Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atomic layer deposition of nickel sulfide thin films and their thermal and electrochemical stability†
Miika
Mattinen
 *ab,
Johanna
Schröder‡
*ab,
Johanna
Schröder‡
 bc,
Timo
Hatanpää
bc,
Timo
Hatanpää
 a,
Georgi
Popov
a,
Georgi
Popov
 a,
Kenichiro
Mizohata
d,
Markku
Leskelä
a,
Kenichiro
Mizohata
d,
Markku
Leskelä
 a,
Thomas F.
Jaramillo
a,
Thomas F.
Jaramillo
 bce,
Michaela Burke
Stevens
bce,
Michaela Burke
Stevens
 c,
Stacey F.
Bent
c,
Stacey F.
Bent
 be and
Mikko
Ritala
be and
Mikko
Ritala
 *a
*a
aDepartment of Chemistry, University of Helsinki, P. O. Box 55, FI-00014, Finland. E-mail: miika.mattinen@helsinki.fi; mikko.ritala@helsinki.fi
bDepartment of Chemical Engineering, Stanford University, 443 Via Ortega, Stanford, California 94305, USA
cSUNCAT Center for Interface Science and Catalysis, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, California 94025, USA
dDivision of Materials Physics, Department of Physics, University of Helsinki, P. O. Box 43, FI-00014, Finland
eDepartment of Energy Science and Engineering, Stanford University, 443 Via Ortega, Stanford, California 94305, USA
First published on 10th July 2025
Abstract
Nickel sulfides (NiSx) show promise for a range of energy and other applications, but their (in)stability under processing and operating conditions is scarcely studied. Herein, we have developed a new NiSx atomic layer deposition process using an easily synthesized NiCl2(TMPDA) precursor (TMPDA = N,N,N′,N′-tetramethyl-1,3-propanediamine) with H2S. Thin films deposited at 165–225 °C consist mostly of the β-NiS phase and display low resistivity (∼40–120 μΩ cm), high purity (<3 at% impurities), and a rough morphology. The thermal stability of the NiSx thin films is studied using high-temperature X-ray diffraction, revealing that structural and compositional changes occur in reducing, inert, and oxidizing atmospheres at approximately 300–400 °C. Under electrochemical water splitting conditions, the films are unstable in acid due to dissolution, especially at oxidizing potentials. In an alkaline electrolyte, we do not observe Ni dissolution, but β-NiS transforms to Ni3S2 under HER conditions, possibly supplemented with Ni and/or Ni(OH)2 species. Under alkaline OER, all sulfur is lost and NiOOH is formed. In addition to offering an attractive, scalable route to the synthesis of NiSx thin films, our work highlights the importance of thermal and electrochemical (in)stability of sulfides as a crucial step for understanding and engineering materials for energy and other applications.
Introduction
Nickel sulfides (NiSx) form a diverse group of materials with different compositions and crystal structures, including five that are observed as minerals: rhombohedral Ni3S2, orthorhombic Ni9S8, rhombohedral β-NiS, cubic Ni3S4, and cubic NiS2 as well as high-temperature phases orthorhombic Ni7S6 and hexagonal α-NiS.1,2 Most of these phases are highly conductive metallic or degenerate semiconducting materials.3–12 Their electronic properties together with the low toxicity and earth abundance of nickel and sulfur make NiSx promising electrode materials for a range of energy applications, including supercapacitors,13,14 lithium-ion batteries,15–17 and dye-sensitized solar cells,5,7,18 as well as electrochemical6,19–23 and photoelectrochemical14 water splitting.The stability of NiSx under processing and operating conditions encountered in different applications has received relatively little attention. The processing of devices from microelectronics to solar cells and beyond often includes annealing steps, which can lead to changes in film composition and structure. In operation of electrocatalysts, the electric potential together with the presence of the electrolyte challenge the stability of the catalyst. Nickel sulfides are regarded as promising water splitting electrocatalysts.6,19–25 However, Pourbaix diagrams predict NiSx to be unstable under typical operating conditions of both the reducing (i.e. hydrogen evolution reaction, HER) and oxidizing (i.e. oxygen evolution reaction, OER) half-reactions of water splitting.25–27 Commercial electrolyzer technologies operate under alkaline (alkaline electrolyzers28,29 and anion exchange membrane (AEM) electrolyzers28,30) or acidic conditions (proton exchange membrane (PEM) electrolyzers31). While numerous studies have reported NiSx to be active for both the HER and OER, investigations into the stability and the actual catalytic species remain rather scarce. Under alkaline OER conditions, NiSx has been reported to transform to NiOOH prior to the OER, making NiSx a precatalyst to the actual NiOOH catalyst.22,25,32–34 Regardless, consensus on the rate and extent (depth) of this transformation has not been reached.25 For the less harsh HER conditions, it remains common to assume NiSx to be stable. However, recent reports have suggested that NiSx may transform to either another NiSx phase,35,36 Ni metal,37 NiOxSy,38 or Ni(OH)2 (ref. 39 and 40) under alkaline HER conditions. A recent review by Kawashima et al.41 suggested that the majority of metal chalcogenides, including NiSx, may undergo some degree of structural and/or compositional change under alkaline HER conditions. For the HER in acid, good stability appears to be the prevailing view, yet dissolution of NiSx has also been observed.19,42 For both the OER and HER, there is limited understanding of the effects of NiSx compositions as well as the preparation method. Thus, questions on the stability of NiSx under water splitting conditions and the identity of the actual catalytic species remain largely unanswered.
A variety of methods have been used to deposit NiSx thin films and nanoparticles, including solid-state,17 hydrothermal,23 solvothermal,13,21 and colloidal synthesis,15 electrodeposition,18 sulfurization,19,20 chemical vapor deposition (CVD),43 pulsed laser deposition,44 and molecular beam epitaxy.45 Nevertheless, existing methods often fail to meet one or more of the following desirable aspects: high purity and crystallinity, thickness control, scalability, uniform coating of three-dimensional substrates, and low processing temperatures. To overcome these challenges, we use atomic layer deposition (ALD), an advanced CVD technique relying on self-limiting surface reactions of alternately supplied precursors. ALD offers unmatched thickness control, reproducibility, scalability and the ability to coat both large and complexly shaped substrates with uniform layers of thin films or nanoparticles.46–48 The advantageous characteristics of ALD along with the rising interest in NiSx have led to the development of several NiSx ALD processes. β-NiS films have been deposited using β-diketonates Ni(thd)2 (ref. 5) and Ni(acac)2 (ref. 49) with H2S, while amorphous NiS (ref. 50) and crystalline Ni9S8 (ref. 22) have been reported using an amidinate Ni(tBuAMD)2 with H2S. Ni(tBuAMD)2 can also be used with di-tert-butyl-disulfide51 and H2S plasma52 to deposit crystalline Ni9S8 and NiS2 films, respectively. An aminoalkoxide, Ni(dmamb)2, combined with H2S has been reported to result in either crystalline Ni3S2 (ref. 6 and 7) or β-NiS (ref. 53) films. Despite several published processes, controlling the deposited NiSx phase remains difficult, and none of the processes can deposit highly pure and conductive NiSx films with a high growth rate from affordable precursors. Conductivity is key to its application as an electrode, while growth rate and precursor cost have a significant effect on the industrial scalability of the process. Recently, NiCl2(TMPDA) (TMPDA = N,N,N′,N′-tetramethyl-1,3-propanediamine) was introduced as a promising low-cost alternative to the existing nickel ALD precursors54–56 that provides sufficient volatility, thermal stability, and reactivity, but NiSx has not yet been deposited using this precursor.
In this work, we used NiCl2(TMPDA) with H2S at 165–225 °C, yielding crystalline films consisting of mainly the β-NiS phase. The film properties including crystallinity, morphology, impurities, and electrical properties were assessed. We investigated the stability of the deposited films in reducing, inert, and oxidizing atmospheres at elevated temperatures to mimic conditions encountered during processing of various devices. We then evaluated β-NiS for both half reactions of electrochemical water splitting (HER and OER) under both alkaline and acidic conditions. Thin films are well suited for investigating stability, which is a crucial step in the process of engineering advanced electrodes for electrocatalysis and other applications.
Results and discussion
ALD process development
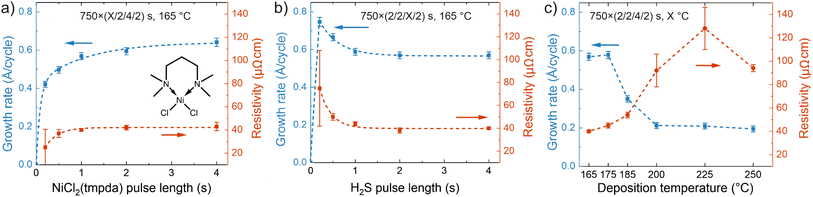
Nickel sulfide films were deposited by ALD using a new precursor combination of NiCl2(TMPDA) and H2S. The precursor pulses were separated by inert nitrogen (N2) purges and the NiCl2(TMPDA)–N2–H2S–N2 ALD cycle was repeated until the desired film thickness was reached. The growth characteristics were initially evaluated on silicon substrates at a temperature of 165 °C using 750 ALD cycles. With an increase of the NiCl2(TMPDA) pulse length, the growth rate leveled off by 1.0 s pulse time to approximately 0.6 Å per cycle (Fig. 1a), indicating the self-limiting precursor adsorption characteristic of ALD. A further increase of the pulse length from 1.0 to 4.0 s increased the growth rate slightly to approximately 0.7 Å per cycle, which we attribute to minor changes in phase composition, crystalline orientation, and morphology (see the section Effects of deposition temperature and film thickness on crystallinity, morphology, and composition and Note S3 in the ESI†). Pulsing only NiCl2(TMPDA) repeatedly resulted in negligible film growth, ruling out precursor decomposition (Fig. S1 in the ESI†). A low resistivity of 40–45 μΩ cm (using a four-point probe) and high uniformity (1–3% standard deviation of sheet resistance over 5 × 5 cm2 substrates) were obtained using a NiCl2(TMPDA) pulse length of at least 1.0 s. The films deposited on both Si and glass substrates had a shiny, metallic appearance.When the H2S pulse length was increased from the shortest value of 0.2 s, the growth rate first decreased before it stabilized at 0.6 Å per cycle using H2S pulse lengths of at least 1.0 s (Fig. 1b). At the same time, the resistivity of the films decreased reaching approximately 40 μΩ cm at 2.0 s H2S pulse length. The film uniformity was good except for the shortest 0.2 s H2S pulse. The S/Ni ratio of the films remained unchanged at approximately 1.0 regardless of the pulse length (Fig. S2†). Regarding the purge steps, we observed that 1.0 s N2 purges after each precursor pulse were sufficient to remove unreacted precursors and byproducts (Fig. S3†). Based on these experiments, pulse lengths of 2.0 s for both NiCl2(TMPDA) and H2S and 2.0 s purges were chosen for further depositions to ensure operation in the saturated ALD regime.
As seen in Fig. 1c, the growth rate remained unchanged when the deposition temperature was increased from 165 to 175 °C, but a further increase led to a decrease in the growth rate to approximately 0.4 Å per cycle at 185 °C and 0.2 Å per cycle at 200–250 °C. Concurrently, the film resistivity increased from 40 μΩ cm at 165 °C to approximately 100 μΩ cm at 200–250 °C, and the non-uniformity estimated from the standard deviation of sheet resistance increased from <3% at 165 °C to ∼15% at 200–225 °C. The lowest deposition temperature of 165 °C was limited by the temperature of 157 °C required to reach a vapor pressure of 0.1 mbar for NiCl2(TMPDA), whereas visible decomposition of NiCl2(TMPDA) was observed at 250 °C, limiting the highest ALD temperature to 225 °C in accordance with the earlier studies of NiCl2(TMPDA).55 In summary, NiCl2(TMPDA) and H2S afford a well-behaved ALD process in the 165–225 °C temperature range.
Effects of deposition temperature and film thickness on crystallinity, morphology, and composition
Next, the morphology and crystallinity of the films deposited on silicon at different temperatures were studied. Since the deposition temperature affects the growth rate, the number of ALD cycles was adjusted to reach a similar thickness (44–48 nm). At 165 °C, relatively large features ranging from approximately 100 to 300 nm in width were observed on the surface of the films by scanning electron microscopy (SEM, Fig. 2b). With increasing deposition temperature, smaller, more rod-like features became more prominent on the film surface in comparison to the flatter, larger structures dominant at 165 °C. X-ray diffraction (XRD, Fig. 2a) showed the films to consist primarily of the rhombohedral β-NiS phase (millerite, powder diffraction file (PDF) 12-41) with a few additional peaks that were indexed to the orthorhombic Ni9S8 phase (godlevskite, PDF 22-1193).57,58 With increasing deposition temperature, the intensities of the Ni9S8 peaks increased and those of the β-NiS peaks decreased. The β-NiS phase was observed to have a preferred (300) orientation for all of the deposition temperatures, while the Ni9S8 phase became (111) textured at 225 °C and above, with no clear texture at lower temperatures (Note S2 and Fig. S7†). The changes in phase composition, preferred orientation, and morphology may at least partially explain the rather strong temperature-dependence of the growth rate (Fig. 1c). Raman spectra of the films deposited at 185–225 °C confirmed the presence of β-NiS (Fig. S5†). No other phases were detected, which may be due to their Raman inactivity (Note S1†).The elemental composition of the films was analyzed by time-of-flight elastic recoil detection analysis (ToF-ERDA; Table S1†). The S/Ni ratio remained at 1.00–1.02 in all of the measured films deposited at 165–225 °C, in agreement with the main β-NiS phase. The films were highly pure, containing less than 2 at% of O, C, N, and H impurities in total that originated from the TMPDA ligands and the atmosphere. Accurate determination of the chlorine content was difficult due to its similar atomic mass with sulfur, but an upper limit of approximately 1 at% was estimated for the chlorine concentration. The high film purity indicates facile and complete surface reactions between NiCl2(TMPDA) and H2S. Thus, we conclude that pure, crystalline films consisting mainly of the β-NiS phase can be deposited within the temperature range of 165–225 °C. At the higher end of the temperature range, the contribution of the additional Ni9S8 phase increases and the size of surface features decreases.
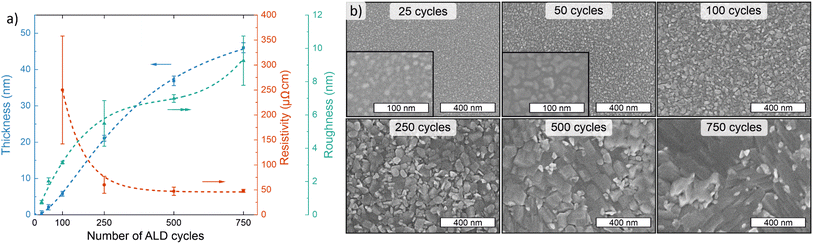
To obtain information on the film nucleation and growth, we evaluated the evolution of thickness, resistivity, roughness, and morphology as a function of film thickness at 165 °C. This deposition temperature was selected as it yields the highest growth rate, as well as the lowest resistivity and best uniformity. After a slight nucleation delay (<25 cycles), film growth was linear up to at least 250 ALD cycles, after which it seemed to slightly slow down (Fig. 3a). The decrease in the growth rate may be due to changes in morphology, namely formation of larger plate-like crystallites (Fig. 3b), or crystalline phase and orientation (Fig. S12†). The thinnest deposited films (25 and 50 cycles) were non-conductive and likely non-continuous, whereas using 100 cycles an approximately 6 nm thick conductive NiSx film (270 μΩ cm) was deposited. With increasing thickness, the film resistivity decreased towards a stable level, reaching 64 μΩ cm at 22 nm (250 cycles) and 51 μΩ cm at 49 nm (750 cycles). The surface roughness of the NiSx film on silicon increased rapidly from 0.16 nm for the bare substrate to 0.76 nm for the discontinuous 25 cycle film, 3.1 nm for the thinnest continuous 100 cycle film, and 9.3 nm for the 49 nm film deposited using 750 cycles (Fig. 3a; atomic force microscopy (AFM) images in Fig. S13†).
Compared to the other published NiSx ALD processes, our NiCl2(TMPDA) + H2S process combines good ALD growth characteristics (saturation with a reasonably high growth rate and low deposition temperatures) with favorable film properties (low resistivity and impurity content), while using an affordable, easily synthesized nickel precursor (see Note S4 and Table S2† for a detailed comparison).
High-temperature stability in different atmospheres
To understand the stability of NiSx under reducing, inert, and oxidizing conditions that are encountered in various applications and processing steps, we performed in situ high-temperature XRD (HTXRD) measurements in different atmospheres. Approximately 50 nm thick films deposited on Si at 165 °C that consist predominantly of β-NiS with a minor Ni9S8 phase present were used. A visual summary of the crystalline phases formed in inert, reducing, and oxidizing atmospheres is shown in Fig. 4, while the XRD data is shown in Fig. S14, S16, S18, S20 and S22.†In a reducing forming gas environment (10% H2/90% N2, atmospheric pressure), β-NiS began to reduce at 300 °C forming Ni9S8 and gaseous H2S (ref. 59) (Fig. 4 and S14†). At 350 °C, metallic fcc Ni (and more H2S) started to form yielding a single phase of Ni metal from 425 °C until the end of the measurement at 750 °C. After cooling down from 750 °C, a discontinuous metallic Ni film was obtained (Fig. S15†). For comparison, an ALD Ni3N film deposited using the same nickel precursor could be reduced to metallic nickel at a temperature as low as 150 °C in 10% H2/N2 (ref. 54) and an ALD NiO film at 260 °C in 5% H2/N2.60 This comparison suggests that β-NiS is more difficult to reduce to Ni metal than Ni3N and NiO.
Under an inert N2 atmosphere at atmospheric pressure, the β-NiS phase was retained up to 400 °C and Ni9S8 up to 475 °C (Fig. 4 and S16†). Above this temperature, phases with a lower S/Ni ratio, first α-Ni7S6 and later Ni3S2, formed. Additionally, NiSi was observed, which is attributed to a reaction with the Si substrate (Note S5†). In a dynamic vacuum at approximately 10−5 mbar, similar phase transformations occurred but at ∼50–150 °C lower temperatures compared to the N2 atmosphere (Fig. 4, S18 and Note S6†). Although Ni3S2 was not observed during the HTXRD measurement in a vacuum, it appeared after cooling, showing that not all the sulfur was lost upon heating in a vacuum to 750 °C (Fig. S19†).
In oxidizing ambient air, Ni9S8 disappeared by 250 °C, leaving only the β-NiS phase present up to 300 °C. At 300 °C, a minor Ni3S4 component started to form (Fig. 4, S20 and Note S7†). Hexagonal α-NiS, a high-temperature form of NiS, began to form at 325 °C. Broad peaks originating from cubic NiO emerged at approximately 350 °C, although an amorphous oxide may have formed at even lower temperatures. The last sulfide phase α-NiS disappeared by 450 °C, followed by an emergence of NiSO4, an oxidation product of NiS.61 Above 550 °C only NiO was observed and its crystallinity improved with increasing temperature (Fig. S20 and S21†). When pure O2 was used instead of ambient air, similar phase transitions were observed at 25–50 °C lower temperatures (Fig. 4, S22 and S23†).
In summary, in a reducing atmosphere, β-NiS loses S and forms metallic Ni at 300–400 °C. This is a relatively high temperature compared to that of other nickel compounds including NiO, showing on one hand the stability of β-NiS (and other NiSx phases) and on the other hand making reduction of β-NiS a rather inconvenient route to metallic Ni. In an inert atmosphere, S loss occurs at higher temperatures compared to the reducing conditions and remains incomplete at 750 °C. Annealing in inert atmospheres is a potential route to produce S poor phases that may be difficult to deposit directly, including Ni9S8, α-Ni7S6, and Ni3S2. Annealing in an oxidizing atmosphere yields NiO after several intermediate phases. Besides phase control by annealing, it is important to note that the temperature ranges where phase transitions occur in all of the atmospheres are similar to typical processing temperatures encountered in, for example, back end-of-line semiconductor62 and solar cell manufacturing (∼400 °C).63 Furthermore, catalysts containing NiSx are employed for hydrotreating petroleum products, where operating temperatures of around 300–400 °C may be used.64
Stability under electrochemical water splitting conditions
Besides thermal catalysis, nickel-based materials including NiSx are considered promising catalysts for electrochemical water splitting.65,66 However, NiSx is thermodynamically unstable under both acidic and alkaline water splitting conditions (see Pourbaix diagrams in Fig. S27 and S28†). The effect of electrolyte impurities is also important, especially for the OER.34,67,68 We evaluated both the reducing (HER) and oxidizing (OER) half reactions of water splitting in acidic (0.5 M H2SO4, pH = 0.3; cf. PEM electrolyzers) as well as alkaline electrolytes (0.1 M KOH, pH = 13; cf. alkaline and AEM electrolyzers). We deposited NiSx thin films on fluorine-doped tin oxide (FTO) at 165 °C. The thickness of the NiSx film was ∼15 nm for alkaline and ∼30 nm for acidic testing. The higher thickness selected in the latter case was due to thermodynamically favored dissolution in acid. The same β-NiS phase was obtained on both the Si and FTO substrates, albeit with some differences in morphology (Note S8†).We began the electrochemical cyclic voltammetry (CV) testing with the HER in acid (0.5 M H2SO4, between 0.0 and −0.45 V vs. RHE at 10 mV s−1). During the first CVs, relatively good catalytic activity was observed. An overpotential η of ∼300 mV (i.e. −0.3 V vs. RHE) was sufficient to reach the typical benchmark current density of 10 mA cmgeo−2 (Fig. 5a). However, after 10 CVs (∼15 min), the HER current started to decrease and hysteresis during the CVs increased. After 30 CVs (∼45 min), the HER current was negligible, and the catalyst was removed for characterization. X-ray photoelectron spectroscopy (XPS) was used to characterize changes in the material. Although the Ni 2p3/2 spectra are complex, the main spectral features described in the literature allow different nickel compounds to be distinguished.69–71 Accordingly, the as-deposited surface was found to consist of NiSx (Ni 2p3/2 binding energy (BE) ≈ 853.1 eV) and Ni(OH)2 (BE ≈ 856.0 eV), the latter arising from surface oxidation. After the HER experiment, Ni(OH)2 was observed almost exclusively (Fig. 6a). The observed S species changed from S2− (S 2p3/2 BE ≈ 161.5 eV) to mostly SO42− (S 2p3/2 BE ≈ 168.5 eV),72 which is attributed to residual H2SO4 electrolyte rather than the film itself (Fig. 6b). Compared to the as-deposited sample, the intensities of both the Ni and S features were significantly weaker in relation to the Sn features from the FTO substrate (Fig. S31b†). Inductively coupled plasma optical emission spectroscopy (ICP-OES) showed that approximately two thirds of the nickel atoms were dissolved during the experiment. Furthermore, SEM showed that the surface coverage of the catalyst decreased such that the FTO substrate was exposed after the experiment (Fig. 6c). We hypothesize that the remaining NiSx was electrically poorly connected or isolated, explaining the negligible HER current at the end of the experiment. Because a rather broad potential range was scanned during the experiments and the films were also observed to slowly dissolve under open circuit potential (OCP), a question remained whether β-NiS may still be stable under HER potentials. To this end, we applied a constant current density of 10 mA cm−2, which initially required an overpotential of ∼350 mV. However, η increased to 600 mV within 2 h (Fig. S29†), showing that the film was unstable also under constant HER operation. Thus, β-NiS appears unstable under the acidic HER conditions.
 | ||
| Fig. 6 Characterization before and after electrochemistry. X-ray photoelectron spectra of (a) Ni 2p3/2 and (b) S 2p regions, (c) SEM images (all images at the same scale. Insets: bare FTO substrate and NiSx films deposited using 500 ALD cycles for acidic and 250 cycles for alkaline conditions), and (d) grazing incidence XRD data of NiSx films as deposited and after electrochemical CV experiments. Samples and electrochemical conditions for panels (a–c) are identical to Fig. 5. For (d), 400 ALD cycles were used followed by 50 CVs in the HER or OER region in 0.1 M KOH. The position and height of the lines in (d) indicate peak positions and intensities of powder references (PDF 12-41 for β-NiS and COD 9000564 for Ni3S2), while peaks from the FTO substrate and sample stage of the XRD instrument are marked with the indicated symbols. | ||
Under oxidizing conditions in acid, we observed a broad oxidation feature from 1.0 to 1.6 V vs. RHE and no OER current as seen in Fig. 5b. The scan was started at 0.25 V and continued up to 1.75 V vs. RHE; following CVs were scanned between 1.15 and 1.75 V vs. RHE. The oxidation feature is attributed to oxidation of sulfur (from nominally S2− in β-NiS to S6+ in HSO4−) and its subsequent dissolution. Oxidation of Ni may also occur (cf. the OER under alkaline conditions), but Ni2+ is soluble and the thermodynamically stable species under these conditions (Fig. S27 and S28†). No significant current was observed in the following CVs, so the sample was removed for characterization after 10 CVs. ICP-OES showed that only 2% of the initial Ni remained and the sample appeared identical to the bare FTO substrate under SEM (Fig. 6c), supporting the rapid film dissolution suggested by the CVs and Pourbaix diagram. XPS showed very little Ni and S remaining that is attributed to electrically isolated domains (Fig. 6a and b). Thus, β-NiS cannot be used for the OER in acid due to its extreme instability.
Subjecting β-NiS to alkaline (0.1 M KOH) HER conditions yielded stable CVs (scanned between 0.0 and −0.55 V vs. RHE at 10 mV s−1). In the first CV, a slightly higher current and stronger hysteresis were observed (Fig. 5c), which we attribute to material changes discussed below. The CVs from the 5th to 50th CV (∼2 h) were practically identical. An overpotential of ∼500 mV was required to reach a current density of 10 mA cmgeo−2, indicating reasonable HER activity. Post-HER characterization of the catalyst by XPS showed that the S/Ni ratio had decreased from 0.9 to 0.2 near the surface. This decrease together with a slight shift of the initial NiSx Ni 2p3/2 feature (BE ≈ 853.1 eV) to 852.7 eV is attributed to formation of Ni metal and/or a sulfur-deficient Ni3S2 phase as discussed below (Fig. 6a). Furthermore, the Ni 2p3/2 hydroxide feature (BE ≈ 856.0 eV) increased in intensity, suggesting Ni(OH)2 formed either directly during the HER or when the species generated under HER conditions were exposed to air. Besides strongly decreasing in intensity, the S 2p peak shifted to a ∼0.7 eV higher BE after the HER (Fig. 6b), which may be linked to the change in the NiSx phase or other changes in the film composition. We note that at OCP (∼0.85 V vs. RHE) in 0.1 M KOH, Ni(OH)2 (but not Ni) formation and nearly complete S loss have also been observed.34 The O 1s spectra showed only a single hydroxide feature that ruled out the formation of NiO (Fig. S31a†). As XPS probes surface composition, we also used EDS to determine the S/Ni ratio in the bulk of the films. The measurement showed that a significant fraction of the sulfur was lost throughout the films, resulting in a S/Ni ratio of 0.5. Grazing incidence XRD indicated that the initial β-NiS phase had completely transformed to Ni3S2 during the 50 CVs applied (Fig. 6d). As the S/Ni ratio in Ni3S2 is 0.67 compared to the observed values of 0.5 (EDS, averaging throughout the whole film) and 0.2 on the surface (XPS), the film likely also contained a non-sulfide phase, such as Ni or Ni(OH)2 that was too weakly crystalline to be observed by XRD. SEM also hints at the presence of two phases, as the overall plate-like morphology was retained after the HER, but small (∼10 nm) particles formed on the edges of the larger ∼100 nm wide crystallites (Fig. 6c). HER measurements of reference samples presented in Fig. S30† revealed that evaporated Ni metal was more active than β-NiS (η ≈ 250 mV at 10 mA cmgeo−2), while Ni(OH)2 and NiO were less active than β-NiS (η ≈ 510 and >600 mV at 10 mA cmgeo−2). The Pourbaix diagram suggests Ni to be the stable species under alkaline HER conditions (Fig. S27 and S28†). Resolving the HER active species likely requires a multi-technique in situ/operando structural and compositional investigation.41 Regardless, our results highlight the instability of β-NiS and its bulk transformation to Ni3S2 and potentially additional surface Ni metal or Ni(OH)2 species under alkaline conditions.
Finally, we explored alkaline OER conditions (0.1 M KOH, CVs in 0.85 to 1.65 V vs. RHE range at 10 mV s−1). During the forward (anodic) scan of the first CV, a large irreversible oxidation feature was observed at ∼1.2–1.6 V vs. RHE (Fig. 5d). We attribute this feature to oxidation of both nickel (from Ni2+ in β-NiS to a mixture of Ni3+ and Ni4+ in “NiOOH”) and sulfur (from S2− in β-NiS to S6+ in SO42−). The integrated peak area determined after deduction of the OER current was within 10% of the theoretically expected amount of transferred charge for the proposed oxidation reaction. The generated sulfate is soluble, while the NiOOH is not and functions as an OER catalyst.67,68,73 On the reverse (cathodic) scan, the reduction feature observed at ∼1.35 V is attributed to reversible NiOOH → Ni(OH)2 transformation. During repeated CVs, the reversible Ni(OH)2/NiOOH redox features remain, although they shift to higher potentials and decrease in area from CV 10 to CV 150, implying changes in the structure of NiOOH and likely incorporation of iron impurities (see below).67,73,74 Regarding the Ni(OH)2/NiOOH phase, the position (1.38 V vs. RHE for the oxidation peak) and area (1.7 e− per Ni atom for the reduction peak) at CV 10 are in agreement with the α-Ni(OH)2/γ-NiOOH redox couple.34,68,73,74 The decrease in area to 1.0 e− per Ni atom by CV 150 corresponds to formation of the β-Ni(OH)2/β-NiOOH couple, which is spontaneous in an alkaline electrolyte,68,73 possibly accompanied by trace Fe incorporation (see below). A slight decrease in the OER current was observed during the experiment, but the current stabilized to approximately 3 mA cm−2 at 1.63 V vs. RHE (i.e. η = 400 mV). XPS showed that only Ni(OH)2 was present after the OER experiment (Ni 2p3/2 BE ≈ 856.0 eV, Fig. 6a; O 1s BE = 531.3 eV, Fig. S31a†), which is in line with ending the measurement below the NiOOH/Ni(OH)2 reduction potential as well as the instability of NiOOH in air.75 No sulfur remained in the films according to XPS (Fig. 6b) and EDS that probe the surface and the whole film, respectively. In contrast, no signs of dissolution of Ni were observed, in accordance with the previous ICP-OES studies.34 No reflections from the film were detected by XRD (only those from the substrate) after the OER, which suggests that the formed (oxy)hydroxide has low crystallinity and is in line with the complete disappearance of NiSx. In terms of the morphology, SEM suggested the formation of a more porous structure that retained the general plate shape of the initial NiSx crystallites (Fig. 6c). We note that iron impurities are known to easily incorporate into nickel-based OER catalysts and have a beneficial effect on their activity.67,68,76 Although the concentration of Fe was below our XPS detection limit of ∼2 cation-% (based on scans in the Fe 3p region), we believe Fe impurities to play a role in supporting a relatively stable OER current as our experiments were performed in reagent grade KOH.67,68 The observed shift of the Ni redox features to higher potentials and the decrease in area during consequent CVs being stronger compared to Ni(OH)2 and NiSx measured in purified KOH in the literature also support Fe incorporation (Fig. 5d).34,67,68,77 Thus, under the alkaline OER conditions β-NiS transforms to Ni(Fe)OOH, a well-known OER catalyst. Its OER activity can be significantly improved by introducing an elevated level of Fe impurities into the alkaline electrolyte.34
In summary, both the electrochemical potential and electrolyte pH play crucial roles in defining the stability of the NiSx electrocatalyst. Importantly, structural changes occurred under all of the studied conditions (HER, OER, and OCP in acid and base). Our β-NiS films were found to be unstable under acidic conditions, ranging from slow dissolution under reductive potentials (HER) and OCP to very fast dissolution under oxidizing conditions (OER). Thus, the results suggest that β-NiS is a poor choice for acidic water splitting, i.e. PEM electrolyzers. Our results are in line with the solubility of Ni2+ in acid. Under alkaline conditions, dissolution of Ni was not observed but sulfur loss occurred. Under reducing conditions, β-NiS “bulk” transformed to Ni3S2 with nickel metal and/or Ni(OH)2 potentially forming at least on the surface, while under oxidizing conditions the whole film transformed to (oxy)hydroxide. The oxyhydroxide formation from NiSx under OER conditions has been observed in several studies.22,25,32,33 While at least partial sulfur loss under HER conditions has been found in multiple studies,35–40 no consensus has been reached on what species are formed with suggestions including Ni3S2,35,36 Ni metal,37 NiOxSy,38 and Ni(OH)2 (ref. 39 and 40). The different species observed may stem from differences in the precatalyst properties (composition, structure, morphology etc.), testing conditions (different potentials in cyclic voltammetry, chronoamperometry, and chronopotentiometry), and characterization methods (both ex situ and in situ, each with different capabilities to observe different materials). We observed the formation of Ni3S2 in agreement with ref. 35 and 36. From the post-HER characterization we infer that a fraction of Ni and/or Ni(OH)2 may also form as suggested in ref. 37, 39 and 40. Factors such as the catalyst phase and morphology as well as the substrate affect the electrochemical stability; however, both the calculated27,78 and experimental thermodynamic data suggest that all of the NiSx phases are slightly and strongly unstable under the HER and OER conditions, respectively (Fig. S27 and S28†). The observed Ni3S2 is the most stable (or least unstable) sulfide under reducing conditions, but Ni metal is thermodynamically the most stable species under alkaline HER conditions. Under OER conditions, NiOOH is predicted to be stable, in line with our observations.
We can now compare the stability of NiSx under electrochemical water splitting conditions to annealing in oxidizing and reducing atmospheres (see the section High-temperature stability in different atmospheres). Under both kinds of reducing conditions, i.e. HER and annealing in H2, we observed the reduction of Ni and decrease of S content, which during H2 annealing produced Ni metal. During the alkaline HER, mostly Ni3S2 formed with hints of Ni metal and Ni(OH)2 formation. Under oxidizing conditions, i.e. OER and annealing in air or O2, NiSx oxidized to NiOx(Hy). The electrochemical oxidation in the presence of water results in the (oxy)hydroxide instead of the thermally formed oxide. Furthermore, the OER conditions present a stronger driving force, forming Ni3+/4+ instead of Ni2+ during annealing in air or O2. Solubility and evaporation are additional key factors that are present in aqueous environments and annealing only, respectively. Thermal annealing has dramatic effects on morphology due to fast diffusion processes, while this is usually absent in aqueous solutions. Finally, we note that our approach of using thin film catalysts coupled with pre/post characterization is broadly applicable to different materials and applications.
Conclusions
We have developed a new nickel sulfide ALD process using easily synthesized NiCl2(TMPDA) and H2S at 165–225 °C. The process compares favorably with previously reported processes, depositing uniform and conformal, highly conductive films with low impurity levels that consist mainly of the β-NiS phase with a minor Ni9S8 component. The effects of the deposition temperature and film thickness on film properties were characterized. The highest growth rate, lowest resistivity, and highest amount of the β-NiS phase were observed at 165 °C. Continuous, conductive films were obtained from a thickness of 6 nm at 165 °C, with an increase in thickness decreasing resistivity and increasing roughness. With increasing deposition temperature, the amount of the Ni9S8 phase increased. High-temperature XRD measurements in reducing, inert, and oxidizing atmospheres revealed that the films undergo structural and compositional changes starting from 300–400 °C, i.e. temperatures relevant to processing and operation of various applications. The material stability was systematically assessed under electrochemical water splitting conditions. In acid, β-NiS was found to dissolve under the HER and OER, as well as OCP conditions. Under alkaline conditions, rather stable HER and OER currents were obtained, but material characterization and electrochemical data suggested the sulfide to transform to Ni(OH)2 at OCP and NiOOH during the OER. During the HER, Ni3S2 formed, possibly with additional Ni and/or Ni(OH)2 species. These observations highlight the importance of characterizing the stability of sulfides and other materials under application-relevant processing and operating conditions. Beyond fundamental stability studies, our ALD process provides a scalable method for engineering advanced NiSx nanostructures on high surface area substrates for various thermal and electrochemical catalytic processes and beyond.Experimental
Film deposition
Nickel sulfide films were deposited using a commercial, hot-wall, cross-flow ALD reactor (F120, ASM Microchemistry) operated at approximately 5 mbar pressure.79 Nitrogen (N2, 99.999%, AGA) was further purified using a SAES Microtorr MC1-902F purifier and used as the carrier and purge gas at a flow rate of 400 sccm. The dichloro(N,N,N′,N′-tetramethyl-1,3-propanediamine)nickel(II) [NiCl2(TMPDA)] precursor synthesized in house53 was heated to 157 °C in an open boat held inside the ALD reactor and pulsed by inert gas valving. The vapor pressure of NiCl2(TMPDA) was estimated to be 0.1 mbar at 157 °C.55 The moderately air sensitive precursor was stored and handled in a N2 glovebox before transferring to the ALD reactor. The reactant hydrogen sulfide (H2S, 99.5%, Linde) was further purified using a SAES Microtorr MC1-302F purifier. The flow rate of H2S was set to 14 sccm under a continuous flow using a mass flow meter and a needle valve and it was pulsed into the reactor using an external solenoid valve.Caution! Safe use of highly toxic and flammable H2S gas requires a properly designed ALD reactor and laboratory space. The H2S bottle was stored in a ventilated gas cabinet and the H2S lines were designed to be compatible with H2S, using VCR metal and EPDM polymer seals. The reactor exhaust was bubbled through an aqueous Cu(NO3)2 solution to remove the H2S downstream of the vacuum pump by precipitation. Reactor modifications required for H2S compatibility have been discussed by Dasgupta et al.80
The NiSx films were mostly deposited on 5 × 5 cm2 Si(100) and soda lime glass (SLG) substrates. FTO coated glass (15 × 20 mm2, TEC 8, Ossila) was used to prepare samples for electrocatalysis. The silicon substrates with a native oxide layer were used as supplied. The SLG substrates were cleaned using successive ultrasonic baths of alkaline detergent (Industrial Strength Cleaner, Branson), tap water, deionized water, and ethanol (10 minutes each, room temperature) followed by careful rinsing using deionized water and a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v%) deionized water/ethanol solution and blown dry using pressurized air. The FTO substrates were cleaned analogously, except that isopropanol was used in the last ultrasonication and rinsing steps.
50 (v/v%) deionized water/ethanol solution and blown dry using pressurized air. The FTO substrates were cleaned analogously, except that isopropanol was used in the last ultrasonication and rinsing steps.
Film characterization
The film morphology was examined by SEM (Hitachi S-4800 and FEI Magellan 400 XHR) and AFM (Veeco Multimode V). Tapping mode AFM imaging was performed in air using silicon probes with a nominal radius of less than 10 nm (Bruker). The AFM images were flattened with no other image processing and film roughness was calculated as a root-mean-square (Rq) value.Film thicknesses were measured by EDS (Oxford INCA 350 connected to the Hitachi S-4800 SEM). GMRFilm software81 was used to convert the measured Ni and S Kα k-ratios to film thicknesses assuming bulk density of β-NiS (5.5 g cm−3).82 Attempts to use X-ray reflectivity to confirm the thickness or density of the films were unsuccessful due to their high roughness. Sheet resistance was measured using a four-point-probe (CPS Probe station connected to a Keithley 2400 SourceMeter). The sheet resistance was converted to resistivity using the thickness measured by EDS.
Crystallinity was studied by XRD (Rigaku SmartLab) using Cu Kα radiation (λ = 1.54 Å) in both grazing incidence (ω = 1°) and θ–2θ geometries. Raman spectroscopy was also used to evaluate phase composition. An NT-MDT Ntegra instrument was used in the back-scattering geometry using a 100× objective and 532 nm laser with a nominal power of 20 mW.
Film composition was analyzed by ToF-ERDA using a 40 MeV 127I7+ ion beam. The incident beam-sample and sample-recoiled beam angles were 16 and 24°. The film surface and film/substrate interfaces were excluded from the analysis. The surface composition and chemical state and changes in them after the electrochemical measurements were analyzed by XPS (PHI VersaProbe 3) using monochromatized Al Kα radiation (hν = 1486.6 eV). The photoelectron take-off angle and incoming X-ray angle were 45° and 90° with respect to the sample surface. No sputtering was performed. A charge neutralizer was used during the measurements. The spectra were referenced to the C 1s C–C component of adventitious carbon at 284.8 eV. Pass energy was set to 55 and 224 eV for the core and survey scans. The S/Ni ratios were calculated using PHI Multipak software by integrating the measured Ni 2p3/2 and S 2p spectra using Shirley backgrounds and relative sensitivity factors provided with the software.
Selected samples were digested before and after the electrochemical measurements followed by analysis of the nickel concentration using ICP-OES (Thermo Scientific ICAP 6300 Duo View). The digestion was done in a Teflon compression cell where the analysis area was limited using an O-ring (9 mm internal diameter), adding 400 μL of concentrated HNO3 (TraceMetal grade, Fisher Chemical), which was let to react for at least 2 h followed by collection and dilution to 5% HNO3 using ultrapure H2O. Standards were prepared from 1000 mg per L stock solutions to the desired concentrations (∼10–1000 μg L−1) in 5% HNO3.
High-temperature XRD
The stability of the NiSx films under different atmospheres was studied by in situ HTXRD measurements using an Anton-Paar HTK1200N oven connected to a PANalytical X'Pert Pro MPD diffractometer using Cu Kα radiation (λ = 1.54 Å) in the grazing incidence (ω = 1°) geometry. Different atmospheres were used, including atmospheric pressure of laboratory air, O2 (AGA, 99.999%), N2 (AGA, 99.999%, further purified using an Entegris Gatekeeper purifier), and forming gas (10% H2 in N2, AGA), as well as dynamic vacuum (approximately 10−5 mbar). The studied NiSx films (deposited at 165 °C using 750 cycles) were first heated from room temperature up to 125 °C at a heating rate of 10 °C min−1. Thereafter, the heating was paused every 25 °C to record a diffractogram, which took 27 min. The measurements were finished at 750 °C, resulting in a total measurement time of approximately 15 h.Electrochemical measurements
Electrochemical measurements were performed using a three-electrode poly(tetrafluoroethylene) (PTFE) compression cell83,84 and a BioLogic SP-200 potentiostat. ALD NiSx deposited on FTO-coated glass was used as a working electrode and placed underneath the cell such that an O-ring exposed an area of 0.5 cm2 to the electrolyte. Electrical connection was made using Cu tape to a section of the working electrode not exposed to the electrolyte. A coiled Pt wire was stored in 60% HNO3, rinsed with ultrapure water (Milli-Q, resistivity 18.2 MΩ cm), flame-annealed, and then used as a counter electrode. A glass-body Ag/AgCl reference electrode (4 M KCl with saturated Ag, Fisherbrand Accumet 13-620-53) was used. The potential of the Ag/AgCl reference electrode was referenced to a commercial reversible hydrogen electrode (RHE, Gaskatel HydroFlex).Measurements were performed under both alkaline and acidic conditions. For the alkaline measurements, a 0.1 M KOH electrolyte (pH = 13) was prepared from granular KOH (ACS Reagent, Sigma-Aldrich, <0.001% Fe, which corresponds to <70 ppb after dilution to 0.1 M) and ultrapure water with the help of a pH meter and a 0.1 M KOH standard (Titripur, Supelco). No additional electrolyte purification was performed. Although no Fe was observed on the samples by XPS after the electrochemical measurements, we expect some iron to incorporate into the samples.67,74 For the acidic measurements, a 0.5 M H2SO4 electrolyte (pH = 0.3) was prepared from concentrated H2SO4 (TraceMetal grade, Fisher) and ultrapure water.
Prior to starting the electrochemical measurements, approximately 20 mL of the electrolyte was added to the cell described above, the electrodes were installed, and the cell was closed with a lid and sparged with N2 using a glass gas dispersion tube for 15 min before starting the measurement. The sparging was continued throughout the experiment, while no additional stirring was performed.
Typical electrochemical measurements consisted of cyclic voltammetry (CV) scans at 10 mV s−1. The potential ranges were 0 to −0.7 V vs. Ag/AgCl (HER in 0.5 M H2SO4), −0.9 to −1.6 V vs. Ag/AgCl (HER in 0.1 M KOH), 0.9 to 1.5 V (OER in 0.5 M H2SO4), and −0.1 to 0.7 V vs. Ag/AgCl (OER in 0.1 M KOH). Depending on the film stability, 10 (OER in 0.5 M H2SO4) to 150 CVs (OER in 0.1 M KOH) were applied. The CVs compensated for 85% of the iRu drop during the measurements, while the remaining 15% was compensated for during post-processing. All the shown data are therefore 100% iRu drop compensated. Ru was measured every 3 CVs using EIS at −0.1 V vs. Ag/AgCl, 5 kHz frequency (chosen for phase angle close to 0), and 10 mV root-mean-square amplitude. The measured Ru was ∼40 Ω in 0.1 M KOH and ∼10 Ω in 0.5 M H2SO4. After the measurements, the samples were rinsed with ultrapure H2O and stored in air for characterization.
Data availability
The data supporting this article have been included as part of the ESI.† Additional data may be requested from the authors.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
M. M. acknowledges a postdoctoral fellowship of the Research Council of Finland (decision 347100). J. S. acknowledges the Leopoldina postdoc fellowship program (grant number LPDS 2022-02) of the German National Academy of Sciences Leopoldina and the Postdoc.Mobility fellowship program (grant number P500PN_210769) of the Swiss Nation Science Foundation. This research was funded in part by the Swiss National Science Foundation (SNSF) [P500PN_210769]. J. S., T. F. J., M. B. S., and S. F. B. were supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science Program to the SUNCAT Center for Interface Science and Catalysis. The use of ALD Center Finland research infrastructure is acknowledged. XPS and part of SEM measurements were performed at the Stanford Nano Shared Facilities (SNSF) supported by the National Science Foundation (award ECCS-2026822). Dr Guangchao Li is acknowledged for ICP-OES measurements. Anton Vihervaara is thanked for AFM measurements of the FTO substrate.References
- H. Okamoto, J. Phase Equilibria Diffus., 2009, 30, 123 CrossRef CAS.
- M. E. Fleet, Rev. Mineral. Geochem., 2006, 61, 365–419 CrossRef CAS.
- P. A. Metcalf, B. C. Crooker, M. McElfresh, Z. Kakol and J. M. Honig, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 2055–2060 CrossRef CAS PubMed.
- G. V. Gibbs, R. T. Downs, C. T. Prewitt, K. M. Rosso, N. L. Ross and D. F. Cox, J. Phys. Chem. B, 2005, 109, 21788–21795 CrossRef CAS PubMed.
- N. Mahuli and S. K. Sarkar, J. Vac. Sci. Technol., A, 2016, 34, 01A142 CrossRef.
- T. A. Ho, C. Bae, H. Nam, E. Kim, S. Y. Lee, J. H. Park and H. Shin, ACS Appl. Mater. Interfaces, 2018, 10, 12807–12815 CrossRef CAS PubMed.
- S. Cho, H. Kim and M. Mo Sung, J. Ind. Eng. Chem., 2019, 77, 470–476 CrossRef CAS.
- T. Ohtani, Electrical properties of Ni1-xS, J. Phys. Soc. Jpn., 1974, 37, 701–710 CrossRef CAS.
- A. Manthiram and Y. U. Jeong, J. Solid State Chem., 1999, 147, 679–681 CrossRef CAS.
- S. R. Krishnakumar, N. Shanthi and D. D. Sarma, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 115105 CrossRef.
- R. L. Kautz, M. S. Dresselhaus, D. Adler and A. Linz, Phys. Rev. B, 1972, 6, 2078–2082 CrossRef CAS.
- C. I. Pearce, R. A. D. Pattrick and D. J. Vaughan, Rev. Mineral. Geochem., 2006, 61, 127–180 CrossRef CAS.
- J. Yang, X. Duan, Q. Qin and W. Zheng, J. Mater. Chem. A, 2013, 1, 7880–7884 RSC.
- H. Pang, C. Wei, X. Li, G. Li, Y. Ma, S. Li, J. Chen and J. Zhang, Sci. Rep., 2014, 4, 3577 CrossRef PubMed.
- K. Aso, A. Sakuda, A. Hayashi and M. Tatsumisago, ACS Appl. Mater. Interfaces, 2013, 5, 686–690 CrossRef CAS.
- H. Fan, H. Yu, X. Wu, Y. Zhang, Z. Luo, H. Wang, Y. Guo, S. Madhavi and Q. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 25261–25267 CrossRef CAS PubMed.
- T. Matsumura, K. Nakano, R. Kanno, A. Hirano, N. Imanishi and Y. Takeda, J. Power Sources, 2007, 174, 632–636 CrossRef CAS.
- H. Sun, D. Qin, S. Huang, X. Guo, D. Li, Y. Luo and Q. Meng, Energy Environ. Sci., 2011, 4, 2630–2637 RSC.
- L. L. Feng, G. Yu, Y. Wu, G. D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS PubMed.
- D. Kong, J. J. Cha, H. Wang, H. R. Lee and Y. Cui, Energy Environ. Sci., 2013, 6, 3553–3558 RSC.
- N. Jiang, Q. Tang, M. Sheng, B. You, D. E. Jiang and Y. Sun, Catal. Sci. Technol., 2016, 6, 1077–1084 RSC.
- H. Li, Y. Shao, Y. Su, Y. Gao and X. Wang, Chem. Mater., 2016, 28, 1155–1164 CrossRef CAS.
- P. Luo, H. Zhang, L. Liu, Y. Zhang, J. Deng, C. Xu, N. Hu and Y. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 2500–2508 CrossRef CAS PubMed.
- S. Anantharaj, S. Kundu and S. Noda, J. Mater. Chem. A, 2020, 8, 4174–4192 RSC.
- K. Kawashima, R. A. Marquez, L. A. Smith, R. R. Vaidyula, O. A. Carrasco-Jaim, K. Kawashima, Z. Wang, Y. J. Son, C. L. Cao and C. B. Mullins, Chem. Rev., 2023, 123, 12795–13208 CrossRef CAS PubMed.
- B. R. Wygant, K. Kawashima and C. B. Mullins, ACS Energy Lett., 2018, 3, 2956–2966 CrossRef CAS.
- K. A. Persson, B. Waldwick, P. Lazic and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 235438 CrossRef.
- M. Chatenet, B. G. Pollet, D. R. Dekel, F. Dionigi, J. Deseure, P. Millet, R. D. Braatz, M. Z. Bazant, M. Eikerling, I. Staffell, P. Balcombe, Y. Shao-Horn and H. Schäfer, Chem. Soc. Rev., 2022, 51, 4583–4762 RSC.
- J. C. Ehlers, A. A. Feidenhans'l, K. T. Therkildsen and G. O. Larrazábal, ACS Energy Lett., 2023, 8, 1502–1509 CrossRef CAS.
- N. Du, C. Roy, R. Peach, M. Turnbull, S. Thiele and C. Bock, Chem. Rev., 2022, 122, 11830–11895 CrossRef CAS PubMed.
- K. Zhang, X. Liang, L. Wang, K. Sun, Y. Wang, Z. Xie, Q. Wu, X. Bai, M. S. Hamdy, H. Chen and X. Zou, Nano Res. Energy, 2022, 1, 1–8 Search PubMed.
- W. Chen, H. Wang, Y. Li, Y. Liu, J. Sun, S. Lee, J. S. Lee and Y. Cui, ACS Cent. Sci., 2015, 1, 244–251 CrossRef CAS PubMed.
- O. Mabayoje, A. Shoola, B. R. Wygant and C. B. Mullins, ACS Energy Lett., 2016, 1, 195–201 CrossRef CAS.
- M. Mattinen, J. Schröder, G. D'Acunto, M. Ritala, T. F. Jaramillo, M. B. Stevens, S. F. Bent, M. B. Stevens and S. F. Bent, Cell Rep. Phys. Sci., 2024, 5, 102284 CrossRef CAS.
- X. Ding, D. Liu, P. Zhao, X. Chen, H. Wang, F. E. Oropeza, G. Gorni, M. Barawi, M. García-Tecedor, V. A. de la Peña O'Shea, J. P. Hofmann, J. Li, J. Kim, S. Cho, R. Wu and K. H. L. Zhang, Nat. Commun., 2024, 15, 5336 CrossRef CAS PubMed.
- Y. Sun, J. Wu, Z. Zhang, Q. Liao, S. Zhang, X. Wang, Y. Xie, K. Ma, Z. Kang and Y. Zhang, Energy Environ. Sci., 2022, 15, 633–644 RSC.
- Q. Ma, C. Hu, K. Liu, S. F. Hung, D. Ou, H. M. Chen, G. Fu and N. Zheng, Nano Energy, 2017, 41, 148–153 CrossRef CAS.
- X. Liu, H. Liao, S. Zhang, M. Liu, Y. Zhang, X. He, F. Liu, H. Cui, P. Tan and J. Pan, Appl. Catal., B, 2024, 357, 124270 CrossRef CAS.
- C. Karakaya, N. Solati, U. Savacı, E. Keleş, S. Turan, S. Çelebi and S. Kaya, ACS Catal., 2020, 10, 15114–15122 CrossRef CAS.
- N. Solati, C. Karakaya, K. Şimşek and S. Kaya, Mater. Today Chem., 2024, 38, 102081 CrossRef CAS.
- K. Kawashima, A. E. F. Milton, J. S. Archer, D. T. Collins, N. L. Serrat, C. E. Chukwuneke, R. A. Marquez, L. A. Smith and C. B. Mullins, ACS Energy Lett., 2024, 9, 6126–6143 CrossRef CAS.
- N. Jiang, L. Bogoev, M. Popova, S. Gul, J. Yano and Y. Sun, J. Mater. Chem. A, 2014, 2, 19407–19414 RSC.
- P. O'Brien, J. H. Park and J. Waters, Thin Solid Films, 2003, 431–432, 502–505 CrossRef.
- L. Heayeon, M. Kanai, T. Kawai and S. Kawai, Jpn. J. Appl. Phys., 1993, 32, 2100–2101 CrossRef.
- H. Nozaki, M. Onoda and K. Kosuda, in Progress in Solid State Chemistry Research, ed. R. W. Buckley, Nova Science Publishers, New York, 2007, ch. 6, pp. 239–284 Search PubMed.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed.
- R. W. Johnson, A. Hultqvist and S. F. Bent, Mater. Today, 2014, 17, 236–246 CrossRef CAS.
- M. Ritala and J. Niinistö, in Chemical Vapour Deposition: Precursors, Processes and Applications, ed. A. C. Jones and M. L. Hitchman, Royal Society of Chemistry, Cambridge, 2009, ch. 4, pp. 158–206 Search PubMed.
- R. Singh and M. M. Ayyub, ACS Appl. Electron. Mater., 2021, 3, 1912–1919 CrossRef CAS.
- Y. Çimen, A. W. Peters, J. R. Avila, W. L. Hoffeditz, S. Goswami, O. K. Farha and J. T. Hupp, Langmuir, 2016, 32, 12005–12012 CrossRef PubMed.
- H. Li, R. Zhao, J. Zhu, Z. Guo, W. Xiong and X. Wang, Chem. Mater., 2020, 32, 8885–8894 CrossRef CAS.
- Z. Guo and X. Wang, Angew. Chem., Int. Ed., 2018, 57, 5898–5902 CrossRef CAS PubMed.
- M. H. Ko, B. Shong and J. H. Hwang, Ceram. Int., 2018, 44, 16342–16351 CrossRef CAS.
- K. Väyrynen, T. Hatanpää, M. Mattinen, K. Mizohata, K. Meinander, J. Räisänen, J. Link, R. Stern, M. Ritala and M. Leskelä, Adv. Mater. Interfaces, 2018, 6, 1801291 CrossRef.
- K. Väyrynen, T. Hatanpää, M. Mattinen, M. J. Heikkilä, K. Mizohata, J. Räisänen, J. Link, R. Stern, M. Ritala and M. Leskelä, Phys. Status Solidi A, 2019, 216, 1900058 CrossRef.
- K. Väyrynen, A. Vihervaara, T. Hatanpää, M. Mattinen, M. J. Heikkilä, K. Mizohata, J. Räisänen, M. Ritala and M. Leskelä, Chem. Mater., 2019, 31, 5314–5319 CrossRef.
- M. E. Fleet, Can. Mineral., 1988, 26, 283–291 CAS.
- M. E. Fleet, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1987, 43, 2255–2257 CrossRef.
- T. Chida and J. D. Ford, Can. J. Chem. Eng., 1977, 55, 313–316 CrossRef CAS.
- M. Utriainen, M. Kröger-Laukkanen, L.-S. Johansson and L. Niinistö, Appl. Surf. Sci., 2000, 157, 151–158 CrossRef CAS.
- J. G. Dunn and C. E. Kelly, J. Therm. Anal., 1977, 12, 43–52 CrossRef CAS.
- P. Batude, L. Brunet, C. Fenouillet-Beranger, F. Andrieu, J. P. Colinge, D. Lattard, E. Vianello, S. Thuries, O. Billoint, P. Vivet, C. Santos, B. Mathieu, B. Sklenard, C. M. V. Lu, J. Micout, F. Deprat, E. A. Mercado, F. Ponthenier, N. Rambal, M. P. Samson, M. Cassé, S. Hentz, J. Arcamone, G. Sicard, L. Hutin, L. Pasini, A. Ayres, O. Rozeau, R. Berthelon, F. Nemouchi, P. Rodriguez, J. B. Pin, D. Larmagnac, A. Duboust, V. Ripoche, S. Barraud, N. Allouti, S. Barnola, C. Vizioz, J. M. Hartmann, S. Kerdiles, P. A. Alba, S. Beaurepaire, V. Beugin, F. Fournel, P. Besson, V. Loup, R. Gassilloud, F. Martin, X. Garros, F. Mazen, B. Previtali, C. Euvrard-Colnat, V. Balan, C. Comboroure, M. Zussy, Mazzocchi, O. Faynot and M. Vinet, Technical Digest – International Electron Devices Meeting, 3.1.1–3.1.4, Intitute of Electrical and Electronics Engineers, San Diego, 2017 Search PubMed.
- K. Lauer, C. Möller, K. Neckermann, M. Blech, M. Herms, T. Mchedlidze, J. Weber and S. Meyer, Energy Procedia, 2013, 38, 589–596 CrossRef CAS.
- M. F. Wagenhofer, H. Shi, O. Y. Gutiérrez, A. Jentys and J. A. Lercher, Sci. Adv., 2020, 6, eaax5331 CrossRef CAS PubMed.
- Y. Tsubonouchi, Z. N. Zahran, D. Chandra, N. Hoshino and M. Yagi, Catal. Sci. Technol., 2024, 14, 3287–3319 RSC.
- Y. Zhao, J. You, L. Wang, W. Bao and R. Yao, Int. J. Hydrogen Energy, 2021, 46, 39146–39182 CrossRef CAS.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- S. Klaus, Y. Cai, M. W. Louie, L. Trotochaud and A. T. Bell, J. Phys. Chem. C, 2015, 119, 7243–7254 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau, A. Gerson and R. S. C. Smart, Surf. Interface Anal., 2009, 41, 324–332 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- D. L. Legrand, H. W. Nesbitt and G. M. Bancroft, Am. Mineral., 1998, 83, 1256–1265 CrossRef CAS.
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1, https://srdata.nist.gov/xps/, accessed, September, 2024 Search PubMed.
- H. Bode, K. Dehmelt and K. Witte, Electrochim. Acta, 1966, 11, 1079–1087 CrossRef CAS.
- R. A. Marquez, K. Kawashima, Y. J. Son, G. Castelino, N. Miller, L. A. Smith, C. Chukwuneke and C. B. Mullins, ACS Energy Lett., 2023, 8, 1141–1146 CrossRef CAS.
- J. Gallenberger, H. M. Fernández, A. Alkemper, M. Li, C. Tian, B. Kaiser and J. P. Hofmann, Catal. Sci. Technol., 2023, 13, 4693–4700 RSC.
- D. A. Corrigan, J. Electrochem. Soc., 1987, 134, 377 CrossRef CAS.
- M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng and S. W. Boettcher, J. Am. Chem. Soc., 2017, 139, 11361–11364 CrossRef CAS PubMed.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- T. Suntola, Thin Solid Films, 1992, 216, 84–89 CrossRef CAS.
- N. P. Dasgupta, J. F. Mack, M. C. Langston, A. Bousetta and F. B. Prinz, Rev. Sci. Instrum., 2010, 81, 044102 CrossRef PubMed.
- R. A. Waldo, in Microbeam Analysis, ed. D. E. Newbury, San Francisco Press, San Francisco, 1988, pp. 310–314 Search PubMed.
- Handbook of Mineralogy, ed. J. W. Anthony, R. A. Bideaux, K. W. Bladh and M. C. Nichols, Mineralogical Society of America, Chantilly, VA, 2005, https://www.handbookofmineralogy.org/pdfs/millerite.pdf, accessed, October, 2023 Search PubMed.
- K. L. Nardi, N. Yang, C. F. Dickens, A. L. Strickler and S. F. Bent, Adv. Energy Mater., 2015, 5, 1500412 CrossRef.
- J. G. Baker, J. R. Schneider, J. A. Garrido Torres, J. A. Singh, A. J. M. Mackus, M. Bajdich and S. F. Bent, ACS Appl. Energy Mater., 2019, 2, 3488–3499 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experiments on ALD characteristics and additional characterization, comparison of NiSx ALD processes, HTXRD measurements in different atmospheres, film growth and characteristics on Si and FTO, Ni–S Pourbaix diagrams, additional data and characterization related to electrochemical experiments. See DOI: https://doi.org/10.1039/d5ta00663e |
| ‡ Present address: Institute for Chemical Technology and Polymer Chemistry (ITCP), Karlsruhe Institute of Technology (KIT), Engesserstraβe 18, 76131 Karlsruhe, Germany. |
| This journal is © The Royal Society of Chemistry 2025 |