Next-generation biopolymer gels: innovations in drug delivery and theranostics
Danish Ahmad
Shergujri
a,
Murtaza Ahmad
Khanday
 a,
Aisha
Noor
a,
Mohd
Adnan
a,
Aisha
Noor
a,
Mohd
Adnan
 b,
Iqra
Arif
a,
Syed Naiem
Raza
a,
Reyaz Hassan
Mir
b,
Iqra
Arif
a,
Syed Naiem
Raza
a,
Reyaz Hassan
Mir
 *c and
Nisar Ahmad
Khan
*a
*c and
Nisar Ahmad
Khan
*a
aPharmaceutics Division, Department of Pharmaceutical Sciences, University of Kashmir, Hazratbal, Srinagar-190006, Jammu and Kashmir, India. E-mail: nakhan2008@gmail.com
bDepartment of Biology, College of Science, University of Ha’il, Ha’il, P.O. Box 2440, Saudi Arabia
cPharmaceutical Chemistry Division, Department of Pharmaceutical Sciences, University of Kashmir, Hazratbal, Srinagar-190006, Jammu and Kashmir, India. E-mail: mirreyaz.phscct@uok.edu.in
First published on 22nd January 2025
Abstract
Biopolymers or natural polymers like chitosan, cellulose, alginate, collagen, etc. have gained significant interest recently due to their remarkable tunable properties that make them appropriate for a variety of applications & play a crucial role in everyday life. The features of biopolymers which include biodegradability, biocompatibility, sustainability, affordability, & availability are vital for creating products for use in biomedical fields. Apart from these characteristics, smart or stimuli-responsive biopolymers also show a distinctive property of being susceptible to various factors like pH, temperature, light intensity, & electrical or magnetic fields. The current review would present a brief idea about smart biopolymer gels along with their biomedical applications. The use of smart biopolymers gels as theranostic agents are also discussed in the present review. This review also focuses on the application of biopolymers in the fields of drug delivery, cancer treatment, tissue engineering & wound healing. These areas demonstrate the development and utilization of different types of biopolymers in current biomedical applications.
1. Introduction
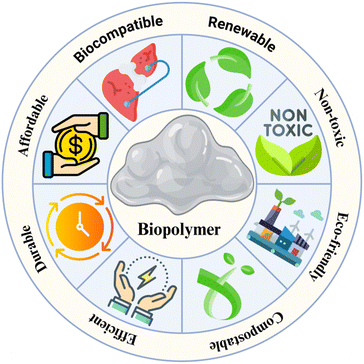
The term “biopolymers” describes polymeric materials entirely or partially synthesized by living organisms by chemical synthesis of biological ingredients from natural resources.1 Similar to other polymers, biopolymers consist of interconnected repeating units, called monomers.2 The primary benefit of biological polymers is their capacity to degrade in the presence of living microorganisms.3 Through the enzymatic action of microbes, biopolymers disintegrate into CO2, CH4, H2O, inorganic chemicals, etc.4 The benefits of biopolymers include reduced carbon emission, biocompatibility, renewability, & affordability.5 Additionally, biopolymers have the extremely significant benefit of being ecologically friendly. Biopolymers have been shown to be risk-free, non-thrombogenic, non-carcinogenic, and easy to extract.6,7Fig. 1 summarizes the main characteristics of biopolymers. Biopolymers are being widely employed in medical, pharmaceutical & industrial fields.3In the pharmaceutical sector, polymeric gels are acknowledged as possibly useful delivery techniques for handling medication delivery issues. Gels are usually homogeneous, semi-solid formulations that include a medication dispersion in a three-dimensional (3D) network that is either hydrophilic or hydrophobic.8,9 The simplicity of preparation has led to the popularity of these formulations. Additionally, they provide a close connection between the therapeutic component & the site of action, along with controlled delivery via several pathways. Gels are commonly divided into two categories: hydrogels (a) & organogels (b). Large quantities of biological fluids or water may bond to hydrogels, a form of hydrophilic three-dimensional polymeric network, without the polymer dissolving.10 As a cross-linked network of hydrophilic polymers, hydrogels may swell when they come into contact with biological fluids or aqueous fluids and contract (de-swell) when the water is released.11–13 Hydrogel possesses the capacity to hold a large amount of water by virtue of capillary action & osmotic pressure. Because of these properties, hydrogels mimic real tissues' extracellular matrix.14 The hydrogels which are sensitive to external stimuli contain functional groups that are ionizable. The sensitivity of these hydrogels is determined by the type, content, and quantity of functional groups on the polymer backbone.15,16 Organogels or oleogels are the result of immobilizing gelator fibers with an organic liquid phase and then creating a three-dimensional network.17
Hydrogel particles with a submicron size range are called microgels, or nanogels.18,19 In aqueous solutions, hydrophilic, hydrophobic, or amphiphilic polymers are combined chemically or physically to create nanogels.20–22 Synthetic, natural, or an amalgam of the two types of polymers can be used to generate nanogels. Depending on the synthesis techniques used, nanogels can take on a variety of morphologies, including spherical particles, core–shell structures, & core–shell–corona structures.23 In the biomedical field, nanogels are particularly advantageous over other types of nanomaterials because of their ability to react rapidly to changes in temperature, ionic strength, light, & pH.22,24 Because of its beneficial features, which merge those of hydrogels & nanoparticles, nanogels are considered as a promising drug delivery system. Many bioactive molecules, such as proteins, drugs, & vaccines, have been extensively studied and delivered via nanogels.21,25,26
Environmentally-sensitive or intelligent gels are other names for smart polymer gels. Researchers are becoming more and more interested in stimuli-responsive gel systems, especially in delivery systems that are controlled or self-regulated. In reaction to slight variations in their surroundings, such as pH, temperature, light, magnetic field, & ionic components, stimuli-responsive or “smart” gels exhibit an exceptional physiochemical change. Since these are reversible alterations, when the signal or trigger is withdrawn, the system may go back to its original state.27,28 Polymer gels' response to external stimuli is governed by several factors, including the material's shape or dimensional change (strain), its force-exerting capability, response speed, shape recovery, and viscoelastic loss resulting from heat dissipation.29 These days, stimuli-responsive polymers may sense two or more stimulating events in addition to a single one because of their unique design.30 Hydrogels that respond to changes in temperature or pH are most widely used.31–33 Targeting cells or tissues with controlled drug release, biopolymer gels may be engineered to react to environmental signals therefore augmenting therapeutic effects. Research has demonstrated that these gels are a flexible and efficient drug delivery platform because they can be tailored to increase drug characteristics, release mechanisms, and targeting abilities in addition to being strengthened mechanically for tissue regeneration.5
Biopolymers can be modified using a range of chemical and physical methods to greatly improve their performance and qualities, opening up a larger range of uses. Among other qualities, these changes can enhance biodegradability, thermal stability, and mechanical strength. The various physiochemical medications include mechanical processing, thermal treatment, crosslinking, grafting, etc. Biopolymers' mechanical qualities can be improved by changing their physical structure through processes like extrusion and molding. Biopolymers become stronger and more flexible as a result, which makes them appropriate for a range of applications in construction and packaging. Biopolymers can also be heated to enhance their processing properties and thermal stability. Applications like automobile parts, where heat resistance is essential, may benefit from this alteration. Crosslinking leads to the formation of bonds among polymer chains, which improve the thermal stability and mechanical strength of the biopolymer. These biopolymers are frequently utilized in coatings and adhesives, besides other applications that call for long-lasting materials. Another modification is grafting, grafting is the technique of adding various groups of chemicals to the core of a biopolymer, which could enhance its lipophilicity or hydrophilicity and increase its compatibility with various other substances. These biopolymers are used in medication delivery systems (bio medical field). Modified biopolymers have a wide range of significant biomedical uses, from tissue engineering & wound healing to drug delivery systems. They are a potential area for medical research and development because of their capacity to be customized for certain purposes.34–36Fig. 2 demonstrates statistics about the number of publications in PubMed since 2000 until 2024 regarding this subject. These data explain the urgent need to explore the enigma of favorable biopolymer gels for biomedical applications.
2. Properties of gels
2.1 Swelling
When the gelling agent and solubilizing solvent come into contact, the gelling agent absorbs a lot of solvent and increases in volume. This process is referred to as swelling. The process is brought about by a substantial amount of solvent penetrating the matrix. The strength, number, and kind of links among the individual molecules of the gelling agent often determine the degree of swelling.37 Hydrogels' ability to swell when exposed to H2O or physiological fluids relies on the osmotic pressure created by the hydrophilicity of the constituent polymers, the static charges on the polymer, and the counter ions in the hydrogel matrix. Three stages are involved in the swelling of hydrogels: the diffusion of liquid into the hydrogel, the relaxation of the chains of polymers upon hydration, & the enlargement of the network as the chains relax.38 Absorption resulting from the hydrophilic & polar groups' attraction to water molecules is known as primary bound water. Consequently, the hydrogels expand, and the hydrophobic components that are exposed bind with water molecules, resulting in secondary bound water.39 Hydrogels' water capacity is often determined by the equilibrium swelling ratio (SR) or swelling degree (SD), which may be computed using the following equation.where M is mass of hydrogel.
The swelling index (SD) of some biopolymer based hydrogels is indicated in Table 1.
2.2 Syneresis
Certain gels instantly release a certain amount of fluid as they contract upon standing. This phenomenon is called syneresis. This process shows that the polymeric gel was unstable from a thermodynamic perspective. Decreasing the gelling agent concentration in a gel increases the degree of syneresis. The process of contraction arises from the relaxation of elastic stress that is created during the gels' settling.37 Syneresis is generally linked to the stiffness of the gel and has been ascribed to disproportion within the forces on each side of the gel–fluid interface.46 Additionally, aging-related chemical changes to the polymer can also cause syneresis. For example, at elevated temperatures, the acrylamide-based polymers employed in H2O cutoff treatments are almost susceptible to hydrolysis. The carboxylate moieties created by the hydrolysis of the polymer's acrylamide groups can additionally combine with divalent cations, resulting in gel syneresis. Another explanation for this syneresis is that divalent ions & carboxylates are over-crosslinked.472.3 Aging
Colloidal systems often exhibit slow aggregation. This phenomenon is called ageing. Aging in gels results in the steady development of a denser structure of the gelling agent.37 Aging has a major effect on the biopolymer composites. Aging affects performance under many circumstances. Composites can deteriorate chemically, thermally, mechanically, organically, or by photo-oxidation as they undergo aging. Additionally, the bio composites' ability to efficiently absorb oxygen at high temperatures speeds up the biopolymer composites' degradation.482.4 Structure
The network that is created when the particles of the gelling agents bind together is what gives a gel its stiffness. Particle characteristics and stress straighten them & thus reduce flow resistance.37 It is believed that the type of force and the nature of the gelling component or components are accountable for the interactions that define the gel characteristics and networking structure.492.5 Rheology
Gelling agent solutions and flocculated solid dispersions have a pseudoplastic nature, meaning that they obey non-Newtonian law and exhibit a viscosity decrease as shear rate increases.37 A commercial fluorosilicone gel (Dow Corning DC4-8022) serves as an example to illustrate several facets of gel rheology. Despite its softness, this gel is nonetheless regarded as strong due to the persistent chemical crosslinks. These soft gels' viscoelastic nature is easily noticeable and adds to their rich dynamic responsiveness.50 Viscosity and shear-thinning behavior are important rheological characteristics in the setting of hydrogels for 3D printing. Proper extrusion via the printer nozzle is ensured by an appropriate viscosity, and shear-thinning behavior enables the hydrogel to decrease viscosity under shear stress throughout printing, promoting layer deposition & smooth flow.51 Kim et al.52 focused on using carrageenan to improve the rheological properties of alginate hydrogels, particularly for extrusion-based bioprinting techniques.3. Biopolymers employed in smart drug delivery
3.1 Chitosan
Chitosan is an extensively researched harmless biopolymer that is widely used for a range of medical uses.53,54 This cationic polymer is made from the naturally existing polymer chitin and is made up of N-acetyl & D-glucosamine. Chitosan is naturally present within the exoskeletons of fungi,55 insects, mollusks, annelids, and crustaceans. Moreover, chitosan has hemostatic, mucoadhesive, adhesive, and biocompatible properties.56,57Chitosan has been included in DDSs in a variety of forms, including oral tablets, gels, films, beads, and microspheres for transdermal, ocular, nasal, and oral routes.58,59 Chenite et al.60 documented the application of chitosan-β-glycerophosphate (C-β-GP) water-based solutions as pH-sensitive and thermoresponsive gelling systems. The ammonium groups in chitosan are neutralized by the phosphates in the glycerophosphate salt, which increases the hydrophobic & hydrogen bonding within the chitosan chains at higher temperatures and forms a more cohesive gel compared to that formed at lower temperatures. The same gel was examined for the in vitro administration of drugs61 and the in vivo distribution of physiologically active growth factors.60 Dang et al.62 examined the process of conjugating hydroxybutyl groups to chitosan, with the aim of producing a polymer that is both thermoresponsive as well as soluble in water. Another method that has been investigated is the modification of chitosan using synthetic polymers to produce thermoresponsive materials.63–65 Carrageenan and chitosan together create a pH-sensitive system where the SO4 groups of carrageenan & the NH2 groups of chitosan interact electrostatically in order to control drug release in an alkaline environment.66
3.2 Hyaluronic acid
HA is a linear anionic polysaccharide that occurs naturally. Its natural chemical structure is composed of 2 disaccharide units, N-acetyl-D-glucosamine and D-glucuronic acid, which polymerize into large macromolecules (approx 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 repeating units). HA is neither immunogenic, thrombogenic, inflammatory or poisonous, but biodegradable in nature. Because of its innately advantageous natural properties, HA is utilized in various pharmaceutical and biomedical applications. Efficiency is enhanced by the amount of hydroxyl and carboxylic acid groups. These functional groups may be helpful to form new functional groups by crosslinking conjugation, and chemical bonding. A simple method for producing the beneficial microgel or nanogel from HA biopolymers is to use a functional crosslinker.67–70 Liang et al.71 synthesized a novel nanogel via self-assembly of granzyme B (GzmB) linear polyethylenimine (PEI), and hyaluronic acid–epigallocatechin gallate conjugates (HA–EGCG), in order to introduce GzmB into cancerous cells. Studies demonstrated that epigallocatechin gallate (EGCG) moieties improved the binding of proteins through physical bonds, resulting in strong nanogels. The use of HA–EGCG as an effective intracellular protein carrier for specific cancer therapy is underlined by this study.72 Nitric oxide (NO) & antimicrobial peptide (AMP) can be delivered simultaneously via a nanogel that was created by Fasiku et al. to fight microbial or biofilm associated infections. The NO-releasing nanogel was made by crosslinking a hyaluronic acid solution with divinyl sulfone. The resultant nitric oxide (NO)-antimicrobial peptide (AMP) showed great anti-bacterial or anti biofilm activity in in vitro release studies.73,74 The properties of hyaluronic acid are highly intriguing, and its applications in biomedicine seem endless. Because of its great swelling capacity, elasticity, and viscosity, it is being used as an injectable hydrogel to improve human soft tissue in regenerative medicine and cosmetology. Its poor mechanical qualities and quick deterioration after injection underneath living tissue are its drawbacks. Various modifications were done in order to improve its hydrogel quality such as photopolymerization, crosslinking with epihalohydrin, divinyl sulfone, epoxides, etc.75
000 repeating units). HA is neither immunogenic, thrombogenic, inflammatory or poisonous, but biodegradable in nature. Because of its innately advantageous natural properties, HA is utilized in various pharmaceutical and biomedical applications. Efficiency is enhanced by the amount of hydroxyl and carboxylic acid groups. These functional groups may be helpful to form new functional groups by crosslinking conjugation, and chemical bonding. A simple method for producing the beneficial microgel or nanogel from HA biopolymers is to use a functional crosslinker.67–70 Liang et al.71 synthesized a novel nanogel via self-assembly of granzyme B (GzmB) linear polyethylenimine (PEI), and hyaluronic acid–epigallocatechin gallate conjugates (HA–EGCG), in order to introduce GzmB into cancerous cells. Studies demonstrated that epigallocatechin gallate (EGCG) moieties improved the binding of proteins through physical bonds, resulting in strong nanogels. The use of HA–EGCG as an effective intracellular protein carrier for specific cancer therapy is underlined by this study.72 Nitric oxide (NO) & antimicrobial peptide (AMP) can be delivered simultaneously via a nanogel that was created by Fasiku et al. to fight microbial or biofilm associated infections. The NO-releasing nanogel was made by crosslinking a hyaluronic acid solution with divinyl sulfone. The resultant nitric oxide (NO)-antimicrobial peptide (AMP) showed great anti-bacterial or anti biofilm activity in in vitro release studies.73,74 The properties of hyaluronic acid are highly intriguing, and its applications in biomedicine seem endless. Because of its great swelling capacity, elasticity, and viscosity, it is being used as an injectable hydrogel to improve human soft tissue in regenerative medicine and cosmetology. Its poor mechanical qualities and quick deterioration after injection underneath living tissue are its drawbacks. Various modifications were done in order to improve its hydrogel quality such as photopolymerization, crosslinking with epihalohydrin, divinyl sulfone, epoxides, etc.75
3.3 Alginate
Alginate is an established linear anionic polyelectrolyte polysaccharide composed of α-L-guluronic acid (G units) & β-D-mannuronic acid (M units) (linked via 1–4 glycosidic bond) and is being widely used in the synthetic hydrogel in artificial ECM (extracellular matrix).76 The monosaccharide sequences of alginate can be organized through blocks of repeating M regions (MM blocks), repeating G regions (GG blocks), or hybrid M & G regions (MG blocks). Gels made from alginates with a larger percentage of G blocks are notably stronger than those made from alginates having a higher percentage of M blocks. This is owing to the fact that G residues are more suitable than M residues to bind divalent ions. Therefore, the primary factors influencing the physiochemical properties of alginate include the M/G ratio & the order of monosaccharide repetitions.77,78 As soon as counter ions are added, alginic acid or its derivatives begin to form a polymeric network, which results in the hydrogel delivery system. An alginate reaction can be initiated by any type of cationic species, although studies have shown that CaCO3 and alginate reactions are the most effective and desirable.79 Initially, the food industry employed alginate as a component and adjuvant, owing to its qualities such as biocompatibility, elasticity, nontoxicity, biodegradability, affordability, etc. it is being widely employed as a major biomaterial in numerous cutting-edge uses in the pharmaceutical, medical, & cosmetics sectors. Like various other naturally occurring polymers, alginate has several drawbacks, like poor dimensional stability, limited biocompatibility, and poor mechanical properties.In order to produce biodegradable colloidal structures like nanoparticles, gels, biofilms, microcapsules, & beads, alginate is used. These biodegradable structures are used in regenerative medicine, orthodontic use, drug delivery, wound healing, etc.75 Valentino et al. demonstrated the formation of nanogels via ionotropic gelation between the cationic spermidine (SP) and the anionic alginate.80 In light of the results, the formulation containing 0.017% (w/w) SP & 0.17% (w/w) alginate (less viscous) was determined to be the most suitable sample. The antioxidant and anti-inflammatory qualities of the formulation, as well as its biocompatibility, were confirmed by the in vitro study conducted on Schwann cells. The element that contributes to the work's uniqueness is the application of SP as both a crosslinker and a neuroprotective agent.73
3.4 Cellulose
Cellulose is the most common polymer occurring naturally on Earth, and it may be found in a broad variety of sources, such as plants (such as wood, cotton, etc.), microorganisms (including amoeba, algae and fungi), and marine life (like tunicates). Cellulose is a polysaccharide composed of anhydro D-glucopyranose units linked with each other via glycosidic linkage (β-1–4 linkage). The fundamental component of many biological compounds is cellulose. Cellulose's hydroxyl groups give it the natural tendency to organize itself and form extensive networks by connecting individual molecules with hydrogen bonds.Cellulose and its derived substances are now being thoroughly investigated for possible use in medicine,81 biomedicine,82 the pharmaceutical field,83,84etc., due to its cost effectiveness, extensive availability, good mechanical properties, biocompatibility, biodegradability, and low cytotoxicity.81 A lot of study has been done on cellulose-based nano gels; just as it has been done with different polysaccharides. A study conducted recently produced responsive and functional nanosilver by in situ reduction of AgNO3 using oxidized carboxymethyl cellulose (OCMC). Nanosilver is confined inside the OCMC gel system. The oxidized carboxymethyl cellulose (OCMC) polymer chain stabilizes nanoparticles in addition to the reduction process. The potent antibacterial property of the silver nanogels effectively eliminated Gram-positive as well as Gram-negative bacteria. The produced Nanogels destroyed the bacterial cell wall & generated ROS within the cell, resulting in bacterial cell death.73
Hydrogels made from cellulose have widespread use in various applications such as biosensors, heavy metal adsorbers, wastewater treatment dyes, and more.75 The hydrogels derived from cellulose and its related substances showcase benefits in structure and morphology, such as pore diameters and swelling ratio increment, owing to the repellent effects of intramolecular carboxyl groups.85 For instance, novel hydrogels made from carboxymethyl cellulose that were physically cross-linked using phytic acid (an uncommon crosslinking agent) showed antibacterial activity.86
3.5 Gelatin
Gelatin refers to a protein that is produced by hydrolyzing collagen in a regulated, partial, and irreversible manner. The collagen comes from animal tissues (such as bone, skin, & cartilage)—typically from fish, bovine or pigs. Two varieties of gelatin are often produced, notably type A (by acid hydrolysis) & type B (by alkaline hydrolysis), based on the method employed as well as the types of animals.75 Gelatin is composed of peptide sequences and is soluble in water (warm). It retains the ability to form basic gel structures through hydrophobic crosslinking at cold temperatures. Due to its distinct physical and chemical properties, gelatin is a biocompatible and non-immunogenic protein that is used as a medication and cell carrier. Gelatin can only be applied at body temperature or higher because of its melting point, which is between 30 and 35 °C. Because of this restriction, it is typically chemically altered in a variety of creative ways, such as by further crosslinking procedures.87,88 Hydrogels based on gelatin are soluble in water, non-immunogenic and non-toxic substances. They were used for a variety of biomedical applications, including cell encapsulation, bone repair,89 nerve regeneration,90 3D bioprinting91 and wound healing due to their remarkable biodegradability and biological compatibility properties.92,93 Gelatin's availability and wide range of commercial applications have made it an important component for the formation of nanogels, for example fish gelatin methacryloyl (GelMA)-based nanogels were created using a water-in-oil nanoemulsion.73 The main drawbacks of hydrogels based on a gelatin are their low melting point and weak mechanical strength.753.6 Collagen
Collagen is an extracellular structural protein that occurs primarily in mammalian connective tissues and essentially gives the body its mechanical strength. Fibroblast cells are the primary producers of collagen, which is mostly found in tendons, skin and ligaments. The triple-helix structure of collagen contains hydrogen bonds, which give it extraordinary tensile strength. Its cationic flexible polymer structure is made up mostly of peptide sequences that are hydrophobic. Collagen finds widespread application in various areas of delivering drugs, tissue engineering, biomedical engineering, and nowadays it is used as a carrier for nucleic acids, proteins, and genes. In cell cultures and biomedical research, collagen serves as one of the most widely utilized biopolymers. The primary benefits of collagen include its strong cell adhesion substrate, low immunogenicity and chemostatic properties. The limitations of collagen's use can be seen in its drawbacks, which include a significant shrinkage, rapid rate of disintegration, poor mechanical strength, and opacity. Collagen's use can be increased by modifying it.73,75Table 2 summarizes the characteristics of different biopolymers.| Biopolymer | Source | Characteristics | Application | Ref. |
|---|---|---|---|---|
| Chitosan | Fungi, crustaceans, etc. | Polysaccharide, moderate mechanical strength | Wound dressings, drug delivery | 55 and 94 |
| Hyaluronic acid | Animal connective tissue | Polysaccharide, excellent mechanical properties | Biomedical field and cosmetic field | 95 |
| Alginate | Brown algae and some bacterial species | Polysaccharide, outstanding mechanical qualities | wound dressings, agricultural industry (seed coating) | 96 and 97 |
| Cellulose | Plant, microorganisms, marine life | Polysaccharide, good mechanical properties | Drug delivery, packaging, textiles | 94 and 98 |
| Gelatin | Animal tissues (collagen) | Protein, weak mechanical qualities | Tissue engineering, 3D bioprinting | 99–101 |
| Collagen | Animal (mammalian connective tissues) | Protein, extraordinary mechanical strength | Biomedical research, cell culture, gene carrier | 99 and 102 |
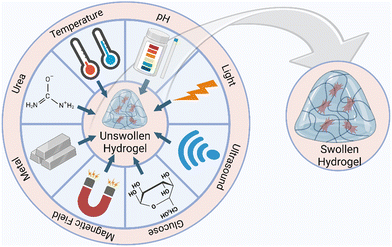
4. Classification of smart biopolymers
The last two decades have seen a sharp increase in the application of functional polymers. These polymers react to variations in pH, electric or magnetic fields, temperature and other factors in a desirable manner (Fig. 3).103 The literature extensively documents the methods by which these stimuli induce structural modifications in the polymer chain and subsequently modulate drug release.11 External stimuli are generated using various stimuli generating devices, while internal stimulus is created inside the body to regulate the structural modifications in the polymer network and achieve the required medication release.104Table 3 highlights the various hydrogel types, their responses to various stimuli, and possible uses in the biomedical industry.| Gel type | Stimulus | Application | Ref. |
|---|---|---|---|
| pH responsive | Acid or base environment | Delivery of drugs. Delivery of cells. | 105 and 106 |
| Thermo-responsive | Heating or cooling, possible electromagnetic waves, including infrared. | Tissue engineering, drug administration, wound care, and imaging methods. | 107 and 108 |
| Electro-responsive | Electrical | Regulated release of drugs | 109 |
| Light-responsive | Electromagnetic waves | Regulation of biological materials, biosensors, and stimulating factors | 110 |
| Ultrasound responsive | Sound or US | Imaging, drug delivery and cancer therapy | 111 |
| Glucose-responsive | Glucose | Insulin release, blood glucose monitoring, | 112 |
| Enzyme responsive | Enzyme | Delivery of drugs | 113 |
4.1 pH responsive biopolymers
The ability of naturally occurring polymers to respond smartly to pH variations has been widely researched. Because of the variations in pH that might occur naturally or as a result of physiological or pathological conditions in the body, pH-responsive materials have become particularly interesting.114 For example, variations in pH are seen in several physiological locations, including the gastrointestinal system, vagina, and blood arteries. These variations may provide as a good foundation for medication release that responds to changes in pH.13 Since tumor tissues have been found to have a significantly more acidic environment (pH range of 5–6) than healthy tissues (pH 7.4), pH-sensitive hydrogels are also being considered in the treatment of cancer targeting.Any polymer that is pH-sensitive must possess an ionizable basic or acidic functional group that reacts when the pH of the surrounding environment changes.105 Since these polymers have basic groups (amino salts) or acidic groups (carboxylic or sulphonic) in their structure, they may accept or release protons in reaction to pH variations.107 Depending on their degree of (de)protonation, these polymers respond to pH changes by self-assembling into vesicles, micelles, gels, unimers, swelling/deswelling phenomena, etc.115 Examples of pH-sensitive biopolymers are gum-tragacanth, chitosan, dextran, hyaluronic acid, alginate & xanthan (Table 4).116,117
| Biopolymer | Source | Ionic nature | Advantages | Ref. |
|---|---|---|---|---|
| Chitosan | Exoskeleton of fungi | Cationic | Minimal levels of toxicity, biologically active, biodegradability, hemostatic action, and mucoadhesive properties. | 55, 118 and 119 |
| Alginate | Brown seaweeds | Anionic | Produces a gel with varying swelling properties when divalent cations like Ca2+, Ba2+, Sr2+, and Zn2+ are present. | 120–122 |
| Hyaluronic acid | Connective tissue, skin and synovial fluid. | Anionic | Non-toxic, biodegradable, non-immunogenic and biocompatible. | 123 and 124 |
| Gelatin | Animal tissues | Cationic | Water soluble, non-toxic, cell adhesion capacity, low immunogenic. | 125 and 126 |
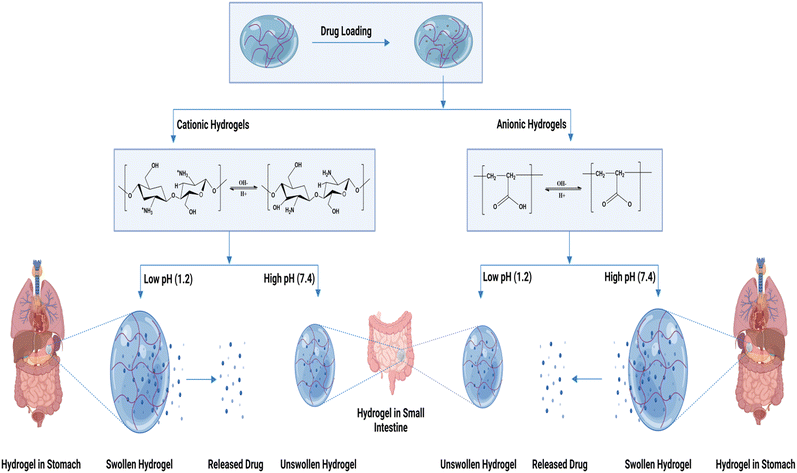
Polymers having basic functional groups are called polycations, and those with acidic functional groups are called polyanions. When the pH of the medium is higher than the pKa, polyanions exhibit an enlarged state; when the pH is less than pKa, they disintegrate. Similarly, for a polybase, polymer chain disintegration takes place when pH > pKb; as a result, they expand when pH < pKb. Chitosan (cationic polymer) expands in pH environments below pKb because of the protonation of the amine or imine functional groups. Sodium alginate (anionic polymer) expands in a basic environment due to the ionization of the acidic functional group. Fig. 4 shows a schematic representation of the pH-responsive hydrogel's drug delivery mechanism.
4.2 Thermo-sensitive biopolymers
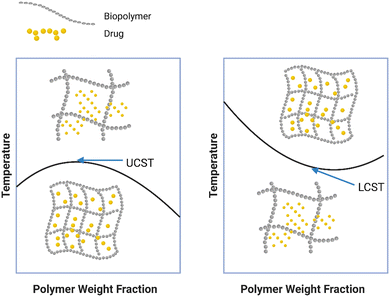
The thermo-sensitive smart polymers adjust their microstructural characteristics in reaction to temperature changes. These polymers are the most researched, widely utilized, and safest in biomaterials and drug delivery systems.127,128 Hydrogels make up the majority of thermo-responsive biopolymers; in these materials, changes in external temperature can control the gelation, swelling, & water affinity.129 The two primary categories of these hydrogels are as follows: (1) hydrogels with an upper critical solution temperature (UCST) and (2) hydrogels with a lower critical solution temperature (LCST). The primary constituents of UCST hydrogels are hydrophilic groups, and as the temperature rises, so does their capacity to expand into an appropriate solvent. Below a certain temperature, called the critical temperature, the polymer matrix contracts & collapses (Fig. 5a). Consequently, at temperatures under the UCST, they're in a gel form. Gelatin and agarose are examples of natural polymers in this category.129–131The LCST type of hydrogels, on the other hand, also experience temperature-dependent sol–gel transitions and are made up of hydrophobic as well as hydrophilic groups. The gel turns into a very viscous liquid as the temperature drops beyond the LCST (Fig. 5b). Certain cellulose derivatives are typical examples of biopolymers that show LCST.129–134 The most commonly used polymers in drug delivery systems are those with LCST. In order to treat an injury or subcutaneous layer, the polymer may be merged with therapeutic agents like medicines, proteins, or cells and injected into the body. As a result, a gel depot develops at the injection site when the temperature is raised.135 Human C-peptide mixed with a biopolymer resembling elastin may be injected subcutaneously to form a hydrogel depot, which would enable the release of human C-protein into the circulation gradually over a 19-day period, according to the study by Lee et al.132 In order to produce a hybrid scaffold, Dong et al.136 created an injectable thermo-responsive chitosan hydrogel and integrated it into a 3D-printed poly(ε-caprolactone). The resulting materials retained their long-lasting compressive strength & offered a favorable microenvironment beneficial for osteogenesis & cell proliferation. Xiao et al.137 created a biodegradable hydrogel using thermoresponsive PNIPAAm, cleavable lactic acid, & dextran groups. In order to create hydrogels, Merten et al.135 modified xyloglucan polymers & reported that this might modify the polymer's LCST by eliminating galactose, which would increase its hydrophobicity.
Temperature-responsive polymeric systems have the following benefits: they can deliver both lipophilic & hydrophilic drugs; they can target particular sites for drug delivery; they help in avoiding the use of hazardous organic solvents; and they have sustained release qualities with fewer adverse effects. However, they also have a number of drawbacks, including rapid drug release, a polymeric system that is not biocompatible, and an abrupt decrease in pH as a result of acidic degradation.134,138Table 5 summarizes common thermo-sensitive biopolymers employed in medical applications.
| Biopolymer | Hydrogel type | Characteristics | Application | Ref. |
|---|---|---|---|---|
| Collagen | UCST | It is non-toxic, biodegradable, and cytocompatible. | Drug administration wound healing tissue engineering | 114 and 139 |
| Gelatin | UCST | Biodegradable, biocompatible, less expensive and easy functionalization. | Tissue engineering bio-sensing drug delivery wound healing | 114, 139 and 140 |
| Cellulose | — | Easily accessible, mild cytotoxic, biodegradable, & biocompatible. | Drug delivery | 139 |
| Agarose | UCST | Biocompatible, biodegradable, non-toxic. | Drug administration, tissue engineering. | 141 |
4.3 Stimuli-responsive biopolymers
Light-sensitive hydrogels are synthesized by adding photosensitive groups to the polymeric structure. The response might be either reversible or irreversible depending on the nature of the photosensitizer used.142 In addition to causing partial or total decrosslinking, degradation, swelling, and/or shrinking of the hydrogel structure, light can also cause photosensitive moieties of the hydrogels to cleave, isomerize, or dimerize.143 Three main types of light-responsive drug delivery systems are: drug release platforms based on photoisomerization, photochemical reactions, and photothermal reactions. When hydrogels are exposed to light, photoisomerization usually results in a conformational shift from trans to cis. Drugs can diffuse out of hydrogels' matrixes during this period due to the opening of the pores of the hydrogels.144 Drug release can be induced via photochemical reactions that alter the hydrogel's network structure & configuration.145 By using materials that can transform light energy into heat energy, the photothermal reaction causes a disruption in a drug carrier that is sensitive to heat.146,147 Anugrah et al.'s146 NIR-sensitive polymer hydrogel, which is made of alginate cross-linked with tetrazine through the Diels–Alder process, serves as a regulated drug carrier. To the polymer hydrogel matrix, doxorubicin and an NIR-responsive indocyanine green were introduced by gelation. The resulting hydrogels showed a fast release pattern for doxorubicin upon NIR irradiation & a regulated release pattern under physiologically simulated settings.
Three common synthesis methods—grafting, blending, and co-precipitation or embedding—are used to create magnetic-sensitive hydrogels. Magnetic nanoparticles vibrate and the temperature rises in the presence of an alternating magnetic field (AMF). This accelerates the drug's particle motion and the polymer's breakdown, which facilitates drug release.159 Moreover, the magnetic-sensitive hydrogel experiences a magnetocaloric effect when exposed to a magnetic field, which might cause alteration in the diffraction peaks & the hydrogel's discolouration.160 Zhang and colleagues synthesised hydrogels by mixing PEG and hyaluronic acid, then adding type II collagen and magnetic nanoparticles to the gel. This hydrogel might move to the sites of tissue defects in physiological fluids under magnetic direction by reacting to an applied magnetic field.161 Emulsion polymerization techniques may be used to trap harmless, biocompatible iron oxide nanoparticles in nanogels, which makes them suitable for the delivery of pharmaceuticals. Magnetic-sensitive nanogels allow for remote management of the medication distribution.162,163 In order to achieve multiresponsive characteristics, using Fe3O4 nanosystems modified with oleic acid, Li et al. polymerized NIPAM and chitosan. These magnetically sensitive chitosan-based devices might potentially guide the delivery of medication via magnetic means.164 In a nutshell, minimal invasiveness, excellent tissue permeability, quick reaction, and outstanding controllability are some benefits of these hydrogels. However, there are certain drawbacks as well, including limited mechanical strength, material toxicity, & poor biocompatibility.165,166
Another class of bioresponsive hydrogels is enzyme-responsive hydrogels. They may be utilized to create hydrogels that respond to enzymes and serve as a natural trigger.171,172 An enzyme-catalyzed reaction is one of the most widely used processes to generate enzyme-responsive hydrogels.172 These systems differ from conventional catalyzed or noncatalyzed chemical processes due to their great selectivity along with enzyme specificity. Because of these exceptional properties, these hydrogels may be employed as extremely beneficial systems for tumor tissue-targeted administration & site-specific triggered release, which are strenuous to accomplish with standard stimuli polymers that are sensitive to light, pH, or temperature.173–175 The extremely selective and precise association of an antibody with its antigen results in an antigen-responsive hydrogel.167 The non-covalent interaction between the antigen and the antibody causes certain kinds of hydrogels to crosslink reversibly. Antigens present in the surrounding environment that are free of charge might compete with the hydrogel's antigens (Fig. 6). As a result, the contact between the antibody & antigen becomes weaker and the hydrogel's crosslinking density is decreased, leading to swelling or osmosis.176 These responsive materials have practical applications in the fields of clinical diagnostics, remote-regulated vaccination depots, and drug administration.167
 | ||
| Fig. 6 Diagram demonstrating the hydrogels that respond to antigens. The hydrogel swells as a result of the unbound antigens present in the surroundings competing with the hydrogel's binding antigens to reduce crosslinking density.159 | ||
5. Significance of biopolymer gels as theranostic systems
A noteworthy development in the world of medical technology is theranostic gels. They are a particular kind of hydrogel that integrates diagnostic and therapeutic features into one unit. These gels have the capacity to give real-time monitoring or imaging of the treatment's success in addition to delivering therapeutic chemicals to specific areas within the body. So theranostic agents are nanoparticles that can function as both a diagnostic and a therapeutic agent on the same platform.182 The following favorable attributes of this system are well known: (a) thermo-sensitivity; (b) injectability, because of a reversible sol-to-gel phase transition at or near body temperature; (c) localizability through quick gelation; (d) extremely low cytotoxicity of dissolved elements; (e) biodegradability with adjustable biodegradation periods; and (f) sustainability and long-term drug release.183 These kinds of small-sized molecules have a number of disadvantages, including inadequate blood circulation times, cytotoxicity, poor biodistribution, etc. It is crucial to use nanotechnology and create novel approaches for concurrent diagnosis as well as treatment systems because of this.184An interesting advancement in biomedicine, theranostic gels have important ramifications for diagnosis and treatment. Since a single nanocarrier can combine both therapeutic and imaging modalities, the field of theranostics has drawn a lot of attention as combined therapy and diagnosis,185 creating methods based on precision medicine to increase the effectiveness of cancer,186,187 targeted delivery,188,189 responsive properties (like stimuli of pH change, temperature or light),190,191 imagining compatibilities,192,193etc.
5.1 Integration of diagnostics and theranostics
This exciting field of study, which focuses on the fusion of therapeutic drug delivery vehicles with diagnostic detecting agents, has given rise to the word theranostics.194 In order to improve results, theranostics fundamentally combines diagnostic and therapeutic methods that are linked to the same exact molecular targets. This allows for more precise patient selection, treatment response & tissue toxicity prediction, and response evaluation.195 Theranostics and diagnostics combined constitute a major breakthrough in customized medicine. The process of diagnosing includes determining whether an illness or condition exists using a variety of tests, imaging methods, or other strategies. The goal of traditional diagnostics is to identify the ailment or condition that a patient has and theranostics combines medicine and diagnostics. In addition to providing a diagnosis, theranostics also advises on the best course of action for a given patient based on their individual biology. It entails adjusting the course of treatment to the unique features of each patient's illness. The materials used to create theranostic gels are usually those that have the ability to encapsulate medications or imaging agents and react to external stimuli to release them in a controlled manner. Both chemical and biological medications (i.e., proteins and peptides) are considered therapeutic agents, while radionuclides, heavy elements like iodine, superparamagnetic iron oxides, fluorescent dyes or quantum dots, etc. are frequently used as diagnostic agents196,197 and the polymers used are both natural and synthetic like poly(N-isopropylacrylamide),198 PAA–PEG poly(aspartic acid)–poly(ethylene glycol); polyglutamic acid; HPMA N-(2-hydroxypropyl) methacrylamide; PLA–PEG poly(lactic acid)–poly(ethylene glycol); PLGA–PEG poly(lactic-co-glycolic acid)–poly(ethylene glycol); and PAMAM poly(amido amine).1995.2 Biomedical applications of theranostic biopolymer gels
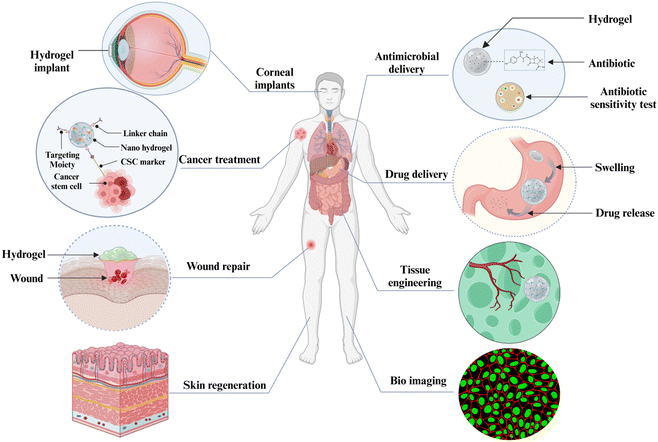
6. Applications of smart biopolymer gels
Considering their unique patterns and their capacity to be used and function in a variety of situations, polymer hydrogels have many uses. Polymer hydrogels' water content makes them sufficiently versatile for use in a wide range of biological, pharmacological, & industrial applications.209 The various applications of smart hydrogels are depicted in Fig. 7.6.1 Drug delivery
Owing of the observed regulated or pulsatile drug release pattern, which resembles biological demand, the use of intelligent polymers for drug delivery holds immense promise.210 Polymer hydrogels must possess the following characteristics in order to be used as drug delivery systems: (i) a porous structure,211 (ii) a sufficient release rate,212 (iii) the capacity to safeguard the drug,212 & (iv) be biodegradable & biocompatible.11,213,214 Polymeric compounds are generally a great option for application as a medication delivery agent. On the other hand, bio-polymers along with their derivatives demonstrate superior characteristics, including water solubility, non-hazardous, biodegradability, & biocompatibility.215,216 Additionally, with regulated drug release, these biopolymers can lessen drug toxicity. Furthermore, they lessen any enzymatic breakdown prior to a therapeutic substance being released at the target areas. Many stimuli, including physical, chemical, environmental, or a combination of many stimuli, can cause the release of a therapeutic drug.217Biodegradable hybrid hydrogels were created using chitosan, a natural polymer, & polyurethane, a synthetic polymer containing azomethine, for the regulated release of medications such as 5-fluorouracil. These hydrogels demonstrated favorable drug release characteristics of 50% 5-fluorouracil, indicating their suitability for this usage.218 Hydrogels based on guar chitosan di-aldehyde that were cross-linked in situ for double drug release were created for use in colorectal cancer treatment. These were intended to treat colorectal cancer while simultaneously providing chemotherapy and pain relief.219
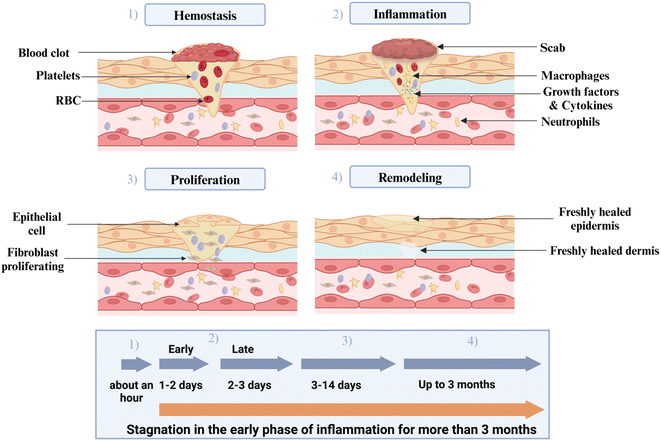
6.2 Wound repair
There are four steps in the healing process of wounds (Fig. 8).220 An excellent wound dressing needs a few characteristics in order to function well and be effective: (a) to shield the wound against microbial infection; (b) to have biocompatibility; (c) to possess the ability to retain moisture; (d) to possess gas permeability; and (e) to supply a moist atmosphere in order to lessen the development of scars.221 By virtue of their outstanding hydrophilicity, biological compatibility, and 3D porous frameworks that mimic the extracellular matrix, hydrogels have specific advantages in wound healing.222Stimulus-sensitive hydrogels, which are created depending on changes in surface pH & temperature, can significantly speed up wound healing. Temperature and pH are significant physicochemical parameters in the human body. Aiming to produce a highly ROS-responsive hydrogel, Hu et al.223 added boronic acid moieties upon alginate polymer chains, resulting in a smart injectable hydrogel exhibiting self-healing capabilities. The hydrogel became both antibacterial along with anti-inflammatory when micelles of the antibiotic amikacin & the anti-inflammatory drug naphthalene propylamine were assembled into it at the same time. In addition to retaining the hydrogels' outstanding rheological qualities & structural integrity, this formulation allowed for controlled medication release at inflammatory areas, which successfully aided in wound healing. A wound dressing based on dynamic imine bonding was created by Ding et al. using collagen, chitosan, & di-aldehyde terminated PEG.224 The resulting hydrogels have demonstrated excellent injectability, remarkable hemostatic capacity, antibacterial activity, thermal stability, & healing potential. Yu et al.225 created an injectable hydrogel consisting of carboxymethyl chitosan, γ-polyglutamic acid, & polydopamine hydrogel, intended for antimicrobial purposes and preventing tumor recurrence. This hydrogel demonstrated an excellent biocompatibility.
6.3 Tissue engineering
Tissue engineering is another field where smart biopolymers have been utilized. In order to heal organs & tissues injured by illness or trauma, tissue engineering has emerged as an emerging area. It works to repair or regenerate wounded or diseased biological tissue or produce replacement organs for a variety of illnesses, including diabetes, heart disease, cirrhosis, osteoarthritis & spinal cord injury.226 The method of creating a scaffold structure with the correct mechanical, chemical, & physical characteristics to allow for cell penetration & three-dimensional tissue growth is the essence of tissue engineering. The primary goal is the development of new tissue within the scaffold to merge with the tissue of the host. Conversely, the scaffold acts as a transient route for regeneration & will disintegrate either during or after healing, obviating the necessity for eliminating the material later and eradicating possible adverse effects linked to the body's retention of materials.210 In the area of tissue engineering, thermoresponsive polymers are frequently utilized in two situations: first, as substrates that support cell growth and proliferation and second, as injectable gels for in situ scaffolding.210 Owing to hydrogels special qualities—such as hydrophilicity, transparency, adjustable mechanical strength, and biocompatibility—hydrogels are becoming essential materials in corneal tissue engineering. Because of these properties, hydrogels can be used to simulate the corneal environment and promote tissue healing. Negin Khoshnood et al. show how hydrogels that can be customized for certain ocular medication delivery applications can be made via 3D bioprinting. The use of betamethasone-loaded gellan gum (GG) and polyethyleneimine (PEI) composite hydrogel shows how sophisticated production techniques might improve hydrogels' performance in corneal applications. Furthermore, the hydrogel demonstrated appropriate mechanical strength, transparency (80%), and rates of deterioration, indicating its potential as a corneal repair material.227 Hydrogels—more especially, polyethyleneglycol (PEG)-cyclodextrin polyrotaxane microgels—are used in ocular tissue engineering because of their moldable and injectable qualities, which promote corneal tissue regeneration and increase the efficiency of tissue engineering techniques.228 As scaffolds, eye drops and films, hydrogels derived from protein-polysaccharide bio materials are used in corneal tissue engineering. Their promotion of ocular permeability, cell adhesion, and proliferation helps heal corneal lesions and associated conditions.229 Therapeutic hydrogels improve tissue repair by acting as vehicles for therapeutic genes that surpass cellular barriers. Because of its slow-release nature, gene therapy in tissue engineering is more effective.230 Injectable hydrogels in cardiac tissue engineering give cardiomyocytes and stem cells a supporting matrix, which is essential for healing injured heart muscle. They facilitate the repair of cardiac wall stress and preserve cell viability.2316.4 Cancer treatment
Currently, surgery, radiation, chemotherapy, immunotherapy, & targeted molecular therapy are used to treat cancer at different stages.232 Owing to the adverse effects linked to the high cytotoxicity of chemotherapy drugs, there has been considerable interest in the development and production of novel, efficient cancer treatment methods. Polymer hydrogels are the most suitable drug delivery mechanism for cancer therapy among a wide range of different formulations, such as films, suspensions, etc. Hydrogels that are sensitive to pH, temperature, & ions are effective drug release systems.233 Thermo-sensitive hydrogel systems have been proven to be effective as they alter states at various temperatures to aid in the loading & releasing of cancer medications.234 Hydrogel loaded with chitosan/disulfiram that released the anticancer medication disulfiram is a good example of a thermo-sensitive hydrogel. Compared to the disulfiram drug alone, this system exhibited more cellular uptake and sustained administration, which may aid in cancer therapy.235 A hybrid, injectable, thermo-responsive hydrogel was created by Gao et al.236 for concurrent administration of doxorubicin & co-encapsulated norcantharidin NPs to patients with hepatocellular carcinoma through intratumoral injection. Tumor growth along with angiogenesis were suppressed during in vivo testing using a mice tumor model.6.5 Antimicrobial applications
Current medical care relies on antimicrobial medications, such as antibiotics, to prevent infections since they can either work to kill or stop the development of pathogens. The emergence of multiresistant microorganisms in chronic non-healing wounds sometimes renders antibiotic therapy completely useless in eliminating infections.237,238 Gupta et al.239 generated Ag nanoparticles encapsulated in a bio-synthetic bacterial cellulose hydrogel in order to create a wound hydrogel dressing. The acquired hydrogel dressing exhibited a wide range of antibacterial effects towards three pathogenic microorganisms that are commonly found in wounds: Staphylococcus aureus, Candida auris, & Pseudomonas aeruginosa. Vaishali et al.240 created a chitosan hydrogel system in which the antimicrobial drug cefuroxime was covalently conjugated with chitosan polymers through ester bonds, giving the hydrogel matrix a long-lasting antibiotic action at localized areas. The obtained hydrogels demonstrated strong cell & hemocompatibility, which may increase the options for treating wound infections.6.6 Bio-sensing applications
Bio-sensing is an emerging analytical discipline for the detection of biological markers by employing transducing systems.241 Polymer hydrogel-based biosensors have drawn a lot of interest recently because of their high sensitivity, simplicity of fabrication, and versatility in a variety of domains (drug detection, disease diagnosis, and environmental contamination detection). Because of their similarity to biological tissue & biocompatibility, they are used in the biomedical field.242 For electrochemical bio-sensing, it has been reported that hydrogels in the shape of porous, cell-like films were created by merging nanoparticles of Prussian blue & several enzymes on electrode surfaces. They were created by drop casting composite hydrogels onto the surface of SPE-type screen printed flexible electrodes. The two amperometric bio-sensors that were created using hydrogel composites that included alcohol dehydrogenase & glucose oxidase demonstrated excellent sensitivity for the quick detection of ethanol & glucose in serum. Hydrogels have the potential to serve as electrochemical bio-sensing systems since their matrix may effectively immobilize certain enzymes & nanomaterials for the identification of an array of analytes.243 A novel, extremely sensitive, and affordable DNA hydrogel sensor has been developed by Wang et al. for the quantitative visual detection of miRNAs, with possible applications in the bio-sensing of nucleic acids.2447. Conclusion and future perspectives
Research on bio-responsive polymers has grown rapidly in recent decades and has a major impact on materials science, molecular pharmaceutics, and nanobiotechnology. The majority of stimulus-responsive systems are engineered for controlled delivery of drugs in order to accomplish programmed release and overcome the drawbacks of systemic drug administration, and new materials for tissue engineering and diagnostics are receiving more and more attention.245 This review addresses the vast array of beneficial natural polymers that are utilized to create various smart biopolymer gel formulations that entrap active ingredients and are intended for use in biological applications. The most prominent characteristics, such as biocompatibility & biodegradability, allow their use in biological, biomedical, & pharmaceutical applications. It's also imperative to conduct more research on the identification & development of novel biopolymers. In the realm of drug delivery, smart biopolymer gels represent an important breakthrough. Biopolymers can be employed to synthesize hydrogels for specific benefits & unique characteristics. Biopolymer gels sensitive to external stimuli (pH, temperature, ultrasound, enzymes, etc.) are currently used for targeted drug delivery, which has proved to be important in the treatment of diseases like cancer, diabetes, brain injury, etc. To increase the response efficiency even more, bio-responsive systems that are triggered by several stimuli have been developed. By altering the physic-chemical properties of hydrogels, these systems can become very specific for targeted delivery of drugs. In addition to drug delivery and various applications, these hydrogels hold potential for the development of ultrasensitive fluorescent & electrically conductive variants, which can be utilized in wearable, implantable, and disposable biosensors for quantitative measurements. The theranostic use of these smart gels has drawn significant attention since it can allow precise treatment and drug delivery. With further developments in biomedical engineering, materials science, and imaging technologies, biopolymer gels have a promising future in theranostic applications. These developments might enhance imaging sensitivity and resolution, improve clinical application and regulatory concerns, and assist biopolymer gels in reaching their full potential in biocompatibility along with stability difficulties generally. Although theranostic uses of nanoparticles coated with biopolymers have been studied, further study is required to determine their long-term safety and toxicity.246 The present study on smart biopolymer gels has advanced significantly, but there are still several drawbacks, including limited mechanical strength, UV carcinogenesis, thermal denaturation, & material toxicity. Hydrogels exhibit fragility and present difficulties in the handling process during the loading of therapeutic agents. They often exhibit spatial heterogeneity as well as an inhomogeneous distribution of crosslink density. The mechanical characteristics of hydrogels are diminished by the unwanted spatial heterogeneities. Over time, hydrogels undergo hydrolytic and enzymatic biodegradation, and hydrogels composed of biopolymers are often biodegradable.247,248 Numerous smart hydrogels have been created & introduced thus far, but their commercialization as drug delivery systems remains unconvincing; just a small number of them have been used in clinical settings.249 In fact, there are currently no precise legal guidelines or criteria for the application of smart drug-loaded hydrogels in therapeutic activities, even with recent developments in the pharmaceutical sector. Furthermore, before they are commercialized, the release characteristics must be modeled in order to make significant advances in in vivo release.250 Therefore, it is crucial to improve the features of these gels in order to ensure the safety as well as efficacy of these formulations. In a nutshell, biopolymers hold great promise for developing novel disease models, creating innovative formulations, and producing medical devices that are safe and effective for the general public.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge Department of Pharmaceutical Sciences, University of Kashmir for providing the necessary facilities to carry out this work.References
- A. M. Smith, S. Moxon and G. Morris, Biopolymers as wound healing materials, Wound healing biomaterials, Elsevier, 2016, pp. 261–287 Search PubMed.
- J. Wroblewska-Krepsztul, T. Rydzkowski, I. Michalska-Pożoga and V. K. Thakur, Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning, Nanomaterials, 2019, 9(3), 404 CrossRef CAS PubMed.
- I. Vroman and L. Tighzert, Biodegradable polymers, Materials, 2009, 2(2), 307–344 CrossRef CAS.
- X. Ao, A. Vazquez-Lopez, D. Mocerino, C. González and D. Y. Wang, Flame retardancy and fire mechanical properties for natural fiber/polymer composite: A review, Composites, Part B, 2024, 268, 111069 CrossRef CAS.
- O. Opriş, C. Mormile, I. Lung, A. Stegarescu, M. L. Soran and A. Soran, An overview of biopolymers for drug delivery applications, Appl. Sci., 2024, 14(4), 1383 CrossRef.
- F. Jummaat, E. B. Yahya, A. Khalil HPS, A. S. Adnan, A. M. Alqadhi, C. K. Abdullah, A. S. AK, N. G. Olaiya and M. Abdat, The role of biopolymer-based materials in obstetrics and gynecology applications: A review, Polymers, 2021, 13(4), 633 CrossRef CAS PubMed.
- M. S. Reddy, D. Ponnamma, R. Choudhary and K. K. Sadasivuni, A comparative review of natural and synthetic biopolymer composite scaffolds, Polymers, 2021, 13(7), 1105 CrossRef CAS PubMed.
- S. D. Paul, H. Sharma, G. Jeswani and A. K. Jha, Novel gels: implications for drug delivery, Nanostructures for drug delivery, Elsevier, 2017, pp. 379–412 Search PubMed.
- G. Aggarwal and M. Nagpal, Pharmaceutical polymer gels in drug delivery, Polymer Gels: Perspectives and Applications, 2018, pp. 249–284 Search PubMed.
- N. A. Peppas, P. Bures, W. S. Leobandung and H. Ichikawa, Hydrogels in pharmaceutical formulations, Eur. J. Pharm. Biopharm., 2000, 50(1), 27–46 CrossRef CAS PubMed.
- P. Gupta, K. Vermani and S. Garg, Hydrogels: from controlled release to pH-responsive drug delivery, Drug Discovery Today, 2002, 7(10), 569–579 CrossRef CAS PubMed.
- L. Yu and J. Ding, Injectable hydrogels as unique biomedical materials, Chem. Soc. Rev., 2008, 37(8), 1473–1481 RSC.
- P. Gupta, K. Vermani and S. Garg, Hydrogels: from controlled release to pH-responsive drug delivery, Drug Discovery Today, 2002, 7(10), 569–579 CrossRef CAS PubMed.
- S. H. Aswathy, U. Narendrakumar and I. Manjubala, Commercial hydrogels for biomedical applications, Heliyon, 2020, 6(4), 3719 CrossRef PubMed.
- X. Huang and T. L. Lowe, Biodegradable thermoresponsive hydrogels for aqueous encapsulation and controlled release of hydrophilic model drugs, Biomacromolecules, 2005, 6(4), 2131–2139 CrossRef CAS PubMed.
- Y. N. Dai, P. Li, J. P. Zhang, A. Q. Wang and Q. Wei, Swelling characteristics and drug delivery properties of nifedipine-loaded pH sensitive alginate–chitosan hydrogel beads, J. Biomed. Mater. Res., Part B, 2008, 86(2), 493–500 CrossRef PubMed.
- A. Vintiloiu and J. C. Leroux, Organogels and their use in drug delivery—a review, J. Controlled Release, 2008, 125(3), 179–192 CrossRef CAS PubMed.
- S. V. Vinogradov, Colloidal microgels in drug delivery applications, Curr. Pharm. Des., 2006, 12(36), 4703–4712 CrossRef CAS PubMed.
- S. Uthaman, S. Maya, R. Jayakumar, C. S. Cho and I. K. Park, Carbohydrate-based nanogels as drug and gene delivery systems, J. Nanosci. Nanotechnol., 2014, 14(1), 694–704 CrossRef CAS PubMed.
- S. Hajebi, N. Rabiee, M. Bagherzadeh, S. Ahmadi, M. Rabiee, H. Roghani-Mamaqani, M. Tahriri, L. Tayebi and M. R. Hamblin, Stimulus-responsive polymeric nanogels as smart drug delivery systems, Acta Biomater., 2019, 92, 1–8 CrossRef CAS PubMed.
- G. Soni and K. S. Yadav, Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art, Saudi Pharm. J., 2016, 24(2), 133–139 CrossRef PubMed.
- K. S. Soni, S. S. Desale and T. K. Bronich, Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation, J. Controlled Release, 2016, 240, 109–126 CrossRef CAS PubMed.
- A. J. Dehchani, A. Jafari and F. Shahi, Nanogels in Biomedical Engineering: Revolutionizing Drug Delivery, Tissue Engineering, and Bioimaging, Polym. Adv. Technol., 2024, 35(10), e6595 CrossRef CAS.
- D. Li, C. F. van Nostrum, E. Mastrobattista, T. Vermonden and W. E. Hennink, Nanogels for intracellular delivery of biotherapeutics, J. Controlled Release, 2017, 259, 16–28 CrossRef CAS PubMed.
- F. Sultana, M. Imran-Ul-Haque, M. Arafat and S. Sharmin, An overview of nanogel drug delivery system, J. Appl. Pharm. Sci., 2013, 3(8), S95–S105 Search PubMed.
- P. Baipaywad, N. Udomluck, S. G. Pyo, H. H. Park and H. Park, Fabrication of nanogels for delivery of molecules, J. Nanosci. Nanotechnol., 2014, 14(10), 7363–7373 CrossRef CAS PubMed.
- H. Almeida, M. H. Amaral and P. Lobão, Temperature and pH stimuli-responsive polymers and their applications in controlled and selfregulated drug delivery, J. Appl. Pharm. Sci., 2012, 01–10 CAS.
- H. Almeida, M. H. Amaral, P. Lobão and J. M. Lobo, Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): main features and their applications in topical and transdermal administration of drugs, J. Pharm. Pharm. Sci., 2012, 15(4), 592–605 Search PubMed.
- N. R. Boase, E. R. Gillies, R. Goh, R. E. Kieltyka, J. B. Matson, F. Meng, A. Sanyal and O. Sedláček, Stimuli-responsive polymers at the interface with biology, Biomacromolecules, 2024, 25(9), 5417–5436 CrossRef CAS PubMed.
- Z. Yuan, J. Ding, Y. Zhang, B. Huang, Z. Song, X. Meng, X. Ma, X. Gong, Z. Huang, S. Ma and S. Xiang, Components, mechanisms and applications of stimuli-responsive polymer gels, Eur. Polym. J., 2022, 177, 111473 CrossRef CAS.
- J. Jagur-Grodzinski, Polymeric gels and hydrogels for biomedical and pharmaceutical applications, Polym. Adv. Technol., 2010, 21(1), 27–47 CrossRef CAS.
- A. K. Anal, Stimuli-induced pulsatile or triggered release delivery systems for bioactive compounds, Recent Pat. Endocr., Metab. Immune Drug Discovery, 2007, 1(1), 83–90 CrossRef CAS.
- T. H. Yang, Recent applications of polyacrylamide as biomaterials, Recent Pat. Mater. Sci., 2008, 1(1), 29–40 CrossRef CAS.
- N. Patra, P. Ramesh, V. Donthu and A. Ahmad, Biopolymer-based composites for sustainable energy storage: recent developments and future outlook, J. Mater. Sci.:Mater. Eng., 2024, 19(1), 34 Search PubMed.
- S. A. Ganie, A. Ali, T. A. Mir and Q. Li, Physical and chemical modification of biopolymers and biocomposites, Advanced Green Materials, Woodhead Publishing, 2021, pp. 359–377 Search PubMed.
- R. M. Cherian, H. Kargarzadeh, N. A. Rosli, C. Jose and S. Thomas, Tuning the Hydrophilic/Hydrophobic Behavior of Biopolymers: Surface Modifications. Handbook of Biopolymers, Springer Nature Singapore, Singapore, 2023, pp. 367–401 Search PubMed.
- S. Faheem, H. Hameed, A. C. Paiva-Santos, M. Zaman, H. S. Sarwar and I. Majeed, Niosome-based gels: a smart nano-carrier for effective and advanced transdermal drug delivery, Int. J. Polym. Mater. Polym. Biomater., 2025, 74(4), 317–335 CrossRef CAS.
- M. Rizwan, R. Yahya, A. Hassan, M. Yar, A. D. Azzahari, V. Selvanathan, F. Sonsudin and C. N. Abouloula, pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications, Polymers, 2017, 9(4), 137 CrossRef PubMed.
- N. Chirani, L. H. Yahia, L. Gritsch, F. L. Motta, S. Chirani and S. Farè, History and applications of hydrogels, J. Biomed. Sci., 2015, 4(02), 1–23 Search PubMed.
- R. Sharma, J. Bajpai, A. K. Bajpai, S. Acharya, R. B. Shrivastava and S. K. Shukla, Designing slow water-releasing alginate nanoreserviors for sustained irrigation in scanty rainfall areas, Carbohydr. Polym., 2014, 102, 513–520 CrossRef CAS PubMed.
- K. Hemvichian, A. Chanthawong and P. Suwanmala, Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals, Radiat. Phys. Chem., 2014, 103, 167–171 CrossRef CAS.
- M. E. Abdel-Raouf, S. M. El-Saeed, E. G. Zaki and A. M. Al-Sabagh, Green chemistry approach for preparation of hydrogels for agriculture applications through modification of natural polymers and investigating their swelling properties, Egypt. J. Pet., 2018, 27(4), 1345–1355 CrossRef.
- S. C. Pang, S. F. Chin, S. H. Tay and F. M. Tchong, Starch–maleate–polyvinyl alcohol hydrogels with controllable swelling behaviors, Carbohydr. Polym., 2011, 84(1), 424–429 CrossRef CAS.
- D. França, Â. F. Medina, L. L. Messa, C. F. Souza and R. Faez, Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable− controlled release materials, Carbohydr. Polym., 2018, 196, 47–55 CrossRef PubMed.
- C. Chang, B. Duan, J. Cai and L. Zhang, Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery, Eur. Polym. J., 2010, 46(1), 92–100 CrossRef CAS.
- B. Brattekås, Å. Haugen, A. Graue and R. S. Seright, Gel dehydration by spontaneous imbibition of brine from aged polymer gel, SPE J., 2014, 19(01), 122–134 CrossRef.
- H. Guo, J. Ge, Q. Wu, Z. He, W. Wang and G. Cao, Syneresis behavior of polymer gels aged in different brines from gelants, Gels, 2022, 8(3), 166 CrossRef CAS PubMed.
- S. Nagaraja, P. Bindiganavile Anand, R. N. Mahadeva Naik and S. Gunashekaran, Effect of aging on the biopolymer composites: Mechanisms, modes and characterization, Polym. Compos., 2022, 43(7), 4115–4125 CrossRef CAS.
- A. Garg, R. Agrawal, C. S. Chauhan and R. Deshmukh, In-situ gel: a smart carrier for drug delivery, Int. J. Pharm., 2024, 123819 CrossRef CAS PubMed.
- A. M. Grillet, N. B. Wyatt and L. M. Gloe, Polymer gel rheology and adhesion, Rheology, 2012, 3, 59–80 Search PubMed.
- A. Agrawal and C. M. Hussain, 3D-printed hydrogel for diverse applications: A review, Gels, 2023, 9(12), 960 CrossRef CAS PubMed.
- J. M. Townsend, E. C. Beck, S. H. Gehrke, C. J. Berkland and M. S. Detamore, Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting, Prog. Polym. Sci., 2019, 91, 126–140 CrossRef CAS PubMed.
- M. T. Yen, J. H. Yang and J. L. Mau, Physicochemical characterization of chitin and chitosan from crab shells, Carbohydr. Polym., 2009, 75(1), 15–21 CrossRef CAS.
- B. K. Heragh, S. Javanshir, G. R. Mahdavinia and M. R. Jamal, Hydroxyapatite grafted chitosan/laponite RD hydrogel: Evaluation of the encapsulation capacity, pH-responsivity, and controlled release behavior, Int. J. Biol. Macromol., 2021, 190, 351–359 CrossRef CAS PubMed.
- M. J. Machodi and M. O. Daramola, Synthesis and performance evaluation of PES/chitosan membranes coated with polyamide for acid mine drainage treatment, Sci. Rep., 2019, 9(1), 17657 CrossRef PubMed.
- Y. H. Lin, P. L. Kang, W. Xin, C. S. Yen, L. C. Hwang, C. J. Chen, J. T. Liu and S. J. Chang, Preparation and evaluation of chitosan biocompatible electronic skin, Comput. Ind., 2018, 100, 1–6 CrossRef.
- E. Szymańska and K. Winnicka, Stability of chitosan—a challenge for pharmaceutical and biomedical applications, Mar. Drugs, 2015, 13(4), 1819–1846 CrossRef PubMed.
- J. Varshosaz, The promise of chitosan microspheres in drug delivery systems, Expert Opin. Drug Delivery, 2007, 4(3), 263–273 CrossRef CAS PubMed.
- P. S. Bakshi, D. Selvakumar, K. Kadirvelu and N. S. Kumar, Chitosan as an environment friendly biomaterial–a review on recent modifications and applications, Int. J. Biol. Macromol., 2020, 150, 1072–1083 CrossRef CAS PubMed.
- A. Chenite, C. Chaput, D. Wang, C. Combes, M. D. Buschmann, C. D. Hoemann, J. C. Leroux, B. L. Atkinson, F. Binette and A. Selmani, Novel injectable neutral solutions of chitosan form biodegradable gels in situ, Biomaterials, 2000, 21(21), 2155–2161 CrossRef CAS PubMed.
- E. Ruel-Gariepy, A. Chenite, C. Chaput, S. Guirguis and J. C. Leroux, Characterization of thermosensitive chitosan gels for the sustained delivery of drugs, Int. J. Pharm., 2000, 203(1–2), 89–98 CrossRef CAS PubMed.
- J. M. Dang, D. D. Sun, Y. Shin-Ya, A. N. Sieber, J. P. Kostuik and K. W. Leong, Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells, Biomaterials, 2006, 27(3), 406–418 CrossRef CAS PubMed.
- J. Wu, W. Wei, L. Y. Wang, Z. G. Su and G. H. Ma, A thermosensitive hydrogel based on quaternized chitosan and poly (ethylene glycol) for nasal drug delivery system, Biomaterials, 2007, 28(13), 2220–2232 CrossRef CAS PubMed.
- J. Wu, Z. G. Su and G. H. Ma, A thermo-and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate, Int. J. Pharm., 2006, 315(1–2), 1 CrossRef CAS PubMed.
- X. He, Y. Zhao, Z. Jin, Y. Su, H. An, L. Ge, D. Wei and L. Chen, Design and cytocompatibility of chitosan-based thermoresponsive cell culture plates, J. Appl. Biomater. Biomech., 2016, 14(4), 404–412 Search PubMed.
- L. Li, L. Wang, J. Li, S. Jiang, Y. Wang, X. Zhang, J. Ding, T. Yu and S. Mao, Insights into the mechanisms of chitosan–anionic polymers-based matrix tablets for extended drug release, Int. J. Pharm., 2014, 476(1–2), 253–265 CrossRef CAS PubMed.
- P. Ilgin, G. Avci, C. Silan, S. Ekici, N. Aktas, R. S. Ayyala, V. T. John and N. Sahiner, Colloidal drug carries from (sub) micron hyaluronic acid hydrogel particles with tunable properties for biomedical applications, Carbohydr. Polym., 2010, 82(3), 997–1003 CrossRef CAS.
- N. Sahiner, C. Silan, S. Sagbas, P. Ilgin, S. Butun, H. Erdugan and R. S. Ayyala, Porous and modified HA particles as potential drug delivery systems, Microporous Mesoporous Mater., 2012, 155, 124–130 CrossRef CAS.
- S. Ekici, P. Ilgin, S. Yilmaz, N. Aktas and N. Sahiner, Temperature and magnetic field responsive hyaluronic acid particles with tunable physical and chemical properties, Appl. Surf. Sci., 2011, 257(7), 2669–2676 CrossRef CAS.
- N. Sahiner, A. K. Jha, D. Nguyen and X. Jia, Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration, J. Biomater. Sci., 2008, 19(2), 223–243 CrossRef CAS PubMed.
- K. Liang, S. Ng, F. Lee, J. Lim, J. E. Chung, S. S. Lee and M. Kurisawa, Targeted intracellular protein delivery based on hyaluronic acid–green tea catechin nanogels, Acta Biomater., 2016, 33, 142–152 CrossRef CAS PubMed.
- S. Coninx, G. Kalot, A. Godard, E. Bodio, C. Goze, L. Sancey and R. Auzély-Velty, Tailored hyaluronic acid-based nanogels as theranostic boron delivery systems for boron neutron cancer therapy, Int. J. Pharm.:X, 2022, 4, 100134 CAS.
- E. Altuntaş, B. Özkan, S. Güngör and Y. Özsoy, Biopolymer-based nanogel approach in drug delivery: basic concept and current developments, Pharmaceutics, 2023, 15(6), 1644 CrossRef PubMed.
- V. O. Fasiku, C. A. Omolo, L. W. Kiruri, N. Devnarain, M. Faya, C. Mocktar and T. Govender, A hyaluronic acid-based nanogel for the co-delivery of nitric oxide (NO) and a novel antimicrobial peptide (AMP) against bacterial biofilms, Int. J. Biol. Macromol., 2022, 206, 381–397 CrossRef CAS PubMed.
- S. Ilic-Stojanovic, L. Nikolic and S. Cakic, A review of patents and innovative biopolymer-based hydrogels, Gels, 2023, 9(7), 556 CrossRef CAS PubMed.
- A. M. Jonker, D. W. Loowik and J. C. Van Hest, Peptide-and protein-based hydrogels, Chem. Mater., 2012, 24(5), 759–773 CrossRef CAS.
- S. Chander, G. T. Kulkarni, N. Dhiman and H. Kharkwal, Protein-based nanohydrogels for bioactive delivery, Front. Chem., 2021, 9, 573748 CrossRef CAS PubMed.
- S. N. Pawar and K. J. Edgar, Alginate derivatization: A review of chemistry, properties and applications, Biomaterials, 2012, 33(11), 3279–3305 CrossRef CAS PubMed.
- A. Setia and P. Ahuja, Nanohydrogels: Emerging trend for drug delivery, Organic materials as smart nanocarriers for drug delivery, 2018, pp. 293–368 Search PubMed.
- C. Valentino, B. Vigani, I. Fedeli, D. Miele, G. Marrubini, L. Malavasi, F. Ferrari, G. Sandri and S. Rossi, Development of alginate-spermidine micro/nanogels as potential antioxidant and anti-inflammatory tool in peripheral nerve injuries. Formulation studies and physico-chemical characterization, Int. J. Pharm., 2022, 626, 122168 CrossRef CAS PubMed.
- S. Kamel, N. Ali, K. Jahangir, S. M. Shah and A. A. El-Gendy, Pharmaceutical significance of cellulose: A review, eXPRESS Polym. Lett., 2008, 2(11), 758–778 CrossRef CAS.
- M. G. Fuster, I. Moulefera, M. N. Muñoz, M. G. Montalbán and G. Víllora, Synthesis of cellulose nanoparticles from ionic liquid solutions for biomedical applications, Polymers, 2023, 15(2), 382 CrossRef CAS PubMed.
- M. Yang, S. Y. Abdalkarim, H. Y. Yu, R. A. Asad, D. Ge and Y. Zhou, Thermo-sensitive composite microspheres incorporating cellulose nanocrystals for regulated drug release kinetics, Carbohydr. Polym., 2023, 301, 120350 CrossRef CAS PubMed.
- P. Kumari, R. Seth, A. Meena and D. Sharma, Enzymatic synthesis of cellulose nanocrystals from lemongrass and its application in improving anti-cancer drug release, uptake and efficacy, Ind. Crops Prod., 2023, 192, 115933 CrossRef CAS.
- S. F. Kabir, P. P. Sikdar, B. Haque, M. R. Bhuiyan, A. Ali and M. N. Islam, Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications, Prog. Biomater., 2018, 7, 153–174 CrossRef CAS PubMed.
- A. Ghilan, L. E. Nita, D. Pamfil, N. Simionescu, N. Tudorachi, D. Rusu, A. G. Rusu, M. Bercea, I. Rosca, D. E. Ciolacu and A. P. Chiriac, One-step preparation of carboxymethyl cellulose—phytic acid hydrogels with potential for biomedical applications, Gels, 2022, 8(10), 647 CrossRef CAS PubMed.
- Y. Lu, Q. Luo, Y. Chu, N. Tao, S. Deng, L. Wang and L. Li, Application of gelatin in food packaging: A review, Polymers, 2022, 14(3), 436 CrossRef CAS PubMed.
- Z. N. Hanani, Y. H. Roos and J. P. Kerry, Use and application of gelatin as potential biodegradable packaging materials for food products, Int. J. Biol. Macromol., 2014, 71, 94–102 Search PubMed.
- R. Liang, Y. Gu, Y. Wu, V. Bunpetch and S. Zhang, Lithography-based 3D bioprinting and bioinks for bone repair and regeneration, ACS Biomater. Sci. Eng., 2020, 7(3), 806–816 CrossRef PubMed.
- J. Li, F. Gao, S. Ma, Y. Zhang, J. Zhang, F. Guan and M. Yao, Control the fate of human umbilical cord mesenchymal stem cells with dual-enzymatically cross-linked gelatin hydrogels for potential applications in nerve regeneration, J. Tissue Eng. Regener. Med., 2020, 14(9), 1261–1271 CAS.
- Y. G. Ko and O. H. Kwon, Reinforced gelatin-methacrylate hydrogels containing poly(lactic-co-glycolic acid) nanofiber fragments for 3D bioprinting, J. Ind. Eng. Chem., 2020, 89, 147–155 CrossRef CAS.
- R. V. Shevchenko, M. Eeman, B. Rowshanravan, I. U. Allan, I. N. Savina, M. Illsley, M. Salmon, S. L. James, S. V. Mikhalovsky and S. E. James, The in vitro characterization of a gelatin scaffold, prepared by cryogelation and assessed in vivo as a dermal replacement in wound repair, Acta Biomater., 2014, 10(7), 3156–3166 CrossRef CAS PubMed.
- H. R. Matheus, H. Hadad, J. L. Monteiro, T. Takusagawa, F. Zhang, Q. Ye, Y. He, I. A. Rosales, Y. Jounaidi, M. A. Randolph and F. P. Guastaldi, Photo-crosslinked GelMA loaded with dental pulp stem cells and VEGF to repair critical-sized soft tissue defects in rats, J. Stomatol. Oral Maxillofac. Surg., 2023, 124(1), 101373 CrossRef PubMed.
- G. P. Gopalan and S. Anas, Structural, Morphological, and Textural Properties of Biopolymers, Handbook of Biopolymers, Springer Nature Singapore, Singapore, 2022, pp. 1–41 Search PubMed.
- M. Yu, X. Guo, K. Zhang, X. Kang, S. Zhang and L. Qian, Hyaluronic Acid Unveiled: Exploring the Nanomechanics and Water Retention Properties at the Single-Molecule Level, Langmuir, 2024, 40(5), 2616–2623 CrossRef CAS PubMed.
- A. M. Jonker, D. W. Löwik and J. C. Van Hest, Peptide-and protein-based hydrogels, Chem. Mater., 2012, 24(5), 759–773 CrossRef CAS.
- M. Zabihi, Applications of Alginate in the Fields of Research Medicine, Industry and Agriculture, Alginate-Applications and Future Perspectives, IntechOpen, 2024 Search PubMed.
- S. Kamel, N. Ali, K. Jahangir, S. M. Shah and A. A. El-Gendy, Pharmaceutical significance of cellulose: A review, eXPRESS Polym. Lett., 2008, 2(11), 758–778 CrossRef CAS.
- S. Ilic-Stojanovic, L. Nikolic and S. Cakic, A review of patents and innovative biopolymer-based hydrogels, Gels, 2023, 9(7), 556 CrossRef CAS PubMed.
- Y. G. Ko and O. H. Kwon, Reinforced gelatin-methacrylate hydrogels containing poly(lactic-co-glycolic acid) nanofiber fragments for 3D bioprinting, J. Ind. Eng. Chem., 2020, 89, 147–155 CrossRef CAS.
- M. S. Azmir, M. N. Moni, A. Gobetti, G. Ramorino and K. Dey, Advances in modulating mechanical properties of gelatin-based hydrogel in tissue engineering, Int. J. Polym. Mater. Polym. Biomater., 2025, 74(3), 215–250 CrossRef CAS.
- E. Altuntas, B. Ozkan, S. Gungor and Y. Ozsoy, Biopolymer-based nanogel approach in drug delivery: basic concept and current developments, Pharmaceutics, 2023, 15(6), 1644 CrossRef CAS PubMed.
- N. A. Pattanashetti, G. B. Heggannavar and M. Y. Kariduraganavar, Smart biopolymers and their biomedical applications, Procedia Manuf., 2017, 12, 263–279 CrossRef.
- A. Gutowska, J. S. Bark, I. C. Kwon, Y. H. Bae, Y. Cha and S. W. Kim, Squeezing hydrogels for controlled oral drug delivery, J. Controlled Release, 1997, 48(2–3), 141–148 CrossRef CAS.
- K. Na, K. H. Lee and Y. H. Bae, pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate, J. Controlled Release, 2004, 97(3), 513–525 CrossRef CAS PubMed.
- A. K. Bajpai, J. Bajpai, R. Saini and R. Gupta, Responsive polymers in biology and technology, Polym. Rev., 2011, 51(1), 53–97 CrossRef CAS.
- Y. Qiu and K. Park, Environment-sensitive hydrogels for drug delivery, Adv. Drug Delivery Rev., 2001, 53(3), 321–339 CrossRef CAS PubMed.
- J. E. Chung, M. Yokoyama, M. Yamato, T. Aoyagi, Y. Sakurai and T. Okano, Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate), J. Controlled Release, 1999, 62(1–2), 115–127 CrossRef CAS PubMed.
- Z. Liu, Y. D. Liu, Q. Shi and Y. Liang, Electroactive dielectric polymer gels as new-generation soft actuators: A review, J. Mater. Sci., 2021, 56(27), 14943–14963 CrossRef CAS.
- S. Liang and L. Zhang, Fluorescent Mechanism and Optical Switching of Fluorophore-Free Organogel, Macromol. Rapid Commun., 2023, 44(5), 2200752 CrossRef CAS PubMed.
- N. Huebsch, C. J. Kearney, X. Zhao, J. Kim, C. A. Cezar, Z. Suo and D. J. Mooney, Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(27), 9762–9767 CrossRef CAS PubMed.
- M. Q. Tong, L. Z. Luo, P. P. Xue, Y. H. Han, L. F. Wang, D. L. Zhuge, Q. Yao, B. Chen, Y. Z. Zhao and H. L. Xu, Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats, Acta Biomater., 2021, 122, 111–132 CrossRef CAS PubMed.
- P. Bilalis, D. Skoulas, A. Karatzas, J. Marakis, A. Stamogiannos, C. Tsimblouli, E. Sereti, E. Stratikos, K. Dimas, D. Vlassopoulos and H. Iatrou, Self-healing pH-and enzyme stimuli-responsive hydrogels for targeted delivery of gemcitabine to treat pancreatic cancer, Biomacromolecules, 2018, 19(9), 3840–3852 CrossRef CAS PubMed.
- L. Altomare, L. Bonetti, C. E. Campiglio, L. De Nardo, L. Draghi, F. Tana and S. Farè, Biopolymer-based strategies in the design of smart medical devices and artificial organs, Int. J. Artif. Organs, 2018, 41(6), 337–359 CrossRef CAS PubMed.
- G. Kocak, C. A. Tuncer and V. J. Bütün, pH-Responsive polymers, Polym. Chem., 2017, 8(1), 144–176 RSC.
- A. Belščak-Cvitanović, D. Komes, S. Karlović, S. Djaković, I. Špoljarić, G. Mršić and D. Ježek, Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium, Food Chem., 2015, 167, 378–386 CrossRef PubMed.
- H. Garshasbi, S. Salehi, S. M. Naghib, S. Ghorbanzadeh and W. Zhang, Stimuli-responsive injectable chitosan-based hydrogels for controlled drug delivery systems, Front. Bioeng. Biotechnol., 2023, 10, 1126774 CrossRef PubMed.
- A. R. Costa-Pinto, R. L. Reis and N. M. Neves, Scaffolds based bone tissue engineering: the role of chitosan, Tissue Eng., Part B, 2011, 17(5), 331–347 CrossRef CAS PubMed.
- A. Verma, J. Dubey, N. Verma and A. Kumar Nayak, Chitosan-hydroxypropyl methylcellulose matrices as carriers for hydrodynamically balanced capsules of moxifloxacin HCl, Curr. Drug Delivery, 2017, 14(1), 83–90 CrossRef CAS PubMed.
- A. H. King, Brown seaweed extracts (alginates), Food hydrocolloids, CRC Press, 2019, pp. 115–188 Search PubMed.
- A. B. Nazlı and Y. S. Açıkel, Loading of cancer drug resveratrol to pH-Sensitive, smart, alginate-chitosan hydrogels and investigation of controlled release kinetics, J. Drug Delivery Sci. Technol., 2019, 53, 101199 CrossRef.
- S. Khot, S. U. Rawal and M. M. Patel, Dissolvable-soluble or biodegradable polymers. Drug Delivery Devices and Therapeutic Systems, 2021, pp. 367–394 Search PubMed.
- D. Silcock, Collagen-based dressings as therapeutic agents for wound healing, Drug-device combination products, Woodhead Publishing, 2010, pp. 280–310 Search PubMed.
- S. M. Han, J. C. Kim, Y. Shin, D. Lee, T. Sim, C. Lim, K. Kang, E. S. Lee, Y. S. Youn and K. T. Oh, Development of a pH-responsive polymer based on hyaluronic acid conjugated with imidazole and dodecylamine for nanomedicine delivery, Macromol. Res., 2022, 30(8), 547–556 CrossRef CAS.
- J. Gautam and H. Schott, Interaction of anionic compounds with gelatin. II: Effect on some physicochemical properties of gelatin, J. Pharm. Sci., 1994, 83(3), 316–321 CrossRef CAS PubMed.
- S. Boughriba, N. Souissi, R. Nasri, M. Nasri and S. Li, pH sensitive composite hydrogels based on gelatin and reinforced with cellulose microcrystals: In depth physicochemical and microstructural analyses for controlled release of vitamin B2, Mater. Today Commun., 2021, 27, 102334 CrossRef CAS.
- M. Nakayama, T. Okano, T. Miyazaki, F. Kohori, K. Sakai and M. Yokoyama, Molecular design of biodegradable polymeric micelles for temperature-responsive drug release, J. Controlled Release, 2006, 115(1), 46–56 CrossRef CAS PubMed.
- K. Soleimani, H. Derakhshankhah, M. Jaymand and H. Samadian, Stimuli-responsive natural gums-based drug delivery systems for cancer treatment, Carbohydr. Polym., 2021, 254, 117422 CrossRef CAS PubMed.
- L. Klouda, Thermoresponsive hydrogels in biomedical applications: A seven-year update, Eur. J. Pharm. Biopharm., 2015, 97, 338–349 CrossRef CAS PubMed.
- L. Klouda, Thermoresponsive hydrogels in biomedical applications: A seven-year update, Eur. J. Pharm. Biopharm., 2015, 97, 338–349 CrossRef CAS PubMed.
- M. A. Ward and T. K. Georgiou, Thermoresponsive polymers for biomedical applications, Polymers, 2011, 3(3), 1215–1242 CrossRef CAS.
- B. Jeong, S. W. Kim and Y. H. Bae, Thermosensitive sol–gel reversible hydrogels, Adv. Drug Delivery Rev., 2012, 64, 154–162 CrossRef.
- N. A. Peppas, P. Bures, W. S. Leobandung and H. Ichikawa, Hydrogels in pharmaceutical formulations, Eur. J. Pharm. Biopharm., 2000, 50(1), 27–46 CrossRef CAS PubMed.
- E. Ruel-Gariepy and J. C. Leroux, In situ-forming hydrogels—review of temperature-sensitive systems, Eur. J. Pharm. Biopharm., 2004, 58(2), 409–426 CrossRef CAS PubMed.
- B. Jeong and A. Gutowska, Lessons from nature: stimuli-responsive polymers and their biomedical applications, Trends Biotechnol., 2002, 20(7), 305–311 CrossRef CAS PubMed.
- L. Dong, S. J. Wang, X. R. Zhao, Y. F. Zhu and J. K. Yu, 3D-printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering, Sci. Rep., 2017, 7(1), 13412 CrossRef PubMed.
- X. Huang, B. R. Nayak and T. L. Lowe, Synthesis and characterization of novel thermoresponsive-co-biodegradable hydrogels composed of N-isopropylacrylamide, poly(L-lactic acid), and dextran, J. Polym. Sci., Part A: Polym. Chem., 2004, 42(20), 5054–5066 CrossRef CAS.
- D. Schmaljohann, Thermo-and pH-responsive polymers in drug delivery, Adv. Drug Delivery Rev., 2006, 58(15), 1655–1670 CrossRef CAS PubMed.
- K. J. Hogan and A. G. Mikos, Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering, Polymer, 2020, 211, 123063 CrossRef CAS.
- R. Song, M. Murphy, C. Li, K. Ting, C. Soo and Z. Zheng, Current development of biodegradable polymeric materials for biomedical applications, Drug Des., Dev. Ther., 2018, 3117–3145 CrossRef CAS PubMed.
- S. Graham, P. F. Marina and A. Blencowe, Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks, Carbohydr. Polym., 2019, 207, 143–159 CrossRef CAS PubMed.
- M. Bustamante-Torres, D. Romero-Fierro, B. Arcentales-Vera, K. Palomino, H. Magaña and E. Bucio, Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials, Gels, 2021, 7(4), 182 CrossRef CAS PubMed.
- Y. Xing, B. Zeng and W. Yang, Light responsive hydrogels for controlled drug delivery, Front. Bioeng. Biotechnol., 2022, 10, 1075670 CrossRef PubMed.
- I. Tomatsu, K. Peng and A. Kros, Photoresponsive hydrogels for biomedical applications, Adv. Drug Delivery Rev., 2011, 63(14–15), 1257–1266 CrossRef CAS PubMed.
- K. Iwaso, Y. Takashima and A. Harada, Fast response dry-type artificial molecular muscles with [c2] daisy chains, Nat. Chem., 2016, 8(6), 625–632 CrossRef CAS PubMed.
- A. Bordbar-Khiabani and M. Gasik, Smart hydrogels for advanced drug delivery systems, Int. J. Mol. Sci., 2022, 23(7), 3665 CrossRef CAS PubMed.
- M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang, X. Chen, D. K. Sang, C. Xing, Z. Li, B. Dong and F. Xing, Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(3), 501–506 CrossRef CAS PubMed.
- T. Distler and A. R. Boccaccini, 3D printing of electrically conductive hydrogels for tissue engineering and biosensors–A review, Acta Biomater., 2020, 101, 1–3 CrossRef CAS PubMed.
- M. J. Garland, T. R. Singh, A. D. Woolfson and R. F. Donnelly, Electrically enhanced solute permeation across poly(ethylene glycol)–crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels: Effect of hydrogel crosslink density and ionic conductivity, Int. J. Pharm., 2011, 406(1–2), 91–98 CrossRef CAS PubMed.
- H. Shi, Z. Dai, X. Sheng, D. Xia, P. Shao, L. Yang and X. Luo, Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications, Sci. Total Environ, 2021, 786, 147430 CrossRef CAS PubMed.
- M. M. Fitzgerald, K. Bootsma, J. A. Berberich and J. L. Sparks, Tunable stress relaxation behavior of an alginate-polyacrylamide hydrogel: comparison with muscle tissue, Biomacromolecules, 2015, 16(5), 1497–1505 CrossRef CAS PubMed.
- S. Murdan, Electro-responsive drug delivery from hydrogels, J. Controlled Release, 2003, 92(1–2), 1–7 CrossRef CAS PubMed.
- T. Aoki, M. Muramatsu, A. Nishina, K. Sanui and N. Ogata, Thermosensitivity of optically active hydrogels constructed with N-(L)-(1-hydroxymethyl) propylmethacrylamide, Macromol. Biosci., 2004, 4(10), 943–949 CrossRef CAS PubMed.
- A. D. Grief and G. Richardson, Mathematical modelling of magnetically targeted drug delivery, J. Magn. Magn. Mater., 2005, 293(1), 455–463 CrossRef CAS.
- L. Tetard, A. Passian, R. H. Farahi, B. H. Davison, A. L. Lereu and T. Thundat, Optical and plasmonic spectroscopy with cantilever shaped materials, J. Phys. D: Appl. Phys., 2011, 44(44), 445102 CrossRef.
- E. Amstad and E. Reimhult, Nanoparticle actuated hollow drug delivery vehicles, Nanomedicine, 2012, 7(1), 145–164 CrossRef CAS PubMed.
- R. P. Blakemore and R. B. Frankel, Magnetic navigation in bacteria, Sci. Am., 1981, 245(6), 58–65 CrossRef.
- R. Hernandez, J. Sacristan, L. Asin, T. E. Torres, M. R. Ibarra, G. F. Goya and C. Mijangos, Magnetic hydrogels derived from polysaccharides with improved specific power absorption: potential devices for remotely triggered drug delivery, J. Phys. Chem. B, 2010, 114(37), 12002–12007 CrossRef CAS PubMed.
- X. Li, L. Duan, M. Kong, X. Wen, F. Guan and S. Ma, Applications and mechanisms of stimuli-responsive hydrogels in traumatic brain injury, Gels, 2022, 8(8), 482 CrossRef CAS PubMed.
- W. Wang, X. Fan, F. Li, J. Qiu, M. M. Umair, W. Ren, B. Ju, S. Zhang and B. Tang, Magnetochromic photonic hydrogel for an alternating magnetic field-responsive color display, Adv. Opt. Mater., 2018, 6(4), 1701093 CrossRef.
- N. Zhang, J. Lock, A. Sallee and H. Liu, Magnetic nanocomposite hydrogel for potential cartilage tissue engineering: synthesis, characterization, and cytocompatibility with bone marrow derived mesenchymal stem cells, ACS Appl. Mater. Interfaces, 2015, 7(37), 20987–20998 CrossRef CAS PubMed.
- S. F. Medeiros, A. M. Santos, H. Fessi and A. Elaissari, Stimuli-responsive magnetic particles for biomedical applications, Int. J. Pharm., 2011, 403(1–2), 139–161 CrossRef CAS PubMed.
- Y. Murakami and M. Maeda, DNA-responsive hydrogels that can shrink or swell, Biomacromolecules, 2005, 6(6), 2927–2929 CrossRef CAS PubMed.
- P. Li, A. M. Zhu, Q. L. Liu and Q. G. Zhang, Fe3O4/poly(N-isopropylacrylamide)/chitosan composite microspheres with multiresponsive properties, Ind. Eng. Chem. Res., 2008, 47(20), 7700–7706 CrossRef CAS.
- J. Zhang, Q. Huang and J. Du, Recent advances in magnetic hydrogels, Polym. Int., 2016, 65(12), 1365–1372 CrossRef CAS.
- P. Geetha, M. S. Latha, S. S. Pillai, B. Deepa, K. S. Kumar and M. Koshy, Green synthesis and characterization of alginate nanoparticles and its role as a biosorbent for Cr(VI) ions, J. Mol. Struct., 2016, 1105, 54–60 CrossRef CAS.
- G. Sharifzadeh and H. Hosseinkhani, Biomolecule-responsive hydrogels in medicine, Adv. Healthcare Mater., 2017, 6(24), 1700801 CrossRef PubMed.
- M. Constantin, S. Bucatariu, P. Ascenzi, M. Butnaru and G. Fundueanu, Smart drug delivery system activated by specific biomolecules, Mater. Sci. Eng., C, 2020, 108, 110466 CrossRef CAS PubMed.
- J. Yang, W. Zeng, P. Xu, X. Fu, X. Yu, L. Chen, F. Leng, C. Yu and Z. Yang, Glucose-responsive multifunctional metal–organic drug-loaded hydrogel for diabetic wound healing, Acta Biomater., 2022, 140, 206–218 CrossRef CAS PubMed.
- D. Shen, H. Yu, L. Wang, X. Chen, J. Feng, C. Li, W. Xiong and Q. Zhang, Glucose-responsive hydrogel-based microneedles containing phenylborate ester bonds and N-isopropylacrylamide moieties and their transdermal drug delivery properties, Eur. Polym. J., 2021, 148, 110348 CrossRef CAS.
- Y. Zhu, J. Jia, G. Zhao, X. Huang, L. Wang, Y. Zhang, L. Zhang, N. Konduru, J. Xie, R. Yu and H. Liu, Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation, J. Nanobiotechnol., 2021, 19(1), 198 CrossRef CAS PubMed.
- S. Kim, Z. K. Cui, B. Koo, J. Zheng, T. Aghaloo and M. Lee, Chitosan–lysozyme conjugates for enzyme-triggered hydrogel degradation in tissue engineering applications, ACS Appl. Mater. Interfaces, 2018, 10(48), 41138–41145 CrossRef CAS PubMed.
- N. Kamaly, Z. Xiao, P. M. Valencia, A. F. Radovic-Moreno and O. C. Farokhzad, Targeted polymeric therapeutic nanoparticles: design, development and clinical translation, Chem. Soc. Rev., 2012, 41(7), 2971–3010 RSC.
- J. Shi, Z. Xiao, N. Kamaly and O. C. Farokhzad, Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation, Acc. Chem. Res., 2011, 44(10), 1123–1134 CrossRef CAS PubMed.
- A. Kumar, A. Srivastava, I. Y. Galaev and B. Mattiasson, Smart polymers: Physical forms and bioengineering applications, Prog. Polym. Sci., 2007, 32(10), 1205–1237 CrossRef CAS.
- A. M. Rosales and K. S. Anseth, The design of reversible hydrogels to capture extracellular matrix dynamics, Nat. Rev. Mater., 2016, 1(2), 1–5 Search PubMed.
- S. Sershen and J. West, Implantable, polymeric systems for modulated drug delivery, Adv. Drug Delivery Rev., 2002, 54(9), 1225–1235 CrossRef CAS PubMed.
- P. Wei, E. J. Cornel and J. Du, Ultrasound-responsive polymer-based drug delivery systems, Drug Delivery Transl. Res., 2021, 11, 1323–1339 CrossRef CAS PubMed.
- Z. G. Gao, H. D. Fain and N. Rapoport, Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound, J. Controlled Release, 2005, 102(1), 203–222 CrossRef CAS PubMed.
- S. Yamaguchi, K. Higashi, T. Azuma and A. Okamoto, Supramolecular polymeric hydrogels for ultrasound-guided protein release, Biotechnol. J., 2019, 14(5), 1800530 CrossRef PubMed.
- J. H. Kuo, M. S. Jan and K. C. Sung, Evaluation of the stability of polymer-based plasmid DNA delivery systems after ultrasound exposure, Int. J. Pharm., 2003, 257(1–2), 75–84 CrossRef CAS PubMed.
- V. M. Vijayan, P. N. Vasudevan and V. Thomas, Polymeric Nanogels for theranostic applications: a mini-review, Curr. Nanosci., 2020, 16(3), 392–398 CrossRef CAS.
- J. I. Kim, B. S. Lee, C. Chun, J. K. Cho, S. Y. Kim and S. C. Song, Long-term theranostic hydrogel system for solid tumors, Biomaterials, 2012, 33(7), 2251–2259 CrossRef CAS PubMed.
- K. Podgorna, K. Szczepanowicz, M. Piotrowski, M. Gajdosová, F. Dtpanek and P. Warszynski, Gadolinium alginate nanogels for theranostic applications, Colloids Surf., B, 2017, 153, 183–189 CrossRef CAS PubMed.
- L. R. Jaidev, D. R. Chellappan, D. V. Bhavsar, R. Ranganathan, B. Sivanantham, A. Subramanian, U. Sharma, N. R. Jagannathan, U. M. Krishnan and S. Sethuraman, Multi-functional nanoparticles as theranostic agents for the treatment & imaging of pancreatic cancer, Acta Biomater., 2017, 49, 422–433 CrossRef CAS PubMed.
- D. Caballero, C. M. Abreu, A. C. Lima, N. M. Neves, R. L. Reis and S. C. Kundu, Precision biomaterials in cancer theranostics and modelling, Biomaterials, 2022, 280, 121299 CrossRef CAS PubMed.
- J. R. Clegg, A. S. Irani, E. W. Ander, C. M. Ludolph, A. K. Venkataraman, J. X. Zhong and N. A. Peppas, Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications, Sci. Adv., 2019, 5(9), eaax7946 CrossRef CAS PubMed.
- H. Koo, M. S. Huh, I. C. Sun, S. H. Yuk, K. Choi, K. Kim and I. C. Kwon, In vivo targeted delivery of nanoparticles for theranosis, Acc. Chem. Res., 2011, 44(10), 1018–1028 CrossRef CAS PubMed.
- L. Chambre, A. Degirmenci, R. Sanyal and A. Sanyal, Multi-functional nanogels as theranostic platforms: exploiting reversible and nonreversible linkages for targeting, imaging, and drug delivery, Bioconjugate Chem., 2018, 29(6), 1885–1896 CrossRef CAS PubMed.
- Y. Wang, M. S. Shim, N. S. Levinson, H. W. Sung and Y. Xia, Stimuli-responsive materials for controlled release of theranostic agents, Adv. Funct. Mater., 2014, 24(27), 4206–4220 CrossRef CAS PubMed.
- P. Mi, Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics, Theranostics, 2020, 10(10), 4557 CrossRef CAS PubMed.
- A. Gupta, A. Sood, E. Fuhrer, K. Djanashvili and G. Agrawal, Polysaccharide-based theranostic systems for combined imaging and cancer therapy: Recent advances and challenges, ACS Biomater. Sci. Eng., 2022, 8(6), 2281–2306 CrossRef CAS PubMed.
- S. P. Victor, W. Paul, M. Jayabalan and C. P. Sharma, Cucurbituril/hydroxyapatite based nanoparticles for potential use in theranostic applications, CrystEngComm, 2014, 16(30), 6929–6936 RSC.
- M. E. Caldorera-Moore, W. B. Liechty and N. A. Peppas, Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers, Acc. Chem. Res., 2011, 44(10), 1061–1070 CrossRef CAS PubMed.
- J. F. Gomes Marin, R. F. Nunes, A. M. Coutinho, E. C. Zaniboni, L. B. Costa, F. G. Barbosa, M. A. Queiroz, G. G. Cerri and C. A. Buchpiguel, Theranostics in nuclear medicine: emerging and re-emerging integrated imaging and therapies in the era of precision oncology, Radiographics, 2020, 40(6), 1715–1740 CrossRef PubMed.
- M. S. Muthu, L. Mei and S. S. Feng, Nanotheranostics: advanced nanomedicine for the integration of diagnosis and therapy, Nanomedicine, 2014, 9(9), 1277–1280 CrossRef CAS PubMed.
- M. A. Ackun-Farmmer, C. T. Overby, B. E. Haws, R. Choe and D. S. Benoit, Biomaterials for orthopedic diagnostics and theranostics, Curr. Opin. Biomed. Eng., 2021, 19, 100308 CrossRef CAS PubMed.
- S. L. Qiao, M. Mamuti, H. W. An and H. Wang, Thermoresponsive polymer assemblies: from molecular design to theranostics application, Prog. Polym. Sci., 2022, 131, 101578 CrossRef CAS.
- Z. Wang, G. Niu and X. Chen, Polymeric materials for theranostic applications, Pharm. Res., 2014, 31, 1358–1376 CrossRef CAS PubMed.
- T. Peng, Z. Deng, J. He, Y. Li, Y. Tan, Y. Peng, X. Q. Wang and W. Tan, Functional nucleic acids for cancer theranostics, Coord. Chem. Rev., 2020, 403, 213080 CrossRef CAS.
- N. Liu, X. Chen, X. Sun, X. Sun and J. Shi, Persistent luminescence nanoparticles for cancer theranostics application, J. Nanobiotechnol., 2021, 19, 1–24 CrossRef PubMed.
- P. Ran, H. Zheng, W. Cao, X. Jia, G. Zhang, Y. Liu and X. Li, On-demand changeable theranostic hydrogels and visual imaging-guided antibacterial photodynamic therapy to promote wound healing, ACS Appl. Mater. Interfaces, 2022, 14(43), 49375–49388 CrossRef CAS.
- H. Huang, Y. Su, C. Wang, B. Lei, X. Song, W. Wang, P. Wu, X. Liu, X. Dong and L. Zhong, Injectable tissue-adhesive hydrogel for photothermal/chemodynamic synergistic antibacterial and wound healing promotion, ACS Appl. Mater. Interfaces, 2023, 15(2), 2714–2724 CrossRef CAS.
- Y. He, F. Sun, M. Li, T. Ji, Y. Fang, G. Tan, C. Ma and Y. Huang, A Gel/fiber composite formulation achieves sequential delivery based on multimodal analgesia reducing chronic pain, Mater. Des., 2023, 225, 111541 CrossRef CAS.
- M. A. Grimaudo, A. Concheiro and C. Alvarez-Lorenzo, Nanogels for regenerative medicine, J. Controlled Release, 2019, 313, 148–160 CrossRef CAS.
- H. K. Patra, M. Azharuddin, M. M. Islam, G. Papapavlou, S. Deb, J. Osterrieth, G. H. Zhu, T. Romu, A. K. Dhara, M. J. Jafari and A. Ghaderi, Rational Nanotoolbox with Theranostic Potential for Medicated Pro-Regenerative Corneal Implants, Adv. Funct. Mater., 2019, 29(38), 1903760 CrossRef.
- P. Hassanzadeh, F. Atyabi and R. Dinarvand, Application of modelling and nanotechnology-based approaches: The emergence of breakthroughs in theranostics of central nervous system disorders, Life Sci., 2017, 182, 93–103 CrossRef CAS PubMed.
- C. Montoya, L. Roldan, M. Yu, S. Valliani, C. Ta, M. Yang and S. Orrego, Smart dental materials for antimicrobial applications, Bioact. Mater., 2023, 24, 1–9 CAS.
- K. Varaprasad, G. M. Raghavendra, T. Jayaramudu, M. M. Yallapu and R. Sadiku, A mini review on hydrogels classification and recent developments in miscellaneous applications, Mater. Sci. Eng., C, 2017, 79, 958–971 CrossRef CAS PubMed.
- N. A. Pattanashetti, G. B. Heggannavar and M. Y. Kariduraganavar, Smart biopolymers and their biomedical applications, Procedia Manuf., 2017, 12, 263–279 CrossRef.
- D. Loessner, C. Meinert, E. Kaemmerer, L. C. Martine, K. Yue, P. A. Levett, T. J. Klein, F. P. Melchels, A. Khademhosseini and D. W. Hutmacher, Functionalization, preparation and use of cell-laden gelatin methacryloyl–based hydrogels as modular tissue culture platforms, Nat. Protoc., 2016, 11(4), 727–746 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Designing hydrogels for controlled drug delivery, Nat. Rev. Mater., 2016, 1(12), 1–7 Search PubMed.
- Y. Qiu and K. Park, Environment-sensitive hydrogels for drug delivery, Adv. Drug Delivery Rev., 2001, 53(3), 321–339 CrossRef CAS.
- Q. Chai, Y. Jiao and X. Yu, Hydrogels for biomedical applications: their characteristics and the mechanisms behind them, Gels, 2017, 3(1), 6 CrossRef PubMed.
- R. Rebelo, M. Fernandes and R. Fangueiro, Biopolymers in medical implants: a brief review, Procedia Eng., 2017, 200, 236–243 CrossRef CAS.
- R. Bhola, S. M. Bhola, H. Liang and B. Mishra, Biocompatible denture polymers-a review. Trends Biomater, Artif. Organs, 2010, 23(3), 129–136 Search PubMed.
- M. C. Biswas, B. Jony, P. K. Nandy, R. A. Chowdhury, S. Halder, D. Kumar, S. Ramakrishna, M. Hassan, M. A. Ahsan, M. E. Hoque and M. A. Imam, Recent advancement of biopolymers and their potential biomedical applications, J. Polym. Environ., 2022, 1–24 Search PubMed.
- M. Kamaci and I. Kaya, Chitosan based hybrid hydrogels for drug delivery: Preparation, biodegradation, thermal, and mechanical properties, Polym. Adv. Technol., 2023, 34(2), 779–788 CrossRef CAS.
- G. Dalei, S. Das, S. R. Jena, D. Jena, J. Nayak and L. Samanta, In situ crosslinked dialdehyde guar gum-chitosan Schiff-base hydrogels for dual drug release in colorectal cancer therapy, Chem. Eng. Sci., 2023, 269, 118482 CrossRef CAS.
- V. Brumberg, T. Astrelina, T. Malivanova and A. Samoilov, Modern wound dressings: Hydrogel dressings, Biomedicines, 2021, 9(9), 1235 CrossRef CAS PubMed.
- J. P. Junker, R. A. Kamel, E. J. Caterson and E. Eriksson, Clinical impact upon wound healing and inflammation in moist, wet, and dry environments, Adv. Wound Care, 2013, 2(7), 348–356 CrossRef PubMed.
- Y. Liang, Y. Liang, H. Zhang and B. Guo, Antibacterial biomaterials for skin wound dressing, Asian J. Pharm. Sci., 2022, 17(3), 353–384 CAS.
- C. Hu, F. Zhang, L. Long, Q. Kong, R. Luo and Y. Wang, Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing, J. Controlled Release, 2020, 324, 204–217 CrossRef CAS PubMed.
- C. Ding, M. Tian, R. Feng, Y. Dang and M. Zhang, Novel self-healing hydrogel with injectable, pH-responsive, strain-sensitive, promoting wound-healing, and hemostatic properties based on collagen and chitosan, ACS Biomater. Sci. Eng., 2020, 6(7), 3855–3867 CrossRef CAS PubMed.
- Y. Yu, X. Zheng, X. Liu, J. Zhao and S. Wang, Injectable carboxymethyl chitosan-based hydrogel for simultaneous anti-tumor recurrence and anti-bacterial applications, Int. J. Biol. Macromol., 2023, 230, 123196 CrossRef CAS PubMed.
- M. A. Ward and T. K. Georgiou, Thermoresponsive polymers for biomedical applications, Polymers, 2011, 3(3), 1215–1242 CrossRef CAS.
- N. Khoshnood, J. P. Frampton, A. Badri and A. Zamanian, 3D bioprinting of betamethasone-loaded gellan gum–polyethyleneimine composite hydrogels for ocular drug delivery, Int. J. Bioprint., 2024, 10(4), 3440 CrossRef.
- A. J. Feliciano, Y. A. Selsouli, P. Habibovic, Z. N. Birgani, L. Moroni and M. B. Baker, Granular polyrotaxane microgels as injectable hydrogels for corneal tissue regeneration, Biomater. Sci., 2024, 12(19), 4993–5009 RSC.
- M. Prasathkumar, C. Dhrisya, F. H. Lin and S. Sadhasivam, The Design and Developments of Protein-Polysaccharide Biomaterials for Corneal Tissue Engineering, Adv. Mater. Technol., 2023, 8(15), 2300171 CrossRef CAS.
- C. Su, D. Lin, X. Huang, J. Feng, A. Jin, F. Wang, Q. Lv, L. Lei and W. Pan, Developing hydrogels for gene therapy and tissue engineering, J. Nanobiotechnol., 2024, 22(1), 182 CrossRef CAS PubMed.
- R. Patel and D. Patel, Injectable Hydrogels in Cardiovascular Tissue Engineering, Polymers, 2024, 16(13), 1878 CrossRef CAS.
- J. Zhao, L. Wang, H. Zhang, B. Liao and Y. Li, Progress of research in in situ smart hydrogels for local antitumor therapy: A review, Pharmaceutics, 2022, 14(10), 2028 CrossRef CAS PubMed.
- A. Hanf, M. Oelze, A. Manea, H. Li, T. Münzel and A. Daiber, The anti-cancer drug doxorubicin induces substantial epigenetic changes in cultured cardiomyocytes, Chem.-Biol. Interact., 2019, 313, 108834 CrossRef CAS.
- D. Rafael, M. M. Melendres, F. Andrade, S. Montero, F. Martinez-Trucharte, M. Vilar-Hernandez, E. F. Durán-Lara, S. Schwartz Jr and I. Abasolo, Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art, Int. J. Pharm., 2021, 606, 120954 CrossRef CAS PubMed.
- A. Ahsan, M. A. Farooq and A. Parveen, Thermosensitive chitosan-based injectable hydrogel as an efficient anticancer drug carrier, ACS Omega, 2020, 5(32), 20450–20460 CrossRef CAS PubMed.
- B. Gao, J. Luo, Y. Liu, S. Su, S. Fu, X. Yang and B. Li, Intratumoral administration of thermosensitive hydrogel co-loaded with norcantharidin nanoparticles and doxorubicin for the treatment of hepatocellular carcinoma, Int. J. Nanomed., 2021, 4073–4085 CrossRef PubMed.
- Y. K. Wu, N. C. Cheng and C. M. Cheng, Biofilms in chronic wounds: pathogenesis and diagnosis, Trends Biotechnol., 2019, 37(5), 505–517 CrossRef CAS PubMed.
- H. Williams, L. Campbell, R. A. Crompton, G. Singh, B. J. McHugh, D. J. Davidson, A. J. McBain, S. M. Cruickshank and M. J. Hardman, Microbial host interactions and impaired wound healing in mice and humans: defining a role for BD14 and NOD2, J. Invest. Dermatol., 2018, 138(10), 2264–2274 CrossRef CAS PubMed.
- A. Gupta, S. M. Briffa, S. Swingler, H. Gibson, V. Kannappan, G. Adamus, M. Kowalczuk, C. Martin and I. Radecka, Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications, Biomacromolecules, 2020, 21(5), 1802–1811 CrossRef CAS PubMed.
- V. Pawar, M. Dhanka and R. Srivastava, Cefuroxime conjugated chitosan hydrogel for treatment of wound infections, Colloids Surf., B, 2019, 173, 776–787 CrossRef CAS PubMed.
- A. P. Turner, Biosensors–sense and sensitivity, Science, 2000, 290(5495), 1315–1317 CrossRef CAS PubMed.
- M. Chelu and A. M. Musuc, Polymer gels: Classification and recent developments in biomedical applications, Gels, 2023, 9(2), 161 CrossRef CAS PubMed.
- R. Baretta, A. Raucci, S. Cinti and M. Frasconi, Porous hydrogel scaffolds integrating Prussian Blue nanoparticles: A versatile strategy for electrochemical (bio) sensing, Sens. Actuators, B, 2023, 376, 132985 CrossRef CAS.
- H. Wang, H. Wang, Y. Li, C. Jiang, D. Chen, Y. Wen and Z. Li, Capillarity self-driven DNA hydrogel sensor for visual quantification of microRNA, Sens. Actuators, B, 2020, 313, 128036 CrossRef CAS.
- J. Zhang, X. Jiang, X. Wen, Q. Xu, H. Zeng, Y. Zhao, M. Liu, Z. Wang, X. Hu and Y. Wang, Bio-responsive smart polymers and biomedical applications, J. Phys.: Mater., 2019, 2(3), 032004 CAS.
- P. Jirvankar, S. Agrawal, N. Chambhare and R. Agrawal, Harnessing Biopolymer Gels for Theranostic Applications: Imaging Agent Integration and Real-Time Monitoring of Drug Delivery, Gels, 2024, 10(8), 535 CrossRef CAS PubMed.
- C. B. Highley, C. B. Rodell and J. A. Burdick, Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels, Adv. Mater., 2015, 27(34), 5075–5079 CrossRef CAS PubMed.
- M. C. Giano, D. J. Pochan and J. P. Schneider, Controlled biodegradation of Self-assembling β-hairpin Peptide hydrogels by proteolysis with matrix metalloproteinase-13, Biomaterials, 2011, 32(27), 6471–6477 CrossRef CAS PubMed.
- A. Shabsigh, N. Kleinmann, A. B. Smith, D. Scherr, E. Seltzer, M. Schoenberg and S. P. Lerner, Pharmacokinetics of UGN-101, a mitomycin-containing reverse thermal gel instilled via retrograde catheter for the treatment of low-grade upper tract urothelial carcinoma, Cancer Chemother. Pharmacol., 2021, 87, 799–805 CrossRef CAS PubMed.
- A. Bordbar-Khiabani and M. Gasik, Smart hydrogels for advanced drug delivery systems, Int. J. Mol. Sci., 2022, 23(7), 3665 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |