Multi-responsive shape memory and self-healing hydrogels with gold and silver nanoparticles†
Hüsna
Kılıç
a and
Deniz
Ceylan
 *b
*b
aBezmialem Vakıf University, Health Sciences Institute, Department of Biotechnology, 34093 Istanbul, Turkey
bBezmialem Vakif University, Faculty of Pharmacy, 34093 Istanbul, Turkey. E-mail: deniz.ceylan@bezmialem.edu.tr
First published on 18th November 2024
Abstract
Nanocomposite smart gels (Nc-x) with self-healing and shape memory properties were designed in different types and size nano particles with temperature or light stimuli. Nc-x networks were prepared by bulk polymerization of stearyl methacrylate (SM) and vinyl pyrrolidone (VP) in the presence of gold and silver nanoparticles. The structure, which does not contain any chemical cross-linkers, is held together by hydrophobic interactions while consisting of dipole–dipole bonds of the VP units and long alkyl groups in the side chains of the SM. Thanks to their crystalline regions, shape memory gels can self-heal with the presence of long hydrophobic chains, and furthermore, the nanoparticles (NPs) incorporated into the structure facilitate the controlled tuning of hydrophilic and hydrophobic properties. Nc-x gels have the ability to self-heal by repairing mechanical damage independently or in the presence of a stimulus, as well as transforming from a temporary form to a permanent form. In vitro experiments on human skin fibroblast cells revealed that cell viability was over 100% after 48 hours and almost complete recovery was observed in scratch experiments at the end of this period. Based on the results obtained, Nc-x gels have been shown to have the potential to be used as a non-invasive wound dressing material alternative to traditional wound closure methods.
1. Introduction
Hydrogels, defined as cross-linked three-dimensional polymer networks that can absorb large amounts of water, are generally prepared through electrostatic interactions/hydrogen bonding or physical/chemical bonding between the polymer chains that form them.1,2 Their responsiveness to stimuli combined with tunable degrees of biocompatibility, biodegradability, mechanical strength, and stability make smart gels ideal candidates for applications in various fields, from tissue engineering to medicine and soft robotic materials.3 Self-healing and shape memory properties are some of the features seen in new generation smart hydrogels and have been the subject of numerous studies. Self-healing ability is one of the superior properties of natural materials that extend the life of living organisms. Self-healing polymers, in which mechanical damage is repaired independently or in the presence of a stimulus, have been used as inspiration for the development of smart materials by mimicking the extraordinary properties of biological systems. The shape memory property is defined as the ability of a material to “remember” its original shape and regain it in response to an external stimulus (usually temperature and light).4 SMPs can initially deform at a high temperature, generally above the glass transition (Tg) or crystal melting temperature (Tm), and then can be converted to a temporary shape at a low (<Tcry) temperature.3,5,6 Incorporating nanoscale inorganic particles into the hydrogel structure improves the physical and mechanical properties, making them not only strong and elastic but also stimuli-responsive.7,8By using hydrogels to mimic tissues, excellent regenerative 3D networks are obtained, and their adhesive properties bind defective tissues together, allowing the wound to heal properly. Wound dressings, used to support various stages of wound healing and create better healing conditions, often cover the wound surface to accelerate healing.9 The use of hydrogels in wound dressings was first reported in 1989, and today, hydrogel wound dressings are commercially available.10,11 Nevertheless, some hydrogel dressings cannot meet ideal dressing requirements due to their low strength and elasticity and may adhere to the wound surface or crush under high stress.12 Therefore, hydrogel with good strength and flexibility is expected to be better as a wound covering material. It is of great importance that the materials to be used in wound closure are tightly bonded to the skin and do not restrict movement artifacts.13–15 Moreover, since skin has dynamic, low surface energy (25–29 mJ m−2) and multiscale surface textures, stable and robust adhesion to the skin is difficult. For this purpose, ultrathin hydrogels with a flexible and adhesive configuration with a thickness of less than 10 μm have been designed.16 Such thin gels can be tightly laminated to the skin, reducing bending stiffness based on van der Waals forces.17,18 However, when the thickness of gels decreases, they become difficult to use and become mechanically weaker. Moreover, once applied to the skin, they cannot be reused.16
In this context, SM-x gels were obtained by bulk polymerization of the hydrophobic monomer stearyl methacrylate (SM) and the hydrophilic monomer vinyl pyrrolidone (VP) in the presence of a photoinitiator. The properties of the structures obtained by gradually changing the SM-VP ratios in gels where the hydrophobic interactions of the long alkyl side chains of the SM units and the dipole–dipole interactions resulting from the VP structure act as cross-linking points were examined in detail in our previous article.19 Among the gels prepared by varying the x value between 30 and 90, SM-70 gel was thought to have optimum properties to be used in potential applications such as wound dressing. Within the scope of this study, smart Nc-x gels that respond to both temperature and different wavelengths of light were obtained by adding different concentrations of gold nanospheres (AuNS) and silver nanocubes (AgNC) into the SM-70 network structure. In bioapplications, AuNS and AgNC have often been preferred to benefit from the plasmonic effect of Au and Ag nanoparticles.20 The thermoplasmonic effect of nanoparticles, which will trigger the heating process in the presence of light, has enabled the design of biocompatible and multifunctional gels with shape memory and self-healing properties. This feature is highly valuable for developing biocompatible and multifunctional materials. By providing precise thermal control, gold and silver nanoparticles enable shape memory hydrogels to change shape in response to a stimulus and then revert to their original form. Additionally, gold nanoparticles are biocompatible, making them safe for use in biomedical applications, allowing for the creation of smart materials that can be applied without harming the body. Plasmonic nanoparticles also enhance self-healing properties, broadening the potential of smart hydrogel applications. Furthermore, these nanoparticles improve the physical and mechanical properties of hydrogels, resulting in stronger and more flexible materials. These advantages make gold and silver nanoparticles ideal for shape memory regulation, especially in fields like biomedical engineering and tissue engineering.21,22 In order to demonstrate the potential of Nc-x gels as a non-invasive wound dressing material as an alternative to traditional wound closure methods, properties such as their ability to adhere to clean/undamaged tissue without sticking to the wet wound area and reusability, biocompatibility and water vapor permeability were investigated.
2. Materials and methods
2.1. Materials
1-Vinyl-2-pyrrolidone 99% (VP), stearyl methacrylate (SM), a mixture of 65% n-octadecyl methacrylate and 35% n-hexadecyl methacrylate, and 2-hydroxy-40-(2-hydroxyethoxy)-2 methylpropiophenone (Irgacure 2959), hydrogen tetrachloroaurate nonahydrate (HAuCI4·9H2O), trisodium citrate dihydrate (C6H5Na3O7·2H2O), polyvinyl pyrrolidone (PVP10-Ma 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), ethylene glycol (EG), polyvinyl pyrrolidone (PVP-Mw ∼ 55
000), ethylene glycol (EG), polyvinyl pyrrolidone (PVP-Mw ∼ 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and silver nitrate (AgNO3) were all purchased from Sigma-Aldrich, sodium sulfide nonahydrate (Na2S, 9H2O) was purchased from Carlo Erba and used without purification. Distilled water was used throughout this study. DMEM/F12 medium, fetal bovine serum, penicillin/streptomycin, and trypsin were purchased from Gibco (Thermo Fisher Scientific, US).
000) and silver nitrate (AgNO3) were all purchased from Sigma-Aldrich, sodium sulfide nonahydrate (Na2S, 9H2O) was purchased from Carlo Erba and used without purification. Distilled water was used throughout this study. DMEM/F12 medium, fetal bovine serum, penicillin/streptomycin, and trypsin were purchased from Gibco (Thermo Fisher Scientific, US).
2.2. Preparation of nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min after dilution with acetone to free the AgNC from the solvent and unreacted PVP chains. After decantation, the particles resuspended with distilled water are stored at +4 °C ready for use after resting for a day.
000 rpm for 15 min after dilution with acetone to free the AgNC from the solvent and unreacted PVP chains. After decantation, the particles resuspended with distilled water are stored at +4 °C ready for use after resting for a day.
2.3. Preparation of nanocomposite gels
In the synthesis of nanoparticle loaded hydrogels, 1-vinyl-2-pyrrolidone (VP) and stearyl methacrylate (SM) were used as hydrophilic and hydrophobic monomers, respectively. Monomer/metal ratios were changed in the gel(s) whose SM-VP ratio was kept constant as 70 mol%. After nanoparticles with different w/v ratios were added to the VP monomer and homogenized in the sonicator, SM was included in the system. In the presence of Irgacure 2959, nanocomposite gels were obtained after exposure to UV light for 24 hours in the photoreactor (Kerman brand equipped with 9 Philips 6 W lamps emitting light at λ = 350 nm). While gels without particles were coded as Nc-0, gels containing Au and Ag nanoparticles with different geometries participating in the structure at two different concentrations (x) were named as AuNc-x and AgNc-x, respectively.2.4. Characterization of nanocomposite gels
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values with time, frequency and strain sweeps were performed. In addition, the photo unit of the device was used to monitor the changes in the network structure properties in the presence of Au and Ag nanoparticles and to quantitatively determine the response of the gels to the light stimulus. To check the repeatability of the system, each measurement was performed in triplicate, and all analyzes were performed at 37 °C. The time sweep was measured at a constant frequency of f = 1 Hz and a strain amplitude of γ0 = 0.1 to ensure that the oscillating strain was within the linear viscoelastic region (LVER).
δ values with time, frequency and strain sweeps were performed. In addition, the photo unit of the device was used to monitor the changes in the network structure properties in the presence of Au and Ag nanoparticles and to quantitatively determine the response of the gels to the light stimulus. To check the repeatability of the system, each measurement was performed in triplicate, and all analyzes were performed at 37 °C. The time sweep was measured at a constant frequency of f = 1 Hz and a strain amplitude of γ0 = 0.1 to ensure that the oscillating strain was within the linear viscoelastic region (LVER).
UV light source (equipped with Omnicure S2000 spot UV curing system with filters of various wavelengths) was used for the gels placed between the plates of the Rheometer. For the time sweep under UV irradiation, storage modulus (G′) and loss modulus (G′′) values were followed under constant frequency and deformation for 5, 10 and 10 minutes, respectively, with UV light turned off, on and off. Strain sweep was performed at a fixed frequency of 1 Hz in the 0.1–100% strain range to determine the LVER of the samples. In UV deformation step, Nc-x gels were exposed to UV light for 10 minutes under UV with a light source suitable for the wavelength of nanoparticles. After the UV was turned off, back deformation was applied between 100% and 0.1%. Frequency sweep was performed at 0.1% strain in the 0.1–100 Hz frequency range. To examine the temperature profiles, the prepared gels were kept at 50 °C for 5 minutes and cooled from 50 °C to 15 °C at a rate of 0.5 °C min−1 to monitor the temperature-dependent change of G′ and G′′ values.
For monitoring the gel fractions, the gels were kept in distilled water until they reached swelling equilibrium after UV irradiation and their water was changed frequently. The gels that came to the swelling equilibrium were dried in a freeze-dryer. The gel fractions were calculated by considering the weight ratio of the insoluble polymer remaining in the network to the initial sample. Each test was repeated three times independently with similar results.
To measure the bioadhesive bond strength of the cylindrical gel samples, a 5 N load cell was used on a commercially purchased collagen placed between two acrylic plates. During the adhesion test, the probe is lowered downwards to touch the collagen layer to begin the bonding process and applied to the layer for a predetermined compression force dwell time. After this time, the probe is withdrawn from the collagen layer and its adhesive properties are measured. The force required to separate the hydrogel is recorded as a function of elongation. The maximum force and area under the curve were obtained along the force/time curve. Results were converted to adhesion work (mJ cm−2) and then displayed as a mean value with standard deviation. For reproducibility, at least seven samples of each gel were measured, and the results averaged.
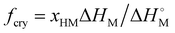 ; where xHM is the mole fraction of hydrophobic monomer in the comonomer feed and
; where xHM is the mole fraction of hydrophobic monomer in the comonomer feed and  is the melting enthalpy of crystal SM units.
is the melting enthalpy of crystal SM units.  was taken as 71.2 kJ mol−1 from previous studies on the melting behavior of long n-alkyl chains exhibiting a hexagonal crystal structure.15,23,24
was taken as 71.2 kJ mol−1 from previous studies on the melting behavior of long n-alkyl chains exhibiting a hexagonal crystal structure.15,23,24
| WVTR = (Mk − Mt)/(A × t) | (1) |
| Wound healing% = (Area·t0 − Area·tx)/Area·t0 | (2) |
2.5. Self-healing tests
In order to determine the heat induced self-healing properties of the hydrogels (n = 5), gel samples of 15 × 5 × 1 mm in initial length, diameter (preliminary condition) and thickness, respectively, were cut in half, and then the ends of the gel were brought together by hand. The self-healing ratio was calculated by comparing with the elongation test of the uncut material. For the UV stimulated self-healing measurements after making an incision (2 mm) in the middle of the gels, ends were joined with forceps. The elongation test was performed by exposing the gels to UV light suitable for the wavelength of the nanoparticles for a certain period of time. The elongation results of the uncut material were used to calculate the self-healing ratio of the gels.2.6. Shape memory tests
To examine the shape memory properties of nanocomposite gels (n = 5), firstly the gels were softened in hot water (T > Tm) to a temporary shape (U shape) and then cooled (T > Tm) to fix the U shape. The light corresponding to the wavelength of each nanoparticle in the composite samples was applied at an angle of 90°. Transformation processes from temporary to permanent shape were photographed to determine the equilibrium angle θT. The time it took for the gel to regain permanent shape was recorded. The recovery rate, R, of the U-fixed gels was calculated:| R = (θT/90) | (3) |
2.7. Biocompatibility studies
The direct contact test was performed according to19,26 CCD cells (1 × 105 cells per well) were cultured in 24-well plates under the same condition; the medium in the cultured wells was then discarded. The Nc-0 and Au/AgNc-x networks were placed directly on the cells adhered to the plate and incubated for 24 and 48 hours at 37 °C under 5% CO2 with the addition of fresh medium. Three replicates were run for each gel sample and gel-free wells were used as the control group.After the incubation period, the viability of CCD cells was determined by MTT assay. In this test, yellow 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT, Sigma, USA) was reduced by metabolically active cells. Gels placed in the wells were removed after certain times and fresh medium containing MTT reagent (final concentration 0.5 mg mL−1) was added to the plates; then incubated at 37 °C for 4 hours. At the end of the application period, the MTT reagent was removed from the wells and 250 μL of dimethyl sulfoxide (DMSO, Sigma, USA) solution was added to dissolve the precipitated formazan crystals.27 To assess cell metabolism, absorbance/optical density (OD) was measured at 570 nm using a microplate reader (BioTek Synergy H1, USA) and the recorded OD results were evaluated according to the following equation:
| Percent cell viability = (OD of experimental group/OD of control group) × 100 | (4) |
2.8. Statistical analysis
Jamovi 2.6.13 program was used for statistical analysis and the suitability of the parameters for normal distribution was evaluated with Shapiro Wilks. Data analysis was performed using a t-test and Repeated Measured Anova. Each sample was tested in triplicate unless specified otherwise. Error bars represent the mean ± standard deviation (SD) of measurements (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).283. Result and discussion
Mechanically strong, temperature and light responsive smart nanocomposite gels with shape memory and self-healing functions were designed and characterized. The synthesis of nanoparticle-loaded gels was carried out by free radical bulk polymerization in the presence of a photoinitiator by adding the hydrophobic monomer stearyl methacrylate (SM) to the structure after incorporation of nanoparticles with different w/v ratios on hydrophilic monomer vinyl pyrrolidone (VP). According to the previous study of our group, SM/VP ratios were gradually changed to examine the effect of hydrophobic and hydrophilic components on the properties of the final gels.19 The hydrogels denoted by SM-x were prepared at a SM/VP molar ratio of x/100-x. In this article, SM-70 gels with the best adhesion and cell properties were preferred, especially considering its potential applications as a wound closure. As shown in Scheme 1, the SM-70 network structure was combined with different concentrations of PVP-coated gold nanospheres (AuNS@PVP) and silver nanocubes (AgNC) to obtain temperature- and light-sensitive nanocomposite hydrogels. The optimized SM-70 will be referred to as Nc-0 in this article. Nanocomposite Nc-x gels were developed by incorporating 0.02 and 0.08 w/v AuNS@PVP and AgNC particles into the gel networks with a constant SM-VP ratio. Thus, variation of the gel properties as a function of nanoparticle amount and also geometry was examined. | ||
| Scheme 1 Schematic representation of the AuNc-x and AgNc-x network structures. Reaction vials before UV irradiation and swollen state AuNc-x and AgNc-x networks. | ||
3.1. Preparation and structural characterization of the Nc-x Gels
Nc-x networks prepared in the photoreactor for 24 h were immersed in water at 50 °C until the swelling equilibrium was reached. While the water around the gels was changed at regular intervals, the weight changes were followed to monitor the swelling kinetics of the networks. In addition to observing a maximum in the swelling behavior of the Nc-x gels on the fourth day of measurements, the gels reached swelling equilibrium within a week. Calculated Wg value which is around 1 indicate that all monomers in the feed are included in the Nc-x networks with and without nanoparticles.In Scheme 1, where the components in the Nc-x networks are illustrated schematically, there are photographs showing the reaction solutions in the vials before UV irradiation, and the gels synthesized on the plates and brought to swelling equilibrium. It is evident that AuNS@PVP and AgNC structures are homogeneously dispersed both in the reaction solutions before UV irradiation and also in the swollen AuNc-x and AgNc-x networks, there is no phase separation, and the particles do not move away and remain in the structure even after swelling.
As it is known, the conversion of kinetic energy of light into thermal energy by nanoparticles with localized surface plasmon resonance (LSPR) included in the gel structure triggers the photothermal effect.29 Supported by metallic nanostructures, LSPR triggers heating in the presence of light, enabling the design of biocompatible and multifunctional gels with shape memory and self-healing properties. The type, geometry and size of the metal used can be manipulated to obtain LSPR with specific wavelength and energy.30–33 In this direction, the synthesis of PVP-coated gold nano-spheres (AuNS@PVP) and silver nano-cubes (AgNC) was carried out within the scope of the study.
DLS and UV-Vis spectra and TEM images were examined to determine the dimensions and geometries of localized surface plasmon resonances of PVP-coated gold nanoparticles synthesized and modified by the Turkevich method (Fig. S1(A–C), ESI†). While the size of the synthesized AuNS@PVPs was measured to be 15 ± 2 nm by DLS, an absorption peak was obtained at 527 nm in UV-Vis analysis. According to the measurements made with Image J on TEM images at different magnifications, the particle size was determined to be around 13 ± 3 nm. When Fig. S1(D–F) (ESI†), which includes DLS, UV-Vis and TEM analyzes of AgNC particles synthesized by the Polyol method, is analyzed, the particle size is 55 nm and an absorption peak at 482 nm was detected, similar to the literature data.34,35 On the TEM images, AgNC particles were seen to be cube-shaped geometry as planned and their dimensions were measured as 55 ± 5 nm consistent with DLS data. Since AgNC particles synthesized according to the Polyol method has an oily structure, homogeneous distribution in the network structure is achieved without requiring any coating or modification on its surface.
To evaluate the potential release of nanoparticles and photoinitiator from the hydrogels in Fig. S2 (ESI†), UV-Vis spectroscopy was performed on the supernatants collected after sample immersion in water at 3, 24, 48 and 72 h. No characteristic absorption peaks corresponding to gold (520–550 nm) or silver (∼400 nm) nanoparticles were observed at any time point, indicating the absence of nanoparticle release. However, an absorption peak was observed at 292 nm, which corresponds to Irgacure 2959, indicating the presence of trace amounts of photoinitiator in the supernatant. This suggests a minimal release of Irgacure 2959 from the hydrogel matrices over time.
When the SEM images of gels without nanoparticles (Nc-0) and containing the highest concentration of Ag (Ag-0.08%) are examined, it is seen that the Ag nanoparticles are homogeneously distributed in the gel structure (Fig. S3, ESI†). To confirm the presence of nanoparticles in the nanocomposite hydrogel, the EDX spectra of unloaded and silver loaded hydrogel were studied. The EDX spectrum for Nc-0 hydrogel did not show the characteristic gold/silver peak, while the EDX spectrum of silver loaded (AgNc-0.08) hydrogel showed a clear peak of silver. The intensity of the silver peak is proportional to the metal concentration in the composite hydrogel.
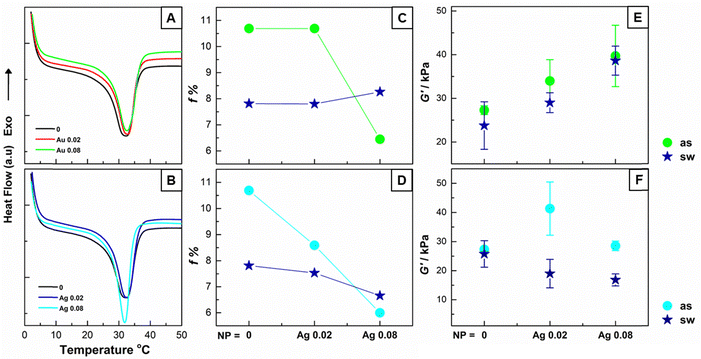
When nanoparticles were incorporated into the Nc-0 networks, the melting enthalpies in the DSC thermograms of the gels with and without AuNS@PVP and AgNC given in Fig. 1(A) and (B) were used to calculate the crystallinity, f%, which gives the ratio of the crystalline structures formed by the long alkyl chains of the SM (Fig. 1(C) and (D)). When the graphs showing the f % values of the network structures after synthesis and in the swollen state are examined, it is seen that the crystallinity decreases in parallel with the amount of both AuNS@PVP and AgNC in the after-synthesis systems.17 With the swelling of the matrix, a decrease in the crystallinity were observed.
In order to follow the viscoelastic behavior of nanocomposite gels, after 1 day reaction in the photoreactor, disk-shaped gels of about 1 mm thickness were subjected to time, frequency and amplitude sweeps at 37 °C. While determining the temperature for the measurement, care was taken to have a temperature above Tm, considering the DSC data. When Fig. 1(E), where the G′ storage modules of the gels are given, is examined, it is seen that the AuNS@PVP added to the structure increases the G′ and increasing the AuNS@PVP concentration from 0.02% to 0.08% causes the G′ value to increase proportionally. Since the measurements are made above the Tm value, the alkyl crystals acting as crosslink sites are in molten state. It is thought that the increase in modulus observed with the addition of nanoparticles is due to the increase in hydrophilic properties by the preparation of AuNS@PVP in an aqueous system, which in turn strengthens the dipole–dipole interactions in the structure and the formation of H-bonds. When the same measurements were applied to the swollen state gels, it was observed that the G′ increased due to the increasing amount of AuNS@PVP. Another striking point in the plot is that the G′ values of swollen networks are relatively decreased at all AuNc-x values. According to the rubber elasticity theory, the decrease in the elastic modulus values is an expected result since the increase in the degree of swelling decreases the crosslink density.36 Unlike the Au-containing system, when 0.02% AgNC was included in the after-synthesis gels, the G′ value of AgNC-0.02 gels increased compared to Nc-0 gels, while G′ decreased with the increase in AgNC amount (Fig. 1(F)). Since the synthesis of AgNC is carried out in an oily system, there is an increase in hydrophobic properties when AgNC is added to the SM-70 network structure. Similar to the previous study, the increase of hydrophobic units in the network structure resulted in a decrease in the G′ value.19 In other words, AgNC particles entering the structure cause a decrease in the modulus value as they disrupt the crystalline structure. In addition, the removal of some of the AgNC particles from the structure by swelling is thought to be a reason for the decrease in the modulus.
The microstructures of nanocomposite gels were analyzed with SAXS and XRD instruments (Fig. 2). The upward-pointing peaks in Fig. 2(A), where SAXS analyzes of AuNC-0.08, AgNc-0.02, and AgNc-0.08 composite gels are presented, indicate the clustering of AuNS@PVP and AgNC nanoparticles. According to the graph, no clusters were found in AuNc-0.02 gels with low concentration and smaller sizes (∼13 nm) compared to Ag nanoparticles (∼55 nm). In the swollen state gels, the disappearance of the upturn of the intensity at low-Q suggests that the after-synthesis clustered nanoparticles can be individually dispersed in the structure after the swelling process (Fig. 2(B)). Since the particle size of the gels containing AuNS@PVP is comparatively smaller and the analysis is performed within limited values, a sharp slope similar to that of AgNc-x could not be observed. Nc-0 data was subtracted from the SAXS patterns of AuNc-0.08 and AgNc-0.02 gels to ignore the peaks of the crystalline structures formed by the side alkyl chains of the polymer and to interpret only the peaks belonging to nanoparticles in Nc-x gels (Fig. S4, ESI†). Considering that the thicknesses of the gels are relatively different, the upturn plateau structure observed in Fig. S4(A) (ESI†) is also seen in the subtracted Fig. S4(B) (ESI†), proving that these peaks originate from nanoparticles and that the particles are individually dispersed. There may be a secondary correlation. In a larger scale, the interparticle spacing of some particles may not be homogeneous. Fig. 2(C) shows the XRD pattern of nanoparticles added at different concentrations onto the SM-70 networks. All gel samples have a peak at approximately 2θ = 21°, corresponding to a Bragg d-spacing of 0.41 ± 0.06 nm between the long alkyl chains of SM. This value is in agreement with the value of long chain alkyl crystal.37–40
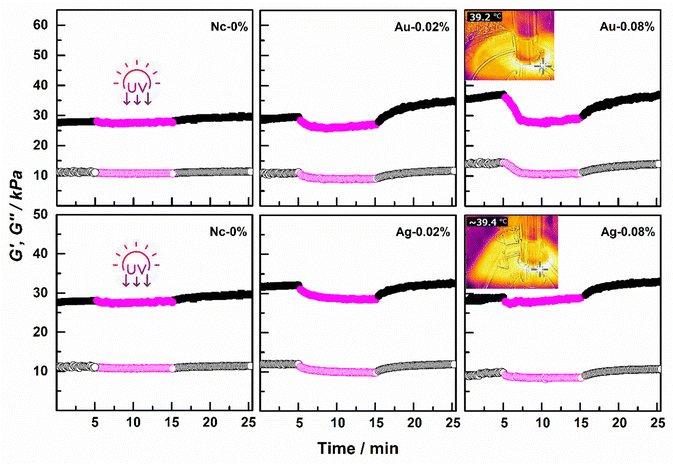
Based on the idea that when AuNS@PVP and AgNC are included in Nc-0 gels, the network structure will be responsive to light, the change of modulus before/after UV irradiation was monitored in the rheometer (Fig. 3 and Fig. S5, ESI†). Measurements were performed at 37 °C on the gels both after-synthesis and swollen states. In this direction, the modulus of the gels placed between the plates were monitored for 5–10–10 min with UV off–on–off, respectively. As given in Fig. 3, there was no significant change in the modules of Nc-0 gels after synthesis with UV irradiation, but when nanoparticles were included in the structure, the module values decreased with the photothermal effect known as heating with light. This elbow-like modulus decreases, which is thought to be due to the melting of the crystalline structures formed by the side alkyl chains, became more evident with the increase in the amount of AuNS@PVP from 0.02% to 0.08%. When the UV was turned off, the temperature of the gels decreased as the photothermal effect disappeared, and upon cooling, the network hardened, causing the modulus to increase. When the composite structures containing AgNC are examined, an elbow-type modulus reduction is observed in low AgNc-0.02 gels with the application of UV, while this decrease is negligible in gels containing more AgNC. As indicated in Fig. 1, the amount of melting to occur with heating decreased proportionally, since increasing AgNC units disrupted the crystalline structure. Analyzes in which the change of module values with UV stimulus were followed were also applied to swollen gels and similar results were obtained with post-synthesis gels (Fig. S5, ESI†).
During the analyzes carried out in the rheometer, the temperature changes were recorded with a FLIR (E6) thermal camera after 10 minutes of UV irradiation. When the after-synthesis gels were examined, it was determined that although the system was 37 °C, it warmed up to about 2 units under the light. It was observed that the temperature increase in the swollen gels was slightly higher compared to the post-synthesis gels, considering that the water in the network structure causes an increase in temperature. The temperatures recorded in the gels containing AuNS@PVP particles increased from 39.2 °C after synthesis to 39.7 °C in the swollen state. While the temperature value of after-synthesis AgNc-0.08 gels containing the highest Ag was recorded as 39.4 °C, it increased to 42.2 °C in the swollen state.
The properties of polymer composites (such as mechanical properties, degradation rate, and drug release) largely depend on molecular-level structural morphology, specifically crystallinity fraction, crystallite size, and polymer chain structure. The polymer structure is characterized by temperature-dependent properties. When surface plasmon resonance (SPR) is induced in polymer composites, the inclusion of inorganic nanofillers allows energy transfer from the particles to the surrounding polymer matrix, leading to localized polymer melting and subsequent structural changes.
Gold and silver nanoparticles embedded in polymer matrices act as localized heat sources that enable modifications in crystallinity, cross-linking, and chemical reactions. Using this nature-inspired cascade approach, as shown in Fig. 3, the internal structure of polymer matrix composites can be gradually altered by photothermal processes at any stage of their lifecycle. Indeed, light responses serve as intermediary signals that produce structural transformations in the material, providing additional functionalities such as controlled on–off release of bioactive molecules.41
When the graphs in Fig. 4, in which after-synthesis Nc-0, AuNc-x and AgNc-x gels were deformed from 0.1% to 100%, were examined, it was observed that the gels had an LVER covering almost the entire range of deformation measured, regardless of nanoparticle content. When the return curves from 100% deformation to 0.1% are examined, it is clearly seen that the gels have completely returned to their initial storage modules. Dynamic interactions that sustain the structure in AuNc-x nanocomposite gels; the dipole–dipole interactions from the PVP-coated gold nanosphere and VP units and the hydrophobic interactions of the alkyl groups in the side chains of the SM are effective in returning to their initial properties after partial deformation. In AgNc-x gels, the interactions of AgNC and SM units, which are relatively hydrophobic, are effective in the recovery of the material. Amplitude sweep tests were also performed in the swollen state (Fig. S6, ESI†). It was observed that LVERs in AuNc-x gels narrowed after swelling, which would lead to a decrease in the mechanical strength of the gels. Unlike AgNc-x gels, there was no change after swelling throughout the studied deformation range.
Temperature profiles were created by measuring the temperature-dependent modulus changes of Nc-0, AuNc-x and AgNc-x gels after synthesis and in the swollen states (Fig. 5 and Fig. S7, ESI†). It is known from the previous work that the viscoelastic behavior of hydrogels changes depending on the melting and recrystallization of the crystalline regions with temperature variations.8 While G′ in after-synthesis gels is between 8 and 25 MPa at 15 °C, it decreases 100–1000 times and becomes 21–27 kPa at 55 °C when the system is raised to a temperature above Tm. Similar graphs were also obtained for the swollen gels (Fig. S7, ESI†). It was observed that the melting temperatures of the gels determined from the DSC thermograms were within the G′ transition range of the temperature profiles obtained in the rheometer for after-synthesis and swollen state gels. Furthermore, regardless of the nanoparticle content, a small shoulder within the transition temperatures is noticeable in the temperature profiles of the swollen gels. The reason for this shoulder structure, which partially shifts to higher temperatures with the increase of Au/AgNP ratio, is not understood.
 | ||
| Fig. 5 Temperature dependent G′ (filled symbols) and G′′ (open symbols) values of the after-synthesis Nc-0, AuNc-x ve AgNc-x networks. | ||
Fig. 6(A) shows a digital photograph of a cylindrical shaped AuNc-0.02 gel during the compression test and stress–strain plots of Nc-0, AuNc-x and AgNc-x networks taken at 37 °C. To determine the fracture stress (σf) and deformation (λf) values, the stress is presented with nominal σnom or actual stress values σtrue (= λσnom), which are forces per cross-sectional area of the undeformed and deformed gel sample.42 When Fig. 6(B), in which the data obtained from the compression graphs are collected, is examined, the initial slope of the curves, E, σf, W and λf, increases as a function of increasing AuNc-x and AgNc-x ratio. This shows that the mechanical properties can be controlled by the amount of gold/silver nanoparticles introduced into the reaction medium. An increase in the modulus values is observed with the increase of Au and AgNP concentrations added to the network structure in the gels after synthesis. Similar to the results of rheological analysis, measurements above Tm cause the crystal regions to melt, revealing the effect of VP units in the network more.
Uniaxial tensile analyzes were performed on Nc-0, AuNc-x and AgNc-x nanocomposite gels using both temperature and a light source of appropriate wavelength (Fig. S8, ESI† and Fig. 7). To make an appropriate comparison, the virgin samples were exposed to the stimulus in the same way as the self-healed gels. The elongation plots of virgin (solid lines) and self-healed (dashed lines) gels are given together in Fig. S8(A) (ESI†), and their blue and pink lines indicate that temperature and light stimuli were used in the measurements, respectively. The elongation at break value of 375 ± 36 obtained for Nc-0 increased to 682 ± 12 for AgNc-0.08 in the measurements taken from the gels kept at 37 °C for 3 minutes in the presence of temperature stimulus. In the measurements made on gels exposed to UV light as a stimulus for 90 seconds, the elongation at break value of 534 ± 16% recorded for Nc-0 increased to 680 ± 14% for AgNc-0.08. In the measurements made under light, the gels with the longest elongation at break were found to be AuNc-0.08 with 770 ± 20%.
 | ||
| Fig. 7 (A) and (B) Healing ratio of Nc-0, AuNc-x, and AgNc-x gels as a function of temperature and light, (C) and (D) images from stress–strain analysis of healed gels. | ||
In addition, to monitor the self-healing properties of the gels, the part cut with a razor blade in the middle of the gel was brought together with the help of forceps and subjected to tensile tests after it was kept under heat at 37 °C for 3 minutes and under the light for 90 seconds (Fig. 7(A)–(C)). Self-healing tests were also performed at body temperature above the Tm value. As given in Fig. 7(A) and (B), the most striking point in the healing tests is that the Nc-0 gels show the highest healing behavior in the presence of both temperature and light stimuli. AuNc-0.02, AgNc-0.02 and AgNc-0.08 gels showed similar self-healing properties in the presence of temperature stimulus as 66 ± 20, 67 ± 21 and 67 ± 30, respectively. Compared to other gels, it was determined that AuNc-0.08 was the gel with the lowest recovery rate. In this system, it has been observed that the increase in hydrophilic properties in the network structure in connection with the increase in nanoparticle concentration reduces the rate of self-healing.40
The self-healing and shape memory properties of hydrogels are crucial for wound healing applications. Self-healing allows the hydrogel to repair itself after mechanical damage or deformation, maintaining the integrity of the wound dressing and ensuring the wound remains effectively sealed, which helps reduce the risk of infection. This feature also enhances the durability and reliability of the dressing, as it can automatically mend tears or damage caused by body movements or during dressing changes. While self-healing is indeed an important parameter for injectable systems, the study suggests that this feature may also be advantageous in hydrogel systems designed as patches. It is believed that self-healing may also increase the durability and functionality of patch-based systems.43 Shape memory, on the other hand, enables the hydrogel to return to its original form in response to an external stimulus, such as temperature or light. This property is particularly beneficial in adapting to the dynamic nature of wounds, as the hydrogel can conform to changes in the wound's shape or size, ensuring a snug and comfortable fit. Additionally, shape memory hydrogels can retain the desired form when applied to the wound and revert to their original shape when needed, optimizing wound closure and accelerating the healing process. Regarding the shape memory behavior, we are aware that its benefit is limited to certain wound shapes. The aim of the shape memory feature is to have the gel apply a force to close the wound as it attempts to change from temporary to permanent shape, and to easily remove the dressing from the skin without causing discomfort to the patient. It provides a controlled, painless separation from the skin by adjusting the transition of the material from its temporary form to its permanent form at temperatures close to body temperature. Thanks to this intelligent temperature-sensitive system, patient comfort is increased during wound dressing changes. Together, these properties make hydrogels ideal materials for wound care, offering both resilience and adaptability to support effective and efficient healing.44–47
3.2. Potentials of Nc-x gels in wound closure application
As mucoadhesive materials, which are important in wound healing systems, become a vital part of minimally invasive treatments, evaluation of the material's adhesive properties is important for potential bioapplications such as dressings.48 In adhesion test studies, the formulation is contacted with tissue and/or an imitation layer (cellulose) and the force required to break the adhesive bond is measured. To test the separation of the gel from the tissue (substrate), the biological tissue/cellulose sheet can be fixed to the adhesion equipment. The separation assay performed on an automated stretching machine allows to record a separation profile, i.e., changes in the force applied versus the distance between the gel and the tissue. Separation is a complex physical process that depends not only on the adhesiveness but also on the deformation and mechanical properties of both the gel and the cellulose layer. The adhesive properties of the gel samples were determined by contacting the cellulose layer for 60 seconds at 37 °C using the mucoadhesion probe of the texture analyzer. In Fig. S9(A) (ESI†), where a typical adhesion graph is given, α and the area indicated by β corresponds to the maximum separation force and the total adhesion work, respectively. Considering the studies indicating that there is a linear relationship between the maximum release force and the total adhesion work, it was determined that the prepared samples had sufficient adhesive properties. When a comparison was made based on the addition of nanoparticles to the structure and the particle concentration, it was revealed that AuNc-0.02 gels showed the lowest adhesion properties (Fig. S9(B), ESI†).49–53 The hydrogel's self-healing and adhesive properties allow it to maintain coverage on the wound site, even when slightly damaged. This ensures a continuous protective barrier over the wound, which is essential for healing and minimizes the need for frequent replacements.54Water vapor transmission rate (WVTR) is another important parameter for the wound healing process. Because low WVTR can delay the healing process and cause the accumulation of exudates that can increase the risk of bacterial growth, while high WVTR results in wound dehydration and subsequent scar formation.9 The evaporation of water from the Nc-0 and Nc-x networks was determined by measuring weight loss through a consecutive monitoring for 3 days. When Fig. S9(A) (ESI†) is examined, which shows the WVTR of Nc-0 and Nc-x gels at the end of the 24, 48 and 78 h periods, it is clearly seen that the WVTR gradually increases as the number of days increases. Evaporative water loss for normal skin, first-degree burn, and granulation wound has been reported to be 204 ± 12, 279 ± 26, and 5138 ± 202 g m−2 h−1, respectively. Commercially available hydrogel wound dressings have been shown to cover a wide range of WVTR, ranging from 120 (Duoderm CGF) to 9360 g m−2 h−1 (Geliperm 1).55 Time-dependent WVTR values of Nc-x gels were obtained as 3025 ± 1103 and 7291 ± 179 g m−2 h−1, which are within the satisfactory range for the targeted wound closure application and can be compared with the commercial products. The repeated measures ANOVA analysis revealed statistically significant differences for each group (p < 0.05), indicating that there were significant variations across the different measurement time points.
To evaluate the cell migration effects of Nc-x on wound closure, an in vitro scratch experiment was performed on a monolayer fibroblast. Fibroblasts are commonly chosen for in vitro scratch (wound healing) experiments due to their critical role in the body's natural wound healing and tissue repair processes. These cells are essential for the synthesis of extracellular matrix (ECM) and collagen, which are essential components for wound closure and tissue repair. During wound healing, fibroblasts migrate to the wound site, where they actively participate in the formation of granulation tissue—a crucial step in wound recovery. Additionally, fibroblasts have a high capacity for both proliferation and migration, two key cellular behaviors observed in scratch assays to assess wound healing.
Their responsiveness to different biomaterials, such as the biocompatible hydrogels, makes fibroblasts an ideal model to examine cell adhesion, spreading, and migration. In vitro evaluation of cell behaviors provides valuable insights into how materials can support or accelerate the wound healing process. Furthermore, fibroblasts are abundant in skin tissue, making them directly relevant to dermal wound healing applications. Given the biocompatibility and responsive properties of the hydrogels developed in this study, assessing their effects on fibroblast behavior helps indicate the material's potential to enhance cell migration and proliferation. This relevance to real wound healing scenarios underscores the choice of fibroblasts for evaluating the smart nanocomposite hydrogels’ effectiveness in promoting cell migration and proliferation, thus showcasing the material's potential in therapeutic applications. MTT showed that optimum concentrations of Nc-0, Au-0.02, and Ag-0.02 gels were applied to the cells separately for 24 and 48 h. Wound thickness and area were measured at 0, 24 and 48 h to expose the contribution of the formulation to cell proliferation and their migration through the cell-free scratch zone (Fig. S10, ESI†). After 24 and 48 hours, the closure rate of untreated cells was calculated as 77% and 99%, respectively. Au-0.02 and Ag-0.02 nanocomposite gels significantly reduced wound thickness, and almost 100% of wounds healed completely within 48 h. Nanocomposite gels closed wounds better than Nc-0 networks. In the presence of Au-0.02 gel, the wound closed at a rate of 99.56 ± 2.83% and was almost completely healed at the end of 24 h. Healing was delayed in cells treated with Nc-0 hydrogel. This is due to the superior pharmacokinetic properties of the nanostructures compared to the wild form and their ability to achieve therapeutic efficacy at lower doses. The hydrogel's self-healing and adhesive properties allow it to maintain coverage on the wound site, even when slightly damaged. This ensures a continuous protective barrier over the wound, which is essential for healing and minimizes the need for frequent replacements. Additionally, in vivo tests demonstrated enhanced wound closure rates and accelerated epithelialization, indicating that the hydrogel promotes faster tissue regeneration and repair in burn injuries.54 Statistically significant differences were observed in the comparison of the control group with the Au-0.02 and Ag-0.02 groups, with t-test results showing p < 0.05.
The effects of AuNPs and AgNPs on cell viability may vary depending on cell type and concentration. Gold (Au) and silver (Ag) nanoparticles support the viability of CCD (Human Dermal Fibroblast) cells through several mechanisms. Gold nanoparticles exhibit antioxidant properties that reduce oxidative stress, preventing damage from free radicals and promoting healthier cell conditions. Both gold and silver nanoparticles can enhance cell proliferation by stimulating growth signals, accelerating the cell cycle, and aiding in tissue regeneration. Silver nanoparticles, known for their anti-inflammatory properties, help reduce inflammation in CCD cells, creating a more favorable environment for cell survival. Moreover, gold and silver nanoparticles improve cell adhesion, enabling CCD cells to better anchor to surfaces, which stabilizes their environment and encourages proliferation. Together, these effects contribute to the enhanced viability, growth, and overall health of CCD cells, making these nanoparticles particularly valuable in applications such as wound healing and tissue engineering.56–58
The starting point of the study is the preparation of a multifunctional potential dressing that will contribute to wound healing without the need for any invasive processes. In this regard, it has been shown that the prepared composite gels adhere tightly to the intact skin tissue around the wound and, more importantly, do not adhere to the wet wound surface. It has been determined that water vapor transmission rates are at optimum levels. The most unique part is that the shape memory feature of self-healing Nc-x gels is planned to be used in wound healing. In this direction, materials with a temporary shape in the size of an open wound and a permanent shape in the size of an ideally healing wound will be prepared. As the gels remember their permanent shape in the presence of a stimulus, a force will be applied to the tissues to close the wound, allowing the healing process to proceed more quickly and in a more controllable manner. Since it is important to determine the shape memory properties of Nc-x gels, bending tests were applied to the swollen networks to analyze the shape memory behavior of the gels. Flat rod-shaped gel samples were given a temporary U-shape above Tm, and this shape was fixed at a temperature below Tm. Nc-0, AuNc-x and AgNc-x gels were exposed to light at wavelengths of 365 nm, 200–700 nm and 400–500 nm, respectively, at a 90° angle, and their recovery rates to permanent rod shape were monitored. It was observed that AuNc-x and AgNc-x gels with photothermal effect returned to permanent rod form in less than one minute (Fig. 8(A)). Shape recovery ratios, % R, of Nc-x networks using the quantitative data obtained plotted against time in Fig. 8(B). In Nc-0 gels, 365 nm light source caused the side alkyl chains to melt by heating with light, and after 3 min, it was observed that the Nc-0 gel recovered itself, while this time decreased to 1 min in Nc-x gels. Thanks to the LSPR formed on the surface of the nanoparticles, heating was realized with light. Accordingly, due to the melting of the crystalline side chains in the network due to heating, nanocomposite gels reach 100% recovery rate in a much shorter time compared to Nc-0 gels with increasing temperature.59
 | ||
| Fig. 8 (A) Digital photographs showing shape memory behavior of swollen-sw state Nc-0, AuNc-x and AgNc-x networks, (B) time dependent shape recovery ratio, R%, under suitable light stimuli. | ||
To examine the biocompatibility of the synthesized gels, their cytotoxicity was evaluated in vitro by a colorimetric MTT test using human skin fibroblast cells CCD-986Sk. It was observed that the Nc-0 and Au/AgNc-x hydrogels did not show significant cytotoxicity, and the viability of the incubated cells was over 82% at all x values (Fig. 9). The increase in cell viability in AuNc-x and AgNc-x gels is remarkable. More than 100% cell viability was obtained after 24 h in AuNc-0.02, AgNc-0.02 and AgNc-0.08 gels. Similar to the literature, a relative decrease in cell viability was observed as the amount of nanoparticles increased.60,61 It was observed that the adhesion and bonding of the CCD cells, followed by light microscopy, increased with time in the presence of nanoparticles after 48 h. Moreover, in 24 h of incubation, the cells can reach localized at the edges of the gel samples, and at the end of 48 h it spreads towards the middle of the gel samples. A t-test was performed on each group in the system with a parametric distribution, and the results were calculated as p < 0.05 These findings indicate that the differences between groups are statistically significant.
The loading percentage and release of Ag+ (silver ions) and Au3+ (gold ions) have a significant effect on the efficacy and safety of hydrogels in biomedical applications. The loading percentage was calculated as 0.02% and 0.08%. High loading percentages can increase antimicrobial activity and plasmonic properties, but excessive loading can be toxic and negatively affect biocompatibility. The release profile indicates how long and in what quantities Ag+ and Au3+ ions are released in the biological environment. Controlled release provides slow and continuous release of ions, reducing long-term antimicrobial efficacy and the risk of toxicity. Slow release minimizes harmful effects on cells while supporting the healing process. For example, sustained release of Ag+ ions reduce the risk of infection, while release of Au3+ ions can promote cell proliferation. Therefore, the loading percentage and release profile of Ag+ and Au3+ should be carefully balanced for optimal performance of hydrogels in medical applications. In light of this information, antibacterial effect and release studies were conducted to observe the release efficiency of Ag+ and Au3+ ions. For the determination of antimicrobial activity, Gram positive bacteria, Staphylococcus aureus, Staphylococcus epidermidis and Gram-negative bacteria, Pseudomonas aeruginosa and Escherichia coli strains were used. After 24 hours of incubation of Nc-0 and Au/Ag-x gels, no inhibition diameter was detected in any of the gel samples. The absence of antibacterial properties was performed as an indirect analysis as evidence that the gold/silver nanoparticles entering the network were not released from the structure. In addition, despite the release profile study, no release of Ag+ and Au3+ ions occurred. This situation shows that the nanoparticles are trapped in the network structure.62–66
For further development of the material, the dressing can be supported to exhibit photothermal-assisted antibacterial activity. Thus, when exposed to near-infrared light, AgNPs only exhibit antibacterial effects It produces localized heat that not only increases the temperature but also supports tissue repair by creating a warm environment conducive to cell migration and wound closure.54,67 By adding adjuvant substances such as collagen, it can be ensured that infection prevention, rapid wound contraction and tissue regeneration are actively promoted through their supportive effects on angiogenesis.68 These combined properties make the hydrogel dressing a promising candidate for effective wound healing in burn treatment applications. In addition, this developed material offers a potentially effective solution not only for wound healing but also in various biomedical fields such as drug delivery, antimicrobial surfaces and biosensor applications.69–71
4. Conclusions
In this study, smart nanocomposite gels that are temperature and light sensitive, have shape memory and self-healing functions were prepared. Firstly, nanoparticles and then nanoparticle-loaded gels were synthesized and both structural and application-oriented characterizations were completed.The size of AuNS@PVPs synthesized by the Turkevich method was measured as 15 ± 2 nm by DLS and 13 ± 3 nm in TEM analysis, while the absorption peak was obtained at 527 nm in UV-Vis analysis. An absorption peak at 482 nm was detected for AgNC particles, which were determined to have a size of 55 nm by both DLS and TEM. Since AgNC particles synthesized according to the Polyol method have an oily structure, homogeneous distribution in the network structure is achieved without requiring any coating or modification on the surface. Photographs of the reaction solutions and swollen state gels prove that AuNS@PVP and AgNC structures are distributed homogeneously in both reaction solutions and swollen AuNc-x and AgNc-x gels, there is no phase separation, and the particles remain in the structure even after swelling. In SAXS analysis, it was determined that there were no clusters in AuNc-0.02 gels with low concentration and smaller size (∼13 nm) compared to Ag nanoparticles (∼55 nm). The disappearance of the upturn slope proves that the after-synthesis clustered nanoparticles can be individually dispersed in the structure after the swelling process. Considering that the thicknesses of the gels are relatively different, the upturn plateau structure proving that these peaks originate from nanoparticles and that the particles are individually dispersed.
As for the characterization of the gels, according to the DSC measurements, the crystallinity decreases in parallel with the amount of both AuNS@PVP and AgNC in the after-synthesis systems. With the swelling of the matrix, a decrease in the crystallinity were observed. As a result of rheological analysis, it was seen that AuNS@PVP added to the structure increased the G′ value and as the AuNS@PVP concentration increased from 0.02% to 0.08%, the G′ value increased proportionally. It is thought that this increase is due to the increase in hydrophilic properties by preparing AuNS@PVP in an aqueous system, which strengthens the dipole–dipole interactions and H-bond formation in the structure. Unlike the Au-containing system, when AgNC was added to the after-synthesis gels, the G′ value increased compared to Nc-0 gels, while G′ decreased with the increase in the amount of AgNC synthesized in an oily system. It is thought that this result may be due to the oily AgNC particles disrupting the crystal structure, as well as the removal of some of the AgNC particles from the structure by swelling. According to the uniaxial compression results, the mechanical properties of the gels can be controlled by the amount of gold/silver nanoparticles added to the reaction medium. The elongation at break value of 375 ± 36 obtained for Nc-0 increased to 682 ± 12 for AgNc-0.08 in the measurements taken from the gels kept at 37 °C for 3 min in the presence of temperature stimulus. In measurements made on gels exposed to UV light as a stimulus for 90 seconds, it was determined that AuNc-0.08 gels had the longest elongation at break with 770 ± 20%.
Water vapor transmission rate, which is an important parameter of the systems recommended to be used for the potential wound healing process, was measured as 3025 ± 1103 and 7291 ± 179 g m−2 h−1 in Nc-x gels, and these values are within the satisfactory range and are comparable to commercial products. It was determined that Nc-x gels, designed to close the wound noninvasively thanks to the shape memory function, reached a 100% healing rate in a much shorter time than light-stimulated Nc-0 gels. In tests performed with human skin fibroblast cells CCD-986Sk, more than 100% cell viability was achieved after 24 hours in AuNc-0.02, AgNc-0.02 and AgNc-0.08 gels. Similar to the literature, a relative decrease in cell viability was observed as the number of nanoparticles increased. According to the results obtained here, it is recommended to be used and developed as a non-invasive alternative material for wound dressing alternative to traditional wound closure methods.
Furthermore, the inclusion of AuNS and AgNC does not significantly increase the overall system cost. Even at minimal concentrations, these additives enhance the system's performance, providing a cost-effective method for functional improvement.
Data availability
All relevant data are within the manuscript and ESI.† The data are available from the corresponding author on reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is a part of Hüsna Kılıç's PhD dissertation. The authors acknowledge resources and support from the Drug Application and Research Center (ILMER) at Bezmialem Vakif University. D. C. T. thanks the Turkish Academy of Sciences (TUBA) for the support. H. K. thanks the Scientific and Technological Research Council of Turkey (TÜBİTAK) for the scholarship support. The authors many thank Asst. Prof. Erkan Şenses and Saeid Darvishi from Koç University (n2STAR-Koç University Nanofabrication and Nanocharacterization Center for Scientific and Technological Advanced Research) for the SAXS analysis.References
- A. Motealleh and N. S. Kehr, Nanocomposite hydrogels and their applications in tissue engineering, Adv. Healthcare Mater., 2017, 6(1), 1600938 CrossRef.
- B. Khurana, et al., Hydrogels: soft matters in photomedicine, Photochem. Photobiol. Sci., 2019, 18(11), 2613–2656 CrossRef CAS.
- A. Vashist, et al., Advances in carbon nanotubes–hydrogel hybrids in nanomedicine for therapeutics, Adv. Healthcare Mater., 2018, 7(9), 1701213 CrossRef.
- J. Hu and S. Chen, A review of actively moving polymers in textile applications, J. Mater. Chem., 2010, 20(17), 3346–3355 RSC.
- Q. Zhao, H. J. Qi and T. Xie, Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding, Prog. Polym. Sci., 2015, 49, 79–120 CrossRef.
- J. Ban, et al., New stimulus-responsive shape-memory polyurethanes capable of UV light-triggered deformation, hydrogen bond-mediated fixation, and thermal-induced recovery, J. Mater. Chem. A, 2017, 5(28), 14514–14518 RSC.
- N. Moini, A. Jahandideh and G. Anderson, Inorganic Nanocomposite Hydrogels: Present Knowledge and Future Challenge, 2019. pp. 805–853 Search PubMed.
- W. Xing and Y. Tang, On mechanical properties of nanocomposite hydrogels: Searching for superior properties, Nano Mater. Sci., 2022, 4(2), 83–96 CrossRef CAS.
- M. Kokabi, M. Sirousazar and Z. M. Hassan, PVA–clay nanocomposite hydrogels for wound dressing, Eur. Polym. J., 2007, 43(3), 773–781 CrossRef CAS.
- J. Rosiak, A. Rucinska-Rybus and W. Pekala, Method of manufacturing hydrogel dressings, Google Pat., 1989 Search PubMed.
- M. T. Razzak, et al., Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing, Radiat. Phys. Chem., 2001, 62(1), 107–113 CrossRef CAS.
- F. Yoshii, et al., Electron beam crosslinked PEO and PEO/PVA hydrogels for wound dressing, Radiat. Phys. Chem., 1999, 55(2), 133–138 CrossRef CAS.
- J. Kimling, et al., Turkevich Method for Gold Nanoparticle Synthesis Revisited, J. Phys. Chem. B, 2006, 110(32), 15700–15707 CrossRef CAS.
- M. A. Mahmoud and M. A. El-Sayed, Comparative Study of the Assemblies and the Resulting Plasmon Fields of Langmuir–Blodgett Assembled Monolayers of Silver Nanocubes and Gold Nanocages, J. Phys. Chem. C, 2008, 112(37), 14618–14625 CrossRef CAS.
- C. Bilici, et al., Melt-Processable Shape-Memory Hydrogels with Self-Healing Ability of High Mechanical Strength, Macromolecules, 2016, 49(19), 7442–7449 CrossRef CAS.
- H. Yi, et al., Bioinspired reversible hydrogel adhesives for wet and underwater surfaces, J. Mater. Chem. B, 2018, 6(48), 8064–8070 RSC.
- S. Kabiri Ameri, et al., Graphene Electronic Tattoo Sensors, ACS Nano, 2017, 11(8), 7634–7641 CrossRef CAS PubMed.
- M. Kaltenbrunner, et al., An ultra-lightweight design for imperceptible plastic electronics, Nature, 2013, 499(7459), 458–463 CrossRef CAS.
- H. Kılıç, et al., Design of Biocompatible Multifunctional Hydrogels with Stearyl Methacrylate and Vinylpyrrolidone, ACS Appl. Polym. Mater., 2022, 4(3), 1717–1727 CrossRef.
- F. Esmailzadeh, et al., Bonding states of gold/silver plasmonic nanostructures and sulfur-containing active biological ingredients in biomedical applications: a review, Phys. Chem. Chem. Phys., 2024, 26, 16407–16437 RSC.
- Y. Hang, A. Wang and N. Wu, Plasmonic silver and gold nanoparticles: shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy, Chem. Soc. Rev., 2024, 53(6), 2932–2971 RSC.
- A. Dhaka, et al., A review on biological synthesis of silver nanoparticles and their potential applications, Results Chem., 2023, 6, 101108 CrossRef CAS.
- H. S. Bisht, P. P. Pande and A. K. Chatterjee, Docosyl acrylate modified polyacrylic acid: synthesis and crystallinity, Eur. Polym. J., 2002, 38(12), 2355–2358 CrossRef CAS.
- Z. Mogri and D. Paul, Gas sorption and transport in side-chain crystalline and molten poly(octadecyl acrylate), Polymer, 2001, 42(6), 2531–2542 CrossRef CAS.
- Z. Raheem, Standard Test Methods for Water Vapor Transmission of Materials 1. 2019 Search PubMed.
- M. E. Smithmyer, L. A. Sawicki and A. M. Kloxin, Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease, Biomater. Sci., 2014, 2(5), 634–650 RSC.
- R. A. Franco, et al., Fabrication and biocompatibility of novel bilayer scaffold for skin tissue engineering applications, J. Biomater. Appl., 2013, 27(5), 605–615 CrossRef PubMed.
- M. Tavafoghi, et al., Multimaterial bioprinting and combination of processing techniques towards the fabrication of biomimetic tissues and organs, Biofabrication, 2021, 13(4), 042002 CrossRef CAS.
- I. Pastoriza-Santos, et al., Plasmonic polymer nanocomposites, Nat. Rev. Mater., 2018, 3(10), 375–391 CrossRef.
- M. A. Mahmoud, P. Szymanski and M. A. El-Sayed, Different Methods of Increasing the Mechanical Strength of Gold Nanocages, J. Phys. Chem. Lett., 2012, 3(23), 3527–3531 CrossRef CAS.
- M. Mahmoud, B. Snyder and M. El-Sayed, Surface plasmon fields and coupling in the hollow gold nanoparticles and surface-enhanced Raman spectroscopy. Theory and experiment, J. Phys. Chem. C, 2010, 114(16), 7436–7443 CrossRef CAS.
- V. Myroshnychenko, et al., Modelling the optical response of gold nanoparticles, Chem. Soc. Rev., 2008, 37(9), 1792–1805 RSC.
- Y. Sun and Y. Xia, Shape-controlled synthesis of gold and silver nanoparticles, Science, 2002, 298(5601), 2176–2179 CrossRef CAS.
- Y. Sun and Y. Xia, Gold and silver nanoparticles: a class of chromophores with colors tunable in the range from 400 to 750 nm, Analyst, 2003, 128(6), 686–691 RSC.
- B. Wiley, Y. Sun and Y. Xia, Synthesis of Silver Nanostructures with Controlled Shapes and Properties, Acc. Chem. Res., 2007, 40(10), 1067–1076 CrossRef CAS PubMed.
- H. Yin, et al., Double network hydrogels from polyzwitterions: high mechanical strength and excellent anti-biofouling properties, J. Mater. Chem. B, 2013, 1(30), 3685–3693 RSC.
- A. Matsuda, et al., Order-Disorder Transition of a Hydrogel Containing an n-Alkyl Acrylate, Macromolecules, 1994, 27(26), 7695–7698 CrossRef CAS.
- N. A. Platé, et al., Structure of crystalline polymers with unbranched long side chains, J. Polym. Sci., Part A-1: Polym. Chem., 1971, 9(8), 2291–2298 CrossRef.
- C. Bilici, S. Ide and O. Okay, Yielding Behavior of Tough Semicrystalline Hydrogels, Macromolecules, 2017, 50(9), 3647–3654 CrossRef CAS.
- O. Okay, Semicrystalline physical hydrogels with shape-memory and self-healing properties, J. Mater. Chem. B, 2019, 7(10), 1581–1596 RSC.
- F. Pierini, et al., Polymer-based nanomaterials for photothermal therapy: from light-responsive to multifunctional nanoplatforms for synergistically combined technologies, Biomacromolecules, 2018, 19(11), 4147–4167 CrossRef CAS PubMed.
- A. Argun, et al., Surfactant-induced healing of tough hydrogels formed via hydrophobic interactions, Colloid Polym. Sci., 2014, 292(2), 511–517 CrossRef CAS.
- D. Rybak, et al., Injectable and self-healable nano-architectured hydrogel for NIR-light responsive chemo- and photothermal bacterial eradication, J. Mater. Chem. B, 2024, 12(7), 1905–1925 RSC.
- P. Wang, et al., Emerging trends in the application of hydrogel-based biomaterials for enhanced wound healing: A literature review, Int. J. Biol. Macromol., 2024, 129300 CrossRef CAS PubMed.
- O. Okay, Self-Healing and Shape-Memory Hydrogels, Hacettepe J. Biol. Chem., 2020, 48(5), 507–525 CrossRef.
- H. Omidian, R. L. Wilson and E. J. Gill, Advancements and Challenges in Self-Healing Hydrogels for Wound Care, Gels, 2024, 10(4), 241 CrossRef CAS PubMed.
- W. Feng and Z. Wang, Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications, Adv. Sci., 2023, 10(28), 2303326 CrossRef CAS.
- A. Sreedevi Madhavikutty, et al., pH responsive cationic guar gum-borate self-healing hydrogels for muco-adhesion, Sci. Technol. Adv. Mater., 2023, 24(1), 2175586 CrossRef PubMed.
- S. A. Mortazavi and J. D. Smart, An investigation of some factors influencing the in vitro assessment of mucoadhesion, Int. J. Pharm., 1995, 116(2), 223–230 CrossRef CAS.
- V. V. Khutoryanskiy, Advances in Mucoadhesion and Mucoadhesive Polymers, Macromol. Biosci., 2011, 11(6), 748–764 CrossRef CAS.
- T. F. R. Alves, et al., Bilayer Mucoadhesive Buccal Film for Mucosal Ulcers Treatment: Development, Characterization, and Single Study Case, Pharmaceutics, 2020, 12(7), 657 CrossRef CAS.
- Y. Yi, et al., Self-adhesive hydrogels for tissue engineering, J. Mater. Chem. B, 2021, 9(42), 8739–8767 RSC.
- Y. Zhang, et al., Nanocomposite adhesive hydrogels: from design to application, J. Mater. Chem. B, 2021, 9(3), 585–593 RSC.
- J. Chen, et al., Multifunctional On-Demand Removability Hydrogel Dressing Based on in Situ Formed AgNPs, Silk Microfibers and Hydrazide Hyaluronic Acid for Burn Wound Healing. Advanced Healthcare, Materials, 2024, 13(8), 2303157 CAS.
- M. Goh, Y. Hwang and G. Tae, Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing, Carbohydr. Polym., 2016, 147, 251–260 CrossRef CAS PubMed.
- Ł. Niżnik, et al., Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review., Int. J. Mol. Sci., 2024, 25(7), 4057 CrossRef PubMed.
- M. M. G. Fouda, et al., Carboxymethyl cellulose supported green synthetic features of gold nanoparticles: Antioxidant, cell viability, and antibacterial effectiveness, Synth. Met., 2020, 269, 116553 CrossRef CAS.
- A. Ávalos, et al., Effects of silver and gold nanoparticles of different sizes in human pulmonary fibroblasts, Toxicol. Mech. Methods, 2015, 25, 1–9 CrossRef.
- D. N. Heo, et al., Enhanced bone regeneration with a gold nanoparticle–hydrogel complex, J. Mater. Chem. B, 2014, 2(11), 1584–1593 RSC.
- S. Vijayakumar and S. Ganesan, In Vitro Cytotoxicity Assay on Gold Nanoparticles with Different Stabilizing Agents, J. Nanomater., 2012, 2012, 734398 CrossRef.
- T. Li, et al., Thermosensitive Hydrogel Co-loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy, AAPS J., 2015, 18, 146–155 CrossRef PubMed.
- Y. P. Moreno Ruiz, et al., Advanced Hydrogels Combined with Silver and Gold Nanoparticles against Antimicrobial Resistance, Antibiotics, 2023, 12(1), 104 CrossRef CAS PubMed.
- O. Kapusta, et al., Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges-A Concise Review, Int. J. Mol. Sci., 2023, 24(3), 2191 CrossRef CAS PubMed.
- F. M. Aldakheel, et al., Silver Nanoparticles Loaded on Chitosan-g-PVA Hydrogel for the Wound-Healing Applications, Molecules, 2023, 28(7), 3241 CrossRef CAS.
- Y. Wang, et al., Metal nanoparticle hybrid hydrogels: the state-of-the-art of combining hard and soft materials to promote wound healing, Theranostics, 2024, 14, 1534–1560 CrossRef CAS PubMed.
- K. Yang, et al., Antimicrobial hydrogels: promising materials for medical application, Int. J. Nanomed., 2018, 2217–2263 CrossRef CAS.
- M. A. Haghighat Bayan, et al., Solar-to-NIR Light Activable PHBV/ICG Nanofiber-Based Face Masks with On-Demand Combined Photothermal and Photodynamic Antibacterial Properties, Adv. Mater. Technol., 2024, 2400450 CrossRef.
- Y. Huang, et al., Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing, J. Colloid Interface Sci., 2022, 608, 2278–2289 CrossRef CAS PubMed.
- P. Nakielski, et al., Multifunctional platform based on electrospun nanofibers and plasmonic hydrogel: A smart nanostructured pillow for near-infrared light-driven biomedical applications, ACS Appl. Mater. Interfaces, 2020, 12(49), 54328–54342 CrossRef CAS.
- M. A. Haghighat Bayan, et al., Near-infrared light activated core–shell electrospun nanofibers decorated with photoactive plasmonic nanoparticles for on-demand smart drug delivery applications, J. Polym. Sci., 2023, 61(7), 521–533 CrossRef CAS.
- L. Amer, et al., Mechanism of hierarchical plasmonic biomaterials engineered through peptide-directed self-assembly, Aggregate, 2024, e677 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01720j |
| This journal is © The Royal Society of Chemistry 2025 |