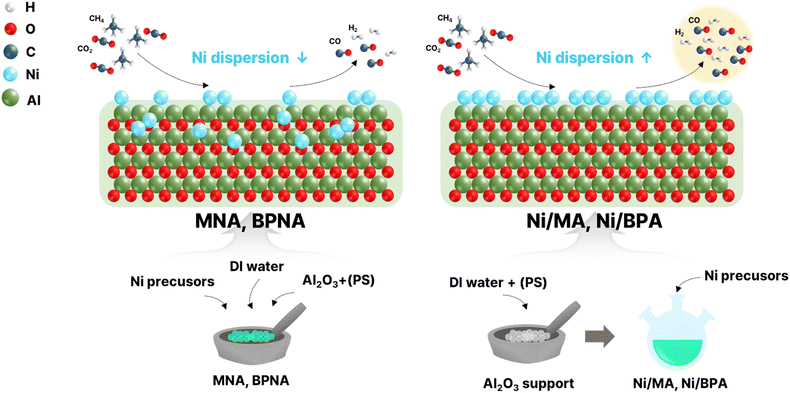
Enhanced catalytic performance of Ni/Al2O3 for the carbon dioxide reforming reaction by controlling the pore structure and Ni addition in catalyst design
Ji-Won
Son†
a,
Hak-Min
Kim†
b,
Jae-Min
Song
a,
Beom-Su
Cheon
a and
Dae-Woon
Jeong
 *c
*c
aDepartment of Environmental Engineering, Changwon National University, 20 Changwondaehak-ro, Changwon, Gyeongnam 51140, Republic of Korea
bDepartment of Smart Mobility, Dongseo University, 47 Jurye-ro, Sasang-gu, Busan 47011, Republic of Korea
cDepartment of Environment & Energy Engineering, Changwon National University, 20 Changwondaehak-ro, Changwon, Gyeongnam 51140, Republic of Korea. E-mail: dwjeong@changwon.ac.kr
First published on 21st October 2025
Abstract
Carbon dioxide reforming (CDR) of methane is a promising technology for the production of hydrogen and the removal of greenhouse gases. However, deactivation of catalysts via carbon deposition and sintering remains a limitation for the commercialization of CDR reactions. In this study, Ni/Al2O3 catalysts were prepared using different methods to investigate the effects of pore properties, as well as the interaction between Ni and supports, on the catalytic performance. Mesoporous and bimodal porous Ni/Al2O3 catalysts were prepared to confirm the relationship between the pore properties and catalytic performance. The addition of Ni was controlled to determine the effect of the interaction between Ni and Al2O3 on the catalytic performance. Various techniques were applied to understand the pore and interaction properties of Ni/Al2O3 catalysts prepared by different methods. The performance of Ni/Al2O3 catalysts prepared by different preparation methods and their pore properties were analyzed at a CH4/CO2 ratio of 1.0, gas hourly space velocity of 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1, and reaction temperature of 700 °C. The highest catalytic performance was demonstrated by the bimodal porous Ni/Al2O3 catalyst, in which Ni was added after the calcination of the Al2O3 support. This result was owing to the high mass transfer due to the bimodal pore structure and proper interaction between Ni and Al2O3. This study will contribute to informing the importance of the pore structure and the addition of Ni for the development of catalysts that are both highly active and stable.
000 mL g−1 h−1, and reaction temperature of 700 °C. The highest catalytic performance was demonstrated by the bimodal porous Ni/Al2O3 catalyst, in which Ni was added after the calcination of the Al2O3 support. This result was owing to the high mass transfer due to the bimodal pore structure and proper interaction between Ni and Al2O3. This study will contribute to informing the importance of the pore structure and the addition of Ni for the development of catalysts that are both highly active and stable.
1. Introduction
Climate change threatens the safety of human life and maintenance of ecosystems on Earth. One of the main contributors to climate change is the use of fossil fuels, which emit greenhouse gases.1,2 Therefore, international efforts, such as the Paris Agreement and Glasgow Climate Pact, have been made to cope with the global climate crisis.3,4 In particular, the high dependence on fossil fuels is a significant barrier to overcoming the problems generated by climate change.5,6 The establishment of a hydrogen society can be a solution to decrease the dependence on fossil fuel because hydrogen is a clean energy carrier with a high energy density per unit mass, emitting only water during combustion.7 Hydrogen can be produced by reforming hydrocarbons, water electrolysis, and nuclear power.8 The dominant method is hydrocarbon reforming, which can be classified into steam reforming (SR), carbon dioxide reforming (CDR), combined steam and carbon dioxide reforming (CSCDR), and autothermal reforming (ATR) depending on the type of oxidizing agent added.9,10 Among the various reforming reactions, CDR has the advantage of producing hydrogen from carbon dioxide and methane, which are greenhouse gases.11–13 In addition, it is easy to produce high-value fuels, which consist of long-chain hydrocarbons, from the syngas generated by the CDR reaction, owing to its low H2/CO ratio.14,15A catalyst is an important factor for the energy-effective performance of CDR because it decreases energy consumption by reducing the activation energy. Precious-metal (Ru, Rh, and Pt)-based catalysts were initially used because of their high activity and stability.16 Recently, Ni-based catalysts have been actively studied by developing design technologies for cost-effective catalysts using nonprecious metals. In particular, the catalytic performance of Ni-based catalysts is affected by the metal loading. M. R. B. Pirshahid et al. reported the effect of nickel loading (5, 10, 15, and 20 wt%) of Ni-based catalysts in dry methane reforming.17–20 It was reported that the catalyst with 15 wt% showed the highest activity and stability. This is because the appropriate level of Ni content increased exposure to the active site to increase accessibility to the reactants, and excessive Ni loading lowered the catalyst performance. W.-S. Dong et al. investigated the effect of Ni content on methane reforming in NiCe–ZrO2 catalysts and reported that a 15 wt% Ni loading catalyst exhibited the best activity, selectivity, and stability.21 This was interpreted as due to an appropriate balance of active sites for methane and oxidants, and therefore, a 15 wt% Ni loading catalyst was used in this study. Nevertheless, Ni-based catalysts still suffer from deactivation due to carbon deposition and sintering at high temperatures. In addition, it is required to design strategies to inhibit the deactivation of Ni-based catalysts by carbon deposition on the catalyst surface and sintering Ni particles. Many studies have reported improvements in resistance to sintering and carbon deposition. In particular, the use of Al2O3 as a support to overcome the limitations of Ni-based catalysts in the CDR reaction has garnered significant attraction.22–24
Al2O3 supports are widely used because of their excellent pore size dispersion, high specific surface area, high mechanical strength, strong chemical inertness, and low cost.22 Wang et al. studied the effect of active metal dispersion on the specific surface area characteristics and pore structure of an Al2O3 support and confirmed that a high specific surface area and large pore volume are helpful for the deposition of NiO particles inside mesopores, thereby resulting in stronger metal–support interactions.23 Bychkov et al. compared the activity and carbon accumulation of Ni catalysts supported by α-Al2O3, γ-Al2O3, and θ-Al2O3 support.24 The activity of the Ni/γ-Al2O3 catalyst was the highest, with low carbon accumulation. Zhong, et al. reported that when the NiAl2O4 spinel phase formed after firing at a high temperature, it was advantageous for maintaining high stability owing to the formation of small Ni particles and strong metal–support interactions.22
The selection of a preparation method is important for preparing highly active and stable Ni/Al2O3 catalysts because the properties of the catalyst, which are directly related to catalytic performance, are strongly influenced by the preparation method.25 Among the various properties, the pore properties, which are controlled by the preparation method, contribute to the improvement of the mass transfer ability, which is related to the contact between the reactant and active sites on the surface of the catalysts.26,27 Recently, bimodal porous materials, including mesopores and macropores, have been considered promising supports for catalytic reactions because the bimodal porous structure compensates for the weaknesses of single-porous materials.28–31 In addition, physicochemical properties were affected by preparation methods such as interaction between Ni and Al2O3, Ni dispersion, and Ni reducibility.
However, this is not sufficient to understand the impacts of the bimodal pore on the performance of catalysts in the CDR reaction. Therefore, it is necessary to investigate the preparation methods of Ni/Al2O3 catalysts that affect the pore and physicochemical properties to design highly active and stable catalysts. In this study, Ni/Al2O3 catalysts with bimodal porous and mesoporous structures were synthesized for understanding the effects of the bimodal structure on the performance of catalysts. In addition, Ni, an active metal, was loaded onto the supports using different methods to optimize the catalytic performance. Various techniques were used to investigate the properties of Ni/Al2O3 catalysts synthesized using different preparation methods. This study is expected to provide a guide for the selection of preparation methods and pore properties to design uniform porous catalysts for chemical reactions.
2. Experimental
2.1. Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ ratio = 5
Al3+ ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Aluminum isopropoxide (3 g) and the synthesized PS particles (1.5 μm, 1.5 g) were mixed with water using a mortar and pestle until a gel-like intermediate was formed. Subsequently, the material was calcined at 700 °C (ramping rate: 0.5 °C min−1) for 10 h to remove the PS template and crystallize γ-alumina. For comparison, mesoporous γ-alumina was prepared by the same procedure but without the PS template.
1). Aluminum isopropoxide (3 g) and the synthesized PS particles (1.5 μm, 1.5 g) were mixed with water using a mortar and pestle until a gel-like intermediate was formed. Subsequently, the material was calcined at 700 °C (ramping rate: 0.5 °C min−1) for 10 h to remove the PS template and crystallize γ-alumina. For comparison, mesoporous γ-alumina was prepared by the same procedure but without the PS template.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3 = 15
Al2O3 = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 wt%) in 100 mL of DI water and stirred at 500 rpm for 1 h. This solution was placed in the oven at 120 °C for 24 h to evaporate the DI water. Finally, to form NiO or NiAl2O4, the materials prepared from two routes were calcined at 700 °C (ramping rate: 10 °C min−1) for 5 h. The difference in the catalyst preparation methods is summarized in Scheme 2.
85 wt%) in 100 mL of DI water and stirred at 500 rpm for 1 h. This solution was placed in the oven at 120 °C for 24 h to evaporate the DI water. Finally, to form NiO or NiAl2O4, the materials prepared from two routes were calcined at 700 °C (ramping rate: 10 °C min−1) for 5 h. The difference in the catalyst preparation methods is summarized in Scheme 2.
The Ni/Al2O3 catalysts prepared by the first method are denoted as MNA with a mesoporous structure and BPNA with a bimodal porous structure. The Ni/Al2O3 catalysts prepared by the second method were denoted as Ni/MA with a mesoporous structure and Ni/BPA with a bimodal porous structure.
2.2. Characterization of catalysts
Various analytical techniques were applied to understand the characteristics of the prepared samples. The various techniques were Brunauer–Emmett–Teller (BET) measurements, field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), H2-temperature programmed reduction (H2-TPR), H2-chemisorption, X-ray photoelectron spectroscopy (XPS), field-emission transmission electron microscopy (FE-TEM), thermogravimetric analysis (TGA), and Raman spectroscopy. The reduction of catalysts was performed to obtain the information of metallic Ni as an active species prior to FE-SEM, FE-TEM, XRD and XPS analyses. N2 adsorption/desorption was performed at −196 °C using an ASAP 2020 (Micromeritics, USA) instrument, and the BET surface area was calculated based on the amount of nitrogen adsorbed by the sample obtained. The microstructures and morphologies of the catalyst surfaces were investigated using FE-SEM (JSM-7900F, JEOL, Japan). The XRD patterns of the catalysts were obtained using an X'Pert PRO MPD diffractometer (PANalytical, Netherlands; Ni filtered Cu-Kα radiation, 40 kV, 30 mA). The patterns were obtained in a diffraction angle range (2θ) of 20° to 80°. The crystallite size was calculated using the Debye–Scherrer equation and XRD patterns. H2-TPR was performed using a chemisorption AutoChem II 2920 (Micromeritics, USA) instrument. The catalyst was heated in a 10% H2/Ar atmosphere from 25 °C to 1000 °C at a rate of 10 °C min−1. The dispersion of Ni in the catalyst was measured using an AutoChem II 2920 instrument (Micromeritics, USA) via H2-chemisorption. Prior to H2-chemisorption, reduction was performed at 700 °C for 3 h in 10 vol% H2/Ar. Thereafter, hydrogen was added at a catalyst bed temperature of 50 °C until the catalyst was saturated. The XPS analysis was conducted using an X-ray microprobe (ESCALAB 250XI, Thermo Fisher Scientific, USA). The results were plotted after calibration, based on the binding energy peak C 1s at 284.8 eV. An FE-TEM analysis was performed at an operating voltage of 200 kV using a JEM-F200 instrument (JEOL, Japan). All samples were sonicated, dispersed in ethanol, dropped onto a copper grid, and deposited with a carbon film. Subsequently, FE-TEM measurements were performed. The amount of carbon accumulated in the catalyst was measured by TGA (SDT 650, TA Instruments, USA). The catalyst was heat-treated in air from 30 °C to 800 °C at a heating rate of 10 °C min−1. Raman spectroscopy was performed to determine the binding state of carbon deposited in the catalyst recovered after the reaction. The analysis was performed using NRS-3000 Series Raman spectrometers (JASCO, Japan) with a laser excitation wavelength of 532 nm at wavenumber 57 cm−1.2.3. Carbon dioxide reforming reaction
CDR of methane was conducted in a fixed-bed reactor with a diameter of 4 mm at 1 atm to evaluate its ability for hydrogen production. A quartz cylindrical reactor was fixed to the heat jacket, and 10 mg of catalyst was loaded over the quartz wool in the center of it. The reaction temperature was monitored using a K-type thermocouple (Omega Eng., Stamford, CT, USA) installed in the catalyst layer. The temperature of the catalyst layer was controlled using a thermal controller (Hanyoung Nux Co., Daegu, Republic of Korea). The catalyst before the catalytic activity test was reduced to 5% H2/N2 gas for 3 h at 700 °C. The gas hourly space velocity (GHSV) was fixed at 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The feed gases were CH4 and CO2, supplied at a ratio of 1
000 mL g−1 h−1. The feed gases were CH4 and CO2, supplied at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The flow rate of the feed gas was 50 NmL min−1 of CH4, CO2, and N2. N2 was used as a balanced (or internal standard) gas to calculate the conversion rate of CH4 and CO2 and yield of H2. The outflow gases were measured using an online micro-gas chromatograph (Micro-GC 490, Agilent Technologies, CA, USA), which was equipped with a thermal conductivity detector (Micro-GC 490, Agilent Technologies, CA, USA). The CH4 and CO2 conversion rates, along with the H2 and CO yields, were calculated as follows:
1. The flow rate of the feed gas was 50 NmL min−1 of CH4, CO2, and N2. N2 was used as a balanced (or internal standard) gas to calculate the conversion rate of CH4 and CO2 and yield of H2. The outflow gases were measured using an online micro-gas chromatograph (Micro-GC 490, Agilent Technologies, CA, USA), which was equipped with a thermal conductivity detector (Micro-GC 490, Agilent Technologies, CA, USA). The CH4 and CO2 conversion rates, along with the H2 and CO yields, were calculated as follows: | (1) |
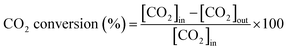 | (2) |
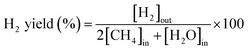 | (3) |
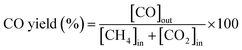 | (4) |
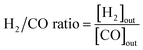 | (5) |
2.4. Kinetic measurements
Kinetic experiments were conducted under various reaction temperatures and feed gas compositions to evaluate the reaction rate characteristics of the CDR reaction. This experiment was carried out in the same manner as the CDR reaction until the catalytic reduction step, and then the reaction was performed by varying the reaction temperature and the flow rate of N2 and CH4 or CO2. It was carried out under reaction temperatures ranging from 650 to 800 °C, and the reaction was carried out by varying the flow rate of N2 and CH4 or CO2 in the range of 10 to 50 mL min−1 at a fixed flow rate of CH4 or CO2. As shown in Table S1, the reaction gas was composed of N2, CH4, and CO2, and the total flow rate was fixed at 70 sccm. Accordingly, GHSV was designed to maintain 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. The generated gas was analyzed using gas chromatography. The reaction rate according to the reactants and products was calculated as follows:
000 mL g−1 h−1. The generated gas was analyzed using gas chromatography. The reaction rate according to the reactants and products was calculated as follows: | (6) |
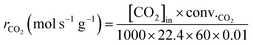 | (7) |
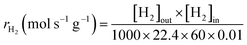 | (8) |
 | (9) |
The Mears criterion related to the external mass transfer was calculated by eqn (10).32
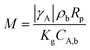 | (10) |
The Weisz–Perater number assigned to the internal mass transfer was calculated by eqn (11).32
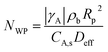 | (11) |
3. Results and discussion
3.1. Characterization of Ni/Al2O3 catalysts
 | ||
| Fig. 1 N2 adsorption/desorption isotherms of the Ni/Al2O3 catalysts: (a) MNA, (b) Ni/MA, (c) BPNA, and (d) Ni/BPA. | ||
| Catalyst | Pore volumea (cm3 g−1) | Pore sizea (nm) | Micropore areaa (m2 g−1) | BET surface areaa (m2 g−1) | Mears criterionb | Weisz–Prater numberb |
|---|---|---|---|---|---|---|
| a Estimated from N2 adsorption at −196 °C. b Estimated from kinetic experiments. | ||||||
| MNA | 0.67 | 7.3 | 29 | 202 | 316 × 105 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 × 105 116 × 105 |
| Ni/MA | 0.35 | 2.6 | 21 | 207 | 520 × 105 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 899 × 105 899 × 105 |
| BPNA | 0.69 | 9.4 | 24 | 186 | 379 × 105 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 531 × 105 531 × 105 |
| Ni/BPA | 0.29 | 2.6 | 19 | 190 | 569 × 105 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 702 × 105 702 × 105 |
Fig. S1 shows the pore diameter distribution curves of Ni/Al2O3 catalysts prepared by different preparation methods. The Ni/MA and Ni/BPA catalysts exhibited more uniform pore structures than the MNA and BPNA catalysts. The characteristics of the MNA and BPNA catalysts were consistent with those of the H3 type, which had primary irregular pores with large diameters.23 This means that the Ni species added when manufacturing the support was inserted into the Al2O3 bulk and thus interfered with uniform pore generation.23,41 Therefore, it was confirmed that the pore structure was deformed according to the preparation method. Because uniform pores suppress carbon deposition and improve mass transfer, the Ni/MA and Ni/BPA catalysts are expected to exhibit higher activities.42,43
Table 1 lists the mesoporous properties of the Ni/Al2O3 catalysts prepared by different methods, as obtained from the N2 adsorption at −196 °C. The surface areas of the Ni/Al2O3 catalysts were similar regardless of the preparation method. The mesoporous properties of the Ni/BPA catalyst were similar to those of the Ni/MA catalyst because the Ni/BPA catalyst included mesopores and macropores. Interestingly, the mesoporous properties were significantly affected by the addition of Ni. The pore size values presented in Table 1 correspond to the x-axis (pore diameter) of Fig. S1. The MNA and BPNA catalysts exhibited a broad pore diameter distribution in the range of 5–20 nm, whereas the Ni/MA and Ni/BPA catalysts showed a narrow and uniform pore diameter of approximately 2.6 nm. This observation is consistent with the pore diameter distribution results shown in the corrected Fig. S1. In addition, it should be noted that the N2 adsorption desorption analysis employed in this study only characterizes pores which have a diameter smaller than 50 nm. The reduced pore size and narrowed distribution in the Ni/MA and Ni/BPA samples are attributed to the destruction of mesopores during the Ni loading process on porous Al2O3. According to previous research, the higher the BET surface area, the faster the reaction rate increases by promoting mass transfer and the adsorption of reactants.26 Therefore, Ni/MA and Ni/BPA catalysts are expected to exhibit high catalytic activities.
The Mears criterion values are summarized in Table 1 when the average particle size diameter of catalysts was 300 μm. The external mass transfer effects can be minimized if the Mears criterion is less than 0.15.44 In addition, the Weisz–Prater numbers are also summarized in Table 1.
Although the presence of macropores in the bimodal catalysts (Ni/BPA and BPNA) was expected to facilitate mass transfer, the Weisz–Prater analysis showed that Ni/BPA exhibited the largest NWP. This is primarily because the intrinsic rate (γA) of Ni/BPA was substantially higher than that of the other catalysts, while its overall pore volume (0.29 cm3 g−1) was the smallest among the series, leading to a lower effective diffusivity (Deff). As a result, the ratio NWP ∝ γA/Deff increased, even though macropores were present. In contrast, MNA, which exhibited a lower intrinsic activity and larger pore volume, showed the smallest NWP. It should also be noted that our N2 sorption only quantifies pores <50 nm, so the contribution of large macropores (>50 nm) is underestimated. Therefore, the bimodal structure still enhances transport qualitatively, but under the current kinetic regime the very high activity of Ni/BPA accentuates the intraparticle concentration gradients in the Weisz–Prater criterion. In addition, the Weisz–Prater criterion effect is neglected when it is lower than 1.44
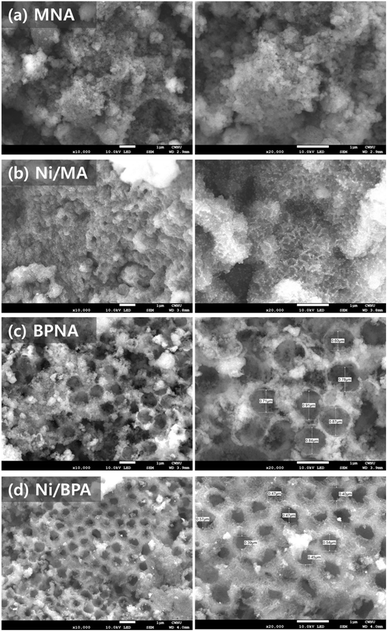
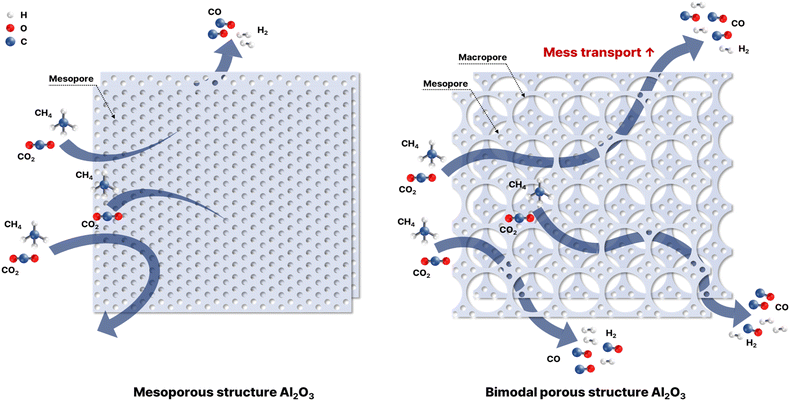
Fig. 2 shows the FE-SEM images of reduced Ni/Al2O3 catalysts at 700 °C prepared using different methods. Mesopores were detected in all the prepared catalysts, whereas macropores were detected in the catalysts with added PS. The presence of mesopores and macropores negates the disadvantages of each type of pore structure. Mesoporous structures can suppress metal agglomeration because uniformly sized pores are regularly arranged. This phenomenon leads to the formation of well-dispersed Ni in the support, which improves its thermal stability.45,46 However, this mesoporous structure can be easily blocked by the deposited carbon produced during the reforming reaction. Macropores show strong resistance to carbon deposition because of their large pore diameter, enhancing the movement of reactants and products and causing low Ni dispersion, which determines the number of active sites.47,48 Therefore, we investigated the preparation of Al2O3 supports with bimodal porous structures composed of mesopores and macropores.26,47,49,50 The MNA and BPNA catalysts had irregular pore structures, consistent with the characteristics of the H3 type, similar to the nitrogen adsorption/desorption isotherms.
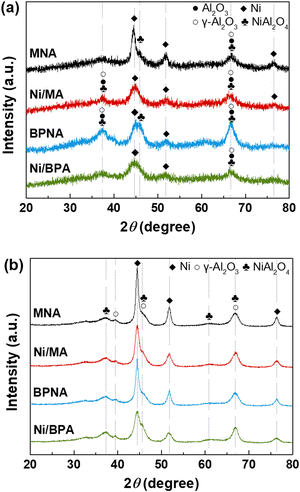
Table 2 shows the characteristics of Ni/Al2O3 catalysts prepared using different methods. It was difficult to calculate the crystallite size of Ni using the Debye–Scherrer equation because the diffraction peak of Ni was small and broad. Therefore, for the comparison of the crystallite sizes of the Ni/Al2O3 catalysts prepared via different methods, XRD analyses were conducted for the Ni/Al2O3 catalysts reduced at 900 °C. The diffraction peak of Ni/Al2O3 catalysts reduced at 900 °C was sharp compared to that of the Ni/Al2O3 catalysts reduced at 700 °C because the higher calcination temperature led to an increase in the crystallite size.22 The Ni crystallite size for reduced Ni/Al2O3 catalysts at 900 °C was as follows: Ni/BPA (3.1 nm) < Ni/MA (3.8 nm) < BPNA (5.0 nm) < MNA (5.2 nm). This result indicates that the Ni/MA and Ni/BPA catalysts have smaller Ni crystal sizes unlike the prepared MNA and BPNA catalysts. Generally, Ni dispersion is inversely proportional to the Ni crystallite size. The trend in Ni dispersion in the Ni/Al2O3 catalysts prepared using different methods, as confirmed by H2-chemisorption, was as follows: MNA (0.42%) < Ni/BPA (0.63%) < Ni/MA (0.70%) < BPNA (1.08%). When considering the reduction degree (RD), the trend of Ni dispersion (RD) was observed to be: MNA (1.43%) < BPNA (1.90%) < Ni/BPA (1.94%) < Ni/MA (2.68%). The BPNA catalyst showed the highest Ni dispersion, but the Ni dispersion considering the reduction degree (RD) was higher in the Ni/MA and Ni/BPA catalysts. It is important to consider the reduction degree of catalysts because metallic Ni is an active site. Especially, NiO in Ni/Al2O3 which is not fully reduced when it is exposed in reaction condition as confirmed by TPR analysis. In addition, the amount of metal Ni formed during the reaction process varies depending on the reduction characteristics of the catalyst. In particular, in catalysts where Ni is introduced sequentially, the strong interaction between Ni and Al2O3 makes reduction relatively difficult, which may lead to a lower H2-chemisorption based Ni dispersion as confirmed by XPS and TPR. Therefore, when analyzing the dispersion, the reduction characteristics must be considered together. This is consistent with the crystal size trend measured by XRD. Dispersion is a key property determining the catalytic activity of Ni-based catalysts for hydrocarbon reforming.54 In addition, highly dispersed Ni enhances carbon deposition resistance and catalytic activity.38,47,55 Therefore, Ni/MA and Ni/BPA catalysts are expected to exhibit excellent catalytic performance.
| Catalyst | Ni crystallite sizea (nm) | Ni dispersionb (%) | Ni dispersion (RD)c (%) |
|---|---|---|---|
| a Calculated from the XRD diffraction peak at 2θ = 52.0° of reduced Ni/Al2O3 catalysts at 900 °C. b Calculated from H2-chemisorption. c Calculated from H2-chemisorption considering the reduction degree. | |||
| MNA | 5.2 | 0.42 | 1.43 |
| Ni/MA | 3.8 | 0.70 | 2.68 |
| BPNA | 5.0 | 1.08 | 1.90 |
| Ni/BPA | 3.1 | 0.63 | 1.94 |
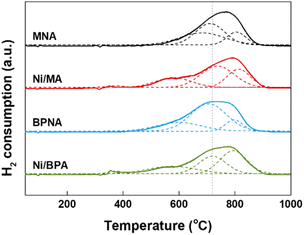
Fig. 4 shows the H2-TPR patterns of the prepared catalysts, which are related to the redox properties, including the reducibility and interaction between Ni and Al2O3. This interaction originates from the existing Ni–O–Al bridges.56,57 Four reduction peaks were observed for the Ni/MA and Ni/BPA catalysts, whereas three peaks were detected for the MNA and BPNA catalysts. According to the literature, the first reduction peak is observed below 500 °C due to the reduction of free NiO, which does not interact with Al2O3.58,59 The second reduction peak appearing around 550 °C is due to the reduction of NiO, which weakly interacts with Al2O3. The third reduction peak appearing around 700 °C was assigned to the reduction of NiO, which strongly interacts with Al2O3. The last reduction peak appearing around 800 °C was related to the reduction of spinel NiAl2O4.58,59 In addition, a reduction peak related to NiO, which strongly interacted with Al2O3 of Ni/MA and Ni/BPA catalysts, was detected at higher temperatures than those for the MNA and BPNA catalysts. This indicates that the addition of Ni is important for determining the interactions between Ni and Al2O3. The intimate interaction between Ni and Al2O3 improves stability by enhancing the resistance of the Ni particles to carbon deposition and sintering.60,61 Therefore, the Ni/MA and Ni/BPA catalysts are expected to be more stable and durable.
Fig. S2 shows the Ni 2p3/2 spectra of the prepared catalysts, which were collected via XPS to explain the chemical properties of the catalyst surfaces. The Ni/Al2O3 catalyst was reduced at 700 °C. Three peaks related to various Ni species and one peak assigned to the satellite peak were observed for all the prepared catalysts. These three peaks can be divided into those of metallic Ni (851.9–852.6 eV), NiO (853–855 eV), and NiAl2O4 (855.6–856.2 eV).52,62–66 In particular, all Ni 2p3/2 peaks were detected at higher binding energies when the Ni/MA and Ni/BPA catalysts were compared with MNA and BPNA. This result indicates that a strong interaction between the active metal and support occurs in the Ni/MA and Ni/BPA catalysts, regardless of the pore structure.46,47,67 This metal–support interaction is known to be an essential factor for enhancing catalytic stability and activity.68,69 In addition, the strong interactions between Ni and Al2O3 in the Ni/MA and Ni/BPA catalysts matched well with the H2-TPR analysis. Table 3 summarizes the amount of each Ni species on the catalyst surface, which was calculated using the peak area of the Ni 2p3/2 spectra. The amount of Ni and NiO species was low when Ni was added to the porous Al2O3 supports, regardless of the pore structure, because the Ni species were inserted into the Al2O3 bulk during the preparation procedure.60 In particular, the proportion of Ni0 species was higher in Ni/MA and Ni/BPA catalysts than in other catalysts. Chen et al. reported that when the proportion of Ni2+ species is excessively high, an excessively strong metal–support interaction (MSI) can encapsulate metal nanoparticles on the support, reducing the exposure of active metal sites. An appropriate level of MSI can promote the generation of Ni0 species during the reduction process, whereas an excessively strong MSI can suppress the reduction of NiO, thereby reducing the amount of metallic Ni.70 Therefore, the high content of Ni0 species and high binding energy observed in the Ni/MA and Ni/BPA catalysts indicate that an appropriate level of MSI was formed that balanced structural stability and reducibility, which is considered to have contributed to the improvement of catalyst performance.
Fig. 5 shows the morphologies of Ni/Al2O3 catalysts of the Ni/Al2O3 catalyst reduced at 700 °C at 200 kV. The EDS elemental mapping results of the Ni/Al2O3 catalysts show the distribution of elements in the catalyst. The analysis revealed that each element was uniformly distributed in all catalysts. However, the Ni distributions of the MNA and BPNA catalysts were weaker than those of the Ni/MA and Ni/BPA catalysts. This was due to the insertion of the Ni species into the Al2O3 bulk during catalyst preparation, as confirmed by the XPS analysis. Among the prepared Ni/Al2O3 catalysts, only rod-like crystallites were observed in the Ni/MA catalyst. The rod-like crystallites of Al2O3 originated from the contact between Al3+ and H2O. For MNA and BPNA, the Ni precursor disrupted the contact between Al3+ and H2O, whereas for Ni/BPA, this contact was inhibited by the PS template. It appears that the addition of the PS template influences the hydrolysis and condensation reactions between aluminum alkoxide and water, leading to the formation of mesopores.26 Therefore, the morphology of the porous Ni/Al2O3 catalyst depends on the amount of H2O added.
3.2. Catalytic performance
Ni/Al2O3 catalysts have been chosen as the general commercial catalysts for the steam reforming (SR) reaction due to their high activity and cost efficiency. In addition, most Ni/Al2O3 catalysts exhibited a high activity in the CDR reaction as shown in Table S2. However, it is performed under severe conditions for the catalysts compared to the SR reaction. A higher reaction temperature is needed due to the dissociation of stable CO2 leads to sintering of the catalyst. In addition, the condition without steam promotes the carbon formation phenomenon. Therefore, it is important to control pore properties which can increase the structural stability and can improve mass transfer to enhance the resistance to sintering and carbon formation. Accordingly, this study is of great significance in that it experimentally verified the effects of high GHSV conditions and double pore structures and preparation methods on CH4 and CO2 conversion, which were not covered in previous studies.To investigate the catalytic performance, the CDR reaction was carried out in a time-on-stream manner, as shown in Fig. 6. The results show higher CO2 conversion compared to CH4 conversion resulting from the reverse water gas shift reaction (CO2 + H2 ⇌ CO + H2O), which is a side reaction to produce CO and H2O by consuming CO2 and H2.71,72 All of the prepared catalysts exhibited stable performance over 20 hours without significant deactivation. As shown in Fig. 6a, the CH4 conversion decreased by approximately 11%, 10%, 10%, and 8% for MNA, Ni/MA, BPNA, and Ni/BPA, respectively. Although MNA appeared relatively stable, it actually exhibited the highest loss in CH4 conversion over time. On the other hand, Ni/BPA showed the smallest decrease and the highest stability. In addition, compared to the deactivation relative rate of catalysts in the literature as summarized in Table S2, the Ni/BPA showed a high ranking. This result indicates that the developed catalyst can be a promising candidate as commercial catalysts for the CDR reaction.
 | ||
| Fig. 6 (a) CH4 conversion, (b) CO2 conversion, and (c) H2/CO ratio of the Ni/Al2O3 catalysts at 700 °C (CDR reaction). | ||
This excellent stability was mainly due to the strong interaction between Ni and support based on the spinel NiAl2O4 structure. Ni/BPA shows the best activity among the prepared catalysts. The initial CH4 and CO2 conversions over the catalyst were 82% and 85%, respectively. This excellent performance resulted from the highly dispersed Ni active sites owing to the strong interaction between Ni and the support, which enhanced the catalytic activity and resistance to carbon deposition and sintering, as confirmed by the various characterization techniques mentioned above. However, the CH4 and CO2 conversions over this catalyst decreased slightly by 75% and 78%, respectively. Therefore, further studies are required to improve the catalytic stability. H2/CO ratio was proportional to CH4 and CO2 conversion. In addition, the CO2 conversion rates of the BPNA and MNA catalysts showed relatively large fluctuations. This is because the CO2 conversion rate can fluctuate more easily than the CH4 conversion rate due to the influence of various side reactions such as reverse water gas shift and methanation reactions.73–75 On the other hand, since the H2/CO ratio is mainly determined by the main CDR reaction, it tends to show a similar tendency to the CH4 conversion rate. The CO2 conversion rate can be varied due to side reactions such as the RWGS reaction, but the overall H2/CO ratio remains relatively stable. This result might be due to the reverse water gas shift reaction and methanation when CH4 conversion was low.76–78
To evaluate the long-term stability of the Ni/BPA catalyst, which exhibited the highest initial activity, the time-on-stream experiment was extended to 100 h, as shown in Fig. S3. The CH4 and CO2 conversion rates decreased by approximately 16% and 14%, from initial conversions of 82% and 85% to 66% and 71% after 100 h, respectively. In the long-term stability test, the activity of the Ni/BPA catalyst tended to decrease slightly with the time on stream despite the high GHSV condition of 900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL g−1 h−1. This indicates that the catalyst has excellent stability even under long-term operating conditions of an industrial scale reforming process.
000 mL g−1 h−1. This indicates that the catalyst has excellent stability even under long-term operating conditions of an industrial scale reforming process.
Fig. S4 shows the XRD patterns of spent Ni/Al2O3 catalysts which were recovered after the CDR reaction for 20 h. The diffraction peaks assigned to the metallic Ni, Al2O3, and NiAl2O4 species are observed in the same position as shown in Fig. 3. The Ni crystallite size of spent catalysts was calculated by the Debye–Scherrer equation. The crystallite size trend of spent catalysts was as follows: Ni/BPA (3.1 nm) < Ni/MA (3.5 nm) < MNA (5.4 nm) < BPNA (6.4 nm). As above mentioned, the Ni crystallite size which was impossible to calculate due to the small size was detected in all the prepared catalysts. Therefore, the sintering phenomenon is observed in all the catalysts. This increasing trend of crystallite size results from the interaction between Ni and Al2O3, as verified by H2-TPR analysis. Interestingly, characteristic peak at 2θ = 26° was detected for all catalysts, which was attributed to graphite carbon species deposited from the methane in the CDR reaction.79,80 In addition, it was confirmed that the presence of graphite carbon species peaks in all catalysts contributed to the decrease in CH4 and CO2 conversion rates from the CDR reaction by covering the active sites in the surface of catalysts. The peak relating to the carbon species of Ni/MA and Ni/BPA catalysts was relatively smaller than that of MNA and BPNA catalysts. The smaller peak of carbon species is due to the small amount of deposited carbon. Among the prepared catalysts, the Ni/BPA catalyst with the smallest graphite carbon peak showed great resistance to carbon deposition due to its bimodal porous structure, improving catalyst stability and activity for the CDR reaction.
TGA analysis was performed to quantify the amount of deposited carbon on the surface of used Ni/Al2O3 catalysts after the CDR reaction and the results are shown in Fig. S5. According to the literature, the weight loss observed at about 220 °C is mainly due to moisture removal.22,72,81,82 The slight increase from 220 °C to about 400 °C is due to the generation of NiO by oxidation of the reduced metallic Ni. The reduction in weight loss above 600 °C is due to the oxidation of graphite carbon. The weight loss decreased in the following order: MNA (25.5%) > BPNA (19.1%) > Ni/MA (12.8%) > Ni/BPA (5.6%). The low weight loss of Ni/BPA and Ni/MA catalysts is because the Ni active point is highly dispersed by the strong interaction between Ni and Al2O3, improving carbon deposition resistance.28 As a result of comparing the catalysts prepared by the same preparation method, the catalyst with the bimodal structure showed lower weight loss than the catalyst with the mesoporous structure. This is because the adsorption of –OH2 or –OH molecules occurred smoothly and interfered with the adsorption of carbon species, and it is confirmed that relatively little carbon deposition occurred.83 As a result, the bimodal structure and the addition of Ni are important to design the Ni/Al2O3 catalyst with high resistance to carbon deposition. Furthermore, the resistance to carbon deposition is strongly influenced by the Ni crystallite size and the strength of the metal–support interaction. It is confirmed that the Ni introduction played a crucial role in determining these characteristics using XRD, H2-TPR, and H2 chemisorption analyses. A smaller Ni crystal size leads to higher dispersion of active sites, while a stronger Ni–Al2O3 interaction suppresses the sintering of Ni particles and contributes to improved catalytic stability in the CDR reaction. Therefore, by optimizing the Ni incorporation strategy, these critical structural parameters can be controlled, thereby effectively minimizing carbon deposition even under severe reaction conditions.
Fig. S6 shows Raman spectra used to analyze the type of carbon species deposited on the surface of the catalyst after the CDR reaction. The graphite carbon causes more serious deactivation problems in the catalysts, compared to disordered carbon. The D band and G band are observed at 1300–1350 cm−1 and 1500–1600 cm−1, respectively.29 The D band is related to disordered carbon, and the G band is related to the stretching vibration of sp2 of ordered carbon and graphite carbon species.84,85 Therefore, the ID/IG representing the intensity ratio of the D band and the G band represents the degree of disorder and graphitization, and the higher the ID/IG, the higher the degree of disorder and the lower the graphitization degree.47 The ID/IG ratios for all catalysts were in the following order: Ni/BPA (1.54) > Ni/MA (1.43) > BPNA (1.38) > MNA (1.37). It was confirmed that the carbon species deposited on the Ni/BPA catalyst after the reaction had a relatively low graphitization degree compared to other catalysts. Lyu et al. reported that the effect of the bimodal porous structure improves mass transfer and quickly removes carbon deposition, reducing the conversion of amorphous carbon to graphite carbon.46 In addition, the low peak intensity of the carbon species of the Ni/BPA catalyst after the CDR reaction is lower than that of other catalysts, which means that the content of carbon deposition is small.47 Therefore, it was confirmed that the bimodal porous structure and Ni addition control are influencing factors in the design of Ni/Al2O3 catalysts with high resistance to carbon deposition.
The FE-TEM images of the spent catalysts after TOS studies were obtained and are shown in Fig. S7. In Fig. S7(a–c), numerous deposited carbons were observed in the spent catalysts.22 In particular, the encapsulated carbon which covered with Ni particles was detected in the MNA and BPNA catalysts, which causes the deactivation of the catalyst. In addition, it can be clearly confirmed that the size of the Ni particles has increased compared to before the reaction, and it is considered that sintering has occurred. On the other hand, no carbonaceous deposits were observed in the Ni/BPA catalyst. This result is not fully consistent with the TGA and Raman results. This might be due to the small amount of deposited carbon which is difficult to detect with TEM analysis.
When carbon atoms diffuse to the interface adjacent to the support through Ni particles, a filamentous carbon structure is formed.86 The proper growth of the filament can prevent encapsulation and prolong catalytic activity.86 However, encapsulation blocks the active sites, resulting in final catalytic deactivation.86 This is related to the metal–support interaction, and it is considered that the Ni/BPA catalyst blocked the growth of carbon due to the strong interaction between Ni and alumina support.87 Also, most deposited carbon was filamentous carbon as confirmed by TEM images. It was reported that the effect of this carbon is not bigger than that of encapsulated carbon or sintering phenomenum.86,88,89 When large amount of it was generated, the activity can be dramatically decreased by blocking gas flow in the reactor. In our study, the main reason for the deactivation might be sintering of active sites.
Fig. S8 shows the reaction rates at different CH4 and CO2 partial pressures at various reaction temperatures. It was clearly found that the higher the reaction temperature, the higher the CH4 and CO2 consumption rates. This result is mainly because the CDR reaction is thermodynamically preferred at a higher reaction temperature. The higher CO2 consumption rate compared to CH4 might be partly attributed to the contribution of the RWGS reaction and the relatively easier activation of CO2. Furthermore, the difference between CO2 and CH4 consumption rates became more pronounced at higher temperatures, possibly due to the enhanced RWGS activity and faster CO2 activation kinetics under such conditions.90 The consumption rates of both CH4 and CO2 increased with an increase in either CH4 or CO2 partial pressure, indicating that higher reactant concentrations promoted the CDR reaction. The CO2 consumption rate tended to increase more rapidly than that of CH4 with increasing CH4 or CO2 partial pressure, which can be attributed to the relatively easier activation of CO2 compared to the dissociation of CH4.91
3.3. Proposed effect of the porous structure and addition of Ni on the catalytic performance
4. Conclusions
In this study, different preparation methods were used to determine the pore structure and interactions between Ni and Al2O3. Mesoporous and bimodal porous Ni/Al2O3 catalysts were prepared to compare their pore properties, and the addition of the Ni precursor was controlled to compare the interactions between Ni and Al2O3. The Ni/Al2O3 catalysts with Ni added after the calcination of the support (Ni/MA, Ni/BPA) exhibited higher catalytic performance than those with Ni added during support preparation (MNA, BPNA). Furthermore, the bimodal porous catalyst (Ni/BPA) outperformed its mesoporous counterpart (Ni/MA). In addition, the bimodal porous Ni/Al2O3 catalyst (Ni/BPA) showed better catalytic performance than the mesoporous Ni/Al2O3 catalyst (Ni/MA). This result indicates that the macropores in bimodal porous Ni/Al2O3 contribute to enhanced catalytic performance by improving the mass transfer. This was because the amount of Ni inserted into bulk Al2O3 was higher when the precursors of Ni and Al2O3 were mixed during the preparation procedure. The Ni/BPA catalyst exhibited the highest catalytic performance for the CDR reaction. The remarkable performance of this catalyst was mainly due to the high mass transfer and proper interaction between Ni and Al2O3. However, the catalytic activity decreased slightly with time-on-stream. Therefore, further studies are required to control the pore properties of the bimodal porous Ni/Al2O3 catalysts. In addition, the Ni loading effect should be investigated because Ni loading affects the pore properties such as surface area, pore size, and pore volume and reduction properties including interaction between Ni and Al2O3.Author contributions
Ji-Won Son: writing – original draft, investigation, data curation. Hak-Min Kim: writing – original draft, writing – review & editing, investigation, validation. Jae-Min Song: methodology, visualization. Beom-Su Cheon: methodology, visualization. Dae-Woon Jeong: conceptualization, supervision, resources, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional tables and figures presenting the kinetic experiment design, catalyst characterization results (BET, XPS, XRD, TGA, Raman, and TEM), and stability test data supporting the findings of this study. See DOI: https://doi.org/10.1039/d5ta07037f.Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20214000000090, Fostering human resources training in advanced hydrogen energy industry). This work was supported by the National Research Foundation of Korea (NRF) and the Commercialization Promotion Agency for R&D Outcomes (COMPA) grant funded by the Korea government (Ministry of Science and ICT) (RS-2024-00432910). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00214068).Notes and references
- S. Sun, C. Zhang, Y. Wang, X. Zhao, H. Sun and C. Wu, Chem. Eng. J., 2023, 468, 143712 CrossRef CAS
.
- J. Wang and W. Azam, Geosci. Front., 2024, 15, 101757 CrossRef CAS
.
- L. Aleluia Reis and M. Tavoni, iScience, 2023, 26, 105933 CrossRef
.
- D. J. van de Ven, S. Mittal, A. Gambhir, R. D. Lamboll, H. Doukas, S. Giarola, A. Hawkes, K. Koasidis, A. C. Köberle, H. McJeon, S. Perdana, G. P. Peters, J. Rogelj, I. Sognnaes, M. Vielle and A. Nikas, Nat. Clim. Change, 2023, 13, 570–578 CrossRef
.
- E. Hille, Energy Econ., 2023, 127, 107061 CrossRef
.
- D. Eze Ekechukwu, O. Ogundipe, A. Esiri, A. Heavens Ikevuje, J. Mensah Kwakye, O. Benjamin Ogundipe and A. Emuobosa Esiri, Artic. Open Access Res. J. Multidiscip. Stud., 2024, 2024, 94–104 Search PubMed
.
- T. Capurso, M. Stefanizzi, M. Torresi and S. M. Camporeale, Energy Convers. Manag., 2022, 251, 114898 CrossRef CAS
.
- M. Younas, S. Shafique, A. Hafeez, F. Javed and F. Rehman, Fuel, 2022, 316, 123317 CrossRef CAS
.
- M. J. Park, H. M. Kim, Y. J. Gu and D. W. Jeong, Energy, 2023, 265, 126273 CrossRef CAS
.
- M. J. Park, H. M. Kim, Y. H. Lee, K. W. Jeon and D. W. Jeong, Waste Manag., 2022, 144, 272–284 CrossRef CAS
.
- H. Wang, X. Zhu, A. Adogwa, Y. Shen, M. Yang and T. B. Lu, Chem. Eng. J., 2024, 493, 152501 CrossRef CAS
.
- D. H. Le Phuong, M. Alsaiari, C. Q. Pham, N. H. Hieu, T. P. T. Pham, N. Rajamohan, D. D. Pham, D. V. N. Vo, T. H. Trinh, H. D. Setiabudi, D. L. T. Nguyen and T. M. Nguyen, J. Taiwan Inst. Chem. Eng., 2024, 155, 105253 CrossRef
.
- R. Gao, C. Zhang, Y. J. Lee, G. Kwak, K. W. Jun, S. K. Kim, H. G. Park and G. Guan, J. Clean. Prod., 2020, 272, 122552 CrossRef CAS
.
- P. K. Gupta, V. Kumar and S. Maity, J. Chem. Technol. Biotechnol., 2020, 96, 853–868 CrossRef
.
- J. J. Torrez-Herrera, S. A. Korili and A. Gil, Chem. Eng. J. Adv., 2021, 5, 100080 CrossRef CAS
.
- S. Yuan, Y. Ge, P. Su, H. Cheng, Z. Wang and F. Yu, J. Environ. Chem. Eng., 2024, 12, 112308 CrossRef CAS
.
- M. R. B. Pirshahid, S. M. Alavi, M. Rezaei, E. Akbari, M. Varbar and K. Khosravi, J. Energy Inst., 2023, 110, 101361 CrossRef CAS
.
- A. Choya, B. de Rivas, M. L. No, J. I. Gutiérrez-Ortiz and R. López-Fonseca, Fuel, 2024, 358, 130166 CrossRef CAS
.
- M. Dan, M. Mihet, G. Borodi and M. D. Lazar, Catal. Today, 2021, 366, 87–96 CrossRef CAS
.
- C. A. Schwengber, F. A. Da Silva, R. A. Schaffner, N. R. C. Fernandes-Machado, R. J. Ferracin, V. R. Bach and H. J. Alves, J. Environ. Chem. Eng., 2016, 4, 3688–3695 CrossRef CAS
.
- W. S. Dong, H. S. Roh, K. W. Jun, S. E. Park and Y. S. Oh, Appl. Catal., A, 2002, 226, 63–72 CrossRef CAS
.
- Z. Bian, W. Zhong, Y. Yu, Z. Wang, B. Jiang and S. Kawi, Int. J. Hydrogen Energy, 2021, 46, 31041–31053 CrossRef CAS
.
- H. Yi, Q. Xue, S. Lu, J. Wu, Y. Wang and G. Luo, Fuel, 2022, 315, 123262 CrossRef CAS
.
- V. Y. Bychkov, Y. P. Tyulenin, A. A. Firsova, E. A. Shafranovsky, A. Y. Gorenberg and V. N. Korchak, Appl. Catal., A, 2013, 453, 71–79 CrossRef CAS
.
- S. Hu, M. Xue, H. Chen and J. Shen, Chem. Eng. J., 2010, 162, 371–379 CrossRef CAS
.
- E. Im, H. J. Seo, D. I. Kim, D. C. Hyun and G. D. Moon, Chem. Eng. J., 2021, 416, 129147 CrossRef CAS
.
- Z. Y. Lim, J. Tu, F. Zhou, B. Chen and K. L. Choy, Appl. Catal., B, 2024, 350, 123891 CrossRef CAS
.
- K. Tao, Y. Zhang, S. Terao, Y. Yoneyama, T. Kawabata, K. Matsuda, S. Ikeno and N. Tsubaki, Chem. Eng. J., 2011, 170, 258–263 CrossRef CAS
.
- L. Lyu, Y. Han, Q. Ma, S. Makpal, J. Sun, X. Gao, J. Zhang, H. Fan and T.-S. Zhao, Catalysts, 2020, 10, 1220 CrossRef CAS
.
- A. H. K. Owgi, A. A. Jalil, I. Hussain, N. S. Hassan, H. U. Hambali, T. J. Siang and D. V. N. Vo, Environ. Chem. Lett., 2021, 19, 2157–2183 CrossRef CAS
.
- F. Wen, C. Xu, N. Huang, T. Wang, X. Sun, H. Li, R. Zhang and G. Xia, Int. J. Hydrogen Energy, 2024, 69, 1481–1491 CrossRef CAS
.
- I. C. Sophiana, S. Steven, A. Revieri, A. Permatasari, R. Andika, N. Nishiyama and B. H. Susanto, Case Stud. Chem. Environ. Eng., 2025, 11, 101078 CrossRef CAS
.
- M. S. Mel’gunov, Adsorption, 2023, 29, 199–208 CrossRef
.
- D. W. Jeong, A. Jha, W. J. Jang, W. B. Han and H. S. Roh, Chem. Eng. J., 2015, 265, 100–109 CrossRef CAS
.
- J. Wang, X. Wang and X. Zhang, J. Mater. Chem. A, 2017, 5, 4308–4313 RSC
.
- L. Xu, J. Zhang, J. Ding, T. Liu, G. Shi, X. Li, W. Dang, Y. Cheng and R. Guo, Miner, 2020, 10, 72 CAS
.
- S. Jayaprakash, N. Dewangan, A. Jangam, S. Das and S. Kawi, Int. J. Hydrogen Energy, 2021, 46, 18338–18352 CrossRef CAS
.
- K. Tao, L. Shi, Q. Ma, D. wang, C. Zeng, C. Kong, M. Wu, L. Chen, S. Zhou, Y. Hu and N. Tsubaki, Chem. Eng. J., 2013, 221, 25–31 CrossRef CAS
.
- R. Daroughegi, F. Meshkani and M. Rezaei, Int. J. Hydrogen Energy, 2017, 42, 15115–15125 CrossRef CAS
.
- C. Italiano, J. Llorca, L. Pino, M. Ferraro, V. Antonucci and A. Vita, Appl. Catal., B, 2020, 264, 118494 CrossRef CAS
.
- N. D. Charisiou, L. Tzounis, V. Sebastian, S. J. Hinder, M. A. Baker, K. Polychronopoulou and M. A. Goula, Appl. Surf. Sci., 2019, 474, 42–56 CrossRef CAS
.
- X. Dong, W. Wang, G. Yang and L. Wang, Ind. Eng. Chem. Res., 2022, 61, 4255–4263 CrossRef CAS
.
- Z. Song, H. Chen, S. Bao, Z. Xie, Q. Kuang and L. Zheng, J. Mater. Chem. A, 2020, 8, 3754–3762 RSC
.
- R. Kumar, S. Shah, J. Bahadur, Y. B. Melnichenko, D. Sen, S. Mazumder, C. P. Vinod and B. Chowdhury, Microporous Mesoporous Mater., 2016, 234, 293–302 CrossRef CAS
.
- W. Gao, Q. Yin, X. Meng, X. He and Z. Xin, Fuel, 2022, 310, 121813 CrossRef CAS
.
- L. Lyu, M. Shengene, Q. Ma, J. Sun, X. Gao, H. Fan, J. Zhang and T. S. Zhao, Fuel, 2022, 310, 122375 CrossRef CAS
.
- Q. Ma, Y. Han, Q. Wei, S. Makpal, X. Gao, J. Zhang and T. S. Zhao, J. CO2 Util., 2020, 35, 288–297 CrossRef CAS
.
- C. Ma, J. Gong, S. Zhao, X. Liu, X. Mu, Y. Wang, X. Chen and T. Tang, Green Energy Environ., 2022, 7, 818–828 CrossRef CAS
.
- B. Li, X. Lin, Y. Luo, X. Yuan and X. Wang, Fuel Process. Technol., 2018, 176, 153–166 CrossRef CAS
.
- Y. Chai, Y. Fu, H. Feng, W. Kong, C. Yuan, B. Pan, J. Zhang and Y. Sun, ChemCatChem, 2018, 10, 2078–2086 CrossRef CAS
.
- Z. Bao, Y. Lu, J. Han, Y. Li and F. Yu, Appl. Catal., A, 2015, 491, 116–126 CrossRef CAS
.
- N. Pham-Ngoc, A. Jamsaz, M. Wang and E. Woo Shin, Chem. Eng. J., 2024, 494, 153207 CrossRef CAS
.
- J. Zhang, Z. Xiao, L. Wang, X. Zhang and G. Li, ChemistrySelect, 2022, 7, e202203339 CrossRef CAS
.
- J. J. Torrez-Herrera, S. A. Korili and A. Gil, Catal. Rev., 2023, 65, 1300–1357 CrossRef CAS
.
- F. Rahbar Shamskar, M. Rezaei and F. Meshkani, Int. J. Hydrogen Energy, 2017, 42, 4155–4164 CrossRef CAS
.
- W. Han, P. Yuan, Y. Fan, G. Shi, H. Liu, D. Bai and X. Bao, J. Mater. Chem., 2012, 22, 25340–25353 RSC
.
- F. E. Massoth, J. Catal., 1975, 36, 164–184 CrossRef CAS
.
- S. V. Moghaddam, M. Rezaei and F. Meshkani, Energy
Technol., 2020, 8, 1900778 CrossRef CAS
.
- Y. Bae and J. Hong, Chem. Eng. J., 2022, 446, 136978 CrossRef CAS
.
- S. Xu, T. J. A. Slater, H. Huang, Y. Zhou, Y. Jiao, C. M. A. Parlett, S. Guan, S. Chansai, S. Xu, X. Wang, C. Hardacre and X. Fan, Chem. Eng. J., 2022, 446, 137439 CrossRef CAS
.
- Z. Wang, Z. Mei, L. Wang, Q. Wu, C. Xia, S. Li, T. Wang and C. Liu, J. Mater. Chem. A, 2024, 12, 24802–24838 RSC
.
- P. Liu, J. Ran, B. Xia, S. Xi, D. Gao and J. Wang, Nano–Micro Lett., 2020, 12, 1–12 CrossRef PubMed
.
- H. J. Byeon, K. W. Jeon, H. M. Kim, Y. H. Lee, Y. S. Heo, M. J. Park and D. W. Jeong, Fuel Process. Technol., 2022, 231, 107229 CrossRef CAS
.
- S. Ali, M. M. Khader, M. J. Almarri and A. G. Abdelmoneim, Catal. Today, 2020, 343, 26–37 CrossRef CAS
.
- D. Gallego-García, U. Iriarte-Velasco, M. A. Gutiérrez-Ortiz and J. L. Ayastuy, Appl. Catal., B, 2024, 344, 123671 CrossRef
.
- S. Yuan, Y. Yang, Z. Xiong, P. Guo, S. Sun, Z. Li, J. Du and Y. Gao, Green Energy Environ., 2024, 9, 321–332 CrossRef CAS
.
- Z. Liu, W. Han, D. Hu, S. Sun, A. Hu, Z. Wang, Y. Jia, X. Zhao and Q. Yang, J. Catal., 2020, 387, 62–72 CrossRef CAS
.
- H. Wang, Z. Fang, Y. Wang, K. Meng and S. Sun, J. Alloys Compd., 2024, 986, 174006 CrossRef CAS
.
- J. Chen, Y. Zhang, Z. Zhang, D. Hou, F. Bai, Y. Han, C. Zhang, Y. Zhang and J. Hu, J. Mater. Chem. A, 2023, 11, 8540–8572 RSC
.
- Y. Chen, S. Zhu, J. Zhou, Y. Wang, Z. Zhang, N. Liu, R. Meng and S. He, Int. J. Hydrogen Energy, 2024, 96, 309–320 CrossRef
.
- M. G. Jeong, S. Y. Kim, D. H. Kim, S. W. Han, I. H. Kim, M. Lee, Y. K. Hwang and Y. D. Kim, Appl. Catal., A, 2016, 515, 45–50 CrossRef CAS
.
- M. J. Park, J. H. Kim, Y. H. Lee, H. M. Kim and D. W. Jeong, Int. J. Hydrogen Energy, 2020, 45, 30188–30200 CrossRef CAS
.
- V. A. Panchenko, Y. V. Daus, A. A. Kovalev, I. V. Yudaev and Y. V. Litti, Int. J. Hydrogen Energy, 2023, 48, 4551–4571 CrossRef CAS
.
- M. Tommasi, S. N. Degerli, G. Ramis and I. Rossetti, Chem. Eng. Res. Des., 2024, 201, 457–482 CrossRef CAS
.
- M. Ayoub, C. C. Chong, A. Zamir, Y. W. Cheng, S. Farrukh, S. R. Naqvi, H. D. Setiabudi, N. Riaz and N. Ramzan, E3S Web Conf., 2021, 287, 1–8 CrossRef
.
- S. Tada, A. Yanagita, N. Shimoda, T. Honma, M. Takahashi, A. Nariyuki and S. Satokawa, J. Jpn. Pet. Inst., 2018, 61, 80–86 CrossRef CAS
.
- S. Tada, D. Shoji, K. Urasaki, N. Shimoda and S. Satokawa, Catal. Sci. Technol., 2016, 6, 3713–3717 RSC
.
- N. Shimoda, D. Shoji, K. Tani, M. Fujiwara, K. Urasaki, R. Kikuchi and S. Satokawa, Appl. Catal., B, 2015, 174–175, 486–495 CrossRef CAS
.
- S. S. Negi, H. M. Kim, B. S. Cheon, C. H. Jeong, H. S. Roh and D. W. Jeong, ACS Appl. Mater. Interfaces, 2023, 15, 51013–51024 CrossRef CAS PubMed
.
- Y. Shi, S. Wang, Y. Li, F. Yang, H. Yu, Y. Chu, T. Li and H. Yin, Materials, 2022, 15, 3044 CrossRef CAS PubMed
.
- J. Niu, S. E. Liland, J. Yang, K. R. Rout, J. Ran and D. Chen, Chem. Eng. J., 2019, 377, 119763 CrossRef CAS
.
- Y. Gao, Q. Li, C. Wang, D. Yan, J. Chen and H. Jia, J. Mater. Chem. A, 2022, 10, 16016–16028 RSC
.
- Q. Zhang, Z. Jiang, Y. Zhang, X. Xu, Y. Yang, Y. Qin, L. Song, Y. Mei and Y. Zu, Appl. Catal., B, 2025, 362, 124722 CrossRef CAS
.
- S. Sun, Y. Wang, Y. Xu, H. Sun, X. Zhao, Y. Zhang, X. Yang, X. Bie, M. Wu, C. Zhang, Y. Zhu, Y. Xu, H. Zhou and C. Wu, Appl. Catal., B, 2024, 348, 123838 CrossRef CAS
.
- F. Rosenburg, E. Ionescu, N. Nicoloso and R. Riedel, Mater, 2018, 11, 93 CrossRef
.
- M. A. Abdel-Fatah and A. Amin, Int. J. Hydrogen Energy, 2025, 137, 236–246 CrossRef CAS
.
- P. K. Chaudhary and G. Deo, Colloids Surf., A, 2022, 646, 128973 CrossRef CAS
.
- A. Serrano-Lotina and L. Daza, Appl. Catal., A, 2014, 474, 107–113 CrossRef CAS
.
- J. Xu, W. Zhou, J. Wang, Z. Li and J. Ma, Chin. J. Catal., 2009, 30, 1076–1084 CrossRef CAS
.
- W. J. Jang, D. W. Jeong, J. O. Shim, H. M. Kim, H. S. Roh, I. H. Son and S. J. Lee, Appl. Energy, 2016, 173, 80–91 CrossRef CAS
.
- Z. Bao, L. Yongwu and Y. Fei, AIChE J., 2017, 63, 2019–2029 CrossRef CAS
.
Footnote |
| † This author contributed equally as the first author. |
| This journal is © The Royal Society of Chemistry 2026 |