Open Access Article
Open Access ArticleElectroless-deposited NiFeP catalyst-coated-membrane cathodes for anion exchange membrane water electrolysis†
Kaiming
Guo
 a,
Masahiro
Kunimoto
b and
Takayuki
Homma
a,
Masahiro
Kunimoto
b and
Takayuki
Homma
 *ac
*ac
aDepartment of Applied Chemistry, Waseda University, Shinjuku, Tokyo 169-8555, Japan. E-mail: t.homma@waseda.jp
bGlobal Center for Science and Engineering, Waseda University, Shinjuku, Tokyo 169-8555, Japan
cResearch Organization for Nano & Life Innovation, Waseda University, Shinjuku, Tokyo 162-0041, Japan
First published on 7th February 2025
Abstract
The rational design of membrane electrode assemblies is crucial for anion exchange membrane (AEM) water electrolysis. In this study, a series of NiFeP catalyst-coated-membranes (CCMs) were synthesized using a Pd-catalyzed electroless deposition method, which served as a cathode for an AEM water electrolyzer. This electroless deposition method enables the in situ growth of NiFeP electrocatalysts on the AEM surface, thus mitigating the degradation of the ionomer/binder during long-term water electrolysis and decreasing the resistance of the membrane electrode assembly. The Ni0.79Fe0.08P0.13 electrocatalyst delivered −500 mA cm−2 at an overpotential of −0.47 V in 1.0 M K2CO3, exhibiting outstanding hydrogen evolution reaction (HER) performance. Furthermore, the Ni0.79Fe0.08P0.13 electrocatalyst demonstrated excellent stability by maintaining −500 mA cm−2 for over 100 h with only a 5.7% increase in overpotential. The exceptional HER catalytic performance of the Ni0.79Fe0.08P0.13 electrocatalyst was attributed to the optimized electronic structure resulting from Fe incorporation and the formation of amorphous regions, which enhanced the catalytic performance and facilitated hydrogen diffusion. This work offers new insights into the fabrication and analysis of ionomer/binder-free CCMs for improving the performance of AEM water electrolysis devices.
Introduction
The contradiction between increasing energy demands and depletion of fossil fuels creates great challenges for modern society, thus emphasizing the importance of developing reliable sustainable energy resources.1 Hydrogen (H2), which is advantageous due to its high energy content and environment friendliness, emerges as a versatile energy carrier.2,3 It facilitates the storage of renewable resources like solar and wind in the form of hydrogen, which can conveniently release energy in fuel cell systems to meet diverse energy consumption requirements. All these advantages contribute to the attractiveness of industrial hydrogen production technologies, for example, like low-temperature water electrolysis.Low-temperature water electrolysis encompasses three major technologies: proton exchange membrane (PEM) water electrolysis, alkaline water electrolysis and anion exchange membrane (AEM) water electrolysis.4 The key advantage of PEM water electrolysis is the usage of PEM, which not only sufficiently separates the cathode side and anode side to avoid the mixing of H2 and O2, but offers a fast proton transportation. Alkaline water electrolysis offers the advantage of employing an alkaline solution, which allows the usage of non-noble metal materials, resulting in lower costs. Anion exchange membrane (AEM) water electrolysis is gaining increasing interest for combining the advantages of proton exchange membrane (PEM) water electrolysis and alkaline water electrolysis.4,5 As a result, AEM water electrolyzers are expected to efficiently generate high-purity H2 at a relatively low cost by employing an alkaline electrolyte. However, research on AEM water electrolysis is still in the early stages, with several challenges remaining to achieve highly active and stable electrolyzers.
The design of membrane electrode assemblies (MEAs), incorporating optimized catalysts and configurations, plays an essential role in the performance of AEM water electrolyzers.6–8 It is vital to select an electrocatalyst for MEAs which is expected to give a high current density (over 0.5 A cm−2) at low potential, for industrial applications.9,10 NiFeP has been widely reported as an efficient catalyst for the hydrogen evolution reaction (HER). For example, Wei et al. synthesized a NiFeP catalyst on Cu foam via optimized electrodeposition using a deep eutectic solvent. This process involves the dissolution of a Fe anode (FA) to supply Fe. Ni nitrite (NN) is also added as a Ni source for electrodeposition. The resulting NiFeP_FA_NN electrocatalyst demonstrated excellent HER activity by delivering −10 mA cm−2 at an overpotential of 56 mV.11 Zhang et al. synthesized the (Fe0.048Ni0.952)2P catalyst via phosphorization of FeNi-LDH, which demonstrated enhanced HER activity in acidic, neutral, alkaline media by achieving −10 mA cm−2 at overpotentials of 81, 90, and 103 mV, respectively.12 These studies highlight the potential of the NiFeP catalyst as an excellent HER catalyst. However, the underlying mechanism behind its high activity toward the HER remains unclear and requires further investigation.
Regarding the configuration of MEAs, a recent study indicates that using a catalyst-coated substrate (CCS) anode paired with a catalyst-coated membrane (CCM) cathode yields optimal performance in the AEM water electrolyzer, particularly with regard to cell voltage.13 However, synthesizing a metal phosphorous film on the AEM for the fabrication of CCM cathodes is challenging because of the unfavorable physical properties of AEMs during fabrication processes, such as their nonconductive nature and limited thermal stability.14 Traditionally, ionomers/binders are crucial to combine the catalyst with the AEM. However, they can restrict the exposure of active sites and degrade during prolonged water electrolysis.15–17 Identifying strategies to avoid the disadvantages of using ionomers/binders is necessary for advancing AEM water electrolyzer technology. In this context, electroless deposition is a powerful technique that enables the in situ growth of metallic materials on the surface of a nonconductive substrate without the need for an ionomer/binder. In 2021, our group reported a novel process to form Pd nuclei on the AEM surface, catalyzing electroless Ni deposition.18 More recently, Kong et al. reported an efficient electroless-deposited NiFe CCM for the oxygen evolution reaction with reduced cell resistance and enhanced durability, attributed to its ionomer-free structure, further validating the superiority of electroless-deposited CCMs.19 However, research on fabricating CCMs via electroless deposition for AEM water electrolysis remains limited, and the properties of CCMs fabricated via Pd-catalyzed electroless deposition are not fully explored.
Considering the membrane stability and corrosion risks of AEM water electrolyzers, the pH value of the electrolyte should be maintained as low as possible. Faraj et al. and Pavel et al. reported stable water electrolysis with a combination of K2CO3 solution (pH = 10–12) and AEM.20,21 Ito et al. suggested that the performance of the electrolyzer with K2CO3 solution (pH value around 12) was more stable and superior than that with KOH at a low pH value of 12.22 Meanwhile, they demonstrated that the utilization of less-corrosive K2CO3 solution enables the utilization of general purpose materials like stainless steel for pipes or tanks, thus decreasing the possibility of electrolyte leakage. Based on the insights from studies above, 1.0 M K2CO3 solution (pH value around 12) appears to be a promising electrolyte for AEM water electrolyzers.
Herein, a series of NiFeP electrocatalysts were synthesized on AEMs via a facile Pd-catalyzed electroless deposition to unveil the origin of their superior HER performance in an AEM water electrolyzer fed with 1.0 M K2CO3 solution. Electrochemical measurements were performed to evaluate the catalytic performance and stability of the as-prepared catalysts. The underlying reasons for the high activity and excellent stability of the as-prepared NiFeP electrocatalysts were explored via characterization studies. Benefiting from the Fe introduction, the NiFeP electrocatalyst's electronic structure was significantly tailored, potentially altering the role of the Ni sites during the HER. Moreover, the Fe content significantly influenced the crystallinity of the NiFeP electrocatalysts, increasing the number of amorphous/crystalline interfaces. This increased the number of active sites and optimized HER performance.
Experimental section
Materials and reagents
Ammonium sulfate ((NH4)2SO4, ≥99.5%), citric acid monohydrate (C6H6Na3O7, ≥99.5%), iron(II) sulfate heptahydrate (FeSO4·7H2O, ≥99.0%), nickel(II) sulfate hexahydrate (NiSO4·6H2O, ≥99.0%), sodium phosphate monohydrate (NaH2PO2·H2O, ≥82.0% as NaH2PO2), palladium(II) chloride (PdCl2, ≥99.0%), hydrochloric acid (HCl, 36%), and sodium hydroxide (NaOH, ≥97.0%) were purchased from Kanto Chemical Co., Inc. (Japan). Dimethylamine borane ((CH3)2NH·BH3, DMAB, ≥97.0%) was purchased from Fujifilm Wako Pure Chemical Corp. (Japan). All reagents were used without further purification. Ultrapure water (18.2 MΩ cm @ 25 °C) was generated using a Milli-Q IQ 7003 (MERCK Co. Ltd.) system.Preparation of the CCM cathode
NiFeP electrocatalysts (10 mm × 10 mm) were synthesized on the AEM surface via electroless deposition, as shown in Scheme 1. The detailed procedure is as follows. First, a 30 mm × 30 mm AEM (A-201, Tokuyama Corp.) was masked with polytetrafluoroethylene (PTFE) tape, leaving only one side of the central region (10 mm × 10 mm) exposed. Subsequently, the masked AEM was rinsed with ultrapure water for 15 s, followed by immersion in a 0.2 g L−1 PdCl2 solution (dissolved in 2.5 mL L−1 of 36% HCl) and 0.02 M DMAB for 30 s and 10 s, respectively. During this step, a change in color from yellow to dark brown was observed in the exposed area (Fig. S1a and b†), indicating the adsorption of Pd species and the formation of Pd nuclei on the AEM surface. Next, FeSO4·7H2O (2.5 mmol, 5 mmol, 7.5 mmol), NiSO4·6H2O (7.5 mmol, 5 mmol, 2.5 mmol), NaH2PO2·H2O (20 mmol), (NH4)2SO4 (40 mmol), and C6H5Na3O7 (10 mmol) were completely dissolved in 50 mL of ultrapure water with continuous stirring. The pH was adjusted to 10 using NaOH, and the solution volume was brought up to 100 mL with ultrapure water. Notably, the total molar amount of FeSO4·7H2O and NiSO4·6H2O was kept constant at 10 mmol. Finally, the pretreated AEM was immersed in the bath at 70 °C for 15 min, forming NiFeP electrocatalysts on the exposed area (Fig. S1c†). For comparison, another electrocatalyst was fabricated using the same process with 5 mmol NiSO4·6H2O but without using FeSO4·7H2O in the electroless deposition bath. The Pt/C noble electrocatalyst (3.0 mg cm−2, Fig. S1d†) on the AEM used in this study was fabricated via the spraying method, as described in previous studies.13,23Characterization and electrochemical tests
The surface morphology and composition of the as-prepared electrocatalysts were analyzed using scanning electron microscopy (SEM, SU 8240, Hitachi) equipped with energy-dispersive X-ray spectroscopy (EDS). Cross-sectional SEM was employed to measure the thickness of the deposits. A focused ion beam (FIB, JIB-4000, JEOL, Ltd.) was used to cut the metal films into small flakes. Transmission electron microscopy (TEM, JEM2100F, JEOL Ltd.) images with selected area electron diffraction (SAED) patterns were obtained to investigate lattice fringes and defects in the as-prepared electrocatalysts, while mapping images provided insights into the elemental distribution across the metal film. X-ray diffraction (XRD, SmartLab 9 kW, Rigaku) analysis was conducted to determine the phases present in the electrocatalyst samples. To evaluate the species and composition of both the bulk material and the interface between the metal film and AEM, depth profiling of X-ray photoelectron spectroscopy (XPS) was performed using an X-ray photoelectron spectrometer (Micro-XPS, VersaProbe II, ULVAC-PHI).Electrochemical measurements were conducted using an HZ-pro electrochemical workstation (Meiden Hokuto Co., Ltd.) in a 1.0 M K2CO3 solution. The previously reported YNU cell configuration is shown in Scheme 2 (picture shown in Fig. S2†).24 In the YNU cell setup, the as-prepared electrocatalyst, a saturated Ag/AgCl electrode, and Ni foam (NF) served as the working, reference, and counter electrodes, and the MEA structure is denoted as electrocatalyst|AEM|NF. Carbon paper was used as the gas diffusion layer (GDL) on the cathode side, and the as-prepared NiFeP electrocatalyst served as the cathode. Ni foam acted as both the anode and the GDL. The electrolyte was circulated only on the anode side to ensure relatively dry hydrogen production on the cathode side. Linear sweep voltammetry (LSV) was conducted at a scan rate of 10 mV s−1. The Tafel plots were generated by applying the following equation to convert the polarization curves:
η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|i| + a. log|i| + a. | (1) |
| E (vs. RHE) = E (vs. Ag/AgCl) + 0.197 + 0.059 pH. | (2) |
 | ||
| Scheme 2 Structure of the test cell and membrane electrode assemblies (MEAs) for anion exchange membrane (AEM) water electrolysis. | ||
The faradaic efficiency (FE) was calculated using the following equation:
| ηFE = (m × z × F)/Q. | (3) |
Results and discussion
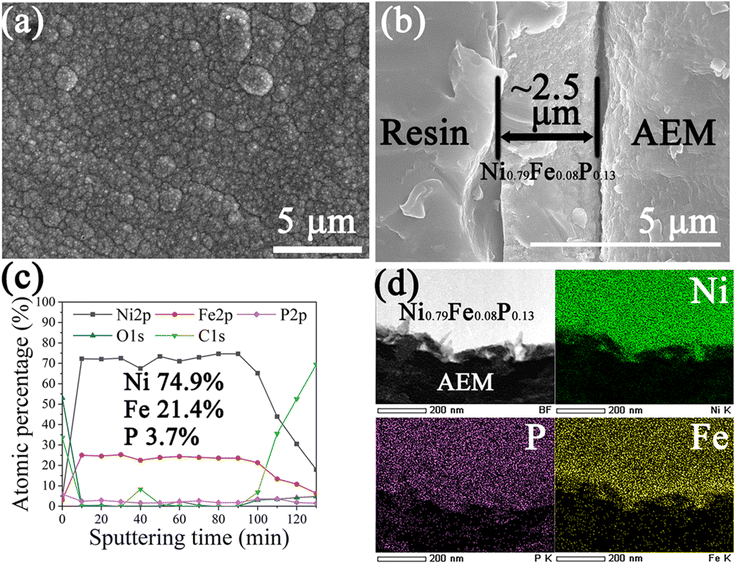
The precise composition of the as-prepared NiFeP electrocatalyst was determined using EDS (Table S1†). The electrocatalysts Ni0.74Fe0.12P0.14, Ni0.79Fe0.08P0.13, Ni0.84Fe0.07P0.09, and Ni0.85P0.15 correspond to electroless deposition baths containing 2.5 mmol of NiSO4·6H2O and 7.5 mmol of FeSO4·7H2O, 5 mmol of NiSO4·6H2O and 5 mmol of FeSO4·7H2O, 7.5 mmol of NiSO4·6H2O and 2.5 mmol of FeSO4·7H2O, and 5 mmol of NiSO4·6H2O without FeSO4·7H2O, respectively. The EDS results reveal that the proportions of Ni and Fe in the NiFeP electrocatalysts exhibit a positive correlation with the concentration of the respective salts in the electroless deposition bath.The catalytic performance and electrochemical properties of the as-prepared catalyst for the HER were assessed electrochemically in a 1.0 M K2CO3 solution (Fig. 1). The LSV curves of the as-prepared catalyst, shown in Fig. 1a, demonstrate the excellent catalytic HER performance of the Ni0.79Fe0.08P0.13 electrocatalyst, achieving overpotentials of 237 and 470 mV to supply current densities of −100 and −500 mA cm−2, respectively. This performance is comparable to that of state-of-the-art Pt/C coatings on AEMs, which exhibit overpotentials of 259 and 427 mV to deliver current densities of −100 and −500 mA cm−2, respectively. Notably, the catalytic performance of the Ni0.79Fe0.08P0.13 electrocatalyst is superior to that of Ni0.74Fe0.12P0.14, Ni0.84Fe0.07P0.09, and Ni0.85P0.15 electrocatalysts. This enhanced activity of the Ni0.79Fe0.08P0.13 electrocatalyst can be attributed to its optimal Fe content, which fine-tunes the electronic structure to be ideal for the HER and adjusts the crystallinity to create beneficial amorphous/crystalline interfaces, thus introducing numerous active sites.25 The electronic structure and crystallinity of the as-prepared electrocatalysts will be discussed in more detail later in this work. To further investigate the HER kinetics of the as-prepared CCM cathode, the Tafel slopes were calculated from the LSV curves near the onset potential range (Fig. 1b). Compared with the Tafel slope of the Ni0.85P0.15 electrocatalyst (280 mV dec−1), the Tafel slopes of the Ni0.74Fe0.12P0.14, Ni0.79Fe0.08P0.13, and Ni0.84Fe0.07P0.09 electrocatalysts decrease to 171, 178, and 205 mV dec−1, respectively, indicating a faster HER kinetics. Interestingly, the overall HER kinetics of the NiFeP electrocatalysts exhibit a negative correlation with the Ni/Fe ratio. The state-of-the-art Pt/C demonstrates a larger Tafel slope of 205 mV dec−1 than that of the Ni0.79Fe0.08P0.13 electrocatalyst, validating the favorable kinetics of the Ni0.79Fe0.08P0.13 electrocatalyst for the HER. In addition, the faradaic efficiency (FE) of the as-prepared electrocatalysts on the AEM was calculated from the ratio of the experimentally quantified H2 gas to the theoretically calculated H2 gas, as shown in Fig. S3.† All as-prepared electrocatalysts on AEMs showed a high FE, and the FEs of Pt/C, Ni0.74Fe0.12P0.14, Ni0.79Fe0.08P0.13, and Ni0.84Fe0.07P0.09, and Ni0.85P0.15 electrocatalysts are 99.8%, 98.8%, 98.9%, 97.4% and 99.5%, respectively. The EIS results of the Ni0.79Fe0.08P0.13 and Ni0.85P0.15 electrocatalysts (Fig. S4 and Table S2†) were obtained using a membrane test machine to assess the resistivity of the electroless-deposited metal films. For comparison, the EIS results of the pure AEM were also measured. The Rohm of the Ni0.85P0.15 electrocatalyst (1.5 Ω) is smaller than that of the Ni0.79Fe0.08P0.13 electrocatalyst (2.4 Ω), demonstrating the lower electrical resistivity of the Ni0.79Fe0.08P0.13 electrocatalyst. This result suggests an increase in electric resistivity with the introduction of Fe, consistent with previous findings.26 The CP measurements reveal the long-term stability of the Ni0.79Fe0.08P0.13 electrocatalyst and Pt/C for the HER at a current density of −500 mA cm−2 (Fig. 1c). The overpotential of Pt/C changes from its best value of 310 mV to 385 mV over 100 h, exhibiting an overpotential increase of 24.2%. The overpotential of the Ni0.79Fe0.08P0.13 electrocatalyst changes from its best value of 470 mV to 497 mV by the end, illustrating an overpotential increase of 5.7% over the same period. These results highlight the excellent stability of the Ni0.79Fe0.08P0.13 electrocatalyst for the HER. During the stability test, the overpotential fluctuations at approximately 40 h and 65 h are attributed to the refreshment of the electrolyte. The improved stability of the Ni0.79Fe0.08P0.13 electrocatalyst for the HER compared to Pt/C films is attributed to its ionomer/binder-free structure.19 The LSV and CP curves of the YNU cell are shown in Fig. S5.† In Fig. S5a,† the voltages with Pt/C and Ni0.79Fe0.08P0.13-integrated cells at a current density of 0.5 A cm−2 are 2.25 V and 2.46 V, respectively. As shown in Fig. S5b,† the resulting potential in the durability test was achieved when the current density was constant at 0.5 A cm−2. During the first 4.5 h, the cell voltage of the Pt/C|AEM|NF cell severely increased from 2.24 V to 2.4 V. The voltage of the Ni0.79Fe0.08P0.13|AEM|NF cell increased from 2.27 V to 2.38 V during 100 h, revealing the good durability of the Ni0.79Fe0.08P0.13 electrocatalyst towards the HER. It is noted that the cell voltages of the YNU cell can be significantly improved, as no electrocatalyst was applied on anode side in the setup.
 | ||
| Fig. 1 Linear sweep voltammetry curves (a), Tafel slopes (b) and chronopotentiometry (c) of the as-prepared catalyst-coated membrane (CCM) cathode at a current density of −500 mA cm−2. | ||
To further investigate the electrochemical surface area (ECSA), the cyclic voltammetry experiments were conducted at scan rates ranging from 20 mV s−1 to 100 mV s−1 (Fig. S6a–c†), and the double-layered capacity (Cdl) was calculated (Fig. S6d†). The Pt/C fabricated via the spraying method exhibits the highest Cdl of 1.65 mF cm−2, compared to 1.31 and 1.55 mF cm−2 for the Ni0.79Fe0.08P0.13 and Ni0.85P0.15 electrocatalysts, respectively. The lower Cdl values of the Ni0.79Fe0.08P0.13 and Ni0.85P0.15 electrocatalysts can be attributed to the relatively dense nature of the electroless deposits. The electrochemical measurement results are summarized in Table S3.† To determine the origin of the excellent catalytic performance of the Ni0.79Fe0.08P0.13 electrocatalyst, further characterization was conducted.
Fig. 2a and b demonstrate the surface and cross-sectional views, respectively, of the Ni0.79Fe0.08P0.13 electrocatalyst. As shown in Fig. 2a, several spherical particles, each composed of small grains, are observed on the Ni0.79Fe0.08P0.13 electrocatalyst surface. The boundaries of the particles serve as a channel for hydrogen diffusion during the HER.27 The acceleration effects of grain boundaries on hydrogen diffusion through Ni-based metal membranes have been reported in previous studies.28–31 During the HER, water molecules diffuse through the AEM from the anode to the cathode, reaching the interface between the AEM and the catalyst layer, where the HER occurs. The morphology formed during the initial stage of electroless deposition influences the number of active sites, thereby playing a crucial role in the catalytic performance of the Ni0.79Fe0.08P0.13 electrocatalyst for the HER. The early stage of the electroless deposition of the Ni0.79Fe0.08P0.13 electrocatalyst was investigated by varying the deposition time, followed by SEM analysis (Fig. S7†). The catalyst grains are evenly distributed on the AEM surface and grow into particles by merging with the surrounding grains. The entire surface of the exposed AEM is covered with the Ni0.79Fe0.08P0.13 electrocatalyst within 30 s of electroless deposition. The SEM images of the electroless-deposited electrocatalysts are shown in Fig. S8.† Fig. S8a† illustrates the morphology of the Ni0.74Fe0.12P0.14 electrocatalyst, where several spherical particles are observed. In contrast to Fig. 2a, no small grains are observed on the surface of an individual particle, resulting in a smoother particle surface. The images of the Ni0.84Fe0.07P0.09 (Fig. S8b†) and Ni0.85P0.15 (Fig. S8c†) surfaces exhibit a morphology similar to that of the Ni0.79Fe0.08P0.13 surface, which corresponds to a combination of spherical particles and small grains. The resin-immobilized Ni0.79Fe0.08P0.13 electrocatalyst was polished to expose the cross-sectional side of the films for SEM evaluation (Fig. 2b). The thickness of the electroless-deposited Ni0.79Fe0.08P0.13 electrocatalyst is approximately 2.5 μm. A magnified cross-sectional image (Fig. S9†) reveals a porous structure near the AEM, which is consistent with the SEM images of the initial deposition stage (Fig. S7†) and is expected to enhance hydrogen diffusion. To confirm the homogeneity and measure the composition of the Ni0.79Fe0.08P0.13 electrocatalyst, XPS depth profiles were obtained (Fig. 2c). Ar sputtering was performed from the surface to the bottom of the Ni0.79Fe0.08P0.13 electrocatalyst every 10 min. The atomic percentages of Ni, Fe, P, O, and C were calculated based on the peak areas in the high-resolution XPS spectra obtained after each sputtering step. Initially, the Ni0.79Fe0.08P0.13 electrocatalyst surface exhibits significantly higher C and O percentage contents compared to Ni, Fe, and P. This is primarily attributed to the adsorption of CO2 and the unavoidable surface oxidation in air. From a sputtering time of 10 min to 100 min, the calculated contents refer to the bulk area of the Ni0.79Fe0.08P0.13 electrocatalyst. In the bulk region, the concentration of C and O nearly disappeared, leaving the average contents of Ni, Fe, and P measuring 74.9%, 21.4%, and 3.7%, respectively. The contents throughout the entire bulk area remain consistent, confirming the homogeneity of the Ni0.79Fe0.08P0.13 electrocatalyst composition. At a sputtering time of 110 min, the C content increases, whereas the Ni, Fe, and P contents decrease, indicating the presence of an interface area between the Ni0.79Fe0.08P0.13 electrocatalyst and the AEM. At this interface (sputtering time of 110 min), the calculated Ni, Fe, and P contents are 71.9%, 25%, and 3.1%, respectively, comparable to those in the bulk area. This result demonstrates the compositional homogeneity of the Ni0.79Fe0.08P0.13 electrocatalyst across both the interface and bulk areas, facilitating the accurate analysis of the active sites by examining the bulk area, which exhibits characteristics similar to those of the interface area. The elemental distribution of Ni, Fe, and P in the Ni0.79Fe0.08P0.13 electrocatalyst was measured by EDS mapping equipped with TEM (Fig. 2d). The uniform distribution of these elements indicates the homogeneity of the Ni0.79Fe0.08P0.13 electrocatalyst near the AEM.
Crystallinity is a key factor influencing the catalytic performance of metal phosphorus toward the HER.32,33 To investigate the crystallinity of the as-prepared NiFeP electrocatalysts, XRD and TEM analyses were conducted (Fig. 3). The XRD patterns of the pristine NiFeP electrocatalysts are shown in Fig. 3a. For the NiFeP electrocatalysts, the diffraction peaks located at 2θ = 21.55° and 24.05° can be ascribed to the (110) and (200) planes of the (CH2)n phase (JCPDS card no. 40-1995), which correspond to the AEM substrate. For Ni0.79Fe0.08P0.13 and Ni0.84Fe0.07P0.09 electrocatalysts, the broad peak observed near 2θ = 44.65° corresponds to the (110) plane of the (Fe, Ni) phase (JCPDS card no. 37-0474), indicating that the primary phases of the Ni0.79Fe0.08P0.13 and Ni0.84Fe0.07P0.09 electrocatalysts correspond to the (Fe, Ni) alloys with low crystallinity. Other phases cannot be ruled out because of the high intensity of the relative peaks for (CH2)n. Furthermore, the (Fe, Ni) phases can obscure weaker peaks associated with phases with low proportions, such as the peaks for the oxidation layer and P species.34 However, the peak for the (110) plane of the (Fe, Ni) phase is hardly observed in the Ni0.74Fe0.12P0.14 electrocatalyst, revealing its amorphous nature. Comparing the peak intensity and full width at half maximum (FWHM) near 2θ = 44.65° for the as-prepared NiFeP electrocatalysts, the crystallinity exhibits a negative correlation with the Fe content, consistent with previous studies.35,36 The Ni0.79Fe0.08P0.13 and Ni0.84Fe0.07P0.09 electrocatalysts exhibit similar intensities and FWHM owing to their comparable Fe contents. The introduction of Fe induces the formation of an amorphous phase, creating several defects and significantly enhancing the catalytic performance of the HER.32,37 Interestingly, the Ni0.79Fe0.08P0.13 electrocatalyst exhibits significantly better catalytic performance than the Ni0.84Fe0.07P0.09 electrocatalyst despite their comparable crystallinities and Fe contents. This is attributed to the synergistic effects of the introduced Fe and P, as the beneficial effects of P species on the HER activities of the electrocatalysts have been widely reported.38–40 Partially enlarged XRD patterns of the as-prepared NiFeP electrocatalysts are shown in Fig. S10a.† A negative shift in the peaks of the (110) plane is observed for the as-prepared NiFeP electrocatalysts, with 2θ angles of 44.34°, 44.4°, and 44.54° for Ni0.74Fe0.12P0.14, Ni0.79Fe0.08P0.13, and Ni0.84Fe0.07P0.09 electrocatalysts, respectively. The negative peak shift is attributed to the substitution of Ni with Fe, which has a larger atomic radius than Ni.35,41,42 A comparison of the XRD patterns of the Ni0.79Fe0.08P0.13 electrocatalyst before and after the prolonged HER durability test is shown in Fig. S10b.† No significant changes are observed, indicating the stability of the Ni0.79Fe0.08P0.13 electrocatalyst during the HER. A high-resolution TEM (HRTEM) image of the area near the interface between the AEM and Ni0.79Fe0.08P0.13 electrocatalyst is shown in Fig. 3b, revealing a mixture of crystalline and amorphous features of the Ni0.79Fe0.08P0.13 electrocatalyst. To further analyze the species of lattice fringes shown in the HRTEM image, the distances between the lattice fringes were measured using software. The calculated distances of 0.201 and 0.204 nm correspond well with the lattice fringe distance of the (110) plane of the (Fe, Ni) phase, in accordance with the XRD results. The orange-labelled square magnified area (Fig. 3c) illustrates the amorphous area generated in the Ni0.79Fe0.08P0.13 electrocatalyst. The amorphous structures are advantageous to the performance of Ni-based material toward the HER as they provide a larger ECSA, better diffusion ability of electrolyte, higher corrosion resistance in alkaline medium, and more defects in the structure.32 The magnified yellow-labeled area (Fig. 3d) highlights three distinct structural features in the crystalline region: the aggregated adjacent lattice fringes (area labeled 1), the vacancies of lattice fringes (area labeled 2), and the bent lattice fringes (area labeled 3). These specific structures are beneficial for HER performance, as they provide a high number of defect sites.43 Such defects can enhance catalytic activity toward the HER by enriching the coordinatively unsaturated atoms, modulating the electronic structures, and supporting the potential catalytically active species during the HER.44 To further understand the TEM image, the SAED pattern of the analyzed TEM area is shown in Fig. 3e. The pattern displays an indistinct diffraction halo and relatively distinct rings, corresponding to the (110), (200), (211), and (222) planes, respectively. This indicates the coexistence of crystalline and amorphous (Fe, Ni) phases.45 To further investigate the crystallinity, FIB-TEM was conducted to obtain a cross-sectional image of a Ni0.79Fe0.08P0.13 electrocatalyst flake (Fig. S11a†). For comparison, the HR-TEM image and SAED pattern of the area far from the AEM are shown in Fig. S11b.† Interestingly, Fig. S11b† depicts a more amorphous region compared to Fig. 3b, which demonstrates decreased crystallinity from the bottom to the surface during the electroless deposition process. The inset (SAED pattern) in Fig. S11b† also shows less distinct rings, confirming the change in crystallinity with depth. These results suggest the presence of a significant number of amorphous/crystalline interfaces at the interface of the AEM and Ni0.79Fe0.08P0.13 electrocatalyst, which have been demonstrated to enhance the HER performance of Ni-based materials.46–48 Moreover, the region farther from the AEM exhibits lower crystallinity, which promotes easier and faster hydrogen diffusion owing to the disordered structure. This variation in crystallinity of the Ni0.79Fe0.08P0.13 electrocatalyst with depth aligns with the ideal conditions for achieving better catalytic performance toward the HER.28 In summary, the electroless-deposited Ni0.79Fe0.08P0.13 electrocatalyst illustrates favorable crystallinity by creating a significant number of amorphous/crystalline interfaces near the AEM, which enhances its catalytic performance towards the HER. Additionally, hydrogen diffusion is facilitated by the reduced crystallinity farther from the AEM.
The survey and high-resolution XPS profiles of the bulk area (sputtering time of 70 min) of the Ni0.79Fe0.08P0.13 and Ni0.85P0.15 electrocatalysts are shown in Fig. 4. The survey spectrum of the Ni0.79Fe0.08P0.13 electrocatalyst (Fig. 4a) demonstrates the Ni 2p, Fe 2p, and P 2p peaks for the Ni0.79Fe0.08P0.13 electrocatalyst, validating the successful synthesis of the Ni0.79Fe0.08P0.13 electrocatalyst on the AEM surface. The relatively low intensity of the P 2p spectrum is attributed to the low P content in the Ni0.79Fe0.08P0.13 electrocatalyst. Understanding the detailed species and electronic structure of the Ni0.79Fe0.08P0.13 electrocatalyst is crucial to enhance its catalytic performance in the HER. In the Ni 2p spectrum of the Ni0.79Fe0.08P0.13 electrocatalyst (Fig. 4b), the peak observed at a binding energy (BE) of 852.65 eV can be attributed to the metallic Ni in the (Fe, Ni) alloy.49 The peak located at a binding energy of 854.53 eV can be ascribed to the existence of Ni(II) species.50 The satellite peak of Ni 2p3/2 is observed at a binding energy of 859.28 eV. The Ni 2p spectrum of the Ni0.85P0.15 electrocatalyst was compared with that of the Ni0.79Fe0.08P0.13 electrocatalyst, showing the peaks of metallic Ni and Ni(II) species, and satellite peaks at binding energies of 853.02, 854.9 eV, and 859.77 eV, respectively. The Ni 2p spectrum of the Ni0.79Fe0.08P0.13 electrocatalyst shows a negative shift of 0.37 eV compared to the Ni0.85P0.15 electrocatalyst, indicating that the Ni sites receive electrons upon the introduction of Fe. In Fig. 4c, the Fe 2p spectrum for the Ni0.79Fe0.08P0.13 electrocatalyst illustrates the existence of metallic Fe and Fe(III), and satellite peaks at the binding energies of 707.28 eV, 712.28 eV, and 720.4 eV, respectively.49,51,52 The standard binding energy for metallic Fe is 707.0 eV, which confirms the positive shift of binding energy by 0.28 eV in the Fe 2p spectrum of the Ni0.79Fe0.08P0.13 electrocatalyst. This indicates the loss of electrons from the Fe sites in the Ni0.79Fe0.08P0.13 electrocatalyst. The high-resolution XPS spectra of P 2p for the Ni0.79Fe0.08P0.13 and Ni0.85P0.15 electrocatalysts are presented in Fig. 4d. For the Ni0.79Fe0.08P0.13 electrocatalyst, a prominent peak at a binding energy of 129.9 eV and a smaller peak at a binding energy of 133.4 eV are observed, which can be attributed to the metal–phosphide bond and phosphate, respectively.50 The intensity of the phosphate peak is significantly weaker compared to that of the phosphide, which is attributed to the low content of phosphate species in the Ni0.79Fe0.08P0.13 electrocatalyst. Comparing the binding energies of the phosphide peaks for the Ni0.79Fe0.08P0.13 electrocatalyst (129.9 eV) and Ni0.85P0.15 electrocatalyst (130.02 eV) reveals that the P sites receive electrons upon Fe introduction. The modulation of the electronic structure is caused by the combined effects of the electronegativity differences between elements and defect structures, which can effectively induce alterations in the localized crystal structure, composition, and chemical states.44,53,54 As a result, the introduction of Fe alters the electronic structure of the Ni0.79Fe0.08P0.13 electrocatalyst, with electrons generally being transferred from the Fe sites to the Ni and P sites. In Ni–P-based materials, the Ni sites are usually positively charged, the P sites are negatively charged, and the Ni and P sites act as hydride and proton acceptors to enhance HER performance.38,53 For the Ni0.79Fe0.08P0.13 electrocatalyst, the introduction of Fe slightly increases the negative charge on the P sites, which enhances their ability to trap protons and facilitates their further reaction during the HER. Interestingly, the Ni sites are slightly negatively charged, and the introduced Fe sites are positively charged. The altered electronic structure suggests that the Fe sites in the Ni0.79Fe0.08P0.13 electrocatalyst play an essential role as hydride acceptors during the HER, which facilitates the adsorption and dissociation of water molecules. Additionally, Ni sites can partially function as proton acceptors, contributing to the increased HER activity.55–57
Finally, the catalytic performance of the Ni0.79Fe0.08P0.13 electrocatalyst towards the HER at a current density of −100 mA cm−2 is compared with that of other Ni-based electrocatalysts reported in recent years, as shown in Fig. 5.58–63 Catalytic performances of other electrocatalysts are reported in 1.0 M KOH or NaOH solutions. The Ni0.79Fe0.08P0.13 electrocatalyst exhibits superior catalytic performance compared with other electrocatalysts in solutions with lower alkalinity, demonstrating its potential as a promising candidate for HER electrocatalysis.
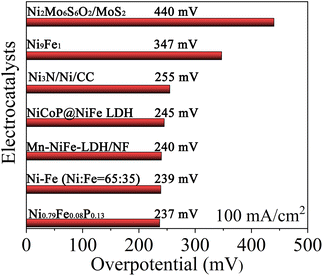 | ||
| Fig. 5 Comparison of the overpotentials required to drive a current density of −100 mA cm−2 for Ni0.79Fe0.08P0.13 and other reported HER electrocatalysts. | ||
Conclusions
The NiFeP electrocatalysts were successfully synthesized on an AEM surface as CCM cathodes via a facile Pd-catalyzed electroless deposition process. The Ni0.79Fe0.08P0.13 electrocatalyst exhibited enhanced catalytic performance for the HER in 1.0 M K2CO3 solution, achieving an overpotential of 470 mV at a current density of −500 mA cm−2. The enhanced catalytic performance is attributed to two main factors. First, the introduction of Fe altered the electronic structure of the Ni0.79Fe0.08P0.13 electrocatalyst, which effectively enhanced its intrinsic activity by absorbing water molecules and protons and transforming them into other intermediates. Second, the Ni0.79Fe0.08P0.13 electrocatalyst exhibited an optimal crystallinity distribution after introducing Fe. The increased number of amorphous/crystalline interfaces between the AEM and Ni0.79Fe0.08P0.13 electrocatalyst significantly improved the HER activity. Lower crystallinity was observed farther from the interface, which could effectively facilitate hydrogen diffusion. The Ni0.79Fe0.08P0.13 electrocatalyst exhibited excellent stability toward the HER with only a 5.7% increase in overpotential after 100 h, which was attributed to the ionomer-/binder-free structure of the as-prepared Ni0.79Fe0.08P0.13 electrocatalyst. This work advances the design and synthesis of non-noble metal-based ionomer-/binder-free CCM cathodes for AEM water splitting devices. It contributes to the development of large-scale AEM water electrolysis systems.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Kaiming Guo: investigation, methodology, conceptualization, formal analysis, and writing of the original draft. Masahiro Kunimoto: conceptualization, investigation, methodology, writing, reviewing, and editing. Takayuki Homma: conceptualization, writing, reviewing and editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.References
- M. Ashraf, N. Ullah, I. Khan, W. Tremel, S. Ahmad and M. N. Tahir, Chem. Rev., 2023, 123, 4443–4509 CrossRef CAS PubMed.
- H. Wang, X. Cheng and Y. Tong, J. Colloid Interface Sci., 2023, 629, 155–164 CrossRef CAS PubMed.
- J. He, Y. Tong, Z. Wang, G. Zhou, X. Ren, J. Zhu, N. Zhang, L. Chen and P. Chen, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2405846121 CrossRef CAS PubMed.
- C. Liu, Z. Geng, X. Wang, W. Liu, Y. Wang, Q. Xia, W. Li, L. Jin and C. Zhang, J. Energy Chem., 2024, 90, 348–369 CrossRef CAS.
- H. A. Miller, K. Bouzek, J. Hnat, S. Loos, C. I. Bernäcker, T. Weißgärber, L. Röntzsch and J. Meier-Haack, Sustain. Energy Fuels, 2020, 4, 2114–2133 RSC.
- M. Riemer, S. Duval-Dachary and T. M. Bachmann, Sustain. Energy Technol. Assessments, 2023, 56, 103086 CrossRef.
- B. Motealleh, Z. Liu, R. I. Masel, J. P. Sculley, Z. R. Ni and L. Meroueh, Int. J. Hydrogen Energy, 2021, 46, 3379–3386 CrossRef CAS.
- Y. Zheng, W. Ma, A. Serban, A. Allushi and X. Hu, Angew. Chem., Int. Ed., 2024, e202413698 Search PubMed.
- Y. Luo, Z. Zhang, M. Chhowalla and B. Liu, Adv. Mater., 2022, 34, 2108133 CrossRef CAS PubMed.
- Y. Chen, Q. Li, Y. Lin, J. Liu, J. Pan, J. Hu and X. Xu, Nat. Commun., 2024, 15, 7278 CrossRef CAS PubMed.
- Z. Wei, M. Guo and Q. Zhang, Appl. Catal., B, 2023, 322, 122101 CrossRef CAS.
- W. Zhang, Y. Zou, H. Liu, S. Chen, X. Wang, H. Zhang, X. She and D. Yang, Nano Energy, 2019, 56, 813–822 CrossRef CAS.
- H. Ito, N. Miyazaki, S. Sugiyama, M. Ishida, Y. Nakamura, S. Iwasaki, Y. Hasegawa and A. Nakano, J. Appl. Electrochem., 2018, 48, 305–316 CrossRef CAS.
- M. Faraj, E. Elia, M. Boccia, A. Filpi, A. Pucci and F. Ciardelli, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3437–3447 CrossRef CAS.
- J. E. Park, S. Y. Kang, S.-H. Oh, J. K. Kim, M. S. Lim, C.-Y. Ahn, Y.-H. Cho and Y.-E. Sung, Electrochim. Acta, 2019, 295, 99–106 CrossRef CAS.
- S. Koch, P. A. Heizmann, S. K. Kilian, B. Britton, S. Holdcroft, M. Breitwieser and S. Vierrath, J. Mater. Chem. A, 2021, 9, 15744–15754 RSC.
- Y. Leng, G. Chen, A. J. Mendoza, T. B. Tighe, M. A. Hickner and C.-Y. Wang, J. Am. Chem. Soc., 2012, 134, 9054–9057 CrossRef CAS PubMed.
- T. Fujimura, M. Kunimoto, Y. Fukunaka, H. Ito and T. Homma, Electrochemistry, 2021, 89, 192–196 CrossRef CAS.
- T.-H. Kong, P. Thangavel, S. Shin, S. Kwon, H. Choi, H. Lee, N. Park, J.-J. Woo and Y. Kwon, ACS Energy Lett., 2023, 8, 4666–4673 CrossRef CAS.
- C. C. Pavel, F. Cecconi, C. Emiliani, S. Santiccioli, A. Scaffidi, S. Catanorchi and M. Comotti, Angew. Chem., Int. Ed., 2014, 53, 1378–1381 CrossRef CAS PubMed.
- M. Faraj, M. Boccia, H. Miller, F. Martini, S. Borsacchi, M. Geppi and A. Pucci, Int. J. Hydrogen Energy, 2012, 37, 14992–15002 CrossRef CAS.
- H. Ito, N. Kawaguchi, S. Someya, T. Munakata, N. Miyazaki, M. Ishida and A. Nakano, Int. J. Hydrogen Energy, 2018, 43, 17030–17039 CrossRef CAS.
- H. Ito, N. Kawaguchi, S. Someya and T. Munakata, Electrochim. Acta, 2019, 297, 188–196 CrossRef CAS.
- K. Nagasawa, T. Ishida, H. Kashiwagi, Y. Sano and S. Mitsushima, Int. J. Hydrogen Energy, 2021, 46, 36619–36628 CrossRef CAS.
- Y. Li, M. Li, C. Xie, Z. Ling, Y. Lv and K. Chen, J. Alloys Compd., 2024, 1002, 175328 CrossRef CAS.
- N. V. Myung and K. Nobe, J. Electrochem. Soc., 2001, 148, C136 CrossRef CAS.
- H. Iwaoka, M. Arita and Z. Horita, Acta Mater., 2016, 107, 168–177 CrossRef CAS.
- A. Oudriss, J. Creus, J. Bouhattate, E. Conforto, C. Berziou, C. Savall and X. Feaugas, Acta Mater., 2012, 60, 6814–6828 CrossRef CAS.
- A. M. Brass and A. Chanfreau, Acta Mater., 1996, 44, 3823–3831 CrossRef CAS.
- S. K. Lawrence, Y. Yagodzinskyy, H. Hänninen, E. Korhonen, F. Tuomisto, Z. D. Harris and B. P. Somerday, Acta Mater., 2017, 128, 218–226 CrossRef CAS.
- G. S. Mogilny, V. N. Shyvaniuk, S. M. Teus, L. M. Ivaskevich and V. G. Gavriljuk, Acta Mater., 2020, 194, 516–521 CrossRef CAS.
- S. Anantharaj and S. Noda, Small, 2020, 16, 1905779 CrossRef CAS PubMed.
- X. Zhao, X. Chen, Y. Wang, P. Song and Y. Zhang, Sustain. Energy Fuels, 2020, 4, 4733–4742 RSC.
- K. Guo, F. Shaik, J. Yang, X. Ren and B. Jiang, J. Electrochem. Soc., 2021, 168, 062512 CrossRef CAS.
- C. Cui, Y. Liu, S. Mehdi, H. Wen, B. Zhou, J. Li and B. Li, Appl. Catal., B, 2020, 265, 118612 CrossRef CAS.
- O. Dragos, H. Chiriac, N. Lupu, M. Grigoras and I. Tabakovic, J. Electrochem. Soc., 2016, 163, D83–D94 CrossRef CAS.
- G. Shao, Q. Wang, F. Miao, J. Li, Y. Li and B. Shen, Electrochim. Acta, 2021, 390, 138815 CrossRef CAS.
- P. Liu and J. A. Rodriguez, J. Am. Chem. Soc., 2005, 127, 14871–14878 CrossRef CAS PubMed.
- D. Liu, G. Xu, H. Yang, H. Wang and B. Y. Xia, Adv. Funct. Mater., 2023, 33, 2208358 CrossRef CAS.
- Y. Pan, Y. Liu, J. Zhao, K. Yang, J. Liang, D. Liu, W. Hu, D. Liu, Y. Liu and C. Liu, J. Mater. Chem. A, 2015, 3, 1656–1665 RSC.
- M. Miao, R. Hou, R. Qi, Y. Yan, L. Q. Gong, K. Qi, H. Liu and B. Y. Xia, J. Mater. Chem. A, 2019, 7, 18925–18931 RSC.
- J. Zhao, N. Liao and J. Luo, J. Mater. Chem. A, 2023, 11, 9682–9690 RSC.
- S. He, C. Li, H. Chen, D. Su, B. Zhang, X. Cao, B. Wang, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2013, 25, 1040–1046 CrossRef CAS.
- J. Xie, X. Yang and Y. Xie, Nanoscale, 2020, 12, 4283–4294 RSC.
- L. Cai, B. Yan, Q. Xue, J. Li, P. Liu, X. Qi and G. Yang, J. Mater. Chem. A, 2022, 10, 18939–18949 RSC.
- X. Xu, T. Guo, J. Xia, B. Zhao, G. Su, H. Wang, M. Huang and A. Toghan, Chem. Eng. J., 2021, 425, 130514 CrossRef CAS.
- B. Zhong, S. Wan, P. Kuang, B. Cheng, L. Yu and J. Yu, Appl. Catal., B, 2024, 340, 123195 CrossRef CAS.
- S. Yang, T. Wen and Y. Gong, Int. J. Hydrogen Energy, 2024, 68, 834–842 CrossRef CAS.
- F. Li, Y. Li, Q. Zhuo, D. Zhou, Y. Zhao, Z. Zhao, X. Wu, Y. Shan and L. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 11479–11488 CrossRef CAS PubMed.
- A. Kafle, M. Kumar, D. Gupta and T. C. Nagaiah, J. Mater. Chem. A, 2021, 9, 24299–24307 RSC.
- Z. Zhang, Y. Bai, Y. He, H. Li, T. He, R. Song, Y. He, J. Song and B. Liu, Surf. Coat. Technol., 2022, 448, 128934 CrossRef CAS.
- B. Li, T. Mei, S. Du and W. Zhang, Mater. Chem. Phys., 2020, 243, 122595 CrossRef CAS.
- Y. Shi, M. Li, Y. Yu and B. Zhang, Energy Environ. Sci., 2020, 13, 4564–4582 RSC.
- C. Xie, D. Yan, W. Chen, Y. Zou, R. Chen, S. Zang, Y. Wang, X. Yao and S. Wang, Mater. Today, 2019, 31, 47–68 CrossRef CAS.
- Q. Fu, X. Wang, J. Han, J. Zhong, T. Zhang, T. Yao, C. Xu, T. Gao, S. Xi, C. Liang, L. Xu, P. Xu and B. Song, Angew. Chem., 2021, 133, 263–271 CrossRef.
- V. Vij, S. Sultan, A. M. Harzandi, A. Meena, J. N. Tiwari, W.-G. Lee, T. Yoon and K. S. Kim, ACS Catal., 2017, 7, 7196–7225 CrossRef CAS.
- X. Chen, J. Wan, J. Wang, Q. Zhang, L. Gu, L. Zheng, N. Wang and R. Yu, Adv. Mater., 2021, 33, 2104764 CrossRef CAS PubMed.
- Z. Zhang, Y. Wu and D. Zhang, Int. J. Hydrogen Energy, 2022, 47, 1425–1434 CrossRef CAS.
- Y. Ma, L. Li, Y. Zhang, N. Jian, H. Pan, J. Deng and J. Li, J. Colloid Interface Sci., 2024, 663, 971–980 CrossRef CAS PubMed.
- H. Zhang, X. Du, X. Zhang and Y. Wang, J. Alloys Compd., 2023, 937, 168412 CrossRef CAS.
- L. Xiong, Y. Qiu, H. Dong, B. Gao, X. Zhang, P. K. Chu and X. Peng, Int. J. Hydrogen Energy, 2024, 59, 400–407 CrossRef CAS.
- Z. Chen, X. Liu, T. Shen, C. Wu, L. Zu and L. Zhang, Int. J. Hydrogen Energy, 2021, 46, 37736–37745 CrossRef CAS.
- H. Cheng, Y. Diao, Q. Liu, L. Wei, X. Li, J. Chen and F. Wang, Chem. Eng. J., 2022, 428, 131084 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06868h |
| This journal is © The Royal Society of Chemistry 2025 |