Electron donation of violet phosphorene nanosheets to sustain the oxygen vacancies of BiOBr for excellent photocatalytic nitrogen fixation†
Abstract
Solar-driven nitrogen fixation is a sustainable method for synthesizing ammonia, helping mitigate global warming and the energy crisis. However, the extreme reduction potential required for the first proton-coupled electron transfer process presents a huge challenge for most semiconductors without active sites. N2 molecules can be captured and activated by oxygen vacancies on the surface of bismuth oxobromide-based semiconductors, providing an alternative pathway to overcome these limitations. However, bismuth oxobromide materials have been found to suffer from photocorrosion, where surface oxygen vacancies are easily oxidized, leading to the loss of activity. Herein, violet phosphorene nanosheets (VPNS), which exhibit good stability and broad optical absorption, were introduced to prepare heterostructures with oxygen vacancy-rich BiOBr nanosheets, forming VPNS-BiOBr(OVs) heterojunctions. The electrons of VPNS were found to transfer to BiOBr(OVs) after the formation of the heterojunction, where the oxygen vacancies were significantly enhanced. The introduction of VPNS was found to not only increase visible light absorption but also prevent the oxidation of oxygen vacancies in the catalysts during photocatalytic nitrogen reduction. The VPNS-BiOBr(OVs) was demonstrated to have an extremely high photocatalytic nitrogen reduction rate of 867 μmol g−1 h−1 with excellent cycling stability, without any cocatalyst. This rate was about 4 times higher than that of BiOBr(OVs) and 10 times higher than that of VPNS. The photocatalytic ammonia production rate of VPNS-BiOBr(OVs) was found to be higher than those reported under both xenon and visible light. The intermediates 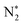 , NH–NH*,
, NH–NH*, 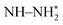 and
and 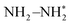 were detected for VPNS-BiOBr(OVs) via in situ FTIR spectroscopy, while the structure of BiOBr(OVs) was found to degrade without ammonia production. The photocatalytic nitrogen reduction of VPNS-BiOBr(OVs) was found to follow an alternating pathway. The electrons of VPNS were photoexcited and transferred to the oxygen vacancies of BiOBr(OVs), enhancing the adsorption of N2 to yield
were detected for VPNS-BiOBr(OVs) via in situ FTIR spectroscopy, while the structure of BiOBr(OVs) was found to degrade without ammonia production. The photocatalytic nitrogen reduction of VPNS-BiOBr(OVs) was found to follow an alternating pathway. The electrons of VPNS were photoexcited and transferred to the oxygen vacancies of BiOBr(OVs), enhancing the adsorption of N2 to yield 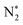 . The first proton-coupled electron transfer process was realized by the oxygen vacancies of VPNS-BiOBr(OVs) to obtain N–NH*, followed by lower reduction potential steps. NH3 was then dissociated from
. The first proton-coupled electron transfer process was realized by the oxygen vacancies of VPNS-BiOBr(OVs) to obtain N–NH*, followed by lower reduction potential steps. NH3 was then dissociated from 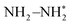 to yield ammonia. The adsorbed ammonia on VPNS-BiOBr(OVs) was found to be easily removed by washing and drying, confirming its photocatalytic cycling stability. This work provides a facile pathway to obtain efficient photocatalysts for nitrogen fixation.
to yield ammonia. The adsorbed ammonia on VPNS-BiOBr(OVs) was found to be easily removed by washing and drying, confirming its photocatalytic cycling stability. This work provides a facile pathway to obtain efficient photocatalysts for nitrogen fixation.



 Please wait while we load your content...
Please wait while we load your content...