A supramolecular assembly of a novel green fluorescent protein chromophore-based analogue and its application in fluorescence anti-counterfeiting†
Yifei
Ren
and
Chusen
Huang
 *
*
The Education Ministry Key Laboratory of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, and Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Shanghai Frontiers Science Research Base of Biomimetic Catalysis, Department of Chemistry, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: huangcs@shnu.edu.cn
First published on 7th January 2025
Abstract
Supramolecular fluorescent materials with switchable behavior and induced luminescence enhancement are a new class of special materials for constructing fluorescence anti-counterfeiting materials. Since these materials are constructed by self-assembly through supramolecular host–guest interactions of non-covalent bonds, such fluorescent materials can regulate their optical properties through a reversible assembly–disassembly process. Inspired by the role of the β-barrel scaffold in activating strong fluorescence of a green fluorescent protein (GFP) chromophore, we designed a supramolecular system based on a novel GFP analogue (CA) and cucurbit[7]uril (CB[7]). CA molecules are encapsulated by CB[7] to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest assembly, thereby the fluorescence brightness of CA can be tuned. The reversible regulation of fluorescence intensity was additionally realized by controlling the dynamic assembly–disassembly process in the presence of a higher binding competitor, amantadine hydrochloride. The CA-CB[7] system was successfully used for information anti-counterfeiting through the reversible fluorescence readout on A4 paper, which enables the GFP chromophore analogue and cucurbituril system to become a potential candidate for constructing intelligent information encryption and anti-counterfeiting materials.
2 host–guest assembly, thereby the fluorescence brightness of CA can be tuned. The reversible regulation of fluorescence intensity was additionally realized by controlling the dynamic assembly–disassembly process in the presence of a higher binding competitor, amantadine hydrochloride. The CA-CB[7] system was successfully used for information anti-counterfeiting through the reversible fluorescence readout on A4 paper, which enables the GFP chromophore analogue and cucurbituril system to become a potential candidate for constructing intelligent information encryption and anti-counterfeiting materials.
1. Introduction
In recent years, stimulus-responsive fluorescent materials (SFMs) have been widely used in biosensing, bioimaging, anti-counterfeiting and information encryption due to their sensitivity to environmental stimuli.1–10 Various stimuli such as light, temperature, pressure, ions, acid–base and humidity have been studied for SFMs.11–19 Supramolecular fluorescent materials are new specialised materials in the field of fluorescent materials compared to other SFMs. Such fluorescent materials are constructed by self-assembly via non-covalent supramolecular host–guest interactions, allowing their optical properties to be controlled by a reversible interaction process. This type of fluorescent system exhibits desirable properties such as autonomous control, leanness, and high spatial and temporal resolution.20–24Shimomura et al. first discovered and extracted the green fluorescent protein (GFP) in Aequorea victoria in 1962. Since then it has been used for bioimaging, protein tracking and super-resolution fluorescence microscopy.25–31 The structure of the GFP β-barrel contains 11 chains of aligned buildings that are connected by an α-helix.32 The GFP chromophore forms a network structure of barrels via non-covalent bonds of hydrogen in the β-barrels. This natural structure enhances fluorescence emission (mainly by inhibiting the internal conversion pathway).33 Protein folding is essential for the effect of fluorescence because the denatured GFP is non-fluorescent, and the GFP fluoresces again after renaturation. The three-dimensional GFP structure strongly inhibits the conformation of the chromophore and thus restricts its intramolecular movement, leading to fluorescence by non-radiative processes, so that isolated chromophores can have unrestricted intramolecular movement without fluorescence.34,35
Due to their excellent reversibility and sensitive response to external stimuli, supramolecular polymers are widely used in a variety of applications such as displays, sensors, and information coding.21,22,36–45 In addition, there are many supramolecular interactions such as host–guest interactions, ligand–metal bonds, π–π-guided ion interactions, and hydrogen bonds, which are important for optical flexibility as well as self-organization and healing potential.46–52 By encapsulating chromophores in a host–guest complex, not only can the water solubility of the fluorescence probe be improved, but also the complexation can maintain or even improve its photophysical properties, such as fluorescence quantum yield, photoisomerization ability and photostability.23,24,53–56 Among the various host macrocycles, cucurbit[7]uril (CB[7]) has attracted our attention because cucurbituril molecules are supramolecular macrocycles condensed from glycoluril to form a barrel-like structure, which is very similar to the β-barrel of the GFP, with a large hydrophobic cavity inside. At the same time, the carbonyl group formed along the upper and lower outer edges of the macrocycle can provide a certain non-covalent bond, which is very similar to the force provided by the carbonyl group at the amino acid site in the GFP. Therefore, cucurbituril molecules have a high binding force with guest molecules, and can encapsulate a large number of organic guest molecules.57 The high affinity of CB[7] for guest molecules with the right geometry, such as ferrocene and adamantane, is similar to the binding between natural proteins and their homologous ligands, such as avidin and biotin.58 In addition, CB[7] is water-soluble and has low cytotoxicity (IC50 = 0.53 mM).59 This would make cucurbituril-based supramolecular materials ideal for building new cryptographic materials.60–65 Despite numerous works on CB-based assembly having been reported, there is still increasing interest in the development of fluorescent dyes which show a high affinity to cucurbiturils in aqueous media and a concomitant fluorescence change upon host–guest complexation.57
Herein, a new GFP chromophore analogue (CA) with good water solubility was designed and synthesized. It exhibited a fluorescence “off–on” property after formation of a complex with CB[7] through the host–guest interactions since CB[7] works like the β-barrel of GFP to inhibit the motion of CA (Scheme 1). Based on the CA–CB[7] system, a new type of fluorescent anti-counterfeiting material was prepared by using a host–guest interaction strategy. To the best of our knowledge, there is little literature reported for the GFP chromophore analogues which have high affinity towards cucurbiturils.
2. Experimental
2.1. Materials and methods
All chemical reagents were commercially available and used directly without any further purification in our experiments. Column chromatography was performed using silica gel (300–400 mesh, Haiyang, Qingdao). Thin-layer chromatography (TLC) was performed on silica gel plates and visualized using a hand-held ultraviolet (UV)-lamp. UV-Vis spectra were obtained with a Hitachi U-3900 UV-Vis spectrophotometer and quartz cuvettes (1 cm × 1 cm). The fluorescence spectrum was recorded with a Hitachi F-7000 fluorescence spectrometer. The excitation and emission slit widths were modified to adjust the fluorescence intensity to a suitable range. A Bruker AM-400 spectrometer was employed for 1H and 13C NMR, with Me4Si as the internal standard. Electrospray ionization (ESI) mass spectrometry was performed using a HP 1100 LC-MS spectrometer. Cell Counting Kit-8 (CCK-8) was purchased from SIGMA-Aldrich. All pH measurements were carried out using a pH meter (Leici PHB-4) which was calibrated with pH 4.00 and pH 9.18 buffers before use.2.2. Synthesis of target probe CA
For detailed synthetic procedures, please see the ESI.† Compound 2 (381.9 mg, 1.52 mmol), 4-pyridine-formaldehyde (220 μL, 2.34 mmol) and zinc chloride (0.6 g, 4.4 mmol) were dissolved in 1,4-dioxane (5 mL). The reaction was stirred at 80 °C for 3 hours under nitrogen protection. Until the TLC indicated that the starting compound 2 had disappeared, the reaction was not stopped. The solvent was removed using a rotary evaporator to obtain the crude products, which were further purified by column chromatography with an eluent of PE/EA (v/v = 10/1). The final pure product CA was obtained (301.7 mg, yield 57%). 1H NMR (DMSO-d6, 400 MHz) δH 8.71 (1H, s), 8.69 (2H, d, J = 6.0 Hz), 8.63 (1H, d, J = 8.8 Hz), 8.06 (1H, d, J = 15.8 Hz), 8.02 (1H, s), 7.98 (1H, d, J = 8.4 Hz), 7.94 (1H, d, J = 6.8 Hz), 7.86 (2H, d, J = 6.0 Hz), 7.62–7.56 (2H, m), 7.53 (1H, d, J = 15.8 Hz), 7.26 (1H, s), 3.32 (3H, s); 13C NMR (DMSO-d6, 101 MHz) δC 170.4, 162.6, 150.8, 142.6, 140.1, 138.2, 133.9, 133.8, 133.3, 132.6, 129.3, 128.7, 128.3, 127.2, 126.7, 122.6, 119.1, 27.1. HRMS (ES+, m/z): calc. for C22H17N3O: 340.1405, found: 340.1446.2.3. Isothermal titration calorimetry
The affinity between CA and CB[7] was determined by NANO-ITC isothermal titration calorimetry (ITC). All titrations were performed in HEPES (100 mM) solution at 25 °C. 2 μL of CB[7] (100 μM) was added into 300 μL of CA solution (10 μM), the addition was repeated for 25 times. And the addition interval was 120 seconds, and the stirring speed was 350 rpm. As a reference run, CB[7] was titrated into a HEPES solution of CA (containing 10% DMSO HEPES solution), and CB[7] was titrated into a blank solvent for the control group (containing 10% DMSO HEPES solution). The resulting thermograms were baseline corrected and the binding signal was corrected for control measurements. The test data were fitted with an independent model, and the corresponding dissociation constant Kd, stoichiometric ratio (n), enthalpy change (ΔH), entropy change (ΔS) and other parameters were obtained.2.4. Fluorescence lifetime test
Fluoromax+ (Horiba Instrument Incorporated) is used to measure the fluorescence lifetime. The test was performed in a HEPES solution (10% DMSO) at 25 °C. CA solution concentration is 50 μM. For the CA–CB[7] system, 25 μM CB[7] was added to HEPES solution containing 50 μM CA. For the CA–CB[7]–amantadine hydrochloride system, 50 μM amantadine hydrochloride was added into the CA–CB[7] system. Time-dependent fluorescence experiments with time-correlated single photon counting (TCSPC) were performed to obtain the lifetime.182.5. Fluorescence quantum yield
The fluorescence quantum yields of CA and CA–CB[7] in HEPES were determined using rhodamine 6G in pure water (Φ = 0.90) as reported in ref. 66 and 67. The detailed calculation process is provided in the ESI.†2.6. Fluorescence anti-counterfeiting image acquisition equipment
The detailed parameters are: a semiconductor laser (λ = 445 ± 5 nm, 10 W) was used for irradiation, a Huawei nova 5z smart phone camera (pixel 2340 × 1080) was used for photography, and the optical filter was an optical longpass filter that was used for transmission wavelength more than 500 nm, and the cut-off rate is OD5.3. Results and discussion
3.1. Design strategy and synthesis
Our main goal is to design a cucurbituril-based reusable fluorescent anti-counterfeiting system that can be used as a highly selective cryptographic fluorescent anti-counterfeiting material. To achieve this goal, two main prerequisites are required: one is the use of appropriate organic fluorophores, no fluorescence or weak fluorescence before interaction with cucurbiturils. And the other is a special cucurbituril that has a strong binding ability to the fluorophore and can induce remarkable fluorescence changes after it forms a supramolecular assembly with the guest fluorophore. Additionally, the fluorophore can be replaced by guest molecules with higher binding affinity such as amantadine.Based on the above considerations, our molecular design is based on the encapsulation of GFP chromophore analogues as a “guest” into a host–guest supramolecular structure similar to a natural β-barrel protective structure. Using GFP chromophore p-hydroxybenzene-2,3-dimethylimidazolinone (p-HBDI) as the core structure a novel GFP chromophore analogue (probe CA) was designed and synthesized by introducing a naphthalene ring into the imidazolinone ring. CA has an expanding π conjugated system by Knoevenagel condensation with 4-aldehyde pyridine and imidazolinone. The purpose of this design has three advantages: (i) the introduction of naphthalene rings can prolong the fluorescence emission wavelength of the whole molecule. Furthermore, the introduction of a naphthalene ring ensures a highly selective host–guest interaction with CB[7]. (ii) The introduction of the pyridine component further enhances the push–pull electron system of the entire molecule, allowing molecules to emit longer wavelength fluorescence. (iii) Thus, once the entire GFP analogue molecule CA is included into CB[7] through a strong host–guest interaction, its free rotation is inhibited and fluorescence is greatly enhanced. CB[7] is chosen as the host molecule because it is highly water-soluble and biocompatible. We anticipate that the self-assembled CA and CB[7] system is a promising supramolecular material that can be used for achieving highly reversible fluorescence optical switching, and thus can be further used for fluorescence anti-counterfeiting.
3.2. Fluorescence response of CA towards CB[6], CB[7] and CB[8]
Initially, the pH effect on CA was investigated. As shown in Fig. S1 (ESI†), there is no significant effect on the absorption and fluorescence of CA. Next, we compared the fluorescence emission spectra of probe CA before and after its interaction with CB[6], CB[7] and CB[8]. It was found that CA (50 μM) exhibited weak fluorescence intensity (its maximum fluorescence emission was at 535 nm), while the addition of cucurbiturils (CB[6], CB[7] or CB[8]) induced the enhancement in fluorescence intensity. Specifically, CB[7] induced the highest fluorescence intensity compared to CB[6] and CB[8] (Fig. 1a and b). The results showed that CB[6], CB[7] and CB[8] could work as the host to induce the fluorescence increasement. The absorption spectra further confirmed the interaction between CA and CB[6], CB[7] and CB[8], respectively (Fig. S2, ESI†). We deduced that this phenomenon could be ascribed to the encapsulation of CA into cucurbiturils and its intramolecular motion was restricted, resulting in the increase of fluorescence.Additionally, we carried out an assay to evaluate the fluorescence enhancement of CA after addition of β-cyclodextrin (and β-CD) or γ-cyclodextrin (γ-CD), respectively. As shown in Fig. S7 (ESI†), β-CD (or γ-CD) cannot enhance the fluorescence intensity of CA. There were no remarkable changes observed from the absorption spectra. This result suggests that there is a highly selective interaction between CA and cucurbiturils. The fluorescence quantum yields of CA and the CA–CB[7] complex were further investigated. As shown in Fig. S10 and Table S2 (ESI†), the fluorescence quantum yield of CA was 0.02. The fluorescence quantum yield of the CA–CB[7] complex is 0.13 which is an increase of about 7 times compared to that of CA, suggesting the fluorescence activation in the presence of CB[7].
3.3. Exploration of interactions between CA and CB[7] with NMR titration
In order to elucidate the proposed mechanism, 1H NMR titration was performed by adding CB[7] to DMSO-d6 containing CA. As shown in Fig. 2, compared with CA solution without CB[7], the addition of CB[7] can induce changes in the proton signals at positions i, j, k, l, m, and n of the pyridine ring and its connected double bonds, and these protons are affected and displaced after entering the CB[7] cavity, and allocated to the ethylene matrix and aromatic protons in the naphthenic ring part. Due to the redshift of protons at the i and j positions and on the naphthalene ring, the probe CA as a whole enters the cavity of CB[7], and the pyridine part forms a strong intermolecular dipole interaction with the carbonyl group on the pocket of the CB[7] molecule, making the designed molecule have a strong host–guest binding ability for CB[7]. The mechanism was further analysed using the HSQC and NOESY spectra (Fig. S24 and S25, ESI†).3.4. Further exploration of interactions between CA and cucurbiturils
To further explore the interaction between CA and cucurbiturils (CB[6], CB[7] and CB[8]), we performed ITC measurements (Fig. 3). In the process of CA titration by CB[7], there is an obvious heat change (endothermic), indicating the existence of a binding effect. The ITC curve shows that the stoichiometric ratio of probe CA to CB[7] is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CA/CB[7] = 2/1). The binding constant is determined to be Ka = 2.33 × 107 (the dissociation constant (Kd) = 4.3 × 10−8), which demonstrates a high binding affinity for the resulting CA–CB[7] complex.
1 (CA/CB[7] = 2/1). The binding constant is determined to be Ka = 2.33 × 107 (the dissociation constant (Kd) = 4.3 × 10−8), which demonstrates a high binding affinity for the resulting CA–CB[7] complex.
 | ||
| Fig. 3 The ITC isotherm for the titration of CB[7] (100 μM) into a solution of CA (10 μM), in 100 mM HEPES solution at 25 °C. | ||
For the CA–CB[7] complex, ΔH is 100 kJ mol−1, ΔS is 476.4 J mol−1 K−1, and ΔG is −42.038 kJ mol−1. Similarly, the binding free energy for CA and CB[6] is determined to be −25.998 kJ mol−1 which is smaller than the ΔG value of CA and CB[7] (Fig. S3, ESI†). While ΔG of CA and CB[8] can not be obtained from the ITC assay (Fig. S4 and Table 1, ESI†). Thus, CB[7] exhibited the highest binding affinity towards CA compared to CB[6] and CB[8]. We deduced that the binding process is driven by hydrophobic action. Additionally, from the ITC results, we could deduce that the binding mechanism for CB[7] and CA is through the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex, while CB[6] and CA formed a 1
2 host–guest complex, while CB[6] and CA formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex. It is difficult to deduce the binding mechanism of CA towards CB[8] from the ITC assay (Fig. S4, ESI†) despite there being fluorescence enhancement as shown in Fig. 1.
1 host–guest complex. It is difficult to deduce the binding mechanism of CA towards CB[8] from the ITC assay (Fig. S4, ESI†) despite there being fluorescence enhancement as shown in Fig. 1.
| CA + CB[7] | CA + CB[6] | CA + CB[8] | |
|---|---|---|---|
| n is the binding molar ratio between CA and cucurbituril, Kd is the dissociation constant, ΔH is the change of enthalpy, ΔS is the change of entropy, and ΔG is the change of Gibbs free energy. NA: not available. | |||
n (CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB[n]) CB[n]) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA |
| K d (M) | 4.30 × 10−8 | 2.77 × 10−5 | NA |
| ΔH (kJ mol−1) | 100 | 100 | NA |
| ΔS (J mol−1 K−1) | 476.4 | 422.6 | NA |
| ΔG (kJ mol−1) | −42.038 | −25.998 | NA |
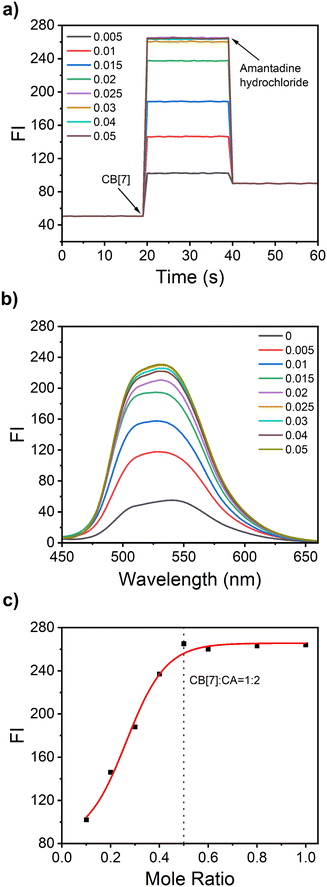
3.5. Dynamics interaction between CA and CB[7]
The real-time dynamics monitoring of CA and CB[7] is conducted using a fluorometer through the time-scan in time-scan mode. Initially, CB[7] was not added to the CA solution, and the scan was performed for 20 seconds. A very weak fluorescence signal was observed. When different concentrations of CB[7] were added to the CA solution at a 20-second time interval, the fluorescence immediately increased and reached a plateau, respectively. After amantadine hydrochloride was added, the fluorescence intensity immediately decreased and did not change with time (Fig. 4a). Because the binding constant Ka of amantadine hydrochloride for CB[7] can reach 1012, which is higher than the Ka value for binding of CA towards CB[7], the addition of amantadine hydrochloride will replace the CA from the cavity of CB[7]. Thus, the fluorescence is weakened. A similar phenomenon was observed in the presence of CA and CB[6] solution (Fig. S5a, ESI†). Then the fluorescence response of CA towards different concentrations of CB[7] was further evaluated. As shown in Fig. 4b, the fluorescence intensity gradually increases with the CB[7] concentration increasing from 5 μM to 25 μM, and finally reaches a plateau above the concentration of CB[7] of 25 μM. There are no remarkable changes in fluorescence intensity with the CB[7] concentration increasing from 25 μM to 50 μM. The fluorescence scatter plot of fluorescence intensity versus the CB[7]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA molar ratio was obtained from the data shown in Fig. 4a. A similar result was observed from the UV-Vis spectra (Fig. S6, ESI†). It can be concluded that CB[7] and CA formed a 1
CA molar ratio was obtained from the data shown in Fig. 4a. A similar result was observed from the UV-Vis spectra (Fig. S6, ESI†). It can be concluded that CB[7] and CA formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex, which is consistent with the ITC test results.
2 host–guest complex, which is consistent with the ITC test results.
Similarly, CB[6] and CA formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex according to the fluorescence scatter plot of fluorescence intensity versus CB[6]
1 host–guest complex according to the fluorescence scatter plot of fluorescence intensity versus CB[6]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA molar ratio (Fig. S5b, ESI†). Next, the effects of adamantane, 1-adamantane methylamine, methylamine, ethylenediamine and 3-hydroxytyramine on the CA–CB[7] complex were investigated. The results showed that both adamantane and 1-adamantane methylamine have a little effect on the disassembly process of CA–CB[7], while 1-adamantanamine hydrochloride can greatly decrease the fluorescence of the CA–CB[7] complex, suggesting the high specificity of 1-adamantanamine hydrochloride for the disassembly process (Fig. S8, ESI†). In addition, fluorescence lifetime studies were performed on self-assembled systems to gain a deeper understanding of the effects of alterations of CB[7] on the photophysical properties of CA (Fig. S9, ESI†).
CA molar ratio (Fig. S5b, ESI†). Next, the effects of adamantane, 1-adamantane methylamine, methylamine, ethylenediamine and 3-hydroxytyramine on the CA–CB[7] complex were investigated. The results showed that both adamantane and 1-adamantane methylamine have a little effect on the disassembly process of CA–CB[7], while 1-adamantanamine hydrochloride can greatly decrease the fluorescence of the CA–CB[7] complex, suggesting the high specificity of 1-adamantanamine hydrochloride for the disassembly process (Fig. S8, ESI†). In addition, fluorescence lifetime studies were performed on self-assembled systems to gain a deeper understanding of the effects of alterations of CB[7] on the photophysical properties of CA (Fig. S9, ESI†).
3.6. Reversible interaction between CA and CB[7]
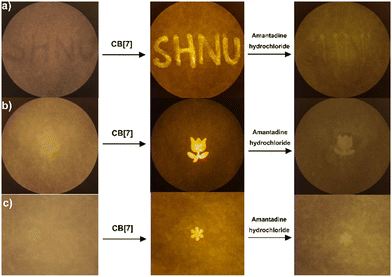
Next, the reversibility between CA and CB[7] is investigated. As shown in Fig. 5, the CA solution displayed a weak fluorescence signal, which could also be observed from the fluorescence of a cuvette under the hand-held UV lamp at 365 nm. The addition of CB[7] induced a strong fluorescence, which could be reversed following the addition of amantadine hydrochloride. Thus, this result further suggested the possibility of using the CA–CB[7] system for constructing reversible fluorescence anti-counterfeiting materials.3.7. Fluorescence anti-counterfeiting test based on the CA–CB[7] system
Inspired by the reversible fluorescence response of the CA–CB[7] system in solution, we next explore the use of CA–CB[7] as a fluorescent anti-counterfeiting material. On A4 paper, the characters of “SHNU” are written with CA solution. After drying in the air, it can be observed that the written paper has no significant fluorescence signal from the characters. After the CB[7] solution is sprayed onto the A4 paper, a strong fluorescence signal displayed by characters “SHNU” is observed. It is very interesting that the fluorescence signal displayed by the characters “SHNU” disappears after the amantadine hydrochloride solution was used for writing following the trace of “SHNU” characters (Fig. 6a). In Fig. 6b and c, rose and snowflake patterns were firstly drawn with CA solution on the A4 paper. No relevant fluorescence patterns were observed after drying. Then the CB[7] solution was sprayed onto these papers, the relevant fluorescence patterns were observed. When these patterns were soaked in the amantadine hydrochloride solution, the fluorescence patterns of rose and snowflake disappeared. This result suggests that the CA–CB[7] system could be used for preparing reusable and fast response fluorescence anti-counterfeiting materials that can directly capture signal changes in fluorescence intensity through portable devices of fluorescence acquisition camera systems. In addition, we conducted an assay to investigate the anti-counterfeiting effect of a single compound (CA) without CB[7], and found that there was no obvious anti-counterfeiting effect (Fig. S11, ESI†). We next measured the stability and durability of the fluorescence of the CA–CB[7] complex under actual conditions, such as exposing the visualized patterns to light, rinsing them repeatedly with water, and subjecting them to physical abrasion. All the results showed that the stability and durability of the CA–CB[7] complex were good (Fig. S12–S15, ESI†). Meanwhile, we explored the fluorescence stability of a “star” pattern after exposing it to continuous irradiation with ultraviolet lamps or white light of a LED lamp, and under the conditions of varying temperatures. The test results showed that the pattern visualized using the CA–CB[7] system had high fluorescence stability and durability under real-world conditions (Fig. S16–S18, ESI†). Finally, we carried out the fluorescence anti-counterfeiting test of the CA–CB[7] system on a glossy coated paper. The result suggested that the CA–CB[7] system can also be used for visualizing the “bear” pattern on the glossy coated paper (Fig. S19, ESI†).4. Conclusions
In summary, we designed and synthesized a reversible fluorescent anti-counterfeiting material based on the supramolecular host–guest interaction. The guest is a new GFP chromophore analogue (CA), and the protective cavity opened by the “guest” is provided by the host molecule CB[7], which is similar to the protective polypeptide coil of a natural fluorescent protein. The host–guest interaction between supramolecular compound CB[7] and probe CA was explored to construct novel fluorescent anti-counterfeiting materials. The experimental results showed that CB[7] could highly selectively encapsulate CA into its cavity by forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex, meanwhile inducing great fluorescence enhancement since the free rotation of CA was inhibited after it was encapsulated. The CA–CB[7] complex can be disassembled by adding a higher binding competitor, amantadine hydrochloride. Interestingly, the disassembly process could be further reversed by adding another aliquot of CB[7], which enables the reversible fluorescence response of CA–CB[7]. Finally, the CA–CB[7] system was successfully used for a fluorescence anti-counterfeiting assay on A4 paper. This proof of concept design presented a new design strategy for constructing fluorescence anti-counterfeiting materials by using the GFP analogue dye-based supramolecular system.
2 host–guest complex, meanwhile inducing great fluorescence enhancement since the free rotation of CA was inhibited after it was encapsulated. The CA–CB[7] complex can be disassembled by adding a higher binding competitor, amantadine hydrochloride. Interestingly, the disassembly process could be further reversed by adding another aliquot of CB[7], which enables the reversible fluorescence response of CA–CB[7]. Finally, the CA–CB[7] system was successfully used for a fluorescence anti-counterfeiting assay on A4 paper. This proof of concept design presented a new design strategy for constructing fluorescence anti-counterfeiting materials by using the GFP analogue dye-based supramolecular system.
Author contributions
Y. R. conducted the experiments and prepared the manuscript. C. H. revised the manuscript and gave the instruction.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grants 21672150 and 21302125), Shanghai Rising-Star Program (19QA1406400), Shanghai Municipal Education Commission, and Shanghai Engineering Research Center of Green Energy Chemical Engineering.Notes and references
- J. Du, L. Sheng, Q. Chen, Y. Xu, W. Li, X. Wang, M. Li and S. X.-A. Zhang, Mater. Horiz., 2019, 6, 1654–1662 RSC.
- H. Jia, Y. Teng, N. Li, D. Li, Y. Dong, D. Zhang, Z. Liu, D. Zhao, X. Guo, W. Di and W. Qin, ACS Mater. Lett., 2022, 4, 1306–1313 CrossRef.
- T. Jiang, F. Zhu, C. Zhang, J. Zhu, M. Zhang and J. Qiu, Adv. Funct. Mater., 2019, 29, 1906068 CrossRef.
- O. Kuksenok and A. C. Balazs, Mater. Horiz., 2016, 3, 53–62 RSC.
- Z. Lin, H. Pan, Y. Tian, J. Tang, C. Zhang, P. Zhang, H. Yang and J. Chen, Dyes Pigm., 2022, 205, 110588 CrossRef.
- Y. Ma, L. Shen, P. She, Y. Hou, Y. Yu, J. Zhao, S. Liu and Q. Zhao, Adv. Opt. Mater., 2019, 7, 1801657 CrossRef.
- J. Zhang, B. He, Y. Hu, P. Alam, H. Zhang, J. W. Y. Lam and B. Z. Tang, Adv. Mater., 2021, 33, 2008071 CrossRef CAS.
- X. Zhou, L. Wang, Z. Wei, G. Weng and J. He, Adv. Funct. Mater., 2019, 29, 1903543 CrossRef.
- S. T. Zimmermann, D. W. R. Balkenende, A. Lavrenova, C. Weder and J. Brugger, ACS Appl. Mater. Interfaces, 2017, 9, 41454–41461 CrossRef CAS.
- A. Li, Z. Li, M. Zhang, B. Wu, Y. Xing and L. Zhu, Adv. Opt. Mater., 2022, 10, 2102146 CrossRef CAS.
- C. Carrillo-Carrion and W. J. Parak, Small Methods, 2017, 1, 1700153 CrossRef.
- D. Hu, W. Xu, G. Wang, K. Liu, Z. Wang, Q. Shi, S. Lin, Z. Liu and Y. Fang, Adv. Funct. Mater., 2022, 32, 2207895 CrossRef CAS.
- Z. Man, Z. Lv, Z. Xu, Q. Liao, J. Liu, Y. Liu, L. Fu, M. Liu, S. Bai and H. Fu, Adv. Funct. Mater., 2020, 30, 2000105 CrossRef CAS.
- Y. Ren, S. Xie, E. Svensson Grape, A. K. Inge and O. Ramström, J. Am. Chem. Soc., 2018, 140, 13640–13643 CrossRef CAS.
- V. K. Singh, R. K. Chitumalla, S. K. Ravi, Y. Zhang, Y. Xi, V. Sanjairaj, C. Zhang, J. Jang and S. C. Tan, ACS Appl. Mater. Interfaces, 2017, 9, 33071–33079 CrossRef CAS PubMed.
- M. Yu, P. Zhang, B. P. Krishnan, H. Wang, Y. Gao, S. Chen, R. Zeng, J. Cui and J. Chen, Adv. Funct. Mater., 2018, 28, 1804759 CrossRef.
- S. Paul, R. S. Fernandes and N. Dey, New J. Chem., 2022, 46, 18973–18983 RSC.
- R. S. Fernandes and N. Dey, J. Mater. Chem. B, 2024, 12, 11789–11799 RSC.
- N. Dey, B. Maji and S. Bhattacharya, Chem. – Asian J., 2018, 13, 664–671 CrossRef CAS.
- K. Jin, X. Ji, T. Yang, J. Zhang, W. Tian, J. Yu, X. Zhang, Z. Chen and J. Zhang, Chem. Eng. J., 2021, 406, 126794 CrossRef CAS.
- H. Wu, Y. Wang, L. O. Jones, W. Liu, B. Song, Y. Cui, K. Cai, L. Zhang, D. Shen, X. Chen, Y. Jiao, C. L. Stern, X. Li, G. C. Schatz and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 16849–16860 CrossRef CAS.
- T. Jiang, X. Wang, J. Wang, G. Hu and X. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 14399–14407 CrossRef.
- W. Guo, T. Peng, W. Zhu, S. Ma, G. Wang, Y. Li, B. Liu and H. Peng, Aggregate, 2023, 4, e297 CrossRef.
- X. Sun, A. Liu, K. Xu, Z. Zheng, K. Xu, M. Dong, B. Ding, J. Li, Z. Zhang and C. Li, Aggregate, 2024, 5, e607 CrossRef.
- D. M. Chudakov, V. V. Belousov, A. G. Zaraisky, V. V. Novoselov, D. B. Staroverov, D. B. Zorov, S. Lukyanov and K. A. Lukyanov, Nat. Biotechnol., 2003, 21, 191–194 CrossRef PubMed.
- T. Grotjohann, I. Testa, M. Leutenegger, H. Bock, N. T. Urban, F. Lavoie-Cardinal, K. I. Willig, C. Eggeling, S. Jakobs and S. W. Hell, Nature, 2011, 478, 204–208 CrossRef PubMed.
- T. Grotjohann, I. Testa, M. Reuss, T. Brakemann, C. Eggeling, S. W. Hell and S. Jakobs, eLife, 2012, 1, e00248 CrossRef PubMed.
- S. W. Hell, Nat. Biotechnol., 2003, 21, 1347–1355 CrossRef PubMed.
- G. Patterson, M. Davidson, S. Manley and J. Lippincott-Schwartz, Annu. Rev. Phys. Chem., 2010, 61, 345–367 CrossRef.
- M. J. Rust, M. Bates and X. Zhuang, Nat. Methods, 2006, 3, 793–796 CrossRef.
- K. I. Willig, S. O. Rizzoli, V. Westphal, R. Jahn and S. W. Hell, Nature, 2006, 440, 935–939 Search PubMed.
- J. Kong, Y. Wang, W. Qi, M. Huang, R. Su and Z. He, Adv. Colloid Interface Sci., 2020, 285, 102286 CrossRef.
- T. D. Craggs, Chem. Soc. Rev., 2009, 38, 2865–2875 RSC.
- S. J. Remington, Protein Sci., 2011, 20, 1509–1519 CrossRef PubMed.
- H. Deng and X. Zhu, Mater. Chem. Front., 2017, 1, 619–629 RSC.
- D. Li, Z. Feng, Y. Han, C. Chen, Q. W. Zhang and Y. Tian, Adv. Sci., 2022, 9, 2104790 CrossRef CAS.
- J. Manigrasso, I. Chillón, V. Genna, P. Vidossich, S. Somarowthu, A. M. Pyle, M. De Vivo and M. Marcia, Nat. Commun., 2022, 13, 2837 CrossRef.
- Y. Sasaki, X. Lyu, W. Tang, H. Wu and T. Minami, J. Photochem. Photobiol., C, 2022, 51, 100475 Search PubMed.
- A. F. Sierra, D. Hernández-Alonso, M. A. Romero, J. A. González-Delgado, U. Pischel and P. Ballester, J. Am. Chem. Soc., 2020, 142, 4276–4284 Search PubMed.
- A. Tittl, A. John-Herpin, A. Leitis, E. R. Arvelo and H. Altug, Angew. Chem., Int. Ed., 2019, 58, 14810–14822 CrossRef CAS PubMed.
- H. Wu, Y. Chen and Y. Liu, Adv. Mater., 2016, 29, 1605271 CrossRef.
- Z. Xu, D. Gonzalez-Abradelo, J. Li, C. A. Strassert, B. J. Ravoo and D.-S. Guo, Mater. Chem. Front., 2017, 1, 1847–1852 RSC.
- A. Hennig and W. M. Nau, Front. Chem., 2020, 8, 00806 CrossRef CAS.
- H. Liu, M. Zhang, J. Yu and Y. Liu, Angew. Chem., Int. Ed., 2020, 60, 3870–3880 Search PubMed.
- E. Masson, X. Ling, R. Joseph, L. Kyeremeh-Mensah and X. Lu, RSC Adv., 2012, 2, 1213–1247 RSC.
- K. Aratsu, R. Takeya, B. R. Pauw, M. J. Hollamby, Y. Kitamoto, N. Shimizu, H. Takagi, R. Haruki, S.-I. Adachi and S. Yagai, Nat. Commun., 2020, 11, 1623 Search PubMed.
- H. Huang, Y. Jin, S. Yan, H. Cong and Z. Tao, ChemistrySelect, 2018, 3, 4705–4711 Search PubMed.
- H. Ju, N. Zhu, H. Wang, Z. A. Page, Z. L. Wu, J. L. Sessler and F. Huang, Adv. Mater., 2021, 34, 2108163 Search PubMed.
- Z. Li, W. Kang, H. Yang, B. Zhou, H. Jiang, D. Liu, H. Jia and J. Wang, Adv. Colloid Interface Sci., 2022, 301, 102617 CrossRef CAS PubMed.
- B. V. K. J. Schmidt, M. Hetzer, H. Ritter and C. Barner-Kowollik, Macromolecules, 2013, 46, 1054–1065 Search PubMed.
- D.-X. Wang and M.-X. Wang, Acc. Chem. Res., 2020, 53, 1364–1380 Search PubMed.
- M. Zhang, H. Liu and Y. Liu, Adv. Mater., 2019, 32, 1806158 Search PubMed.
- M. Canton, A. B. Grommet, L. Pesce, J. Gemen, S. Li, Y. Diskin-Posner, A. Credi, G. M. Pavan, J. Andréasson and R. Klajn, J. Am. Chem. Soc., 2020, 142, 14557–14565 CrossRef CAS PubMed.
- J. Mohanty and W. M. Nau, Angew. Chem., Int. Ed., 2005, 44, 3750–3754 CrossRef CAS.
- D. Sun, Y. Wu, X. Han and S. Liu, Chem. – Eur. J., 2021, 27, 16153–16160 CrossRef CAS PubMed.
- H. Wu, Y. Chen, X. Dai, P. Li, J. F. Stoddart and Y. Liu, J. Am. Chem. Soc., 2019, 141, 6583–6591 CrossRef CAS PubMed.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 Search PubMed.
- D. Shetty, J. K. Khedkar, K. M. Park and K. Kim, Chem. Soc. Rev., 2015, 44, 8747–8761 RSC.
- V. D. Uzunova, C. Cullinane, K. Brix, W. M. Nau and A. I. Day, Org. Biomol. Chem., 2010, 8, 2037–2042 Search PubMed.
- H. Jung, K. M. Park, J.-A. Yang, E. J. Oh, D.-W. Lee, K. Park, S. H. Ryu, S. K. Hahn and K. Kim, Biomaterials, 2011, 32, 7687–7694 CrossRef CAS PubMed.
- C. Kim, S. S. Agasti, Z. Zhu, L. Isaacs and V. M. Rotello, Nat. Chem., 2010, 2, 962–966 CrossRef CAS.
- H. Wang, K.-F. Xue, Y. Yang, H. Hu, J.-F. Xu and X. Zhang, J. Am. Chem. Soc., 2022, 144, 2360–2367 CrossRef CAS PubMed.
- Y. Ma, B. Guo, J.-Y. Ge, L. Chen, N. Lv, X. Wu, J. Chen and Z. Chen, Anal. Chem., 2022, 94, 12383–12390 CrossRef CAS PubMed.
- H. Wang, X. Ji, Z. Li and F. Huang, Adv. Mater., 2017, 29, 1606117 CrossRef.
- Y. Huang, L. Ning, X. Zhang, Q. Zhou, Q. Gong and Q. Zhang, Chem. Soc. Rev., 2024, 53, 1090–1166 RSC.
- C. Würth, M. Grabolle, J. Pauli, M. Spieles and U. Resch-Genger, Nat. Protoc., 2013, 8, 1535–1550 CrossRef.
- D. Magde, R. Wong and P. G. Seybold, Photochem. Photobiol., 2002, 75, 327–334 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb02112f |
| This journal is © The Royal Society of Chemistry 2025 |