Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pcu topology metal–organic framework, Ni(1,4-bib)(inca)2, that exhibits high CO2/N2 selectivity and low water vapour affinity†
Samuel M.
Shabangu
,
Alan C.
Eaby
 ,
Sousa
Javan Nikkhah
,
Sousa
Javan Nikkhah
 ,
Lilia
Croitor
,
Tao
He
,
Andrey A.
Bezrukov
,
Lilia
Croitor
,
Tao
He
,
Andrey A.
Bezrukov
 ,
Matthias
Vandichel
,
Matthias
Vandichel
 and
Michael J.
Zaworotko
and
Michael J.
Zaworotko
 *
*
Department of Chemical Sciences, Bernal Institute, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: xtal@ul.ie
First published on 29th April 2025
Abstract
Herein we report the synthesis of a new metal–organic framework, Ni(1,4-bib)(inca)2 or pcu-1-Ni, where 1,4-bib = 1,4-bis(imidazole-1-yl)benzene, inca = indazole-5-carboxylic acid, through the crystal engineering strategy of using an N-donor linker to pillar a square lattice, sql, topology net. pcu-1-Ni adopts pcu topology and features two types of hydrophobic pore, small pore A and large pore B. The biporous nature of pcu-1-Ni is reflected in its stepped CO2 and H2O adsorption isotherms, highlighting the influence of pore size and chemistry on gas and water vapour sorption properties. pcu-1-Ni exhibits the unusual combination of high CO2/N2 selectivity (IAST selectivity 100–250) and low water affinity at low RH (an S-shaped water vapour isotherm with an inflection point at 45–65% RH). Whereas pcu-1-Ni degrades upon repeated exposures to water vapour, its structure–property relationships can provide guidance for design of the next generation of CO2-selective sorbents. In this context, Canonical Monte Carlo simulations provide insight into the preferential adsorption of CO2 over N2 and H2O.
Introduction
Metal–organic materials (MOMs),1,2 including metal–organic frameworks (MOFs)3,4 and porous coordination polymers (PCPs),5,6 are a class of porous materials that have attracted attention due to their amenability to design based on crystal engineering7,8 principles. This strategy has enabled the development of MOFs with potential utility for direct air capture of CO2,9,10 separation of CO2 from N2 (e.g. flue gas remediation),11,12 atmospheric water harvesting13,14 as well as indoor humidity control (IHC).15,16 Flue gas remediation is often mitigated by the presence of other components in gas feeds.17 For example, the presence of water vapour in downstream feedstocks poses a challenge because water adsorption can overwhelm CO2 affinity in MOFs,18 even in the most CO2 selective sorbents, such as hybrid ultramicroporous materials, HUMs.19 This is largely because most binding sites that are highly selective for CO2 over N2 also bind to H2O.20 Therefore, designing MOFs with high CO2 selectivity while controlling water adsorption remains a critical design challenge in crystal engineering.The node-and-linker approach to design of coordination networks introduced by Hoskins and Robson8 provides an effective strategy to crystal engineering (design) of structural motifs that are suitable for the control and fine-tuning of properties. This approach offers several prominent structural motifs with square lattice (sql, 2D)21 and primitive cubic (pcu, 3D)22 topologies being amongst the most prevalent families of coordination networks.23 Many pcu networks are constructed from mixed-linker systems, where two (or more) linkers are integrated within a structure, thereby providing much more compositional diversity than is possible with a single linker.24,25 Commonly occurring networks are those comprised of sheets with composition ML2 (L = linker ligand) pillared by inorganic26 or ditopic N-donor linkers,27 L′, resulting in 3D networks of formula ML2L’. An archetypal example of the former is SIFSIX-1-Zn,28 a HUM comprised from sql layers pillared by hexafluorosilicate (SiF62−, “SIFSIX”) dianionic linkers.26,28,29 By systematically varying the inorganic pillar, second generation materials with improved properties such as NbOFFIVE-1-Ni (ref. 30) and TIFSIX-3-Ni (ref. 31) were readily generated. Another commonly exploited type of pillar is represented by N-donor linker ligands such as pyrazine and dipyridyl-type linker ligands (e.g. 4,4′-bipyridine).32,33 The prototypical example of such “pillared-layered” MOFs is DMOF-1,32 in which the dicarboxylate linker, 1,4-benzenedicarboxylate, forms sql layers from paddlewheel dinuclear Zn2 units that are pillared by diazabicyclooctane to generate the resulting pcu topology MOF. By varying the organic N-donor pillar linkers, numerous variants of DMOF-1 have been developed such as MOF-508 (ref. 34) and X-pcu-n-Zn (ref. 33) (X = extended ligand or “X-ligand”), examples of which have demonstrated potential utility in hydrocarbon separation.35
The linker ligands used for pcu frameworks not only affect pore chemistry and sorption properties, they can also facilitate interpenetrated or non-interpenetrated structures.36 Interpenetration can be enabled by the use of longer linkers, and necessarily reduces porosity.36,37 Conversely, when one or both linkers are relatively short, this can reduce the level or even the existence of interpenetration.36 Nevertheless, reduced porosity does not necessarily equate to poor properties as ultramicroporous interpenetrated materials can offer enhanced gas separation properties that surpass the performance of non-interpenetrated analogues as seen for SIFSIX-2-Cu and SIFSIX-2-Cu-i (i = interpenetrated).36,38
N-donor linker ligands are not limited to those based upon pyridyl moieties. 1,4-Bis(imidazole-1-yl)benzene, 1,4-bib, is of interest to us and others as it can adopt both cis and trans configurations, which result in unusual network structures and enhanced sorption properties.39–42 1,4-Bib can also afford 3D porous structures with dia (ref. 40 and 41) or pcu (ref. 42 and 43) topology, both being widely studied platforms for the evaluation of sorption properties.44,45 However, the use of 1,4-bib in the construction of 3D pcu MOFs remains underexplored. Indeed, our survey of the 3D MOF subset of the Cambridge Structural Database (CSD), focusing on 1,4-bib coordinated to transition metal ions, identified 238 3D network structures with pcu topology, among which only 34 exhibit potential porosity. Notably, the water vapour sorption properties of these MOFs have not been evaluated, including key performance parameters such as sorption kinetics and recyclability, representing a significant gap in the study of these pcu topology MOFs.
Herein we report on the use of 1,4-bib with indazole-5-carboxylic acid (inca). The latter features both carboxyl and azole groups and has been previously used for the design of sql layers that can be pillared into pcu MOFs.46 H2inca can exist as either a monoanionic linker (Fig. 1a) or a dianionic linker (Fig. 1b), facilitating the construction of pcu architectures when paired with pillaring N-donor linkers as exemplified by {[Zn(inca)(pbptz) and Zn(inca)(4,4-bipy)], pbptz = 3,6-bis(4-pyridyl)-1,2,4,5-tetrazine, 4,4-bipy = 4,4-bipyridine}.46 Our survey of the MOF subset of the CSD revealed only 10 hits based on inca, 6 of which form sql networks when inca is a monoanionic linker (REFCODES: RUHKEN, RUHKEN01, ZIJHUZ, ZIJJEL, NUYNED and SEGBIR)(Table S1†). When present as a dianionic linker, inca can facilitate the creation of 3D porous structures as a single linker (REFCODE: SEGBOX and ELENOB) or sql layers pillared by N-donor linkers (REFCODES: BUTZUO and BUVBAY) (Table S1†). To date, previous reports based on this ligand have addressed gas sorption properties (Table S1†).46–49 Analysis of these studies revealed that only high pressure 273 K CO2 isotherms have been reported in BUTZUO and BUVBAY (Table S1†). To the best of our knowledge, no studies have reported combination of inca and 1,4-bib to construct 3D ‘pillared sheet’ platforms and evaluate their gas and water vapour sorption properties, the matter we address herein.
Results and discussion
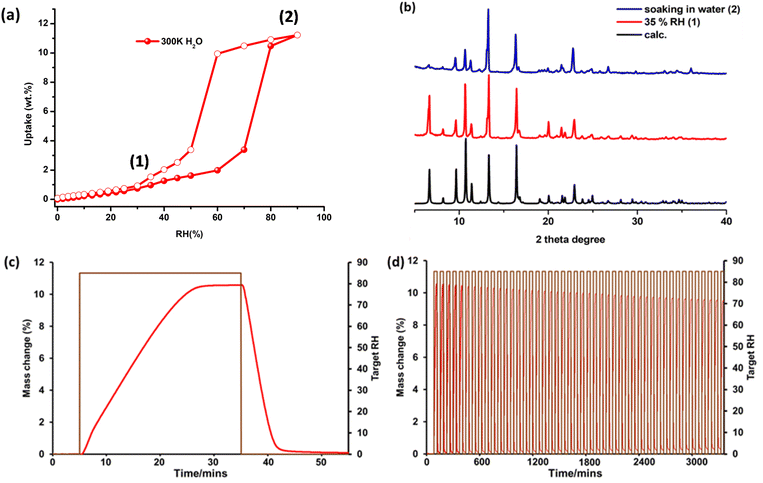
Solvothermal reaction of 1,4-bib, inca and Ni(NO3)2·6H2O afforded green block-shaped single crystals of the as-synthesised form, pcu-1-Ni (see ESI† for Experimental details). Single-crystal X-ray diffraction (SCXRD) experiments revealed that pcu-1-Ni had crystallised in the monoclinic space group P21/c (Table S2†). The asymmetric unit is comprised of two (inca)− ligands and one 1,4-bib ligand (Fig. 2a). The structure can be described as an sql network formed by Ni(II) atoms linked by monoanionic inca ligands (Fig. 2b) that is pillared by 1,4-bib linker ligands to form a 3D pcu topology framework (Fig. 2c) with 2-fold interpenetration (Fig. 2d). Along the c-axis, there are two types of ultramicropore, A and B, with effective pore windows of 3.0 Å × 6.8 Å and 5.3 Å × 5.8 Å, respectively, and a calculated guest-accessible void volume of ca. 33.7% (Fig. S1†). SCXRD analysis revealed that pore B is occupied by N,N-dimethylformamide (DMF) and H2O. (Fig. S1†). Phase purity was supported by comparison of the calculated and experimental PXRD diffractograms (Fig. S2†). Thermogravimetric (TG) analysis (Fig. S3†) revealed that solvent loss occurred below 500 K. A methanol exchanged sample of pcu-1-Ni (3 × 72 h) revealed 11% weight loss at an onset temperature of 353 K. In situ variable temperature powder X-ray diffraction (VT-PXRD) measurements demonstrated that heating pcu-1-Ni under N2 flow did not induce a phase change, consistent with structural rigidity (Fig. S4†).Gas sorption and in situ PXRD
To evaluate gas sorption properties, a methanol-exchanged sample of pcu-1-Ni was activated at 373 K under dynamic vacuum for 10 h. We conducted CO2 (195 K) and N2 (77 K) gas sorption measurements and pcu-1-Ni revealed CO2 uptake of 6.02 mmol g−1 at 1 bar and negligible uptake of N2, an indication that it could be a potential CO2/N2 selective sorbent (Fig. 3a). The CO2 sorption isotherms at 273 and 298 K revealed a step in the adsorption branch of the isotherm. Specifically, CO2 sorption at 273 K follows a Type-I50 isotherm profile until 0.05 bar, with CO2 uptake of 0.7 mmol g−1, followed by an inflection and subsequent sorption of 5.3 mmol g−1 at 1 bar (Fig. 3b). Similarly, at 298 K an initial Type-I isotherm profile is observed, with step onset at 0.18 bar and CO2 uptake of 1.1 mmol g−1, reaching a maximum uptake of 4.5 mmol g−1 at 1 bar (Fig. 3b). Negligible N2 uptake at 298 K was observed (Fig. 3b). A comparison with literature reported values revealed that the gravimetric uptake of pcu-1-Ni at 1 bar is among the highest in the context of CO2 sorbents (Table S3†). The selectivity of pcu-1-Ni for CO2 suggested to us the possibility of separating CO2 from N2. Whereas ideal absorbed solution theory (IAST)51 calculations based upon 298 K single-component adsorption isotherms can serve as an indicator of separation performance, care must be taken in the case of stepped isotherms. Nevertheless, IAST calculations afforded selectivities of 250 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) and 118 (1
85) and 118 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) at 298 K and 1 bar for CO2/N2 (Fig. 3c and Table S4†), based on the initial slope of the 298 K isotherm. This selectivity is relatively high among CO2/N2 selective sorbents at 15
99) at 298 K and 1 bar for CO2/N2 (Fig. 3c and Table S4†), based on the initial slope of the 298 K isotherm. This selectivity is relatively high among CO2/N2 selective sorbents at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 composition (Fig. 3d) but lower than benchmark sorbents. To better understand the CO2 isotherm profile, in situ variable pressure PXRD measurements were performed under CO2 at 298 K. The PXRD diffractograms remained unchanged upon exposure to CO2 up to 1 bar (Fig. 3e), indicating that no phase change had occurred. This suggests that the pcu-1-Ni retains its structure and does not undergo any significant structural rearrangement or swelling induced by CO2, indicating that there are multiple CO2 binding sites.
85 composition (Fig. 3d) but lower than benchmark sorbents. To better understand the CO2 isotherm profile, in situ variable pressure PXRD measurements were performed under CO2 at 298 K. The PXRD diffractograms remained unchanged upon exposure to CO2 up to 1 bar (Fig. 3e), indicating that no phase change had occurred. This suggests that the pcu-1-Ni retains its structure and does not undergo any significant structural rearrangement or swelling induced by CO2, indicating that there are multiple CO2 binding sites.
Water vapour sorption
To evaluate water vapour sorption properties, dynamic vapour sorption (DVS) experiments were conducted at 300 K on a 9 mg sample of pcu-1-Ni and they revealed an S-shaped isotherm profile. The isotherm exhibits gradual uptake reaching 2 wt% at 55% RH with a step between 55 and 65% RH, resulting in uptake of 11 wt% at saturated humidity (Fig. 4a). Moderate hysteresis upon desorption was observed. The shape of the isotherm profile (i.e., S-shaped at 45–65% RH range) is in accordance with the recommended working humidity range for IHC (45–65% RH) to ensure comfortable moisture levels in an indoor environment. This kind of S-shaped water vapour sorption isotherm, wherein the adsorption and desorption branches are centred between 45 and 65% RH, has only been reported in a few sorbents, namely rigid MOF-101,52Cr-soc-MOF-1,53UiO-67,16Y-shp-MOF-5 (ref. 15) and flexible Znbtca (ref. 54) and sql-(azpy)(pdia)-Ni.55 To gain a deeper understanding of the nature of the water vapour sorption, an attempt was made to perform H2O solvent exchange using as-synthesized crystals suitable for SCXRD analysis. Unfortunately, the crystals underwent a reduction in crystallinity after soaking for 3 days at 308 K, rendering them unsuitable for SCXRD analysis. Alternatively, analysis of PXRD patterns after exposure at various levels of RH was conducted. As revealed in Fig. 4b, incubation of an activated powder sample of pcu-1-Ni in a 35% (1) RH chamber for 48 h and soaking in H2O (3 × 2 days) (2) did not result in any changes in the PXRD pattern, further suggesting structural rigidity and some degree of hydrolytic stability. Overall, these data indicate that the S-shaped water vapour isotherm profile is consistent with a pore-filling56 (type V) mechanism that is typical of rigid water sorbents.57 The sorption kinetics of pcu-1-Ni were studied by subjecting a sample (9 mg) to humidity swing conditions at full loading and unloading (0 to 85%). As shown in Fig. 4c, pcu-1-Ni underwent 28 min of adsorption followed by 15 min of desorption. The slow adsorption rate for pcu-1-Ni is expected as kinetics of adsorption is often dependent on the position of the inflection point in the water vapour isotherm, as explained by our recently reported sorption kinetics isotherm determination (SKID) model.58 To evaluate recyclability, humidity swing conditions at 300 K (0–85% RH) were applied to a 9 mg sample of pcu-1-Ni for 50 cycles of 43 min each (28 min adsorption, 15 min desorption). Unfortunately, pcu-1-Ni exhibited a loss of working capacity during the test (11.3 wt% at cycle 1 vs. 8.6 wt% at cycle 50) (Fig. 4d).Computational studies
To better understand the gas and water vapour sorption behaviour in pcu-1-Ni, we employed Canonical Monte Carlo (CMC) simulations and Grand Canonical Monte Carlo (GCMC) simulations. The CMC experiments generated density maps that highlight the probable adsorption sites for CO2, N2, and H2O within the framework (Table S6 and Section S9†). These simulated probability density maps reveal sorbate–sorbent interactions between in both pores A and B (as represented in Fig. S6†). However, the larger pore B features the most plausible adsorption sites for all adsorbates, therefore, it is no surprise that CO2 and H2O exhibit stepped isotherms, suggesting both pore types are filled sequentially during sorption (see Movies S1–S3†).59 In contrast, in both pores, the distribution density of N2 is lower than CO2 at 1 bar and 298 K, demonstrating a weaker adsorption affinity for N2 (Tables S6 and S7†). This trend is also reflected in the experimental and GCMC simulated adsorption isotherms (Fig. 3b and S7†), further corroborating the experimentally observed selectivity. The high CO2 uptake can be attributed to the strong adsorption binding sites (see Table S7†). The higher CO2 uptake observed at 273 K compared to 298 K (see Fig. 3b and S7†) can be attributed to the exothermic nature of adsorption, where lower temperatures favour stronger interactions between CO2 molecules and the framework. The preferential adsorption of CO2 can be attributed to its higher quadrupole moment and polarizability, which facilitate stronger electrostatic and van der Waals interactions with the framework.In the case of water, the hydrophobic nature of the framework results in minimal water adsorption at low RH, as the pore surface offers limited interaction with water molecules. However, as RH exceeds 55%, water molecules begin to form clusters via hydrogen bonding. Pore B, with its more accessible geometry, is filled first as water molecules preferentially occupy this pore. Once pore B is filled, water proceeds to occupy the more confined pore A. These clusters facilitate the adsorption of larger aggregates, enabling water to penetrate the confined pores (both A and B) of the framework. Despite the hydrophobic surface, water's small kinetic diameter (2.65 Å) allows it to access these pores, and the aggregation of water molecules is energetically favourable. This resulted in the steep uptake observed in the isotherm at >55% RH (Fig. 4a), illustrating that even in a moderately hydrophobic environment, water can exploit the framework's pore geometry.
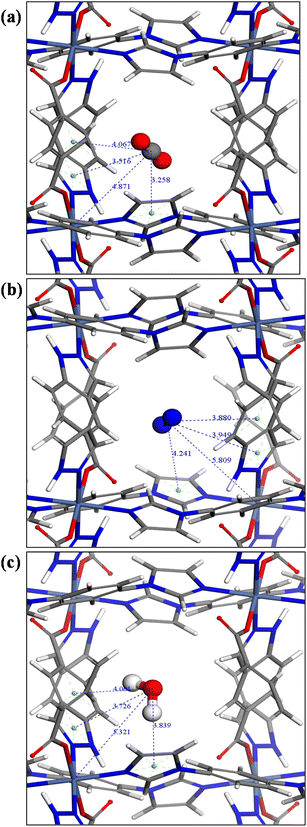
Analysis of the strongest adsorbate binding sites by CMC, in particular binding interactions and distances, further provides insight into preferential adsorption behaviour (Fig. 5). CO2 has a smaller kinetic diameter of 3.30 Å, compared to 3.64 Å for N2.60 The smaller kinetic diameter allows CO2 to bridge multiple binding sites simultaneously, enhancing its overall interaction strength. In Fig. 5a, the Ni–C(CO2) distance of 4.87 Å indicates the possibility of electrostatic interactions between the nickel centre and CO2. The distance between the centroid of the imidazole ring in the linker and C(CO2) is 3.26 Å, suggesting electrostatic forces or hydrogen bonding. Similarly, the distance between the centroid of the imidazole ring in purine and C(CO2) is 3.52 Å, indicative of π–π interactions. Additionally, the distance between the centroid of the pyrimidine ring of purine and C(CO2) is 4.07 Å, reflecting weaker van der Waals forces. In contrast, N2 has a slightly larger kinetic diameter which limits its ability to access these sites effectively, resulting in weaker adsorption. N2 indeed exhibits weaker binding interactions (Fig. 5b), the distance from Ni to the N2-centroid is 5.81 Å, signifying a reduction in electrostatic interaction strength compared to CO2. The distance between the centroid of the imidazole ring and the N2-centroid is 4.24 Å, while the distance between the centroid of the imidazole ring in purine and the centroid of N2 is 3.949 Å, both suggesting weaker π–π or van der Waals interactions. These longer distances also reflect the lower polarity of N2 compared to CO2, resulting in less favourable binding. In summary, CO2 demonstrates stronger binding interactions, driven by electrostatic and π–π interactions, while N2 binding is predominantly governed by weaker van der Waals interactions. For H2O (Fig. 5c), the Ni–H2O distance of 5.32 Å indicates weak electrostatic interactions between the nickel centre and the H2O molecule. The distance between the centroid of the imidazole ring in the linker and H2O is 3.84 Å, suggesting electrostatic or hydrogen bonding. The distance between the centroid of the imidazole ring in purine and H2O is 3.73 Å, indicative of π–π interactions. Additionally, the distance between the centroid of the pyrimidine ring of purine and H2O is 4.00 Å, reflecting weaker van der Waals forces. The water vapour sorption in pcu-1-Ni occurs through a pore filling mechanism that is influenced by the material's moderate hydrophobicity.
Conclusions
In summary, this study introduces pcu-1-Ni, a new MOF derived by pillaring of sql nets through N-donor linkers. pcu-1-Ni exhibited strong CO2 adsorption and weak N2 adsorption, resulting in good CO2/N2 selectivity comparable to CO2/N2 selective sorbents other than HUMs. Computational studies provide insight into why pcu-1-Ni preferentially adsorbs CO2 over N2, validating its potential for CO2 capture. Additionally, pcu-1-Ni shows an S-shaped water vapour isotherm in the 45–65% RH range, making it suitable for indoor humidity control (IHC) applications. In addition, the low water uptake in the 0–50% RH range enhances its potential for flue gas separation, where moisture management is essential. This distinctive balance of strong CO2 selectivity, minimal water adsorption at low RH coupled with water uptake at optimal RH range for IHC is unusual. Whereas hydrolytic stability was found to be unsuitable for practical utility, we anticipate that pcu-1-Ni can serve as a prototype for the generation of a broader family of related sorbents through metal and/or pillar substitution to enable improvements to stability and separation performance.Data availability
The data supporting the findings of this study are available in the ESI† or from the authors upon request.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. CRediT: S. M. S, A. A. B, A. C. E: conceptualization, investigation, methodology, writing-original draft, review and editing; L. C, T. H.: investigation, writing-review and editing; S. J. N, M. V: formal analysis, writing-review and editing; M. Z.: funding acquisition, formal analysis, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. J. Z. gratefully acknowledges the support from the European Research Council (ADG 885695) and Research Ireland (21/US/3760). S. J. N. and M. V. acknowledge the Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and support. S. J. N. is grateful for the support by Enterprise Ireland and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement no. 847402, project ID: MF20210297).Notes and references
- J. J. Perry Iv, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 Search PubMed.
- T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- H.-C. J. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- S. R. Batten, S. M. Neville and D. R. Turner, Coordination Polymers: Design, Analysis and Application, The Royal Society of Chemistry, 2008 Search PubMed.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space and L. Wojtas, Nature, 2013, 495, 80–84 CrossRef CAS.
- S. Mukherjee, N. Sikdar, D. O'Nolan, D. M. Franz, V. Gascón, A. Kumar, N. Kumar, H. S. Scott, D. G. Madden, P. E. Kruger, B. Space and M. J. Zaworotko, Sci. Adv., 2019, 5, eaax9171 CrossRef CAS.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356, 430–432 CrossRef CAS.
- H. Kim, S. R. Rao, E. A. Kapustin, L. Zhao, S. Yang, O. M. Yaghi and E. N. Wang, Nat. Commun., 2018, 9, 1191 CrossRef.
- R. G. Abdulhalim, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, K. Adil, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2017, 139, 10715–10722 CrossRef CAS.
- N.-X. Zhu, Z.-W. Wei, C.-X. Chen, X.-H. Xiong, Y.-Y. Xiong, Z. Zeng, W. Wang, J.-J. Jiang, Y.-N. Fan and C.-Y. Su, Angew. Chem., Int. Ed., 2022, 61, e202112097 CrossRef CAS.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS.
- N. Chanut, S. Bourrelly, B. Kuchta, C. Serre, J.-S. Chang, P. A. Wright and P. L. Llewellyn, ChemSusChem, 2017, 10, 1543–1553 CrossRef CAS.
- A. Kumar, D. G. Madden, M. Lusi, K. J. Chen, E. A. Daniels, T. Curtin, J. J. Perry and M. J. Zaworotko, Angew. Chem., Int. Ed., 2015, 54, 14372–14377 CrossRef CAS.
- S. Ullah, K. Tan, D. Sensharma, N. Kumar, S. Mukherjee, A. A. Bezrukov, J. Li, M. J. Zaworotko and T. Thonhauser, Angew. Chem., Int. Ed., 2022, 61, e202206613 CrossRef CAS PubMed.
- R. W. Gable, B. F. Hoskins and R. Robson, J. Chem. Soc. Chem. Commun., 1990, 1677–1678, 10.1039/C39900001677.
- D. J. O'Hearn, A. Bajpai and M. J. Zaworotko, Small, 2021, 17, 2006351 CrossRef PubMed.
- L. T. Glasby, J. L. Cordiner, J. C. Cole and P. Z. Moghadam, Chem. Mater., 2024, 36, 9013–9030 CrossRef CAS.
- B.-Q. Ma, K. L. Mulfort and J. T. Hupp, Inorg. Chem., 2005, 44, 4912–4914 CrossRef CAS PubMed.
- F. ZareKarizi, M. Joharian and A. Morsali, J. Mater. Chem. A, 2018, 6, 19288–19329 RSC.
- S. Mukherjee and M. J. Zaworotko, Trends Chem., 2020, 2, 506–518 CrossRef CAS.
- J.-S. Qin, S. Yuan, Q. Wang, A. Alsalme and H.-C. Zhou, J. Mater. Chem. A, 2017, 5, 4280–4291 RSC.
- S. Subramanian and M. J. Zaworotko, Angew Chem. Int. Ed. Engl., 1995, 34, 2127–2129 CrossRef CAS.
- N. Kumar, S. Q. Wang, S. Mukherjee, A. A. Bezrukov, E. Patyk-Kaźmierczak, D. O'Nolan, A. Kumar, M. H. Yu, Z. Chang, X. H. Bu and M. J. Zaworotko, Chem. Sci., 2020, 11, 6889–6895 RSC.
- P. M. Bhatt, Y. Belmabkhout, A. Cadiau, K. Adil, O. Shekhah, A. Shkurenko, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 9301–9307 CrossRef CAS PubMed.
- A. Kumar, C. Hua, D. G. Madden, D. O'Nolan, K.-J. Chen, L.-A. J. Keane, J. J. Perry and M. J. Zaworotko, Chem. Commun., 2017, 53, 5946–5949 RSC.
- D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033–5036 CrossRef CAS PubMed.
- A.-X. Zhu, Q.-Y. Yang, S. Mukherjee, A. Kumar, C.-H. Deng, A. A. Bezrukov, M. Shivanna and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 18212–18217 CrossRef CAS PubMed.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390–1393 CrossRef CAS.
- J. Tan, Y. Wang, Y. Ren, J. Li, Y. Chen and L. Li, Eur. J. Inorg. Chem., 2025, 28, e202400583 CrossRef CAS.
- R. Haldar, N. Sikdar and T. K. Maji, Mater. Today, 2015, 18, 97–116 CrossRef CAS.
- J. Zhang, L. Wojtas, R. W. Larsen, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2009, 131, 17040–17041 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- D.-S. Zhang, Y.-Z. Zhang, J. Gao, H.-L. Liu, H. Hu, L.-L. Geng, X. Zhang and Y.-W. Li, Dalton Trans., 2018, 47, 14025–14032 RSC.
- K. Koupepidou, V. I. Nikolayenko, D. Sensharma, A. A. Bezrukov, M. Shivanna, D. C. Castell, S.-Q. Wang, N. Kumar, K.-i. Otake, S. Kitagawa and M. J. Zaworotko, Chem. Mater., 2023, 35, 3660–3670 CrossRef CAS PubMed.
- K. Koupepidou, A. Subanbekova and M. J. Zaworotko, Chem. Commun., 2025, 61, 3109–3126 RSC.
- B.-Q. Song, Q.-Y. Yang, S.-Q. Wang, M. Vandichel, A. Kumar, C. Crowley, N. Kumar, C.-H. Deng, V. GasconPerez, M. Lusi, H. Wu, W. Zhou and M. J. Zaworotko, J. Am. Chem. Soc., 2020, 142, 6896–6901 CrossRef CAS PubMed.
- Q. Y. Yang, P. Lama, S. Sen, M. Lusi, K. J. Chen, W. Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS PubMed.
- Q.-Y. Yang, P. Lama, S. Sen, M. Lusi, K.-J. Chen, W.-Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry IV, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS PubMed.
- K. Koupepidou, V. I. Nikolayenko, D. Sensharma, A. A. Bezrukov, M. Vandichel, S. J. Nikkhah, D. C. Castell, K. A. Oyekan, N. Kumar, A. Subanbekova, W. G. Vandenberghe, K. Tan, L. J. Barbour and M. J. Zaworotko, J. Am. Chem. Soc., 2023, 145, 10197–10207 CrossRef CAS PubMed.
- A. A. García-Valdivia, M. Pérez-Mendoza, D. Choquesillo-Lazarte, J. Cepeda, B. Fernández, M. Souto, M. González-Tejero, J. A. García, G. M. n. Espallargas and A. Rodríguez-Diéguez, Cryst. Growth Des., 2020, 20, 4550–4560 CrossRef.
- W.-F. Zhang, Y. Du, X.-Y. Sun, H.-M. Pan, Y.-Y. Ma, D.-Y. Li, S. Wu, T. Yan and Z.-H. Jing, Inorg. Chem. Commun., 2021, 126, 108469 CrossRef CAS.
- X. Liang, S. Wang, J. Feng, Z. Xu, Z. Guo, H. Luo, F. Zhang, C. Wen, L. Feng, C. Wan and M.-M. Titirici, Inorg. Chem. Front., 2023, 10, 2961–2977 RSC.
- C. S. Hawes, R. Babarao, M. R. Hill, K. F. White, B. F. Abrahams and P. E. Kruger, Chem. Commun., 2012, 48, 11558–11560 RSC.
- K. S. W. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- S. Lee, J. H. Lee and J. Kim, Korean J. Chem. Eng., 2018, 35, 214–221 CrossRef CAS.
- G. Akiyama, R. Matsuda, H. Sato, A. Hori, M. Takata and S. Kitagawa, Microporous Mesoporous Mater., 2012, 157, 89–93 CrossRef CAS.
- S. M. Towsif Abtab, D. Alezi, P. M. Bhatt, A. Shkurenko, Y. Belmabkhout, H. Aggarwal, Ł. J. Weseliński, N. Alsadun, U. Samin, M. N. Hedhili and M. Eddaoudi, Chem, 2018, 4, 94–105 CAS.
- S. M. Shabangu, A. A. Bezrukov, A. C. Eaby, S. J. Nikkhah, S. Darwish, V. I. Nikolayenko, D. Sensharma, S.-Q. Wang, M. Vandichel and M. J. Zaworotko, ACS Mater. Lett., 2025, 433–441, DOI:10.1021/acsmaterialslett.4c02019.
- X. Li, D. Sensharma, V. I. Nikolayenko, S. Darwish, A. A. Bezrukov, N. Kumar, W. Liu, X.-J. Kong, Z. Zhang and M. J. Zaworotko, Chem. Mater., 2023, 35, 783–791 CrossRef CAS PubMed.
- M. J. Kalmutzki, C. S. Diercks and O. M. Yaghi, Adv. Mater., 2018, 30, 1704304 CrossRef.
- X. Li, A. A. Bezrukov, W. Graham, D. Sensharma, X.-J. Kong, T. Thonhauser and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2024, 16, 34402–34408 CrossRef CAS.
- A. A. Bezrukov, D. J. O’Hearn, V. Gascon-Perez, S. Darwish, A. Kumar, S. Sanda, N. Kumar, K. Francis and M. J. Zaworotko, Cell Rep. Phys. Sci, 2023, 4, 101252 CrossRef CAS.
- N. Ko, P. G. Choi, J. Hong, M. Yeo, S. Sung, K. E. Cordova, H. J. Park, J. K. Yang and J. Kim, J. Mater. Chem. A, 2015, 3, 2057–2064 RSC.
- N. Mehio, S. Dai and D.-e. Jiang, J. Phys. Chem. A, 2014, 118, 1150–1154 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2429367. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ta01995h |
| This journal is © The Royal Society of Chemistry 2025 |