Interface and defect engineering strategies toward high-performance blue perovskite light-emitting diodes
Yachun
Guo
a,
Taifei
Zhou
a,
Shuguang
Zhang
 *ab,
Jiaming
Yu
a,
Yue
Liang
ab,
Jiangshan
Chen
*ab,
Jiaming
Yu
a,
Yue
Liang
ab,
Jiangshan
Chen
 ab,
Linfeng
Lan
ab and
Junbiao
Peng
ab
ab,
Linfeng
Lan
ab and
Junbiao
Peng
ab
aSchool of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: mssgzhang@scut.edu.cn
bState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China
First published on 28th October 2025
Abstract
Perovskite light-emitting diodes (PeLEDs) hold great promise for full-color displays and solid-state lighting due to their high efficiency and low fabrication cost. Among the primary colors, blue PeLEDs lag behind because of intrinsic challenges, including high defect densities in the emissive layer and inefficient interfacial charge transport, which limit device performance. Here, we report a dual-strategy approach combining defect passivation and interface engineering to realize high-efficiency quasi-2D blue PeLEDs. Incorporation of dimethylphosphine oxide (DMPO) into the PPA0.8(Cs0.7FA0.3)1.1Pb(Br0.8Cl0.2)3.9 precursor effectively passivates undercoordinated Pb2+ sites, reducing trap density by 30.75% and yielding a maximum external quantum efficiency (EQE) of 6.92%. Introducing a propionamide (PAM) interlayer on the hole transport layer further improves film crystallinity, suppresses nonradiative recombination, accelerates the radiative carrier dynamics, and optimizes energy-level alignment for more efficient hole injection. The synergistic combination of DMPO and PAM enhances brightness, efficiency, and operational stability of PeLEDs. Consequently, the optimized quasi-2D sky-blue PeLEDs achieve a maximum luminance of 2135 cd m−2 and a peak EQE of 12.94% at the electroluminescence (EL) emission peak of 486 nm. This study demonstrates the effectiveness of DMPO for defect passivation and establishes PAM as a promising interlayer strategy, proving a viable route toward high-performance blue PeLEDs.
1. Introduction
Since the first demonstration of room-temperature electroluminescent metal halide perovskites in 2014,1 these materials have garnered significant attention due to their exceptional properties, including high color purity, narrow emission spectra, excellent charge carrier mobility, and wide color gamut.2–12 Perovskite light-emitting diodes (PeLEDs) have shown great potential in full-color displays and lighting applications due to their high efficiency and cost-effectiveness.13–18 Red and green PeLEDs have achieved remarkable progress, with external quantum efficiency (EQE) advancing from less than 0.1% to over 30%.1,19–21 However, blue PeLEDs, a key part of the three primary colors, still lag behind their red and green counterparts in brightness, efficiency, and stability, restricting their use in full-color displays. The performance of blue PeLEDs is limited by high defect density in perovskite films, which increases nonradiative recombination and promotes ion migration, reducing film stability.14,22,23 Additionally, suboptimal interface energy alignment hinders efficient carrier injection,24 further impacting device performance.In recent years, significant progress has been made in boosting the performance of PeLEDs through defect passivation and interfacial engineering. Molecular additives with carbonyl, phosphoryl, or ionic functional groups have been employed to coordinate undercoordinated Pb2+ ions and suppress halide migration, effectively reducing trap-assisted recombination and enhancing radiative efficiency.25–27 At the same time, interface modification strategies-such as introducing ionic liquids, polymeric interlayers, or metal oxide layers-have improved film crystallinity, optimized energy-level alignment, and facilitated more balanced carrier injection, leading to record-high EQE and luminance.21,27 Despite these advances, blue PeLEDs still face persistent challenges. Defect passivation often remains incomplete, interfacial charge injection imbalances limit efficiency, and device stability continues to be a major bottleneck. Approaches that focus exclusively on either bulk defect suppression or interfacial tuning are insufficient to simultaneously address these issues. This situation underscores the need for a synergistic strategy that integrates defect passivation with interfacial engineering to unlock both high efficiency and long-term operational stability in blue PeLEDs.
In this study, we incorporate dimethylphosphine oxide (DMPO) into the PPA0.8(Cs0.7FA0.3)1.1Pb(Br0.8Cl0.2)3.9 precursor solution to passivate undercoordinated Pb2+ ions in the perovskite film. This strategy effectively reduces defect density, thereby enhancing both film quality and device performance, and resulting in a maximum EQE of 6.92%. Furthermore, we introduce a propionamide (PAM) interlayer on top of the hole transport layer (HTL) to promote perovskite crystallization, suppress nonradiative pathways and accelerate radiative dynamics. The PAM interlayer also reduces the hole injection barrier, optimizing energy-level alignment. Through the synergistic strategies of defect passivation and interface engineering, we significantly enhance the brightness, efficiency, and operational lifetime of PeLEDs. Consequently, we fabricate highly efficient quasi-2D sky-blue PeLEDs, achieving a peak brightness of 2135 cd m−2 and a champion EQE of 12.94% at an EL emission peak of 486 nm. These findings underscore the efficacy of DMPO in defect passivation and the innovative role of PAM as an interlayer, paving the way for the development of high-efficiency blue PeLEDs.
2. Experimental section
2.1 Materials
L-Arginine (Arg., 99%) was supplied by Tokyo Chemical Industry. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS), formamidinium bromide (FABr), lead bromide (PbBr2, 99.99%), phenethylammonium bromide (PPABr, 99%), and 1,3,5-tris(N-phenylbenzimidazol-2-yl)benzene (TPBi, >99.8%) were obtained from Xi'an Polymer Light Technology Corp. Chlorobenzene (CB, 99.9%), dimethyl sulfoxide (DMSO, 99.9%), lithium fluoride (LiF, ≥99.99%), and molybdenum trioxide (MoO3, 99.97%) were purchased from Sigma-Aldrich. Propionamide (PAM, 97%) and dimethylphosphine oxide (DMPO, 95.0%) were provided by Shanghai Macklin Biochemical Corp., while isopropanol (IPA, 99.7%) was sourced from Nanjing Chemical Reagent Corp.2.2 Preparation of perovskite precursor solution
The HTL solution was prepared by mixing PEDOT:PSS with an aqueous Arg solution (4 mg ml−1) in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The quasi-2D blue perovskite precursor with the composition PPA0.8(Cs0.7FA0.3)1.1Pb(Br0.8Cl0.2)3.9 was synthesized as follows: PPABr, FABr, PbBr2, and PbCl2 were each dissolved in DMSO at 100 mg ml−1 and stirred at 60 °C for 30 min to ensure complete dissolution. CsBr was dissolved in DMSO at the same concentration but remained partially undissolved and was reserved for subsequent use. PPABr, FABr, PbBr2, and PbCl2 solutions were then added according to a precise volume ratio of PPABr
2. The quasi-2D blue perovskite precursor with the composition PPA0.8(Cs0.7FA0.3)1.1Pb(Br0.8Cl0.2)3.9 was synthesized as follows: PPABr, FABr, PbBr2, and PbCl2 were each dissolved in DMSO at 100 mg ml−1 and stirred at 60 °C for 30 min to ensure complete dissolution. CsBr was dissolved in DMSO at the same concentration but remained partially undissolved and was reserved for subsequent use. PPABr, FABr, PbBr2, and PbCl2 solutions were then added according to a precise volume ratio of PPABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CsBr
CsBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FABr
FABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbBr2
PbBr2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbCl2 = 1.055
PbCl2 = 1.055![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.252
0.252![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.366
1.366![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.662. The mixture was heated and stirred at 60 °C for 1 hour to ensure complete dissolution and then filtered through a 0.45 μm PTFE membrane to obtain the perovskite precursor solution. For defect passivation, DMPO was introduced into the precursor at a concentration of X mg per mL (DMPOX solution). Likewise, PAM was dissolved in isopropanol (X mg mL−1) to yield the PAMX solution.
0.662. The mixture was heated and stirred at 60 °C for 1 hour to ensure complete dissolution and then filtered through a 0.45 μm PTFE membrane to obtain the perovskite precursor solution. For defect passivation, DMPO was introduced into the precursor at a concentration of X mg per mL (DMPOX solution). Likewise, PAM was dissolved in isopropanol (X mg mL−1) to yield the PAMX solution.
2.3 Device fabrication
ITO-coated glass substrates were sequentially cleaned with tetrahydrofuran, isopropanol, and deionized water, followed by drying in an oven at 80 °C for 8 h. Prior to use, the cleaned substrates were treated with ultraviolet ozone for 20 min. The HTL was deposited by spin-coating the Arg-modified PEDOT:PSS solution at 2000 rpm for 30 s, followed by annealing at 150 °C for 15 min in ambient air. For the PAM interlayer, after annealing and cooling the Arg-modified PEDOT (m-PEDOT) layer, the PAM solution was spin-coated at 5000 rpm for 30 s and subsequently annealed at 150 °C for 15 min in air. After cooling, the substrates were transferred into a nitrogen-filled glovebox for further processing. The perovskite precursor solution was spin-coated at 3000 rpm for 70 s, with 100 μL of CB dripped at 31 s to induce crystallization. The resulting films were then annealed at 80 °C for 15 min in a nitrogen atmosphere. Subsequently, the blue PeLEDs were completed by sequential thermal evaporation of TPBi (40 nm), LiF (1 nm), and Al (1 μm) electrodes through a shadow mask under a vacuum of 4 × 10−4 Pa. Each device had an active area of 0.1 cm2 and a perovskite layer thickness of approximately 50 nm. Finally, the devices were encapsulated with epoxy resin and cured under UV light for 5 min to prevent degradation from oxygen and moisture.2.4 Characterization
Ultraviolet-visible (UV-Vis) absorbance spectra were recorded using a spectrophotometer (Shimadzu, UV 2006, Japan), with perovskite layers deposited on quartz glass/HTL substrates. Steady-state PL and EL spectra were collected with a fiber-optic spectrometer (Ocean Optics, USB2000, USA) on perovskite films coated onto ITO/HTL substrates. The PLQY of the films was measured using a quantum efficiency system (C11347-11), with samples prepared on quartz glass/HTL substrates. Fourier transform infrared spectroscopy (FTIR) spectra were obtained using an infrared spectrophotometer (Thermo Fisher Scientific, Nicolet IS50, USA). X-ray diffraction (XRD) was carried out on a diffractometer (Panalytical Netherlands) using Cu Kα radiation. X-ray photoelectron spectroscopy (XPS) together with ultraviolet photoelectron spectroscopy (UPS) measurements were performed on an X-ray photoelectron spectrometer (Thermo Fisher Scientific, ESCALAB XI+, USA), with perovskite films deposited on ITO/HTL substrate. The XPS spectra were analyzed using Avantage software. The surface morphology of the perovskite films was characterized by scanning electron microscopy (SEM, Hitachi S-4800, Japan). Conductive atomic force microscopy (c-AFM) measurements were carried out using a Bruker Dimension Icon system under ambient conditions, with a Pt/Ir-coated conductive tip (radius <25 nm) to map the local current distribution of the perovskite films. Time-resolved photoluminescence (TRPL) spectra were acquired using time-correlated single-photon counting (Edinburgh FLS980) with a 370 nm pulsed laser, while transient absorption spectroscopy (TAS) was performed using a HARPIA TA spectrometer (Ultrafast System) with a 25 kHz repetition rate, both on perovskite films deposited on quartz glass/HTL substrates. Electrostatic potential (ESP) simulations were carried out using Gaussian 16 software to evaluate charge distribution and molecular interactions. All PeLEDs and space charge limited current (SCLC) measurements were conducted at room temperature under ambient air using a Keithley 2400 source meter and a colorimeter (Cs-200). Device lifetimes were initially measured under various luminance conditions (at a current density of 5 mA cm−2) and subsequently converted into T50 lifetimes at 100 cd m−2 using the equation of L0n × T50 = C, where n = 1.5 in this study.283. Results and discussion
The performance improvement of perovskite thin films through defect passivation strategies is remarkable. In this study, we introduce DMPO as a novel additive to the perovskite precursor solution. The simulated surface electrostatic potential (ESP) of DMPO, presented in Fig. 1a, reveals a pronounced negative potential localized around the oxygen atom of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating a highly electron-rich site. In contrast, the methyl groups exhibit positive potentials. This distribution indicates that the oxygen atom is electron-rich and can effectively interact with undercoordinated Pb2+ ions, as illustrated in Fig. 1b. To confirm these interactions, we performed FTIR on pure DMSO, a DMSO–DMPO mixture, and a DMSO–DMPO–perovskite composite. As shown in Fig. S1, the FTIR spectrum of the DMSO–DMPO mixture exhibits a peak at 1166.9 cm−1, corresponding to the stretching vibration of the P
O, indicating a highly electron-rich site. In contrast, the methyl groups exhibit positive potentials. This distribution indicates that the oxygen atom is electron-rich and can effectively interact with undercoordinated Pb2+ ions, as illustrated in Fig. 1b. To confirm these interactions, we performed FTIR on pure DMSO, a DMSO–DMPO mixture, and a DMSO–DMPO–perovskite composite. As shown in Fig. S1, the FTIR spectrum of the DMSO–DMPO mixture exhibits a peak at 1166.9 cm−1, corresponding to the stretching vibration of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in DMPO. This peak shifts to 1161.1 cm−1 upon incorporation of perovskite. This red shift suggests a weakening of the P
O group in DMPO. This peak shifts to 1161.1 cm−1 upon incorporation of perovskite. This red shift suggests a weakening of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond strength, typically associated with coordination interactions between the electron-rich oxygen atom and metal cations such as Pb2+, consistent with prior studies.29,30 XPS analysis provides further insight into the interaction mechanism between DMPO and perovskite films (Fig. 1c). The Pb 4f5/2 and 4f7/2 peaks exhibit a redshift from 142.15 eV and 137.25 eV in the pristine sample to 141.65 eV and 136.75 eV upon DMPO incorporation, respectively. This redshift indicates an increased electron cloud density around Pb atoms, suggesting that the P
O bond strength, typically associated with coordination interactions between the electron-rich oxygen atom and metal cations such as Pb2+, consistent with prior studies.29,30 XPS analysis provides further insight into the interaction mechanism between DMPO and perovskite films (Fig. 1c). The Pb 4f5/2 and 4f7/2 peaks exhibit a redshift from 142.15 eV and 137.25 eV in the pristine sample to 141.65 eV and 136.75 eV upon DMPO incorporation, respectively. This redshift indicates an increased electron cloud density around Pb atoms, suggesting that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in DMPO coordinate with undercoordinated Pb2+ ions, facilitating enhanced electron donation.31,32 Similarly, this electron donation from P
O groups in DMPO coordinate with undercoordinated Pb2+ ions, facilitating enhanced electron donation.31,32 Similarly, this electron donation from P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups to Pb increases the electron cloud density of Br 3d and Cl 2p, resulting in a corresponding redshift in their binding energies, as observed in Fig. 1d and e.
O groups to Pb increases the electron cloud density of Br 3d and Cl 2p, resulting in a corresponding redshift in their binding energies, as observed in Fig. 1d and e.
To assess the impact of DMPO on perovskite film quality, we employed SEM to examine changes in film morphology influenced by the modified chemical environment. The results are presented in Fig. S2. The untreated perovskite films exhibit a rough, porous morphology, “whereas DMPO-treated films are smoother and more compact”. This optimized morphology, induced by DMPO incorporation, enhances the optical performance of the films. The PL spectra (Fig. 1f) illustrate that the untreated films exhibit weak fluorescence and low luminescence intensity, likely due to poor morphology and abundant pores. Conversely, DMPO doping leads to a notable increase in PL intensity, which we attributed to improved film quality. The uniform and compact morphology could potentially reduce defect states, facilitating more efficient charge carrier dynamics. Notably, despite the enhanced PL intensity with DMPO doping, the luminescence peak remains stable at 484 nm without a redshift, indicating that the phase distribution of the perovskite remains unchanged. This observation aligns with the UV-Vis absorption spectra (Fig. S3), which show no noticeable shift in the absorption onset with increasing DMPO concentration, indicating that the bandgap remains largely unaffected. This stability in the absorption edge implies that the DMPO incorporation does not substantially alter the phase distribution or dimensionality of the perovskite films, thereby preserving the overall crystal structure.
In line with the observed improvements in film morphology and luminescence, the PLQY and TRPL measurements demonstrate a clear dependence with DMPO concentration. As depicted in Fig. 1g, the PLQY of perovskite films increases markedly upon DMPO addition, rising from 9.4% for the undoped film to 16.7% at a DMPO concentration of 20 mg ml−1 and reaching a peak of 35.9% at 30 mg ml−1. However, further increasing the DMPO concentration to 40 mg ml−1 results in a slight decrease in PLQY to 31.1%. Although DMPO effectively passivates defects, excessive concentrations may disrupt charge transport pathways due to its insulating properties, leading to elevated nonradiative recombination losses. The c-AFM current maps (Fig. S4) and the I–V characteristics (Fig. S5) consistently reveal a non-monotonic variation in conductivity with increasing DMPO concentration. The conductivity enhancement up to DMPO30 can be attributed to improved film compactness and reduced trap-assisted scattering, whereas the subsequent decline at DMPO40 suggests that excess DMPO molecules introduce additional insulating domains or disturb charge percolation network, ultimately impeding efficient carrier transport across the film. To elucidate the underlying cause of the observed luminescent enhancement, we conducted TRPL measurements. The photoluminescence decay curves were fitted using a triple-exponential model,33–35 with the fitting results summarized in Table S1. As shown in Fig. 1h, the average fluorescence lifetime (τavg) increases with rising DMPO concentration. The τavg of the undoped perovskite film is 4.91 ns, which progressively extends to 10.25 ns with increasing DMPO doping level to 30 mg ml−1. The parameter τ2 corresponds to non-radiative recombination processes associated with defect states in the perovskite. With the introduction of DMPO, τ2 increases, indicating a suppression of non-radiative recombination caused by defect states. The prolonged τ2 implies that charge carriers take longer to recombine through non-radiative pathways, reflecting a lower density or weaker activity of trap states. This suggests that DMPO effectively passivates defects within the perovskite lattice, thereby mitigating trap-assisted recombination and facilitating more efficient radiative processes. As shown in Table S1, the radiative recombination rate (kr) increases from 1.91 × 107 s−1 (undoped) to 3.33 × 107 s−1 at 30 mg per ml DMPO, indicating that DMPO incorporation facilitates more efficient radiative transitions. This enhancement likely results from the effective passivation of trap states, which minimizes carrier losses through non-radiative pathways and enables a higher proportion of charge carriers to recombine radiatively. The improved radiative efficiency aligns well with the observed increase in PLQY and extended carrier lifetimes, further confirming DMPO's beneficial role in improving the optoelectronic properties of perovskite films.
To further investigate the impact of DMPO on PeLED performance, we fabricated blue PeLEDs with a conventional device structure of ITO/m-PEDOT/perovskite/TPBi/LiF/Al, employing perovskite films with varying DMPO concentrations. As shown in Fig. 2a, the devices without DMPO exhibit suboptimal performance, achieving a maximum luminance of only 253 cd m−2 and a peak EQE of 2.95%. With increasing DMPO concentration, both maximum luminance and current efficiency improve, reaching optimal values at a DMPO concentration of 30 mg ml−1. At this point, the devices achieved a maximum luminance of 954 cd m−2, a maximum current efficiency of 8.06 cd A−1, and a peak EQE of 6.92% (Fig. 2a and b). However, when the DMPO concentration increases to 40 mg ml−1, device performance declines, consistent with the reduced PLQY at the same concentration. This degradation may stem from excessive DMPO forming insulating domains within the perovskite matrix, which impede charge transport and partially counteract the benefits of defect passivation. As a result, both the maximum EQE and luminance decreased, dropping from 6.92% to 6.34% and from 954 cd m−2 to 785 cd m−2, respectively. These results indicate that an optimal DMPO concentration is critical for balancing film quality improvement with efficient charge transport, thereby enabling high-performance PeLEDs. Fig. 2c presents the EL spectra of the PeLED measured under a constant voltage of 4 V, with the emission peak centered at 484 nm. The EL intensity increases progressively with increasing DMPO concentration, reaching a maximum at 30 mg mL−1. Further analysis of the normalized EL spectra (Fig. S6) reveals that from DMPO0 to DMPO40, the emission peak positions exhibit a slight redshift, while the FWHM decreases by about 2 nm, indicating that the phase distribution of the perovskite films remains largely stable with varying DMPO concentrations.36,37 Moreover, the EL peak position remains unchanged under various applied voltages (Fig. 2d), further confirming the stability of the emissive phase. The passivation effect of DMPO on the perovskite emissive layer is quantitatively evaluated via the SCLC method.38 As shown by the J–V characteristics of single-carrier devices, the trap-filled limit voltage (VTFL) decreases from 1.27 V (Fig. 2e) to 0.88 V (Fig. 2f) after DMPO doping. The trap density of the perovskite film can be calculated based on VTFL. According to the eqn (1):
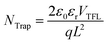 | (1) |
In addition to defect passivation, interface engineering offers an effective strategy to tune energy level alignment and further optimize device performance. Here, we introduce PAM as a novel interface layer between the HTL and the perovskite emitting layer, aiming to synergistically enhance the efficiency and stability of the devices. PAM is a small organic molecule containing both carbonyl and amino groups, which can interact with perovskite materials. The molecular structure of PAM and its electrostatic potential distribution are shown in Fig. 3a. The carbonyl region exhibits a high electron cloud density and pronounced negative polarity, whereas the amino group shows relatively lower electron density and carries partial positive polarity. This bipolar charge distribution enables PAM molecules to form directional interactions with different ionic sites in perovskite materials. As illustrated in Fig. 3b, the carbonyl group in PAM can coordinate with Pb2+ ions, effectively passivating undercoordinated sites or defect states near the metal centers. Meanwhile, the amino group may interact with halide anions through hydrogen bonding or electrostatic attraction, contributing to the stabilization of the perovskite surface.39 Furthermore, both the carbonyl and amino groups are polar and hydrophilic, enhancing the surface wettability of the material (Fig. S7).
XPS analysis of perovskite films on m-PEDOT and PAM-modified substrates (Fig. S8) shows that the core-level peaks of Pb, Br, and Cl shift to lower binding energies in the presence of PAM, indicating increased local electron density and electronic interactions at the interface. This effect arises from the electron-rich carbonyl groups in PAM, which donate lone-pair electrons to undercoordinated Pb2+ ions, thereby enhancing electron density around Pb and neighboring halides and explaining the observed binding energy reduction. To further explore the interactions between PAM and perovskite, FTIR spectroscopy was performed on DMSO–PAM and DMSO–PAM–perovskite solutions. As shown in Fig. 3c, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration exhibits a noticeable blue shift from 1665.9 cm−1 to 1680.1 cm−1, indicating coordination between the carbonyl group and Pb2+ ions. This coordination withdraws electron density from the carbonyl oxygen, thereby enhancing its double-bond character.40 Simultaneously, the amino peak shows a red shift from 3482 to 3472 cm−1 (Fig. 3d), reflecting a weakening of the N–H bond and a corresponding decrease in vibrational frequency. This shift is commonly attributed to hydrogen bond formation,34,41 such as N–H⋯Br or N–H⋯Cl, which elongates and weakens the N–H bond. Strong hydrogen bonding interactions generally result in a more significant redshift in vibrational frequency, sometimes accompanied by broadening of the absorption band. Overall, the carbonyl (C
O stretching vibration exhibits a noticeable blue shift from 1665.9 cm−1 to 1680.1 cm−1, indicating coordination between the carbonyl group and Pb2+ ions. This coordination withdraws electron density from the carbonyl oxygen, thereby enhancing its double-bond character.40 Simultaneously, the amino peak shows a red shift from 3482 to 3472 cm−1 (Fig. 3d), reflecting a weakening of the N–H bond and a corresponding decrease in vibrational frequency. This shift is commonly attributed to hydrogen bond formation,34,41 such as N–H⋯Br or N–H⋯Cl, which elongates and weakens the N–H bond. Strong hydrogen bonding interactions generally result in a more significant redshift in vibrational frequency, sometimes accompanied by broadening of the absorption band. Overall, the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and amino (–NH2) groups in PAM interact with Pb2+ and halide (Br−/Cl−) ions in the perovskite via coordination and hydrogen bonding. These interactions effectively passivate interfacial defects, stabilize the perovskite structure, and enhance the adhesion between the emissive layer and the underlying substrate. Such improvements in film quality provide a foundation for examining the crystalline properties of the perovskite films, which are further investigated by XRD.
O) and amino (–NH2) groups in PAM interact with Pb2+ and halide (Br−/Cl−) ions in the perovskite via coordination and hydrogen bonding. These interactions effectively passivate interfacial defects, stabilize the perovskite structure, and enhance the adhesion between the emissive layer and the underlying substrate. Such improvements in film quality provide a foundation for examining the crystalline properties of the perovskite films, which are further investigated by XRD.
XRD measurements further reveal that increasing the PAM concentration enhances the crystallinity of the perovskite films (Fig. 3e). The diffraction peak at approximately 30.9°, corresponding to the (200) plane of the 3D perovskite phase, becomes markedly intensified when the PAM concentration reaches 15 mg ml−1. In addition, the emergence of a new diffraction peak at 15.5° indicates the formation of the (100) plane. The improved crystallinity suggests an increase in grain size and a reduction in grain boundary density, which together suppress nonradiative recombination both in the bulk and at grain boundaries, thereby contributing to improved PL performance of the films. When the perovskite film is deposited on the PAM15-modified interface, the PL intensity and PLQY increase by a factor of 3 (Fig. 3f) and 1.2-fold (Fig. 3g), respectively, compared with the PAM0 sample. Specifically, the PLQY improves from 35.9% for PAM0 to 43.2% for PAM15. This enhancement can be attributed to the multifaceted role of PAM, which effectively passivates surface and interfacial defects, and suppresses nonradiative recombination. Moreover, the PL emission peak remains nearly unchanged across all samples (Fig. S9), suggesting that the PAM incorporation does not alter the phase composition of the films, consistent with the UV-Vis absorption spectra (Fig. S10).42,43
To further evaluate the effect of PAM on carrier dynamics, we analyzed the radiative and non-radiative recombination rates of the films using TRPL measurements. The decay curves were fitted with a triple-exponential model, and the detailed parameters are summarized in Table S2. As shown in Fig. 3h, in the absence of the PAM interfacial layer, the average carrier lifetime (τavg) of the PAM0 film is 10.25 ns. With increasing PAM concentration, the τavg rises from 10.25 ns for PAM0 to 13.16 ns for PAM15, while the non-radiative recombination rate (knr) decreases from 5.95 × 107 s−1 to 4.31 × 107 s−1, indicating effective defect passivation and suppression of nonradiative pathways. The radiative recombination rate (kr) shows only a minor decrease, confirming that the lifetime extension is primarily due to reduced nonradiative losses. Additionally, the fast decay component (τ1), associated with interfacial or surface defects, increases from 1.76 ns to 2.23 ns, further demonstrating that PAM passivates high-energy trap states at the surface and interface, thereby enhancing carrier transport efficiency.
To gain deeper insight into the charge carrier dynamics, transient absorption (TA) spectroscopy was performed on perovskite films with and without the PAM interfacial layer. Prominent ground-state bleaching (GSB) signals are observed at 443 nm (n = 3 phase) and 467 nm (n ≥ 4 phase). In the PAM-free film (Fig. 4a), carriers are initially generated in both phases, with the n = 3 bleaching intensity rising within the first picosecond, indicating rapid carrier accumulation in the higher-energy phase. Subsequently, the n = 3 signal decays while the n ≥ 4 signal increases, reaching its maximum at ∼2.0 ps, evidencing energy transfer from the n = 3 to the n ≥ 4 phase. In contrast, the PAM-modified film (Fig. 4b) shows accelerated carrier transfer, with the n ≥ 4 peak reaching maximum intensity at ∼1.5 ps. Furthermore, to gain deeper insight into the carrier dynamics, the TA kinetics were analyzed at representative probe wavelengths corresponding to the n = 3 and n ≥ 4 phases (Table S3). Where the fast decay constant (τ1) corresponds to the energy transfer process and τet represents the formation time constant.23 As shown in Fig. 4c and d, τ1 decreases from 0.294 ps in the PAM-free film to 0.256 ps after PAM incorporation, indicating accelerated inter-phase transfer that reduces carrier residence in the high-energy phase and lowers the likelihood of trapping at interfacial or bulk defects. Meanwhile, the formation time constant τet of the n ≥ 4 phase decreases from 0.421 ps to 0.252 ps, demonstrating enhanced energy transfer efficiency from low-n to high-n domains. This improved energy funneling promotes more efficient charge carrier migration, thereby suppressing nonradiative recombination.44
While TA measurements reveal the carrier dynamics and energy transfer processes within the perovskite films, understanding the surface and interfacial electronic structure is equally essential for elucidating charge injection and extraction. For this reason, UV-vis absorption spectroscopy (Fig. S11) and UPS (Fig. S12) were performed to probe the energy level alignment induced by the PAM interfacial layer. As shown in Fig. S12, the valence band maximum (VBM) levels, derived from the secondary electron cutoff and the valence band edge relative to the Fermi level, are estimated as 5.36 eV for m-PEDOT, 5.58 eV for PAM, and 5.70 eV for perovskite on PAM. Considering the p-type nature of the perovskite emissive layer, such energy level alignment is expected to promote more efficient hole injection from the HTL.45,46Fig. 5a and b present the device architecture and the corresponding energy level diagram, which is constructed from the UPS measurements together with reported energy levels of other functional layers in the literature (need citation). Analysis of this diagram indicates that introducing the PAM interfacial layer establishes a more favorable energy alignment. The VBM of PAM is positioned between that of m-PEDOT and the perovskite, thereby functioning as an energy-level bridge. This positioning reduces the hole injection barrier from m-PEDOT to the perovskite emissive layer and facilitates more efficient hole transport across the interface. In addition, the higher WF of the perovskite film deposited on PAM reveals that PAM modifies the surface dipole and strengthens band bending at the interface, further promoting charge injection and suppressing recombination losses. Overall, PAM not only passivates interfacial defects but also optimizes energy-level alignment, which proves essential for achieving balanced carrier injection and improved device performance.47,48
Fig. 5a and b illustrate the device structure and energy level diagram after introducing the PAM interfacial layer. After confirming the beneficial effects of PAM on defect passivation and energy-level alignment, we next examine how different PAM concentrations influence device performance. Fig. 5c presents the current density–voltage–luminance (J–V–L) characteristics of PeLEDs with varying PAM-treated interfaces. The results reveal that the incorporation of PAM significantly enhances both the current density and luminance while reducing the turn-on voltage, indicating improved conductivity and electroluminescent efficiency. Specifically, the device without PAM (PAM0) exhibits a maximum luminance of 954 cd m−2, a peak current efficiency of 8.06 cd A−1, and a maximum EQE of 6.92%. Upon increasing the PAM concentration to 15 mg ml−1, the device achieves a substantially higher maximum luminance of 2135 cd m−2, a peak current efficiency of 15.84 cd A−1 (Fig. 5d), and a maximum EQE of 12.94% (Fig. 5e), representing the optimal device performance. Table S4 summarizes several recent studies, showing that our device performance ranks at an intermediate level among the recently reported sky-blue perovskite emitters operating around 485 nm. We next investigate the effect of PAM concentration on the EL spectra (Fig. 5f). With increasing PAM content, the emission intensity progressively rises, reaching a maximum at 15 mg ml−1. Normalized EL spectra (Fig. S13) show minimal impact on peak positions, with PAM0, PAM5, and PAM10 remaining at ∼484 nm, while PAM15 and PAM20 show a slight redshift to 486 nm. This trend is consistent with the UV-Vis absorption spectra (Fig. S10), which reveal negligible change in phase distribution and a minor redshift of the absorption edge.49Fig. 5g displays the EL spectra of the PAM15 device under different operating voltages. As the voltage increases, the luminance rises markedly, exhibiting bright and stable blue emission (inset), while the emission peak remains constant at 486 nm until the maximum luminance is reached, demonstrating excellent spectral stability. In terms of color purity, the PAM15 device exhibits CIE chromaticity coordinates of (0.073, 0.265) (Fig. 5h), confirming a sky-blue emission. To assess practical applicability, device operational lifetimes were measured (Fig. S14). Under constant current driving, devices with different PAM concentrations exhibit T50 of 139 s, 69 s, 72 s, 125 s, and 106 s, respectively. Considering the variations in initial luminance, the lifetimes are normalized to 100 cd m−2 using the equation L0n × T50 = C and the calculated results are presented in Fig. 5i. Notably, the PAM15 device demonstrates the longest T50 of 617.71 s, indicating substantially enhanced operational stability. These results highlight that incorporating PAM not only improves device efficiency but also substantially enhances stability, offering a practical pathway toward reliable next-generation perovskite optoelectronic devices.
4. Conclusions
This study presents a robust strategy for enhancing the performance of quasi-2D blue PeLEDs through the combined optimization of bulk and interfacial properties. Incorporation of DMPO into the perovskite precursor effectively passivates undercoordinated Pb2+ ions, reducing trap densities and facilitating more efficient radiative recombination. Concurrently, the introduction of a PAM interlayer refines film morphology, enhances crystallinity, suppresses non-radiative recombination, and establishes favorable energy level alignment within the perovskite/HTO interface. These synergistic modifications result in markedly improved electroluminescent performance, with sky-blue PeLEDs (emission peak at 486 nm) achieving a maximum luminance of 2135 cd m−2 and a peak EQE of 12.94%. Overall, this work highlights the dual functionality of DMPO and PAM in defect passivation and interfacial engineering, offering a viable design strategy for the development of high-efficiency blue perovskite optoelectronic devices.Conflicts of interest
The authors declare no conflict of interest.Data availability
All data supporting the findings of this study are available within the article and its supporting information (SI). Additional raw data, including characterization results, device performance measurements, and analysis files, are available from the corresponding author upon reasonable request. Supplementary information: FTIR spectra; SEM images; c-AFM images; UV-Vis absorption spectra; statistical results of TRPL fitted data; normalized PL spectra; contact angles images; XPS; TA kinetics fitted data; UPS results; normalized EL spectra; luminance decay curves. See DOI: https://doi.org/10.1039/d5ta07534c.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (62474070, 61404051), in part by the National Key R&D Program of China under Grant 2024YFF1504501, partially by National Science and Technology Major Project under Grant 2024ZD0604100, and in part by Key-Area Research and Development Program of Guangdong Province (2020B010180001).References
- Z.-K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- Y. Huang, L. Qiao, Y. Jiang, T. He, R. Long, F. Yang, L. Wang, X. Lei, M. Yuan and J. Chen, Angew. Chem., Int. Ed., 2019, 58, 17834–17842 CrossRef CAS PubMed.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 RSC.
- S. Cho, S. Kim, J. Kim, Y. Jo, I. Ryu, S. Hong, J.-J. Lee, S. Cha, E. B. Nam, S. U. Lee, S. K. Noh, H. Kim, J. Kwak and H. Im, Light: Sci. Appl., 2020, 9, 156 CrossRef PubMed.
- J.-N. Yang, Y. Song, J.-S. Yao, K.-H. Wang, J.-J. Wang, B.-S. Zhu, M.-M. Yao, S. U. Rahman, Y.-F. Lan, F.-J. Fan and H.-B. Yao, J. Am. Chem. Soc., 2020, 142, 2956–2967 CrossRef CAS PubMed.
- S. U. Rahman, Y.-F. Lan, F.-J. Fan and H.-B. Yao, J. Am. Chem. Soc., 2020, 142, 2956–2967 CrossRef.
- H. Shao, Y. Zhai, X. Wu, W. Xu, L. Xu, B. Dong, X. Bai, H. Cui and H. Song, Nanoscale, 2020, 12, 11728–11734 RSC.
- C. Zou, Y. Liu, D. S. Ginger and L. Y. Lin, ACS Nano, 2020, 14, 6076–6086 CrossRef CAS PubMed.
- M. You, H. Wang, F. Cao, C. Zhang, T. Zhang, L. Kong, L. Wang, D. Zhao, J. Zhang and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 43018–43023 CrossRef CAS PubMed.
- L. N. Quan, B. P. Rand, R. H. Friend, S. G. Mhaisalkar, T.-W. Lee and E. H. Sargent, Chem. Rev., 2019, 119, 7444–7477 CrossRef CAS PubMed.
- T. He, Y. Jiang, X. Xing and M. Yuan, Adv. Mater., 2020, 32, 1903937 CrossRef CAS PubMed.
- L. Wang, Y. Xue, M. Cui, Y. Huang, H. Xu, C. Qin, J. Yang, H. Dai and M. Yuan, Angew. Chem., Int. Ed., 2020, 132, 6504–6512 CrossRef.
- J. Chen, J. Wang, X. Xu, J. Li, J. Song, S. Lan, S. Liu, B. Cai, B. Han, J. T. Precht, D. Ginger and H. Zeng, Nat. Photonics, 2021, 15, 238–244 CrossRef CAS.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- Z. Luo, B. Liu, T. Zheng, X. Luo, L. Lu, B. Tian, P. Xu, H. S. Kwok and G. Li, ACS Photonics, 2022, 9, 2422–2430 CrossRef CAS.
- X.-K. Liu and F. Gao, J. Phys. Chem. Lett., 2018, 9, 2251–2258 CrossRef CAS PubMed.
- Y. Cao, H. Ke, Y. Xu, M. Wang, Y. Yang, M. Du, K. Fu, Z. Kong, D. Dai, D. Jin, Y. Li, G. Li, H. Peng, Q. Wang, J. Huang and W. Wang, Nature, 2018, 562, 249–253 CrossRef CAS PubMed.
- K. Lin, J. Xing, L. N. Quan, F. P. G. De Arquer, X. Gong, J. Lu, L. Xie, W. Zhao, D. Zhang, C. Yan, W. Li, X. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. Xiong and Z. Wei, Nature, 2018, 562, 245–248 CrossRef CAS PubMed.
- P. Chen, Y. Xiao, S. Li, X. Jia, D. Luo, W. Zhang, H. J. Snaith, Q. Gong and R. Zhu, Chem. Rev., 2024, 124, 10623–10700 CrossRef CAS PubMed.
- Y. Liu, S. Wang, Z. Yu, G. Chen, C. Wang, T. Wang, W. Ke and G. Fang, Adv. Mater., 2023, 35, 2302161 CrossRef CAS PubMed.
- M. Li, Y. Yang, Z. Kuang, C. Hao, S. Wang, F. Lu, Z. Liu, J. Liu, L. Zeng, Y. Cai, Y. Mao, J. Guo, H. Tian, G. Xing, Y. Cao, C. Ma, N. Wang, Q. Peng, L. Zhu, W. Huang and J. Wang, Nature, 2024, 630, 631–635 CrossRef CAS PubMed.
- W. Bai, T. Xuan, H. Zhao, H. Dong, X. Cheng, L. Wang and R. Xie, Adv. Mater., 2023, 35, 2302283 CrossRef CAS PubMed.
- S. Teale, M. Degani, B. Chen, E. H. Sargent and G. Grancini, Nat. Energy, 2024, 9, 779–792 CrossRef CAS.
- F. Zhang, Y. Yang, Y. Gao, D. Wang, W. Dong, P. Lu, X. Wang, M. Lu, Y. Wu, P. Chen, J. Hu, X. Yang, D. Zhou, D. Liu, L. Xu, B. Dong, Z. Wu, Y. Zhang, H. Song and X. Bai, Nano Lett., 2024, 24, 1268–1276 CrossRef CAS PubMed.
- K. Yang, F. Li, H. Hu, T. Guo and T. W. Kim, Nano Energy, 2019, 65, 104029 CrossRef CAS.
- Z. Yu, X. Shen, X. Fan, Y.-K. Jung, W. H. Jeong, M. Kober-Czerny, P. Caprioglio, S. H. Park, H. Choi, S. D. Stranks and B. R. Lee, ACS Energy Lett., 2023, 8, 4296–4303 CrossRef CAS.
- W. Zhou, Y. Shen, L. Cao, Y. Lu, Y. Tang, K. Zhang, H. Ren, F. Xie, Y. Li and J. Tang, Adv. Funct. Mater., 2023, 33, 2301425 CrossRef CAS.
- K. Yang, B. Xu, Q. Lin, Y. Yu, H. Hu and F. Li, Chem. Eng. J., 2024, 483, 149291 CrossRef CAS.
- X. Dai, Z. Zhang, Y. Jin, Y. Niu, H. Cao, X. Liang, L. Chen, J. Wang and X. Peng, Nature, 2014, 515, 96–99 CrossRef CAS PubMed.
- Y. Dong, Y.-K. Wang, F. Yuan, A. Johnston, Y. Liu, D. Ma, M.-J. Choi, B. Chen, M. Chekini, S.-W. Baek, L. K. Sagar, J. Fan, Y. Hou, M. Wu, S. Lee, B. Sun, S. Hoogland, R. Quintero-Bermudez, H. Ebe, P. Todorovic, F. Dinic, P. Li, H. T. Kung, M. I. Saidaminov, E. Kumacheva, E. Spiecker, L.-S. Liao, O. Voznyy, Z.-H. Lu and E. H. Sargent, Nat. Nanotechnol., 2020, 15, 668–674 CrossRef CAS.
- M. Yu, X. Mei, T. Qin, R. Zhuang, Y. Hua and X. Zhang, Chem. Eng. J., 2022, 450, 138021 CrossRef CAS.
- Z. Ren, J. Yu, Z. Qin, J. Wang, J. Sun, C. C. S. Chan, S. Ding, K. Wang, R. Chen, K. S. Wong, X. Lu, W. Yin and W. C. H. Choy, Adv. Mater., 2021, 33, 2005570 CrossRef CAS PubMed.
- A. Liu, C. Bi and J. Tian, Adv. Funct. Mater., 2022, 32, 2207069 CrossRef CAS.
- R. Khan, S. Chu, Z. Li, K. O. Ighodalo, W. Chen and Z. Xiao, Adv. Funct. Mater., 2022, 32, 2203650 CrossRef CAS.
- J. Liu, J. Leng, K. Wu, J. Zhang and S. Jin, J. Am. Chem. Soc., 2017, 139, 1432–1435 CrossRef CAS PubMed.
- S. Peng, Y. Wang, M. Braun, Y. Yin, A. C. Meng, W. Tan, B. Saini, K. Severson, A. F. Marshall, K. Sytwu, J. D. Baniecki, J. Dionne, W. Cai and P. C. McIntyre, Matter, 2023, 6, 2052–2065 CrossRef CAS.
- C. Zhang, L. K. Ono and Y. Qi, Adv. Funct. Mater., 2024, 34, 2314762 CrossRef CAS.
- B. Han, S. Yuan, B. Cai, J. Song, W. Liu, F. Zhang, T. Fang, C. Wei and H. Zeng, Adv. Funct. Mater., 2021, 31, 2011003 CrossRef CAS.
- E. A. Duijnstee, J. M. Ball, V. M. Le Corre, L. J. A. Koster, H. J. Snaith and J. Lim, ACS Energy Lett., 2020, 5, 376–384 CrossRef CAS.
- Z. Yu, X. Shen, X. Fan, Y.-K. Jung, W. H. Jeong, A. Dasgupta, M. Kober-Czerny, P. Caprioglio, S. H. Park, H. Choi, H. J. Snaith, S. D. Stranks and B. R. Lee, ACS Energy Lett., 2023, 8, 4296–4303 CrossRef CAS.
- S. Li, Y. Yun, W. Wei, J. Du, S. Jiang, Y. Tian, H. Luo, K. Huang, C. Li, M. Chen and R. Zhang, ACS Photonics, 2024, 11, 4716–4724 CrossRef CAS.
- Q. Wang, X. Wang, Z. Yang, N. Zhou, Y. Deng, J. Zhao, X. Xiao, P. Rudd, A. Moran, Y. Yan and J. Huang, Nat. Commun., 2019, 10, 5633 CrossRef CAS PubMed.
- Z. Liu, P. Liu, T. He, L. Zhao, X. Zhang, J. Yang, H. Yang, H. Liu, R. Qin and M. Yuan, ACS Appl. Mater. Interfaces, 2020, 12, 26670–26679 CrossRef CAS PubMed.
- M. Pylnev, A. M. Barbisan and T.-C. Wei, Appl. Surf. Sci., 2021, 541, 148559 CrossRef CAS.
- Z. Guo, Y. Zhang, B. Wang, L. Wang, N. Zhou, Z. Qiu, N. Li, Y. Chen, C. Zhu, H. Xie, T. Song, L. Song, H. Xue, S. Tao, Q. Chen, G. Xing, L. Xiao, Z. Liu and H. Zhou, Adv. Mater., 2021, 33, 2102246 CrossRef CAS PubMed.
- N. Jiang, Z. Wang, Y. Zheng, Q. Guo, W. Niu, R. Zhang, F. Huang and D. Chen, Nano Energy, 2022, 97, 107181 CrossRef CAS.
- Y.-K. Wang, D. Ma, F. Yuan, K. Singh, J. M. Pina, A. Johnston, Y. Dong, C. Zhou, B. Chen, B. Sun, H. Ebe, J. Fan, M.-J. Sun, Y. Gao, Z.-H. Lu, O. Voznyy, L.-S. Liao and E. H. Sargent, Nat. Commun., 2020, 11, 3674 CrossRef CAS PubMed.
- Y. Ahn, S. Lee, D.-H. Kwak, M. Kim, D. Y. Kim, J. Kim, Y. Park and M. C. Suh, Appl. Surf. Sci., 2020, 507, 145071 CrossRef CAS.
- S. Li, Y. Yun, W. Wei, J. Du, S. Jiang, Y. Tian, H. Luo, K. Huang, C. Li, M. Chen and R. Zhang, ACS Photonics, 2024, 11, 4716–4724 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |





