubated for 6 h. The cells were then exposed to a 650 nm laser (1 W cm−2) for 10 min. The culture medium was withdrawn after an extra 24 h of incubation, and the cells were stained with a Calcein-AM/PI live-dead cell staining kit. The state of the cells was assessed using a fluorescence microscope, where live cells were visualized as green and dead cells as red.4.15. Apoptosis assay
B16F10 and H1975 cells were plated in 6-well plates and allowed to incubate overnight. HA-MOFs (TCPP concentrations equivalent to 25 μg mL−1) were added and incubation was continued for 4 h. Then, the cells were irradiated with a 650 nm laser (1 W cm−2) for 10 min and incubation was continued. Following a 24 h period, the cells were stained in accordance with the Annexin V-FITC/PI apoptosis detection kit instructions, and flow cytometry was used to determine the apoptosis rate.4.16. In vivo biosafety
Male C57BL/6J mice (6–8 weeks, 20 g) were subcutaneously injected with HA-MOFs (TCPP concentrations equivalent to 5 mg kg−1). The identical amount of saline was put into the control group. Body weights of mice were recorded at 1, 7, 14, and 21 days after injection. Ocular vein blood sampling was performed after different time points, and blood samples from mice were collected for routine blood tests. The upper serum layer was centrifuged and the biochemical parameters were determined. The indicators measured include: white blood cell (WBC), neutrophil (NEUT), lymphocyte (LYMPH), monocytes (MONO), red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean erythrocyte hemoglobin concentration (MCHC), albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), uric acid (UA), and urea nitrogen (UREA). Mice were euthanised 14 days after injection and major tissues were excised for H&E staining.4.17. Construction of tumor mouse models
Male C57BL/6J mice (6–8 weeks, 20 g) and BALB/c nude mice (6–8 weeks, 15 g) were selected to construct subcutaneous tumor mouse models. 5 × 106 tumor cells were injected subcutaneously on the right side of the back of the mice. BALB/c nude mice were inoculated with B16F10 cells and C57BL/6J mice with H1975 cells. After 7 days of inoculation, the mice were used for in vivo distribution and antitumor treatment experiments with HA-MOFs.4.18. In vivo biodistribution of HA-MOFs
B16F10-tumor bearing mice were subcutaneously injected with HACy5.5-MOFs (TCPP concentrations equivalent to 5 mg kg−1). The inguinal LNs, tumors and major organs of mice at 0, 12, 24 and 48 h after injection were collected. Ex vivo LN imaging and fluorescence intensity analysis were performed using the IVIS® spectrum imaging system (PerkinElmer, USA). Inguinal LNs from mice 24 h after injection were collected, cryosectioned and immunofluorescently stained with the LYVE-1 antibody. The co-localisation of HACy5.5-MOFs in inguinal LNs was observed using an Olympus BX-51 optical system. Tumors and remaining major organs were weighed and tissue homogenized, and the supernatant was centrifuged to detect fluorescence intensity (λex = 680 nm, λem = 710 nm).4.19. Pharmacokinetics
Male C57BL/6J mice (6–8 weeks, 20 g) were injected subcutaneously with 5 mg kg−1 TCPP concentration of HA-MOFs, and serum was collected at 0, 1, 2, 4, 6, 8, 12, and 24 h. The samples were incubated with aqua regia at 80 °C for 2 h. The content of Zr was measured by ICP-MS.4.20. In vivo PDT efficacy of B16F10-tumor bearing mice
Mice bearing B16F10 tumors were randomly grouped and named as follows: control group, light group, HA-MOFs group, and HA-MOFs + light group. HA-MOFs were administrated subcutaneously into subjects in the HA-MOFs group and HA-MOFs + light group at a concentration of TCPP of 5 mg kg−1 every three days. The same volume of saline was also injected into the subjects in control and light groups. The light and HA-MOFs + light groups were was subjected to a 650 nm laser (1 W cm−2) for 10 min, 12 h after subcutaneous injection. Body weights of all subjects in this section were recorded daily throughout the treatment period. Mice were euthanized and tumors along with major organs were harvested one-week post-treatment. Tumor slices were handled with H&E, TUNEL, and Ki67 staining, and the remaining organs were also stained with H&E for further analysis.4.21. In vivo PDT efficacy of H1975-tumor bearing mice
Mice bearing H1975 tumors were also randomly grouped into four groups: control group, light group, MOFs group, HA-MOFs group, MOFs + light group, and HA-MOFs + light group. Tumor-bearing mice were subcutaneously injected every three days with MOFs and HA-MOFs at a TCPP concentration of 5 mg kg−1, as described previously. The same volume of saline was also injected into the subjects in control and light groups. The light, MOFs + light and HA-MOFs + light groups were subjected to a 650 nm laser (0.5 W cm−2) for 10 min, 12 h after subcutaneous injection. Body weights of all subjects in this section were recorded daily throughout the treatment period. Mice were euthanized and tumors along with major organs were harvested two-week post-treatment. Tumor were handled weighed and photographed before being stained with H&E, TUNEL, and Ki67, and the remaining organs were also stained with H&E for further analysis.4.22. Survival curves for mice
All tumor-bearing mice were grouped randomly into the control group, light group, HA-MOFs group and HA-MOFs + light group. Tumor-bearing mice were subcutaneously injected twice weekly with HA-MOFs at a TCPP concentration of 5 mg kg−1. Subjects in control and light groups received injections of the same volume of saline. For B16F10 tumor-bearing mice, the light and HA-MOFs + light groups were exposed to a 650 nm laser (1 W cm−2) for 10 min, 12 h post-subcutaneous injection. For H1975 tumor-bearing mice, the light and HA-MOFs + light groups were irradiated with a 650 nm laser (0.5 W cm−2) for 10 min, 12 h after subcutaneous injection. Mice were considered dead with tumors exceeding 20 mm in any dimension, and 40-day survival was recorded.4.23. Statistical analysis
Data analysis was done using GraphPad Prism (8.0.2). For numerous comparisons, paired t-tests and one-way ANOVA were employed, unless specified otherwise. Disparities with a p-value of less than 0.05 were deemed statistically significant.Author contributions
Qiuhong Ouyang: methodology, data curation, software, writing – original draft. Xin Li: methodology, software, data curation. Yuechen He: methodology, data curation. Yuxin Bai: methodology, data curation. Xunhuan Song: methodology, data curation. Xinglv Chen: methodology, data curation. Yuzhou Xiao: methodology, data curation. Lili Mao: methodology, data curation. Min Chen: methodology, data curation. Xiaodan Pan: methodology, data curation. Weihong Kuang: data curation, review & editing. Feng Qin: supervision, resources, review & editing. Meng Qin: supervision, project administration, review & editing. Xiaoai Wu: supervision, resources, project administration, writing – review & editing. All authors reviewed the manuscript and discussed the results.Data availability
The data that support the findings of this study are available from the authors on reasonable request, see author contributions for specific data sets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52473137) and the 135 Project for Disciplines of Excellence of West China Hospital Sichuan University (ZYGD23011, ZYGD23016 and ZYJC21029). The figures were created in BioRender.com. The authors would also like to extend gratitude to Qiuxiao Shi from the Histology and Imaging Platform, Core Facilities of West China Hospital, Sichuan University, for her assistance in transmission electron microscopy imaging.Notes and references
- WHO, Global Cancer Observatory: Cancer Today, https://gco.iarc.fr/today, (accessed February, 2021).
- X. Jin, Z. Demere, K. Nair, A. Ali, G. B. Ferraro, T. Natoli, A. Deik, L. Petronio, A. A. Tang, C. Zhu, L. Wang, D. Rosenberg, V. Mangena, J. Roth, K. Chung, R. K. Jain, C. B. Clish, M. G. Vander Heiden and T. R. Golub, Nature, 2020, 588, 331–336 CrossRef CAS PubMed.
- P. S. Steeg, Nat. Rev. Cancer, 2016, 16, 201–218 CrossRef CAS PubMed.
- M. Esposito, S. Ganesan and Y. Kang, Nat. Rev. Cancer, 2021, 2, 258–270 CrossRef PubMed.
- K. J. Hiam-Galvez, B. M. Allen and M. H. Spitzer, Nat. Rev. Cancer, 2021, 21, 345–359 CrossRef CAS PubMed.
- S. Gerstberger, Q. Jiang and K. Ganesh, Cell, 2023, 186, 1564–1579 CrossRef CAS PubMed.
- C. K. Lee, S. H. Jeong, C. Jang, H. Bae, Y. H. Kim, I. Park, S. K. Kim and G. Y. Koh, Science, 2019, 363, 644–649 CrossRef CAS PubMed.
- J. M. Ubellacker, A. Tasdogan, V. Ramesh, B. Shen, E. C. Mitchell, M. S. Martin-Sandoval, Z. Gu, M. L. McCormick, A. B. Durham, D. R. Spitz, Z. Zhao, T. P. Mathews and S. J. Morrison, Nature, 2020, 585, 113–118 CrossRef CAS PubMed.
- B. M. Gruner and S. M. Fendt, Nature, 2020, 585, 36–37 CrossRef CAS PubMed.
- L. Munoz-Erazo, J. L. Rhodes, V. C. Marion and R. A. Kemp, Cell Mol. Immunol., 2020, 17, 570–575 CrossRef CAS PubMed.
- T. N. Schumacher and D. S. Thommen, Science, 2022, 375, eabf9419 CrossRef CAS PubMed.
- S. Feins, W. Kong, E. F. Williams, M. C. Milone and J. A. Fraietta, Am. J. Hematol., 2019, 94, 3–9 CrossRef PubMed.
- B. C. Creelan, C. Wang, J. K. Teer, E. M. Toloza, J. Yao, S. Kim, A. M. Landin, J. E. Mullinax, J. J. Saller, A. N. Saltos, D. R. Noyes, L. B. Montoya, W. Curry, S. A. Pilon-Thomas, A. A. Chiappori, T. Tanvetyanon, F. J. Kaye, Z. J. Thompson, S. J. Yoder, B. Fang, J. M. Koomen, A. A. Sarnaik, D. T. Chen, J. R. Conejo-Garcia, E. B. Haura and S. J. Antonia, Nat. Med., 2021, 27, 1410–1418 CrossRef CAS PubMed.
- J. Chen, L. Wang, Q. Yao, R. Ling, K. Li and H. Wang, Breast Cancer Res., 2004, 6, 474–477 CrossRef PubMed.
- A. J. Harvey, S. A. Kaestner, D. E. Sutter, N. G. Harvey, J. A. Mikszta and R. J. Pettis, Pharm. Res., 2011, 28, 107–116 CrossRef CAS PubMed.
- S. N. Thomas and A. Schudel, Curr. Opin. Chem. Eng., 2015, 7, 65–74 CrossRef PubMed.
- G. M. Ryan, L. M. Kaminskas and C. J. Porter, J. Controlled Release, 2014, 193, 241–256 CrossRef CAS PubMed.
- A. L. St John, C. Y. Chan, H. F. Staats, K. W. Leong and S. N. Abraham, Nat. Mater., 2012, 11, 250–257 CrossRef CAS PubMed.
- X. Hong, X. Zhong, G. Du, Y. Hou, Y. Zhang, Z. Zhang, T. Gong, L. Zhang and X. Sun, Sci. Adv., 2020, 6, eaaz4462 CrossRef CAS PubMed.
- Z. Yan, F. Wang, Z. Wen, C. Zhan, L. Feng, Y. Liu, X. Wei, C. Xie and W. Lu, J. Controlled Release, 2012, 157, 118–125 CrossRef CAS PubMed.
- H. Qin, R. Zhao, Y. Qin, J. Zhu, L. Chen, C. Di, X. Han, K. Cheng, Y. Zhang, Y. Zhao, J. Shi, G. J. Anderson, Y. Zhao and G. Nie, Adv. Mater., 2021, 33, 2006007 CrossRef CAS PubMed.
- H. Kang, Z. Zuo, R. Lin, M. Yao, Y. Han and J. Han, Drug Delivery, 2022, 29, 3087–3110 CrossRef CAS PubMed.
- J. Wu, T. Zhai, J. Sun, Q. Yu, Y. Feng, R. Li, H. Wang, Q. Ouyang, T. Yang, Q. Zhan, L. Deng, M. Qin and F. Wang, J. Colloid Interface Sci., 2022, 624, 307–319 CrossRef CAS PubMed.
- H. M. Kim, J. H. Park, Y. J. Choi, J. M. Oh and J. Park, RSC Adv., 2023, 13, 5529–5537 RSC.
- L. Gong, H. Zhou, S. Zhang, C. Wang, K. Fu, C. Ma, Y. Zhang, C. Peng and Y. Li, Adv. Healthcare Mater., 2023, 12, 2202228 CrossRef CAS PubMed.
- P. Zhang, Y. Cui, J. Wang, J. Cheng, L. Zhu, C. Liu, S. Yue, R. Pang, J. Guan, B. Xie, N. Zhang, M. Qin, L. Jing, Y. Hou and Y. Lan, J. Nanobiotechnol., 2023, 21, 4 CrossRef CAS PubMed.
- X. Cai, W. Liu, J. Zhang, Z. Li, M. Liu, S. Hu, J. Luo, K. Peng, B. Ye, Y. Wang and R. Yan, Small, 2024, 20, 2309086 CrossRef CAS PubMed.
- Y. Wang, H. Li, G. Niu, Y. Li, Z. Huang, S. Cheng, K. Zhang, H. Li, Q. Fu and Y. Jiang, ACS Nano, 2024, 18, 5828–5846 CAS.
- X. Hu, R. Li, J. Liu, K. Fang, C. Dong and S. Shi, Adv. Healthcare Mater., 2024, 13, 2302333 CrossRef CAS PubMed.
- S. Banerji, J. Ni, S. X. Wang, S. Clasper, J. Su, R. Tammi, M. Jones and D. G. Jackson, J. Cell Biol., 1999, 144, 789–801 CrossRef CAS PubMed.
- D. G. Jackson, R. Prevo, S. Clasper and S. Banerji, Trends Immunol., 2001, 22, 317–321 CrossRef CAS PubMed.
- S. Mizrahy, S. R. Raz, M. Hasgaard, H. Liu, N. Soffer-Tsur, K. Cohen, R. Dvash, D. Landsman-Milo, M. G. Bremer, S. M. Moghimi and D. Peer, J. Controlled Release, 2011, 156, 231–238 CrossRef CAS PubMed.
- T. Liu, G. Wu, J. Cheng, Q. Lu, Y. Yao, Z. Liu, D. Zhu, J. Zhou, J. Xu, J. Zhu and D. He, Nano Res., 2016, 9, 473–489 CrossRef CAS.
- D. G. Jackson, Matrix Biol., 2019, 78–79, 219–235 CrossRef CAS PubMed.
- G. Wu, H. Zhang, Z. Zhan, Q. Lu, J. Cheng, J. Xu and J. Zhu, Chin. J. Chem., 2015, 33, 1153–1158 CrossRef CAS.
- X. Liang, L. Fang, X. Li, X. Zhang and F. Wang, Biomaterials, 2017, 132, 72–84 CrossRef CAS PubMed.
- H. Wang, D. Yu, J. Fang, C. Cao, Z. Liu, J. Ren and X. Qu, ACS Nano, 2019, 13, 9206–9217 CrossRef CAS PubMed.
- M. He, M. Zhang, T. Xu, S. Xue, D. Li, Y. Zhao, F. Zhi and D. Ding, J. Controlled Release, 2024, 368, 233–250 CrossRef CAS PubMed.
- P. Huang, L. Kong, F. Zhang, L. Chen, Y. Zhang, X. Shi, T. Lawson, S. Chou, Y. Liu and W. Wu, Small, 2024, 20, 2312211 CrossRef CAS PubMed.
- X. Chen, D. Cheng, N. Yu, J. Feng, J. Li and L. Lin, J. Mater. Chem. B, 2024, 12, 1296–1306 RSC.
- M. Xu, Y. Qi, G. Liu, Y. Song, X. Jiang and B. Du, ACS Nano, 2023, 17, 20825–20849 CrossRef PubMed.
- L. Guo, Y. Chen, T. Wang, Y. Yuan, Y. Yang, X. Luo, S. Hu, J. Ding and W. Zhou, J. Controlled Release, 2021, 330, 119–131 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb02199a |
| ‡ These authors contributed to the work equally. |
| This journal is © The Royal Society of Chemistry 2025 |