Recent status, key strategies and challenging perspectives of smart batteries for next-generation batteries
Lei
Wang†
a,
Zhipeng
Su†
a,
Rui
Wang
a,
Han
Liang
a,
Biao
Fang
a and
Runwei
Mo
*ab
aSchool of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai, 200030, China. E-mail: rwmo@ecust.edu.cn
bShanghai Key Laboratory of Intelligent Sensing and Detection Technology, East China University of Science and Technology, Shanghai 200237, China
First published on 10th June 2025
Abstract
Lithium-ion batteries are a very important energy storage device, which has led to a wide range of applications and excellent performance making them important in many industries. However, the development of rechargeable batteries has been slow in recent decades due to the inherent properties of the material. With the advent of the fourth industrial revolution, the development of high technology, such as artificial intelligence, is changing rapidly. The development of smart batteries is an effective strategy to improve battery life and operational safety by integrating smart concepts into battery design, manufacturing and management. This review comprehensively describes the current development of smart batteries. Based on different perspectives of battery design, manufacturing and management, smart batteries can be divided into three parts: smart materials, smart manufacturing and intelligent sensing. The mechanism of action and application principles of each part are also discussed in detail for in-depth understanding. In addition, we have analyzed the challenges and issues facing the development of smart batteries to facilitate their practical development.
1 Introduction
It is well known that battery technology is a key supporting technology for the energy, information and transportation revolutions. Batteries have brought endless benefits to mankind, which has propelled the development of laptops, cell phones, and electric cars. A review of the development of batteries is shown in Fig. 1. As early as 1799, Italian physicist Alessandro Volta laid the foundation for the development of batteries by creating the first battery using zinc (anode) and copper (cathode) sheets and sheets of paper moistened with brine (electrolyte). In 1850, French physicist Gaston Plante prepared a rechargeable lead-acid battery capable of delivering 12 volts using low-cost lead as the anode, lead oxide as the cathode, and a sulfuric acid solution as the electrolyte. This battery was used in automobiles, early electric cars, and so on. In 1899, Swedish Waldemar Jungner invented the chromium–nickel battery by using nickel as the cathode and cadmium as the anode and utilizing a liquid electrolyte. However, it was gradually eliminated from the market due to the defect of “memory effect”. In 1953, Bell Labs successfully developed the first silicon solar cell with practical value. Since then, solar cells have gradually moved to the industrial field.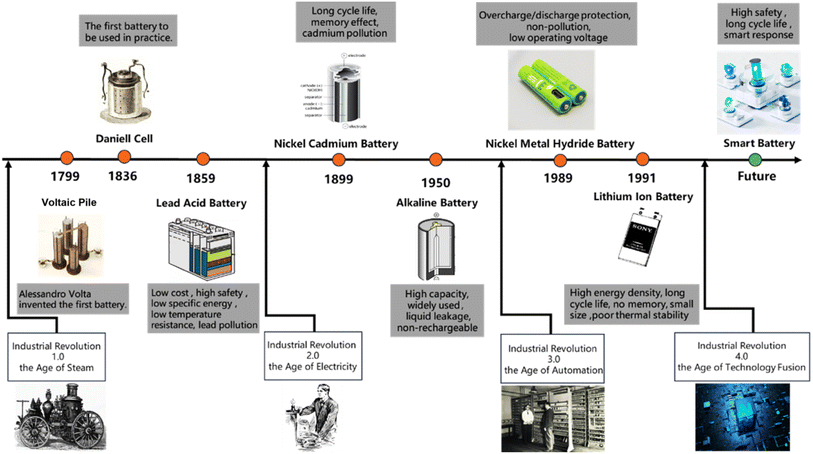 | ||
| Fig. 1 Battery development history based on the first to fourth industrial revolutions and the future of smart batteries. | ||
In 1970, the National Aeronautics and Space Administration (NASA) and Panasonic Corporation of Japan developed a primary lithium battery with solid fluorocarbon as the cathode, active lithium metal as the anode and a non-aqueous electrolyte. This battery has the advantages of high voltage, high energy density, small size, light weight and so on. The successful commercialization of primary lithium batteries has brought lithium batteries into the limelight for the first time.1 In 1989, the first commercially available nickel-metal hydride (NiMH) batteries were introduced, which used a metal hydride or hydrogen storage alloy as the anode and nickel hydroxide as the cathode. Compared with nickel–cadmium batteries, NiMH batteries have a higher energy density and are less polluting. Therefore, NiMH batteries are used in a large number of digital products. In 1991, Sony introduced a commercial secondary lithium battery with a graphite anode, lithium cobalt oxide cathode and LiPF6-PC electrolyte. Lithium-ion batteries are widely used commercially due to their high energy density and rechargeable characteristics.2 After more than 200 years, battery technology has evolved rapidly, which has resulted in lighter weight, smaller size batteries and higher energy density.
From their birth, lithium-ion batteries have rapidly gained great attention from all walks of life due to their advantages such as high output voltage, high capacity, and stable embedded material structure.3 However, the development of rechargeable batteries has been slow in recent decades due to the inherent properties of the material. It is worth noting that there are many problems with conventional lithium-ion batteries. For example, irreversible deformation occurs during battery operation. High-capacity electrode materials undergo large volume changes due to concentration changes within the particles during charging and discharging, which leads to deformation of the electrode materials and generates stress. This stress leads to fragmentation, disintegration, fracture and loss of contact between the collector and active electrode material, which exposes the surface to the electrolyte. These degradation processes ultimately lead to a decay in the electrochemical cycling capacity of the electrode materials, which results in cracked or even broken electrodes.4
Electrode degradation is a major factor affecting the performance improvement of conventional batteries. Therefore, research on conventional batteries over the years has focused on optimizing the quality, reliability, lifetime and safety (QRLS) of electrode materials. However, it is limited by the difficulty of electrode material innovation technology and the challenge of practical application.5 For electrode volume changes, commonly used improvement strategies mainly target electrode structure, elemental doping, composite modification, binder, and electrolyte modification to extend the electrode lifetime by enhancing bond strength or stabilizing the solid electrolyte interfacial layer (SEI).6 However, all these means can only improve the stability of the electrode to a certain extent, and cannot detect the electrode stress and respond to it in time. Due to the high negative oxidation–reduction potential of lithium metal, it spontaneously reacts with the electrolyte to form a harmful SEI layer. During the cycling process, this unstable SEI layer continuously breaks down, producing new exposed lithium that reacts with the electrolyte repeatedly. The continuous consumption of electrolyte and the electrochemical corrosion of lithium metal result in low coulombic efficiency and capacity decay.7 However, the microscopic mechanism of corrosion behavior and its correlation with the interface are not yet clear. The existing corrosion quantitative detection technologies need to be enriched, and effective strategies for solving the persistent lithium corrosion problem need to be developed.8
In addition, the internal temperature of the battery rises dramatically in the presence of mechanical, thermal and electrical abuse, which leads to the onset of thermal runaway due to the inability to dissipate heat in a timely manner.9 The traditional solution is to optimize the composition and content of the electrolyte and electrodes. In terms of electrolyte design, non-flammable solvents, highly concentrated electrolytes or locally concentrated electrolytes can be chosen. For electrodes, a straightforward solution is to use more stable cathodes, e.g., LiFePO4 and its analogs. However, these improvement strategies affect to some extent the electrochemical performance of the battery as well as provide a one-time protection. Therefore, it is imperative to explore reversible and harmless methods to build safer batteries. In short, there is little room for the development of new battery technology based on traditional concepts. Therefore, there is an urgent need to break the functional limits of the original battery, which promotes the development of battery technology in a new direction through intelligent means.
2 Smart batteries
During the “13th Five-Year Plan” period, the global new energy vehicle market is growing rapidly, and China will focus its investment on supporting the development and manufacturing of automotive batteries. During the “14th Five-Year Plan” period, China will increase its support for the development of energy storage batteries to achieve the goal of carbon peak and carbon neutrality. The R&D of advanced batteries is part of the national key development program, which includes “high-end functional and smart materials”, “energy storage and smart grid technology”, “new energy vehicles” and “smart sensors”.10 As shown in Fig. 2, the R&D of advanced batteries will mainly focus on the development of smart materials and smart technologies. The EU's “Battery 2030+” (Battery 2030+) points out the R&D focus of EU battery technology in the next 10 years, aiming at the development of ultra-high performance batteries that are smart, safe, sustainable and cost-competitive, and future work will be centered on six key areas: development of smart materials, research on battery interfaces/phases, smart sensors, self-repair functions, manufacturing and recycling.12 | ||
| Fig. 2 China's key R&D project plan for the 14th five-year plan: batteries reproduced with permission.11 Copyright 2022, Institute of Electrical and Electronics Engineers. | ||
Researchers have now introduced the concept of artificial intelligence (AI) into battery design, development and management,13 as shown in Fig. 3. The battery manufacturing process includes electrode manufacturing, cell assembly and cell finishing. The battery manufacturing process determines the initial performance and health of the battery, and AI-based battery manufacturing can strictly control and manage this process, which can improve the initial capacity of the battery, and reduce impedance and first cycle loss. On the other hand, battery management plays a key role in determining the lifetime of the battery. After the battery is manufactured, it will work in different operating modes to provide power or store power, which requires various management control schemes. However, smart batteries are able to access battery health parameters through various sensor devices and materials to detect the state of health (SOH) of the battery and predict the battery life, and algorithms are used to develop a model for battery control and optimize the charging process of the battery in order to prolong its life.
Current research has demonstrated the advantages of AI in measuring battery strain, thermal response, optical characterization, and electrode potential. A smart battery is an integrated system that combines real-time sensing, dynamic response, and AI technologies such as smart materials, smart sensing, smart manufacturing, and automatic control. For example, Kuznetsov et al.14 prepared self-supporting battery electrodes by in situ mixing atomized electrode actives with grown carbon nanotube (SWNT) aerosols, which eliminated the need for electrochemically inactive metal collectors, additives, and binders, and increased energy density by 40% using emerging smart materials. The electrodes can withstand about twice the tensile strain compared to conventional batteries. They also found that electrode sheets with a high concentration of nanotubes (≥5 wt%) are mechanically robust, flexible, and have higher electrical conductivity (103–104 Ω m−1). In addition, the embedded SWNT network has a piezoresistive effect and can operate as an intrinsic strain sensor, providing information about changes in the cell structure. When the battery is running, mechanical perturbations are translated into sharp changes in potential difference, thus reflecting the health of the battery. By “smartening” the battery beyond the basic performance of conventional rechargeable batteries, it is possible to improve the electrochemical performance, and structural and thermal stability of the battery and extend its cycle life.
Classification based on intelligent characteristics, the functions of the smart battery can be summarized into three kinds, namely, real-time sensing, dynamic response, and self-regulation. Real-time monitoring and prediction of the operating state of lithium-ion (Li-ion) batteries is essential to improve the operating life and safety of Li-ion batteries. Compared to traditional battery management strategies that rely only on module-level voltage, current, and temperature, real-time sensing capabilities are enabled by implanting multidimensional physical sensors in the battery, which can monitor the physical information inside the battery in real time and realize the conversion of physical-chemical-digital information. These batteries can accurately sense signals such as battery temperature, strain, decomposition gas, and pressure. This monitoring is critical for solving complex microelectrochemical reactions within the battery, as well as detecting changes in strain and pressure. Recently, Li et al. integrated fiber Bragg grating (FBG) fiber-optic sensors into batteries to fabricate smart lithium-ion batteries capable of measuring temperature, force and displacement at the battery level through a simple beam structure, which shows great potential for early detection of battery safety incidents. During the actual operation of the battery, the temperature increases due to internal electrochemical reactions, and the embedding/de-embedding of lithium ions between the cathodes and anodes and the growth of the SEI film cause the expansion and contraction of the electrode layer, while both the temperature and the force cause axial deformation of the optical fiber grating. Therefore, the battery volume change acts on the MP-FBG sensor, thus reflecting the expansion force of the battery.15 The researchers performed nail penetration tests at 0% and 100% SOC and recorded the changes in cell voltage, resistance, temperature and force during the period. As shown in Fig. 4(a), the force signal began to change at 274 seconds after the nail contacted the cell at 0% SOC, which was 5 seconds before the voltage signal. And the temperature did not increase significantly until 21 seconds after the force signal changed, with a maximum temperature of about 91 °C. Fig. 4(b) shows the nail penetrating 100% SOC when the nail just touched the cell at 301 seconds. The temperature, voltage and resistance changed only after 6, 5 and 4 seconds, respectively. However, the MP-FBG sensor responds to the force signal at the instant of contact, so the intelligent battery mechanical mode based on the MP-FBG sensor can issue a warning a few to several tens of seconds earlier than traditional battery temperature monitoring methods. In oven temperature testing, the MP-FBG sensor can reflect changes in battery geometry based on the expansion displacement of the battery, thus knowing whether the battery is structurally damaged.
 | ||
| Fig. 4 Variation of voltage, resistance, temperature and force during nail penetration tests at (a) 0% SOC and (b) 100% SOC. Reproduced with permission.16 Copyright 2022, Elsevier. | ||
As a result, smart batteries with integrated smart devices are able to improve battery safety by giving safety warnings in advance before they are in a critical steady state. This smart battery with real-time monitoring grasps information about the battery's internal and external operating environments and provides a more accurate assessment of the state of charge (SOC) and state of health (SOH). Accurate monitoring of the battery's condition can identify potential problems in time and prevent battery failures, thus improving the overall safety of the battery system.
Dynamically responsive smart batteries utilize smart materials that can adapt to changing environments. Smart materials are advanced materials that respond to external stimuli (temperature, humidity, pressure, pH, electric and magnetic fields, etc.).17 They enable an interactive process of stimulus response that allows for timely and accurate feedback based on the state of the battery. Smart materials can be categorized into six main groups: shape memory materials, piezoelectric materials, magnetostrictive materials, electrostrictive and magnetorheological fluids, and self-healing materials. Shape memory materials are a class of smart materials that recover their original shape under stimulation after undergoing plastic deformation.18 Piezoelectric materials are a class of smart materials that are capable of energy conversion, generating an electrical charge when subjected to mechanical stress and deforming mechanically when an electric field is applied.19 Magnetostrictive materials have the ability to convert electrical or magnetic energy into mechanical energy and have the unique characteristic of changing shape or size in response to the application of a magnetic field.20 Electrorheological and magnetorheological materials have the unique ability to change their rheological properties in response to an external magnetic field, providing self-protection in the event of an overcharge of a smart battery.21,22 Self-healing materials are materials that are capable of repairing themselves when damaged.23 Many research and development efforts have shown that smart materials have high potential. These advanced smart materials have smart functions such as shape memory, self-healing and sensing capabilities, which will not only improve the electrochemical performance of the battery, but also confer better environmental compatibility in terms of safety, adaptability and reliability.
Traditional battery management systems (BMSs) are used for battery fault monitoring and protection, but they suffer from unreliability in real-time fault diagnosis due to inaccurate algorithmic modeling, limited computational power, and insufficient data storage, as shown in Fig. 5(a). Compared with the traditional battery system integration and management architecture, an intelligent battery system for smart batteries, as shown in Fig. 5(b) and (c), can simultaneously monitor battery current, voltage, internal/surface temperature, pressure, strain, etc., and can generate more real-time sensing data.24 The self-reconfiguring BMS focuses on the global coordination of the battery, while the self-regulating BMS optimizes the overall performance of the battery pack by communicating and coordinating with multiple local intelligent units.
 | ||
| Fig. 5 Structures and management architectures of different kinds of LIB systems: (a) conventional BMS, (b) self-reconfiguring BMS, (c) self-regulating BMS. Reproduced with permission.24 Copyright 2021, Elsevier. | ||
The intelligent BMS is composed of a self-regulating multi-cell battery pack. The self-regulating multi-cell battery pack is a topology structure that includes cell-level sensors, switching switches, and battery controllers. During daily operation, the sensors in each unit collect current, voltage, and surface temperature and other state information, and then transmit it to the controller via a specific communication interface. After receiving the state information, the battery controller makes decisions based on the embedded various estimation and control algorithms. The operation is then fed back to the individual cells via switches for coordination to achieve real-time regulation of the battery pack. For example, an intelligent BMS adjusts charging and discharging parameters (e.g., current and voltage) in real time through adaptive control algorithms. When the temperature of the battery is predicted to rise, the controller reduces the charging current via a toggle switch to prevent the battery from overheating. When the battery is predicted to be about to be depleted, charging will be initiated in time to avoid over-discharge. When the battery is predicted to be aging, the charging current is reduced by other means to slow down the aging process. The complex topology allows each unit to be equipped with multiple switches for higher configuration flexibility and self-adjustment, enabling real-time switching of connections such as series, parallel, and series-parallel. A reliable BMS is essential to enhance the reliability, efficiency and lifetime of lithium-ion battery systems. Self-regulating smart batteries provide high design and operational flexibility, high fault tolerance, and enhanced battery life management. Therefore, the development of smart batteries with self-regulating functions not only determines the current state of the battery and predicts the future state of the battery with high accuracy, but also realizes autonomous control through the smart BMS.
The in-depth integration of smart materials, smart manufacturing and smart sensing technologies is promoting the battery system from traditional energy storage devices to the adaptive, self-diagnostic, self-repair capabilities of the ‘smart battery’ leap. At the material level, smart materials give the battery structure as well as adaptive characteristics. In the manufacturing dimension, intelligent manufacturing technology achieves accurate control of the whole battery production process through digital and intelligent means. At the system management level, intelligent sensing networks build a real-time sensing nervous system for the battery. Through the closed-loop synergy of material innovation, process innovation and real-time regulation, these three technologies have achieved systematic breakthroughs in the intrinsic safety, manufacturing precision and dynamic management of batteries. In addition, smart batteries are evolving in the direction of deep coupling of smart materials-smart manufacturing-smart sensing. Smart materials achieve real-time feedback of the battery eigenstates, smart manufacturing can achieve dynamic tuning of process parameters, and smart sensing has millisecond fault isolation capability. This biomimetic technology path will enable the battery system to break through the scope of existing energy storage tools, evolving into a ‘cognitive energy organ’ with self-awareness, self-optimization and self-repair characteristics. This lays the foundation for the next-generation energy storage system with high energy density, long life and high safety.
3 Smart materials
With the advent of the smart era, the breakthrough development of smart devices has raised higher standards and demands for energy storage technologies. Although lithium-ion batteries have been widely used in commercial applications, their electrochemical efficacy and broad applicability are still challenged by the inherent limitations of materials and the complexity of technological innovation. In the wave of the fourth industrial revolution led by information technology and artificial intelligence (AI), smart batteries with excellent electrochemical performance, high reliability and adaptability are being constructed based on the fusion of cutting-edge materials and revolutionary technology innovations to meet the urgent demand for efficient and safe energy storage solutions in the smart era. During the operation of lithium-ion batteries, a series of complex electrochemical reactions occur at multiple scales, from the micro-level of the material, to the electrode and electrolyte state, to the macro-state of the battery, such as voltage, current, and temperature. These processes fundamentally determine the safety, cycle life and electrochemical performance of lithium-ion batteries. For example, expansion and contraction of Li-ion battery electrodes during cell lithiation and delithiation, stress/strain energy leading to reduced interparticle contact, and electrode crushing during repeated cycling lead to significant irreversible capacity loss and poor cycle stability.25 Excessive delithiation of the cathode during overcharging of lithium-ion batteries results in side reactions that generate large amounts of heat and oxygen, leading to oxidation of the electrolyte or cathode material and thermal runaway.26 Therefore, in order to extend the cycle life of the battery and improve the safety of the battery, it is necessary to develop smart lithium-ion batteries to realize the multi-scale response to the battery's side reactions or abnormalities and self-protection and healing, as shown in Fig. 6.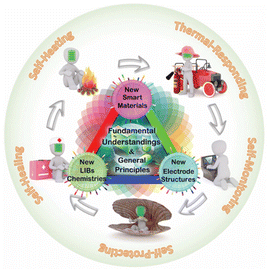 | ||
| Fig. 6 Introduction to smart materials. Reproduced with permission.27 Copyright 2019, Wiley. | ||
Smart materials are a class of advanced materials that respond to external stimuli like biological systems by mimicking biological behavior. Smart materials are able to respond to external factors such as temperature, humidity, pressure, pH, electric and magnetic fields, and can quickly return to their original state after the external stimulus disappears. Smart materials have the advantage of increasing the flexibility of materials, extending their lifespan and reducing maintenance costs. It's worth noting that smart materials can be divided into six categories: shape-memory materials, piezoelectric materials, magnetostrictive materials, electrostatic and magnetorheological fluids, and self-healing materials. By integrating smart materials with conventional lithium-ion batteries, it is possible to obtain smart batteries with different functions that can spontaneously detect or respond to anomalies at an early stage and significantly improve battery safety and cycle life. This section provides an overview of the application of smart materials in the field of lithium-ion batteries, including design principles, various smart materials and structures.
3.1 Self-healing materials
In recent years, with the development of rechargeable electrochemical energy storage devices and their wide application in daily life, their operational stability, safety and service life have gradually attracted attention. In long-term operation, the battery is affected by multiple factors such as chemical substances, heat, and force, which will inevitably lead to cracks or damage, thus affecting the operational life. For example, lithium-ion batteries have the problem of expansion and contraction of the battery during lithiation and delithiation, and changes in the volume of the electrodes will lead to cracks on the surface, or even crushing, thus affecting the conduction of lithium ions and reducing the cycle life of the battery. In addition, in lithium-ion batteries the uneven lithium deposition leads to the growth of lithium dendrites, which can puncture the diaphragm, triggering a battery short circuit, which will produce serious safety hazards.The commercial application of lithium batteries requires not only high capacity, but also safety and long cycle life. If the physical structure of the electrode or electrolyte can be repaired in time when mechanical damage occurs and the performance of the battery can be restored, then the cycle life of the battery can be extended. Therefore, self-healing is an effective means to improve battery life. Self-healing materials are a class of intelligent materials which can automatically restore part or all of their functions after being mechanically, thermally or radiation-damaged. The self-healing ability of these materials not only extends the service life of the product, but also improves the reliability of the product and reduces the waste of resources.28 Based on the advantages of self-healing materials, they have been widely used in various batteries and supercapacitors. Self-healing is one of the important features of next-generation smart materials, and is very helpful for the development of smart batteries.29 So far, self-healing materials are dominated by polymers, as well as a few low melting point metals. The mechanisms of self-healing can be summarized as (a) external additive action and (b) internal interaction. External additive action repairs the damage through pre-embedded healing agents, while internal interactions repair mainly through dynamic reorganization of reversible chemical bonds. In this section, the role and principles of self-repairing materials are discussed separately according to the repair mechanism.
 | ||
| Fig. 7 Working principle of microencapsulated self-healing materials: (a) crack extension, (b) healing agent release, (c) polymerization reaction. Reproduced with permission.31 Copyright 2001, Nature. | ||
Hydrogen bonds are weak dynamic reversible non-covalent bonds with low bond energy that can be broken and reorganized at room temperature. Self-healing materials based on the principle of hydrogen bonding can realize the self-healing process at room temperature. Chen et al.32 developed a crosslinked polymer network as a self-healing binder for silicon anodes. Polyacrylic acid (PAA) was used as the backbone of the polymer network to provide mechanical support, and tannic acid (TA) was used as the physical cross-linking agent, and the two were cross-linked through abundant dynamic hydrogen bonding, as shown in Fig. 8, endowing the binder with unique self-healing properties and strong adhesion to the silicon anode. The bond strength is enhanced due to the formation of abundant hydrogen bonds in the TA-c-PAA binder after the introduction of TA. Upon observing the morphology of the Si@TA-c-PAA electrode before and after 100 cycles, the surface is still dense with only a few tiny cracks, which is attributed to the formation of substitutional hydrogen bonds in the branched chains to effectively transfer the stress, and the abundant dynamic hydrogen bond dissociation/conjugation facilitates the energy dissipation and prevents the accumulation of stress. The self-healing ability of the TA-c-PAA binder was tested. After scratching the Si@TA-c-PAA electrode, the scratches on the surface were reduced from 100 μm to 60 μm within 72 h, while Si@PAA remained unchanged, showing excellent self-healing ability.
 | ||
| Fig. 8 Schematic diagram of the synthesis and mechanism of the TA-c-PAA binder. Reproduced with permission.32 Copyright 2024, Elsevier. | ||
Due to the weak action of single heavy hydrogen bonding, current research focuses on the introduction of multiple hydrogen bonds or synergistic action with other chemical bonds to enhance the self-healing function. The tetrahydrogen-bonded polymeric binder synthesized by hydrolysis after copolymerization of tert-butyl acrylate and ureido-pyrimidinone monomers has excellent self-repairing ability to repair electrode cracks caused by changes in the volume of silicon particles. Compared with the silicon anode using a conventional binder, the silicon electrode with a tetrahydrogen bonding binder was able to maintain a high capacity of 2638 mA h g−1 after 110 cycles, with significantly improved electrochemical performance.33 Metal–ligand bonding has excellent self-healing properties by selecting suitable metal ions and ligands to obtain weak dynamic coordination bonds. Moreover, the metal–ligand bonding energy is easily adjustable, the thermal stability is excellent, and the repair is possible without external stimulation.34 Dou et al.35 prepared self-healing PU elastomers based on coordination bonds, Fe3+ with DAP ligands and amide bonds with two weak bonds between N and O, which are prone to fracture recombination, giving the polymers excellent self-repairing ability. Through fracture repair tests, the repair efficiency of mechanical strength reached 95.2% after 24 h at 50 °C, showing excellent self-healing properties. In addition, subject–guest interactions are also commonly used to prepare self-healing materials, which are based on a reversible cross-linking system between the subject and the guest. Macrocyclic compounds such as crown ethers, cuproaromatics, and cyclodextrins act as host molecules that can accommodate many guest molecules in their inner cavities. β-Cyclodextrin (β-CD) is commonly used as a host with a hydrophobic cavity to accommodate many guest molecules, and is able to form encapsulated compounds with them. On this basis, Wang et al.36 utilized β-CD (host) to crosslink with 18-carbon alkyl chains (guest) to obtain supramolecular networks (PU-CD). The PU-CD network was cut into small pieces. When placed at 80 °C for 1 h, these fragments were reattached as a whole without obvious defects, and the mechanical properties were almost unchanged from those before repair. This is due to the dynamic exchange nature of the host–guest interaction itself, which allows the PU-CD network to be repaired at high temperatures.
Self-repairing materials based on the principle of reversible non-covalent bonding are able to work at room temperature, but the weak intermolecular forces between the non-covalent bonds lead to low mechanical properties and long repair times at room temperature. Dynamic covalent bonding has strong bonding energy, and by introducing dynamic covalent bonding, the two act synergistically to improve the mechanical properties of the materials. Song et al.37 formed PU elastomers with excellent mechanical, self-healing, shape recovery and reprocessing properties by introducing a crosslinked network of quadruple hydrogen and DA bonds in the main chain. Samples with only hydrogen bonding (sHPU-0) showed poor mechanical properties and low elastic elongation (around 500%), as shown in Fig. 9(a). The samples based on the synergistic action of hydrogen and DA bonds (sHPU-x) have reduced chain flexibility due to the formation of multiple hydrogen bonds providing physical cross-linking. The density of DA bonds in the molecular chain is increased, thus replacing some of the quadruple hydrogen bonds and forming a rigid ring structure that restricts the movement of some of the molecular chains. The enhancement of mechanical properties induced by the rigid molecules compensates for the weakening of the hydrogen bonding effect, which improves the stress resistance of the material and further increases the strength of the material as shown in Fig. 9(b), with a strength of about 6.3 MPa and a tensile strain of about 1957%.
 | ||
| Fig. 9 Typical stress–strain curves of the original and repaired (a) sHPU-0 and (b) sHPU-3. Reproduced with permission.37 Copyright 2023, American Chemical Society. | ||
Disulfide bonds possess dynamically reversible properties, and their reaction conditions are relatively mild and responsive to external changes. The materials prepared by such disulfide bond-based reactions are attracting more and more research attention due to their unique low-temperature self-repairing and remodelability. These properties not only bring new possibilities for materials science, but also provide broad prospects for practical applications. Liang et al.39 explored the microcrack self-healing ability of dynamic disulfide-bonded waterborne polyurethane/polyacrylate composites (SWPUA). The film was cut to produce 300 μm microcracks, which were repaired in a high-temperature oven at 75 °C for 3 h. The scratches of the samples containing disulfide bonds became significantly lighter and narrower, as shown in Fig. 10; the self-healing efficiency of tensile strength was 101.03%, and the fracture elongation could be restored to 97.66%. The results of the study showed that the efficiency of a single restoration was high. In addition, the self-repair efficiency for repetitive tensile strength was 91% and elongation at break was 75%, which indicated good self-repair performance.
 | ||
| Fig. 10 Self-repair process of SWPUA at 75 °C for 3 h. Reproduced with permission.39 Copyright 2023, Wiley. | ||
Similar to the borate ester bond, the dynamic covalent boron ester-based bonding between boric acid and diols imparts excellent self-healing properties to hydrogels, organogels, elastomers and plastics.40 However, the complexity of the preparation of self-healing materials based on this principle and the need to introduce chemical modifications limit their applications.
| Self-healing type | Principle | Characteristics |
|---|---|---|
| Microencapsulation | Ring opening translocation polymerization | High cost, slow repair, irreversible |
| Reversible non-covalent bonding | Intermolecular interaction | Mild reaction conditions, low mechanical properties, long repair time |
| Reversible covalent bonds | Fracture and recombination of dynamic covalent bonds | Good stability, need external stimulation |
| Reversible solid–liquid transformation | Metal solid–liquid conversion | Good electrical conductivity, safety, high cost, scarce metal resources |
3.2 Smart response materials
With the development of lithium-ion battery technology, the thermal runaway (TR) problem has attracted widespread attention. Ou et al.43 summarized the causes of the thermal runaway phenomenon, which are mainly in the following four categories: (1) mechanical abuse; (2) overcharging and discharging; (3) short circuit; and (4) external overheating. Mechanical abuse is the most direct cause of thermal runaway. Since mechanical stress strain expansion makes the lithium-ion battery electrode interlayer contact pressure increase, reducing the contact resistance of conductive carbon particles while destroying the physical structure of the battery, irreversible deformation occurs. In addition, mechanical stress can also lead to fatigue of the internal components of the battery, resulting in increased impedance and capacity degradation, and ultimately reducing the cycle life of the battery.44 An internal short circuit means that the positive and negative terminals are directly connected, and a large current flows rapidly through the short circuit path, causing a large amount of heat to be generated inside the battery, leading to thermal runaway. Over-charging and over-discharging can cause the chemical reaction inside the battery to go out of control, generating a large amount of heat, which in turn triggers thermal runaway. External overheating refers to the acceleration of the internal chemical reaction of the battery in the presence of high ambient temperatures or external heat sources, which leads to an increase in the temperature of the battery.45Aiming at addressing the above causes of thermal runaway, relevant research has been carried out around the design of electrolyte and electrode materials. For example, the electrolyte is selected as a non-flammable solvent, a highly concentrated electrolyte, a localized electrolyte, an ionic liquid or a solid electrolyte, etc.46 Electrode materials are selected as cathodes with excellent thermal stability, such as LiFePO4, LiFe0.8 Mn0.2 PO4, LiNiPO4 and so on. Although traditional methods can effectively ensure the safety of lithium-ion batteries, they usually come at the cost of low electrochemical performance and high cost.27 Moreover, these methods can only provide one-time safety protection. Therefore, exploring reversible and responsive methods to build safer Li-ion batteries is a challenge for Li-ion batteries.
Smart materials are modern, high-tech materials that can respond to the external environment (e.g., temperature, force, voltage, electric field, etc.) and change and return to their original state when the external stimulus disappears.47 Combining smart materials with lithium-ion batteries to make them thermally responsive and to improve the safe operating life of the batteries is an effective means to prevent thermal runaway. Responsive materials can be classified into four categories according to their functions: (1) mechanically responsive smart materials, based on the principle of reversible phase change, dynamically adjusting the viscosity to resist physical shocks; (2) voltage-responsive smart materials, controlling the voltage below the critical voltage; (3) thermally responsive smart materials, which reversibly and automatically shut down the circuits at high temperatures to impede the transmission of lithium ions; (4) lithium dendrite responsive smart materials, detecting and dissipating dendrites. The working principles of the four types of smart response materials and their related research will be introduced in the following.
Liquid electrolytes with shear thickening properties can be used as high-performance electrolytes with impact resistance. As shown in Fig. 11, a shear-thickening fluid (STF) is a non-Newtonian fluid in which rigid colloidal particles are suspended in a carrier solution, generally at low shear stresses with low viscosity. As the shear stress increases, the viscosity increases dramatically. When the short-range hydrodynamic interactions between the particles exceed the repulsive forces between the particles, shear thickening behavior occurs, and small particles aggregate into clusters to form water clusters, which increases the viscosity of the suspension. When the shear rate is higher than the critical shear rate, a large number of water clusters combine to form larger aggregates, which impede the flow and increase the viscosity. When the shear force disappears, the water clusters decompose and again form a stable suspension.50
 | ||
| Fig. 11 Schematic diagram of the shear-thickened electrolyte process for the three states of (a) static, (b) shear-thinning, and (c) shear-thickening. Reproduced with permission.49 Copyright 2019, Elsevier. | ||
Non-Newtonian shear-thickened electrolytes (STEs) with high ionic conductivity and good electrode compatibility, which can be reversibly transformed to a solid phase under high shear, are able to effectively resist physical shocks, thus avoiding internal short circuits. Liu et al.49 configured an electrochemically stable shear-thickened electrolyte using surface-modified glass fibers (mGFs) as fillers, and this impact-resistant electrolyte has excellent electrochemical stability and good compatibility with commercial electrode materials (LTO, LFP). The researchers tested the impact resistance of the electrolyte under the low-speed impact of a steel ball and the high-speed impact of a bullet, respectively. The results showed that the electrolyte could easily resist external impacts at speeds ranging from 1.1 to 79 m s−1, demonstrating that the electrolyte viscosity could be increased instantaneously under large shear forces. At the sharp increase in shear rate generated by the high-speed impact, the mGFs in the shear-thickened electrolyte formed water clusters, which limited the Brownian motion of the electrolyte molecules. Furthermore, the mGF filler increases the tortuosity of the lithium transport channel, which facilitates the uniform deposition of lithium ions. This shear-thickened electrolyte performs well against external shocks, which is expected to be a candidate for commercial electrolytes.
In contrast to STEs, Magneto-Rheological Electrolytes (MREs) are advanced smart materials capable of modulating their viscosity through a magnetic field to achieve an active response to impact stress. They are characterized by the presence of internal magnetic particles that are able to rearrange themselves in the presence of a magnetic field, thereby changing the physical properties of the entire material, such as its viscosity. Therefore, by adjusting the strength and direction of the external magnetic field, the viscosity change of the MRE can be precisely controlled so that it can actively adapt and respond to various impact stresses. By adding magnetic nanoparticles (e.g., Fe3O4) to the electrolyte and stabilizing it with a small amount of silica nanoparticles, a reversible magnetorheological electrolyte with excellent electrical conductivity and mechanical properties responsive to the field changes was prepared. The MRE exhibits low viscosity in the absence of a magnetic field and increases in viscosity or transforms into a solid phase in the presence of a magnetic field, and this change from a liquid to a solid does not significantly alter the conductivity of the MRE.51 Since the ionic conductivity does not change with increasing magnetic field strength, MREs have good electrochemical stability. Smart materials for coping with mechanical abuse are still in the research stage, and their commercialization requires consideration of their material cost as well as their impact on electrochemical properties, which makes it difficult to promote their application. Mechanical failures come in various forms, so it is crucial to study the mechanical response and failure mechanisms under the coupled effects of various mechanical abuses.
Over-discharge is the most common electrical abuse and can trigger severe thermal runaway if the heat generated continues to accumulate without being released. However, the exact mechanism of discharge-induced heating and battery failure is uncertain. Li et al.53 investigated the heating effect and failure mechanism of soft-packed lithium-ion batteries under over-discharge in electric vehicles. The results show that over-discharge triggers the dissolution of the anode copper collector, and the dissolved Cu2+ passes through the diaphragm and is deposited on the cathode, as shown in Fig. 12. Specifically, the copper dissolution reaction releases electrons, leading to a sharp voltage drop and generating a large amount of heat. The cumulative effect of copper dissolution (exothermic reaction) and copper deposition (heat-absorbing reaction) culminates in an exothermic reaction.
 | ||
| Fig. 12 Principle of thermal runaway caused by over-discharge. Reproduced with permission.53 Copyright 2024, Elsevier. | ||
The main idea to address the overcharging problem is to control the voltage below the peak voltage by establishing a protective voltage. Effective additives can be used in the design to avoid the dangers caused by overcharging, such as redox shuttle additives, electropolymerizers, and so on. A redox shuttle is a chemical overcharge protection agent commonly used in electrolytes and does not affect the electrochemical performance of the battery. When an overcharge occurs in a lithium-ion battery containing a redox shuttle, the closed-shell shuttle molecules are oxidized near the cathode surface, depleting the excess charge completely and thus controlling the cathode voltage. The resulting radical cations then diffuse through the electrolyte to the anode and are reduced to a neutral state.54 This shuttling mechanism prevents danger by depleting excess current during battery overcharging and preventing sharp voltage increases during overcharging. Commonly used redox shuttle additives include (1) organometallic ferrocene derivatives; (2) dihydrophenazine systems; (3) metallocene and dimethoxybenzene derivatives; (4) thiourea alkene derivatives; (5) 2,5-di-tert-butyl-1,4-dimethoxybenzene derivatives; (6) 2,2,6,6-tetramethylpiperidinium oxides and derivatives thereof; (7) triphenylamine derivatives; and (8) lithium borate cluster salts.
Since ferrocene derivatives and lithium halides can only provide voltage control of 3–3.5 V, they are not applicable to commercial lithium batteries.55 In recent years, researchers have begun to explore redox shuttle additives with oxidation potentials higher than 3.5 V. Many aromatic compounds, such as 1,4-dimethoxybenzene (DMB) and its derivatives, show high oxidation potentials (>3.5 V) as well as good cycling performance. Therefore, DMB and its derivatives have begun to be applied to lithium-ion batteries. Chen et al.56 reported the first aromatic redox shuttle applied to lithium-ion batteries. The compound additive was 2,5-di-tert-butyl-1,4-dimethoxybenzene with a redox potential of about 3.96 V. Battery overcharge and discharge experiments showed that LiFePO4/graphite lithium-ion batteries with the addition of 2,5-di-tert-butyl-1,4-dimethoxybenzene shuttle at a concentration of 0.08 M were able to support more than 300 overcharge cycles, and each overcharge accounted for 100% of the battery capacity.
The higher solubility of redox shuttles not only provides overcharge protection over a large current range, but also extends the service life. However, DMB has a low molecular dipole moment and limited solubility (<0.1 M) in commonly used lithium battery electrolytes. Therefore, Zhang et al.53 proposed a highly soluble (0.6 M) 1,4-dialkoxybenzene (DMMB) shuttle molecule, which contains polyether chains that can provide better ionic coordination and polarity, thus improving solubility. During charging, two voltage plateaus were observed at 3.4 V and 3.8 V. The first plateau corresponds to the normal charging cathode of LiFePO4. When the maximum capacity was reached, the voltage rapidly increased to 3.8 V, initiated the redox shuttle of DMMB and controlled the voltage. The DMMB-containing battery withstood 500 overcharge cycles over 3 × 103 hours and retained 80% of its discharged capacity.
Although redox shuttle additives have been studied for more than a decade, the number of compound additives that can be stabilized in lithium-ion batteries is relatively small. Therefore, in-depth study of the relationship between the performance and structure of redox shuttles and the development of additives with different functional groups are future research directions. An electropolymerizer is another additive that is functionally equivalent to a redox shuttle and also provides overcharge protection for lithium-ion batteries. When the battery is overcharged, at the electrode surface, the monomer molecules are excited by the applied potential and a redox reaction occurs. In the oxidized state, the monomer molecules form positive ions or excited states and then react with other monomer molecules. The excited monomer molecules gradually polymerize on the electrode surface to form polymer chains. This polymerization usually occurs on the electrode surface wetted with electrolyte solution, which in turn induces the attachment of polymer monomers and the gradual growth of polymer chains over time at a certain potential, eventually forming a continuous polymer film.57 As the degree of polymerization increases, the polymer film seals the cathode surface and micropores of the diaphragm, disrupting the electrode reaction and internal ion transport, which prevents decomposition of the electrolyte and thermal runaway in the cell. Unlike the redox shuttle mechanism, the electropolymerization protection is irreversible during overcharge.
Electropolymerizers include biphenyl, xylene, cyclohexylbenzene, pyrrole and thiophene. Biphenyl (BP) was one of the first polymer additives studied for overcharge protection. However, biphenyl does not protect batteries well without a safety valve. Cyclohexylbenzene (CHB) provides better overcharge protection than biphenyl when electropolymerized at 4.75 V. Although BP and CHB are common overcharge protection additives in commercial Li-ion batteries, the combination of these two additives can achieve better performance; the BP and CHB overcharge protection additives promote the decomposition of lithium salts at high temperatures and form a thin film on the surface of the electrodes, which can degrade the performance of Li-ion batteries. Therefore, Gu et al.58 developed an effective and safe electrolyte system with excellent energy storage performance at high temperatures by adding some high temperature additives, tris (trimethylsilyl) phosphite (TMSP) and lithium difluoride (oxalate) borate (LiDFOB), to the electrolyte containing BP and CHB overcharge protection additives. Among them, LiDFOB can enhance the stability of the SEI by growing abundant LiF nanoparticles on the SEI. TMSP can effectively remove water and inhibit the decomposition of lithium salts, thus reducing the generation of HF to significantly improve the high-temperature storage performance of the electrolyte. The results show that 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 LIBs with the addition of the modified electrolyte can be stored at 60 °C for 50 days with a capacity retention of 91.36%, and discharged to 0% after returning to room temperature with a uniform morphology and clear particle edges on the electrode surface.
650 LIBs with the addition of the modified electrolyte can be stored at 60 °C for 50 days with a capacity retention of 91.36%, and discharged to 0% after returning to room temperature with a uniform morphology and clear particle edges on the electrode surface.
It's worth noting that electropolymerization additives have higher and more practical polymerization potentials (4.2–5.5 V) than redox additives. Electropolymerization additives can be used in conjunction with SEI film stabilization additives or flame retardant additives to minimize negative effects. Solubility, diffusivity, operating voltage, and effect on electrochemical properties should be considered when selecting overcharge protection additives. Complex polymers with aromatic functional groups, epoxy resins or propionate esters will become a hot topic in the study of overcharge additives for lithium-ion batteries.59 However, this electro-polymeriser can only provide battery protection once when the battery is overcharged. Once the electrodes are covered by the non-conductive polymer film produced by surface electropolymerisation, loss of conductivity of the electrodes and battery failure occur. In contrast, a diaphragm with voltage-responsive functionality can protect against battery overcharging, relying on a reversible phase transition between the conductive and insulating states of the electroactive polymer used as a coating. By incorporating the electroactive polymer into the porous diaphragm, oxidation of this polymer during overcharging causes an internal short circuit. This allows the overcharge current to pass harmlessly through the cell. Upon termination of the overcharge, subsequent reduction of the polymer restores it to an insulating state, thus facilitating normal battery discharge. Zhang et al.60 prepared voltage-sensitive diaphragms with 3.6 V overcharge protection by doping redox-active poly (3-butylthiophene-2,5-diyl) (P3BT) into the micropores of commercial diaphragms. The diaphragm can reversibly switch between electronically insulating and conducting states to keep the charging voltage of LiFePO4/Li4Ti5O12 below 2.4 V and protect the battery from voltage runaway.
Temperature-sensitive electrode materials have received special attention due to their high reliability, cost-effectiveness, and good compatibility with lithium batteries. Thermally responsive electrode materials usually use positive temperature coefficient (PTC) materials as the conductive component of the active material or the surface coating of the collector, whose resistance value increases rapidly as the temperature rises to a certain level. PTC materials are mainly categorized into inorganic ceramic materials dominated by barium titanate and organic polymer materials filled with conductive fillers. They are in an electrically conductive state at room temperature, and when the temperature rises abnormally to a certain value, the PTC material will rapidly change to an insulating state, cutting off the current flow to the electrodes and preventing further heating and possible runaway reactions, which is considered a promising way to improve battery safety issues.61 Li et al.62 prepared a symmetric all-organic battery with overheating self-protection by using poly(3-octylthiophene) (P3OT) as cathode and anode active materials. The all-organic battery was able to charge and discharge normally at room temperature. When the temperature increased to 110 °C, the cell was deactivated and the voltage was maintained at about 0.3 V below the upper cut-off voltage limit of 2.0 V. The cell was also protected from overheating by the P3OT. Similarly, the thermal response behavior of the battery during discharge at 110 °C was tested, and the discharge voltage dropped to a cutoff voltage of 0.01 V. The all-organic batteries will be rapidly switched off. And after returning to room temperature, the battery was able to operate again. The researchers analyzed the working principle of this thermoresponsive battery: high-temperature PF6− was de-doped from the main skeleton of P3OT, to make all-organic batteries maintain a low power state during the entire charging process, and during over-discharge the battery reaction shut down rapidly. This thermoresponsive material can provide overheating protection for the battery at high temperatures. When the battery temperature rises abnormally, the PTC material inside the battery can block the electron transfer between the collector and the cathode active layer. Another mechanism to block ion transfer between electrodes is through thermo-responsive polymer microspheres coated on the anode or diaphragm of the battery. When thermoresponsive polymer microspheres (polyethylene or paraffin microspheres) are doped onto the battery anode or diaphragm, and a critical temperature is reached inside the battery, the microspheres melt and form a non-conducting barrier between the anode and the diaphragm, blocking lithium-ion transfer and permanently shutting down the battery,63,64 as shown in Fig. 13.
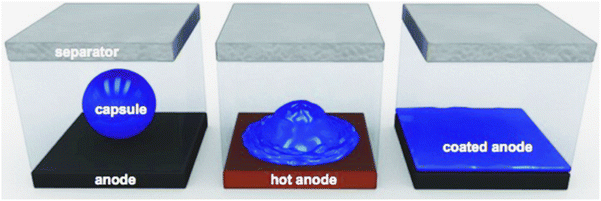 | ||
| Fig. 13 Schematic of a microsphere-based lithium-ion battery shutdown. Reproduced with permission.63 Copyright 2012, Wiley. | ||
Commercial thermal shutdown diaphragms shrink and risk electrode short circuits, and ionic conduction is impeded by the formation of a polymer film on the electrode surface. Unlike commercial thermal shutdown diaphragms, microsphere technology can not only be used in conjunction with multilayer diaphragms, but can also be used to optimize the shutdown response by setting cell-specific trigger temperatures, shutdown rates, and thermo-mechanical stability.
In the liquid electrolyte, organophosphates are generally used as flammability-resistant solvents or additives for the electrolyte. At high temperatures, organophosphates evaporate into gaseous form and decompose to generate phosphorus-containing free radicals, which then combine with hydrogen free radicals to suppress combustion. The working principle of a fluoride electrolyte is similar, where it turns into gaseous form at high temperatures and decomposes to generate fluorine-containing free radicals, which can clear hydrogen free radicals and thus hinder the free radical reaction that sustains combustion. Research has shown that adding phosphazinic fluoride as a flame retardant to the electrolyte can also have good flame-retardant efficiency, and also helps to improve the electrochemical performance of the battery.65Table 2 compares the advantages and disadvantages of non-flammable liquid electrolytes.
| Liquid electrolytes | Advantages | Disadvantages |
|---|---|---|
| Organophosphate electrolytes | Cheap | Low flame retardancy and easy side reactions |
| Fluoride electrolytes | Good electrochemical stability and flame retardancy | Expensive |
| Fluorinated phosphazene flame retardant | Good electrochemical stability, high flame retardant efficiency | Expensive |
Several thermally responsive materials have been developed, such as thermally sensitive materials, thermal shut-off membranes and thermally responsive liquid electrolytes, which have better battery safety under conditions of thermal abuse. However, due to uneven lithium plating/stripping behavior, most of them have poor cyclic stability.66 Compared with liquid electrolytes, solid electrolytes are inherently safe electrolyte systems because of their lack of leakage, high mechanical strength and low reaction heat production. However, solid electrolytes such as polymers, sulfides and oxides still have some problems, such as low ionic conductivity, large interfacial impedance and difficult preparation. Therefore, the development of polymer electrolytes with thermal response characteristics is an effective strategy to avoid thermal runaway and enhance the electrochemical performance of lithium batteries.67 Compared with liquid electrolytes, solid electrolytes are essentially safe electrolyte systems due to their non-flammability, non-leakage and low reaction heat generation characteristics. However, solid electrolytes such as polymers, sulfides, and oxides still suffer from low ionic conductivity, high interfacial impedance, and difficult preparation. Therefore, the development of polymer electrolytes with thermally responsive properties is an effective strategy to avoid thermal runaway and enhance the electrochemical performance of lithium batteries. For example, poly(N-isopropylacrylamide) (PNIPAM), with a critical solution temperature (LCST) of about 32 °C, changes from a swollen, hydrophilic state to a contracted, hydrophobic state at high temperatures due to the disruption of hydrogen bonding between N-isopropyl and water, forming a hydrogel, which inhibits the migration of conductive ions and eliminates the conductive pathway between electrodes.63 The reversible phase transition of PNIPAM can be used for self-protection of electrochemical energy storage devices against overheating. The reversible sol–gel transition of polymers can also be used for thermal protection of electrochemical energy storage devices. At low temperatures, the thermoresponsive electrolyte is in the liquid state and conductive ions can migrate freely through the polymer solution. When the temperature increases, the crosslinking of the polymer chains undergoes a sol–gel transition, which inhibits the free movement of conductive ions in the system, thus preventing thermal runaway.68 Pluronic [poly(ethylene oxide)-block-poly(propylene oxide)-block-poly (ethylene oxide) (PEO-PPO-PEO)] aqueous solutions have been used to develop thermally responsive smart electrolytes. Pluronic-based electrolytes transform into a gel state at elevated temperatures, inhibiting the migration of ions and ultimately shutting down the electrochemical devices.69
Diaphragms play an important role in facilitating the transfer of lithium ions between the cathode and anode of lithium-ion batteries. Conventional commercial diaphragms have a low melting point and poor thermal stability, melting and shrinking between 135 and 170 °C. The melting point of the diaphragm is higher than the battery's internal temperature. When the internal temperature of the battery is higher than its melting point, a large thermal contraction occurs, and eventually the cathode and anode of the battery come into direct contact with each other, leading to internal short circuits and thermal runaway as the temperature continues to rise.70 The result is an internal short circuit and a continuous rise in temperature leading to thermal runaway. Therefore, building a thermally responsive diaphragm is crucial for lithium batteries. Paraffin wax (PW), as a phase change material with suitable melting point (∼40 °C) and high latent heat (∼212 J g−1), can be used as a thermo-responsive material for heat absorption to efficiently regulate the heat in the battery. Huang et al.71 prepared a temperature-regulated nanofiber diaphragm for lithium-sulfur batteries, which effectively solved the problem of elevated internal battery temperature. The diaphragm was prepared by encapsulating PW in polyacrylonitrile (PAN) nanofibers, and then vacuum-filtering UiO66/black phosphorus heterostructure (UiO66@BP) hybrids onto the surface of the electrostatically spun diaphragm. The UiO66@BP heterostructure not only inhibits the dendritic growth and shuttling effect of LiPSs, but also has high ionic conductivity due to the high porosity of the nanofibrous backbone. The melting process of PW can absorb a large amount of heat, so the temperature-regulated diaphragm can alleviate the internal temperature rise in a timely manner, and the heat storage principle is shown in Fig. 14, which absorbs heat when the temperature rises and melts into a liquid state, and releases the heat, and then cools down and solidifies.
 | ||
| Fig. 14 Thermal storage principle of core-sheath nanofibers. Reproduced with permission.72 Copyright 2023, Elsevier. | ||
After exposure to 180 °C for 10 minutes, the commercial diaphragm melted at 163.7 °C and contracted severely. The temperature-modified diaphragm, on the other hand, showed excellent thermal stability with PAN melting at up to 250 °C, which means that the PAN nanofiber shell enables the temperature-modified diaphragm to operate safely at high temperatures. In a long cycling test under variable temperature conditions, the modified diaphragm showed stable overpotentials throughout the cycling time, indicating that the diaphragm can self-regulate internal temperature variations and contribute to a stable SEI layer. Therefore, the temperature-modified diaphragm has promising applications in wearable electronics.
It is difficult to detect the growth of lithium dendrites in conventional batteries, and conventional polyolefin diaphragms with non-uniform pores and moderate modulus are not sufficient to prevent dendrite diffusion in lithium-metal batteries. Therefore, functionalizing the diaphragm by modifying it to give the diaphragm the function of detecting lithium dendrite growth at an early stage is an important strategy to avoid internal short circuits caused by lithium dendrites. Wang et al.74 used red phosphorus (RP) as a coating to modify the diaphragm, and the RP-functionalized coated diaphragm was prepared to give the battery the function of detecting lithium dendrites. No additional electrodes are required, and in situ detection of lithium dendrites can be performed based on the voltage profile, as shown in Fig. 15(a) and (b). Significant voltage changes (>2 V) can be observed when lithium dendrites are exposed to the RP coating at a rate of 5 mA cm−2.
 | ||
| Fig. 15 (a) Full cell test with RP coating and (b) without RP coating. Reproduced with permission.74 Copyright 2019, Wiley. | ||
For detectorized diaphragms, further improvements should focus on the development of diaphragms that are more responsive and reliable, which requires a number of smart designs to enhance the compatibility of the integrated diaphragm with other smart components in the lithium battery. In addition to detecting the formation of lithium dendrites, functional diaphragms that inhibit the formation of lithium dendrites at an early stage are another effective means of avoiding thermal runaway. In recent years, researchers have developed functional diaphragms that inhibit the growth of lithium dendrites based on the following principles: (1) development of polymer diaphragms with high strength and high Young's modulus; and (2) homogenization of lithium flux through precisely designed nanopore structures.75 According to the Monroe–Newman theory, the interfacial strength can only inhibit the growth of lithium dendrites when the Young's modulus of the diaphragm is two times higher than that of lithium metal.76 Therefore, Wang et al.77 prepared high-performance poly(vinyl alcohol) composite diaphragms (OPVA/NHNTs diaphragms) with nanostructured halloysite nanotubes (NHNTs), considering both the importance of Young's modulus and ionic conductivity in inhibiting the growth of lithium dendrites. As shown in Fig. 16(a), the hollow channels created by chemical etching of the nanotubes endowed them with higher ionic conductivity and were able to homogenize the Li+ flux and promote the uniform distribution of lithium ions. Compared with the low ionic conductivity (0.17 mS cm−1) and low tensile strength (5 MPa) of commercial diaphragms, the OPVA/NHNTs diaphragm exhibits both high tensile strength (12.5 MPa) and ionic conductivity (1.14 mS cm−1), which can effectively retard the growth of lithium dendrites and maintain electrochemical performance, as shown in Fig. 16(b–d). Commercial diaphragms exhibit poor Young's modulus distribution, while Fig. 16(e) shows that OPVA/NHNTs diaphragms have a higher average Young's modulus of about 50 GPa. Different diaphragm assemblies were tested for Li‖Li symmetric batteries, and Fig. 16(f) shows that commercial diaphragms exhibit high and unstable voltage distribution and poor constant current cycling performance due to low Young's modulus and ionic conductivity. In contrast, the OPVA/NHNTs diaphragm has an ultra-stable voltage–time profile with the lowest overpotential around 10 mV, indicating that the diaphragm with high Young's modulus and ionic conductivity can effectively inhibit the growth of lithium dendrites and improve the safety of lithium metal batteries.
 | ||
| Fig. 16 (a) Schematic diagram of cell lithium dendrites for OPVA/NHNTs diaphragms. Tensile strength (b) and ionic conductivity (c) of Celgard diaphragms and OPVA/NHNTs diaphragms. (e) Statistical plots of Young's modulus for commercial diaphragms (d) and OPVA/NHNTs diaphragms (e). (f) Cycling performance of Li//Li symmetric cells with commercial and OPVA/NHNTs diaphragms at 1 mA cm−2. Reproduced with permission.77 Copyright 2023, Elsevier. | ||
Kong et al.78 used Co/MoN nanoparticle composites as modifiers to modify commercial diaphragms. Co/MoN@PP diaphragms not only effectively inhibit the shuttling of polysulfides (LiPSs) and accelerate their electrochemical conversion, but also homogeneously regulate Li+ flux and inhibit the growth of lithium dendrites. The cycling stability of Li//Li symmetric cells with various diaphragms was measured at a current density of 1 mA h cm−2, and the results are shown in Fig. 17, where cells with commercial diaphragms show progressively increasing overpotentials and short-circuits after 250 hours. In contrast, the cell with Co/MoN@PP diaphragm exhibited a low overpotential of 26 mV and a long cycle life of 800 h as well as smaller and more stable polarization vibrations. The detector and ablative diaphragms provide methods to inhibit the growth of lithium dendrites. Although many effective designs have been demonstrated, many improvements are needed before these smart materials can be applied, and research should be focused on reversible and self-protective mechanisms for inhibiting lithium dendrites.
 | ||
| Fig. 17 Cycling performance of Li//Li symmetric cells with various diaphragms at 1 mA cm−2. Reproduced with permission.78 Copyright 2023, Wiley. | ||
Many researchers have thoroughly investigated the composition and formation process of the solid electrolyte interface layer (SEI) and studied the effects of different components of the SEI on the lithium metal anode. The results show that the different components of the SEI exhibit different chemical and electrochemical properties, which affect the transport of lithium ions, deposition and inhibition of lithium dendrites.79 The researchers proposed strategies to enhance the performance of the SEI, such as electrolyte additives, solid-state electrolytes, and surface coatings to construct an artificial SEI in situ or ex situ at the interface between the anode and electrolyte.80 Polyvinyl alcohol (PVA) is commonly used to construct in situ SEI protective layers on lithium metal anodes due to its good elastic properties and excellent film-forming properties. However, the low mechanical modulus and ionic conductivity of PVA do not meet the requirements for an ideal artificial protective layer. Lithium fluoride (LiF), which has high mechanical strength and low solubility, is widely used for lithium metal anode protection and can provide lower in-plane Li nucleation barriers and higher interfacial energies, thus guiding layer deposition and inhibiting lithium dendrite growth.81 Therefore, Liu et al.82 constructed a PVA/LiF composite artificial protective layer in situ by introducing LiF particles to improve the ionic conductivity and mechanical strength of the PVA matrix, thereby isolating the lithium metal anode from direct contact with the electrolyte. As shown in Fig. 18(a), the PLF-modified cell exhibited smaller polarization (40 mV) and longer cycle life (more than 800 h) in the symmetric cell test at 1 mA h cm−2, suggesting that PLF provides a more stable interface. In contrast, the symmetric cell with bare copper exhibited a rapidly increasing hysteresis in the overpotential after 170 h, suggesting the formation of a highly resistive interfacial layer that further promotes dendrite growth and electrolyte depletion.
 | ||
| Fig. 18 (a) Electrochemical performance of bare Cu with a Li‖Li symmetric cell having PLF modification at 1 mA h cm−2.83 (b) Electrochemical performance of bare Li with a Li‖Li symmetric cell having TPU coating modification at 1 mA h cm−2. (c) Surface morphology of a bare lithium electrode and (d) lithium electrode with TPU coating modification after the 1st, 50th and 200th cycles. Reproduced with permission.84 Copyright 2023, Elsevier. | ||
The advantage of an artificial SEI layer constructed by ectopic construction is its ability to form under ambient conditions prior to assembly. This helps to better control the thickness, structure and composition of the protective layer without being limited by the inert gas environment. Thermoplastic polyurethane (TPU) is a block polymer consisting of hard and soft segments with high elasticity and high abrasion resistance, and has become one of the important thermoplastic elastomer materials.85 The flexible soft segments give the material rubber-like properties, while the hard segments provide excellent modulus of elasticity, high tensile strength, good chemical stability and the ability to easily form films. It was found that the TPU material can not only inhibit the growth of lithium dendrites and adapt to the volume expansion of the lithium anode, but also promote the uniform deposition of Li+ flux through the high elasticity of the material conferred by the hard segment. Therefore, Zhao et al.86 used TPU materials for the construction of an artificial SEI layer, where the soft segment poly(ethylene oxide) (PEO) in the polymer structure provided ion transport channels, while the hard segment isophorone diisocyanate (IPDI) conferred high elasticity and flexibility to the coating, and the addition of a small amount of LiF salts to the solution inhibited the growth of lithium dendrites. The synergistic effect of the soft and hard segments ensures the long-term stable cycling performance of lithium-ion batteries. As shown in Fig. 18(b), the lithium symmetric battery with TPU coating achieved a stable long-term cycling performance of 1300 h at a current density of 1 mA h cm−2, while the bare lithium electrode started to increase the polarization voltage around 600 h and reached 50 mV at 800 h. The bare lithium after the 1st, 50th and 200th cycles (Fig. 18(c)) was compared to the electrode with TPU coating (Fig. 18(d)), and it was observed that the surface morphology of the bare lithium electrode showed obvious roughness at the end of the 1st cycle, and obvious lithium dendrites and cracks appeared on the lithium surface as the number of cycles increased. In contrast, the surface of the lithium electrode with TPU coating remained smooth, showing the superiority of the artificial SEI layer in inhibiting lithium dendrite growth. Table 3 summarizes the principles and applications of intelligent response materials.
| The type of response | Principle | Main applications |
|---|---|---|
| Mechanical response | Reversible phase change | Shear-thickened electrolytes, magnetorheological electrolytes |
| Voltage response | Establish a protection voltage | Redox shuttle, electropolymerizer |
| Thermal response | Thermal shutdown circuitry | Thermal electrodes, polymer electrolytes |
| Dendrite detection and digestion | Introduce functional groups | Inorganic fillers, thermoplastic polyurethane elastomers |
4 Smart manufacturing in batteries
4.1 3D printing technology
With the rapid development of society, the energy density and cycling performance of batteries can no longer meet the demand; the slow ion diffusion rate, poor long cycle life, high production cost, and other problems in battery materials are becoming more and more prominent. The capacity per unit area of the battery electrode prepared by the traditional process is low, so the energy density is also low. In order to obtain higher energy storage, it is necessary to increase the size of the battery or improve the thickness of the electrode, but in practical applications, the occupied area of the battery is fixed, and increasing the thickness of the electrode will impede the diffusion rate of ions, which seriously affects the rate performance and power density of the battery.87 Therefore, the development of low-cost, high-energy-density,88 rollable89,90 and high-power-density batteries has become a new research hotspot. In addition to traditional processes, battery manufacturing includes 3D printing technology, electrostatic spinning,91 screen printing92 and other new technologies.3D printing is a technology based on digital programming and manufacturing procedures that uses bondable materials such as powdered metals, plastics, or resins to design patterns and shapes by printing them layer by layer, and then realizing them using high-precision equipment. 3D printing technology can control the precise tuning of material geometries from the macro-scale to the nanoscale, and as a result, the technology can break through the limitations of traditional manufacturing processes and print many structures that would not be possible with traditional processes. 3D printing opens new avenues for rapidly building complex, designable structures and is already being used in a variety of fields, including medicine, electronics, industry and aerospace. This precise, comprehensive and designable process is critical to the advancement and commercialization of energy storage devices, including miniature, solid-state and flexible batteries. The application of 3D printing technology in battery material preparation allows the cathode, anode and electrolyte of the battery to present a complex three-dimensional structure in space, ensuring that the thickness of the electrode material can be increased to increase the energy density in an effective volume space, and also shortening the ion transport distance between the cathode and anode, increasing the transfer rate and enhancing the rate performance of the material. Therefore, 3D printing opens up a new way of thinking for the rapid manufacture of three-dimensional structured batteries with complex structure and excellent performance.
The geometry of the battery electrodes plays a key role in determining the application and performance of the battery.95 The two basic performance metrics for batteries are energy density and power density. However, increasing energy density negatively affects power density. This is due to the fact that ions are transported over longer distances within the battery structure, which ultimately hinders the rate of energy transfer. Therefore, cell geometry can be tuned to create a balance between power density and energy density. The geometry of a cell is determined by the structure of its components, including thin films and the design of 3D porous structures.96 As these component architectures are combined, they give rise to different cell–cell structures such as sandwich, in-plane, concentric tubes and fiber arrangements. Among these, thin-film structures and porous frames (grids) stand out as the most common and important forms.95 Thin-film structures are among the most widely recognized structures that are readily available in the market and can be manufactured by conventional methods.97 They are made by stacking rectangular electrodes on top of each other, which improves performance by reducing surface area. This structure has the significant advantage of reducing resistance and shortening the ion diffusion length, which contributes to higher power density.98 This structure has the significant advantage of reducing resistance and shortening the ion diffusion length, contributing to higher power density. In contrast, the energy density of this structure is relatively low, which stems from the limited space in which the ions can move. To increase the overall energy density, conditioning and further modifications in the film are required.99
Porous structures represent an innovative geometry that can be efficiently fabricated by techniques such as 3D printing compared to conventional methods, which often struggle to control complex geometries. Fabricating pores of various scales in the structure and increasing the electrode thickness facilitates ion transport within the structure, balancing energy and increasing power density. Another advantage of the design is its electrolyte penetration capability, which enhances the ability of ions to participate in electrochemical reactions and improves battery performance.100
3D printing can have more precise control over the design and can increase the load of the active material inside the structure in a smaller volume, resulting in a higher energy density.106 The ability of 3D printing to control the geometry of the battery assembly is also important. On the other hand, the ability of 3D printing to finely control the geometry of the cell module plays a key role in increasing the energy conversion rate inside the structure, ultimately leading to higher power density.107 The arrangement of the electrodes and the uniform distribution of the active material affect the charge/discharge cycle of the battery and thus the cycle life. In addition, designing specific geometries through 3D printing technology can improve thermal management, prevent overheating, and enhance safety. In addition, proper diaphragm and electrolyte design, as well as internal pressure management mechanisms, contribute to improved safety and long lifespan.108
One of the advantages of 3D printing is the controllability of the design, which leads to the customizability of the structure. In addition, depending on the method and the resolution of the device, it is possible to control the size and manufacture parts in a wide range of scales for the production of miniaturized batteries.109 3D printing offers significant efficiencies compared to traditional methods, which include paste preparation, cast molding, material drying, calendering, material cutting, assembly, electrolyte filling, and final encapsulation. During the 3D printing process, depending on the chosen 3D printing method, the steps include material preparation, part geometry design, 3D printing, assembly, and optional electrolyte filling. One of the advantages of 3D printing in battery production is the reduction in fabrication time, which is attributed to the simplicity of the process and the small number of steps. Nonetheless, it is worth noting that the overall completed manufacturing time depends on the specific methodology used and post-processing requirements.110
Using computer-driven design, manufacturing batteries through 3D printing methods can minimize material loss, thereby reducing production costs and promoting environmental sustainability. Solid-state batteries use solid-state electrolytes instead of liquid electrolytes, offering high dimensional integrity, excellent mechanical properties, and high safety.111,112 3D printing technology, with its precise accuracy and design control, facilitates the design and fabrication of solid-state electrolytes compatible with electrode structures, where all components can be printed and stacked on top of each other, resulting in an all-solid-state battery. This approach eliminates the need for a glove box, making production more cost effective and environmentally friendly.112 One of the key benefits of 3D printing is the ability to fabricate battery components using novel materials. This unique ability allows researchers to explore cutting-edge materials in battery architectures with high precision, which not only facilitates rapid prototyping, but also opens up the possibility of developing next-generation energy storage solutions using innovative materials.113
4.2 Introduction to battery 3D printing technology
3D printing, as a new manufacturing process, has been widely used in automotive, medical, aerospace and other fields. Depending on the principle of operation, 3D printing can be divided into several types: (1) inkjet printing, (2) direct ink writing, (3) fused deposition modeling, (4) powder laser sintering, (5) photopolymerization and so on. Considering the unique manufacturing requirements of batteries, not all 3D printing techniques are suitable. This chapter describes the above five 3D printing technologies used to fabricate battery components and how they work, and presents the progress and results of researchers' studies on batteries using printing technologies.As an emerging cell preparation process, IJP has the advantages of customizable patterning and high material utilization.115 The composition, fluidity, and curing speed of the ink affect the performance of the printed cell. Currently, IJP inks have realized a wide selection of raw materials, including metallic, organic and inorganic materials.116 However, the ideal ink must meet specific viscosity, surface tension and density requirements. Failure to do so may lead to problems such as nozzle clogging, compositional inhomogeneity or structural instability. In addition, the stability of the ink droplets and the speed of curing also affect the structure of the object in the print cell, which is more pronounced in the case of thick electrodes and high loads.117 The IJP utilizes a print-on-demand ink supply that meets different design requirements and saves ink. As a result, IJP is now used in the fabrication of thin-film electrodes, interface layers and positional spraying. In addition, IJP can directly deposit functional nanomaterials on flexible substrates, an environmentally friendly and low-cost process that has attracted the interest of researchers.
Tao Chen et al.118 obtained zinc metal anode materials with excellent electrochemical properties by printing Ag nanoparticles on a 3D conductive skeleton by an inkjet printing technique, which was used to dynamically guide Zn nucleation and avoid dendrite growth, as shown in Fig. 19(a). The basic idea is to utilize Ag nanoparticles as heterogeneous metal seeds to induce homogeneous Zn nucleation at the initial plating stage. At the same time, Ag can react with Zn to form a Zn-friendly AgZn3 alloy, which can be used as a Zn source to offset the irreversible loss of active Zn during the cycling process. The coulombic efficiency of the half-cell using Ag-modified carbon cloth can be maintained at ≈99.5% when cycling 800 cycles at a current density of 5.0 mA cm−2 and a capacity of 2.0 mA h cm−2. In addition, the full cell assembled with NaV3O8·1.5H2O as an anode pair has remarkable cycling stability as well as high-temperature resistance, as shown in Fig. 19(b) and (c).
 | ||
| Fig. 19 Schematic of inkjet printable (a) AgNPs@CC print synthesis. (b) Temperature distribution image of pure CC film. (c) Temperature distribution image of AgNPs@CC film. Reproduced with permission.118 Copyright 2021, Wiley. | ||
Athanasios Goulas et al.121 fabricated solid electrolytes by using sodium polyaluminate ceramics by direct ink writing addition, and electrolyte samples were tested to show σ = 0.14 ± 0.019 S cm−1 ionic conductivity with a density of ρ = 3.1 ± 0.02 g cm−3, as shown in Fig. 20. This work demonstrates the unique ability of direct ink writing in molding ceramic electrolytes, offering the possibility of one-stop manufacturing for multilayer and multi-material fabrication of all-solid-state battery structures necessary to meet future energy storage needs.
 | ||
| Fig. 20 Direct ink writing type: (a) ionic conductivity graph. (b) Electrolyte density graph. Reproduced with permission.121 Copyright 2024, Elsevier. | ||
FDM is a widely used 3D printing technology with the advantages of fast printing speed and low cost. Currently, FDM is widely used in additive manufacturing of devices in the aerospace, automotive and medical fields. The layer thickness resolution of the first layer of most FDM prints usually reaches 200 μm. The layer thickness resolution of successive layers can often be increased to as high as 50 percent.123 Due to the printability requirements of the FDM process, the active substance loading in the final fabricated electrode pole piece is kept relatively low, which seriously affects the electrochemical performance. For this problem, many researchers have overcome it by introducing plasticizers. Alexis Maurel et al.124 developed and optimized to print complete lithium-ion batteries with LFP-PLA and PLA-SiO2 composite 3D printing filaments by FDM (Fig. 21). In addition, the diaphragm geometry was designed to improve the electrolyte uptake by taking advantage of 3D printing's ability to design patterns. Lithium iron phosphate/polylactic acid (LFP/PLA) and SiO2/PLA filaments were produced by fused deposition technology, which can be used as a cathode and diaphragm, respectively, in lithium-ion batteries. The active material content in the cathode filaments is significantly increased, while adequate mechanical properties can be provided, and the diaphragms show higher conductivity and specific capacity.
 | ||
| Fig. 21 Fused deposition modeling: schematic diagram of fused deposition 3D printing: (1) after mixing the components into a solvent, the slurry is applied to the glass support using the scraping method and finally formed into a film; (2) the composite film homogeneous sheet is introduced in an extruder. A typical 1.75 mm diameter 3D printing filament was obtained and rolled; (3) the fiber filament was introduced into a commercial FDM 3D printer. Reproduced with permission.124 Copyright 2019, Nature Health. | ||
SLS/SLM eliminates the need for high-quality inks, thus avoiding the disadvantages inherent in printing through ink. As a result, SLS/SLM technology can be used to develop cells with high loads and stringent precision parameter requirements. In addition, the degree of sintering and defects are challenging for further diffusion of the laser printing technology. Li Cao et al.126 addressed the challenges of agglomeration and low adhesion strength, which exist in conventional slurry-coated anodes by powder bed melting technology. The work consisted of preparing an Al–Si–Cu alloy layer on a copper foil collector and then dealloying it to form a porous Si–Cu anode, as shown in Fig. 22(a). The experimental results showed that the porous Si–Cu alloy was formed by optimizing the laser spot (55 μm) and powder size (1–5 μm), and the alloy layer was successfully formed. Controlled cooling yielded incipient Si particles ranging from 150 nm to 1 μ m. The Si particles were then deposited on the surface of the substrate. The resulting microstructure enhanced the electrochemical properties, especially by tuning the size of the incipient Si. The resulting porous Si–Cu anode has a metallurgical combination of uniformly distributed incipient Si (200 nm) and Cu network, with a first-time coulombic efficiency of 83% at 2C multiplicity and a capacity retention of 80% after 300 cycles, as shown in Fig. 22(b).
 | ||
| Fig. 22 Powder laser sintering: (a) schematic diagram of the powder laser sintering process for the dealloying process. (b) Long cycle curve of the A-D55-Si27 anode. Reproduced with permission.126 Copyright 2025, Elsevier. | ||
SLA is characterized by rapid prototyping and ultra-high precision. Therefore, high precision 3D structures can be obtained based on the photopolymerization process. In addition, another advantage of SLA is that it has a high resolution of 0.5 μm, which makes it very suitable for the manufacturing of cells with complex geometries.129,130 There is also the problem of printing a single raw material since the printing raw material is a photosensitive resin. Therefore, the active component can be dispersed in the resin or loaded with the active component by direct heat treatment to make the printed structure catalytically active. In general, 3D printing resins are generally mechanically stable. Ishamol Shaji et al.131 prepared electrodes by a photopolymerization 3D printing process using low reactivity diallyl ether functional groups, as shown in Fig. 23(a). The slow kinetics of the allyl portion prepared by the photopolymerization 3D printing process was improved,as shown in Fig. 23(b), and the rate of polymerization was increased by at least 50% within the first 10 min of the photopolymerization reaction compared to the classical UV curing reaction (Fig. 23(c)). SPE integrated electrodes were prepared using an in situ photopolymerization process with C-LiFeO4 and nickel–cobalt–aluminum (NCA) as the anode to achieve high specific capacity and low interfacial resistance. Table 4 summarizes the comparison of 3D printing technologies.
 | ||
| Fig. 23 Photopolymerization type: (a) schematic diagram of the photopolymerization 3D printing process. (b) Thermal images of different stages of the photopolymerization reaction of allyl ether oligomers collected at different time intervals using dual initiators. (c) Monomer conversion versus curing time for free radical photopolymerization. Reproduced with permission.131 Copyright 2022, Wiley. | ||
| 3DP | Precision/μm | Printing material | Key issue |
|---|---|---|---|
| IJP | 5–200 | Viscous liquid (metallic, organic and inorganic materials) | Meet specific viscosity, surface tension and density requirements |
| DIW | 1–400 | Various high-shear thinning fluids | Harsh requirements for inks |
| FDM | 50–400 | Thermoplastic materials (PC, PA, PLA, ABS) | Low precision |
| High material limitation | |||
| SLS | 0.5–20 | Ceramics, metals, or specialty polymers | Poor continuity and densification, rough surface and internal defects |
| SLA | 0.4–30 | Photosensitive resin | Low efficiency, low stability and raw material uniformity |
4.3 Towards a practical 3D printing technology
The use of a single 3D printing technology is often inefficient and the printed objects are unstable. For example, it is impractical for lithium battery manufacturing because layer-by-layer printing requires long curing times, gradient structures tend to collapse during rolling, and multi-system printing requires additional processes. Composite printing technology combines 3D printing technology with traditional processes or other printing techniques to improve the performance of the printed object or optimize the overall process. In addition, cutting-edge printing technologies in other research areas, such as TPL, fixed-point lasers, and composite printing, are inspiring for future LIB manufacturing. Among the printing technologies in the field of LIBs, DIW and IJP are the most widely used at present; however, the printing precision is limited by ink characteristics and printhead size. Therefore, it is important to explore and develop ultra-precision printing technologies. For example, TPL is an advanced lithography technology. Unlike other lithography techniques, TPL relies on a two-photon absorption mechanism less than the diffraction-limited target spot using a focused infrared femtosecond laser. The photoinitiator absorbs two photons up to a specific wavelength and polymerizes, stimulating polymerization only at the target spot without affecting other areas. As a result, TPL can print microstructures with feature sizes smaller than 3 nm with much higher precision than other printing techniques. However, experiments have shown that it takes several hours to print a unit volume of electrodes, and the fast printing speed is an important issue affecting the application of TPL to batteries. In addition, a pioneering 3DP called “Continuous Liquid Interface Printing” achieves printing speeds up to 100 times faster than currently available SLA, relying on an oxygen-permeable transparent window at the bottom of the resin vessel and creating a “dead zone” in which dissolved oxygen inhibits polymerization. By continuously projecting a UV image underneath the resin bath, a reaction occurs within a thin transparent window and the cured print is pulled out. The process does not rely on the layer-by-layer print curing of typical 3D printing processes such as composite lithography, composite nozzle, freeze drying – IJP, electrostatic spinning – DIW, etc., and allows for the continuous formation of solid–liquid interfaces, dramatically accelerating the production process.4.4 3D printing in batteries
This section analyzes in detail the relevant applications of 3D printing for different modules in batteries and discusses the performance of the printed modules, especially the performance impact on lithium metal batteries.Compared with traditional coated electrodes, 3D printed cathodes have significant advantages in terms of structure control precision, which can optimize ion transport, achieve controllable and complex 2D electrodes with high surface activity or homogeneous 3D electrodes with high loading, and make full use of the limited space and create porous structures, which ultimately lead to batteries with high energy density and high power density. Different 3D printing technologies meet different material and performance requirements. 3D printed anodes have been successfully applied to LIBs, LMBs, Li–O2 batteries and zinc batteries. In the field of lithium-ion batteries, several major cathode materials have been successfully printed, including LiFePO4, LiCoO2, lithium manganate and nickel–cobalt–aluminum. When printing to make electrodes, the composition and fluidity of the ink must be carefully regulated, which ensures reliable ink flow through the deposition nozzle, promotes layer-to-layer adhesion, and provides the structural integrity necessary to withstand later drying and sintering without delamination or distortion.
Zhen Liu et al.133 prepared a high-precision MnO2 cathode to overcome the problems of poor cycling stability and ion diffusion of the MnO2 cathode in aqueous zinc ion batteries by a direct ink printing method (Fig. 24). The experimental results show that the customized mesh-layer structure still maintains the original shape after 100 cycles. In addition, the 3D-printed structure with excellent mechanical strength can effectively alleviate internal stress and provide a larger specific surface area. The specific capacity of the 3D-printed cathode was three times higher than that of the 2D-printed cathode after 110 stable cycles at a certain current density. This electrode preparation method provides a new idea for the further application of zinc ion batteries.
 | ||
| Fig. 24 Printed cathode schematic diagram of the MnO2 cathode 3D printing process and photos of printed products. Reproduced with permission.133 Copyright 2023, Elsevier. | ||
Tiansheng Mu et al.135 designed and constructed self-supported silicon-graphene electrodes with ultra-high area capacity by modulating the electrode structure through 3D printing technology. A highly concentrated aqueous graphene oxide solution containing silicon nanoparticles was used as a printable ink, and the mass loading and printing filament spacing in the 3D printed lattice silicon-graphene electrode were controlled by commands from a computer, as shown in Fig. 25. In this structure, the enriched porous hierarchy and 3D porous scaffolds effectively mitigate the volume expansion and contraction of the silicon anode. In addition, the unique edge coaxial and internal disordered structure in the printed wire greatly enhances the mechanical stability of the electrode. The 3D-printed Si/G-1 electrode achieves an ultra-high specific area capacity of 16.2 mA h cm−2. The 3D printing process provides favorable conditions for the commercialization of silicon-anodes.
 | ||
| Fig. 25 Schematic diagram of the 3D-Si/G electrode preparation process. Reproduced with permission.135 Copyright 2022, Elsevier. | ||
Kenny Lee et al.137 prepared solid polymer electrolytes consisting of nanoscale ion-conducting channels embedded in a rigid cross-linked polymer matrix by photopolymerization 3D printing. A visible-light-mediated polymerization reaction-induced microphase separation method was employed to produce materials with two chemically independent nanodomains and highly tunable nanostructures, as shown in Fig. 26(a). By preparing materials containing ionic liquid-solubilized poly(ethylene oxide) domains, materials with excellent room temperature (22 °C), high shear modulus (>108 Pa) and up to σ = 3 × 10−4 S cm−1 ionic conductivity of solid polymer electrolytes were obtained, as shown in Fig. 26(b). This work demonstrates that on-demand fabrication through 3D printing technology can simplify the process steps for solid polymer electrolytes with customized geometries.
 | ||
| Fig. 26 Printed solid-state electrolyte: (a) photographs of the heated plate of the multijet printing system and the 3D printing device printer; the inlays show annular collectors with a single structure, a parallel structure, and a series structure, respectively. (b) Photographs of the flexible cell in different bending states. (c) Ionic conductivities as a function of temperature. Reproduced with permission.137 Copyright 2022, Wiley. | ||
Guanhua Zhang et al.138 fabricated a novel three-dimensional zinc-metal anode with a multi-channel lattice structure by combining 3D printing and electroplating technologies. The preparation process is shown in Fig. 27(a)–(d). The constructed 3D Ni–Zn anode with a multi-channel lattice structure and superhydrophobic surface can effectively improve the electric field distribution, induce uniform deposition of Zn, and retard the growth of Zn dendrites. Due to the low Zn nucleation rate and homogeneous and localized electric field distribution, the 3D Ni–Zn cell exhibits highly reversible Zn deposition/stripping with good coulombic efficiency. Thus, the facile and low-cost fabrication of conductive metal dots with tunable 3D multichannel structures provides new opportunities for the development of other multifunctional cell structures.
 | ||
| Fig. 27 Printing of multifunctional structures: schematic diagram of the preparation process of 3D Ni–Zn dots, (a) schematic diagram of a 3D printer, (b) 3D polymer dot matrix, (c) 3D Ni dot matrix, and (d) 3D Ni–Zn dot matrix. Reproduced with permission.138 Copyright 2021, Wiley. | ||
 | ||
| Fig. 28 Printed full cell: schematic diagram of the printing process and top view of the corresponding SEM images: (a) printed LFP. (b) Schematic diagram of the AJP process printing. (c) Layered cathode/electrolyte formation process. Reproduced with permission.140 Copyright 2023, Wiley. | ||
Compared with classical stacked collectors, the development of novel structured collectors (substrates) with diverse collectors is beneficial for accelerating ion/electron transfer by increasing the effective contact area between the electrode material and the electrolyte, thereby improving the electrochemical performance of MBs. For example, double helix collectors and electrodes were realized as an integrated composite material by FDM printing. The internal collector and external LTO anode can be printed simultaneously. As a result, the morphology of the 3D printed LTO anode is very smooth and the LTO particles and polymer are uniformly distributed in the printed electrode. Chenglong Chen et al.142 prepared three-dimensional structured copper mesh collectors by a 3D printing method. In the first step, copper powder, polyvinylidene fluoride (PVDF) and 1-methyl-2-pyrrolidone were mixed proportionally to prepare a printing slurry, as shown in Fig. 29(a). In the second part, the slurry was printed into a mesh structure using a 3D printer, and finally, the PVDF was removed from the copper mesh through heat treatment to obtain the mesh-structured copper collector, as shown in Fig. 29(b) and (c). The experimental results show that the coulombic efficiency of lithium deposition on the 3D copper mesh collector remains above 98% for 500 cycles and 1000 cycles of depositing or stripping 1 mA h cm−2 of lithium at current densities of 1 mA cm−2 and 10 mA cm−2. The 3D-printed copper mesh collector effectively prevents the growth of dendrites, improves the coulombic efficiency, and mitigates the volumetric changes.
 | ||
| Fig. 29 Printing collector: preparation process of a 3D printed copper mesh; (a) synthesis of printing paste. (b) Schematic diagram of a 3D printing process and post-processing of copper mesh. (c) Mass production of a 3D printed copper mesh. Reproduced with permission.142 Copyright 2022, American Chemical Society. | ||
Ji Qian et al.145 prepared stretchable lithium-ion battery diaphragms by extrusion 3D printing of active materials mixed with nanofibrillated cellulose (NFC), as shown in Fig. 30. The researchers used nanofibrillated cellulose as a surfactant to disperse the CNTs as well as the actives in solution in order to form a stable and homogeneous aqueous ink for 3D printing. The CNTs contribute to the viscosity of the ink due to their high aspect ratio. The resulting diaphragm achieves 50% stretchability and remains electrochemically stable during repeated stretching. The facile 3D printing of patterned diaphragms leads to the low-cost fabrication of high-performance stretchable lithium-ion batteries, demonstrating their great potential for realizing stretchable energy storage devices.
 | ||
| Fig. 30 Printed diaphragm: schematic diagram of a 3D printed stretchable battery diaphragm. Reproduced with permission.145 Copyright 2022, Elsevier. | ||
Chang-Ping Feng et al.147 prepared a 3D-printable flexible and conformal phase change electronic encapsulation material with excellent comprehensive performance by a simple swelling strategy to effectively confine paraffin wax in a robust cross-linked polymer 3D network structure (Fig. 31). The resulting composite phase change material possesses a high latent heat of phase change of up to 145.6 J g−1 and exhibits remarkable thermal stability even at an endurance temperature of 130 °C. In addition, due to its excellent 3D printability, fused jet deposition 3D printing can produce complex two-dimensional patterns or three-dimensional solids. By varying the nozzle diameter, air pressure, and printing speed, the line width of phase change electronic encapsulants can be as thin as 80% of the line width of the phase change electronics package. Finally, the phase change material can be used to encapsulate electronic devices according to the circuit structure using fused jet deposition 3D printing.
 | ||
Fig. 31 Printing package: (a) schematic diagram of accurate packaging of circuits with phase-change-based electronic packaging materials by 3D printing technology. (b) Circuit package diagram (the LED chip on the left side is packaged and the LED chip on the right side is not packaged with phase-change-based electronic packaging materials). (c) Infrared image of the LED chip with and without packaging. (d) Temperature curve of three LED chip which were packaged by sylgard 184 and 70 PW under the same power of 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W. (e) Complex circuit without LED chip package diagram. (f) The schematic diagram of heat dissipation of phase-change-based electronic packaging materials. Reproduced with permission.147 Copyright 2023, Elsevier. W. (e) Complex circuit without LED chip package diagram. (f) The schematic diagram of heat dissipation of phase-change-based electronic packaging materials. Reproduced with permission.147 Copyright 2023, Elsevier. | ||
4.5 3D printed miniature batteries
The rise of portable microelectronics and smart sensors has inspired the pursuit of advanced microscale electrochemical energy storage devices. Microbatteries with features such as being lightweight and customizable, and having seamless integration, high performance and high stability are the best choice as microscale electrochemical energy storage devices. Conventional batteries prepared by the slurry casting method find it difficult to meet the versatility and shape customizability of microelectronics. Notably, microbatteries can address the above issues of versatility and customized structures by 3D printing methods, i.e., direct fabrication of controllable 3D structures using rationally designed inks. 3D printed microbatteries offer the following advantages: (i) significantly increased design freedom and capability on the micro-scale; (ii) they have a higher mass per unit area of material and a larger electrode aspect ratio, which provides a higher surface/body energy density; (iii) increased power density of microcells due to shorter ion diffusion paths; (iv) significantly improved material utilization, thus reducing production costs; (v) directly compatible integration of microcells in microelectronics. Next, the latest advances in 3D printed microbatteries will be summarized based on the charge carriers in the electrolyte.Ke Sun150et al. fabricated lithium-ion microbatteries consisting of anode and anode microarrays with high depth-to-width ratios by 3D printing (Fig. 32(a)). Concentrated anode and cathode inks were prepared by suspending spinel-structured lithium titanate (Li4Ti5O12) (LTO, with an average particle size of 50 nm) and LiFePO4 (LFP, with an average diameter of 180 nm) nanoparticles in a solution consisting of deionized water, ethylene glycol, glycerol, and a cellulose-based viscosity builder through a multistep process of particle dispersion, centrifugation, and homogenization (Fig. 32(b) and (c)). These inks were deposited onto a glass substrate through a cylindrical nozzle to print multilayer electrodes with a high depth-to-width ratio (Fig. 32(d)).
 | ||
| Fig. 32 Schematic illustration of 3D interdigitated microbattery architectures (3D-IMA) fabricated on (a) gold current collector by printing (b) Li4Ti5O12 (LTO) and (c) LiFePO4 (LFP) inks through 30 μm nozzles, followed by sintering and (d) packaging. Reproduced with permission.150 Copyright 2013, Wiley. | ||
Jiaxin Ma152 demonstrated the construction of a fully 3D printed flexible fork-finger sodium-ion microcell with a 3D conductive carrier transport network, showing exceptionally enhanced area/volume capacity and rate capability. Specifically, 3D-printed microelectrode inks with tunable viscosity and excellent rheological properties consisted of well-dispersed high-capacity Na3V2(PO4)2O2F (NVPF) or high-magnification carbon-covered NaTi2(PO4)3 (NTP), as well as high-conductivity additives including 2D electrochemically exfoliated graphene nanosheets and 1D carbon nanotubes (Fig. 33(a)). The resulting dimensional electrodes have a thickness of 300 to 1200 μm, with a 3D interconnected conductive and porous skeleton, which greatly reduces the resistance and polarization during sodium ion embedding/exfoliation. The miniature sodium-ion battery fabricated by 3D imprinting has a high area capacity of 4.5 mA h cm−2 and a corresponding area energy density of 7.33 mW h cm−2 (Fig. 33(b–e)). In addition, the miniature sodium-ion battery shows compatible serial and parallel modularity, can be customized in terms of capacity and voltage outputs, and has good dexterity and flexibility under various bending conditions.
 | ||
| Fig. 33 Printed microzinc batteries: (a) schematic of aqueous NVPF and NTP microelectrode inks and ionic gel electrolyte inks used for 3D printing. (b–e) Photographs of each pattern for 3D printing. Reproduced with permission.152 Copyright 2022, Wiley. | ||
Yujin Ren et al.158 synthesized CNT@MnO2 inks for the first time by coating MnO2 on carbon nanotubes, and a novel microplanar flexible Zn-based battery was designed and constructed using 3D printing technology (Fig. 34(a)). Firstly, the synergistic effect of CNT@MnO2 composites was investigated in button batteries, and the specific capacity was increased by about 100 mA h g−1 after the introduction of CNTs. The incorporation of CNTs led to the formation of a 3D network structure, and the increase of both conductivity and charge transfer kinetics. These factors improved the electrochemical properties of MnO2, and composites with nanoscale particle sizes can be reliably used in 3D printing technology. Zinc powder ink with micron-sized particle size replaces zinc foil, making the cells highly flexible and bendable (Fig. 34(b)).
 | ||
| Fig. 34 Printed microzinc cells: (a) photographs of the heated plates of the multijet printing system and the 3D printing device printer; the inlays show annular collectors with a single structure, a parallel structure, and a series structure, respectively. (b) Photographs of the flexible cell in different bending states. Reproduced with permission.158 Copyright 2023, Elsevier. | ||
5 Intelligent sensing
With the development of battery technology, energy storage batteries with high specific energy, long life and high power are more and more widely used in electric vehicles or energy storage power stations. For batteries, the state of charge (SOC), state of health (SOH) and state of safety (SOS) are important bases for evaluating their work quality, work reliability and cycle life (QRL). With the continuous operation of batteries, frequent safety accidents have cast a shadow on their development. At this stage, the development of the anode, cathode and electrolyte of the battery itself is in a “bottleneck”, and the solution of the battery safety problem is imminent.159 The problems of traditional batteries are: (i) the lithium battery system is highly nonlinear and has multi-spatial scale and multi-time scale aging, so it is difficult to accurately model. (ii) The internal situation of the battery cannot be obtained by direct detection methods, and is susceptible to environmental temperature, pressure, etc. This makes it difficult to accurately predict the internal state of the battery. (iii) The inconsistencies of the cell directly affect the efficiency of the battery pack. Therefore, the self-monitoring and self-management of the battery to improve the safety of the battery have become an effective method, giving rise to an extremely important branch of science – the application of intelligent sensing in the battery field. Different from the traditional battery intelligence at the module level or system level, smart batteries focus on improving the intelligence of individual batteries with the ability of self-monitoring and self-management, which has become one of the mainstream research hotspots.5.1 Acoustic sensors
Acoustic sensing is a class of non-destructive testing technology with strong penetration, non-destructive, high sensitivity, and is now widely used in medical, aerospace, construction and other fields. The essence of a sound wave is a mechanical wave, is a periodic mechanical vibration in the medium of the form of propagation. When the sound wave passes through the object and interacts with it, the observation and determination of the sound speed, attenuation, frequency and other characteristics of the sound wave that passes through the object or is reflected from the object can be used to obtain the change of the elastic modulus of the object material, the internal stress and other state parameters, and then accurately assess the characteristics of the material and its internal structure. According to these characteristics of acoustic sensing, only a probe needs to be laid on the outside of the battery, and one can probe the internal structure of the battery and obtain the internal information of the battery, which fundamentally solves the difficulties encountered by implantable sensors, and it is a kind of ideal non-destructive monitoring of the battery. Acoustic sensing can be categorized into passive acoustic sensing and active acoustic sensing.Zuolu Wang et al.163 developed an active acoustic emission sensing technique for rapid joint SOC/SOH estimation. The developed monitoring technique involves the application of power ultrasound and acoustic emission (AE). Appropriate power and ultrasound are used to excite the battery and trigger an acoustic emission event, while an acoustic emission transducer actively captures elastic waves released from battery materials with different properties to characterize the battery state, as shown in Fig. 35. Experimental studies have shown that RMS is an effective feature to distinguish battery SOC from SOH during charge/discharge cycling and aging. It can be seen that RMS decreases with decreasing battery SOC and SOH. This study provides an idea to develop an independent and fast SOC/SOH estimation method.
 | ||
| Fig. 35 Schematic diagram of ultrasonic inspection of a soft pack battery. Reproduced with permission.163 Copyright 2023, Elsevier. | ||
5.2 Optical sensors
Among all the battery sensing methods, fiber optic sensing, as an emerging technology, has some unique advantages. For most of the sensing methods, only when the sensor is placed inside the battery can it directly obtain the information on the internal parameters of the battery. Fiber optic sensors are tough, small, lightweight, and immune to electromagnetic interference, which allows them to be attached to the surface of the battery as well as embedded inside the battery, and they are expected to be integrated into the battery management system for monitoring the critical information and status of the battery. In addition, the high sensitivity and multiplexing capability of fiber optic sensors can accurately monitor multiple parameters and states of the battery simultaneously with good spatial and temporal resolution.Wenxin Mei et al.165 developed a compact multifunctional fiber optic sensor assembled with FBG and Fabry–Perot (FPI) that can be inserted into the center hole of a crystal cell and operated continuously to continuously monitor internal temperature and pressure effects during the thermal runaway of a battery (Fig. 36). The researchers observed a stable and reproducible correlation between battery thermal runaway and optical effects, and the sensing signal showed two internal pressure peaks corresponding to electrolyte leakage and thermal runaway safety incidents, respectively. Experimental analysis shows that the sensor provides an expandable safety solution that can provide a good warning of the impending thermal runaway before the battery suffers a catastrophic safety leak. This tunable fiber-optic sensor provides an important safeguard in battery monitoring by identifying the risk of unwarranted thermal runaway before it occurs, allowing for early warning or automatic shutdown.
 | ||
| Fig. 36 Principle of the combined FBG/FPI sensor for simultaneous monitoring of temperature and pressure inside the cell. (a) Position of the sensor inside the cell. (b) The cascade structure of the FBG–FPI sensor. (c) The incident broadband spectral line. (d–f) For the FBG configuration (left: cross section, right: side view), the sensing principle (the periodic refractive index modulation of the FBG reflects a narrow resonance) and its spectrum respectively (narrow Bragg wavelength λB). (g–i) FPI configuration (left: cross section, right: side view), sensing principle (the open cavity interferes with light between two surfaces M1 and M2) and its spectra (several relatively broad resonances). Reproduced with permission.165 Copyright 2023, Springer Nature. | ||
Xile Han et al.168 used a TFBG sensor that can be inserted into a battery for in situ and continuous monitoring of mass transfer in the electrolyte near the electrode, as shown in Fig. 37. The sensor was inserted near the electrode surface of an operating lithium-metal battery without disturbing the normal operation of the battery. Thanks to the ultrafine optical resonance of the TFBG, rapid monitoring of mass transport kinetics and lithium dendrite growth at the nanoscale interface of the lithium anode is realized.
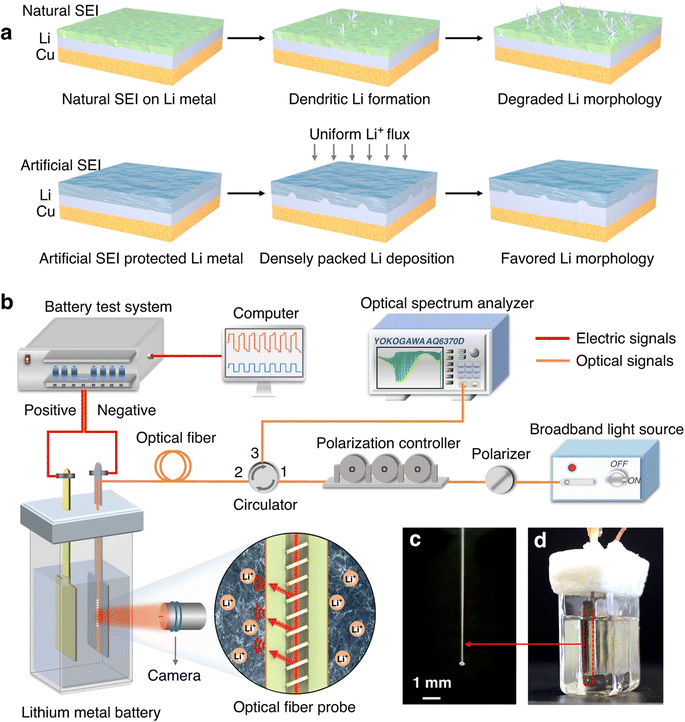 | ||
| Fig. 37 (a) Scheme of the lithium metal morphological evolution with cycling for a natural SEI (top) and artificial SEI (bottom). (b) Experimental setup of an evanescent optical fiber sensing system for monitoring the ionic concentration of lithium metal battery at electrolyte–electrode interface. The zoomed inset presents the idea of capturing the localized ionic concentrations and fast ion transport kinetics over the lithium anode interface using an implanted optical fiber sensor. (c and d) Photographs of the optical fiber sensing probe and lithium metal anode symmetrical cell, respectively. Reproduced with permission.168 Copyright 2024, Springer Nature. | ||
The FOEW signal shows sensitivity to the lithium concentration on the surface of graphite particles. This facilitates the monitoring of the state of health (SOH) of the battery by monitoring the relationship between light transmission amplitude and available capacity.172 Jonas Hedman et al.173 demonstrated that fiber optic swift wave (FOEW) sensors are capable of detecting sodium metal deposition on copper foil and hard carbon electrodes. In the case of hard carbon, plating was detected both due to insufficient intercalation sites (i.e., poorly balanced cells or nodes with reduced capacity) and polarization-induced plating (i.e., excessively high charging rates). Experiments were performed using commercial electrodes, and the cell geometry remained unchanged except for the introduction of a 125 μm diameter optical fiber between the hard carbon electrode and the diaphragm. This work also showed that the cycling performance of the full cell was not significantly affected when the sensor was used, demonstrating the feasibility of detecting plating during the rapid charging of a full cell (Fig. 38).
 | ||
| Fig. 38 Schematic of the optical-electrochemical setup of the FOEW sensor placed on the surface of the copper electrode in a copper–sodium half-cell. Reproduced with permission.173 Copyright 2022, American Chemical Society. | ||
Yifei Yu et al.177 implanted a Rayleigh scattering optical fiber inside an 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 battery (Fig. 39(a) and (b)). After more than 60 cycles, they found that the effect of the optical fiber on the battery was almost negligible. In addition, two different Rayleigh scattering optical fibers showed highly reproducible temperature monitoring in the cell through 20 h of continuous monitoring. These findings demonstrate that the distributed fibers inside the cell can ensure continuous and stable operation over long periods of time, ensuring the long-term performance stability of the embedded sensor (Fig. 39(c)).
650 battery (Fig. 39(a) and (b)). After more than 60 cycles, they found that the effect of the optical fiber on the battery was almost negligible. In addition, two different Rayleigh scattering optical fibers showed highly reproducible temperature monitoring in the cell through 20 h of continuous monitoring. These findings demonstrate that the distributed fibers inside the cell can ensure continuous and stable operation over long periods of time, ensuring the long-term performance stability of the embedded sensor (Fig. 39(c)).
 | ||
| Fig. 39 Rayleigh scattering fiber optic sensor: (a) ε placement of DFOS and T-DFOS in the cell. (b) Schematic diagram of the test system. (c) Battery performance was not affected by DFOSs for 100 cycles at 1C at 25 °C ambient temperature. Reproduced with permission.177 Copyright 2022, Elsevier. | ||
5.3 Temperature sensors
Temperature has an important impact on the mass transfer rate and electrochemical reaction rate inside the battery, which in turn affects battery performance, lifetime and safety. Therefore, the thermal management system is a crucial component of the battery system, and its core function is to control the battery operating temperature in the appropriate zone while ensuring the uniformity of the battery temperature. Currently, thermal management systems usually use thermocouples to measure the temperature on the battery surface or at the pole lugs, and use the external temperature and the battery heat production during charging and discharging as inputs to the thermal model, to calculate the temperature distribution within the battery system to optimize the thermal management design. With the development of batteries in the direction of high energy density and large capacity, the problem of battery temperature inhomogeneity is aggravated. Uneven temperature affects the current distribution within the battery pack and leads to uneven aging. However, research and knowledge on the measurement of temperature inhomogeneity and its coupling effects with electrochemistry and mechanics are still very limited, so sensing the internal temperature is of great significance for understanding the battery mechanism and optimizing the thermal management of the battery. Currently, the main temperature sensors used in the battery field are miniature thermocouple sensors, thermistor sensors, and impedance-based temperature sensors.Wei et al.180 arranged some miniature thermocouples in the axial center plane of an 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cell along the radial direction of the cell to obtain the radial temperature distribution of the cell during the charging and discharging process, as shown in Fig. 40. The results show that the higher discharge multiplicity leads to an increase in the internal temperature of the battery. Increasing the convective heat transfer coefficient can reduce the temperature rise of the battery, but the degree of variation of the internal temperature gradient is large so treating the battery as an isothermal body under forced convection conditions will bring large errors.
650 cell along the radial direction of the cell to obtain the radial temperature distribution of the cell during the charging and discharging process, as shown in Fig. 40. The results show that the higher discharge multiplicity leads to an increase in the internal temperature of the battery. Increasing the convective heat transfer coefficient can reduce the temperature rise of the battery, but the degree of variation of the internal temperature gradient is large so treating the battery as an isothermal body under forced convection conditions will bring large errors.
 | ||
| Fig. 40 Smart battery with DFOS for real-time internal and external temperature monitoring. Reproduced with permission.180 Copyright 2024, Institute of Electrical and Electronics Engineers. | ||
B. Gulsoy et al.181 have embedded a customized ceramic-type K thermocouple sensor into a modified cell for in situ temperature monitoring (Fig. 41(a–c)). Current–voltage variations were observed during matched load cycling of the reference cell and the cell under test (Fig. 41(d and e)). The results show that the thermocouples were not degraded by the harsh chemical environment inside the cell, that the monitored surface temperatures did not match the internal temperature data, and that the thermocouple located inside the cell showed little change in the temperature detected by the surface thermocouple (Fig. 41(f–i)). In addition, the materials used in the construction of the sensors and the instrumentation methods used do not adversely affect cell operation.
 | ||
| Fig. 41 Miniature thermocouple sensing: (a–c) sensor inserted into the battery for testing. (d and e) Cell voltages were recorded during load profile cycling with reference and instrumented cells; current waveforms were applied to the cells during load profile cycling. (f–i) Internal and external cell temperatures were measured during cycling experiments for cells 1, 2, and 3; delta temperature, which shows the difference between internal and external temperatures during cycling, for all three instrumented cells. Reproduced with permission.181 Copyright 2022, Elsevier. | ||
Joe Fleming et al.183 implanted thin-film thermistors in the internal cavity of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cells and in the middle of the thickness direction (between the anode and the diaphragm) of soft-packed batteries, as shown in Fig. 42. Cycling tests and dismantling and characterization of the batteries showed that the implantation of the thermistors would bring potential capacity loss and lithium precipitation risk to soft-packed batteries, and could also cause uneven pressure and mechanical damage after the batteries were assembled. Removing some of the electrode material and implanting a thermistor can mitigate the uneven pressure caused by sensor implantation, but it will reduce the energy density of the battery and increase the complexity of the production process. Rapid temperature changes in the battery were successfully detected without hysteretic response. In addition, the battery electrochemical properties remain stable and the sensor can operate for long periods. Thermistors have high measurement accuracy and fast response in the low-temperature range. However, thermistors are limited in temperature range and accuracy and are not suitable for high-temperature and high-accuracy applications. In contrast, thermistors offer higher accuracy and can be used for higher-temperature testing.
650 cells and in the middle of the thickness direction (between the anode and the diaphragm) of soft-packed batteries, as shown in Fig. 42. Cycling tests and dismantling and characterization of the batteries showed that the implantation of the thermistors would bring potential capacity loss and lithium precipitation risk to soft-packed batteries, and could also cause uneven pressure and mechanical damage after the batteries were assembled. Removing some of the electrode material and implanting a thermistor can mitigate the uneven pressure caused by sensor implantation, but it will reduce the energy density of the battery and increase the complexity of the production process. Rapid temperature changes in the battery were successfully detected without hysteretic response. In addition, the battery electrochemical properties remain stable and the sensor can operate for long periods. Thermistors have high measurement accuracy and fast response in the low-temperature range. However, thermistors are limited in temperature range and accuracy and are not suitable for high-temperature and high-accuracy applications. In contrast, thermistors offer higher accuracy and can be used for higher-temperature testing.
 | ||
| Fig. 42 Thermistor sensor: (a) insert sensors into the soft-pack battery and then display the X-ray images of it. (b) Insert the sensor into the cylindrical battery and then display the X-ray image of it. Reproduced with permission.183 Copyright 2024, Elsevier. | ||
Rengaswamy Srinivasan et al.185 discussed a new approach to predicting and preventing thermal runaway in lithium-ion batteries based on rapid (seconds) monitoring of battery impedance. Winding and patch-heating techniques were used and changes in cell temperature, voltage, and phase shift were measured simultaneously. The phase shift of impedance increased linearly when the cell temperature increased, as shown in Fig. 43. However, the Ecv (slot voltage) and Tsurf (surface temperature) do not reflect the impending temperature runaway in time. ∥z, while also providing the ability to predict temperature runaway, the individual values are too small, and z′ or z′′ are obtained from ∥z and φ measurements and do not provide new information to prevent temperature runaway. The results show that impending temperature runaway can be better predicted by monitoring the phase shift φ.
 | ||
| Fig. 43 Impedance-based temperature sensor: (a) phase shift φ and voltage Ecv curves when heated with two patch heaters toward thermal runaway (cell surface temperature, Tsurf, red; cell voltage Ecv in purple). (b) Phase shift φ and voltage Ecv curves when the surround heater is heated toward thermal runaway (cell surface temperature, Tsurf, red; cell voltage Ecv is purple). Reproduced with permission.185 Copyright 2024, Elsevier. | ||
5.4 Mechanical sensors
During the battery charging and discharging process, the de-embedding of metal ions in the cathode and anode materials is accompanied by changes in the volume of the material particles and the generation of stress/strain, i.e., intercalation strain. Periodic intercalation strain during long battery cycling causes rupture of electrode particles and growth of cracks, which may result in the loss of active materials on the one hand, and on the other hand, the new interfaces formed by the growth of cracks will react with the electrolyte to cause the loss of active materials, resulting in the degradation of the capacity of the battery. Therefore, the study of the evolution law of battery mechanical properties is of great significance to understanding the performance degradation mechanism of batteries. Nowadays, the mechanical sensors used in the field of batteries mainly contain the following three categories: piezoresistive sensors, inductive sensors, and piezoelectric sensors.Steven Green et al.186 created piezoresistive sensors using channel circuit electrodes (CCE) coated with hydrazide-based graphene (HG) via a vaporizer (Fig. 44). Channel circuits generated using paste filling of fine traces is a simple but largely unreported technique used to create electrodes. The CCEs were prepared by molding the electrode patterns into resin and filling them with a conductive paste with a trace width of 100 μ m and a thickness of 4 μ m. Their resistance was 5–10 kΩ per each electrode was 60 cm long and serpentine in shape.
 | ||
| Fig. 44 Piezoresistive sensors: exposure of flexible graphene sensors to continuous flow test and measurement environments in a DNT. Reproduced with permission.186 Copyright 2024, Springer Nature. | ||
 | ||
| Fig. 45 Inductive sensors: inductance-based battery extension sensors' operating principle. Reproduced with permission.187 Copyright 2022, Elsevier. | ||
 | ||
| Fig. 46 Process of integrating internal pressure sensors inside the power cell. Reproduced with permission.188 Copyright 2024, Elsevier. | ||
5.5 Electrochemical sensors
An electrochemical sensor is defined as “a device that converts chemical data, ranging from the concentration of individual sample components to an overall compositional analysis, into an analyzable signal”. In most cases, chemical sensors consist of two basic functional units: the receptor and the physicochemical sensor. The receptor is variable and can range from activated or doped surfaces to complex macroscopic phenomena or molecular manifestations where the analyte produces highly specific interactions. Electrochemical sensors are by far the most commonly used type of sensor because of their high accuracy, rapidity, and low cost. Ideally, electrochemical sensing devices can respond continuously and reversibly without disturbing the sample. Electrical signals such as voltage and current are the most basic signals captured by a battery management system (BMS), which usually monitors the voltage of different parallel-connected single cells at the module level and the current of different series-connected modules at the pack level. Most of the current battery state estimation algorithms for BMS are based on current and voltage signals, but it is difficult to accurately assess the internal state of the battery. Therefore, the development of new battery electrical signal measurement techniques to obtain more battery information is the focus of current research, in which representative works include internal potential measurement, current measurement and impedance measurement.For decades, pH electrodes have been the most widely used potentiostat overall. Thin ion-sensitive glass films are used to fabricate glass electrodes and are the most common type, available in a variety of forms and sizes. Nevertheless, other types of potentiometric sensors utilizing organic polymers (e.g., polymethylene blue) or redox-active molecules (e.g., ferrocene and quinones) can be used to detect pH other than those mentioned above. Jinhai Liu et al.190 developed a lithium-free fast-charging strategy based on monitoring the anode potential to regulate the current multiplication rate, in which a diaphragm-covered reference electrode was placed between the anode and the diaphragm to measure the anode potential. The charging current was adjusted in real time during the charging process so that the anode potential was close to, and slightly higher than, 0 V, thus realizing the maximal charging current in the case of lithium-free precipitation. The authors experimentally showed that the fast-charging strategy can increase the charging speed by 40% compared with the manufacturer's standard fast-charging strategy, and the scanning electron microscope showed that there was no lithium precipitation at the anode after 100 fast-charging cycles.
 | ||
| Fig. 47 Current sensor: structural frame diagram of an HV current sensor. Reproduced with permission.193 Copyright 2016, IET Circuits, Devices & Systems. | ||
 | ||
| Fig. 48 Electrochemical impedance sensing: (a) high-capacity battery with a built-in reference electrode; (b) small EIS detection system and detection battery. Reproduced with permission.195 Copyright 2024, Institute of Electrical and Electronics Engineers. | ||
5.6 Odor sensors
Lithium-ion batteries generate gases when the electrolyte consumes and generates the SEI during the aging process, and also generate gases when the SEI grows, the cathode material crystal phase transforms, or the electrolyte decomposes during the cycling process, as well as generating gases in the process of internal short-circuiting, thermal runaway, and so on. Therefore, gas monitoring is of great significance in understanding the aging mechanism of batteries, improving the battery material system, and evaluating and warning the safety risks of batteries.So far, various types of gas sensors have been used to realize the monitoring of battery status. Currently, the following types of gas sensors are commonly used: catalytic combustion type gas sensors,196 electrochemical gas sensors,197 quartz crystal microbalance type gas sensors,198 thermally conductive gas sensors,199 infrared absorption gas sensors200 and resistive gas sensors.201 Sensitivity is an important characteristic of gas sensors. Sensitivity is an important characteristic of gas sensors, which refers to the minimum volume concentration of the target gas that can be detected and is a general parameter for evaluating the sensitivity of gas sensors to the target gas. In addition, stability refers to the long-term reliability and service life of the gas sensor and also evaluates the stability of the gas sensor to detect the target gas under the influence of other factors. Thermal resistance indicates the ability of the gas sensor to resist high temperature erosion and requires special attention when the cell generates a large amount of heat during thermal runaway. Selectivity is the ability of the gas sensor to prevent interference from other gases under the same conditions. Response time is a parameter reflecting the response speed of the gas sensor in detecting the target gas, which is an important prerequisite for realizing rapid warning. Convenience indicates the cost of the gas sensor and the ease with which the gas sensor can be integrated into a battery system for gas detection and early warning.
Catalytic combustion sensors have poor selectivity for flammable gases and are dangerous when applied to battery detection due to their dark fire operation. Thermal conductivity sensors have the disadvantages of poor detection accuracy and low sensitivity, and are not suitable for rapid detection and early warning. Quartz crystal microbalance gas sensors have low reusability of detection components. Although the electrochemical sensor technology is relatively mature, it suffers from the disadvantages of short service life and high power consumption. Infrared sensors have been used for battery thermal runaway detection and exhibit high stability, excellent selectivity, and fast detection speed, but they still suffer from the disadvantages of complex structure, high cost, and integration difficulties in practical applications. Compared with other types of gas sensors, resistive gas sensors have the advantages of high sensitivity, high stability, low cost, and easy integration, and have been widely used in the field of thermal runaway gas detection and battery warning. Although the poor selectivity and high operating temperature of resistive gas sensors have hindered their wide application in fields such as indoor air detection and medical care, the limited types of gases released during the thermal runaway process of batteries and the existence of inherently high temperature environments have relatively little impact on the detection of thermal runaway gases in batteries. After a comprehensive analysis of the above gas sensors, the next section will focus on the application of pyroelectric infrared gas sensors, metal–semiconductor gas sensors, and catalytic gas sensors in battery thermal runaway detection and early warning.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) . The sensor is particularly highly selective for CO
. The sensor is particularly highly selective for CO![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) and cross-sensitization by other sensors can be largely avoided due to the absorption exclusivity of the chosen wavelength. Atmospheric CO2 concentrations can also be used for routine calibration of the sensor, preventing the sensor from drifting over time and ensuring long-term use without maintenance. When the battery fails, the gas production process is often accompanied by high temperatures and explosions. NDIR gas sensors offer the advantages of low cost, high accuracy and good stability.
and cross-sensitization by other sensors can be largely avoided due to the absorption exclusivity of the chosen wavelength. Atmospheric CO2 concentrations can also be used for routine calibration of the sensor, preventing the sensor from drifting over time and ensuring long-term use without maintenance. When the battery fails, the gas production process is often accompanied by high temperatures and explosions. NDIR gas sensors offer the advantages of low cost, high accuracy and good stability.
Yulu Han et al.202 applied a newly developed non-dispersive infrared spectroscopy (NDIR) gas sensing system consisting of pyroelectric infrared detectors to monitor the thermal runaway (TR) process of lithium-ion batteries in real time (Fig. 49(a)); the gas sensors can detect CO2 and CH4 during the overcharge test of LFP batteries to realize an early warning system for the TR process of batteries (Fig. 49(b)).
 | ||
| Fig. 49 NDIR sensor: (a) schematic diagram of an NDIR gas sensor. (b) NDIR sensor gas concentration test: (1) CO2; (2) CH4. Reproduced with permission.202 Copyright 2023, Energies. | ||
Metal oxide semiconductor (MOS)-based conductive gas sensors are one of the most researched gas sensors because of their low cost, flexible fabrication, ease of use, and wide range of detectable gases. MOS gas sensors are widely used for the detection of reducing gases, such as CO and VOCs, and a number of commercially available products have been developed. The gas sensitization mechanism of the MOS sensors is based on a redox reaction catalyzed on the semiconductor surface.204 As a result, a single material is capable of reacting with many different gases, resulting in low selectivity of individual MOS sensors. The detection accuracy of MOS gas sensors is about a few ppm, which is well below the explosive limit of combustible gases inside lithium-ion batteries. However, commercial MOS gas sensors typically operate at temperatures as high as 200–800 °C; the higher operating temperature limits their use inside LIBs. Also, the high power consumption limits the integration of MOS-type gas sensors in BMS. Xiaoxue Wang et al.205 proposed a gas monitoring solution for early warning of thermal runaway to overcome the limitations of temperature and electrical signal monitoring (Fig. 50(a) and (b)). By analyzing the release of gases during thermal runaway, gases such as CO2, volatile organic compounds (VOCs), hydrocarbons, and CO were identified as suitable indicators for early detection of thermal runaway (Fig. 50(c)).
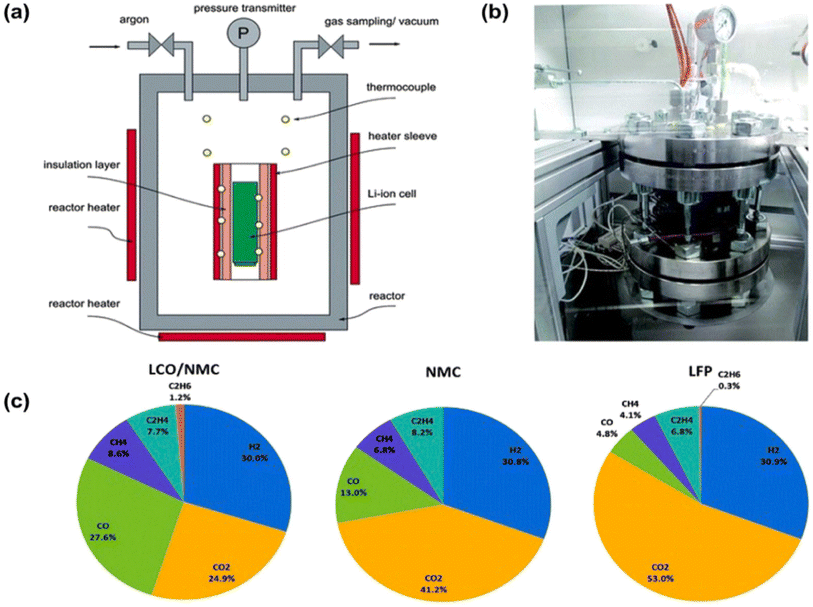 | ||
| Fig. 50 MOS gas sensor: (a) reactor and its main components; (b) photograph of the reactor; (c) detection of the component (mol%) of the gas produced. Reproduced with permission.205 Copyright 2024, Elsevier. | ||
| Sensor type | Installation position | Working mechanism | Function |
|---|---|---|---|
| Acoustic sensors | External battery | By analyzing the acoustic emission signals collected from the battery during cycling | The material structure inside the battery changes |
| Optical sensors | Attached to the battery surface/embedded inside the battery | Change in light signal intensity | Monitor the mechanical/thermodynamic changes during battery charging and discharging |
| Temperature sensors | Embedded outside the battery | Pole lug/embedded outside the battery | Sense internal battery temperature/optimize thermal management |
| Mechanical sensors | Attached to the battery surface | The conductor geometry changes under pressure | It is important to study the evolution law of battery mechanical properties for understanding the degradation mechanism of battery properties |
| Electrochemical sensors | Between positive and negative | The receptor and the physicochemical sensor | Monitor the voltage of different parallel cells and the current of different series modules at the battery pack level |
| Odor sensors | Cover the outside of the battery | Changes in gas types and concentrations | It is important to understand the aging mechanism of batteries, improve the battery material system, and evaluate and warn the safety risks of batteries |
6 Summary and outlook
LIBs are the most commonly used energy storage devices in modern society, featuring high specific capacity, long cycle life and no memory effect for small and lightweight portable devices. These advantages have led to the commercialization of LIBs, gradually replacing previous less efficient batteries such as NiCd and NiMH batteries.209–211 However, in recent decades, the development of LIBs has been slow due to the low intrinsic quality of the materials and bottlenecks in technological innovation. LIB operation suffers from problems such as lithium dendrite growth, electrode volume changes, and thermal runaway, and conventional battery materials have deficiencies such as slow electron diffusion rates, poor rate performance, and high production costs. LIBs inevitably deteriorate during operation, which is mainly attributed to electrode passivation, electrode/electrolyte reaction, internal or external electron leakage, electrolyte leakage, partial dissolution, and mechanical breakdown of active substances. The intelligent 3D printing processes can be used to create components that are better suited to the long-term operation of the battery. Certain battery manufacturing characteristics or external conditions may have an impact on aging patterns. Commercial LIBs include button, cylindrical, spherical, prismatic, and soft-pack battery types. Different battery packs produce different internal mechanical stresses. In addition, battery aging is also affected by external factors such as ambient temperature, charge and discharge current amplitude, discharge depth, and ambient humidity. The generation of many factors makes the intelligent control of batteries increasingly difficult. Therefore, it is necessary to develop sensing equipment with diversity, high sensitivity and high anti-interference strength. Battery status can be monitored more accurately using the new sensing devices, and battery life can be extended by adjusting the state of the battery in terms of charging and discharging, voltage and current. Traditional research ideas focus on optimization and improvement of materials, but cannot solve the core problems to protect battery safety and improve battery performance. With the development of smart technologies, the development of smart LIBs by integrating smart concepts into battery design, manufacturing and management is an effective strategy to improve battery life and operational safety.This review analyzes the deficiencies of traditional batteries and their improvement, and discusses the current status of the practical application of smart batteries integrating smart materials, smart manufacturing and smart sensing, respectively, in view of the problems of battery manufacturing and operation.
Section I summarizes the main applications of smart materials in building safer and durable LIB design strategies. The main classifications are as follows: (1) self-healing materials (SHMs): capable of restoring the mechanical, electrical, and physicochemical properties of materials through self-healing mechanisms, they can repair mechanical and electrical damage, including microcracks, abrasion, pulverization, dendrites, and punctures. Researchers have applied two principles to LIBs, namely external repair based on microencapsulation embedded with healing agents, and internal repair based on dynamic reorganization of reversible chemical bonds, to make them self-repairing; (2) Smart Response Materials (SRMs): capable of responding to changes in the external environment (e.g., temperature, force field, voltage, and electric field, etc.) promptly and restoring the original state when the stimulus disappears. Researchers have developed smart electrode materials with mechanical/electrical/thermal response, electrolytes with reversible self-protection, and smart diaphragms that detect and inhibit the growth of lithium dendrites. Compared with conventional materials, the smart-responsive materials can “turn on” and “turn off” reversibly, which provides better adjustability.
Section II summarizes the main applications of intelligent manufacturing in the preparation of structurally complex and precise electrode materials, describes five 3D printing technologies used to manufacture battery components and their working principles, and introduces the research progress and achievements of printing technology in batteries.3D printing technology is mainly classified into five types: (1) inkjet printing: ink is directly deposited in the form of droplets on flexible or rigid substrates through nozzles, which has the advantages of customizable patterns, easy access to materials and high utilization rate, but the physical properties and structure still need to be optimized and improved; (2) direct ink writing: the ink is stored in a syringe, and is extruded from the syringe nozzle driven by pneumatic or mechanical force, and finally the desired pattern is drawn under the control of a computer. With the advantages of low cost, material diversity, ease of operating, and no need for molds to build a 3D framework, it is considered one of the best printing methods in lithium-ion batteries; (3) Fused deposition modeling: the thermoplastic material is heated and melted in the extrusion head, and then the molten state of the liquid material is extruded onto the substrate to get the pre-determined structure. It is more widely used and has the advantages of fast printing speed and low cost, but due to processability issues, the printed electrode sheet has a low active material loading; (4) powder laser sintering: sintered or melted surface powder is used to make an object by 3D printing technology, which can be used to develop batteries with high loading and stringent precision parameter requirements; (5) photopolymerization type: the cell is made by using a UV laser beam irradiated on the cured resin filled container filled with cured resin, and using a blade to repeatedly add new resin until it cures to form the desired pattern. It is characterized by rapid prototyping and ultra-high precision. Currently, realizing fully printed energy devices remains a challenge, where most printing processes can only create one or two printable components, such as electrodes and electrolytes, without being able to fabricate the entire device by printing methods. There is a need to ensure that all units (electrodes, electrolyte/diaphragm, collector and encapsulation material) in an energy storage device can be printed simultaneously. Printed cathodes offer significant advantages over conventional processes, such as high active substance loading, surface reactivity and efficient permeation channels. Printed electrolytes have been shown to precisely tune the structure, create efficient ion diffusion pathways, reduce interfacial impedance, and increase strength. In addition, 3DP can provide functional architectures for battery modules designed to meet specific performance requirements, such as size, shape, flexibility, sacrificial bodies, and buffer layers. 3D-configured microbatteries are more conducive to ion transport and can be constructed with 3D electron/ion network structures, taking into account low interfacial resistance between the electrodes and electrolyte, as well as integrability with microelectronic devices on the same substrate. The synergistic combination of emerging 3D printing technologies with conventional technologies will be very promising. Thicker and more porous electrodes can achieve higher area energy density and power density, but the rich porous structure affects the stacking density of the electrodes and reduces the volumetric energy density of MBs.
Section III summarizes that the monitoring of acoustic, optical, thermal, force, electrical, and gas signals can effectively sense the internal state of the battery and its evolution, which is of great significance for understanding the occurrence mechanism of processes such as battery decline, failure, and thermal runaway, and for the development of high-reliability and high-safety sensors. Therefore, the development of smart battery technology integrating multiple sensors is expected to solve the current problems of low precision of battery state estimation and difficulty of full life cycle safety control in battery management systems due to the lack of sensing signals. The development of these diagnostic tools combined with advanced BMS methods will provide new possibilities for batteries to approach their theoretical performance during real-time operation. By providing accurate information and a history of important information, batteries are not only more user-friendly, but also more valuable and less interchangeable.
Nonetheless, there are still many significant challenges that need to be addressed. Future research on high-safety smart batteries will focus on the following directions to solve existing problems and promote technological progress. (i) Intelligent design with high performance: under the premise of ensuring that battery performance is not compromised, innovative intelligent design solutions are explored to optimize battery management, and improve cycle life and safety. (ii) Intelligent design for performance improvement: on the basis of the realized intelligence, we continue to explore and improve the design strategy, aiming to further enhance the key performance indexes of the battery, such as energy density, cycle stability, and fast-charging capability. (iii) Deep integration with advanced intelligent sensors: strengthen the integration and application of LIBs with high-precision and highly integrated intelligent sensors to realize real-time monitoring and accurate prediction of battery status, providing powerful data support for the safe operation of the battery system. (iv) Expanded application of the battery system: expand the design concepts and technical achievements of intelligent LIBs to other types of battery systems, such as solid-state batteries, sodium-ion batteries, etc., to promote the intelligent upgrading of the entire battery industry. (v) Development of new processes, materials and innovative architectures: committed to developing new smart manufacturing processes, smart material systems and architecture design to solve the current LIB challenges in cost, safety, environmental adaptability, etc., and to lay a solid foundation for the sustainable development of safe and smart LIBs.
In addition, the commercialization of smart batteries faces multiple technical and economic barriers. The core challenge lies in finding a balance between material innovation and performance optimization, while overcoming the real-world constraints of cost, manufacturability and large-scale production. From a technical point of view, the improvement of battery performance is highly dependent on breakthroughs in material systems to enhance safety, extend cycle life, or improve charging and discharging efficiency. However, these material innovations are often accompanied by high production costs and complex preparation processes, such as the synthesis of high-purity precursors, the precise control of nanoscale structures or the optimization of interfacial stability, which can significantly increase production complexity and equipment investment, leading to a reduced feasibility of large-scale manufacturing. At the same time, there is an inherent contradiction between the performance enhancement objectives: (i) increasing energy density may sacrifice thermal stability; (ii) pursuing fast charging may accelerate the degradation of electrode materials; and (iii) designing for extended life may increase system weight and size, thereby reducing energy efficiency. Such mutual constraints among performance parameters force the technology path selection to make compromises in multi-objective optimization. Furthermore, the impact of integrating smart features on the trade-off between battery performance and cost needs to be considered from multiple dimensions. While the introduction of auxiliary components such as smart management systems (BMS), sensors, and communication modules can improve safety, charging and discharging efficiency, and condition monitoring, they will inevitably take up space and add extra mass to the battery system, resulting in a lower proportion of active materials, which will have a direct impact on the actual energy density of the battery pack (typically by 5–15%). At the same time, the parasitic energy consumption of auxiliary circuits (about 1–3%) will further compromise the available energy output.
In order to solve the problems of high material cost, complex equipment integration and large-scale production difficulties faced by the development of smart batteries, it is necessary to achieve breakthroughs through multi-dimensional innovation. On the one hand, the current technological breakthrough lies in the development of highly integrated chips (e.g., SoC solutions to reduce PCB area by 40%), flexible circuit embedding technology (to reduce the weight of structural components), and self-powered sensors (to eliminate external energy supply losses). On the other hand, through material innovation (e.g. solid electrolyte integrated sensing function), manufacturing process optimization (3D printing multi-layer circuits) and large-scale production, the impossible triangle contradiction of energy density-life-cost is gradually resolved. In addition, for large-scale production, it is necessary to develop continuous manufacturing processes, deploy Industry 4.0 intelligent manufacturing systems, and establish a collaborative innovation platform across the industry chain to reduce marginal costs through the scaling effect. Therefore, the commercialization of smart batteries requires the construction of systematic solutions at the intersection of materials science, engineering and economics to achieve a double breakthrough in performance enhancement and cost control.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
L. W., Z. S., and R. M. proposed the topic of the work. L. W. and Z. S. were responsible for image beautification. B. F., H. L., and R. W. revised the literature and provided the content. Correspondence should be addressed to R. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the Shanghai Pilot Program for Basic Research (grant no. 22TQ1400100-8), Shanghai Pujiang Program (grant no. 20PJ1402500), Natural Science Foundation of Shanghai (grant no. 22ZR1416600) and the Fundamental Research Funds for the Central Universities.References
- N. Watanabe and M. Fukuda, Primary Cell for Electric Batteries, 1970, p. 25115 Search PubMed.
- M. Winter, B. Barnett and K. Xu, Before Li Ion Batteries, Chem. Rev., 2018, 118(23), 11433–11456 CrossRef CAS PubMed.
- S. U. O. Liu-Min and L. I. Hong, The past, present and future of lithium ion batteries, Physics, 2020, 49(1), 17–23 Search PubMed.
- A. Mukhopadhyay and B. W. Sheldon, Deformation and stress in electrode materials for Li-ion batteries, Prog. Mater. Sci., 2014, 63, 58–116 CrossRef CAS.
- Q. Meng, Y. Huang and L. Li, et al., Smart batteries for powering the future, Joule, 2024, 8(2), 344–373 CrossRef CAS.
- J. Tang, F. Wu and X. Dai, et al., Robust MXene adding enables the stable interface of silicon anodes for high-performance Li-ion batteries, Chem. Eng. J., 2023, 452, 139139 CrossRef CAS.
- S. Kang, J. Cheng and W. Gao, et al., Toward safer lithium metal batteries: a review, Energy Mater., 2023, 3(5), 300043 CAS.
- C. Jin, Y. Huang and L. Li, et al., A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries, Nat. Commun., 2023, 14(1), 8269 CrossRef CAS PubMed.
- R. D. Mckerracher, J. Guzman-Guemez and R. G. A. Wills, et al., Advances in Prevention of Thermal Runaway in Lithium-Ion Batteries, Adv. Energy Sustainability Res., 2021, 200059 Search PubMed.
- Q. Li, X. Yu and H. Li, Batteries: From China's 13th to 14th Five-Year Plan, eTransportation, 2022, 14, 100201 CrossRef.
- K. Liu, Z. Wei and C. Zhang, et al., Towards Long Lifetime Battery: AI-Based Manufacturing and Management, IEEE CAA J. Autom. Sinica, 2022, 9(7), 1139–1165 Search PubMed.
- X. Shen, X.-Q. Zhang and F. Ding, et al., Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect, Energy Mater. Adv., 2021, 2021, 1205324 Search PubMed.
- T. Vegge, J. M. Tarascon and K. Edström, Toward Better and Smarter Batteries by Combining AI with Multisensory and Self-Healing Approaches, Adv. Energy Mater., 2021, 11, 2100362 CrossRef CAS.
- O. A. Kuznetsov, S. Mohanty and E. M. Pigos, et al., High Energy Density Flexible and Ecofriendly Lithium-Ion Smart Battery, Energy Storage Mater., 2022, 266–275 Search PubMed.
- Y. Li, W. Wang and X.-G. Yang, et al., A smart Li-ion battery with self-sensing capabilities for enhanced life and safety, J. Power Sources, 2022, 546, 231705 CrossRef CAS.
- Y. Li, W. Wang and X.-G. Yang, et al., A smart Li-ion battery with self-sensing capabilities for enhanced life and safety, J. Power Sources, 2022, 546, 231705 CrossRef CAS.
- M. Yildirim and Z. Candan, Smart materials: The next generation in science and engineering, Mater. Today: Proc., 2023, 2214–7853 Search PubMed.
- Y. Li and D. Lau, Advances in shape memory polymers and their composites: From theoretical modeling and MD simulations to additive manufacturing, Giant, 2024, 18, 100277 CrossRef CAS.
- Y. Yin and J. A. Rogers, Introduction: Smart Materials, Chem. Rev., 2022, 122(5), 4885–4886 CrossRef CAS PubMed.
- K. H. J. Buschow and F. R. De Boer, Physics of Magnetism and Magnetic Materials, Springer US, Boston, MA, 2003, pp. 171–175 Search PubMed.
- Y. D. Liu and H. J. Choi, Electrorheological fluids: smart soft matter and characteristics, Soft Matter, 2012, 8(48), 11961–11978 RSC.
- H. Eshgarf, A. Ahmadi Nadooshan and A. Raisi, An overview on properties and applications of magnetorheological fluids: Dampers, batteries, valves and brakes, J. Energy Storage, 2022, 50, 104648 CrossRef.
- B. Li, P. F. Cao and T. Saito, et al., Intrinsically Self-Healing Polymers: From Mechanistic Insight to Current Challenges, Chem. Rev., 2023, 123(2), 701–735 CrossRef CAS PubMed.
- Z. Wei, J. Zhao and H. He, et al., Future smart battery and management: Advanced sensing from external to embedded multi-dimensional measurement, J. Power Sources, 2021, 489, 229462 CrossRef CAS.
- E. R. Ezeigwe, L. Dong and R. Manjunatha, et al., A review of self-healing electrode and electrolyte materials and their mitigating degradation of Lithium batteries, Nano Energy, 2021, 84, 105907 CrossRef CAS.
- W. Huang, X. Feng and X. Han, et al., Questions and Answers Relating to Lithium-Ion Battery Safety Issues, Cell Rep. Phys. Sci., 2021, 2(1), 100285 CrossRef CAS.
- L. Wen, J. Liang and J. Chen, et al., Smart Materials and Design toward Safe and Durable Lithium Ion Batteries, Small Methods, 2019, 3(11), 1900323 CrossRef CAS.
- W. Zhang, H. Jiang and Z. Chang, et al., Recent achievements in self-healing materials based on ionic liquids: a review, J. Mater. Sci., 2020, 55(28), 13543–13558 CrossRef CAS.
- Y. Wang, Q. Cao and C. Guan, et al., Recent Advances on Self-Supported Arrayed Bifunctional Oxygen Electrocatalysts for Flexible Solid-State Zn–Air Batteries, Small, 2020, 16(33), 2002902 CrossRef CAS PubMed.
- S. R. White, N. R. Sottos and P. H. Geubelle, et al., Autonomic healing of polymer composites, Nature, 2001, 409, 794–797 CrossRef CAS PubMed.
- S. R. White, N. R. Sottos and P. H. Geubelle, et al., Autonomic healing of polymer composites, Nature, 2001, 409(6822), 794–797 CrossRef CAS PubMed.
- J. Chen, Y. Li and X. Wu, et al., Dynamic hydrogen bond cross-linking binder with self-healing chemistry enables high-performance silicon anode in lithium-ion batteries, J. Colloid Interface Sci., 2024, 657, 893–902 CrossRef CAS PubMed.
- G. Zhang, Y. Yang and Y. Chen, et al., A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries, Small, 2018, 14(29), 1801189 CrossRef PubMed.
- C.-H. Li and J.-L. Zuo, Self-Healing Polymers Based on Coordination Bonds, Adv. Mater., 2020, 32(27), 1903762 CrossRef CAS PubMed.
- D. Liuhao, X. Rui and H. Lei, et al., Preparation and properties of self-healing polyurethane based on coordination bond, J. Nanjing Univ., 2022, 58(4), 699–705 Search PubMed.
- H. Wang, J. Xu and J. Hu, et al., Reprocessing and shape recovery of polyurethane enabled by crosslinking with poly(β-cyclodextrin) via host-guest interactions, Polymer, 2023, 281, 126122 CrossRef CAS.
- Y. Song, J. Li and G. Song, et al., Self-Healing Polyurethane Elastomers with High Mechanical Properties Based on Synergistically Thermo-Reversible and Quadruple Hydrogen Bonds, ACS Appl. Polym. Mater., 2023, 5(2), 1302–1311 CrossRef CAS.
- L. Feng, Z. Yu and Y. Bian, et al., Self-healing behavior of polyurethanes based on dual actions of thermo-reversible Diels-Alder reaction and thermal movement of molecular chains, Polymer, 2017, 124, 48–59 CrossRef CAS.
- S. Liang, P. Wang and Z. Sun, et al., Microcrack self-healing capability of waterborne polyurethane/polyacrylate composites with dynamic disulfide bond, J. Appl. Polym. Sci., 2023, 140, 53744 CrossRef.
- S. Cho, S. Y. Hwang and D. X. Oh, et al., Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: boronic/boronate esters, borax, and benzoxaborole, J. Mater. Chem. A, 2021, 9, 14630–14655 RSC.
- R. Agarwal and A. Mohamad, Gallium-based liquid metals as smart responsive materials: Morphological forms and stimuli characterization, Adv. Colloid Interface Sci., 2024, 329, 103183 CrossRef CAS PubMed.
- P. Zhai, L. Liu and Y. Wei, et al., Self-Healing Nucleation Seeds Induced Long-Term Dendrite-Free Lithium Metal Anode, Nano Lett., 2021, 21(18), 7715–7723 CrossRef CAS PubMed.
- Y. Ou, P. Zhou and W. Hou, et al., Smart materials for safe lithium-ion batteries against thermal runaway, J. Energy Chem., 2024, 94, 360–392 CrossRef CAS.
- Y. Zhang, J. Li, Y. Liu, et al., Effect of Mechanical Stress on the Electrical Characteristics of Different Type IGBT Chips; proceedings of the 2019 16th China International Forum on Solid State Lighting & 2019 International Forum on Wide Bandgap Semiconductors China (SSLChina: IFWS), 2019, pp. 25–27 Search PubMed.
- M. Zhi, Q. Liu and Q. Xu, et al., Review of prevention and mitigation technologies for thermal runaway in lithium-ion batteries, Aerosp. Trans. Saf., 2024, 1(1), 55–72 Search PubMed.
- S. Yuan, K. Ding and X. Zeng, et al., Advanced Nonflammable Organic Electrolyte Promises Safer Li-Metal Batteries: From Solvation Structure Perspectives, Adv. Mater., 2023, 35(13), 2206228 CrossRef CAS PubMed.
- W. B. Spillman, J. S. Sirkis and P. T. Gardiner, Smart materials and structures: what are they?, Smart Mater. Struct., 1996, 5(3), 247 CrossRef.
- K. Shu, C. Wang and W. Li, et al., Electrolytes with reversible switch between liquid and solid phases, Curr. Opin. Electrochem., 2020, 21, 297–302 CrossRef CAS.
- K. Liu, C.-F. Cheng and L. Zhou, et al., A shear thickening fluid based impact resistant electrolyte for safe Li-ion batteries, J. Power Sources, 2019, 423, 297–304 CrossRef CAS.
- N. J. Wagner and J. F. Brady, Shear thickening in colloidal dispersions, Phys. Today, 2009, 62, 27–32 CrossRef CAS.
- J. Ding, G. Peng and K. Shu, et al., Novel reversible and switchable electrolytes based on magneto-rheology, Sci. Rep., 2015, 5(1), 15663 CrossRef CAS PubMed.
- N. Mao, T. Zhang and Z. Wang, et al., A systematic investigation of internal physical and chemical changes of lithium-ion batteries during overcharge, J. Power Sources, 2022, 518, 230767 CrossRef CAS.
- Z. Li, M. Gao and X. Zhao, et al., Heat generation effect and failure mechanism of pouch-type lithium-ion battery under over-discharge for electric vehicle, J. Energy Storage, 2024, 76, 109759 CrossRef.
- J. Zhang, I. A. Shkrob and R. S. Assary, et al., An extremely durable redox shuttle additive for overcharge protection of lithium-ion batteries, Mater. Today Energy, 2019, 13, 308–311 CrossRef.
- J. K. Feng, Y. L. Cao and X. P. Ai, et al., Polytriphenylamine: A high power and high capacity cathode material for rechargeable lithium batteries, J. Power Sources, 2008, 177(1), 199–204 CrossRef CAS.
- J. Chen, C. Buhrmester and J. R. Dahn, Chemical Overcharge and Overdischarge Protection for Lithium-Ion Batteries, Electrochem. Solid-State Lett., 2005, 8(1), A59 CrossRef CAS.
- S. Lin, Q. Wu and Y. Lu, Recent Progress of the Application of Electropolymerization in Batteries and Supercapacitors: Specific Design of Functions in Electrodes, ChemElectroChem, 2024, 11(12), e202300776 CrossRef CAS.
- Q. Gu, M. Wang and Y. Liu, et al., Electrolyte Additives for Improving the High-Temperature Storage Performance of Li-Ion Battery NCM523∥Graphite with Overcharge Protection, ACS Appl. Mater. Interfaces, 2022, 14(3), 4759–4766 CrossRef CAS PubMed.
- Y. Chen, Recent advances of overcharge investigation of lithium-ion batteries, Ionics, 2022, 28(2), 495–514 CrossRef CAS.
- H. Zhang, Y. Cao and H. Yang, et al., A redox-active polythiophene-modified separator for safety control of lithium-ion batteries, J. Polym. Sci., Part B, 2013, 51(20), 1487–1493 CrossRef CAS.
- J.-X. Guo, C. Gao and H. Liu, et al., Inherent thermal-responsive strategies for safe lithium batteries, J. Energy Chem., 2024, 89, 519–534 CrossRef CAS.
- T. Li, L. Wang and J. Li, A safer, symmetric all-organic battery based on temperature-responsive polymers as both cathode and anode, Chem. Eng. J., 2022, 442, 136232 CrossRef CAS.
- M. Baginska, B. J. Blaiszik and R. J. Merriman, et al., Autonomic Shutdown of Lithium-Ion Batteries Using Thermoresponsive Microspheres, Adv. Energy Mater., 2012, 2(5), 583–590 CrossRef CAS.
- H. Yang, Z. Liu and B. K. Chandran, et al., Self-Protection of Electrochemical Storage Devices via a Thermal Reversible Sol–Gel Transition, Adv. Mater., 2015, 27(37), 5593–5598 CrossRef CAS PubMed.
- S. Zhang, S. Li and Y. Lu, Designing safer lithium-based batteries with nonflammable electrolytes: A review, eScience, 2021, 1(2), 163–177 CrossRef.
- H. Zhang, L. Huang and H. Xu, et al., A polymer electrolyte with a thermally induced interfacial ion-blocking function enables safety-enhanced lithium metal batteries, eScience, 2022, 2(2), 201–208 CrossRef.
- B. Yang, T. Li and Y. Pan, et al., Design strategy towards flame-retardant gel polymer electrolytes for safe lithium metal batteries, Energy Mater., 2024, 4(5), 400061 CAS.
- H. Yang, W. R. Leow and X. Chen, Thermal-Responsive Polymers for Enhancing Safety of Electrochemical Storage Devices, Adv. Mater., 2018, 30(13), 1704347 CrossRef PubMed.
- Y. Shi, H. Ha and A. Al-Sudani, et al., Thermoplastic Elastomer-Enabled Smart Electrolyte for Thermoresponsive Self-Protection of Electrochemical Energy Storage Devices, Adv. Mater., 2016, 28(36), 7921–7928 CrossRef CAS PubMed.
- S. Zhang, Z. Shen and Y. Lu, Research Progress of Thermal Runaway and Safety for Lithium Metal Batteries, Acta Phys.-Chim. Sin., 2020, 37, 2008065 Search PubMed.
- Y. Huang, Y. Wang and Y. Fu, A Thermoregulating Separator Based on Black Phosphorus/MOFs Heterostructure for Thermo-stable Lithium-Sulfur Batteries, Chem. Eng. J., 2022, 454, 140250 CrossRef.
- Y. Huang, Y. Wang and Y. Fu, A thermoregulating separator based on black phosphorus/MOFs heterostructure for thermo-stable lithium-sulfur batteries, Chem. Eng. J., 2023, 454, 140250 CrossRef CAS.
- J. Deng, X. Yang and G. Zhang, Simulation study on internal short circuit of lithium ion battery caused by lithium dendrite, Mater. Today Commun., 2022, 31, 103570 CrossRef CAS.
- T. Wang, R. V. Salvatierra and J. M. Tour, Detecting Li Dendrites in a Two-Electrode Battery System, Adv. Mater., 2019, 31(14), 1807405 CrossRef PubMed.
- W. Zhang, Z. Tu and J. Qian, et al., Design Principles of Functional Polymer Separators for High-Energy, Metal-Based Batteries, Small, 2018, 14(11), 1703001 CrossRef PubMed.
- C. Monroe and J. Newman, The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces, J. Electrochem. Soc., 2005, 152(2), A396 CrossRef CAS.
- W. Wang, A. C. Y. Yuen and Y. Yuan, et al., Nano architectured halloysite nanotubes enable advanced composite separator for safe lithium metal batteries, Chem. Eng. J., 2023, 451, 138496 CrossRef CAS.
- Y. Kong, L. Wang and M. Mamoor, et al., Co/Mon Invigorated Bilateral Kinetics Modulation for Advanced Lithium–Sulfur Batteries, Adv. Mater., 2024, 36(13), 2310143 CrossRef CAS PubMed.
- S. Shi, P. Lu and Z. Liu, et al., Direct Calculation of Li-Ion Transport in the Solid Electrolyte Interphase, J. Am. Chem. Soc., 2012, 134(37), 15476–15487 CrossRef CAS PubMed.
- Q. Wu, Y. Mei and H. Huang, et al., Dynamic physical network constructed by tripartite H-bonds in artificial SEI to shape ultra-long life dendrite-free lithium-metal anodes, Mater. Today, 2024, 75, 112–124 CrossRef CAS.
- X. Shen, Y. Li and T. Qian, et al., Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery, Nat. Commun., 2019, 10(1), 900 CrossRef PubMed.
- G. Liu, Y. Li and L. Zhang, et al., In Situ Construction of Pva/Lif Composite Artificial Protective Layer to Assist Dendrite-Free Li Metal Anode, SSRN Electron. J., 2023, 620, 156809 CAS.
- G. Liu, Y. Li and L. Zhang, et al., In situ construction of PVA/LiF composite artificial protective layer to assist dendrite-free Li metal anode, Appl. Surf. Sci., 2023, 620, 156809 CrossRef CAS.
- B. Zhao, C. Xing and Y. Shi, et al., Construction of high elastic artificial SEI for air-stable and long-life lithium metal anode, J. Colloid Interface Sci., 2023, 642, 193–203 CrossRef CAS PubMed.
- H. J. Qi and M. C. Boyce, Stress–strain behavior of thermoplastic polyurethanes, Mech. Mater., 2005, 37, 817–839 CrossRef.
- B. Zhao, C. Xing and Y. Shi, et al., Construction of high elastic artificial SEI for air-stable and long-life lithium metal anode, J. Colloid Interface Sci., 2023, 642, 193–203 CrossRef CAS PubMed.
- C. Zhao, R. Wang and B. Fang, et al., Boosting the Lithium Storage Properties of a Flexible Li4Ti5O12/Graphene Fiber Anode via a 3D Printing Assembly Strategy, Batteries, 2023, 9, 493 CrossRef CAS.
- S. Sun, C.-Z. Zhao and H. Yuan, et al., Multiscale understanding of high-energy cathodes in solid-state batteries: from atomic scale to macroscopic scale, Mater. Futures, 2022, 1(1), 012101 CrossRef CAS.
- T. Xiong, B. He and T. Zhou, et al., Stretchable fiber-shaped aqueous aluminum ion batteries, EcoMat, 2022, 4(5), e12218 CrossRef CAS.
- Z. Wang, X. Li and Z. Yang, et al., Fully transient stretchable fruit-based battery as safe and environmentally friendly power source for wearable electronics, EcoMat, 2021, 3(1), e12073 CrossRef CAS.
- C. Ning, S. Xiang and X. Sun, et al., Highly stretchable kirigami-patterned nanofiber-based nanogenerators for harvesting human motion energy to power wearable electronics, Mater. Futures, 2024, 3(2), 025101 CrossRef CAS.
- D. Li, Y. He and B. Chen, et al., Electrically active/inert dual-function architecture enabled by screen printing grid-like SiO on Cu foil for ultra-long life lithium metal anodes, EcoMat, 2024, 6(8), e12478 CrossRef CAS.
- K. Tagawa, M. Yoshio, R. J. Brodd and A. Kozawa, Production Processes for Fabrication of Lithium-Ion Batteries, Lithium-Ion Batteries: Science and Technologies, Springer New York, New York, NY, 2009, pp. 181–194 Search PubMed.
- M. Idrees, S. Batool and M. A. U. Din, et al., Material-structure-property integrated additive manufacturing of batteries, Nano Energy, 2023, 109, 108247 CrossRef CAS.
- Z. Lyu, G. J. H. Lim and J. J. Koh, et al., Design and Manufacture of 3D-Printed Batteries, Joule, 2021, 5(1), 89–114 CrossRef CAS.
- Y. Wang, C. Chen and H. Xie, et al., 3D-Printed All-Fiber Li-Ion Battery toward Wearable Energy Storage, Adv. Funct. Mater., 2017, 27(43), 1703140 CrossRef.
- J. Huang, J. Yang and W. Li, et al., Electrochemical properties of LiCoO2 thin film electrode prepared by ink-jet printing technique, Thin Solid Films, 2008, 516(10), 3314–3319 CrossRef CAS.
- N. J. Dudney and B. J. Neudecker, Solid state thin-film lithium battery systems, Curr. Opin. Solid State Mater. Sci., 1999, 4(5), 479–482 CrossRef.
- B. Clement, M. Lyu and E. Sandeep Kulkarni, et al., Recent Advances in Printed Thin-Film Batteries, Engineering, 2022, 13, 238–261 CrossRef.
- C. Liu, Y. Qiu and Y. Liu, et al., Novel 3D grid porous Li4Ti5O12 thick electrodes fabricated by 3D printing for high performance lithium-ion batteries, J. Adv. Ceram., 2022, 11(2), 295–307 CrossRef CAS.
- Y. Mu, Y. Chu and L. Pan, et al., 3D printing critical materials for rechargeable batteries: from materials, design and optimization strategies to applications, Int. J. Extreme Manuf., 2023, 5(4), 042008 CrossRef CAS.
- J. M. Nyika, F. M. Mwema and R. M. Mahamood, et al., Advances in 3D printing materials processing-environmental impacts and alleviation measures, Adv. Mater. Process. Tech., 2021, 8, 1275–1285 Search PubMed.
- M. Mao, J. He and X. Li, et al., The Emerging Frontiers and Applications of High-Resolution 3D Printing, Micromachines, 2017, 8(4), 113 CrossRef.
- A. Maurel, A. C. Martinez and S. Grugeon, et al., Toward High Resolution 3D Printing of Shape-Conformable Batteries via Vat Photopolymerization: Review and Perspective, IEEE Access, 2021, 9, 140654–140666 Search PubMed.
- M.-G. Zhang, H. Mei and P. Chang, et al., 3D printing of structured electrodes for rechargeable batteries, J. Mater. Chem., 2020, 8, 10670–10694 RSC.
- H. He, D. Luo and L. Zeng, et al., 3D printing of fast kinetics reconciled ultra-thick cathodes for high areal energy density aqueous Li–Zn hybrid battery, Sci. Bull., 2022, 67(12), 1253–1263 CrossRef CAS PubMed.
- J. Wang, Q. Sun and X. Gao, et al., Toward High Areal Energy and Power Density Electrode for Li-Ion Batteries via Optimized 3D Printing Approach, ACS Appl. Mater. Interfaces, 2018, 10(46), 39794–39801 CrossRef CAS PubMed.
- M. H. Cao, J. Su and S. Q. Fan, et al., Wearable piezoresistive pressure sensors based on 3D graphene, Chem. Eng. J., 2021, 406, 126777 CrossRef CAS.
- T. Wei, B. Y. Ahn and J. Grotto, et al., 3D Printing of Customized Li-Ion Batteries with Thick Electrodes, Adv. Mater., 2018, 30, 1703027 CrossRef PubMed.
- P. H. Yang and H. J. Fan, Inkjet and Extrusion Printing for Electrochemical Energy Storage: A Minireview, Adv. Mater. Technol., 2020, 5(10), 2000217 CrossRef CAS.
- A. M. Bates, Y. Preger and L. Torres-Castro, et al., Are solid-state batteries safer than lithium-ion batteries?, Joule, 2022, 6(4), 742–755 CrossRef CAS.
- W. Zaman and K. B. Hatzell, Processing and manufacturing of next generation lithium-based all solid-state batteries, Curr. Opin. Solid State Mater. Sci., 2022, 26(4), 101003 CrossRef CAS.
- Y. Mu, Y. Chu and L. Pan, et al., 3D printing critical materials for rechargeable batteries: from materials, design and optimization strategies to applications, Int. J. Extreme Manuf., 2023, 5, 042008 CrossRef CAS.
- T.-T. Huang and W. Wenzhuo, Scalable nanomanufacturing of inkjet-printed wearable energy storage devices, J. Mater. Chem. A, 2019, 7, 23280–23300 RSC.
- S. Soni, P. Sathe and S. K. Sarkar, et al., Inkjet-printed sub-zero temperature sensor for real-time monitoring of cold environments, Int. J. Biol. Macromol., 2024, 258, 128774 CrossRef CAS PubMed.
- C.-X. Zhao, J.-N. Liu and B.-Q. Li, et al., Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries, Adv. Funct. Mater., 2020, 30(36), 2003619 CrossRef CAS.
- S. Zhou, Y. Wang and H. Lu, et al., Anti-Corrosive and Zn-Ion-Regulating Composite Interlayer Enabling Long-Life Zn Metal Anodes, Adv. Funct. Mater., 2021, 31(46), 2104361 CrossRef CAS.
- T. Chen, Y. Wang and Y. Yang, et al., Heterometallic Seed-Mediated Zinc Deposition on Inkjet Printed Silver Nanoparticles Toward Foldable and Heat-Resistant Zinc Batteries, Adv. Funct. Mater., 2021, 31(24), 2101607 CrossRef CAS.
- M. Wei, F. Zhang and W. Wang, et al., 3D direct writing fabrication of electrodes for electrochemical storage devices, J. Power Sources, 2017, 354, 134–147 CrossRef CAS.
- C. Zhao, R. Wang and H. Liang, et al., Autonomous self-healing strategy for flexible fiber lithium-ion battery with ultra-high mechanical properties and volumetric energy densities, Chem. Eng. J., 2024, 496, 154153 CrossRef CAS.
- A. Goulas, D. Xie and G. Gatzoulis, et al., Rapid manufacture of sodium polyaluminate electrolyte ceramics for solid state batteries via direct ink writing, J. Eur. Ceram. Soc., 2024, 44(8), 5041–5047 CrossRef CAS.
- W. L. Gao, C. Iffelsberger and M. Pumera, Dual polymer engineering enables high-performance 3D printed Zn-organic battery cathodes, Appl. Mater. Today, 2022, 28, 101515 CrossRef.
- F. Zhang, M. Wei and V. V. Viswanathan, et al., 3D printing technologies for electrochemical energy storage, Nano Energy, 2017, 40, 418–431 CrossRef CAS.
- A. Maurel, S. Grugeon and B. Fleutot, et al., Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling, Sci. Rep., 2019, 9(1), 18031 CrossRef CAS PubMed.
- J. C. Ruiz-Morales, A. Tarancón and J. Canales-Vázquez, et al., Three dimensional printing of components and functional devices for energy and environmental applications, Energy Environ. Sci., 2017, 10(4), 846–859 RSC.
- L. Cao, M. Zheng and G. Dong, et al., Tailoring nanoscale primary silicon in laser powder bed fusion for high-performance lithium-ion battery anodes, J. Mater. Sci. Technol., 2025, 211, 278–287 CrossRef CAS.
- Y. J. He, S. J. Chen and L. Nie, et al., Stereolithography Three-Dimensional Printing Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries, Nano Lett., 2020, 20(10), 7136–7143 CrossRef CAS PubMed.
- Y. He, S. Chen and L. Nie, et al., Stereolithography Three-Dimensional Printing Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries, Nano Lett., 2020, 20(10), 7136–7143 CrossRef CAS PubMed.
- D. Ahn, L. M. Stevens and K. Zhou, et al., Rapid High-Resolution Visible Light 3D Printing, ACS Cent. Sci., 2020, 6(9), 1555–1563 CrossRef CAS PubMed.
- Y. Yang, W. Yuan and X. Zhang, et al., Overview on the applications of three-dimensional printing for rechargeable lithium-ion batteries, Appl. Energy, 2020, 257, 114002 CrossRef CAS.
- I. Shaji, D. Diddens and M. Winter, et al., Mechanistically Novel Frontal-Inspired In Situ Photopolymerization: An Efficient Electrode|Electrolyte Interface Engineering Method for High Energy Lithium Metal Polymer Batteries, Energy Environ. Mater., 2023, 6(6), e12469 CrossRef CAS.
- B. S. Lee, A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance, Polymers, 2020, 12(9), 2035 CrossRef CAS PubMed.
- Z. Liu, H.-B. He and Z.-X. Luo, et al., 3D printing of customized MnO2 cathode for aqueous zinc-ion batteries, Trans. Nonferrous Met. Soc. China, 2023, 33(4), 1193–1204 CrossRef CAS.
- B. M. Su, O. Cai, C. Yuan, et al., A study of the interactive effect of cathode material loss, SEI formation and lithium plating in NMC-graphite battery modeling, Proceedings of the IEEE Vehicle Power and Propulsion Conference (VPPC), Merced, CA, 2022 Search PubMed.
- T. Mu, L. Xiang and X. Wan, et al., Ultrahigh areal capacity silicon anodes realized via manipulating electrode structure, Energy Storage Mater., 2022, 53, 958–968 CrossRef.
- X. F. Chen, Z. Q. Guan and F. L. Chu, et al., Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: Challenges, strategies, and prospects, InfoMat, 2022, 4(1), e12248 CrossRef CAS.
- K. Lee, Y. Shang and V. A. Bobrin, et al., 3D Printing Nanostructured Solid Polymer Electrolytes with High Modulus and Conductivity, Adv. Mater., 2022, 34(42), 2204816 CrossRef CAS PubMed.
- G. Zhang, X. Zhang and H. Liu, et al., 3D-Printed Multi-Channel Metal Lattices Enabling Localized Electric-Field Redistribution for Dendrite-Free Aqueous Zn Ion Batteries, Adv. Energy Mater., 2021, 11, 2003927 CrossRef CAS.
- J. W. Long, B. Dunn and D. R. Rolison, et al., Three-Dimensional Battery Architectures, Chem. Rev., 2004, 104(10), 4463–4492 CrossRef CAS PubMed.
- R. Lopez-Hallman, R. Rodriguez and Y.-T. Lai, et al., All-Solid-State Battery Fabricated by 3D Aerosol Jet Printing, Adv. Eng. Mater., 2023, 26, 2300953 CrossRef.
- D. Gibson and C. Macgregor, A Novel Solid State Non-Dispersive Infrared CO2 Gas Sensor Compatible with Wireless and Portable Deployment, Sensors, 2013, 13(6), 7079–7103 CrossRef CAS PubMed.
- C. Chen, S. Li and P. H. L. Notten, et al., 3D Printed Lithium-Metal Full Batteries Based on a High-Performance Three-Dimensional Anode Current Collector, ACS Appl. Mater. Interfaces, 2021, 13(21), 24785–24794 CrossRef CAS PubMed.
- H. Lee, M. Yanilmaz and O. Toprakci, et al., A review of recent developments in membrane separators for rechargeable lithium-ion batteries, Energy Environ. Sci., 2014, 7(12), 3857–3886 RSC.
- K. K. Jana, S. J. Lue and A. Huang, et al., Separator Membranes for High Energy-Density Batteries, ChemBioEng Rev., 2018, 5(6), 346–371 CrossRef CAS.
- J. Qian, Q. Chen and M. Hong, et al., Toward stretchable batteries: 3D-printed deformable electrodes and separator enabled by nanocellulose, Mater. Today, 2022, 54, 18–26 CrossRef CAS.
- E. Maiser, Battery Packaging - Technology Review; Proceedings of the 1st International Freiberg Conference on Electrochemical Storage Materials, TU Bergakademie Freiberg, Freiberg, Germany, 2014 Search PubMed.
- C.-P. Feng, K.-Y. Sun and J.-C. Ji, et al., 3D Printable, form stable, flexible phase-change-based electronic packaging materials for thermal management, Addit. Manuf., 2023, 71, 103586 CrossRef CAS.
- S. H. Zheng, Z. S. Wu and F. Zhou, et al., All-solid-state planar integrated lithium ion micro-batteries with extraordinary flexibility and high-temperature performance, Nano Energy, 2018, 51, 613–620 CrossRef CAS.
- A. Toor, A. Wen and F. Maksimovic, et al., Stencil-printed Lithium-ion micro batteries for IoT applications, Nano Energy, 2021, 82, 105666 CrossRef CAS.
- K. Sun, T. Wei and B. Y. Ahn, et al., 3D Printing of Interdigitated Li-Ion Microbattery Architectures, Adv. Mater., 2013, 25, 4539–4543 CrossRef CAS PubMed.
- S. H. Zheng, H. J. Huang and Y. F. Dong, et al., Ionogel-based sodium ion micro-batteries with a 3D Na-ion diffusion mechanism enable ultrahigh rate capability, Energy Environ. Sci., 2020, 13(3), 821–829 RSC.
- J. Ma, S. Zheng and L. Chi, et al., 3D Printing Flexible Sodium-Ion Microbatteries with Ultrahigh Areal Capacity and Robust Rate Capability, Adv. Mater., 2022, 34(39), 2205569 CrossRef CAS PubMed.
- C. C. Ho, K. Murata and D. A. Steingart, et al., A super ink jet printed zinc–silver 3D microbattery, J. Micromech. Microeng., 2009, 19(9), 094013 CrossRef.
- C. Kim, B. Y. Ahn and T.-S. Wei, et al., High-Power Aqueous Zinc-Ion Batteries for Customized Electronic Devices, ACS Nano, 2018, 12(12), 11838–11846 CrossRef CAS PubMed.
- A. Pendashteh, J. S. Sanchez and J. Palma, et al., Anchored NiCoMnS4 nanoparticles on N-doped rGO: High-performance bifunctional electrocatalysts for rechargeable Zn-Air batteries, Energy Storage Mater., 2019, 20, 216–224 CrossRef.
- L. Fu, J. K. Luo, J. E. Huber, et al., Design and fabrication of a micro zinc/air battery, Proceedings of the International MEMS Conference 2006, Singapore, 2006 Search PubMed.
- Y. Hou, C. Wang and X. Wen, et al., Double-network gel electrolyte with high electrolyte retention for flexible high-voltage Zn-air batteries, Chem. Eng. J., 2024, 496, 153817 CrossRef CAS.
- Y. Ren, F. Meng and S. Zhang, et al., CNT@MnO2 composite ink toward a flexible 3D printed micro-zinc-ion battery, Carbon Energy, 2022, 4(3), 446–457 CrossRef CAS.
- Z. Huang, L. Sugiarto and Y.-C. Lu, Feature–target pairing in machine learning for battery health diagnosis and prognosis: A critical review, EcoMat, 2023, 5(6), e12345 CrossRef.
- T. Ohzuku, N. Matoba and K. Sawai, Direct evidence on anomalous expansion of graphite-negative electrodes on first charge by dilatometry, J. Power Sources, 2001, 97–98, 73–77 CrossRef CAS.
- T. Ohzuku, H. Tomura and K. Sawai, Monitoring of Particle Fracture by Acoustic Emission during Charge and Discharge of Li/MnO2 Cells, J. Electrochem. Soc., 1997, 144(10), 3496 CrossRef CAS.
- A. Hsieh, S. Bhadra and B. Hertzberg, et al., Electrochemical-Acoustic Time of Flight: In Operando Correlation of Physical Dynamics with Battery Charge and Health, Energy Environ. Sci., 2015, 8, 1569–1577 RSC.
- Z. Wang, X. Zhao and H. Zhang, et al., Active acoustic emission sensing for fast co-estimation of state of charge and state of health of the lithium-ion battery, J. Energy Storage, 2023, 64, 107192 CrossRef.
- D. Y. Chen, Q. Zhao and Y. Zheng, et al., Recent Progress in Lithium-Ion Battery Safety Monitoring Based on Fiber Bragg Grating Sensors, Sensors, 2023, 23(12), 5609 CrossRef CAS PubMed.
- W. Mei, Z. Liu and C. Wang, et al., Operando monitoring of thermal runaway in commercial lithium-ion cells via advanced lab-on-fiber technologies, Nat. Commun., 2023, 14(1), 5251 CrossRef CAS PubMed.
- L. Fazzi, N. Dias and M. Holynska, et al., Monitoring of silicone adhesive in space solar cells with an embedded multi-parameter TFBG sensor in a simulated space environment, Meas. Sci. Technol., 2022, 33(8), 085108 CrossRef CAS.
- J. Q. Huang, X. L. Han and F. Liu, et al., Monitoring battery electrolyte chemistry via in-operando tilted fiber Bragg grating sensors, Energy Environ. Sci., 2021, 14(12), 6464–6475 RSC.
- X. Han, H. Zhong and K. Li, et al., Operando monitoring of dendrite formation in lithium metal batteries via ultrasensitive tilted fiber Bragg grating sensors, Light: Sci. Appl., 2024, 13(1), 24 CrossRef CAS PubMed.
- R. Taitt Chris, G. P. Anderson and F. S. Ligler, Evanescent wave fluorescence biosensors: Advances of the last decade, Biosens. Bioelectron., 2016, 76, 103–112 CrossRef PubMed.
- A. Ghannoum, R. C. Norris and K. Iyer, et al., Optical Characterization of Commercial Lithiated Graphite Battery Electrodes and in Situ Fiber Optic Evanescent Wave Spectroscopy, ACS Appl. Mater. Interfaces, 2016, 8(29), 18763–18769 CrossRef CAS PubMed.
- A. Ghannoum, K. Iyer, P. Nieva, et al., Fiber optic monitoring of lithium-ion batteries: A novel tool to understand the lithiation of batteries, Proceedings of the 2016 IEEE Sensors, 2016 Search PubMed.
- A. Ghannoum and P. Nieva, Graphite lithiation and capacity fade monitoring of lithium ion batteries using optical fibers, J. Energy Storage, 2020, 28, 101233 CrossRef.
- J. Hedman, R. Mogensen and R. Younesi, et al., Fiber Optic Sensors for Detection of Sodium Plating in Sodium-Ion Batteries, ACS Appl. Energy Mater., 2022, 5(5), 6219–6227 CrossRef CAS.
- B. Liu, Z. Yu and Z. Tian, et al., Temperature dependence of sapphire fiber Raman scattering, Opt. Lett., 2015, 40(9), 2041–2044 CrossRef CAS PubMed.
- H. H. Kee, G. Lees and T. P. Newson, All-fiber system for simultaneous interrogation of distributed strain and temperature sensing by spontaneous Brillouin scattering, Opt. Lett., 2000, 25(10), 695–697 CrossRef CAS PubMed.
- Y. Yu, E. Vergori and D. Worwood, et al., Distributed thermal monitoring of lithium ion batteries with optical fibre sensors, J. Energy Storage, 2021, 39, 102560 CrossRef.
- Y. Yu, E. Vergori and F. Maddar, et al., Real-time monitoring of internal structural deformation and thermal events in lithium-ion cell via embedded distributed optical fibre, J. Power Sources, 2022, 521, 230957 CrossRef CAS.
- T. J. Bajzek, Thermocouples: a sensor for measuring temperature, IEEE Instrum. Meas. Mag., 2005, 8(1), 35–40 Search PubMed.
- S. Kim, S. Lim and M. H. Jeong, et al., Flexible thermocouple using a thermoelectric graphene fiber with a seamless junction, J. Mater. Sci. Technol., 2024, 172, 15–22 CrossRef CAS.
- Z. Wei, J. Hu and H. He, et al., Embedded Distributed Temperature Sensing Enabled Multistate Joint Observation of Smart Lithium-Ion Battery, IEEE Trans. Ind. Electron., 2023, 70(1), 555–565 Search PubMed.
- B. Gulsoy, T. A. Vincent and J. E. H. Sansom, et al., In-situ temperature monitoring of a lithium-ion battery using an embedded thermocouple for smart battery applications, J. Energy Storage, 2022, 54, 105260 CrossRef.
- F. X. Meng, M. Fan and K. M. Wu, et al., Flexible Liquid Crystal Thermistor and Its Application in Temperature Sensor, IEEE Trans. Electron. Dev., 2022, 69(11), 6298–6303 CAS.
- J. Fleming, T. Amietszajew and J. Charmet, et al., The design and impact of in-situ and operando thermal sensing for smart energy storage, J. Energy Storage, 2019, 22, 36–43 CrossRef.
- D. M. G. Preethichandra and P. Sonar, Electrochemical Impedance Spectroscopy and its Applications in Sensor Development and Measuring Battery Performance, IEEE Sens. J., 2022, 22(11), 10152–10162 CAS.
- R. Srinivasan, P. A. Demirev and B. G. Carkhuff, Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention, J. Power Sources, 2018, 405, 30–36 CrossRef CAS.
- S. Green, S. Song and B. Kim, Fabrication of Channel Circuit Electrodes and Flexible Graphene Resistive Sensors for Detecting Dinitrotoluene 2,4 (DNT), Int. J. Precis. Eng. Manuf., 2020, 21(10), 1943–1953 CrossRef.
- S. Pannala, A. Weng and I. Fischer, et al., Low-Cost Inductive Sensor and Fixture Kit for Measuring Battery Cell Thickness Under Constant Pressure, IFAC-PapersOnLine, 2022, 55(37), 712–717 CrossRef.
- Z. Chen, J. Lin and C. Zhu, et al., Detection of jelly roll pressure evolution in large-format Li-ion batteries via in situ thin film flexible pressure sensors, J. Power Sources, 2023, 566, 232960 CrossRef CAS.
- Y. Lyu, Y. R. Zhang and L. B. Xu, et al., Solid-Contact Ion Sensing Without Using an Ion-Selective Membrane through Classic Li-Ion Battery Materials, Anal. Chem., 2021, 93(21), 7588–7595 CrossRef PubMed.
- J. Liu, Z. Chu and H. Li, et al., Lithium-plating-free fast charging of large-format lithium-ion batteries with reference electrodes, Int. J. Energy Res., 2021, 45, 7918–7932 CrossRef CAS.
- J. Hu, G. Sang and N. Zeng, et al., Amperometric sensor for the detection of hydrogen stable isotopes based on Pt nanoparticles confined within single-walled carbon nanotubes (SWNTs), Sens. Actuators, B, 2022, 356, 131344 CrossRef CAS.
- N. Y. Stasyuk, G. Z. Gayda and A. E. Zakalskiy, et al., Amperometric biosensors for L-arginine and creatinine assay based on recombinant deiminases and ammonium-sensitive Cu/Zn(Hg)S nanoparticles, Talanta, 2022, 238, 122996 CrossRef CAS PubMed.
- C.-C. Wang, Z.-Y. Hou and W.-J. Lu, et al., High-voltage on-chip current sensor design and analysis for battery modules, IET Circuits, Devices Syst., 2016, 10, 492–496 CrossRef.
- J. Olarte, J. M. De Ilarduya and E. Zulueta, et al., A Battery Management System with EIS Monitoring of Life Expectancy for Lead-Acid Batteries, Electronics, 2021, 10(11), 1228 CrossRef CAS.
- M. Crescentini, A. D. Angelis and R. Ramilli, et al., Online EIS and Diagnostics on Lithium-Ion Batteries by Means of Low-Power Integrated Sensing and Parametric Modeling, IEEE Trans. Instrum. Meas., 2021, 70, 1–11 Search PubMed.
- D. Del Orbe Henriquez, I. Cho and H. Yang, et al., Pt Nanostructures Fabricated by Local Hydrothermal Synthesis for Low-Power Catalytic-Combustion Hydrogen Sensors, ACS Appl. Nano Mater., 2021, 4(1), 7–12 CrossRef CAS.
- E.-B. Kim and H.-K. Seo, Highly Sensitive Formaldehyde Detection Using Well-Aligned Zinc Oxide Nanosheets Synthesized by Chemical Bath Deposition Technique, Materials, 2019, 12(2), 250 CrossRef CAS PubMed.
- R. L. Pérez, C. E. Ayala and J.-Y. Park, et al., Coating-Based Quartz Crystal Microbalance Detection Methods of Environmentally Relevant Volatile Organic Compounds, Chemosensors, 2021, 9(7), 153 CrossRef.
- H. Zhang, B. Shen and W. Hu, et al., Research on a Fast-Response Thermal Conductivity Sensor Based on Carbon Nanotube Modification, Sensors, 2018, 18(7), 2191 CrossRef PubMed.
- Y. Chang, D. Hasan and B. Dong, et al., All-Dielectric Surface-Enhanced Infrared Absorption-Based Gas Sensor Using Guided Resonance, ACS Appl. Mater. Interfaces, 2018, 10(44), 38272–38279 CrossRef CAS PubMed.
- Y. Jian, W. Hu and Z. Zhao, et al., Gas Sensors Based on Chemi-Resistive Hybrid Functional Nanomaterials, Nano-Micro Lett., 2020, 12(1), 71 CrossRef CAS PubMed.
- Y. Han, Y. Zhao and A. Ming, et al., Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries, Energies, 2023, 16(9), 3620 CrossRef CAS.
- Z. Li, H. Li and Z. Wu, et al., Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature, Mater. Horiz., 2019, 6(3), 470–506 RSC.
- T. T. Lin, X. Lv and S. Li, et al., The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties, Sensors, 2017, 17(12), 2779 CrossRef PubMed.
- X.-X. Wang, Q.-T. Li and X.-Y. Zhou, et al., Monitoring thermal runaway of lithium-ion batteries by means of gas sensors, Sens. Actuators, B, 2024, 411, 135703 CrossRef CAS.
- L. Xu, T. Li and X. L. Gao, et al., A High-Performance Three-Dimensional Microheater-Based Catalytic Gas Sensor, IEEE Electron Device Lett., 2012, 33(2), 284–286 Search PubMed.
- E. Brauns, E. Morsbach and S. Kunz, et al., Temperature Modulation of a Catalytic Gas Sensor, Sensors, 2014, 14(11), 20372–20381 CrossRef PubMed.
- E. E. Karpov, E. F. Karpov and A. Suchkov, et al., Energy efficient planar catalytic sensor for methane measurement, Sens. Actuators, A, 2013, 194, 176–180 CrossRef CAS.
- L. Wang, S. Xu, Z. Wang, E. Yang, W. Jiang, S. Zhang, X. Jian and F. Hu, A nano fiber–gel composite electrolyte with high Li+ transference number for application in quasi-solid batteries, eScience, 2023, 3, 100090 CrossRef.
- C. Niu, J. Meng, X. Wang and C. Han, et al., General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis, Nat. Commun., 2015, 6, 7402 CrossRef PubMed.
- M. Li, R. Mo, A. Ding, K. Zhang, F. Guo and C. Xiao, Electrochemical technology to drive spent lithium- ion batteries (LIBs) recycling: recent progress, and prospects, Energy Mater., 2024, 4, 400070 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |




