Open Access Article
Open Access ArticleHigh yield ammonia production via glucose oxidation assisted electrochemical nitrate reduction†
Akansha
Chaturvedi‡
,
Sukhjot
Kaur‡
,
Kalpana
Garg
and
Tharamani C.
Nagaiah
 *
*
Department of Chemistry, Indian Institute of Technology Ropar, Rupnagar-140001, Punjab, India. E-mail: tharamani@iitrpr.ac.in
First published on 25th February 2025
Abstract
The electrocatalytic nitrate (NO3−) reduction reaction (NO3RR) provides a potential route for the synthesis of value-added ammonia (NH3) and removal of nitrate pollutants. However, this reaction is limited by nitrate adsorption and slow kinetics involving multiple proton and electron transfer steps. The sluggish OER can hinder NO3RR performance, but replacing it with a more facile oxidation process enhances performance. Herein, we have demonstrated the glucose oxidation assisted NO3RR, utilizing CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S as a bifunctional catalyst demonstrating a high NH3 faradaic efficiency (F.E.) of 93.44% with a yield rate of 4.7 mg h−1 cm−2 or 280.47 mmol gcat−1 h−1 at −0.4 V vs. RHE along with achieving high current densities of ∼118 mA cm−2 and its high activity compared to monometallic variants was explained using the ultraviolet photoelectron spectroscopy (UPS) technique. Furthermore, a mechanistic study using in situ electrochemical Raman spectroscopy, illustrates the hydrogenation of NO3− to NH3via a NO2− intermediate. Moreover, replacing the OER with the glucose oxidation reaction (GOR) in a full cell system could decrease the total energy input by 200 mV and increase the NH3 yield from 120.95 μg h−1 cm−2 to 259.46 μg h−1 cm−2. Furthermore, high value-added products were obtained at both the anode and cathode at a low cell voltage. More interestingly, we demonstrate the practical extraction of high-purity NH4Cl(s) and NH3 aqueous products from electro-reduced NO3− after electrolysis at 100 mA cm−2 for 50 h, with a collection efficacy of 89.84% for the condensed NH3(aq) solution.
2)S as a bifunctional catalyst demonstrating a high NH3 faradaic efficiency (F.E.) of 93.44% with a yield rate of 4.7 mg h−1 cm−2 or 280.47 mmol gcat−1 h−1 at −0.4 V vs. RHE along with achieving high current densities of ∼118 mA cm−2 and its high activity compared to monometallic variants was explained using the ultraviolet photoelectron spectroscopy (UPS) technique. Furthermore, a mechanistic study using in situ electrochemical Raman spectroscopy, illustrates the hydrogenation of NO3− to NH3via a NO2− intermediate. Moreover, replacing the OER with the glucose oxidation reaction (GOR) in a full cell system could decrease the total energy input by 200 mV and increase the NH3 yield from 120.95 μg h−1 cm−2 to 259.46 μg h−1 cm−2. Furthermore, high value-added products were obtained at both the anode and cathode at a low cell voltage. More interestingly, we demonstrate the practical extraction of high-purity NH4Cl(s) and NH3 aqueous products from electro-reduced NO3− after electrolysis at 100 mA cm−2 for 50 h, with a collection efficacy of 89.84% for the condensed NH3(aq) solution.
Introduction
Hydrogen energy is being touted as an appealing substitute for fossil fuels in light of the rising need to reduce carbon emissions.1 However, challenges with H2 storage and transportation hinder its scalability.2 Amidst these challenges, ammonia (NH3) emerges as a key player in clean hydrogen deployment due to its high volumetric energy density (12.92–14.4 MJ L−1), high hydrogen content (17.65%), and easy liquefaction.3–8 NH3 is also crucial for fertilizer production, addressing the UN's Sustainable Development Goal of Zero Hunger.9,10 With an annual demand exceeding 200 million metric tonnes, NH3 is the second most synthesized feedstock globally.11 Currently, NH3 production relies on the energy-intensive Haber–Bosch process, consuming about 1% of the world's fossil-fuel energy and emitting over 400 million metric tons of CO2 annually.4,12–16 Recently, electrochemical nitrogen reduction (NRR) to ammonia has emerged as a sustainable alternative.4,17,18 However, challenges such as the activation of the inert dinitrogen triple bond, low water solubility of N2, and the competing hydrogen evolution reaction (HER) result in low faradaic efficiency (F.E.) and NH3 yield, falling short of the U.S. Department of Energy's targets (DoE, F.E.: 50% and an NH3 yield rate of over 1700 μg h−1 cm−2).19–24 The electrochemical nitrate reduction reaction (NO3RR) to ammonia offers a glimmer of hope, due to the lower bond dissociation energy of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (204 kJ mol−1) compared to N
O bond (204 kJ mol−1) compared to N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (941 kJ mol−1) and higher water solubility (316 g L−1 at 20 °C).25–27 Moreover the NO3RR has the potential to generate NH3 from polluted industrial and agricultural wastewater, aiding in environmental remediation.25,26,28,29
N (941 kJ mol−1) and higher water solubility (316 g L−1 at 20 °C).25–27 Moreover the NO3RR has the potential to generate NH3 from polluted industrial and agricultural wastewater, aiding in environmental remediation.25,26,28,29
Furthermore, the overall nitrate electrolysis pairs up with the NO3RR (NO3− + 7H2O + 8e− → NH4OH + 9OH−) at the cathode and the OER (4OH− → O2 + 2H2O + 4e−) at the anode. However, the slow kinetics associated with the O–O bond formation and the requirement of four proton-coupled electron transfer demand high energy input making NO3− electrolysis in the NO3RR–OER pair an energy intensive process.30,31 Moreover, the OER doesn't provide us with an industrially relevant product. Hence, replacing the OER with alternative anodic reactions which are thermodynamically favourable has become an emerging solution to address the “energy-saving” issue in the NO3RR–OER electrolysis. Therefore, owing to lower overpotential required compared to the OER, the GOR (C6H12O6 + 2OH− → C6H12O7 + 2H2O + 2e− and C6H10O7 + 2OH− → C6H12O8 + 2H2O + 2e−) is a potential candidate to pair with the NO3RR to produce useful chemical commodities such as glucaric acid, gluconic acid and gluconolactone, used in polymer synthesis, the pharmaceutical industry, food and beverage production, and agriculture.32–37 Glucaric acid (GRA) is known as a “top valuable compound” derived from biomass.38 It serves as an essential intermediate in the production of biodegradable polymers, eco-friendly detergents, and metal chelating agents.35,39 A market report from Grand View Research, Inc. indicates that the global GRA market size was approximately USD 550.4 million in 2016, with projections to reach USD 1.30 billion by 2025.35 The substitution of the NO3RR–OER pair with the NO3RR–GOR pair in NO3− electrolysis could be a game changing strategy which provides value-added products in both anodic and cathodic reactions while simultaneously reducing the total energy demand required for efficient NH3 production.40–44 Designing a bifunctional catalyst for the NO3RR and GOR is challenging, as it must facilitate both reactions with distinct mechanisms. Noble metals like Pt, Ru, Rh, and Pd are efficient but costly and scarce, prompting the search for earth-abundant, cost-effective alternatives.40–46 Transition metal catalysts, particularly Cu-based ones, are promising for the NO3RR to NH3 due to the similar energies of the Cu d orbital and the π* (LUMO) molecular orbital of NO3.47–50 Although Cu-based catalysts have weak proton adsorption, which minimizes the competitive HER, insufficient hydrogen adsorption species hinder intermediate (NO2−, NO, etc.) hydrogenation, which is critical for NH3 formation.47–50 Nickel (Ni) exhibits significant O–H activation and a strong affinity for the adsorption of hydrogen (H*) species.51 Incorporating Ni into Cu-based catalysts optimizes the hydrogen species supply for nitrate reduction while minimizing the superfluous HER.52 Additionally, Ni and Cu-based catalysts have shown notable success in electrochemical glucose oxidation.53–55 Herein, we investigated a cost-effective transition metal-based bifunctional catalyst CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S for glucose-assisted electrochemical nitrate reduction for ammonia production at room temperature (Fig. 1). The proposed catalyst demonstrated superior NO3RR electrocatalytic activity for NH3 production with a high NH3 yield rate of 4.76 mg h−1 cm−2 and a F.E. of 93.44%. Under full cell conditions, the NH3 yield rate improved from 120.95 μg h−1 cm−2 to 259.46 μg h−1 cm−2 in the presence of glucose.
2)S for glucose-assisted electrochemical nitrate reduction for ammonia production at room temperature (Fig. 1). The proposed catalyst demonstrated superior NO3RR electrocatalytic activity for NH3 production with a high NH3 yield rate of 4.76 mg h−1 cm−2 and a F.E. of 93.44%. Under full cell conditions, the NH3 yield rate improved from 120.95 μg h−1 cm−2 to 259.46 μg h−1 cm−2 in the presence of glucose.
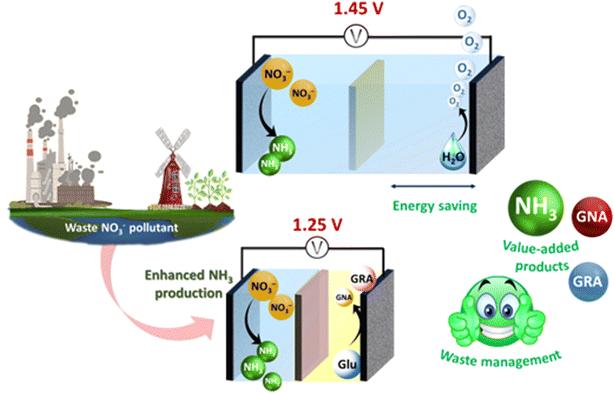 | ||
Fig. 1 Schematic representation of the replacement of anodic OER with GOR during ammonia production using CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) S as a bifunctional electrocatalyst. 2) S as a bifunctional electrocatalyst. | ||
Results and discussion
Synthesis and characterization
The CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S catalyst along with the control catalysts, NiS and CuxS, were synthesized using a one-step hydrothermal method (detailed in the ESI†), and its microstructure was assessed using the powder X-ray diffraction (P-XRD) pattern. The P-XRD pattern (Fig. 2a) of the synthesized NiS demonstrated diffraction peaks at 2θ = 30.168°, 34.743° 46.035°, and 53.547°, which are attributed to the (100), (101), (102) and (110) planes of NiS matching with JCPDS no. 02-1280. Furthermore, the P-XRD pattern of CuxS revealed a combination of peaks that corresponded to CuS (JCPDS no. 06-0464) and Cu2S (JCPDS no. 53-0522), with the most prominent peak at 31.785°, which can be attributed to the (103) plane of CuS and a major peak at 46.105° corresponding to the (220) plane of Cu2S. Thus, the P-XRD pattern of the designed CuNi(x
y)S catalyst along with the control catalysts, NiS and CuxS, were synthesized using a one-step hydrothermal method (detailed in the ESI†), and its microstructure was assessed using the powder X-ray diffraction (P-XRD) pattern. The P-XRD pattern (Fig. 2a) of the synthesized NiS demonstrated diffraction peaks at 2θ = 30.168°, 34.743° 46.035°, and 53.547°, which are attributed to the (100), (101), (102) and (110) planes of NiS matching with JCPDS no. 02-1280. Furthermore, the P-XRD pattern of CuxS revealed a combination of peaks that corresponded to CuS (JCPDS no. 06-0464) and Cu2S (JCPDS no. 53-0522), with the most prominent peak at 31.785°, which can be attributed to the (103) plane of CuS and a major peak at 46.105° corresponding to the (220) plane of Cu2S. Thus, the P-XRD pattern of the designed CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S catalysts demonstrated the coexistence of NiS, CuS and Cu2S in all the synthesised variants, matching well with their respective standard JCPDS data (Fig. 2a and S1†). CuNi(1
y)S catalysts demonstrated the coexistence of NiS, CuS and Cu2S in all the synthesised variants, matching well with their respective standard JCPDS data (Fig. 2a and S1†). CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S, as revealed by the field emission scanning electron microscopy (FE-SEM) image, exhibited a spherical morphology wherein the flakes aggregated into microspheres (Fig. 2b and c). Similarly, NiS and CuxS showed a slightly distorted morphology with microspheres of varying sizes (Fig. S2†). In addition, energy dispersive X-ray spectroscopy (EDS) elemental dot mapping validated the coexistence and uniform distribution of Cu, Ni and S within the sphere (Fig. 2d). Besides, UPS was utilized to understand the interactions between NiS and CuxS in the CuNi(1
2)S, as revealed by the field emission scanning electron microscopy (FE-SEM) image, exhibited a spherical morphology wherein the flakes aggregated into microspheres (Fig. 2b and c). Similarly, NiS and CuxS showed a slightly distorted morphology with microspheres of varying sizes (Fig. S2†). In addition, energy dispersive X-ray spectroscopy (EDS) elemental dot mapping validated the coexistence and uniform distribution of Cu, Ni and S within the sphere (Fig. 2d). Besides, UPS was utilized to understand the interactions between NiS and CuxS in the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst. Fig. 2e reveals the secondary electron cutoff energy56,57 (Ecutoff) at a binding energy (BE) of ca. 17.94 eV, 16.8 eV and 17.11 eV for NiS, CuS and CuNi(1
2)S catalyst. Fig. 2e reveals the secondary electron cutoff energy56,57 (Ecutoff) at a binding energy (BE) of ca. 17.94 eV, 16.8 eV and 17.11 eV for NiS, CuS and CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S, respectively. The work function (WF) of CuNi(1
2)S, respectively. The work function (WF) of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S was computed to be 6.33 eV which lies between the WFs of NiS (5.8 eV) and CuS (6.59 eV) signifying electron transfer from Ni to Cu in the CuNi(1
2)S was computed to be 6.33 eV which lies between the WFs of NiS (5.8 eV) and CuS (6.59 eV) signifying electron transfer from Ni to Cu in the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst (Table S1, detailed calculation in the ESI†). Additionally, X-ray photoelectron spectroscopy (XPS) analysis was carried out to comprehend the oxidation states of the elements in the CuNi(1
2)S catalyst (Table S1, detailed calculation in the ESI†). Additionally, X-ray photoelectron spectroscopy (XPS) analysis was carried out to comprehend the oxidation states of the elements in the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst. The XPS survey spectrum shows the presence of all the expected elements Cu, Ni and S in the CuNi(1
2)S catalyst. The XPS survey spectrum shows the presence of all the expected elements Cu, Ni and S in the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst (Fig. S3a†). The deconvoluted Ni 2p XP spectra exhibited peaks at BEs of 873.3 eV and 855.65 eV, which are separated by 17.65 eV, corresponding to Ni 2p1/2 and Ni 2p3/2, respectively, with satellite peaks at 879.07 eV and 861.3 eV ascribed to Ni in the +2 oxidation state (Fig. S3b†).58 The deconvoluted Cu 2p XPS pattern shown in Fig. S3c† revealed BE peaks at 932.3 eV and 952.2 eV corresponding to 2p3/2 and 2p1/2, respectively, of Cu+. Furthermore, peaks at 933.67 eV and 953.48 eV correspond to 2p3/2 and 2p1/2 of Cu2+.59,60 Thus, we can conclude that Cu is present in both Cu(I) and Cu(II) oxidation states in the CuNi(1
2)S catalyst (Fig. S3a†). The deconvoluted Ni 2p XP spectra exhibited peaks at BEs of 873.3 eV and 855.65 eV, which are separated by 17.65 eV, corresponding to Ni 2p1/2 and Ni 2p3/2, respectively, with satellite peaks at 879.07 eV and 861.3 eV ascribed to Ni in the +2 oxidation state (Fig. S3b†).58 The deconvoluted Cu 2p XPS pattern shown in Fig. S3c† revealed BE peaks at 932.3 eV and 952.2 eV corresponding to 2p3/2 and 2p1/2, respectively, of Cu+. Furthermore, peaks at 933.67 eV and 953.48 eV correspond to 2p3/2 and 2p1/2 of Cu2+.59,60 Thus, we can conclude that Cu is present in both Cu(I) and Cu(II) oxidation states in the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst. Two satellite peaks were also observed at 942.72 eV and 962.42 eV, which are ascribed to Cu2+.59 Furthermore, the deconvoluted S 2p XP spectrum revealed peaks at 161.21 eV and 162.15 eV corresponding to S 2p3/2 and S 2p1/2 bonded with metal, while the peaks at 163.29 eV and 168.02 eV represented divalent sulphide and sulphate, respectively (Fig. S3d†).61 Furthermore, the increase in BE of Ni 2p by 0.3 eV in CuNi(1
2)S catalyst. Two satellite peaks were also observed at 942.72 eV and 962.42 eV, which are ascribed to Cu2+.59 Furthermore, the deconvoluted S 2p XP spectrum revealed peaks at 161.21 eV and 162.15 eV corresponding to S 2p3/2 and S 2p1/2 bonded with metal, while the peaks at 163.29 eV and 168.02 eV represented divalent sulphide and sulphate, respectively (Fig. S3d†).61 Furthermore, the increase in BE of Ni 2p by 0.3 eV in CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S and the decrease in BE of Cu 2p by 0.3 eV in CuNi(1
2)S and the decrease in BE of Cu 2p by 0.3 eV in CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S relative to CuxS indicate successful Cu–Ni binding in CuNi(1
2)S relative to CuxS indicate successful Cu–Ni binding in CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S (Fig. 2f and g). All the aforementioned observations from UPS and XPS indicate close chemical interaction between Cu and Ni, involving the transfer of electrons from Ni to Cu atoms within CuNi(1
2)S (Fig. 2f and g). All the aforementioned observations from UPS and XPS indicate close chemical interaction between Cu and Ni, involving the transfer of electrons from Ni to Cu atoms within CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S.62–64
2)S.62–64
Electrochemical nitrate reduction (NO3RR)
The electrocatalytic activity of the designed CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S catalysts towards the NO3RR was investigated using various electrochemical and spectroscopic techniques. Initially, linear sweep voltammetric (LSV) experiments were conducted in Ar-saturated 1 M KOH + 100 mM KNO3 (Fig. S4A†) at a scan rate of 25 mV s−1. As observed in Fig. 3a, the reduction current was observed at a potential of −0.095 V, reaching the highest current density of 270 mA cm−2 @ −0.6 V vs. RHE. While a shift in the reduction current to more negative potentials was observed in the absence of KNO3 signifying that the designed catalyst is active towards nitrate reduction. To gain a deeper understanding of the role of dual metal catalysts in NO3RR activity, control experiments were performed using monometallic catalysts viz. NiS and CuxS. The comparison of LSV curves (Table S2†) for the synthesized monometallic catalysts viz. NiS and CuxS is shown in Fig. 3a. As expected, the onset potentials were observed at more negative values of −0.25 V and −0.22 for CuxS and NiS, respectively, which are higher than that of the CuNi(1
y)S catalysts towards the NO3RR was investigated using various electrochemical and spectroscopic techniques. Initially, linear sweep voltammetric (LSV) experiments were conducted in Ar-saturated 1 M KOH + 100 mM KNO3 (Fig. S4A†) at a scan rate of 25 mV s−1. As observed in Fig. 3a, the reduction current was observed at a potential of −0.095 V, reaching the highest current density of 270 mA cm−2 @ −0.6 V vs. RHE. While a shift in the reduction current to more negative potentials was observed in the absence of KNO3 signifying that the designed catalyst is active towards nitrate reduction. To gain a deeper understanding of the role of dual metal catalysts in NO3RR activity, control experiments were performed using monometallic catalysts viz. NiS and CuxS. The comparison of LSV curves (Table S2†) for the synthesized monometallic catalysts viz. NiS and CuxS is shown in Fig. 3a. As expected, the onset potentials were observed at more negative values of −0.25 V and −0.22 for CuxS and NiS, respectively, which are higher than that of the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst (−0.095 V). The current density @ −0.6 V vs. RHE was found to be 194 mA cm−2 and 155 mA cm−2 for CuxS and NiS, respectively, both of which are lower than that of the CuNi(1
2)S catalyst (−0.095 V). The current density @ −0.6 V vs. RHE was found to be 194 mA cm−2 and 155 mA cm−2 for CuxS and NiS, respectively, both of which are lower than that of the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst indicating its superior NO3RR activity. Hence, to understand the role of the Cu to Ni ratio in the CuNi(x
2)S catalyst indicating its superior NO3RR activity. Hence, to understand the role of the Cu to Ni ratio in the CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S catalyst, further experiments were performed with other variants. As observed in Fig. S4B,† the onset potential for CuNi(1
y)S catalyst, further experiments were performed with other variants. As observed in Fig. S4B,† the onset potential for CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)S and CuNi(2
1)S and CuNi(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)S was almost similar which is −0.14 V vs. RHE, reaching current densities of 248 mA cm−2 and 237 mA cm−2 respectively @ −0.6 V vs. RHE. Furthermore, to quantify the ammonia produced during nitrate reduction, chronoamperometric measurements were conducted in an Ar-saturated 100 mM KNO3 + 1 M KOH solution at various potentials ranging from −0.2 V to −0.6 V vs. RHE for 1 h each (Fig. 3b). The electrolyte was collected after 1 h of chronoamperometric electrolysis at different potentials and subjected to UV-Vis studies using the indophenol blue method (Fig. S5 and S6†). As observed in Fig. 3c, the NH3 yield rate increased as the potential moved towards more negative values during the NO3RR, indicating higher NH3 synthesis at larger negative potential. The highest NH3 yield rate of 8.05 mg h−1 cm−2 was attained at −0.6 V vs. RHE. Furthermore, the F.E. of NH3 was calculated at various potentials, revealing 93.44% F.E. at −0.4 V vs. RHE with an NH3 yield rate of 4.76 mg h−1 cm−2 (Fig. 3c) and a turn over frequency (TOF) of 11.92 h−1 suggesting the efficient performance of CuNi(1
1)S was almost similar which is −0.14 V vs. RHE, reaching current densities of 248 mA cm−2 and 237 mA cm−2 respectively @ −0.6 V vs. RHE. Furthermore, to quantify the ammonia produced during nitrate reduction, chronoamperometric measurements were conducted in an Ar-saturated 100 mM KNO3 + 1 M KOH solution at various potentials ranging from −0.2 V to −0.6 V vs. RHE for 1 h each (Fig. 3b). The electrolyte was collected after 1 h of chronoamperometric electrolysis at different potentials and subjected to UV-Vis studies using the indophenol blue method (Fig. S5 and S6†). As observed in Fig. 3c, the NH3 yield rate increased as the potential moved towards more negative values during the NO3RR, indicating higher NH3 synthesis at larger negative potential. The highest NH3 yield rate of 8.05 mg h−1 cm−2 was attained at −0.6 V vs. RHE. Furthermore, the F.E. of NH3 was calculated at various potentials, revealing 93.44% F.E. at −0.4 V vs. RHE with an NH3 yield rate of 4.76 mg h−1 cm−2 (Fig. 3c) and a turn over frequency (TOF) of 11.92 h−1 suggesting the efficient performance of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S towards the NO3RR to ammonia (Table S3†). The validation of generated NH3 and reliability of the data for the same have emerged as critical and significant criteria. As a result, a strict methodology was followed, including cleaning of cell components.65 Moreover, prior to the electrochemical experiments, the feeding Ar gas was passed through an alkaline KMnO4 scrubbing solution to remove the impurities of reducible and labile N-containing compounds, namely NOx.66 The use of Ni foam as a substrate for the working electrode was mainly due to its higher surface area and high conductivity.67 Meanwhile, it has been proven that Ni foam is a relatively inert material for the NO3RR, while maintaining the catalyst's performance.67,68 Thus, similar control experiments were performed on the bare Ni foam electrode at −0.4 V vs. RHE and in the absence of NO3− using CuNi(1
2)S towards the NO3RR to ammonia (Table S3†). The validation of generated NH3 and reliability of the data for the same have emerged as critical and significant criteria. As a result, a strict methodology was followed, including cleaning of cell components.65 Moreover, prior to the electrochemical experiments, the feeding Ar gas was passed through an alkaline KMnO4 scrubbing solution to remove the impurities of reducible and labile N-containing compounds, namely NOx.66 The use of Ni foam as a substrate for the working electrode was mainly due to its higher surface area and high conductivity.67 Meanwhile, it has been proven that Ni foam is a relatively inert material for the NO3RR, while maintaining the catalyst's performance.67,68 Thus, similar control experiments were performed on the bare Ni foam electrode at −0.4 V vs. RHE and in the absence of NO3− using CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S. As can be seen in the UV-Vis spectra (Fig. 3d) negligible absorbance was observed in both the cases and the quantification clearly indicates that a negligible amount of NH3 was produced (Fig. S7†). Thus, it is affirmed that the source of NH3 is exclusively from the added NO3− and there are no potential interferences. Furthermore monometallic variants (NiS and CuxS) were also compared with CuNi(1
2)S. As can be seen in the UV-Vis spectra (Fig. 3d) negligible absorbance was observed in both the cases and the quantification clearly indicates that a negligible amount of NH3 was produced (Fig. S7†). Thus, it is affirmed that the source of NH3 is exclusively from the added NO3− and there are no potential interferences. Furthermore monometallic variants (NiS and CuxS) were also compared with CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S under similar experimental conditions (Fig. 3e and S8†). It was observed that CuNi(1
2)S under similar experimental conditions (Fig. 3e and S8†). It was observed that CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S exhibited a considerably enhanced NH3 yield rate (4.76 mg h−1 cm−2) and F.E. (93.44%) compared to monometallic NiS (0.81 μg h−1 cm−2, 25.02%) and CuxS (2.00 mg h−1 cm−2, 48.02%) at a potential of −0.4 V vs. RHE (Fig. 3f). The superior performance of CuNi(1
2)S exhibited a considerably enhanced NH3 yield rate (4.76 mg h−1 cm−2) and F.E. (93.44%) compared to monometallic NiS (0.81 μg h−1 cm−2, 25.02%) and CuxS (2.00 mg h−1 cm−2, 48.02%) at a potential of −0.4 V vs. RHE (Fig. 3f). The superior performance of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S benefited from the advantageous synergy between Cu and Ni in CuNi(1
2)S benefited from the advantageous synergy between Cu and Ni in CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S. More importantly, all the three variants of CuNi(x
2)S. More importantly, all the three variants of CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S with different metal ratios exhibited a high F.E. of more than 80%, with CuNi(1
y)S with different metal ratios exhibited a high F.E. of more than 80%, with CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S showing the highest NH3 yield rate and F.E., indicating its superior NO3RR activity (Fig. S9†). This superior activity of CuNi(1
2)S showing the highest NH3 yield rate and F.E., indicating its superior NO3RR activity (Fig. S9†). This superior activity of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S was further justified by various electrochemical characterization techniques including Tafel analysis, electrochemical impedance spectroscopy (EIS) and electrochemical surface area (ECSA) analysis. First, the Tafel slopes were obtained from LSV curves shown in Fig. 3a for the catalysts in the presence of NO3−. The lower Tafel slope of 102 mV dec−1 for CuNi(1
2)S was further justified by various electrochemical characterization techniques including Tafel analysis, electrochemical impedance spectroscopy (EIS) and electrochemical surface area (ECSA) analysis. First, the Tafel slopes were obtained from LSV curves shown in Fig. 3a for the catalysts in the presence of NO3−. The lower Tafel slope of 102 mV dec−1 for CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S as compared to other controlled synthesized catalyst variants (Fig. S8b, S10 and Table S4†), indicated its faster kinetics towards the NO3RR. Furthermore, the Tafel slope slightly less than 120 mV dec−1 signifies that the rate determining step is the first one electron transfer that occurs during the NO3− to NO2− conversion.69,70 Furthermore, to confirm the conclusions from Tafel analysis, electrochemical impedance spectroscopic (EIS) analysis was conducted by recording Nyquist plots at −0.4 V in the presence of NO3− over the frequency range of 7 mHz to 3 MHz. As shown in Fig. S11,† a lower charge transfer resistance (Rct) was observed for CuNi(1
2)S as compared to other controlled synthesized catalyst variants (Fig. S8b, S10 and Table S4†), indicated its faster kinetics towards the NO3RR. Furthermore, the Tafel slope slightly less than 120 mV dec−1 signifies that the rate determining step is the first one electron transfer that occurs during the NO3− to NO2− conversion.69,70 Furthermore, to confirm the conclusions from Tafel analysis, electrochemical impedance spectroscopic (EIS) analysis was conducted by recording Nyquist plots at −0.4 V in the presence of NO3− over the frequency range of 7 mHz to 3 MHz. As shown in Fig. S11,† a lower charge transfer resistance (Rct) was observed for CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S (Table S5†), signifying a fast charge transfer at the electrode–electrolyte interface during the electrochemical reaction. To further validate this, we estimated the exchange current density and rate constant from the Rct values using the following equations.71–73
2)S (Table S5†), signifying a fast charge transfer at the electrode–electrolyte interface during the electrochemical reaction. To further validate this, we estimated the exchange current density and rate constant from the Rct values using the following equations.71–73| Rct = RT/nFi0 | (1) |
| Rct = RT/n2F2Ack0 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst. These values are higher than those for CuxS (2.80 mA cm−2, 4.56 cm s−1) and NiS (2.72 mA cm−2, 4.43 × 10−9 cm s−1), indicating an enhanced rate of reaction. Furthermore, the i0 and k0 values for CuNi(1
2)S catalyst. These values are higher than those for CuxS (2.80 mA cm−2, 4.56 cm s−1) and NiS (2.72 mA cm−2, 4.43 × 10−9 cm s−1), indicating an enhanced rate of reaction. Furthermore, the i0 and k0 values for CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)S and CuNi(2
1)S and CuNi(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)S catalysts were found to be 3.48 mA cm−2, 5.67 × 10−9 cm s−1 and 3.34 mA cm−2, 5.44 × 10−9 cm s−1 respectively. This facile kinetics could also be attributed to the increased number of accessible electrochemically active sites on the catalyst surface, as measured using the double layer capacitance (Cdl) in the non-faradaic region. The electrochemical surface area (ECSA) of the CuNi(1
1)S catalysts were found to be 3.48 mA cm−2, 5.67 × 10−9 cm s−1 and 3.34 mA cm−2, 5.44 × 10−9 cm s−1 respectively. This facile kinetics could also be attributed to the increased number of accessible electrochemically active sites on the catalyst surface, as measured using the double layer capacitance (Cdl) in the non-faradaic region. The electrochemical surface area (ECSA) of the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst was found to be 37.5 cm2 which is superior to that of other variants (Fig. S12 and Table S6†). Thus, the superior activity of the CuNi(1
2)S catalyst was found to be 37.5 cm2 which is superior to that of other variants (Fig. S12 and Table S6†). Thus, the superior activity of the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst towards the NO3RR was substantially justified by its lower charge transfer resistance (Rct) and higher ECSA.
2)S catalyst towards the NO3RR was substantially justified by its lower charge transfer resistance (Rct) and higher ECSA.
To confirm that the ammonia generated herein is a result of the NO3RR only, the most consistent control isotope labelling experiments were performed using 14NO3− and 15NO3− potassium salts as the N source (Fig. 4a). For the quantitative analysis of 15NH4+/14NH4+ acquired from the isotope labelling studies, the calibration curves were extracted for different known concentrations of 14NH4+ and 15NH4+ using 1H NMR spectra (Fig. S13 and S14†). After electrolysis of the 14NO3− aqueous solution, 1H NMR spectra were acquired exhibiting a triplet with peaks present at 7.06, 6.93 and 6.8 ppm corresponding to a coupling constant of ∼52 Hz, which were attributed to 14NH4+ signals. On the other hand, the 1H NMR spectrum of the 15NO3− aqueous solution upon electrolysis showed a doublet at 7.02 and 6.84 ppm with a coupling constant of ∼72 Hz and no doublet or triplet was observed in the absence of NO3−, indicating that the chemical origin of the produced NH3 during the NO3RR is due to the supplied NO3− (Fig. 4a). Quantification of NH3 for both 15NH4+ and 14NH4+ obtained by 1H NMR is consistent with the indophenol blue method (Fig. 4b and Table S7†). Similarly, quantification of NH3 produced by the electrochemical NO3RR using the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst was further validated by Nessler's reagent test and Fig. 4c shows almost similar NH3 yield values obtained from all the three methods (Fig. 4c, S15 and Table S8†).
2)S catalyst was further validated by Nessler's reagent test and Fig. 4c shows almost similar NH3 yield values obtained from all the three methods (Fig. 4c, S15 and Table S8†).
To corroborate whether any intermediate/by products viz., nitrites (NO2−), hydrazine (N2H4), and hydroxyl amine (NH2OH) were produced during the NO3RR, they were also quantified after chronoamperometric analysis under similar experimental conditions. NO2− was quantified using the Griess method74 at potentials similar to those used for NH3 quantification and it was found that a maximum F.E. of 6.52% was achieved at −0.4 V vs. RHE (Fig. S16 and S17†).
Furthermore, N2H4 and NH2OH by products were also quantified using various colorimetric and UV-Vis spectroscopy techniques (Fig. S18 and S19 detailed in the ESI†). It is worth noting that N2H4 and NH2OH were not detected as revealed by the UV-Vis spectra (Fig. S18c and S19c†), thus indicating a relatively high selectivity for NH3 synthesis by CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S. The total F.E. for the NO3RR at a potential of −0.4 V vs. RHE was found to be 99.96% (∼100%) with the F.E. for NH3, and NO2− being 93.44% and 6.52%, respectively (Fig. S20†). The energy efficiency was found to be 30.95% at a potential of −0.4 V vs. RHE (details in ESI†).
2)S. The total F.E. for the NO3RR at a potential of −0.4 V vs. RHE was found to be 99.96% (∼100%) with the F.E. for NH3, and NO2− being 93.44% and 6.52%, respectively (Fig. S20†). The energy efficiency was found to be 30.95% at a potential of −0.4 V vs. RHE (details in ESI†).
This was further confirmed by in situ electrochemical Raman spectroscopy (Fig. 4d). The Raman spectra was recorded over the range of 980 to 1600 cm−1 under an applied potential of −0.4 V vs. RHE. Initially, the only prominent peak was observed at around 1043 cm−1, which is attributed to the presence of nitrate ions (NO3−).67 As the electrochemical reaction progressed, a new peak emerged at approximately 1056 cm−1, corresponding to the stretching vibrations of the NO3− ions.75 This suggests the formation of an intermediate or a slight change in the chemical environment of the NO3− ions, indicating NO3− reduction. Additionally, peaks at 1340 cm−1 and 1366 cm−1 were observed at the onset of the reaction in the measurements, representing symmetric and antisymmetric stretching of NO2 in NO3−, respectively.76,77 This indicates the reduction of NO3−via NO2. As the reaction time increases, the intensity of the peak corresponding to NO3− (initially at 1043 cm−1) decreases, indicating the consumption or transformation of nitrate ions during the reduction process. Simultaneously, a new peak emerges at around 1575 cm−1. This peak is attributed to the antisymmetric bending vibrations of the HNH moiety in ammonia (NH3).67,78 The increasing intensity of this peak over time suggests a greater formation of ammonia as the reaction progresses. Thus, the Raman spectral changes observed during the electrochemical nitrate reduction provide insights into the formation of intermediate species and the conversion of nitrate ions to ammonia. Thus, based on our electrochemical results and previous studies, we conclude that the electrochemical reaction NO3− + 6H2O + 8e− → NH3 + 9OH− is characterized by a sequence of deoxidation reactions (*NO3− → *NO2 → *NO → *N), followed by hydrogenation steps (*N → *NH → *NH2 → *NH3).25,79
Along with electrochemical activity, stability is an important parameter for evaluating the performance of catalyst. The electrochemical stability of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S towards the NO3RR was then investigated through electrolysis for 10 consecutive cycles (1 h each) at the potential of −0.4 V vs. RHE. The chronoamperometric curves revealed a negligible change in the current response and the corresponding UV-Vis spectra for consecutive 10 cycles of 1 h each remained consistent (Fig. S21†). A steady F.E. and NH3 yield were maintained throughout the 10-h stability test (Fig. 4e). Moreover, post analysis of the catalyst after prolonged chronoamperometric measurement was further investigated by P-XRD, FE-SEM and XPS. The P-XRD pattern and FE-SEM image showed that the crystalline structure and morphology are well preserved (Fig. S22a and b†). Furthermore, post XPS analysis (Fig. S23†) showed no change in the chemical composition of elements present in CuNi(1
2)S towards the NO3RR was then investigated through electrolysis for 10 consecutive cycles (1 h each) at the potential of −0.4 V vs. RHE. The chronoamperometric curves revealed a negligible change in the current response and the corresponding UV-Vis spectra for consecutive 10 cycles of 1 h each remained consistent (Fig. S21†). A steady F.E. and NH3 yield were maintained throughout the 10-h stability test (Fig. 4e). Moreover, post analysis of the catalyst after prolonged chronoamperometric measurement was further investigated by P-XRD, FE-SEM and XPS. The P-XRD pattern and FE-SEM image showed that the crystalline structure and morphology are well preserved (Fig. S22a and b†). Furthermore, post XPS analysis (Fig. S23†) showed no change in the chemical composition of elements present in CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S confirming the high stability of the catalyst towards the NO3RR. The aforementioned results show the appreciable activity and robust nature of CuNi(1
2)S confirming the high stability of the catalyst towards the NO3RR. The aforementioned results show the appreciable activity and robust nature of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S towards the NO3RR in alkaline media and its superiority compared to the reported literature (Table S9†).
2)S towards the NO3RR in alkaline media and its superiority compared to the reported literature (Table S9†).
Glucose oxidation reaction (GOR)
To probe the catalytic activity of CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S towards the glucose oxidation reaction (GOR), LSV curves were recorded for all variants in the absence (i.e., OER) and presence of glucose (i.e., GOR) in 1 M KOH at a scan rate of 5 mV s−1 in a 3-electrode assembly, with catalyst-coated Ni foam serving as the working electrode (WE), graphite rod as the counter electrode (CE), and Hg/HgO/1 M NaOH as the reference electrode (RE). The catalyst was activated prior to the LSV measurements by recording cyclic voltammetry patterns at a scan rate of 50 mV s−1 for 50 cycles. Cyclic voltammetry patterns in Fig. S24† illustrate the behaviour of CuNi(1
y)S towards the glucose oxidation reaction (GOR), LSV curves were recorded for all variants in the absence (i.e., OER) and presence of glucose (i.e., GOR) in 1 M KOH at a scan rate of 5 mV s−1 in a 3-electrode assembly, with catalyst-coated Ni foam serving as the working electrode (WE), graphite rod as the counter electrode (CE), and Hg/HgO/1 M NaOH as the reference electrode (RE). The catalyst was activated prior to the LSV measurements by recording cyclic voltammetry patterns at a scan rate of 50 mV s−1 for 50 cycles. Cyclic voltammetry patterns in Fig. S24† illustrate the behaviour of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S for 50 cycles in 1 M KOH where both the anodic and cathodic peak current displayed an exponential increase in the current w.r.t cycles, indicating the formation of a metal oxide layer. The plausible mechanism involved in the GOR is as follows: Cu and Ni atoms are initially oxidised and then they take part in the GOR as described by reactions (3) and (4):55
2)S for 50 cycles in 1 M KOH where both the anodic and cathodic peak current displayed an exponential increase in the current w.r.t cycles, indicating the formation of a metal oxide layer. The plausible mechanism involved in the GOR is as follows: Cu and Ni atoms are initially oxidised and then they take part in the GOR as described by reactions (3) and (4):55| NiO + CuO + 2OH− → NiO(OH) + CuO(OH) + 2e− | (3) |
| NiO(OH) + CuO(OH) + 2 glucose → NiO + CuO + 2 glucoseox | (4) |
Among the several variants of synthesised catalysts examined, CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S exhibited the highest GOR activity, with an onset potential of 1.35 V vs. RHE as observed in Fig. 5a and S25.† The comparison of LSV for OER and GOR activity of CuNi(1
2)S exhibited the highest GOR activity, with an onset potential of 1.35 V vs. RHE as observed in Fig. 5a and S25.† The comparison of LSV for OER and GOR activity of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S in Fig. 5a depicts that the GOR occurs at a lower potential of 1.35 V vs. RHE as compared to the OER (1.55 V vs. RHE). In addition, a lower Tafel slope value of 78 mV dec−1 in the presence of glucose was obtained, compared to that for OER (146 mV dec−1) for CuNi(1
2)S in Fig. 5a depicts that the GOR occurs at a lower potential of 1.35 V vs. RHE as compared to the OER (1.55 V vs. RHE). In addition, a lower Tafel slope value of 78 mV dec−1 in the presence of glucose was obtained, compared to that for OER (146 mV dec−1) for CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S (Fig. S26†), pointed towards faster kinetics for glucose oxidation in comparison to oxygen evolution.31 To determine the precise onset potential for the GOR, sequential chronoamperometric measurements were performed at potentials ranging from 1.1 V to 1.6 V vs. RHE with a 0.05 V interval for 4 minutes each. As shown in Fig. S27,† the current density showed a sudden increase for the GOR at 1.35 V vs. RHE, whereas for the OER, the current density increased only after 1.55 V vs. RHE. Furthermore, the catalytic performance of CuNi(1
2)S (Fig. S26†), pointed towards faster kinetics for glucose oxidation in comparison to oxygen evolution.31 To determine the precise onset potential for the GOR, sequential chronoamperometric measurements were performed at potentials ranging from 1.1 V to 1.6 V vs. RHE with a 0.05 V interval for 4 minutes each. As shown in Fig. S27,† the current density showed a sudden increase for the GOR at 1.35 V vs. RHE, whereas for the OER, the current density increased only after 1.55 V vs. RHE. Furthermore, the catalytic performance of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S was assessed by recording LSV curves across various concentrations from 0 to 250 mM (Fig. S28†). Interestingly, an increasing trend in current density was observed up to 100 mM glucose concentration and upon further increasing the glucose concentration, a decline in current density was observed which could be potentially attributed to the attainment of complete coverage of the catalyst surface by glucose.31 Furthermore, to examine the products obtained during the GOR, chronopotentiometry measurement at 50 mA cm−2 was performed for 4 h (Fig. 5b) and the electrolyte was analysed using high resolution mass spectrometry (HRMS, Fig. S29†). Table S10† depicts the successful formation of gluconic acid, glucaric acid, guluronic acid and their adducts. Moreover, long-term chronopotentiometry tests were performed at 50 and 100 mA cm−2 for 15 h, displaying good stability of the catalyst with a negligible decrease in current density (Fig. S30†). Table S11† shows the comparable performance of our catalyst to that reported in the literature. Thus, CuNi(1
2)S was assessed by recording LSV curves across various concentrations from 0 to 250 mM (Fig. S28†). Interestingly, an increasing trend in current density was observed up to 100 mM glucose concentration and upon further increasing the glucose concentration, a decline in current density was observed which could be potentially attributed to the attainment of complete coverage of the catalyst surface by glucose.31 Furthermore, to examine the products obtained during the GOR, chronopotentiometry measurement at 50 mA cm−2 was performed for 4 h (Fig. 5b) and the electrolyte was analysed using high resolution mass spectrometry (HRMS, Fig. S29†). Table S10† depicts the successful formation of gluconic acid, glucaric acid, guluronic acid and their adducts. Moreover, long-term chronopotentiometry tests were performed at 50 and 100 mA cm−2 for 15 h, displaying good stability of the catalyst with a negligible decrease in current density (Fig. S30†). Table S11† shows the comparable performance of our catalyst to that reported in the literature. Thus, CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S demonstrates remarkable bifunctional activity towards the electrocatalytic NO3RR and GOR for the production of value added products from both reactions.
2)S demonstrates remarkable bifunctional activity towards the electrocatalytic NO3RR and GOR for the production of value added products from both reactions.
Glucose oxidation assisted NH3 production
The outstanding bifunctional catalytic activity of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S towards both the cathodic NO3RR and anodic GOR, inspired us to investigate its practical applicability for glucose oxidation assisted NH3 generation. We assembled a full cell using CuNi(1
2)S towards both the cathodic NO3RR and anodic GOR, inspired us to investigate its practical applicability for glucose oxidation assisted NH3 generation. We assembled a full cell using CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
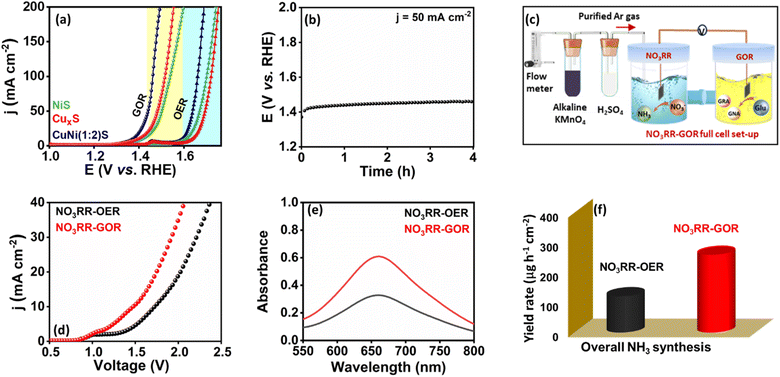
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S as both the anode and cathode electrocatalysts in a homemade H-type cell separated by a Nafion membrane. The anodic chamber was filled with a solution of 1 M KOH + 100 mM glucose. On the other hand, the cathodic chamber was filled with a solution containing 1 M KOH + 100 mM KNO3, to facilitate the reduction of nitrate. Initially the LSV curve was recorded in the presence of glucose, which showed an increase in the current density at a potential of 1.25 V, whereas a voltage of 1.45 V was observed in the absence of glucose, which is 200 mV higher (Fig. 5d). Moreover, the NO3RR–OER cell configuration requires a voltage of 1.72 V to reach a current density of 10 mA cm−2. However, when the OER is substituted with the GOR at the anode during NH3 production, a voltage of 1.48 V is enough to accomplish the same current density. After electrolysis for 1 h at a potential of 1.5 V, a further evaluation of the NH3 generation yield was carried out using UV-Vis spectroscopy (Fig. S31† and 5e). The NH3 yield rate of 259.46 μg h−1 cm−2 for the NO3RR–GOR cell was determined, which is higher than the NH3 yield rate for the NO3RR–OER cell system (120.95 μg h−1 cm−2), as shown in Fig. 5f. This strengthens the relevance of our conceptualization. Therefore, this work may delve into the implementation of metal sulphides for overall NH3 production at decreased cell voltage by substituting the anodic OER with GOR for practical application.
2)S as both the anode and cathode electrocatalysts in a homemade H-type cell separated by a Nafion membrane. The anodic chamber was filled with a solution of 1 M KOH + 100 mM glucose. On the other hand, the cathodic chamber was filled with a solution containing 1 M KOH + 100 mM KNO3, to facilitate the reduction of nitrate. Initially the LSV curve was recorded in the presence of glucose, which showed an increase in the current density at a potential of 1.25 V, whereas a voltage of 1.45 V was observed in the absence of glucose, which is 200 mV higher (Fig. 5d). Moreover, the NO3RR–OER cell configuration requires a voltage of 1.72 V to reach a current density of 10 mA cm−2. However, when the OER is substituted with the GOR at the anode during NH3 production, a voltage of 1.48 V is enough to accomplish the same current density. After electrolysis for 1 h at a potential of 1.5 V, a further evaluation of the NH3 generation yield was carried out using UV-Vis spectroscopy (Fig. S31† and 5e). The NH3 yield rate of 259.46 μg h−1 cm−2 for the NO3RR–GOR cell was determined, which is higher than the NH3 yield rate for the NO3RR–OER cell system (120.95 μg h−1 cm−2), as shown in Fig. 5f. This strengthens the relevance of our conceptualization. Therefore, this work may delve into the implementation of metal sulphides for overall NH3 production at decreased cell voltage by substituting the anodic OER with GOR for practical application.
Extraction of pure ammonia
With our catalyst's noteworthy NO3RR performance, we further illustrate the practical recovery of high-purity ammonia products. A catalyst's good stability is essential at industrially relevant current densities for real applications. Thus, we performed a long-term chronopotentiometry (CP) stability test of CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S in an H-cell at a current density of 100 mA cm−2 for 50 h for the NO3RR (Fig. 6a). After that, ammonia was extracted from the electrolyte solution via the distillation process, which resulted in ammonia condensation in the receiver flask while leaving the remaining reaction mixture in the distilling flask (Fig. 6b and Scheme S1, detailed in the ESI†). The condensed NH3(aq) was verified using 1H NMR spectroscopy as shown in Fig. 6c and the inset shows the condensed NH3. The condensed NH3 was quantified by UV-Vis spectroscopy, with a condensation collection efficiency of 89.84% (detailed in the ESI†). The condensed NH3(aq) solution was further treated with HCl to finally form NH4Cl.80 High-purity NH4Cl(s) powder was obtained through rotary evaporation at a temperature of 40 °C, and its formation was confirmed by the XRD pattern that matches with standard JCPDS ICDD no. 00-007-0007, with no extra peak (Fig. 6d). The collection efficiency of solid NH4Cl was also calculated to be 82.27%. This practical illustration shows the entire process for electrochemically converting nitrate to an advantageous, high-purity ammonia product.
2)S in an H-cell at a current density of 100 mA cm−2 for 50 h for the NO3RR (Fig. 6a). After that, ammonia was extracted from the electrolyte solution via the distillation process, which resulted in ammonia condensation in the receiver flask while leaving the remaining reaction mixture in the distilling flask (Fig. 6b and Scheme S1, detailed in the ESI†). The condensed NH3(aq) was verified using 1H NMR spectroscopy as shown in Fig. 6c and the inset shows the condensed NH3. The condensed NH3 was quantified by UV-Vis spectroscopy, with a condensation collection efficiency of 89.84% (detailed in the ESI†). The condensed NH3(aq) solution was further treated with HCl to finally form NH4Cl.80 High-purity NH4Cl(s) powder was obtained through rotary evaporation at a temperature of 40 °C, and its formation was confirmed by the XRD pattern that matches with standard JCPDS ICDD no. 00-007-0007, with no extra peak (Fig. 6d). The collection efficiency of solid NH4Cl was also calculated to be 82.27%. This practical illustration shows the entire process for electrochemically converting nitrate to an advantageous, high-purity ammonia product.
Conclusion
In summary, we successfully synthesised a CuNi binary metal sulphide CuNi(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y)S catalyst using a one-step hydrothermal approach with flakes aggregated into a microsphere-like morphology for the glucose assisted NO3RR in alkaline media. A high NH3 yield of 4.76 mg h−1 cm−2 (280.47 mmol gcat−1 h−1) and a F.E. of 93.44% at a potential of −0.4 V (vs. RHE) with a high current density of ∼118 mA cm−2 was achieved for the CuNi(1
y)S catalyst using a one-step hydrothermal approach with flakes aggregated into a microsphere-like morphology for the glucose assisted NO3RR in alkaline media. A high NH3 yield of 4.76 mg h−1 cm−2 (280.47 mmol gcat−1 h−1) and a F.E. of 93.44% at a potential of −0.4 V (vs. RHE) with a high current density of ∼118 mA cm−2 was achieved for the CuNi(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)S catalyst, which is superior to the yield obtained in the industrial Haber–Bosch process (<200 mmol gcat−1 h−1).40 Moreover, the successful NO3RR to NH3 conversion was studied by in situ electrochemical Raman spectroscopy. The full-cell studies reveal 2.14 times higher NH3 yield for the NO3RR–GOR system compared to the NO3RR–OER system. This work unveils the synergistic effect of Cu and Ni for efficient NO3RR and GOR and contributes to a better understanding and design of effective bimetallic catalysts for the NO3RR and other applications. This study significantly broadens the area of research for the glucose-assisted NH3 generation at lower cell potential with high NH3 yield, which is accomplished by substituting the sluggish oxygen evolution reaction with the GOR. Furthermore, we extracted a pure NH3 aqueous product and NH4Cl for large-scale industrial application.
2)S catalyst, which is superior to the yield obtained in the industrial Haber–Bosch process (<200 mmol gcat−1 h−1).40 Moreover, the successful NO3RR to NH3 conversion was studied by in situ electrochemical Raman spectroscopy. The full-cell studies reveal 2.14 times higher NH3 yield for the NO3RR–GOR system compared to the NO3RR–OER system. This work unveils the synergistic effect of Cu and Ni for efficient NO3RR and GOR and contributes to a better understanding and design of effective bimetallic catalysts for the NO3RR and other applications. This study significantly broadens the area of research for the glucose-assisted NH3 generation at lower cell potential with high NH3 yield, which is accomplished by substituting the sluggish oxygen evolution reaction with the GOR. Furthermore, we extracted a pure NH3 aqueous product and NH4Cl for large-scale industrial application.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
T. C. Nagaiah thanks the Science and Engineering Research Board (SERB, CRG/2018/004478 and SPG/2021/002415) for funding. A. Chaturvedi acknowledges I.I.T Ropar for the fellowship and research facilities. S. K. thanks the CSIR (09/1005(0029)/2020-EMR-1) for the fellowship. K. G. acknowledges the Prime Minister Research Fellowship (PMRF) scheme for a fellowship. We also thank the team at the Central Research Facility of I.I.T Ropar for the NMR and HRMS facilities and the department's LCMS facility (SR/FST/CS-I/2018/55).Notes and references
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Chem. Rev., 2020, 120, 851–918 Search PubMed.
- R. Lan and S. Tao, Front. Energy Res., 2014, 2, 35 Search PubMed.
- D. Gupta, A. Kafle and T. C. Nagaiah, Faraday Discuss., 2023, 243, 339–353 Search PubMed.
- G. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C.-P. Chen, M. R. Smith and T. W. Hamann, Chem. Rev., 2020, 120, 5437–5516 Search PubMed.
- V. Smil, Nature, 1999, 400, 415 Search PubMed.
- A. Klerke, C. H. Christensen, J. K. Nørskov and T. Vegge, J. Mater. Chem., 2008, 18, 2304–2310 Search PubMed.
- R. Schlögl, Angew. Chem., Int. Ed., 2003, 42, 2004–2008 Search PubMed.
- C. Zamfirescu and I. Dincer, J. Power Sources, 2008, 185, 459–465 Search PubMed.
- M. S. Allahyari and M. Sadeghzadeh, in Zero Hunger, ed. W. Leal Filho, A. M. Azul, L. Brandli, P. G. Özuyar and T. Wall, Springer International Publishing, Cham, 2020, pp. 41–52, DOI:10.1007/978-3-319-95675-6_2.
- S. Ahmad, L. Tang, R. Shahzad, A. M. Mawia, G. S. Rao, S. Jamil, C. Wei, Z. Sheng, G. Shao, X. Wei, P. Hu, M. M. Mahfouz, S. Hu and S. Tang, J. Agric. Food Chem., 2021, 69, 8307–8323 Search PubMed.
- G. Soloveichik, Nat. Catal., 2019, 2, 377–380 Search PubMed.
- G. Leigh, in Catalysts for Nitrogen Fixation, Springer, 2004, pp. 33–54 Search PubMed.
- S. Ghavam, M. Vahdati, I. Wilson and P. Styring, Front. Energy Res., 2021, 9, 34 Search PubMed.
- J. Nørskov, J. Chen, R. Miranda, T. Fitzsimmons and R. Stack, Sustainable Ammonia Synthesis–Exploring the Scientific Challenges Associated with Discovering Alternative, Sustainable Processes for Ammonia Production, US DOE Office of Science, 2016 Search PubMed.
- Y. Li, L. Ouyang, J. Chen, X. Fan, H. Sun, X. He, D. Zheng, S. Sun, Y. Luo, Q. Liu, L. Li, W. Chu, J. Du, Q. Kong, B. Zheng and X. Sun, J. Colloid Interface Sci., 2024, 663, 405–412 Search PubMed.
- C. Ma, L. Bao, X. Fan, X. He, X. Liu, W. Chu, A. Farouk, M. S. Hamdy, S. Sun, Q. Li, M. Wu and X. Sun, Catal. Sci. Technol., 2024, 14, 3007–3011 Search PubMed.
- C. S. Diercks, Y. Liu, K. E. Cordova and O. M. Yaghi, Nat. Mater., 2018, 17, 301–307 Search PubMed.
- F. Chang, W. Gao, J. Guo and P. Chen, Adv. Mater., 2021, 33, 2005721 Search PubMed.
- B. H. R. Suryanto, C. S. M. Kang, D. Wang, C. Xiao, F. Zhou, L. M. Azofra, L. Cavallo, X. Zhang and D. R. MacFarlane, ACS Energy Lett., 2018, 3, 1219–1224 Search PubMed.
- C. J. van der Ham, M. T. Koper and D. G. Hetterscheid, Chem. Soc. Rev., 2014, 43, 5183–5191 Search PubMed.
- Y. Luo, G.-F. Chen, L. Ding, X. Chen, L.-X. Ding and H. Wang, Joule, 2019, 3, 279–289 Search PubMed.
- X. Cui, C. Tang and Q. Zhang, Adv. Energy Mater., 2018, 8(22), 1800369 Search PubMed.
- C. Guo, J. Ran, A. Vasileff and S.-Z. Qiao, Energy Environ. Sci., 2018, 11, 45–56 Search PubMed.
- D. Gupta, A. Kafle, S. Kaur and T. C. Nagaiah, J. Mater. Chem. A, 2023, 11, 22132–22146 Search PubMed.
- Y. Wang, A. Xu, Z. Wang, L. Huang, J. Li, F. Li, J. Wicks, M. Luo, D.-H. Nam, C.-S. Tan, Y. Ding, J. Wu, Y. Lum, C.-T. Dinh, D. Sinton, G. Zheng and E. H. Sargent, J. Am. Chem. Soc., 2020, 142, 5702–5708 Search PubMed.
- S. Ye, Z. Chen, G. Zhang, W. Chen, C. Peng, X. Yang, L. Zheng, Y. Li, X. Ren, H. Cao, D. Xue, J. Qiu, Q. Zhang and J. Liu, Energy Environ. Sci., 2022, 15, 760–770 Search PubMed.
- X. Fan, J. Liang, L. Zhang, D. Zhao, L. Yue, Y. Luo, Q. Liu, L. Xie, N. Li, B. Tang, Q. Kong and X. Sun, Carbon Neutralization, 2022, 1, 6–13 Search PubMed.
- X. Fan, C. Liu, X. He, Z. Li, L. Yue, W. Zhao, J. Li, Y. Wang, T. Li, Y. Luo, D. Zheng, S. Sun, Q. Liu, L. Li, W. Chu, F. Gong, B. Tang, Y. Yao and X. Sun, Adv. Mater., 2024, 36, 2401221 Search PubMed.
- X. He, T. Xie, K. Dong, J. Nan, H. Sun, Y. Yao, X. Fan, D. Zheng, Y. Luo, S. Sun, Q. Liu, L. Li, W. Chu, L. Xie, Q. Kong and X. Sun, Sci. China Mater., 2024 DOI:10.1007/s40843-024-2798-5.
- Z. Lu, J. Wang, S. Huang, Y. Hou, Y. Li, Y. Zhao, S. Mu, J. Zhang and Y. Zhao, Nano Energy, 2017, 42, 334–340 Search PubMed.
- N. Thakur, D. Mehta, A. Chaturvedi, D. Mandal and T. C. Nagaiah, J. Mater. Chem. A, 2023, 11, 15868–15877 Search PubMed.
- A. Chaturvedi, D. Gupta, S. Kaur, K. Garg and T. C. Nagaiah, J. Mater. Chem. A, 2023, 11, 18280–18290 Search PubMed.
- G. Chen, X. Li and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202209014 Search PubMed.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 Search PubMed.
- W.-J. Liu, Z. Xu, D. Zhao, X.-Q. Pan, H.-C. Li, X. Hu, Z.-Y. Fan, W.-K. Wang, G.-H. Zhao and S. Jin, Nat. Commun., 2020, 11, 1–11 Search PubMed.
- Y. Zhang, B. Zhou, Z. Wei, W. Zhou, D. Wang, J. Tian, T. Wang, S. Zhao, J. Liu, L. Tao and S. Wang, Adv. Mater., 2021, 33, 2104791 Search PubMed.
- A. Chaturvedi, S. Gaber, S. Kaur, K. C. Ranjeesh, T. C. Nagaiah and D. Shetty, ACS Energy Lett., 2024, 9, 2484–2491 Search PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 Search PubMed.
- A. Kardan, N. Ashraf, Z. Dabirifar and S. Khadempir, RSC Adv., 2021, 11, 10615–10624 Search PubMed.
- J. Li, G. Zhan, J. Yang, F. Quan, C. Mao, Y. Liu, B. Wang, F. Lei, L. Li, A. W. M. Chan, L. Xu, Y. Shi, Y. Du, W. Hao, P. K. Wong, J. Wang, S.-X. Dou, L. Zhang and J. C. Yu, J. Am. Chem. Soc., 2020, 142, 7036–7046 Search PubMed.
- J. Lim, C.-Y. Liu, J. Park, Y.-H. Liu, T. P. Senftle, S. W. Lee and M. C. Hatzell, ACS Catal., 2021, 11, 7568–7577 Search PubMed.
- Y. Han, X. Zhang, W. Cai, H. Zhao, Y. Zhang, Y. Sun, Z. Hu, S. Li, J. Lai and L. Wang, J. Colloid Interface Sci., 2021, 600, 620–628 Search PubMed.
- G. E. Dima, G. L. Beltramo and M. T. M. Koper, Electrochim. Acta, 2005, 50, 4318–4326 Search PubMed.
- H.-F. Cui, J.-S. Ye, X. Liu, W.-D. Zhang and F.-S. Sheu, Nanotechnology, 2006, 17, 2334 Search PubMed.
- Z. Tang, Z. Bai, X. Li, L. Ding, B. Zhang and X. Chang, Processes, 2022, 10, 751 Search PubMed.
- J. Wang, Y. Wang, C. Cai, Y. Liu, D. Wu, M. Wang, M. Li, X. Wei, M. Shao and M. Gu, Nano Lett., 2023, 23, 1897–1903 Search PubMed.
- H. Xu, Y. Ma, J. Chen, W.-x. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710–2758 Search PubMed.
- M. Teng, J. Ye, C. Wan, G. He, H. J. I. Chen and E. C. Research, J. Ind. Eng. Chem., 2022, 61, 14731–14746 Search PubMed.
- J. Zhao, L. Liu, Y. Yang, D. Liu, X. Peng, S. Liang and L. Jiang, ACS Sustain. Chem. Eng., 2023, 11, 2468–2475 Search PubMed.
- Y. Xu, K. Ren, T. Ren, M. Wang, Z. Wang, X. Li, L. Wang and H. Wang, Appl. Catal., B, 2022, 306, 121094 Search PubMed.
- Y. Li, X. Tan, R. K. Hocking, X. Bo, H. Ren, B. Johannessen, S. C. Smith and C. Zhao, Nat. Commun., 2020, 11, 2720 Search PubMed.
- R. Zhao, Y. Wang, G. Ji, J. Zhong, F. Zhang, M. Chen, S. Tong, P. Wang, Z. Wu, B. Han and Z. Liu, Adv. Mater., 2023, 35, 2205262 Search PubMed.
- B. Barbee, B. Muchharla, A. Adedeji, A. Karoui, K. Kumar Sadasivuni, M. S. Sha, A. M. Abdullah, G. Slaughter and B. Kumar, Sci. Rep., 2022, 12, 7507 Search PubMed.
- X. Lv, R. Tan, X. Xu, Y. Li, C. Geng, Y. Fang, C. Tan, B. Cui and L. Wang, J. Appl. Electrochem., 2022, 52, 895–905 Search PubMed.
- H. Wei, Q. Xue, A. Li, T. Wan, Y. Huang, D. Cui, D. Pan, B. Dong, R. Wei, N. Naik and Z. Guo, Sens. Actuators, B, 2021, 337, 129687 Search PubMed.
- X. Wang, X. Chi, Z. Fu, Y. Xiong, S. Li, Y. Yao, K. Zhang, Y. Li, S. Wang, R. Zhao, Z. Yang and Y.-M. Yan, Appl. Catal., B, 2023, 322, 122130 Search PubMed.
- H.-J. Shin, W. M. Choi, D. Choi, G. H. Han, S.-M. Yoon, H.-K. Park, S.-W. Kim, Y. W. Jin, S. Y. Lee, J. M. Kim, J.-Y. Choi and Y. H. Lee, J. Am. Chem. Soc., 2010, 132, 15603–15609 Search PubMed.
- M. Kumar and T. C. Nagaiah, J. Mater. Chem. A, 2022, 10, 13031–13041 Search PubMed.
- M. Goswami, S. Kumar, N. Singh, N. Sathish, M. Ashiq and S. Kumar, Ionics, 2021, 27, 5277–5285 Search PubMed.
- P. V. F. de Sousa, A. F. de Oliveira, A. A. da Silva and R. P. Lopes, Environ. Sci. Pollut. Res., 2019, 26, 14883–14903 Search PubMed.
- M. Lu, N. Gao, X.-J. Zhang and G.-S. Wang, RSC Adv., 2019, 9, 5550–5556 Search PubMed.
- W. Yu, J. Yu, M. Huang, Y. Wang, Y. Wang, J. Li, H. Liu and W. Zhou, Energy Environ. Sci., 2023, 16, 2991–3001 Search PubMed.
- R. Zhang, Y. Guo, S. Zhang, D. Chen, Y. Zhao, Z. Huang, L. Ma, P. Li, Q. Yang and G. Liang, Adv. Energy Mater., 2022, 12, 2103872 Search PubMed.
- Y. Cao, D. Li, C. Ding, S. Ye, X. Zhang, H. Chi, L. Liu, Y. Liu, J. Xiao and C. Li, ACS Catal., 2023, 13, 11902–11909 Search PubMed.
- D. Gupta, A. Kafle, S. Kaur, T. S Thomas, D. Mandal and T. C. Nagaiah, ACS Appl. Mater. Interfaces, 2023, 15, 4033–4043 Search PubMed.
- J. Choi, B. H. R. Suryanto, D. Wang, H.-L. Du, R. Y. Hodgetts, F. M. Ferrero Vallana, D. R. MacFarlane and A. N. Simonov, Nat. Commun., 2020, 11, 5546 Search PubMed.
- J.-Y. Fang, Q.-Z. Zheng, Y.-Y. Lou, K.-M. Zhao, S.-N. Hu, G. Li, O. Akdim, X.-Y. Huang and S.-G. Sun, Nat. Commun., 2022, 13, 7899 Search PubMed.
- Y. Zhang, Y. Zhao, Z. Chen, L. Wang, P. Wu and F. Wang, Electrochim. Acta, 2018, 291, 151–160 Search PubMed.
- W. He, J. Zhang, S. Dieckhöfer, S. Varhade, A. C. Brix, A. Lielpetere, S. Seisel, J. R. C. Junqueira and W. Schuhmann, Nat. Commun., 2022, 13, 1129 Search PubMed.
- Y. Li, Y. K. Go, H. Ooka, D. He, F. Jin, S. H. Kim and R. Nakamura, Angew. Chem., Int. Ed., 2020, 59, 9744–9750 Search PubMed.
- M. Kumar, N. Thakur, A. Bordoloi, A. Kumar Yadav, S. N. Jha, D. Bhattacharyya, D. Mandal and T. C. Nagaiah, J. Mater. Chem. A, 2022, 10, 11394–11404 Search PubMed.
- E. P. Randviir, Electrochim. Acta, 2018, 286, 179–186 Search PubMed.
- M. Kumar, A. K. Padhan, D. Mandal and T. C. Nagaiah, Energy Storage Mater., 2022, 45, 1052–1061 Search PubMed.
- L. C. Green, D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok and S. R. Tannenbaum, Anal. Biochem., 1982, 126, 131–138 Search PubMed.
- H. Lucas and J.-P. Petitet, J. Phys. Chem. A, 1999, 103, 8952–8958 Search PubMed.
- F. Lei, K. Li, M. Yang, J. Yu, M. Xu, Y. Zhang, J. Xie, P. Hao, G. Cui and B. Tang, Inorg. Chem. Front., 2022, 9, 2734–2740 Search PubMed.
- S.-E. Bae, K. L. Stewart and A. A. Gewirth, J. Am. Chem. Soc., 2007, 129, 10171–10180 Search PubMed.
- D. P. Butcher and A. A. Gewirth, Nano Energy, 2016, 29, 457–465 Search PubMed.
- J.-X. Liu, D. Richards, N. Singh and B. R. Goldsmith, ACS Catal., 2019, 9, 7052–7064 Search PubMed.
- F.-Y. Chen, Z.-Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. A. Cullen, H. Zhou, Y. Han, D. E. Perea, C. L. Muhich and H. Wang, Nat. Nanotechnol., 2022, 17, 759–767 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta07397e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |