 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transparent nature-based luminescent solar concentrator with NIR emission and integrated thermal sensing†
Sandra F. H.
Correia
 *a,
Bruno
P. Falcão‡
*a,
Bruno
P. Falcão‡
 b,
Gonçalo
Figueiredo
b,
Gonçalo
Figueiredo
 bc,
Bárbara M. C.
Vaz
d,
Letícia S.
Contieri
de,
Leonardo M.
de Souza Mesquita
bc,
Bárbara M. C.
Vaz
d,
Letícia S.
Contieri
de,
Leonardo M.
de Souza Mesquita
 de,
Juliana
Almeida
fg,
Joana C.
Fradinho
de,
Juliana
Almeida
fg,
Joana C.
Fradinho
 fg,
Diana C. G. A.
Pinto
fg,
Diana C. G. A.
Pinto
 h,
Lianshe
Fu
h,
Lianshe
Fu
 b,
Paulo S.
André
c,
Sónia P. M.
Ventura
b,
Paulo S.
André
c,
Sónia P. M.
Ventura
 d,
Rute A. S.
Ferreira
d,
Rute A. S.
Ferreira
 b and
Vitor
Sencadas
*i
b and
Vitor
Sencadas
*i
aInstituto de Telecomunicações, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal. E-mail: sandracorreia@av.it.pt
bDepartment of Physics, CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
cDepartment of Electrical and Computer Engineering, Instituto de Telecomunicações, Instituto Superior Técnico, University of Lisbon, 1049-001 Lisbon, Portugal
dDepartment of Chemistry, CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
eMultidisciplinary Laboratory of Food and Health (LabMAS), School of Applied Sciences (FCA), University of Campinas, Rua Pedro Zaccaria 1300, Limeira, Sao Paulo 13484-350, Brazil
fAssociate Laboratory i4HB – Institute for Health and Bioeconomy, NOVA School of Science and Technology, NOVA University of Lisbon, 2829-516 Caparica, Portugal
gUCIBIO – Applied Molecular Biosciences Unit, Department of Chemistry, NOVA School of Science and Technology, NOVA University Lisbon, 2829-516 Caparica, Portugal
hLAQV – REQUIMTE, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal
iDepartment of Materials and Ceramic Engineering, CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: vsencadas@ua.pt
First published on 20th March 2025
Abstract
The engineering of luminescent solar concentrators (LSCs) offers a way to turn windows into energy-generating units while maintaining transparency. Through UV/blue down-shifting materials to the red/near-infrared (NIR) spectral region, the performance of building integrated photovoltaics is maximized without compromising indoor light quality. The most efficient solutions are based on quantum dots, which raise environmental concerns. To address this, natural renewable materials, like bacteriochlorophyll (BChl) from phototrophic bacteria were used to fabricate an LSC prototype dispersed in a styrene–ethylene–butylene–styrene (SEBS) matrix. The LSCs emit in the red/NIR region with an emission quantum yield of ∼7%, demonstrating external photon efficiency and electrical device efficiency values of ∼1.0% and ∼0.04%, respectively. The thermal dependence of the BChl/SEBS emission is used to set two independent thermometric parameters based on the emission and the electrical power generated by the LSC edge-mounted photovoltaic cells with relative sensitivity values up to ∼2% °C−1, which is a remarkable performance. This prototype was scaled up for an active area of 0.1 m2, representing the first large-area LSC using nature-based red/NIR emission centers.
Introduction
Three primary sectors—buildings, industry, and transportation—are global energy consumers, with buildings leading the consumption. Windows, a significant factor in heat dynamics, offer potential for energy transformation via luminescent solar concentrators (LSCs).1,2 Developing efficient LSC transparent devices for natural lighting and energy harvesting requires UV-absorbing and NIR-emitting centers.3,4 However, these centers are scarce and mostly reliant on quantum dots (QDs), which involve complex processing and present significant environmental risks, primarily related to toxicity via aquatic organisms and bioaccumulation.5,6 Meeting Sustainable Development Goals (SDG 7 and 9 affordable and clean energy; and industry, innovation, and infrastructure, respectively)7 demands innovative, efficient LSCs capable of broad solar radiation absorption, minimal re-absorption, and emission compatible with Si-based commercial photovoltaic (PV) cells.In response to these challenges, the spotlight is shifting towards natural molecules, offering a sustainable alternative for LSCs.8–12 Furthermore, cutting-edge developments in LSC technology now incorporate sensing capabilities, enabling these devices to function as sunlight-powered optical temperature sensors integrated with IoT networks.13–16 This integration enhances LSC functionality and drives the advancement of smart, energy-efficient building technologies. By harnessing renewable energy and providing critical environmental data, natural molecules play a pivotal role in the future of LSCs, marking a significant leap towards sustainable energy solutions like supply-less IoT-based windows.15
Theoretical studies have shown the potential of NIR-emitting LSCs using QDs,17–21 indicating an external photon efficiency (ηext, defined as the ratio between the output and input optical power) of up to 14.6%,18 however, few experimental reports quantify the performance of planar NIR-based LSCs.8 These LSCs, using optically active layers with PbS,22,23 PbSe,24 PbS/CdS,25 CISeS/ZnS,6 CZISe/ZSe,26 CuInS2,27 CuInS2/ZnS,28 CuInS2/Zn/Al,29 and Si QDs,30 or synthetic dyes31–34 or even nanocrystals35 typically show ηext values below 8.1%28 (for single edge collection), correspondent to a device efficiency (ηdev, defined as the ratio between the output electrical power and the incident optical power) of 2.18%.28 Higher values, up to 12.6%, when considering all edges in a planar LSC,23 have been reported, both using PbS QDs. To address QDs' limitations—lower quantum yield in the NIR range, toxicity, and photoblinking—alternative NIR-emitting LSCs based on hexanuclear metal halide clusters3 and a cyanine derivative4 have been explored without quantitative performance characterization. The performance of a NIR-emitting LSC based on silicon 2,3-naphthalocyanine bis(trihexylsilyloxide) (SiNc) immobilized in an organic–inorganic triureasil matrix t-U(5000) and coupled to a Si-based PV device revealed ηext ∼ 1.5%.34
Recent studies emphasize the potential of natural renewable materials in LSCs,9–13,36–38 aligning with the growing demand for natural products across various sectors.39 Addressing environmental impact concerns in industries has heightened the push for sustainability. Integrating natural materials into smart, low-waste manufacturing chains aligns with current sustainability concepts. While some LSCs based on natural molecules have emerged,10–13,36–38 none emit in the red/NIR spectral range, vital for maximum absorption by c-Si PV cells. This highlights the need for the search of new nature-derived red/NIR-emitting materials to boost LSC sustainability without compromising efficiency.
Ensuring the photostability and processability of films of bio-based materials is critical alongside optical performance.9 Styrene–ethylene–butylene–styrene (SEBS) emerges as a notable thermoplastic elastomer suitable for smart windows.15 It has polystyrene (hard PS block) at both ends and poly(ethylene-co-1-butene) (soft PEB block) in the middle.40 Its processability, transparency, cost-effectiveness and thermal behavior (glass transition temperature (Tg) of the hard-block around 100 °C),41 that is similar to polymethylmethacrylate (PMMA, Tg ≈ 110 °C),42 a commonly used hosting matrix material for LSC applications, make it a viable alternative. Produced by hydrogenating styrene–butadiene–styrene copolymer, SEBS improves heat stability and weather resistance. Known for its rubber-like properties, high elasticity, and excellent tensile strength, SEBS is widely used in various applications requiring flexibility and durability, including automotive parts, adhesives, and roofing materials. Its high UV resistance43 makes it an ideal choice for outdoor applications like windows, particularly in high-temperature environments.44 Incorporating natural pigments such as bacteriochlorophylls (BChls) with photosynthetic potential, holds promise for smart, highly energy-efficient windows.45
BChl, found in photosynthetic bacteria, features a tetrapyrrole ring structure similar to chlorophylls (Chls) but with a more reduced ring, enabling light absorption at longer wavelengths.46 While BChl does not produce oxygen like chlorophyll-containing organisms during light conversion, its unique ability for anoxygenic photosynthesis is valuable in low-light conditions, showing potential for high-performance energy materials.47 These pigments notably harness light in UV and NIR spectral regions.46 However, the traditional recovery of BChl often involves harmful solvents like acetone or methanol.48–50 Replacing such solvents, especially methanol known for its hazards and lower efficiency, is crucial to reduce environmental and economic impact. Furthermore, the thermal dependence of the BChl optical properties allows the use of luminescence as a method for remote temperature measurement, capitalizing on the thermal dependency of phosphor emission. This innovative approach, initially tailored for conventional spectrometers, has been adapted to mobile optical (mOptical) sensing, leveraging the charge-coupled devices (CCDs) within smartphones and facilitating sensing functions.51,52 Recent findings propose using the inherent temperature-dependent emission effects of LSCs, which cause variations in the electrical output of edge-coupled PV cells, as a basis for thermal sensing.13–15 This work presents the development of a large-area LSC utilizing a nature-based NIR-emitting optically active material with temperature-sensing capabilities. It pioneers the use of a natural molecule emitting in the NIR spectrum for large-area LSC prototypes, where the cultivation of this molecule is a by-product of polyhydroxyalkanoate (PHA) production, thus aligning with the principles of biorefinery and resource optimization. Furthermore, the thermal dependence of the optical properties of BChl is leveraged to transform the LSC prototype into an in situ, sunlight-powered optical temperature sensor.13–15
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, for 30 minutes at room temperature. The supernatant was discharged, and the biomass pellets were stored at −20 °C until further processing. The biomass collection occurred for 2 weeks until 10 g of biomass was recovered. This process is represented in Fig. 1a.
000g, for 30 minutes at room temperature. The supernatant was discharged, and the biomass pellets were stored at −20 °C until further processing. The biomass collection occurred for 2 weeks until 10 g of biomass was recovered. This process is represented in Fig. 1a.
 | (1) |
 | (2) |
Results and discussion
BChl extraction
The structure of Chls comprises a hydrophobic phytol tail, yet it possesses polar functionalities due to C–O and C–N bonds, along with a chelated magnesium ion, mirroring BChl's structural traits.60 Various solvents were tested (Fig. 2a) to evaluate their effectiveness in extracting BChl, with solvent polarity playing a crucial role in influencing the recovery of BChl, evident in Fig. 2b: high-polarity solvents like water and aqueous ethanol (50% v/v), along with low-polarity solvents like dimethyl ether, showed lower performance. Ethanol and acetone, owing to both their polarity and cell membrane disruption capability, demonstrated improved extraction efficiency.61 Furthermore, the impact of different classes of surface-active compounds on BChl release was evaluated. Tension-active compounds, particularly cationic ones, facilitated cell membrane disruption by interacting with phospholipids, with longer alkyl chains showing slightly better extraction efficiency.62,63 Anionic compounds generally had limited effectiveness, except for SDS,53,54 which stood out by enhancing Chl and BChl extraction.53,54 Among the alternative solvents tested, SDS exhibited the highest extraction efficiency, reaching approximately 70%relative, surpassing acetone (around 60%relative). Both SDS and ethanol extracts were analyzed, confirming the presence of Chl, BChl, and various derivatives (Fig. 2c and Tables S1–S3, ESI†). Considering the goal of maximizing pigment content for better film performance in LSCs, ethanol, as a less hazardous solvent, was chosen for further developments.Structural and thermal properties
The films of SEBS with different BChl concentrations (denoted as BChl/SEBS-X, where X = 1, 2, 3, 4 according to the BChl concentration of 2, 6, 10, and 20 wt%, respectively) present high flexibility (Fig. 3a), exhibiting a consistently smooth morphology (average roughness below 69 nm) across different BChl amounts (Fig. S3, ESI†). FT-IR spectra of the SEBS matrix, BChl solid extract, and BChl/SEBS composites pre and post AM 1.5 G solar radiation exposure are shown in Fig. 3b and c. For SEBS, absorption bands were observed at 2960 and 2923 cm−1 that are attributed to C–H asymmetric stretching of –CH3 (in PEB block) and –CH2– (in PEB block and PS block), respectively, and an absorption band at 2852 cm−1 that is associated with C–H symmetric stretching of –CH2– in both PEB block and PS block.40 Bands at 1460 and 1379 cm−1 are the C–H bending of –CH2– and –CH3 in PEB block, respectively. Bands at 760 and 698 cm−1 are due to![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bending vibrating of side benzene ring in PS block, whereas the weak band at 721 cm−1 is from C–H rocking of –CH2– in PEB block. For BChl, bands at 2956 and 2923 cm−1 indicate –CH3 and –CH2– asymmetric stretching vibrations, respectively, while the band at 2854 cm−1 is from symmetric stretching vibration of –CH2–. The strong bands located at 1720 and 1650 cm−1 can be assigned to the C
C–H bending vibrating of side benzene ring in PS block, whereas the weak band at 721 cm−1 is from C–H rocking of –CH2– in PEB block. For BChl, bands at 2956 and 2923 cm−1 indicate –CH3 and –CH2– asymmetric stretching vibrations, respectively, while the band at 2854 cm−1 is from symmetric stretching vibration of –CH2–. The strong bands located at 1720 and 1650 cm−1 can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations of the BChl molecule at different positions.64 Bands at 1190 and 1060 cm−1 tentatively relate to Chl's C–N absorption,65 which can also be discerned in the spectra of BChl/SEBS composites, indicating that BChl was encapsulated into SEBS. Remarkably, no perceptible changes were evident in the FT-IR spectra post-irradiation (Fig. 3c), suggesting that the main chemical structure remains unaffected.
O vibrations of the BChl molecule at different positions.64 Bands at 1190 and 1060 cm−1 tentatively relate to Chl's C–N absorption,65 which can also be discerned in the spectra of BChl/SEBS composites, indicating that BChl was encapsulated into SEBS. Remarkably, no perceptible changes were evident in the FT-IR spectra post-irradiation (Fig. 3c), suggesting that the main chemical structure remains unaffected.
The XRD patterns of BChl, SEBS and BChl/SEBS samples are presented in Fig. 3d. The XRD pattern of BChl shows a weak broad band centered at 2θ of 20°, indicating that BChl is in an amorphous state. The sharp peaks located at 27.3, 31.6, 45.3, and 56.4° are associated with NaCl, matching well the NaCl reference (JCPDS card no. 75-0306), suggesting that some NaCl remained from the mineral medium used in PMC. The XRD pattern of SEBS displays a broad band centered at 18.8° with some small diffraction peaks, corresponding to monoclinic crystals (α-form).66 After incorporation of BChl into SEBS, the main diffraction profile is from the host SEBS, which implies that there are no significant changes of BChl doping on the XRD pattern. The weak sharp peaks are attributed to the NaCl from the medium using in the PMC to extract the BChl.
To investigate the thermal stability of SEBS and BChl/SEBS composites, thermogravimetric studies were performed (Fig. 3e). This analysis reveals that SEBS and BChl/SEBS composites are stable up to 400 °C, followed by gradual degradation culminating at 500 °C, displaying consistent maximum degradation temperatures without residual char formation. To evaluate the stabilization effect of SEBS on BChl, the TGA derivative (DTG) is used to investigate thermal stability, as the peaks of DTG curves can indicate the temperatures at which the maximum change of weight loss occurs.67 The positions of the peaks in DTG curves are 456, 462, 464, 465 and 466 °C for SEBS and BChl/SEBS-X (X = 1, 2, 3 and 4), respectively (Fig. 3e), the same tendency as T50% values (T50% is defined as the temperature at which 50% of mass loss occurs) of 454, 456, 458, 459 and 460 °C for SEBS and BChl/SEBS-X (X = 1, 2, 3 and 4), respectively. Also, there is no significant difference in the elastic modulus between the pure SEBS matrix and the BChl/SEBS sample (Fig. S4 in ESI†), which are 1.1 ± 0.1 MPa and 1.2 ± 0.1 MPa, respectively. These results indicate that compared to the stability of SEBS, the encapsulation of BChl into SEBS is not detrimental to the stability of the BChl/SEBS composites, a behavior akin to SEBS nanocomposites with graphene nanoplatelets prepared via a melting-blending process.68
Optical properties
The BChl/SEBS samples presented a reddish coloration under UV irradiation, corresponding to the emission spectra shown in Fig. 4a, whose energy is independent of the BChl concentration. For all the cases, the spectra show two prominent bands around 690 and 770 nm, which may be attributed to the presence of Chl and BChl from the extract, respectively. The excitation spectra were monitored on the more intense bands (Fig. 4b), showing two main components with peaks around 380 and 420 nm for all the BChl/SEBS samples, which are also present in the absorption spectra (Fig. 4c). These peaks were attributed to the presence of Chl (which typically have characteristic peaks around 415 and 670 nm) and carotenoids.69Both emission and excitation spectra presented a red-shift related to those of the BChl in ethanolic solution (Fig. S5, ESI†), which is due to the polarization interaction between BChl and SEBS, as reported before for other luminescent molecules incorporated into organic–inorganic hybrids.14,70,71
The emission decay curves were investigated, revealing a single exponential behavior (Table 1 and Fig. S6–S9, ESI†), yielding values of 5.1 ± 0.5 ns which are like the ones found for the BChl in ethanolic solution (Table 1 and Fig. S10, ESI†), suggesting that BChl was dispersed in SEBS homogeneously and the local environment of the molecule is preserved after incorporation. The emission properties of the BChl/SEBS materials were further quantified by measuring q values (Table 1). The data produced suggest that the incorporation of BChl into the SEBS matrix did not induce a negative effect on the photon conversion efficiency of these materials, and in some cases was beneficial, with higher q values comparing to those found for the BChl ethanolic solution, with maximum values of 0.07 ± 0.01, further demonstrating the protective effect of SEBS on BChl due to the interaction between them. To assess the potential of BChl in ethanolic solution and BChl/SEBS materials for absorbing solar radiation, the overlap integral (O) between their absorption spectra and the solar irradiation (AM 1.5 G) was estimated (Table 1). The calculations revealed that the BChl/SEBS samples have the ability to absorb up to 18% of the solar photon flux on Earth (4.3 × 1021 photons per s per m2).34 The photostability of the BChl/SEBS films was evaluated by exposing them to continuous AM 1.5 G for ∼27 h with periodic q values measurement, which showed that the variation is within the 10% experimental error providing strong evidence of the material photostability (Fig. S11, ESI†). Also, accelerated aging tests (given the intended application of these LSCs as windows) were performed in a climatic chamber showing that these samples are stable under extreme conditions (Fig. S12, S13 and Table S4, ESI†). Given these results, it is not expected that the LSC devices will undergo significant performance degradation due to environmental conditions over time. One of the main parameters to evaluate the suitability of BChl as an optically active material for large-area LSCs is the re-absorption evaluated by the overlap between its absorption and emission spectra. This may be evaluated by the modified overlap integral OI* defined by:33,72
 | (3) |
| Sample | O (×1020 photons per s per m2) | OI* | τ (ns) | q | (x,y) | CRI | CCT (K) | AVT (%) |
|---|---|---|---|---|---|---|---|---|
| a Excitation at 410 nm. b Excitation at 415 nm. c Excitation at 420 nm. | ||||||||
| BChl (ethanolic solution) | — | — | 5.30 ± 0.01 | 0.03 ± 0.01a | — | — | — | — |
| BChl/SEBS-1 | 2.4 | 0.13 | 5.06 ± 0.04 | 0.07 ± 0.01b | (0.35,0.35) | 95.3 | 4949 | 94.7 |
| BChl/SEBS-2 | 4.4 | 0.35 | 5.58 ± 0.04 | 0.04 ± 0.01c | (0.36,0.36) | 92.6 | 4455 | 87.6 |
| BChl/SEBS-3 | 6.0 | 0.43 | 5.04 ± 0.03 | 0.04 ± 0.01c | (0.38,0.37) | 89.2 | 3971 | 80.7 |
| BChl/SEBS-4 | 7.9 | 0.60 | 4.74 ± 0.01 | 0.02 ± 0.01c | (0.39,0.37) | 86.5 | 3750 | 72.6 |
The Average Visible Transmission (AVT) of the fabricated BChl/SEBS samples lies in the range between 73 and 95% (Table 1) making them suitable for window applications.73,74 To quantify the color appearance of the planar LSCs and their effects on color perception, the transmitted light was also analyzed in terms of CIE 1931 color space diagram coordinates and Color Rendering Index (CRI) which evaluated the ability to accurately render the color of objects (Fig. 4d and S14, ESI†). The light transmitted through the BChl/SEBS films showed coordinates that are very close to ideal white light (0.33, 0.33) (Table 1) and the CRI values indicated minimal distortion of incoming sunlight, further supported by the Color Correlation Temperature (CCT) values of the transmitted light (Table 1), corresponding to warm/neutral light. This makes the material suitable for residential or commercial spaces, highlighting its high potential as solar windows. Based on the data described above, namely the O and q values, and the quality of the transmitted light, the BChl/SEBS-2 sample was selected to be used in the fabrication of the LSC prototype.
Luminescent solar concentrator based on BChl/SEBS
A planar LSC was manufactured by coating a 10.5 × 10.5 × 0.8 cm3 glass substrate and coupling it to an array of c-Si PV cells (Fig. 5a) which harvested the BChl/SEBS emission guided and concentrated along the edges of the substrate. The LSC's performance was evaluated under simulated AM 1.5 G irradiation (Fig. 5c, d and S15, ESI†) and the figures of merit were estimated according to the standard definitions of external photon efficiency (ηext) and device efficiency (ηdev) recently reported:75,76 | (4) |
 | (5) |
These values are similar to those reported for NIR-emitting synthetic dyes8,31–34 and, although lower than those reported for QDs NIR-emitting LSCs with similar emission spectral range,8,26,29,30 this represents the first ever reported LSC based on a NIR-emitting natural optically active centers, serving as a proof of concept and paving the way for further research and innovation in this direction. The devices' output energy was also estimated considering an initial 24 hour period with a maximum recorded solar radiation of 1000 W m−2 after four hours from the start of the test (Fig. 5e).
Additionally, the scale-up of this nature-based NIR LSC was proven by fabricating a large area LSC by coating a 38 × 28 × 0.8 cm3 glass substrate with the BChl/SEBS material, resulting in a transparent glass that could be used as a window in real world applications (Fig. 5b). The haze of the coating was quantified by measuring total and diffuse reflectance, yielding values between ∼0.10 and ∼0.35 across the visible spectrum (Fig. S16, ESI†). This haze is attributed to the unoptimized deposition method rather than the SEBS matrix or dye clusters, as confirmed by the AVT measurements and the assessment of transmitted light quality (Table 1), which indicate minimal distortion. While some haze is present, it does not significantly affect optical clarity and may even be advantageous for LSC performance. Moderate light scattering can enhance light trapping and total internal reflection within the waveguide, improving photon guidance toward the solar cells at the edges while maintaining sufficient transparency.77,78
BChl/SEBS-based LSC as a temperature sensor
The emission spectra of the LSC based on BChl/SEBS under simulated solar irradiation show the two main bands of BChl emission as described above (Fig. S17a, ESI†), confirming its ability to efficiently absorb and convert solar radiation. Moreover, the relative intensity of the aforementioned bands is temperature-dependent (Fig. S17a, ESI†). Thus, we propose the definition of the following ratiometric thermometric parameter:13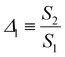 | (6) |
| Optical centre | λ p | Δ | Slope (°C−1) | S r (% °C−1) | δT (°C) | |
|---|---|---|---|---|---|---|
| a Here are included all examples in which the thermometric parameter is based on the ratio between integrated areas of defined regions of the emission spectra. Voc1 – open-circuit voltage values measured at the PV cell attached to the LSC, when coupled to wavelength-discriminating filter; Voc – open-circuit voltage values measured at the PV cell directly attached to the LSC. | ||||||
| Spectrometer | BChl [this work] | 770 | Δ 1 | (−6.2 ± 0.2) × 10−3 | 0.71 ± 0.03 | 0.3 |
| eGFP13 | 510 | (−1.23 ± 0.05) × 10−3 | 1.13 ± 0.01 | 0.9 | ||
| APC13 | 665 | (−0.9 ± 0.1) × 10−3 | 0.91 ± 0.02 | 1.3 | ||
| CDs14 | 535 | (2.98 ± 0.04) × 10−3 | 0.33 ± 0.01 | 1 | ||
| PV cell | BChl [this work] | 770 | Δ 2 | (−1.46 ± 0.04) × 10−2 | 1.96 ± 0.06 | 0.4 |
| CDs14 | 535 | (−8.60 ± 0.02) × 10−2 | 1.03 ± 0.03 | 0.4 | ||
| eGFP13 | 510 |

|
(−1.2 ± 0.4) × 10−2 | 1.23 ± 0.03 | 0.02 | |
| Eu3+ (ref. 15) | 612 | (−2.9 ± 0.2) × 10−3 | 0.29 ± 0.02 | 0.3 | ||
We note that although Sr is commonly used as a figure of merit to compare different thermometers, it depends on experimental conditions (e.g. emission spectra resolution) and the sample characteristics, such as concentration and media.58 Nonetheless, the values here reported are larger than those reported for an environmentally-friendly nature-based thermometer based on enhanced green fluorescent protein (eGFP) (0.23% °C−1)79 and for the one based on carbon dots (0.3% °C−1)14 (Table 2).
The temperature-dependent photoluminescence features of the BChl/SEBS also result in a variation in the optical and electrical performances of the LSC under the solar simulator irradiation (Fig. S17b, ESI†). This analysis showed that the generated electrical power (Pout) decreases as the temperature is elevated (Fig. 5f), allowing the definition of the thermometric parameter:
 | (7) |
Conclusions
Luminescent solar concentrators (LSCs) offer seamless integration of photovoltaics within buildings, preserving aesthetics and indoor light quality. This study presents large-area LSC prototypes utilizing nature-based NIR-emitting molecules—specifically, bacteriochlorophyll embedded within a styrene–ethylene–butylene–styrene (SEBS) matrix. Optical and power conversion efficiencies of 1.0% and 0.04%, respectively, were achieved, highlighting the potential of nature-based LSCs for promoting more sustainable methodologies in device fabrication.This work serves as a proof of concept, emphasizing the need to explore alternatives to conventional luminophores, which have been widely studied and applied. While naturally derived luminophores may not yet be the most cost-effective option for highly efficient LSCs, our primary goal was to demonstrate the potential of sustainable materials, paving the way for further research and innovation. We acknowledge that further improvements in energy efficiency can be achieved through optimization strategies, and future work will focus on refining the optical design via surface patterning, enhancing PV cell coupling techniques, and exploring energy recovery mechanisms such as reflective coatings and secondary optical elements to minimize losses and maximize performance.
Beyond its photovoltaic function, the temperature-dependent emission properties of the material have enabled the establishment of two distinct thermometric parameters, derived from its emission characteristics and the electrical power generated by edge-mounted photovoltaic cells integrated into the LSC. The remarkable performance is underscored by relative sensitivity values reaching up to 1.96% °C−1. In a significant advancement, the prototype has been successfully scaled up to cover an active area of 0.1 m2. This achievement marks a milestone as the first large-area LSC employing NIR emission centers derived from natural materials, offering immense potential for applications in both energy harvesting and precise temperature sensing.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
RASF, PSA and VS conceived the idea of the experiment; SFHC, BPF and GF took experimental data regarding the physics characterization. The bacteriochlorophyll production was accomplished by JCF and JA. The bacteriochlorophyll extraction process, including their concentration and purification, was developed, and optimized by BMCV, LSC, LMSM, SPMV. VS performed its incorporation on SEBS matrix. VS and LF performed the structural characterization and analysis. The UHPLC-MS analysis was performed by DCGAP. All authors have read and contributed to writing, besides agreeing to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the projects CICECO – Aveiro Institute of Materials, UIDB/50011/2020 (https://doi.org/10.54499/UIDB/50011/2020), UIDP/50011/2020 (https://doi.org/10.54499/UIDP/50011/2020) and LA/P/0006/2020 (https://doi.org/10.54499/LA/P/0006/2020) and Instituto de Telecomunicações, UIDB/50008/2020 (https://doi.org/10.54499/UIDB/50008/2020), UIDP/50008/2020 (https://doi.org/10.54499/UIDP/50008/2020) and LA/P/0109/2020 (https://doi.org/10.54499/LA/P/0109/2020), Applied Molecular Biosciences Unit – UCIBIO, UIDP/04378/2020 (https://doi.org/10.54499/UIDP/04378/2020) and UIDB/04378/2020 (https://doi.org/10.54499/UIDB/04378/2020), Associate Laboratory Institute for Health and Bioeconomy – i4HB, LA/P/0140/2020 (https://doi.org/10.54499/LA/P/0140/2020), LAQV-Tecnologia e Processos Limpos (UID/QUI/50006/2019) and projects PLANETa (CENTRO-01-0145-FEDER-181242), and SOLPOWINS – Solar-Powered Smart Windows for Sustainable Buildings (PTDC/CTM-REF/4304/2020) financed by national funds through the FCT/MEC (PIDDAC), and when appropriate co-financed by FEDER under the PT2020 Partnership through European Regional Development Fund (ERDF) in the frame of Operational Competitiveness and Internationalization Programme (POCI). S. F. H. C. thanks FCT (2022.03740.CEECIND) and European Space Agency (ESA STAR AO 2-1790). B. M. C. V. and G. F. thank FCT for the doctoral grants (2022.13816.BD and 2023.00526.BDANA, respectively).References
- R. A. S. Ferreira, S. F. H. Correia, A. Monguzzi, X. Liu and F. Meinardi, Mater. Today, 2020, 33, 105–121 CrossRef.
- T. A. de Bruin and W. G. J. H. M. van Sark, Adv. Photon. Res., 2025, 6, 2400068 CrossRef CAS.
- Y. M. Zhao and R. R. Lunt, Adv. Energy Mater., 2013, 3, 1143–1148 CrossRef CAS.
- Y. M. Zhao, G. A. Meek, B. G. Levine and R. R. Lunt, Adv. Opt. Mater., 2014, 2, 606–611 Search PubMed.
- E. Dumas, C. Gao, D. Suffern, S. E. Bradforth, N. M. Dimitrijevic and J. L. Nadeau, Environ. Sci. Technol., 2010, 44, 1464–1470 CrossRef CAS.
- F. Meinardi, H. McDaniel, F. Carulli, A. Colombo, K. A. Velizhanin, N. S. Makarov, R. Simonutti, V. I. Klimov and S. Brovellii, Nat. Nanotechnol., 2015, 10, 878–885 CrossRef CAS PubMed.
- Transforming our World: The 2030 Agenda for Sustainable Development, https://sdgs.un.org/2030agenda, accessed May 2nd, 2019 Search PubMed.
- R. A. S. Ferreira, S. F. H. Correia, P. Geogieva, L. Fu, M. Antunes and P. S. André, Sci. Data, 2024, 11, 50 CrossRef PubMed.
- M. A. Hernández-Rodríguez, S. F. H. Correia, R. A. S. Ferreira and L. D. Carlos, J. Appl. Phys., 2022, 131, 140901 Search PubMed.
- C. L. Mulder, L. Theogarajan, M. Currie, J. K. Mapel, M. A. Baldo, M. Vaughn, P. Willard, B. D. Bruce, M. W. Moss, C. E. McLain and J. P. Morseman, Adv. Mater., 2009, 21, 3181–3185 CAS.
- R. Bose, M. Gonzalez, P. Jenkins, R. Walters, J. Morseman, M. Moss, C. McLain, P. Linsert, A. Buchtemann, A. J. Chatten and K. W. J. Barnham, Presented in Part at the 35th IEEE Photovoltaic Specialists Conference, Honolulu, USA, 20–25 June, 2010 Search PubMed.
- S. Sadeghi, R. Melikov, H. B. Jalali, O. Karatum, S. B. Srivastava, D. Conkar, E. N. Firat-Karalar and S. Nizamoglu, ACS Appl. Mater. Interfaces, 2019, 11, 8710–8716 Search PubMed.
- S. F. H. Correia, A. R. N. Bastos, M. Martins, I. P. E. Macário, T. Veloso, J. L. Pereira, J. A. P. Coutinho, S. P. M. Ventura, P. S. André and R. A. S. Ferreira, Adv. Sci., 2022, 9, 2104801 CAS.
- S. F. H. Correia, L. Fu, L. M. S. Dias, R. F. P. Pereira, V. de Zea Bermudez, P. S. André and R. A. S. Ferreira, Nanoscale Adv., 2023, 5, 3428–3438 CAS.
- G. Figueiredo, S. F. H. Correia, B. P. Falcão, V. Sencadas, L. Fu, P. S. André and R. A. S. Ferreira, Adv. Sci., 2024, 11, 2400540 Search PubMed.
- F. Meinardi, F. Bruni, C. Castellan, M. Meucci, A. M. Umair, M. La Rosa, J. Catani and S. Brovelli, Adv. Energy Mater., 2024, 14, 2304006 Search PubMed.
- M. Kennedy, S. J. McCormack, J. Doran and B. Norton, Sol. Energy, 2009, 83, 978–981 CAS.
- S. R. Wilton, M. R. Fetterman, J. J. Low, G. J. You, Z. Y. Jiang and J. Xu, Opt. Express, 2014, 22, A35–A43 Search PubMed.
- L. R. Bradshaw, K. E. Knowles, S. McDowall and D. R. Gamelin, Nano Lett., 2015, 15, 1315–1323 CAS.
- X. M. Hu, R. D. Kang, Y. Y. Zhang, L. G. Deng, H. Z. Zhong, B. S. Zou and L. J. Shi, Opt. Express, 2015, 23, A858–A867 CAS.
- K. E. Knowles, T. B. Kilburn, D. G. Alzate, S. McDowall and D. R. Gamelin, Chem. Commun., 2015, 51, 9129–9132 CAS.
- R. H. Inman, G. V. Shcherbatyuk, D. Medvedko, A. Gopinathan and S. Ghosh, Opt. Express, 2011, 19, 24308–24313 Search PubMed.
- G. V. Shcherbatyuk, R. H. Inman, C. Wang, R. Winston and S. Ghosh, Appl. Phys. Lett., 2010, 96, 191901 Search PubMed.
- D. L. Waldron, A. Preske, J. M. Zawodny, T. D. Krauss and M. C. Gupta, Nanotechnology, 2017, 28, 095205 Search PubMed.
- Y. Zhou, D. Benetti, Z. Fan, H. Zhao, D. Ma, A. O. Govorov, A. Vomiero and F. Rosei, Adv. Energy Mater., 2016, 6, 1501913 Search PubMed.
- X. Liu, B. Luo, J. Liu, D. Jing, D. Benetti and F. Rosei, J. Mater. Chem. A, 2020, 8, 1787–1798 Search PubMed.
- A. Anand, M. L. Zaffalon, G. Gariano, A. Camellini, M. Gandini, R. Brescia, C. Capitani, F. Bruni, V. Pinchetti, M. Zavelani-Rossi, F. Meinardi, S. A. Crooker and S. Brovelli, Adv. Funct. Mater., 2020, 30, 1906629 CrossRef CAS.
- M. R. Bergren, N. S. Makarov, K. Ramasamy, A. Jackson, R. Gughelmetti and H. McDaniel, ACS Energy Lett., 2018, 3, 520–525 CrossRef CAS.
- M. B. Zhu, Y. X. Li, S. Q. Tian, Y. Xie, X. J. Zhao and X. Gong, J. Colloid Interface Sci., 2019, 534, 509–517 CrossRef CAS.
- J. Huang, J. J. Zhou, T. Haraldsson, A. Clemments, M. Fujii, H. Sugimoto, B. Xu and I. Sychugov, Sol. RRL, 2020, 4, 2000195 CrossRef CAS.
- V. Sholin, J. D. Olson and S. A. Carter, J. Appl. Phys., 2007, 101, 123114 CrossRef.
- C. H. Yang, M. Moemeni, M. Bates, W. Sheng, B. Borhan and R. R. Lunt, Adv. Opt. Mater., 2020, 8, 1901536 CAS.
- C. C. Yang, J. Zhang, W. T. Peng, W. Sheng, D. Y. Liu, P. S. Kuttipillai, M. Young, M. R. Donahue, B. G. Levine, B. Borhan and R. R. Lunt, Sci. Rep., 2018, 8, 16359 Search PubMed.
- R. Rondão, A. R. Frias, S. F. H. Correia, L. S. Fu, V. de Zea Bermudez, P. S. André, R. A. S. Ferreira and L. D. Carlos, ACS Appl. Mater. Interfaces, 2017, 9, 12540–12546 Search PubMed.
- R. Mazzaro, A. Gradone, S. Angelon, G. Morselli, P. G. Cozzi, F. Romano, A. Vomiero and P. Ceroni, ACS Photonics, 2019, 6, 2303–2311 CAS.
- A. R. Frias, S. F. H. Correia, M. Martins, S. P. M. Ventura, E. Pecoraro, S. L. Ribeiro, P. S. Andre, R. A. S. Ferreira, J. A. P. Coutinho and L. D. Carlos, Adv. Sustain. Syst., 2019, 3, 1800134 Search PubMed.
- A. R. Frias, E. Pecoraro, S. F. H. Correia, L. M. G. Minas, A. R. Bastos, S. Garcia-Revilla, R. Balda, S. J. L. Ribeiro, P. S. Andre, L. D. Carlos and R. A. S. Ferreira, J. Mater. Chem. A, 2018, 6, 8712–8723 CAS.
- C. P. A. Carlos, S. F. H. Correia, M. Martins, O. A. Savchuk, J. A. P. Coutinho, P. S. André, J. B. Nieder, S. P. M. Ventura and R. A. S. Ferreira, Green Chem., 2020, 22, 4943–4951 CAS.
- F. Chemat, M. Abert-Vian, A. S. Fabiano-Tixier, J. Strube, L. Uhlenbrock, V. Gunjevic and G. Cravotto, TrAC, Trends Anal. Chem., 2019, 118, 248–263 CAS.
- T. Zhou, A. Zhang, C. S. Zhao, H. W. Liang, Z. Y. Wu and J. K. Xia, Macromolecules, 2007, 40, 9009–9017 CrossRef CAS.
- J. Rieger, J. Therm. Anal., 1996, 46, 965–972 CrossRef CAS.
- Q. Beuguel, E. Kirillov, J. F. Carpentier and S. M. Guillaume, ACS Appl. Polym. Mater., 2022, 4, 2251–2255 CrossRef CAS.
- C. C. White, K. T. Tan, D. L. Hunston, T. Nguyen, D. J. Benatti, D. Stanley and J. W. Chin, Polym. Degrad. Stab., 2011, 96, 1104–1110 CAS.
- A. K. Pal, A. K. Mohanty and M. Misra, RSC Adv., 2021, 11, 36398–36438 CAS.
- Y. Zhao, A. W. Dunn and D. L. Shi, MRS Commun., 2019, 9, 675–681 CAS.
- B. Grimm, R. J. Porra, W. Rüdiger and H. Scheer, Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications, Springer, Dordrecht, 2006 Search PubMed.
- A. W. D. Larkum, R. J. Ritchie and J. A. Raven, Photosynthetica, 2018, 56, 11–43 CAS.
- R. K. Clayton, Photochem. Photobiol., 1966, 5, 669–677 CAS.
- E. Siefert, R. L. Irgens and N. Pfennig, Appl. Environ. Microbiol., 1978, 35, 38–44 Search PubMed.
- R. U. Meckenstock, R. A. Brunisholz and H. Zuber, FEBS Lett., 1992, 311, 128–134 CAS.
- J. F. C. B. Ramalho, A. N. C. Neto, L. D. Carlos, P. S. André and R. A. S. Ferreira, in Handbook on the Physics and Chemistry of Rare Earths, ed. J.-C. Bünzli and V. K. Pecharsky, Elsevier Science, B. V., Amsterdam, 2022, ch. 324, vol. 61, pp. 31–128 Search PubMed.
- J. F. C. B. Ramalho, L. D. Carlos, P. S. André and R. A. S. Ferreira, Adv. Photonics Res., 2021, 6, 2000211 Search PubMed.
- M. Martins, C. M. Albuquerque, C. F. Pereira, J. A. P. Coutinho, M. G. P. M. S. Neves, D. C. G. A. Pinto, M. A. F. Faustino and S. P. M. Ventura, ACS Sustain. Chem. Eng., 2021, 9, 1772–1780 CrossRef CAS.
- M. Martins, A. P. M. Fernandes, M. A. Torres-Acosta, P. N. Collen, M. H. Abreu and S. P. M. Ventura, Sep. Purif. Technol., 2021, 254, 117589 CrossRef CAS.
- T. E. Sintra, S. S. Bagagem, F. G. Ahsaie, A. Fernandes, M. Martins, I. P. E. Macario, J. L. Pereira, F. J. M. Goncalves, G. Pazuki, J. A. P. Coutinho and S. P. M. Ventura, Sep. Purif. Technol., 2021, 255, 117538 CrossRef CAS.
- C. A. S. Ruiz, M. Martins, J. A. P. Coutinho, R. H. Wijffels, M. H. M. Eppink, C. van den Berg and S. P. M. Ventura, Chem. Eng. J., 2020, 399, 125683 CrossRef.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743–4756 CrossRef CAS.
- C. D. S. Brites, A. Millán and L. D. Carlos, in Handbook on the Physics and Chemistry of Rare Earths, ed. J.-C. Bünzli and V. K. Pecharsky, Elsevier Science, B. V., Amsterdam, 2016, ch. 281, vol. 49, pp. 339–427 Search PubMed.
- IXOLAR™ High Efficiency SolarBIT IXYS KXOB25-01X8F, https://waf-e.dubudisk.com/anysolar.dubuplus.com/techsupport@anysolar.biz/O18Adzl/DubuDisk/www/Gen2/KXOB25-01X8FDATASHEET202007.pdf Search PubMed.
- S. Pareek, N. A. Sagar, S. Sharma, V. Kumar, T. Agarwal, G. A. González-Aguilar and E. M. Yahia, in Fruit and Vegetable Phytochemicals: Chemistry and Human Health, ed. E. M. Yahia, John Wiley & Sons Ltd, 2nd edn, 2017, pp. 269–284, DOI:10.1002/9781119158042.ch14.
- M. Martins, R. Oliveira, J. A. P. Coutinho, M. A. F. Faustino, M. G. P. M. S. Neves, D. C. G. A. Pinto and S. P. M. Ventura, Sep. Purif. Technol., 2021, 255, 117723 CrossRef CAS.
- M. Martins, C. W. Ooi, M. C. Neves, J. F. B. Pereira, J. A. P. Coutinho and S. P. M. Ventura, J. Chem. Technol. Biotechnol., 2018, 93, 1864–1870 CrossRef CAS.
- M. Kholany, P. Trebulle, M. Martins, S. P. M. Ventura, J. M. Nicaud and J. A. P. Coutinho, J. Chem. Technol. Biotechnol., 2020, 95, 1126–1134 CrossRef CAS.
- W. Mantele, A. Wollenweber, F. Rashwan, J. Heinze, E. Nabedryk, G. Berger and J. Breton, Photochem. Photobiol., 1988, 47, 451–455 Search PubMed.
- S. S. Kumar, P. Manoj and P. Giridhar, J. Food Sci. Technol., 2015, 52, 8131–8139 Search PubMed.
- V. Benedetti, M. Scatto, M. Baratieri and P. Riello, Waste Biomass Valorization, 2021, 12, 3485–3496 CAS.
- E. Alikhani, M. Mohammadi and M. Sabzi, Polym. Bull., 2023, 80, 7991–8012 CAS.
- M. A. Paglicawan and J. R. Celorico, Polym. Polym. Compos., 2021, 29, S154–S165 CAS.
- B. M. C. Vaz, M. Martins, L. M. D. Mesquita, M. C. Neves, A. P. M. Fernandes, D. C. G. A. Pinto, M. G. P. M. S. Neves, J. A. P. Coutinho and S. P. M. Ventura, Chem. Eng. J., 2022, 428, 131073 CAS.
- Y. Wang, Y. Q. Liu, G. M. Xie, J. L. Chen, P. Li, Y. H. Zhang and H. R. Li, ACS Appl. Mater. Interfaces, 2022, 14, 5951–5958 CAS.
- M. L. Gómez, D. P. Fasce, R. J. J. Williams, H. A. Montejano and C. M. Previtali, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 289–296 Search PubMed.
- B. S. Richards and I. A. Howard, Energy Environ. Sci., 2023, 16, 3214–3239 Search PubMed.
- T. A. de Bruin and W. G. J. H. M. van Sark, Front. Phys., 2022, 10, 856799 Search PubMed.
- R. R. Lunt, Appl. Phys. Lett., 2012, 101, 043902 CrossRef.
- C. C. Yang, H. A. Atwater, M. A. Baldo, D. Baran, C. J. Barile, M. C. Barr, M. Bates, M. G. Bawendi, M. R. Bergren, B. Borhan, C. J. Brabec, S. Brovelli, V. Bulovic, P. Ceroni, M. G. Debije, J. M. Delgado-Sanchez, W. J. Dong, P. M. Duxbury, R. C. Evans, S. R. Forrest, D. R. Gamelin, N. C. Giebink, X. Gong, G. Griffini, F. Guo, C. K. Herrera, A. W. Y. Ho-Baillie, R. J. Holmes, S. K. Hong, T. Kirchartz, B. G. Levine, H. B. Li, Y. L. Li, D. Y. Liu, M. A. Loi, C. K. Luscombe, N. S. Makarov, F. Mateen, R. Mazzaro, H. McDaniel, M. D. McGehee, F. Meinardi, A. Menendez-Velazquez, J. Min, D. B. Mitzi, M. Moemeni, J. H. Moon, A. Nattestad, M. K. Nazeeruddin, A. F. Nogueira, U. W. Paetzold, D. L. Patrick, A. Pucci, B. P. Rand, E. Reichmanis, B. S. Richards, J. Roncali, F. Rosei, T. W. Schmidt, F. So, C. C. Tu, A. Vahdani, W. G. J. H. M. van Sark, R. Verduzco, A. Vomiero, W. W. H. Wong, K. F. Wu, H. L. Yip, X. W. Zhang, H. G. Zhao and R. R. Lunt, Joule, 2022, 6, 8–15 CrossRef.
- M. G. Debije, R. C. Evans and G. Griffini, Energy Environ. Sci., 2021, 14, 293–301 RSC.
- C. Preston, Y. L. Xu, X. G. Han, J. N. Munday and L. B. Hu, Nano Res., 2013, 6, 461–468 CrossRef CAS.
- D. B. Mahadik, R. V. Lakshmi and H. C. Barshilia, Sol. Energy Mater. Sol. Cells, 2015, 140, 61–68 CrossRef CAS.
- O. A. Savchuk, O. F. Silvestre, R. M. R. Adao and J. B. Nieder, Sci. Rep., 2019, 9, 7535 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta08036j |
| ‡ Present address: i3N and Department of Physics, University of Aveiro, 3810-193 Aveiro, Portugal. |
| This journal is © The Royal Society of Chemistry 2025 |





