Open Access Article
Open Access ArticleFluoride-catalysed reduction of glucose using silicon powder as a reducing agent
Toa Kojoa,
Ken Motokura b and
Atsushi Takagaki
b and
Atsushi Takagaki *b
*b
aDepartment of Chemistry, Chemical Engineering and Life Science, College of Engineering Science, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan
bDivision of Materials Science and Chemical Engineering, Faculty of Engineering, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama 240-8501, Japan. E-mail: takagaki-atsushi-gw@ynu.ac.jp
First published on 17th November 2025
Abstract
As a means of simultaneously utilizing biomass and waste silicon from used solar panels, this work assessed the reduction of glucose to sorbitol using silicon powder as a reducing agent. Tetrabutylammonium fluoride was found to catalyse this process in conjunction with water as a proton donor. The addition of an acid also suppressed the undesired base-catalysed side reactions, resulting in an improved sorbitol yield.
Sustainability spotlightPhotovoltaic power generation is a clean energy source that does not depend on fossil fuels and can contribute to climate change mitigation. Most of the solar panels currently in use are based on silicon, which poses a problem of disposal at the end of their useful life. We have proposed the use of silicon as a chemical reductant, and in this study, we applied it to the reduction of glucose as a model compound for biomass resources. The present work aligns with several sustainable development goals including UN SDG 7 (affordable and clean energy), UN SDG12 (responsible consumption and production), and UN SDG 13 (climate action). |
Introduction
Biomass is considered to be an essentially carbon neutral resource because although these materials emit carbon dioxide when burned, this carbon dioxide was originally removed from the atmosphere through photosynthesis.1 It is also possible to synthesize chemical products from biomass in a process referred to as biorefinement.2 These syntheses are based on reduction reactions, as opposed to the oxidation reactions used in oil refinement. These reduction processes are required because raw biomass compounds such as sugars contain a high proportion of oxygen.3 As an example, glucose (the most abundant monosaccharide obtained from cellulose via hydrolysis) can be reduced to obtain sorbitol.4–7 The latter compound is widely used as a food additive and in cosmetics and pharmaceuticals. In addition, isosorbide can be produced from sorbitol via a cyclodehydration reaction.8–10 A subsequent polymerization reaction can be used to synthesize polycarbonate resins that have commercial applications as bio-based engineering plastics.11,12Solar power has been increasingly used worldwide over the last several decades as a source of renewable energy.13 However, the safe disposal of used solar panels remains problematic. In particular, the high-purity silicon contained in crystalline silicon solar panels has low economic value, and effective recycling methods have yet to be established.14 One possible means of mitigating these issues could be to utilize recovered silicon powder as a powerful reducing agent for a CO2-to-formic acid reaction using a fluoride-based catalyst.15,16 This same general process can also be used to generate methanol from CO2 and to produce formamide from CO2 and an amine.17,18 These reactions typically use tetrabutylammonium fluoride (TBAF·3H2O) as the fluoride-based catalyst to reduce CO2 in an aprotic polar solvent such as N-methyl-2-pyrrolidone (NMP), incorporating water as a source of protons.
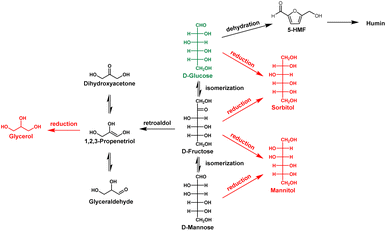
The work reported herein demonstrates that a reaction system using silicon powder is applicable to the reduction of glucose, with the latter compound serving as a model for biomass utilization. Scheme 1 shows a typical reaction pathway for glucose conversion. While the purpose of this study was the reduction of glucose to sorbitol using silicon as a reducing agent, glucose is also very easily converted to other compounds by acids and bases.19 As an example, the isomerization of glucose yields fructose and mannose, both of which can be reduced to mannitol.20,21 In addition, the retro-aldol reactions of these hexoses yield trioses that can subsequently be reduced to glycerol.22 In addition, 5-hydroxymethylfurfural can be generated via a dehydration process23 and can then condense to a solid material known as humin. Thus, in contrast to previous studies in which CO2 was reduced using silicon, in the present work, it was important to control the reaction conditions so that acid–base reactions were minimized as much as possible.
The reduction of glucose was carried out using a fluoride-based catalyst in a solvent containing glucose, silicon powder, water and an organic acid in a stainless-steel reactor vessel. Each reaction was performed at 120 °C for 18 h with stirring, and the liquid supernatant was subsequently analysed using two high-performance liquid chromatography (HPLC) systems (see the SI for details). Monocrystalline silicon wafers (solar grade, Si: >99.9999%) rejected during solar panel production were used. These wafers were crushed to powder in an alumina mortar and then passed through a 40 µm mesh sieve. The Brunauer–Emmett–Teller (BET) surface area of silicon powder was 5 m2 g−1.
Results and discussion
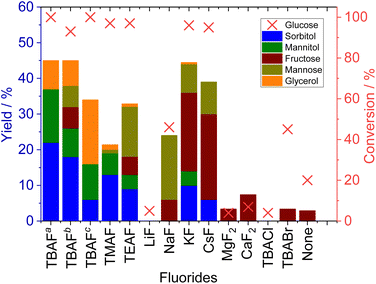
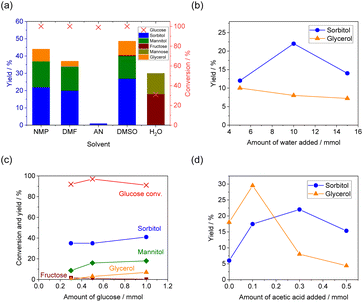
The results of glucose conversion trials using various fluoride-based catalysts are summarized in Fig. 1 (data are available in Table S1). The organic fluoride salt TBAF·3H2O exhibited the highest activity for glucose reduction among the organic and inorganic fluoride-based compounds that were assessed. Similar results were obtained in prior work based on CO2 conversion using metallic silicon as a reducing agent.15–17 The reduction products obtained using TBAF·3H2O included sorbitol, mannitol and glycerol, with the former as the main product. Although the glucose in the reaction mixture was almost 100% converted, the total product yield was only 45%, indicating that other undetected products were also generated. These compounds are thought to have been various polymers formed as a solid residue. Decreasing the amount of TBAF·3H2O in the reaction mixture to 0.05 mmol (TBAFb in Fig. 1) decreased the yields of sorbitol and mannitol and also resulted in the formation of hexose isomers fructose and mannose. Among the inorganic fluorides, LiF provided only a very low conversion of glucose, whereas NaF solely afforded hexose isomers together with approximately 50% glucose conversion. Both KF and CsF exhibited good activity with high levels of glucose conversion. Similar to LiF, MgF2 and CaF2 showed no activity for the reduction of glucose. TBACl and TBABr, which contained halides other than fluoride, were inactive for the reaction, confirming that fluoride anions were required to facilitate the glucose reduction process. Organic fluorides with shorter carbon chains like TMAF and TEAF showed lower activity, while TBAF yielded better results. Similar findings have been reported in the reduction of carbon dioxide.17 As the carbon chain lengthens, the hydrophobicity of the organic cation increases, allowing the fluoride ion to exist more freely, which is thought to enhance the reactivity.The effects of different solvents on the glucose reduction reaction are shown in Fig. 2(a) (Table S2). With the exception of acetonitrile (AN), aprotic solvents were found to be suitable for the reaction based on the ability of these compounds to donate electrons to the activated silane groups as well as to dissolve sugars. While glucose dissolved completely in other solvents shown here, it did not dissolve in acetonitrile. Although trace amount of water was added to the reaction, the failure of glucose to dissolve in the solvent is a major cause of the low activity. Furthermore, previous studies using silicon and TBAF for CO2 reduction reactions have also reported very low activity in acetonitrile.15,17 Dimethyl sulfoxide (DMSO) is sometimes considered as an alternative to more toxic solvents such as NMP and dimethylformamide (DMF) and so was assessed in this work.24 Although a slightly higher yield of sorbitol was obtained in DMSO, significant amounts of polymerized solids were also generated, exceeding the quantities produced when using NMP. Employing water as the solvent provided a low glucose conversion and no reduction products. This outcome can likely be attributed to the hydration and therefore stabilization of fluoride ions in water, as these ions failed to attack the silicon surfaces.
Aprotic solvents such as NMP were suitable for the reaction, and no reduced products were obtained at all in large amounts of water. However, water was necessary as a proton source in this reaction system.15–17 The effects of water as a proton source on the glucose reduction reaction in NMP solvent are shown in Fig. 2(b). The sorbitol yield reached its optimal value when 10 mmol of water was added. However, excess water adversely affected the sorbitol yield. Similar results were observed in the reduction reaction from carbon dioxide to formic acid,15 likely due to the enhanced solvation effect of TBAF·3H2O, which reduced the activity of the fluoride anion as a catalyst. The effects of amount of glucose on the product yield and selectivity are shown in Fig. 2(c). Varying the glucose amount from 0.3 to 1.0 mmol resulted in conversion exceeding 90% in all cases. The yields of sorbitol, mannitol, and glycerol all increased slightly with increasing glucose amounts. For the reaction using 1.0 mmol of glucose, the yield of sorbitol was 41%, mannitol was 18%, and glycerol was 6.9%. Based on the produced sorbitol and mannitol, the catalyst turnover number was 11.8, indicating that the fluoride catalytically promoted the reaction.
It should be noted that the addition of acetic acid significantly increased the production of sorbitol (Fig. 2(d) (Table S3)). As shown in the third trial using TBAF in Fig. 1 (TBAFc), the sorbitol yield was only 5% without acetic acid, with three times as much glycerol formed as sorbitol. However, as the amount of acetic acid was increased, the sorbitol yield increased, whereas the glycerol yield dropped. Adding acetic acid at molar equivalence to the TBAF catalyst increased the sorbitol yield to 20% and reduced the glycerol yield to 5%, whereas further increases in the proportion of acetic acid decreased the sorbitol yield. In the proposed reaction pathway (Scheme 1), glycerol is generated via the reduction of a triose that, in turn, is formed by glucose isomerization and a subsequent retro-aldol reaction.22 The addition of acetic acid inhibited this undesired reaction pathway, resulting in an increased yield of sorbitol. The addition of acid is thought to suppress the conversion of sugar by base, as it promotes a neutralization reaction with either the fluoride anion or hydroxide anion.
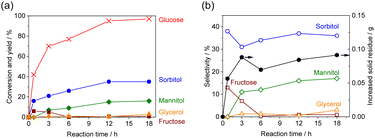
The time course of a reaction is presented in Fig. 3 (Tables S4 and S5). After 45 min, 42% of glucose was converted along with the formation of sorbitol with 38% selectivity and fructose with 13% selectivity. A total of 0.02 g of original glucose was converted to products other than sorbitol and fructose and 0.05 g of solid residue was found, suggesting that the remaining glucose had been converted to various polymers. An attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectrum of the dried solution after the reaction showed two absorption peaks at 1609 and 1714 cm−1 (Fig. S1). These peaks originated from carbonyl groups conjugated with a carbon–carbon double bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, indicating the formation of humin as condensed polymers from glucose.25,26 The discrepancy between 0.02 g and 0.05 g is ascribed to the presence of a small amount of residual solvent in the residue. After 45 min, the sorbitol yield increased with reaction time. It should be noted that the selectivity for sorbitol was nearly constant, indicating that sorbitol was the primary product and that byproducts such as glycerol were not generated from sorbitol. As the reaction time was increased, the yield of fructose decreased, whereas mannitol and glycerol began to be produced. These results confirm that the latter two byproducts were formed from the reduction of fructose, in good agreement with the proposed reaction pathway. In the reduction of glucose to sorbitol, the cyclic glucose ring opens and undergoes direct reduction. Meanwhile, mannose produced as a byproduct is suggested to form via fructose as an intermediate, as indicated by the change in product selectivity over time during the reaction. At 120 °C, most glucose had converted after 12 h, thus reaction at lower temperatures was investigated (Fig. S2). At 95 °C, the glucose conversion was 29% after 18 h and only 66% after 96 h. The sorbitol yield was 18% with the selectivity of 29% after 96 h. The sorbitol selectivity was lower than that observed at 120 °C.
O, indicating the formation of humin as condensed polymers from glucose.25,26 The discrepancy between 0.02 g and 0.05 g is ascribed to the presence of a small amount of residual solvent in the residue. After 45 min, the sorbitol yield increased with reaction time. It should be noted that the selectivity for sorbitol was nearly constant, indicating that sorbitol was the primary product and that byproducts such as glycerol were not generated from sorbitol. As the reaction time was increased, the yield of fructose decreased, whereas mannitol and glycerol began to be produced. These results confirm that the latter two byproducts were formed from the reduction of fructose, in good agreement with the proposed reaction pathway. In the reduction of glucose to sorbitol, the cyclic glucose ring opens and undergoes direct reduction. Meanwhile, mannose produced as a byproduct is suggested to form via fructose as an intermediate, as indicated by the change in product selectivity over time during the reaction. At 120 °C, most glucose had converted after 12 h, thus reaction at lower temperatures was investigated (Fig. S2). At 95 °C, the glucose conversion was 29% after 18 h and only 66% after 96 h. The sorbitol yield was 18% with the selectivity of 29% after 96 h. The sorbitol selectivity was lower than that observed at 120 °C.
The incorporation of acetic acid or other acids was found to increase the sorbitol yield (Fig. 4 (Table S6)). The addition of each organic acid in an amount equimolar to the amount of TBAF resulted in an increase in the sorbitol yield. Interestingly, there was also a correlation between the product selectivity and the pKa value of the acid. Formic acid (pKa = 3.77) was found to exhibit the best performance, providing a 43% sorbitol yield at 98% glucose conversion. The order of sorbitol yields was roughly correlated with the pKa values of acids with the exception of pyruvic acid (pKa = 2.39). These data indicated that both the amount of acid and the acid strength determined the extent to which base-catalysed side reactions were suppressed. Stronger acids were additionally found to provide lower yields of glycerol, suggesting that these acids inhibited the retro-aldol reaction to a greater extent. In this study, the molar amount of acid added was kept constant for all samples. The fact that acids have different pKa values means that even when the same molar amount of acid is added, the amount of protons released varies depending on the pKa. In this study, acid addition suppressed the reactions catalysed by bases, such as isomerization and retro-aldol reactions. Even with the same molar amount of acid, acids with a smaller pKa, which release more protons, are thought to have resulted in a higher sorbitol yield.
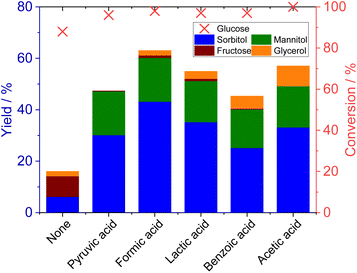 | ||
| Fig. 4 Effects of various acids on glucose reduction. Reaction conditions: glucose (0.5 mmol), TBAF·3H2O (0.05 mmol), NMP (3 mL), Si (5 mmol), water (10 mmol), acid (0.05 mmol), 120 °C, 18 h. | ||
The obtained sorbitol yield of the present study was compared with previous studies using conventional catalytic hydrogenation (Table S7). While noble metals like ruthenium achieved a sorbitol yield of 99%, they required high-pressure hydrogen at 4 MPa.27 Similarly, inexpensive RANEY® nickel-based catalyst also required high-pressure hydrogen, with a reported sorbitol yield of 55%.28 In contrast, a major difference and advantage of the present study is that it did not require such high-pressure hydrogen or metal catalysts like precious metals. Currently, the sorbitol yield is 43%, which is slightly lower than the results obtained with the RANEY® nickel-based catalyst. In catalytic hydrogenation, hydrogen must be produced as the reducing agent. In contrast, this research used unnecessary silicon as the reductant, offering an energy advantage.
Additionally, comparisons were made with catalytic hydrogenation regarding the environmental factor (E-factor, defined by the weight ratio of waste to product) and environmental energy impact (ξ) (see the SI).29,30 The latter was calculated by dividing the E-factor by the energy economy factor, an index obtained by dividing the sorbitol yield by temperature and time. The E-factor for this study was 89, while values for the RANEY® nickel catalyst and Ru/ZSM-5 catalysts were 3, and values for supported Pt catalysts exceeded 400. Calculations for environmental energy impact (ξ) showed that the value for this study was larger than those reported in many of the research papers on catalytic hydrogenation, although some smaller values were also observed in a few references. It should be noted that this metric can be reduced by more than an order of magnitude not only by improving the yield but also by aiming for lower reaction temperatures and shorter reaction times.
After use, the silicon powder was characterized by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), the results of which are presented in Fig. 5 and 6, respectively. Whereas characteristic diffraction peaks ascribed to metallic Si were observed in the XRD patterns for the fresh powder, these sharp peaks decreased in intensity with increasing reaction time. In addition, broad peaks in the range of 20° to 25° related to amorphous SiO2 became more intense. The ATR-FTIR spectrum also indicated the formation of amorphous SiO2 (Fig. S3). Furthermore, scanning electron microscopy (SEM) images of silicon powder before and after the reaction revealed a drastic change in morphology (Fig. S4). Prior to the reaction, most silicon particles were several microns in size, with the average size based on an equivalent circular diameter of approximately 1.5 µm. Among these, larger particles were found to be angular. In contrast, particles with no edges and indistinct outlines were observed after the reaction. Similar to this study, it has been reported that in the CO2 conversion reaction using Si as a reducing agent, the resulting silica is a porous material with a surface area of approximately 300 m2 g−1.18 Its reuse as a catalyst support or adsorbent is considered feasible.
 | ||
| Fig. 5 XRD patterns for fresh silicon powder and solid residue recovered after 3 and 12 h reaction durations. | ||
 | ||
| Fig. 6 (a) Si 2p and (b) F 1s XPS spectra of fresh silicon powder and solid residue recovered after the reaction. | ||
The oxidation of silicon during the reaction was also confirmed by the XPS data. Prior to the reaction, the surface of the silicon powder consisted of both metallic silicon and the product of oxidation because of passivation under ambient air. Regarding the surface oxide layer, XPS data partially confirmed the presence of Si4+. However, since Si0 was the main component, the layer was not very thick and considered to be at least less than the XPS photoelectron escape depth (several nanometers). Following the glucose reduction reaction, the peak related to pure metal was almost completely absent (Fig. 6(a)). The F 1s spectrum of the sample following the reaction exhibited a broad peak indicative of the formation of Si–F bonds.31 These results are in good agreement with those of previous studies.15–18 A previous study on the conversion of CO2 to formamide has shown via DRIFT measurements that heating silicon and TBAF alone to 100 °C generated Si–H species.18 Since the fundamental reaction in the present study also involved silicon and TBAF, it is considered that Si–H species were similarly generated.
Based on the results summarized above, a reaction mechanism was devised and is presented in Scheme 2. In this process, fluoride anions from the TBAF initially attack the silicon surface to cleave Si–Si bonds, followed by the simultaneous formation of Si–H and Si–F bonds, with water acting as a proton donor. The Si–H groups subsequently reduce glucose in conjunction with fluoride or hydroxide anions (shown here generically as A−). The Si–F groups react with hydroxide anions to generate Si–OH groups, which then undergo further reaction to form Si–O–Si linkages. As a result, fluoride anions are regenerated along with the formation of an oxidized silicon surface.
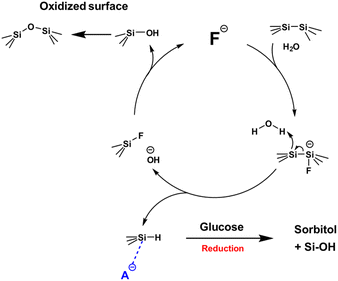 | ||
| Scheme 2 Proposed mechanism for the catalytic cycle involving fluoride anions and resulting in glucose reduction. | ||
While it cannot be ruled out that HF generated within the system exerts some influence, we consider the reaction proceeds primarily via the proposed reaction mechanism involving the reaction of fluorine and silicon. The following are our considerations based on the results of the reaction test.
First, considering that TBAF reacts with water to generate HF, which strongly correlates with the reactivity, the sorbitol yield should increase with the amount of water added. However, in the experiment, adding a large amount of water as a proton source decreased the activity (Fig. 2(b)). This suggests that even assuming HF is formed, it does not directly contribute to the reduction of glucose.
Second, assuming HF is generated, it is considered that it reacts with silicon oxide to form H2SiF6. In that case, F− is consumed and TBAF cannot act catalytically. However, in the experiment, the turnover number reached 11.8 based on the combined yield of sorbitol and mannitol, indicating that TBAF is being used catalytically and that HF formation would be minimal.
Third, even in the absence of acids like acetic acid, sorbitol is still produced, albeit at about 5% yield (Fig. 2(d)), suggesting that it does not appear that HF is an essential chemical species for the activity of this reaction. In the reaction of the present study, the primary role of adding acid is to suppress side reactions such as isomerization. The reaction under conditions without silicon is shown in Table S8. In experiments varying the TBAF amount (0–0.5 mmol) without adding either silicon or acid, the glucose conversion increased with the amount of TBAF, and the yields of fructose and mannose from isomerization also increased. However, under similar conditions in the absence of silicon, adding an equimolar amount of lactic acid to TBAF reduced the fructose yield by half. This indicates that while TBAF alone promoted base-catalysed undesirable side reactions, including isomerization to fructose, adding acid suppressed these reactions. The difference in activity due to acid strength (shown in Fig. 4) is thought to correlate roughly with the degree of suppression of these base-catalysed side reactions.
Conclusions
This work demonstrates the first-ever reduction of glucose to sorbitol using silicon as the reductant. A fluoride-based catalyst was found to be essential to this reaction based on the formation of active Si–H groups on the silicon surface, and the presence of water as a proton donor was also required. The addition of an acid significantly increased the yield of sorbitol (up to 43%) by suppressing undesired base-catalysed side reactions, including the aldose-ketose isomerization and retro-aldol reactions.Author contributions
KM and AT: conceptualization; TK and AT: investigation and methodology; TK and AT: formal analysis; AT: supervision; KM and AT: funding acquisition; TK and AT: writing – original draft; KM and AT: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental. See DOI: https://doi.org/10.1039/d5su00529a.Acknowledgements
This work was supported by JST-ALCA-Next Japan Grant Number JPMJAN23C7.Notes and references
- A. L. Merchan, T. Fischöder, J. Hee, M. S. Lehnertz, O. Osterthun, S. Pielsticker, J. Schleier, T. Tiso, L. M. Blank, J. Klankermayer, R. Kneer, P. Quicker, G. Walther and R. Palkovits, Green Chem., 2022, 24, 9428 RSC.
- H. Kobayashi, T. Sagawa and A. Fukuoka, Chem. Commun., 2023, 59, 6301 RSC.
- X. Wu, M. De bruyn and K. Barta, Chem. Commun., 2023, 59, 9929 RSC.
- A. Fukuoka and P. L. Dhepe, Angew. Chem., Int. Ed., 2006, 45, 5161 CrossRef CAS PubMed.
- I. Bonnin, R. Méreau, T. Tassaing, F. Jérôme and K. De Oliveira Vigier, ACS Sustainable Chem. Eng., 2021, 9, 9240 CrossRef CAS.
- S. Yamaguchi, S. Fujita, K. Nakajima, S. Yamazoe, J. Yamasaki, T. Mizugaki and T. Mitsudome, Green Chem., 2021, 23, 2010 RSC.
- B. García, A. Orozco-Saumell, M. López Granados, J. Moreno and J. Iglesias, ACS Sustainable Chem. Eng., 2021, 9, 14857 CrossRef.
- R. Otomo, T. Yokoi and T. Tatsumi, Appl. Catal., A, 2015, 505, 28 CrossRef CAS.
- P. Che, H. Ma, X. Nie, W. Yu and J. Xu, Green Chem., 2022, 24, 7545 RSC.
- Y. Morita, S. Furusato, A. Takagaki, S. Hayashi, R. Kikuchi and S. T. Oyama, ChemSusChem, 2014, 7, 748 CrossRef CAS PubMed.
- S. Chatti, G. Schwarz and H. R. Kricheldorf, Macromolecules, 2006, 39, 9064 CrossRef CAS.
- H. Wang, F. Xu, Z. Zhang, M. Feng, M. Jiang and S. Zhang, RSC Sustainability, 2023, 1, 2162 RSC.
- A. Chadly, K. Moawad, K. Salah, M. Omar and A. Mayyas, Sustain. Horiz, 2024, 11, 100108 CrossRef.
- S. Preet and S. T. Smith, J. Cleaner Prod., 2024, 448, 141661 CrossRef CAS.
- R. A. Praudita, K. Nakao, C. Nakagawa, R. Wang, T. Mochizuki, H. Takato, Y. Manaka and K. Motokura, Energy Adv., 2022, 1, 385 RSC.
- K. Motokura, Y. Sasaki, Y. Tanimura, T. Shiroshita, S. Hasegawa, K. Arata, R. Takemura, K. Namba and Y. Manaka, ACS Sustainable Resour. Manage., 2025, 2, 1220 CrossRef CAS.
- K. Motokura, K. Nakao and Y. Manaka, Asian J. Org. Chem., 2022, 11, e202200230 CrossRef CAS.
- R. Wang, K. Nakao, Y. Manaka and K. Motokura, Commun. Chem., 2022, 5, 150 CrossRef CAS PubMed.
- A. Takagaki, Catalysts, 2019, 9, 907 CrossRef CAS.
- B. Toukoniitty, J. Kuusisto, J.-P. Mikkola, T. Salmi and D. Y. Murzin, Ind. Eng. Chem. Res., 2005, 44, 9370 CrossRef CAS.
- D. Polidoro, A. Perosa, E. Barbaro, M. Feltracco, E. Argiriadis and M. Selva, ACS Sustainable Chem. Eng., 2022, 10, 2844 CrossRef CAS.
- G. Gao, S. Feng, Z. Jiang, C. Hu, Q. Zhang and D. C. W. Tsang, Ind. Eng. Chem. Res., 2023, 62, 3140 CrossRef CAS.
- A. Takagaki, M. Ohara, S. Nishimura and K. Ebitani, Chem. Commun., 2009, 6276 RSC.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Chem. Rev., 2022, 122, 6749 CrossRef CAS PubMed.
- S. K. R. Patil, J. Heltzel and C. R. F. Lund, Energy Fuels, 2012, 26, 5281 CrossRef CAS.
- M. N. Gey and U. Schröder, RSC Adv., 2025, 15, 25132 RSC.
- X. Guo, X. Wang, J. Guan, X. Chen, Z. Qin, X. Mu and M. Xian, Chin. J. Catal., 2014, 35, 733 CrossRef CAS.
- H. Li, W. Wang and J. F. Deng, J. Catal., 2000, 191, 257 CrossRef CAS.
- Z. Fehér, J. Kiss, P. Kisszékelyi, J. Molnár, P. Huszthy, L. Kárpáti and J. Kupai, Green Chem., 2022, 24, 8447 RSC.
- E. Barnard, J. J. R. Arias and W. Thielemans, Green Chem., 2021, 23, 3765 RSC.
- T. Takahagi, A. Ishitani, H. Kuroda and Y. Nagasawa, J. Appl. Phys., 1991, 69, 803 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |