Vacancy induced dissociation of hydrogenized CO2 for promoted CH4 production in MBenes†
Abstract
In the electrocatalytic CO2 reduction reaction, the first hydrogenation step only forms *COOH or *HCOO, which often limits the catalytic efficiency. Herein, we have extensively investigated the effect of transition metal atom vacancies in a series of M2B2 (M = Mo, Ti, V, Cr, Fe, Hf) MBenes on the catalytic performance of the CO2RR. Our results suggest that an active microregion formed by the Mo vacancy in Mo2B2 directly facilitates the cleavage of the C–O bond in *CO2 during the first hydrogenation step, leading to the formation of *CO/*OH intermediates, resulting in high selectivity for CH4 production (UL = −0.49 V). The modulation mechanism is illustrated by the vacancy-induced local electronic structure reconstruction, which leads to differences in electron filling of the d orbitals of Mo near the vacancy. This facilitates a unique *CO2 adsorption configuration, in which the 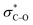 orbital is fully occupied, significantly weakening the C–O bond. And due to the lower energy barrier for hydrogenation occurring on the O atom, the first protonation step leads to the cleavage of *CO2, creating a new CO2RR pathway. This phenomenon expands the methods of CO2 activation and CO2RR pathways, paving the way for constructing vacancies as co-catalytic sites to enhance product selectivity.
orbital is fully occupied, significantly weakening the C–O bond. And due to the lower energy barrier for hydrogenation occurring on the O atom, the first protonation step leads to the cleavage of *CO2, creating a new CO2RR pathway. This phenomenon expands the methods of CO2 activation and CO2RR pathways, paving the way for constructing vacancies as co-catalytic sites to enhance product selectivity.



 Please wait while we load your content...
Please wait while we load your content...