Highly graphitized nitrogen-doped ordered mesoporous carbon supported Ni nanocrystals for efficient hydrazine-assisted CO2 splitting†
Kang
Lian
ab,
Junyang
Ding
c,
Jin
Zhang
*a,
Quan
Zhang
*bc,
Yifan
Liu
d,
Guangzhi
Hu
 e,
Jia
He
b and
Xijun
Liu
e,
Jia
He
b and
Xijun
Liu
 *c
*c
aSchool of Public Health/Key Laboratory of Endemic and Ethnic Diseases, Ministry of Education, Key Laboratory of Medical Molecular Biology of Guizhou Province, Guizhou Medical University, Guiyang 561113, China. E-mail: jzhang@gmc.edu.cn
bInstitute for New Energy Materials & Low-Carbon Technologies, School of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China. E-mail: 18230299580@163.com
cGuangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, MOE Key Laboratory of New Processing Technology for Nonferrous Metals and Materials, School of Resources, Environment and Materials, Guangxi University, Nanning, Guangxi 530004, China. E-mail: xjliu@gxu.edu.cn
dInstitute of Advanced Science Facilities, Shenzhen 518107, Guangdong, China
eInstitute for Ecological Research and Pollution Control of Plateau Lakes, School of Ecology and Environmental Science, Yunnan University, Kunming 650504, China
First published on 26th November 2024
Abstract
The reaction activity and product selectivity of the CO2 electroreduction process are hindered by intense hydrogen evolution and substantial *CO adsorption when nickel particle size is increased to the nanoscale dimension. This study introduces a highly graphitized nitrogen-doped ordered mesoporous carbon (Ni-NC) as a support for dispersing Ni nanocrystals, which not only enhances mass transfer but also exposes abundant catalytic Ni sites. In situ electrochemical measurements, characterization, and density functional theory calculations revealed that the synergistic interaction between nickel nanocrystals and the nitrogen-doped carbon matrix promoted CO2 adsorption and the formation of the *COOH intermediate. Ni-NC achieved a peak CO faradaic efficiency (FECO) of ∼100% at −0.8 V vs. RHE, maintaining FECO above 90% across a wide potential window from −0.7 to −1.0 V vs. RHE. Additionally, Ni-NC efficiently catalyzed hydrazine-assisted CO2 decomposition, offering up to 69% theoretical energy savings compared to traditional water oxidation-coupled systems in MEA while consistently maintaining a cathodic FECO above 90% across a broad cell voltage range, offering valuable insights for the development of more cost-effective CO2 splitting systems.
1 Introduction
In the context of rapid advancements in modern society, excessive anthropogenic CO2 emissions have caused a multitude of climate and environmental crises, thereby posing a substantial threat to the sustainable development of human society.1–5 Fortunately, the advancement of renewable energy utilization has facilitated the adoption of green electricity to drive the CO2 reduction reaction (CO2RR), which has emerged as a pivotal strategy for emission reduction.6–8 This approach effectively converts CO2 into fuels or value-added chemicals under mild conditions with relatively low energy expenditures, thereby contributing to the mitigation of the CO2 crisis and the closure of the anthropogenic carbon cycle.9,10 However, the practical application of the CO2RR remains hindered by the lack of efficient catalysts specifically designed for target products and the absence of suitable low-energy reaction systems.As one of the principal products of CO2RR, CO not only serves as a direct fuel but also plays a pivotal role in various industrial applications, including carbonylation reactions and Fischer–Tropsch synthesis.11 Recent studies have demonstrated that despite the exceptional catalytic performance of precious metal catalysts, particularly Au and Ag, in the electroreduction of CO2-to-CO, their high costs and scarcity significantly impede their practical implementation.12–14 In contrast, non-precious metal nickel-based catalysts have garnered considerable interest due to their abundant resources and impressive catalytic properties, particularly atomic dispersed Ni, which exhibits substantial catalytic activity in converting CO2 to CO.15–17 However, as the metal size increases further, nano-sized Ni-based catalysts, despite demonstrating conductivity and stability comparable to conventional metal catalysts, face limitations in their applications for CO2RR due to their pronounced competitive hydrogen evolution capability and significant adsorption of the reactive intermediate *CO.18–20 To mitigate this limitation, the integration of metal with carbon materials to modulate the active metal sites has emerged as a promising strategy.21,22 This approach facilitates electron transfer between the metal and the carbon substrate, enabling the precise tuning of the electronic interactions between the catalyst surface and reactants.23,24 Such modulation influences the desorption energy during the adsorption reaction process, ultimately enhancing CO2RR activity. Consequently, the rational design of efficient nano-Ni and carbon composite catalysts for CO2RR represents a critical and promising direction for future research.
Besides optimizing electrocatalyst design, enhancing the reaction system has emerged as pivotal for improving CO2RR performance. Traditionally, CO2RR has been coupled with the energy-intensive water oxidation reaction (OER) at the anode, consuming about 90% of total input energy and significantly limiting cathodic CO2 electroreduction efficiency.25–27 To address these challenges, a promising approach involves replacing OER with a more thermodynamically favorable oxidation reaction, thereby forming a hybrid electrocatalytic CO2RR (HECR) system.28 Among potential anode replacement reactions, the hydrazine oxidation reaction (HzOR) stands out due to its substantially lower onset potential compared to OER, enabling significant energy savings.29 This strategy, previously demonstrated in hydrogen electrolysis, also offers environmental benefits by leveraging the hydrazine commonly found in wastewater.30–32
In this work, we introduce nanoscale nickel dispersed on highly graphitized nitrogen-doped ordered mesoporous carbon (Ni-NC). The highly graphitized nitrogen-doped ordered mesoporous carbon provides efficient mass transfer channels and exposes catalytic Ni sites, which, in synergy with Ni nanocrystals (Ni NCs), promote CO2 adsorption and the formation of the *COOH intermediate during CO2RR. Furthermore, the electrocatalytic HzOR on Ni-NC exhibits thermodynamically favorable characteristics. When applied to both the anode and cathode, Ni-NC facilitates an efficient HzOR-assisted CO2RR system, significantly reducing the reaction voltage compared to conventional OER-coupled CO2RR. This leads to improved CO2 reduction activity at a lower cell voltage and, consequently, a substantial increase in the energy utilization efficiency of the CO2 electrolysis system.
2 Results and discussion
The synthesis procedure for Ni-NC is illustrated in Fig. 1a, in which nickel chloride hexahydrate (NiCl2·6H2O), ethylenediamine (EDA), and carbon tetrachloride (CTC) serve as the sources of nickel, nitrogen, and carbon, respectively. The aforementioned precursors were filled into the pore structure of the mesoporous molecular sieve (SBA-15), followed by a series of processes, including refluxing, calcination, and HF etching, ultimately yielding a target catalyst that exhibits a morphology resembling a reverse replica of the SBA-15. Scanning electron microscopy (SEM, Fig. S1†) images demonstrate that both Ni-NC and NC exhibit densely packed carbon nanofibers with a consistent orientation. This arrangement provided Ni-NC with a carbon support possessing a large specific surface area, thereby facilitating the effective dispersion of Ni nanocrystals (Ni NCs). Transmission electron microscopy (TEM, Fig. 1b and S2†) images indicated that the aligned carbon nanofibers in Ni-NC and NC formed ordered mesoporous voids, confirming the successful reverse replication of the SBA-15 template. Additionally, Fig. 1b displays the lattice fringes of Ni NCs on Ni-NC with an interplanar spacing of 0.209 nm, corresponding to Ni (111). Fig. 1c illustrates high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images, which highlight the effective dispersion of Ni NCs on the carbon substrate. From the corresponding particle size distribution graph, as illustrated in Fig. 1d, it is evident that the average particle size of the Ni NCs is approximately 27.40 nm. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping of Ni-NC, shown in Fig. 1e, revealed a uniform distribution of C, N, and O elements, with Ni elements exhibiting distinct aggregation.The crystal phases of the materials were elucidated through X-ray diffraction (XRD) analysis, as depicted in Fig. 2a. Unlike the metal-free NC, the Ni-NC displayed three prominent peaks corresponding to metallic Ni, aligning with the cubic nickel phase (JCPDS PDF #04-0850). Additionally, at approximately 26°, Ni-NC exhibited a diffraction peak with higher intensity and sharpness compared to NC, which corresponded to the (002) plane of graphitic carbon.33 This enhancement is likely attributable to the catalytic effect of Ni during the thermal synthesis process, which increases the graphitization degree of Ni-NC.34–36 Further investigation of the carbon structure via Raman spectroscopy confirmed this finding, as shown in Fig. 2b. The intensity ratios of the D band (sp3 disordered carbon, 1349 cm−1) to the G band (sp2 graphitic carbon, 1583 cm−1) were 0.83 and 1.20 for Ni-NC and NC, respectively.37,38 Furthermore, the pronounced 2D band at 2675 cm−1 in the Ni-NC spectrum corroborated its graphitization characteristics. The increased degree of graphitization is beneficial for enhancing the material's electronic conductivity and chemical stability, which is crucial for electrochemical applications.39,40
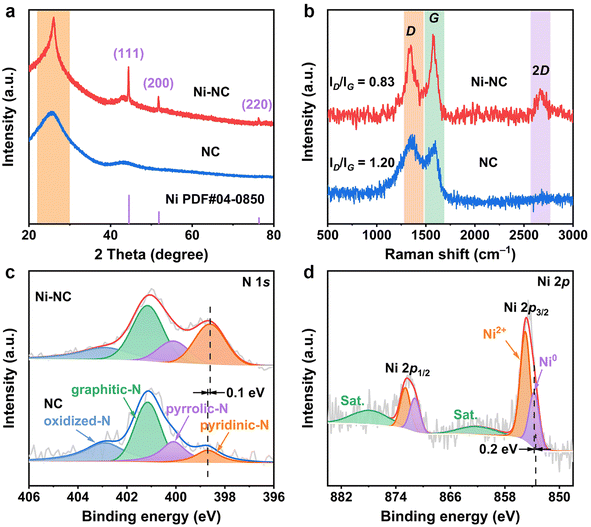 | ||
| Fig. 2 (a) XRD patterns and (b) Raman spectra of Ni-NC and NC. XPS spectra of (c) N 1s for Ni-NC and NC, and (d) Ni 2p for Ni-NC. | ||
The specific surface area and pore size distribution of the two materials were determined using automated gas adsorption methods (Fig. S3 and Table S1†). Both Ni-NC and NC exhibited typical type IV adsorption isotherms. Given that both materials utilized SBA-15 as a template, the Brunauer–Emmet–Teller method was employed to ascertain their specific surface areas, which were found to be similar: 827.3 m2 g−1 for Ni-NC and 816.5 m2 g−1 for NC. Additionally, pore size distribution calculations using density functional theory revealed that both materials exhibited a similar multi-level structure predominantly composed of mesopores, consistent with the results obtained from TEM. It has been reported that this unique porous matrix not only facilitated the dispersion of Ni NCs but also possessed excellent reactant/product mass transfer capabilities and significantly enhanced the accessibility of catalytic active Ni sites.35,41
To investigate the surface chemical states of the materials, X-ray photoelectron spectroscopy (XPS) was conducted. The comprehensive XPS spectra revealed the presence of C, N, and O in both materials, with a distinctive Ni 2p signal observed in Ni-NC (Fig. S4†). As shown in Fig. 2c, the high-resolution N 1s spectra of both materials could be deconvoluted into pyridinic-N (398.7 eV), pyrrolic-N (400.2 eV), graphitic-N (401.2 eV), and oxidized-N (402.9 eV), confirming the successful incorporation of nitrogen into the carbon matrix.42,43 It is noteworthy that the peak intensity of pyridine-N in Ni-NC is significantly enhanced, which, considering its potential as an effective active site for CO2RR, indicates that Ni-NC holds great promise for CO2RR applications.44 Furthermore, the binding energy of pyridine-N in Ni-NC is reduced by 0.1 eV compared to NC. In conjunction with the XPS spectrum of Ni 2p shown in Fig. 2d, the binding energy of Ni0 in Ni-NC (853.7 eV) exhibits a noticeable shift from the reported value of Ni0 (853.5 eV), suggesting a strong interaction between the Ni NCs and the nitrogen-doped carbon matrix.35,45
The electrocatalytic performance of Ni-NC was evaluated through a series of electrochemical measurements utilizing a three-electrode configuration. The material's CO2RR capabilities were assessed in an H-type cell containing 0.5 M KHCO3 electrolyte. Through linear sweep voltammetry (LSV, Fig. 3a) analysis, Ni-NC exhibited an enhanced current density in CO2-saturated electrolyte compared to that in an Ar atmosphere, indicating its substantial electrocatalytic activity for CO2RR.46,47 Additionally, the corresponding Tafel slope (Fig. S5†) in CO2-saturated electrolyte was 242.0 mV dec−1, demonstrating the favorable kinetic advantage of Ni-NC.48 Combined with the performance analysis of NC, this enhanced electrochemical activity could be attributed to the synergistic interaction between the catalytically active Ni sites and the highly graphitized carbon support in Ni-NC. Through gas chromatography and 1H nuclear magnetic resonance analysis of the reaction products (Fig. S6†), only gaseous products CO and H2 were detected, and no liquid-phase formation was observed. Specifically, as shown in Fig. 3b, Ni-NC consistently maintained a FECO above 90% across the broad potential ranging from −0.7 V to −1.0 V vs. RHE, with the highest value approaching 100% at −0.8 V vs. RHE, indicating an exceptionally high selectivity of Ni-NC for the sole carbon product CO. In contrast, NC achieved a maximum FECO of only 70% at −0.5 V vs. RHE. To further optimize the CO2RR performance of Ni-NC, a series of Ni-NC-X (where X represents the multiple of Ni content in the original Ni-NC, taking values of 0.1, 0.5, and 1.5) materials was synthesized with varying nickel contents. Performance evaluations revealed that Ni-NC exhibited the highest current density along with the maximum FECO (Fig. S7†). Consequently, Ni-NC was selected for all subsequent electrochemical measurements of CO2RR under the optimal synthesis conditions.
The jCO (Fig. S8†) of NC exhibited a low and gentle slope in stark contrast to Ni-NC, which maintained 94% FECO at −1.0 V vs. RHE while achieving a jCO of 16.4 mA cm−2. This performance exceeded that of several previously reported electrocatalysts for CO production from CO2RR, as illustrated in Fig. 3c (and Table S2†). The exceptional catalytic performance of Ni-NC was further elucidated through electrochemical impedance spectroscopy (EIS) and electrochemical surface area (ECSA) analysis. It was distinctly observed from the low-frequency region of the EIS (Fig. S9†) that Ni-NC exhibited the lowest charge transfer resistance (Rct), indicating that the exposed Ni NCs and highly graphitized carbon support collectively endowed Ni-NC with superior charge transport properties.49,50 Furthermore, through double-layer capacitance measurements (Fig. S10†), the ECSA values of Ni-NC and NC were determined to be 41.3 cm2 and 12.6 cm2, respectively.51 This indicates that the ordered mesoporous structure provides a substantial active surface area for the Ni NCs, while the introduced Ni NCs may play a critical role in the adsorption and activation of the reactants. Considering the aforementioned characteristics and the significant advantages demonstrated by the Tafel slope, the substantial potential of Ni-NC as a CO2RR electrocatalyst was confirmed. At −0.8 V vs. RHE, a 52 hour durability test was conducted, during which Ni-NC maintained a relatively stable current density and a FECO exceeding 95%, as shown in Fig. 3d. The structural and chemical composition of the material exhibited minimal changes before and after the test (Fig. S11†), confirming the exceptional stability of Ni-NC.
To reduce the anode energy consumption in the CO2RR system, we employed HzOR as a replacement for the sluggish OER.52,53 Simultaneously, to control the catalyst cost, the catalytic performance of Ni-NC for HzOR was evaluated in a single-pool reactor containing 1 M KOH with 0.5 M N2H4 as the electrolyte. Initially, a comparative analysis of the LSV profiles for the Ni-NC and Ni-NC-X series was performed to optimize the nickel content (Fig. S12†). The results demonstrate that Ni-NC exhibits superior HzOR performance. Accordingly, under the optimal synthesis conditions, Ni-NC was utilized for all subsequent electrochemical measurements related to HzOR. As shown in Fig. 3e, the LSV analysis of NC indicates that the ordered mesoporous structure exhibits commendable HzOR activity. The introduction of Ni NCs led to a synergistic interaction with the highly graphitized carbon support, which further enhanced the HzOR activity of Ni-NC, allowing it to achieve a current density of 10 mA cm−2 at only 0.66 V vs. RHE, which was significantly lower than the 1.70 V vs. RHE required to drive the same current density in water oxidation reactions (1 M KOH as the electrolyte). The corresponding Tafel slope demonstrates that Ni-NC has a slope of only 211.3 mV dec−1 (Fig. S13†), highlighting its excellent HzOR kinetics.54,55
The HzOR activity of Ni-NC was further assessed using turnover frequency (TOF), EIS, and ECSA. A comparison of the two sets of TOF curves indicates (Fig. S14†) that the intrinsic HzOR activity of Ni-NC surpasses that of NC, consistent with the HzOR measurement results.56,57 Additionally, EIS analysis (Fig. S15†) reveals that Ni-NC exhibits the smallest curvature radius, confirming its exceptional alkaline HzOR activity.58 Furthermore, the significant differences observed in ECSA (Fig. S16†) are analogous to the results obtained from CO2RR, highlighting that the introduction of Ni NCs can facilitate the rapid occurrence of HzOR.59 A larger ECSA also confirms that it has more catalytic active sites. The long-term stability of the material was assessed through the examination of LSV profiles before and after prolonged electrolysis, as well as the characterization of the Ni-NC's morphology and composition (Fig. 3f and S17†). The results indicated that, at a current density of 10 mA cm−2, Ni-NC provided a relatively stable current response for HzOR over 12 hours, with minimal changes observed in the LSV profiles before and after durability testing. The material retained its original structure and chemical composition even after extended electrolysis, demonstrating that Ni-NC possesses stable catalytic performance.
The application of in situ FTIR spectroscopy enables the continuous monitoring of the behavior of reaction intermediates adsorbed on the Ni-NC catalytic interface. As illustrated in Fig. 4a, all prominent bands between 1275 and 1668 cm−1 is attributed to the *COOH.60 As the negative reduction potential increases, the *COOH signal progressively intensifies, indicating that *CO2 can be continuously converted into *COOH on the Ni-NC. Moreover, the infrared absorption band at 2078 cm−1, associated with the chemisorption of CO/*CO, further elucidated the subsequent transformation process of *COOH.61 For HzOR, as shown in Fig. 4b, the H–N–H bending vibration appears around 1442 cm−1, while the –NH2 is observed at approximately 1281 cm−1.62 With the increase in positive oxidation potential, both signals gradually strengthen, suggesting that *N2H4 can be continuously converted into *N2 at the Ni-NC catalytic interface.
The mechanisms of CO2RR and HzOR on Ni-NC were systematically investigated using DFT calculations. The model for the active site of Ni-NC is constructed with a single Ni NC anchored on the N-doped carbon substrate, whose density of states demonstrates favorable bonding interactions and electrical conductivity (Fig. S18†). Through differential charge density analysis (Fig. S19†), a detailed understanding of the electronic interactions at the interface between the Ni NCs and the N-doped carbon support was gained. The differential charge density distribution revealed a significant charge transfer from the Ni NCs to the N-doped carbon support in the optimized Ni-NC structure, suggesting a strong interfacial interaction between the metal and the support. Subsequently, the free energy changes of steps in the CO2RR and HzOR were evaluated on the Ni-NC and N-doped carbon (NC) model surfaces based on reaction pathways established from the relevant literature and in situ FTIR data.62,63 As shown in Fig. 4c, the rate-limiting step for CO2RR on Ni-NC is the desorption of *CO, with a Gibbs free energy (ΔG) of 0.96 eV, which is lower than the rate-determining reaction (*COOH formation, 1.20 eV) on NC. Furthermore, compared to NC, the synergy between Ni NCs and the N-doped carbon substrate significantly reduces the ΔG for CO2 adsorption and *COOH formation on Ni-NC. For HzOR, extensive model optimization calculations (Fig. S20†) indicate that NC has a weak adsorption affinity for N2H4 and N2.64,65 In contrast, the incorporation of Ni NCs renders the HzOR steps on Ni-NC thermodynamically spontaneous, as illustrated in Fig. 4d, underscoring its superior HzOR activity.
Given the excellent performance of Ni-NC in the independent CO2RR and HzOR processes, it was used as both the anode and cathode materials to assemble a two-electrode HECR system in an H-type cell (Fig. S21†) to verify the feasibility of the energy-efficient HzOR‖CO2RR. Fig. 5a illustrates the polarization curves comparing HzOR‖CO2RR with the conventional OER‖CO2RR. Compared to OER‖CO2RR, the voltage required to drive a current density of 10 mA cm−2 in HzOR‖CO2RR under cathodic CO2 saturation is significantly reduced by 1.1 V, theoretically saving a remarkable 33% of energy consumption.66,67 Further verification through polarization curves under cathodic Ar saturation confirms that HzOR, as an alternative reaction to OER, plays a dominant role in lowering the cell voltage of the HECR system. To ensure the exceptional electrocatalytic performance of the cathodic Ni-NC in HzOR‖CO2RR, precise voltage control for regulating FECO is imperative. As shown in Fig. 5b, HzOR‖CO2RR maintains a cathodic FECO above 83% within the voltage range of 1.2 to 2.2 V, reaching 91% at 1.8 V. In contrast, the FECO of OER‖CO2RR barely reaches 79% at a higher voltage of 2.2 V. The above results confirm that substituting the traditional OER with the thermodynamically more favorable HzOR significantly reduces the overall reaction voltage of CO2RR. This results in improved cathodic CO selectivity at low cell voltages, leading to a substantial increase in the system's CO2 electroreduction efficiency.65 In addition, a HzOR‖CO2RR system in an H-type cell was prepared using NC as both the anode and cathode materials, whose polarization curve showed a significant lag compared to the HECR system employing Ni-NC as the electrode material (Fig. S22†). Furthermore, at the same cell voltage, the FECO of the Ni-NC-based HECR system surpassed that of the NC-based system by about 79%, as illustrated in Fig. 5c. This result strongly demonstrates the superior electrocatalytic activity of Ni-NC as an electrode material for HECR. At a cell voltage of 1.8 V, the durability of Ni-NC as an electrode material in the HECR system was validated (Fig. S23†). Over a prolonged 60 h stability test, its cathodic FECO consistently remained above 90%, with periodic electrolyte refreshes having a negligible impact on this system's stability. To the best of our knowledge, this work is one of the rare documented instances of HzOR-assisted energy-saving CO2RR, thereby validating the feasibility of such HECR systems.68
As shown in Fig. 5d, the HzOR‖CO2RR system was further upgraded by employing Ni-NC as the anode and cathode catalysts, integrated into a membrane electrode assembly (MEA) with a zero-gap configuration, utilizing an anion exchange membrane (AEM). The polarization curves of the conventional OER‖CO2RR and HzOR‖CO2RR systems obtained on this setup, as depicted in Fig. 5e, revealed that under the minimization of ohmic resistance by the MEA, the onset voltage of HzOR‖CO2RR significantly decreased with the introduction of N2H4 into the anode feed, and the current density was much higher than that of OER‖CO2RR. Within a given voltage range, the required cell voltages for OER‖CO2RR to achieve current densities of 10, 20, and 30 mA cm−2 were 3.9, 4.4, and 4.8 V, respectively. In contrast, the HzOR‖CO2RR system reached the same current densities with voltage reductions of 2.7, 2.6, and 2.4 V, achieving a maximum energy savings of 69% at 10 mA cm−2. As shown in Fig. 5f, analysis of the cathodic products of HzOR‖CO2RR indicated that the FECO remained nearly constant across different current densities, maintaining approximately 90%, and reached a peak value of 93.8% at 30 mA cm−2. To assess the feasibility at higher current densities, the electrochemical stability of the HzOR‖CO2RR system was investigated (Fig. S24†). The HzOR‖CO2RR system, with Ni-NC as the catalyst, exhibited excellent stability, maintaining FECO above 91% over 300 minutes at a higher current density of 30 mA cm−2. It is worth noting that the simple implementation of the HzOR‖CO2RR system in the MEA setup in this work did not include the design of other coupling system components, such as the cathodic gas diffusion electrode, anode membrane, solid electrolyte, and interfaces.69,70 Future efforts will focus on addressing these issues.
3 Conclusions
In summary, we report the development of Ni nanocrystals supported on nitrogen-doped ordered mesoporous carbon for CO production via CO2RR. The Ni-NC exhibits exceptional CO2RR performance, achieving nearly 100% FECO at −0.8 V vs. RHE, and demonstrates robust stability over 52 hours of operation. XPS, in situ FTIR, and DFT calculations reveal that the synergistic interaction between Ni nanocrystals and the nitrogen-doped carbon matrix enhances CO2 adsorption and promotes the formation of the *COOH intermediate, facilitating CO2 electroreduction. Additionally, the ordered mesoporous structure and enhanced graphitization of the carbon substrate improve the accessibility of catalytic Ni sites and boost electronic conductivity. Moreover, leveraging the thermodynamically spontaneous properties of HzOR on Ni-NC and considering catalyst cost, we constructed an efficient hydrazine-assisted CO2RR system with Ni-NC as both the anode and cathode. Compared to conventional water oxidation coupled with CO2RR, the HzOR‖CO2RR system achieves 69% energy savings, a FECO of over 90%, and excellent electrochemical stability in MEA. This work provides a valuable strategy for designing efficient CO2-to-CO electrocatalysts and offers a promising solution for profitable CO2 electrolysis systems.Data availability
Data will be made available on request.Author contributions
Kang Lian: writing – original draft, data curation. Kang Lian: data curation. Junyang Ding: data curation. J. Zhang: data curation, investigation, writing – review & editing. Quan Zhang: investigation, writing – review & editing. Yifan Liu: investigation. Guangzhi Hu: validation. Jin Zhang: writing – review & editing, conceptualization. Quan Zhang: writing – review & editing, software. Jia He: data curation, software. Xijun Liu: writing – review & editing, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the Guangxi Natural Science Fund for Distinguished Young Scholars (2024GXNSFFA010008), National Natural Science Foundation of China (22469002), and the Shenzhen Science and Technology Program (JCYJ20230807112503008).References
- L. Li, X. Li, Y. Sun and Y. Xie, Chem. Soc. Rev., 2022, 51, 1234–1252 RSC.
- X. Tan, C. Yu, Y. Ren, S. Cui, W. Li and J. Qiu, Energy Environ. Sci., 2021, 14, 765–780 RSC.
- D. Beillouin, M. Corbeels, J. Demenois, D. Berre, A. Boyer, A. Fallot, F. Feder and R. Cardinael, Nat. Commun., 2023, 14, 3700 CrossRef CAS PubMed.
- K. Chen, D. Ma, Y. Zhang, F. Wang, X. Yang, X. Wang, H. Zhang, X. Liu, R. Bao and K. Chu, Adv. Mater., 2024, 36, e2402160 CrossRef PubMed.
- J. Chen, D. Wang, X. Yang, W. Cui, X. Sang, Z. Zhao, L. Wang, Z. Li, B. Yang, L. Lei, J. Zheng, L. Dai and Y. Hou, Angew. Chem., Int. Ed., 2023, 62, e202215406 CrossRef CAS PubMed.
- J. E. Huang, F. Li, A. Ozden, A. S. Rasouli, F. P. G. d. Arquer, S. Liu, S. Zhang, M. Luo, X. Wang, Y. Lum, Y. Xu, K. Bertens, R. Miao, C. T. Dinh, D. Sinton and E. H. Sargent, Science, 2021, 372, 1074–1078 CrossRef CAS PubMed.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Nørskov, T. F. Jaramillo and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS.
- W. Zheng, X. Yang, Z. Li, B. Yang, Q. Zhang, L. Lei and Y. Hou, Angew. Chem., Int. Ed., 2023, 62, e202307283 CrossRef CAS PubMed.
- R. G. Grim, Z. Huang, M. T. Guarnieri, J. R. Ferrell, L. Tao and J. A. Schaidle, Energy Environ. Sci., 2020, 13, 472–494 RSC.
- N. Han, P. Ding, L. He, Y. Li and Y. Li, Adv. Energy Mater., 2019, 10, 1902338 CrossRef.
- S. M. Han, M. Park, J. Kim and D. Lee, Angew. Chem., Int. Ed., 2024, 63, e202404387 CrossRef CAS.
- Q. Chen, P. Tsiakaras and P. Shen, Catalysts, 2022, 12, 1348 CrossRef CAS.
- D. L. T. Nguyen, H. H. Do, M. T. Nguyen, D. V. N. Vo, V. H. Nguyen, C. C. Nguyen, S. Y. Kim and Q. V. Le, Chem. Eng. Sci., 2021, 234, 116403 CrossRef CAS.
- S. Zheng, Y. Liu, X. Yang, Z. Zhao, Q. Zhang, B. Yang, Z. Li, C. Dong, L. Lei and Y. Hou, Chem. Eng. Sci., 2024, 298, 120314 CrossRef CAS.
- W. Peng, F. Li, S. Kong, C. Guo, H. Wu, J. Wang, Y. Shen, X. Meng and M. Zhang, Carbon Energy, 2024, 6, e498 CrossRef.
- D. M. Koshy, S. Chen, D. U. Lee, M. B. Stevens, A. M. Abdellah, S. M. Dull, G. Chen, D. Nordlund, A. Gallo, C. Hahn, D. C. Higgins, Z. Bao and T. F. Jaramillo, Angew. Chem., Int. Ed., 2020, 59, 4043–4050 CrossRef CAS PubMed.
- Y. Zhou, Q. Zhou, H. Liu, W. Xu, Z. Wang, S. Qiao, H. Ding, D. Chen, J. Zhu, Z. Qi, X. Wu, Q. He and L. Song, Nat. Commun., 2023, 14, 3776 CrossRef.
- J. Yang, Z. Qiu, C. Zhao, W. Wei, W. Chen, Z. Li, Y. Qu, J. Dong, J. Luo, Z. Li and Y. Wu, Angew. Chem., Int. Ed., 2018, 57, 14095–14100 CrossRef CAS PubMed.
- Y. Luo, Z. Zhang, F. Yang, J. Li, Z. Liu, W. Ren, S. Zhang and B. Liu, Energy Environ. Sci., 2021, 14, 4610–4619 RSC.
- L. C. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS.
- W. Zheng, D. Wang, W. Cui, X. Sang, X. Qin, Z. Zhao, Z. Li, B. Yang, M. Zhong, L. Lei, Q. Zheng, S. Yao, G. Wu and Y. Hou, Energy Environ. Sci., 2023, 16, 1007–1015 RSC.
- Y. Tong, J. Liu, L. Wang, B. Su, K. Wu, J. Juang, F. Hou, L. Yin, S. Dou, J. Liu and J. Liang, Adv. Funct. Mater., 2022, 32, 2205654 CrossRef CAS.
- Y. Li, L. Zeng, Z. Zhao, Q. Zhang and Y. Hou, AIChE J., 2024, 70, e18397 CrossRef CAS.
- Y. Zhu, C. Guo, Y. Zheng and S. Qiao, Acc. Chem. Res., 2017, 50, 915–923 CrossRef CAS.
- W. Liu, W. Que, R. Yin, J. Dai, D. Zheng, J. Feng, X. Xu, F. Wu, W. Shi, X. Liu and X. Cao, Appl. Catal., B, 2023, 328, 122488 CrossRef CAS.
- B. Cui, C. Wang, S. Huang, L. He, S. Zhang, Z. Zhang and M. Du, J. Colloid Interface Sci., 2020, 578, 10–23 CrossRef CAS PubMed.
- J. Tian, C. Cao, D. Ma, S. Han, Y. He, X. Wu and Q. Zhu, Small Struct., 2021, 3, 2100134 CrossRef.
- S. Sun, L. Dong, J. Li, J. Shi, J. Liu, Y. Wang, Q. Huang and Y. Lan, Angew. Chem., Int. Ed., 2022, 61, e202207282 CrossRef CAS PubMed.
- T. Wang, X. Cao and L. Jiao, Angew. Chem., Int. Ed., 2022, 61, e202213328 CrossRef CAS PubMed.
- S. Ma, B. Yu, B. Xia and B. You, Sci. China Mater., 2024, 67, 752–761 CrossRef CAS.
- H. Jin, X. Wang, C. Tang, A. Vasileff, L. Li, A. Slattery and S. Qiao, Adv. Mater., 2021, 33, e2007508 CrossRef.
- W. Liu, W. Liu, T. Hou, J. Ding, Z. Wang, R. Yin, X. San, L. Feng, J. Luo and X. Liu, Nano Res., 2024, 17, 4797–4806 CrossRef CAS.
- S. Du, B. Huang, L. Zhu, Y. Wu, J. Huang, Z. Li, H. Yu and J. Xiao, Carbon, 2023, 215, 118451 CrossRef CAS.
- S. Liang, Q. Jiang, Q. Wang and Y. Liu, Adv. Energy Mater., 2021, 11, 2101477 CrossRef CAS.
- L. Wang, Y. Kong, H. Cai, J. Sun, X. Jiang, X. Li, Q. Hu, H. Yang and C. He, J. Mater. Chem. A, 2024, 12, 16403–16409 RSC.
- Y. Li, N. M. Adli, W. Shan, M. Wang, M. J. Zachman, S. Hwang, H. Tabassum, S. Karakalos, Z. Feng, G. Wang, Y. C. Li and G. Wu, Energy Environ. Sci., 2022, 15, 2108–2119 RSC.
- X. Liu, M. Chen, J. Ma, J. Liang, C. Li, C. Chen and H. He, China Powder Sci. Technol., 2024, 30, 35–45 Search PubMed.
- J. Yang, F. Zhang and J. Chen, China Powder Sci. Technol., 2024, 30, 161–170 Search PubMed.
- Z. Li, B. Li, C. Yu, H. Wang and Q. Li, Adv. Sci., 2023, 10, 2206605 CrossRef CAS.
- L. Jiao, W. Yang, G. Wan, R. Zhang, X. Zheng, H. Zhou, S. H. Yu and H. L. Jiang, Angew. Chem., Int. Ed., 2020, 59, 20589–20595 CrossRef CAS PubMed.
- Z. Luo, Z. Yin, J. Yu, Y. Yan, B. Hu, R. Nie, A. F. Kolln, X. Wu, R. K. Behera, M. Chen, L. Zhou, F. Liu, B. Wang, W. Huang, S. Zhang and L. Qi, Small, 2022, 18, e2107799 CrossRef.
- Y. Shu, X. Song, F. Lan, C. Zhao, Q. Guan and W. Li, ACS Catal., 2022, 12, 15386–15399 CrossRef CAS.
- T. Wang, X. Sang, W. Zheng, B. Yang, S. Yao, C. Lei, Z. Li, Q. He, J. Lu, L. Lei, L. Dai and Y. Hou, Adv. Mater., 2020, 32, 2002430 CrossRef CAS PubMed.
- Q. Lu, C. Chen, Q. Di, W. Liu, X. Sun, Y. Tuo, Y. Zhou, Y. Pan, X. Feng, L. Li, D. Chen and J. Zhang, ACS Catal., 2022, 12, 1364–1374 CrossRef CAS.
- C. Jia, S. Li, Y. Zhao, R. K. Hocking, W. Ren, X. Chen, Z. Su, W. Yang, Y. Wang, S. Zheng, F. Pan and C. Zhao, Adv. Funct. Mater., 2021, 31, 2107072 CrossRef CAS.
- Y. Zhang, Z. Li, K. Chen, X. Yang, H. Zhang, X. Liu and K. Chu, Adv. Energy Mater., 2024, 2402309 CrossRef.
- Y. Qi, X. Hou, Z. He, F. He, T. Wei, G. Meng, H. Hu, Q. Liu, G. Hu and X. Liu, Chem. Commun., 2024, 60, 8728–8731 RSC.
- F. Lü, H. Bao, Y. Mi, Y. Liu, J. Sun, X. Peng, Y. Qiu, L. Zhuo, X. Liu and J. Luo, Sustain. Energy Fuels, 2020, 4, 1012–1028 RSC.
- Y. Niu, C. Zhang, Y. Wang, D. Fang, L. Zhang and C. Wang, ChemSusChem, 2021, 14, 1140–1154 CrossRef CAS.
- R. He, A. Zhang, Y. Ding, T. Kong, Q. Xiao, H. Li, Y. Liu and J. Zeng, Adv. Mater., 2018, 30, 1705872 Search PubMed.
- Y. Mi, S. Shen, X. Peng, H. Bao, X. Liu and J. Luo, ChemElectroChem, 2019, 6, 2393–2397 Search PubMed.
- H. Wu, G. Fei, X. Gao, X. Guo, X. Gong, X. Ma, Q. Wang and S. Xu, China Powder Sci. Technol., 2023, 29, 70–80 Search PubMed.
- T. Xie, X. He, L. He, K. Dong, Y. Yao, Z. Cai, X. Liu, X. Fan, T. Li, D. Zheng, S. Sun, L. Li, W. Chu, A. Farouk, M. S. Hamdy, C. Xu, Q. Kong and X. Sun, Chin. Chem. Lett., 2024, 35, 110005 Search PubMed.
- C. Zhang, H. Xu, M. An, Y. Wang, Z. Yuan, W. Zhang, C. Li, M. Guo and D. Su, China Powder Sci. Technol., 2023, 29, 80–93 Search PubMed.
- P. Liu, Y. Liu, K. Wang, S. Shi, M. Jin, J. Liu, T. Qin, Q. Liu, X. Liu and J. He, Nano Res., 2024, 17, 7957–7966 CrossRef CAS.
- X. Liu, J. He, S. Zhao, Y. Liu, Z. Zhao, J. Luo, G. Hu, X. Sun and Y. Ding, Nat. Commun., 2018, 9, 4365 Search PubMed.
- D. Merki, S. Fierro, H. Vrubel and X. Hu, Chem. Sci., 2011, 2, 1262–1267 RSC.
- C. Tang, R. Zhang, W. Lu, Z. Wang, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 842–846 CrossRef CAS PubMed.
- C. Chen, M. Sun, F. Zhang, H. Li, M. Sun, P. Fang, T. Song, W. Chen, J. Dong, B. Rosen, P. Chen, B. Huang and Y. Li, Energy Environ. Sci., 2023, 16, 1685–1696 RSC.
- R. Niu, Q. Liu, B. Huang, Z. Liu, W. Zhang, Z. Peng, Z. Wang, Y. Yang, Z. Gu and J. Li, Appl. Catal., B, 2022, 317, 121727 Search PubMed.
- S. Zhu, B. Jiang, W. B. Cai and M. Shao, J. Am. Chem. Soc., 2017, 139, 15664 CrossRef CAS.
- H. Y. Wang, L. Wang, J. T. Ren, W. W. Tian, M. L. Sun and Z. Y. Yuan, Nano-Micro Lett., 2023, 15, 155 CrossRef CAS.
- J. Li, W. Y. Zan, H. Kang, Z. Dong, X. Zhang, Y. Lin, Y. W. Mu, F. Zhang, X. M. Zhang and J. Gu, Appl. Catal., B, 2021, 298, 120510 CrossRef CAS.
- S. Fu, Y. Li and Y. Wang, China Powder Sci. Technol., 2024, 30, 27–40 Search PubMed.
- X. Luo, Y. Wu, H. Hu, T. Wei, B. Wu, J. Ding, Q. Liu, J. Luo and X. Liu, Small, 2024, 20, 2403399 Search PubMed.
- L. Te, L. Tao, D. Ao and C. Gao, China Powder Sci. Technol., 2024, 30, 46–55 Search PubMed.
- J. Ding, Z. Peng, Z. Wang, C. Zeng, Y. Feng, M. Yang, G. Hu, J. Luo and X. Liu, J. Mater. Chem. A, 2024, 12, 28023–28031 Search PubMed.
- C. Hu, Y. Zhang, A. Hu, Y. Wang, X. Wei, K. Shen, L. Chen and Y. Li, Adv. Mater., 2023, 35, 2209298 Search PubMed.
- W. Ma, T. Jian, J. Ma, X. Li, H. Gao and H. Liu, China Powder Sci. Technol., 2024, 30, 1–14 Search PubMed.
- Q. Cheng, M. Huang, Q. Ye, B. Deng and F. Dong, Chin. Chem. Lett., 2024, 35, 109112 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06503d |
| This journal is © The Royal Society of Chemistry 2025 |




