Open Access Article
Open Access ArticleComparison between two different approaches for the deconstruction of lignocellulosic feedstocks using alkanolamine-based solvents†
Anagha
Krishnamoorthy
a,
Venkataramana
R. Pidatala
ab,
Xueli
Chen
a,
Joseph
M. Palasz
a,
Yinglei
Han
 bc,
Tyrell
Lewis
bc,
Tyrell
Lewis
 ad,
Hemant
Choudhary
ad,
Hemant
Choudhary
 bc,
Alberto
Rodriguez
bc,
Alberto
Rodriguez
 bc,
John M.
Gladden
bc,
John M.
Gladden
 bc,
Chang
Dou
bc,
Chang
Dou
 ad,
Ning
Sun
ad,
Ning
Sun
 ad and
Blake A.
Simmons
ad and
Blake A.
Simmons
 *ab
*ab
aBiological Systems and Engineering Division, Lawrence Berkeley National Laboratory, Berkeley, CA, USA. E-mail: basimmons@lbl.gov
bDeconstruction Division, Joint BioEnergy Institute, Emeryville, CA, USA
cSandia National Laboratories, Livermore, CA, USA
dAdvanced Biofuels and Bioproducts Process Development Unit, Emeryville, CA, USA
First published on 9th June 2025
Abstract
Exploring the feasibility of applying alkanolamines as biomass pretreatment solvents for the deconstruction of biofuels is useful, owing to their ease of accessibility and their potential to serve as a low energy-intense, cost-effective downstream conversion. For the current study, we have selected a wide range of biomass feedstocks and evaluated biomass deconstruction efficiency using dual-functional solvents, ethanolamine and ethanolammonium acetate, and comparing two different solvent recovery approaches – water washing and solvent evaporation. Pretreatment conditions for both processes included 15% solid loading of 2 mm size ground and homogeneously mixed biomass with pure ethanolamine (EA) and ethanolammonium acetate (EAA) at 140 °C for 3 h. For the first solvent removal process, the pretreated biomass was washed until the pH reached 7.0, and for the second solvent removal process, solvent evaporation was performed in a vacuum oven set at 80 °C and 140 °C for EA and EAA respectively, followed by pH adjustment to 5.0. The next step involved saccharification using Cellic® enzymes to liberate glucose and xylose from the pretreated solids. Enzymatic hydrolysis of coconut chips, hay, rice hulls and a pelletized 4-crop blended mix (corn stover, switchgrass, pine, and eucalyptus) revealed significantly higher sugar release through the solvent evaporation route as compared to the washing process as washing led to high solid losses. Through this study, we demonstrated the effective use of alkanolamines as biomass pretreatment solvents relevant to a commercial biorefinery setting, as well as that vacuum-based solvent removal is a better strategy for improved release of fermentable sugars that also enables facile solvent removal.
Sustainability spotlightWhat is the situation and why is it important to address/understand this? This work makes significant contributions to advancing sustainable biofuel production processes by integrating green chemistry principles into the pretreatment of lignocellulosic feedstocks. It serves as a model for developing energy-efficient, resource-conserving, and environmentally friendly biomass conversion technologies. What is the sustainable advancement of the work? This study improves sustainability by significantly reducing water use, improving solvent recovery (up to 98.5%), retaining more biomass (minimizing losses of up to 53%), and enhancing sugar yields (up to 87.34%). These advancements align with sustainability principles and make the processes more affordable and scalable for biofuel production. While this work shows that solvent recovery through vacuum distillation is efficient (90–98.5%), some solvent loss occurs, which could still contribute to resource depletion and environmental impact. It is recommended that future work be focused on developing closed-loop solvent systems with near-100% recovery efficiency. This would include exploring alternative solvent recovery methods, such as membrane separation or adsorption techniques, which could be less energy-intensive than vacuum distillation. Which UN SDG(s) (https://sdgs.un.org/goals) does the work align with? Affordable and clean energy, responsible consumption and production. |
1. Introduction
Lignocellulosic feedstocks, including agricultural residues, forestry waste, and energy crops, have a projected sustainable availability of over a billion dry tons annually in the U.S.1 and represent promising renewable sources for biofuels and bioproducts.2–5 However, their structural complexity leads to biomass recalcitrance, an inherent resistance to deconstruction, which, along with inhibitors formed during pretreatment, hinders efficient conversion.6–12 Addressing these challenges requires costly and energy-intensive pretreatment processes, which can account for up to 20% of total biofuel production costs.13,14In this regard, protic ionic liquids based on alkanolamines and their respective solvent counterparts are increasingly being recognized as effective agents for the pretreatment of lignocellulosic biomass. They can act as both hydrogen bond donor/acceptor and as a Brønsted base (proton/hydrogen ion acceptor) for a variety of chemical reactions. Their usage leads to higher yields of fermentable sugars owing to their unique properties that allow for selective lignin solubilization, thereby preserving the integrity of carbohydrate components.14,15 Additionally, alkanolamines can facilitate the removal of inhibitory compounds formed during pretreatment14 by binding to these harmful substances, especially acidic ones, neutralizing them and making the environment more favorable for enzymes and microbes used in biofuel production. This helps improve sugar yields and overall efficiency of the conversion process. Additionally, their potential for recovery and reuse further contribute to their appeal as sustainable agents in biomass pretreatment, positioning them as valuable tools in the development of renewable biofuels.
Pretreatment involves the effective breakdown of chemical bonds between cellulose, hemicellulose and lignin, leading to the solubilization of sugars and lignin into the pretreated liquor.15–17 After pretreatment, generally the slurry is centrifuged or filtered to separate the solid and liquid fractions. The liquid fraction, consisting of the washed water as well as the pretreated liquor, are often discarded due to their pH incompatibility and presence of inhibitory compounds.15,18,19 Subsequently, the solid residues are washed extensively with water till the pH reaches neutrality and only then it is deemed fit for enzymatic hydrolysis.15,20,21 The washing process is conventional, and in the case of acid and alkali pretreatments, it inevitably results in huge water consumption as well as the loss of large amounts of biomass and chemicals.19,22,23 However, the solvent removal method under vacuum presents a scope for solvent recycling, which in turn enables biorefineries to potentially significantly lower operational costs.24
The objective of this study is to evaluate the two different solvent recovery approaches – water washing and solvent evaporation via vacuum distillation, and also to compare the deconstruction efficiency of a wide variety of lignocellulosic feedstocks using the dual-functional solvent, ethanolamine, and its protic ionic liquid-form, ethanolammonium acetate. The pretreatment effectiveness in terms of chemical compositions (glucan, xylan, solid, and lignin) and their recovery, have been evaluated and presented. Additionally, biophysical techniques including X-ray diffraction (XRD), and Fourier-transform infrared (FTIR) spectroscopy (ESI†) were performed to characterize the changes in structural properties of raw and pretreated biomass post-washing and solvent evaporation. Overall, a practical method to reduce water consumption and chemicals loss during the biomass valorization process is presented.
2. Experimental section
Chemicals: ethanolamine, ethanolammonium acetate, sodium azide, citric acid (ACS reagent ≥99.5%) and sodium citrate tribasic dihydrate (ACS reagent, ≥99.0%) were obtained from Sigma-Aldrich (St. Louis, MO) and used as received. Sulfuric acid (72% and 95–98%) was purchased from VWR. Ethanol (200 proof) was purchased from Decon Labs, Inc. (King of Prussia, PA). Sulfuric acid (72%) was procured from RICCA Chemical Company (Arlington, TX). Analytical standard grade glucose and xylose were also obtained from Sigma-Aldrich (St. Louis, MO) and used for calibration.Glassware: glass pressure tubes were procured from Ace Glass – 30 mL volume, 60 psig @ 120 °C.
Biomass: to understand the deconstruction efficiency and the impact of solvent recovery across biomass types, several different feedstocks and formulations were evaluated. The biomass feedstocks studied here were coconut chips, hay, rice hulls, and 4-crop mix pellets, which were dried for 48 h in a 40 °C oven. Subsequently, they were knife-milled to 2 mm size (Thomas-Wiley Model 4, Swedesboro, NJ) (Fig. 1 bottom panel). The resulting biomass was then stored in a dry cool place in a leak-proof bag to prevent chemical or biological contamination. The moisture content of the dried biomass was determined using the Halogen Moisture Analyzer HC103 (Mettler-Toledo). The 4-crop mix biomass was received in a pelleted form, which was made by compressing different biomasses—like already-ground lodge pine, corn stover, Eucalyptus and switchgrass—into small cylindrical shapes that are easy to store, transport and process further. Pelleted biomass (Fig. 1d) is generally used as such, and need not be further ground for pretreatment and other downstream processes.
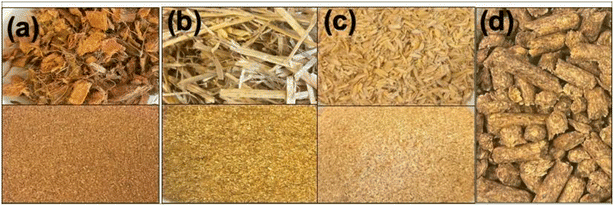 | ||
| Fig. 1 Representative image of the different feedstocks (a) coconut chips, (b) hay, (c) rice hulls, and (d) 4-crop mix as such (top panel), and when ground to 2 mm size (bottom panel). | ||
Enzymes: commercial cellulase (Cellic® CTec3, 1853 BHU-2-HS per g, 1.212 g mL−1) and hemicellulase (Cellic® HTec3, 1760 FXU per g, 1.210 g mL−1) mixtures were provided by Novozymes, North America (Franklinton, NC).
2.1. Compositional analysis
The compositional analysis of the biomass before and after pretreatment was performed using the National Renewable Energy Laboratory (NREL) two-step acid hydrolysis protocol in duplicates.30 Firstly, 300 mg of extractive-free biomass and 3 mL of 72% sulfuric acid (H2SO4) were incubated at 30 °C while shaking at 200 rpm for 1 h. The solution was diluted to 4% H2SO4 with 84 mL of DI water and autoclaved at 121 °C for 1 h. The flasks were cooled down before removing the solids by filtration using filtering crucibles. Subsequently, the filtrates were spectrophotometrically analyzed for the acid-soluble lignin (ASL) (NanoDrop 2000; Thermo Fisher Scientific, Waltham, MA) using the absorbance at 240 nm. Acid insoluble lignin (AIL) was quantified gravimetrically after heating the samples overnight at 105 °C to obtain the weight of AIL and ash. Additionally, monomeric sugars (glucose and xylose) were determined by HPLC using an Agilent 1200 series instrument equipped with a Bio-Rad Aminex HPX-87H column and a refractive index detector. An aqueous solution of 4 mM H2SO4 was used as the mobile phase (column temperature 60 °C, 0.6 mL min−1) for the separation of products. The total run time was set to 20 min and the amount of glucan and xylan was calculated from the glucose and xylose content multiplied by the anhydro correction factors of 162/180 and 132/150, respectively. Due to co-elution under the selected conditions, mannose and galactose were detected as xylose, which does not affect the study's conclusions since detailed sugar profiling is beyond the scope of this study. The moisture content of the raw biomass was evaluated by drying 0.5 to 2 grams of the sample in porcelain crucibles using a conventional oven (Binder GmbH, Germany) for a minimum of 4 hours. The dried biomass was subjected to further heating in a muffle furnace at 575 °C for at least 6 hours to determine the ash content. Crucibles were permitted to cool in desiccators, and their weights were recorded between each heating phase.2.2. Biomass pretreatment with ethanolamine (EA) and ethanolammonium acetate (EAA)
Coconut chips, hay, rice hulls, and 4-crop mix pellet samples of 2 mm were mixed with either ethanolamine or ethanolammonium acetate in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6 ratio (w/w) to achieve a biomass loading of 15 wt% in a 30 mL Ace glass pressure tube and pretreated for 3 h in an oil bath heated at 140 °C. After pretreatment, the samples were removed from the oil bath and allowed to cool.
5.6 ratio (w/w) to achieve a biomass loading of 15 wt% in a 30 mL Ace glass pressure tube and pretreated for 3 h in an oil bath heated at 140 °C. After pretreatment, the samples were removed from the oil bath and allowed to cool.
2.3. Solvent removal by washing
A measure of 10 mL DI water was slowly added to the biomass-IL slurry and mixed well. The mixture was transferred to 50 mL Falcon tubes and the pressure tubes were washed with additional water to obtain a total volume of 40 mL. The mixture was centrifuged at high speed (4000 rpm) to separate solids and remove any residual solvent. This process was repeated until all the solvent was removed and the pH of the pretreated biomass reached neutral (7.0). The water-washed solid was freeze-dried to obtain dried pretreated biomass for further analysis.2.4. Solvent removal by vacuum distillation
In the second set up, the tubes containing the pretreated biomass with the solvent were placed in a vacuum oven chamber set at 80 °C and 140 °C for the removal of EA and EAA respectively, followed by pH adjustment to 5.0.2.5. Enzymatic saccharification
Enzymatic saccharification set up was conducted in duplicates using commercially available enzymes, Cellic® CTec3 and HTec3 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) from Novozymes, at 50 °C in a rotary incubator (Enviro-Genie, Scientific Industries, Inc.). All the reactions were performed at 15 wt% biomass loading in a 30 mL glass tube. The pH of the mixture was adjusted to 5 with 1 M sodium citrate buffer. Additionally, 0.1 wt% sodium azide was added to prevent microbial contamination. The total reaction volume included a total protein content of 30 mg per g solids. The amount of sugar released was measured by HPLC the same way as described above.
1 v/v) from Novozymes, at 50 °C in a rotary incubator (Enviro-Genie, Scientific Industries, Inc.). All the reactions were performed at 15 wt% biomass loading in a 30 mL glass tube. The pH of the mixture was adjusted to 5 with 1 M sodium citrate buffer. Additionally, 0.1 wt% sodium azide was added to prevent microbial contamination. The total reaction volume included a total protein content of 30 mg per g solids. The amount of sugar released was measured by HPLC the same way as described above.
2.6. Powder X-ray diffraction (PXRD) analysis
PXRD was taken at the UC Berkeley Small Molecule X-ray Crystallography Facility (CheXray). Biomass samples were dried in a vacuum oven and run as-is with no further milling or processing. Samples were collected at room temperature using a Rigaku miniflex 6g benchtop powder XRD instrument operating with a CuKα source and a HyPix-400MF Hybrid Pixel Array 0D/1D/2D Detector.2.7. Fourier-transform infrared (FTIR) spectroscopy
Samples for FTIR analysis were prepared by drying them in a vacuum oven. IR spectra were collected with a Bruker VERTEX 70/80 ATR-FTIR system (Billerica, MA, USA) using a germanium probe tip contacting the sample. Each spectrum consisted of 32 averaged scans at a resolution of 4 cm−1. Data for sample spectra were obtained from OPUS software on the instrument and were then transferred into Origin Lab25 and the spectra were baseline-corrected and normalized.3. Results and discussion
3.1. Compositional analysis of raw biomass
Compositional analysis (Tables S1† and 1) of the biomass was determined, and the highest glucan content was observed in hay (39.2% of biomass), followed closely by rice hulls (35.8% of biomass) and 4-crop mix (32.6% of biomass). The lowest glucan content was observed in coconut chips (24.8% of biomass). Xylan contents in coconut chips, hay, rice hulls and the 4-crop mix were 11.5, 25.9, 13.3 and 19.2% of biomass, respectively. Combining both glucan and xylan, the total fermentable sugars for the four biomasses ranged from 36.3 to 65.1% of dry biomass, respectively. The lignin content for coconut chips, hay, rice hulls and the 4-crop mix were 47.2, 25.7, 24.9, and 34.5% of biomass, respectively. According to available literature, high lignin content in a biomass leads to its recalcitrance to breakdown into simple sugars. Therefore, from that aspect the 4-crop mix and coconut chips are expected to be very challenging materials for deconstruction and conversion.26,27 On the contrary, higher lignin content in these feedstocks also paves way to lignin valorization, to produce value-added products like phenols, energy storage materials, and composites.28,29 Therefore, the results show that all the aforementioned biomass feedstocks can be utilized to produce biofuels and platform chemicals. However, variations in their composition could lead to changes in their performance.| Sample | Condition | Solid loss (%) | Composition of biomass (%) | % Removal of cellulosic and lignin components | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucan | Xylan | AIL | Total ash | Glucan | Xylan | AIL | Total ash | |||
| Coconut chips | Raw | 24.8 ± 0.1 | 11.5 ± 0.1 | 43.5 ± 0.2 | 0.04 ± 0.0 | |||||
| PT with ethanolamine | 49.38 | 45.05 ± 0.39 | 25.66 ± 0.01 | 14.36 ± 0.9 | 1.16 ± 0.33 | 8.05 | 0 | 83.29 | 100.00 | |
| PT with ethanolammonium acetate | 34.59 | 29.43 ± 0.43 | 18.75 ± 0.35 | 38.84 ± 1.01 | 0 ± 0.85 | 22.38 | 0 | 41.60 | 100.00 | |
| Hay | Raw | 39.2 ± 1.0 | 25.9 ± 0.8 | 21.4 ± 0.1 | 3.39 ± 0.13 | |||||
| PT with ethanolamine | 40.02 | 53.9 ± 0.24 | 25.13 ± 0.01 | 3.43 ± 0.8 | 1.53 ± 0.14 | 17.53 | 41.80 | 90.39 | 72.93 | |
| PT with ethanolammonium acetate | 31.51 | 39.81 ± 0.08 | 32.09 ± 0.54 | 14.11 ± 1.42 | 2.21 ± 0.40 | 30.44 | 15.14 | 54.84 | 55.35 | |
| Rice hulls | Raw | 35.8 ± 0.6 | 13.3 ± 0.0 | 22.0 ± 0.1 | 18.13 ± 0.15 | |||||
| PT with ethanolamine | 47.3 | 40.56 ± 0.13 | 20.06 ± 0.55 | 9.49 ± 0.48 | 22.96 ± 0.19 | 40.29 | 20.51 | 77.27 | 33.26 | |
| PT with ethanolammonium acetate | 53.35 | 33.99 ± 0.12 | 18.12 ± 0.29 | 19.41 ± 0.15 | 20.58 ± 0.04 | 55.71 | 36.44 | 58.84 | 47.05 | |
| 4-Crop mix | Raw | 32.6 ± 0.2 | 19.20 ± 0.2 | 31.8 ± 0.2 | 1.5 ± 0.13 | |||||
| PT with ethanolamine | 39.6 | 42.92 ± 3.66 | 26.17 ± 1.64 | 19.73 ± 1.64 | 0.07 ± 0.14 | 20.48 | 17.67 | 62.53 | 97.18 | |
| PT with ethanolammonium acetate | 40.2 | 34.66 ± 0.11 | 24.65 ± 0.08 | 29.57 ± 0.19 | 0.78 ± 0.83 | 36.42 | 23.23 | 44.39 | 68.90 | |
3.2. Solid loss by water washing and composition of pretreated and washed biomass
Solid loss percentage is the amount of dried biomass lost post pretreatment with ethanolamine and ethanolammonium acetate and water washing. In a study performed by Cheng et al.30 on NaOH-pretreated and washed rice straw samples, the overall glucose yields were greater in unwashed rice straw samples in comparison with the washed NaOH-pretreated rice straw, as significant amounts of solids and sugars are being lost during the washing step. Similarly, a significant impact on the solids was observed for ethanolamine-based pretreatments, with coconut chips exhibiting the maximum solid loss (49.38%) and the 4-crop mix pellets showing the least solid loss (39.6%) (Tables S2† and 1). In the case of ethanolammonium acetate, ∼53% solids were lost after pretreatment of rice hulls along the course of water washing (Table 1). This could possibly be due to the presence of higher ash content and glucan solubilization.31 The 4-crop mix pellets showed most uniform and lower solid loss percentages (Tables S3† and 1), which is in compliance with Sun et al.32 report stating that its high recovery is due to higher content of glucan.To understand the actual removal of the cellulosic and lignin components, the percentage removal of each component was also calculated as follows:
| % Removal = [100 − {(% solid recovery) × (composition of the pretreated biomass/composition of the untreated biomass)}]. |
The chemical composition of the biomass was determined post washing, and the results are summarized below (Tables S2 and S3†). In the case of ethanolamine-pretreated and washed biomass samples, the highest glucan content was observed in hay (53.9%), followed by coconut chips (45.05%), 4-crop mix (42.92%) and rice hulls (40.56%). The xylan contents across all four feedstocks were comparable, in the order of the 4-crop mix (26.17%), followed closely by coconut chips (25.13%), hay (25.13%) and rice hulls (20.06%) (Table S2†). The Klason lignin of the four feedstocks decreased substantially by pretreatment and washing with ethanolamine in comparison to the raw biomass (Table S1†). The total lignin content of coconut chips, hay, rice hulls and the 4-crop mix reduced significantly, from 47.2% (Table S1†) to 17.56% (Table S2†), 25.7% (Table S1†) to 5.83% (Table S2†), 24.9% (Table S1†) to 11.59 (Table S2†), and 34.5% (Table 1) to 21.73% (Table S2†), respectively.
With respect to the raw biomass, biomass pretreated and washed with ethanolammonium acetate showed glucan content ranging from 39.81% (hay), to 34.66% (4-crop mix), to 33.99% (rice hulls) and 29.43% (coconut chips) (Tables 1 and S3†). The highest xylan content was observed in hay (32.09%), followed by the 4-crop mix (24.65%), coconut chips (18.75%) and rice hulls (18.12%) (Tables 1 and S3†). In the case of ethanolammonium acetate-pretreated and washed biomasses, we could not observe a significant difference in the total lignin content in comparison to the raw biomass, with coconut chips, hay, rice hulls and the 4-crop mix showing 42.64%, 17.63%, 21.59% and 31.65% total lignin, respectively (Tables 1 and S3†).
3.3. Fermentable sugar yields from pretreated biomass using two different solvents and solvent recovery approaches
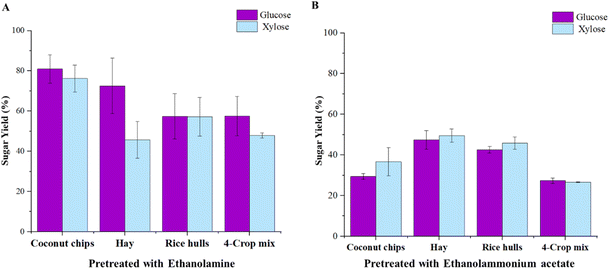
To compare the performance of pretreatment and the different solvent recovery methods, enzymatic hydrolysis of the four biomasses was carried out post pretreatment with ethanolamine and ethanolammonium acetate with an enzyme loading of 30 mg of protein per g of biomass (Fig. 2 and 3). Glucose and xylose yields released from the above-mentioned biomasses after solvent removal by centrifuge separation and washing (Fig. 2A and B), and by solvent distillation in a vacuum chamber were studied (Fig. 3A and B).It is a known fact that the complexity of the biomass type can influence the effectiveness of pretreatment.33 Therefore, developing a feedstock-agnostic pretreatment strategy is an ideal and economical way to produce biomass-derived biofuels.34 Notably, the compatibility of the various feedstock types with the two solvents was taken into consideration to determine the efficiency of pretreatment on these biomass types. The glucose and xylose yields resulting from the enzymatic saccharification of the different biomasses pretreated with ethanolamine and washed, was in compliance with the feedstock-type, and ranged from 57.41% (rice hulls) and 57.52% (4-crop mix) to 80.93% (coconut chips), and 47.9% (4-crop mix pellets) to 76.25% (coconut chips). Rice hulls, an agricultural residue, demonstrated only 42.59% of glucose, and 45.87% of xylose yields by water washing due to massive solid losses, and coconut chips, a tropical biomass rich in lignin content, yielded 80.93% of glucose and 76.25% of xylose, respectively (Fig. 2A). Interestingly, the feedstocks screened represent a wide range of lignocellulosic feedstocks, and the results from enzymatic hydrolysis of the pretreated biomasses with ethanolammonium acetate and washing showed consistently lower glucose and xylose yields across all feedstocks. While hay, an agricultural feedstock, showed 47.42% glucose yields, coconut chips, being a tropical feedstock, only yielded 29.45% of glucose post saccharification as opposed to ethanolamine pretreatment (Fig. 2B). The water-washed 4-crop mix pellets exhibited lower values in glucose and xylose yields as well, with 27.37% of glucose and 26.62% of xylose (Fig. 2B). The relationship between these yields and the process types for each biomass and solvent are further discussed in length through mass balance flowcharts (Fig. 4).
The yields of glucose and xylose resulting from the enzymatic saccharification of the different biomasses pretreated with ethanolamine and solvent removed by distillation was much better to that of solvent removal by washing in terms of the biomass-type, with the 4-crop mix pellets demonstrating around 52.29% glucose and 24.95% xylose, and hay with 77.64% glucose and 44.71% xylose. Coconut chips showed glucose and xylose yields of 69.42% and 39.98% (Fig. 3A). Rice hulls exhibited 57.78% of glucose, and its xylose yields were comparable to that of the 4-crop mix pellets (24.88%) (Fig. 3A). Although the results from enzymatic hydrolysis of the pretreated biomasses with ethanolammonium acetate and solvent removal by distillation showed lower glucose and xylose yields in comparison with the ethanolamine-pretreated feedstocks, the yields were definitely better than the ethanolammonium acetate-pretreated and water-washed biomasses (Fig. 3B and 2B). Hay being an agricultural residue, showed 87.34% glucose and 78.85% xylose yields, however, coconut chips yielded only 31.59% of glucose and 28.44% of xylose post hydrolysis (Fig. 3B). Rice hulls and the 4-crop mix pellets showed similar trends in glucose and xylose yields as with washing, and their glucose and xylose yields were 53.58% of glucose and 53.75% respectively, with the 4-crop mix pellets showing 27.64% of glucose and 16.58% of xylose (Fig. 3B). The lower sugar yields in comparison with washing can be attributed to the fact that washing removes majority of the lignin as well as other impurities,35,36 whereas solvent distillation under vacuum merely removes the solvent and in fact leaves behind some traces of the solvent in the slurry.
The solvent evaporation percentage under vacuum was also tabulated for both ethanolamine and ethanolammonium acetate, and the results are as follows. In the case of ethanolamine, the solvent evaporation percentage varied between 90.18% and 94.44%, with coconut chips and rice hulls exhibiting the lowest and the highest solvent distillation under vacuum, with hay and the 4-crop mix exhibiting 92.35% and 90.92% solvent evaporation (Fig. 3A). The protic ionic liquid form of ethanolamine, ethanolammonium acetate, demonstrated better solvent distillation percentage, with coconut chips, hay, rice hulls and the 4-crop mix pellets showing 95.32, 98.55, 98.27 and 88.21% respectively (Fig. 3B).
3.4. Process mass balance
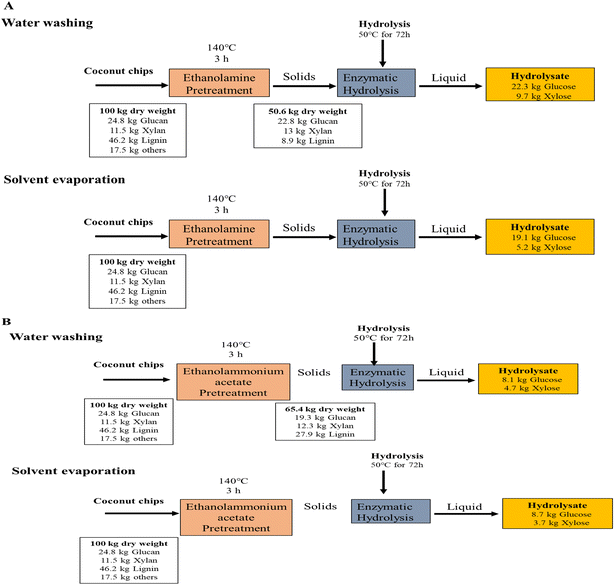
The results obtained from pretreatment and enzymatic hydrolysis were used to develop a process mass balance for each biomass, solvent and pretreatment technology (Fig. 4). Each pretreatment reaction has been represented per 100 kg of the dried biomass, generating a solid stream (pretreated biomass). For the washing process, ethanolamine and ethanolammonium acetate pretreatments with 100 kg of coconut chips, utilized a significant quantity of water at room temperature (Fig. 4A and B). On the contrary, the major advantage of the solvent evaporation process accounts for the solvent being released after vacuum distillation which can then be recycled and reused in an industrial system. Through the water washing process, the recovered solid stream represented about 50.6% of the coconut chips, primarily because of the xylan and other extractives being removed during pretreatment (Fig. 4A). Enzymatic hydrolysis of the pretreated coconut chips was performed using the optimal combination of the commercial enzyme cocktails as mentioned previously. For water-washed ethanolamine-pretreated biomass, 22.8 kg of the glucan was converted to 22.3 kg of monomeric glucose during enzymatic hydrolysis, while 13 kg of the xylan was converted to 9.7 kg of monomeric xylose. However, the total soluble sugars present in the hydrolysate (monomeric and oligomeric) represent only a portion of the initial glucan and xylan, respectively. The residual solids stream is composed of unhydrolyzed carbohydrates and lignin.37,38In the case of hay pretreated with ethanolamine, through the water washing process, 31.6 kg of glucose and 13.4 kg of xylose were released, however solvent evaporation yielded higher glucose conversion (33.8 kg) and xylose (12.2 kg) (Fig. S1(A)†). These results were replicated significantly with ethanolammonium acetate pretreatment of hay, where the solvent evaporation process gave rise to 38 kg of glucose and 22.7 kg of xylose, whereas water washing only led to 27.3 kg of glucan and 22 kg of xylan getting converted to 20.6 kg of glucose and 14.5 kg of xylose, respectively (Fig. S1(B)†).
Similar results were observed in rice hulls pretreated and washed with ethanolammonium acetate, where the sugar losses were as a result of significant solid loss during washing (Table S3†). The solvent evaporation route led to 21.3 kg of glucose and 7.9 kg of xylose, whereas the water washing process only released 16.9 kg of glucose and 6.9 kg of xylose, respectively (Fig. S1(D)†).
The 4-crop mix pellets pretreated using ethanolamine and ethanolammonium acetate yielded comparable simple sugars, through both processes (Fig. S1(E and F)†). This is certainly indicative of the fact that solvent evaporation is a cost-effective step that not only reduces solvent costs through recovery and recycle, but also retains the majority of the solids without sugar losses that are incurred due to water washes.3,39
One of the approaches to achieve economic sustainability of a process is by reducing costs of pretreatment and downstream processing.14 Therefore, we can consider that these results align well with the key criteria accounted for while designing a biorefinery, which is generally the unit operation that includes the processes of pretreatment, enzymatic hydrolysis, recycling of solvents and recovery, as well as fermentation for bioconversion. It is thus mandatory to optimize these processes to obtain the best value from the biomass feedstock being considered.40
To summarize the mass balance flowcharts, although this work does not provide a complete techno-economic evaluation for the various pretreatments from the process point-of-view, it certainly presents meaningful insights on the different aspects of post-pretreatment processing and how they depend on the type of biomass and process chosen for study. To validate the same, a previous technoeconomic analysis (TEA) of distillable amine-based ionic liquids (e.g., ethanolammonium acetate) published by our research group showed that distillability strongly affects process costs.41
3.5. Crystallinity of the raw and pretreated biomass
To explore the differing mechanisms of the washing and vacuum distillation approaches with ethanolamine (EA) and ethanolammonium acetate (EAA) we obtained powder X-ray diffraction (PXRD) spectra for coconut chips, hay, rice hulls and 4-crop mix before and after the different pretreatments (Fig. 5). PXRD can observe the crystalline phases of cellulose within biomass samples and can provide information into the structural changes occurring during pretreatment.42,43 Since the cellulose content of the biomass changes substantially during the washing steps, a calculation of crystallinity index is not an appropriate quantitative comparison, however qualitative observations can be made.44 Across all four biomass samples, the EAA pretreatment with evaporation displayed markedly broader features, indicating a more amorphous character of the pretreated cellulose. This can be attributed to the high polarity of the EAA ionic liquid serving to solubilize some of the carbohydrate fractions, paired with no removal of lignin or hemicellulose in the evaporative solvent removal process resulting in a predominantly amorphous sample. Both washed and evaporated samples treated with EA displayed crystalline cellulose peaks, with the most prominent example appearing in coconut chips, where the intensity of the crystalline cellulose peaks even increased compared to the untreated biomass. In the washed sample this can be attributed to the substantial delignification which occurred during washing. These results paired with the compositional analysis of the washed samples reveal that while the EAA may influence the crystallinity of cellulose through some dissolution mechanism, it is not a necessary step in pretreatment as the removal of crystalline cellulose does not correlate with sugar yields. With the exception of hay with an evaporation protocol, EA performed better as a pretreatment solvent and displayed minimal changes in cellulose structure for both washed and evaporated samples, suggesting its mode of action is centered on the lignin and hemicellulose components rather than the cellulose.4. Conclusion
Post-processing of pretreated biomass has a definitive impact on the overall process efficiency and sugar yields obtained. This study demonstrates that the use of a vacuum-based solvent removal approach post-pretreatment that results in higher yields of fermentable sugars as compared to solvent removal through water washing. Solvent removal through distillation proved to be a more sustainable and efficient strategy than the conventional water washing method, minimizing water consumption and allowing for solvent recycling, critical for the economic viability of commercial biorefineries. This work also achieved outstanding results using ethanolamine and ethanolammonium acetate as dual-functional solvents for biomass pretreatment, successfully processing a variety of feedstocks including coconut chips, hay, rice hulls, and a 4-crop mix. Overall, these findings unequivocally demonstrate the vast potential of alkanolamines as game-changing pretreatment agents, capable of efficiently processing a wide range of biomass feedstocks and paves the way for a significant leap forward in sustainable and cost-effective biofuel production.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
BAS has a financial interest in Illium Technologies, Caribou Biofuels, and Erg Bio. None of the other authors have any outside financial interests to disclose.Acknowledgements
The authors thank Nick Settineri and the UC Berkeley CheXray facility for assistance obtaining PXRD spectra. This work was supported by the DOD Tri-Service Biotechnology for a Resilient Supply Chain (T-BRSC) program through award AWD00007196 to Lawrence Berkeley National Laboratory. The portion of the work conducted at the Joint BioEnergy Institute was supported by the U.S. Department of Energy, Office of Science, Biological and Environmental Research Program, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy. The portion of the work conducted at the Advanced Biofuels and Bioproducts Development Unite was supported by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes.References
- M. Langholtz, M. Davis, C. Hellwinckel, D. Ugarte, R. Efroymson, R. Jacobson and A. Milbrandt, 2023 Billion-Ton Report: An Assessment of US Renewable Carbon Resources, 2024 Search PubMed.
- X. Chen, N. Mosier and M. Ladisch, Trends Biotechnol., 2024, 42, 1348–1362 CrossRef CAS.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- K. D. Jetti and N. S. Kishore, Sugar Technol.,, 2024, 26(2), 562–572 CrossRef CAS.
- K. Ding, D. Liu, X. Chen, H. Zhang, S. Shi, X. Guo, L. Zhou, L. Han and W. Xiao, Renewable Sustainable Energy Rev., 2024, 202, 114692 CrossRef CAS.
- A. K. Kumar and S. Sharma, Bioresour. Bioprocess., 2017, 4, 7 CrossRef.
- M. E. Himmel, S.-Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804–807 CrossRef CAS PubMed.
- V. Ninkuu, Z. Liu, Y. Zhou, E. Guo, X. Song, P. Gao, Y. Xie and X. Sun, Mod. Agric., 2023, 1, 122–141 CrossRef.
- L.-H. Xu, C.-Y. Ma, P.-F. Wang, Y. Xu, X.-J. Shen, J.-L. Wen and T.-Q. Yuan, Bioresour. Technol., 2023, 385, 129415 CrossRef CAS PubMed.
- Y. Wang, Y. Zhang, Q. Cui, Y. Feng and J. Xuan, Molecules, 2024, 29(10), 2275 CrossRef CAS PubMed.
- Y. Yuan, B. Jiang, H. Chen, W. Wu, S. Wu, Y. Jin and H. Xiao, Biotechnol. Biofuels, 2021, 14, 205 CrossRef CAS PubMed.
- W. Fatriasari, F. Nurhamzah, R. Raniya, R. P. B. Laksana, S. H. Anita, A. H. Iswanto and E. Hermiati, J. Korean Wood Sci. Technol., 2020, 48, 651–665 CrossRef.
- B. Yang and C. E. Wyman, Biofuels, Bioprod. Biorefin., 2008, 2, 26–40 CrossRef CAS.
- E. C. Achinivu, S. Frank, N. R. Baral, L. Das, M. Mohan, P. Otoupal, E. Shabir, S. Utan, C. D. Scown, B. A. Simmons and J. Gladden, Green Chem., 2021, 23, 8611–8631 RSC.
- J. Zhao, Y. Yang, M. Zhang and D. Wang, Bioresour. Technol., 2021, 339, 125605 CrossRef CAS.
- A. Limayem and S. C. Ricke, Prog. Energy Combust. Sci., 2012, 38, 449–467 CrossRef CAS.
- W. Wang and D.-J. Lee, Bioresour. Technol., 2021, 339, 125587 CrossRef CAS.
- Y. Song, Y. Gyo Lee, E. Jin Cho and H.-J. Bae, Fuel, 2020, 278, 118247 CrossRef CAS.
- C. C. Santos, W. de Souza, C. Sant'Anna and M. Brienzo, Ind. Crops Prod., 2018, 111, 193–200 CrossRef CAS.
- K. Ninomiya, C. Ogino, M. Ishizaki, M. Yasuda, N. Shimizu and K. Takahashi, Biochem. Eng. J., 2015, 103, 198–204 CrossRef CAS.
- J. Yu, Z. Xu, L. Liu, S. Chen, S. Wang and M. Jin, Bioresour. Technol., 2019, 279, 10–16 CrossRef CAS.
- R. A. Alexander, G. M. Innasimuthu, S. K. Rajaram, P. M. Jeganathan and S. Chellam Somasundarar, Environ. Prog. Sustainable Energy, 2020, 39, 13289 CrossRef CAS.
- Y. Wang, X. Guo, K. Li, Y. Nan, J. Wang, J. Zhang, S. Dou, L. Li, G. Liu and M. Yang, Ind. Crops Prod., 2019, 141, 111806 CrossRef CAS.
- J. L. Wong, S. N. B. A. Khadaroo, J. L. Y. Cheng, J. J. Chew, D. S. Khaerudini and J. Sunarso, Next Mater., 2023, 1, 100012 CrossRef.
- Citing an OriginLab Product, https://www.originlab.com/index.aspx?go=Company%26pid=1130, accessed January 22, 2025.
- C. G. Yoo, X. Meng, Y. Pu and A. J. Ragauskas, Bioresour. Technol., 2020, 301, 122784 CrossRef CAS PubMed.
- L. Das, E. C. Achinivu, C. A. Barcelos, E. Sundstrom, B. Amer, E. E. K. Baidoo, B. A. Simmons, N. Sun and J. M. Gladden, ACS Sustainable Chem. Eng., 2021, 9, 4422–4432 CrossRef CAS.
- Y. Jing, L. Dong, Y. Guo, X. Liu and Y. Wang, ChemSusChem, 2020, 13, 4181–4198 CrossRef CAS.
- D. Stewart, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- Y.-S. Cheng, Y. Zheng, C. W. Yu, T. M. Dooley, B. M. Jenkins and J. S. VanderGheynst, Appl. Biochem. Biotechnol., 2010, 162, 1768–1784 CrossRef CAS PubMed.
- C. Martín and A. B. Thomsen, J. Chem. Technol. Biotechnol., 2007, 82, 174–181 CrossRef.
- N. Sun, F. Xu, N. Sathitsuksanoh, V. S. Thompson, K. Cafferty, C. Li, D. Tanjore, A. Narani, T. R. Pray, B. A. Simmons and S. Singh, Bioresour. Technol., 2015, 186, 200–206 CrossRef CAS.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed.
- A. E. Ray, C. Li, V. S. Thompson, D. L. Daubaras, N. J. Nagle and D. S. Hartley, in Biomass Volume Estimation and Valorization for Energy, ed. J. S. Tumuluru, InTech, 2017 Search PubMed.
- W. B. Betts, R. K. Dart, A. S. Ball and S. L. Pedlar, in Biodegradation, ed. W. B. Betts, Springer London, London, 1991, pp. 139–155 Search PubMed.
- F. Brienza, D. Cannella, D. Montesdeoca, I. Cybulska and D. P. Debecker, RSC Sustainability, 2024, 2(1), 37–90 RSC.
- N. Uppugundla, L. da Costa Sousa, S. P. Chundawat, X. Yu, B. Simmons, S. Singh, X. Gao, R. Kumar, C. E. Wyman, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2014, 7, 72 CrossRef PubMed.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, D. Sexton and D. Dudgeon, Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover, National Renewable Energy Laboratory (NREL), Golden, CO (United States), 2011 Search PubMed.
- X. Li, X. Jin, Y. Wu, D. Zhang, F. Sun, H. Ma, A. Pugazhendhi and C. Xia, Sci. Total Environ., 2023, 876, 162549 CrossRef CAS.
- D. M. Alonso, S. H. Hakim, S. Zhou, W. Won, O. Hosseinaei, J. Tao, V. Garcia-Negron, A. H. Motagamwala, M. A. Mellmer, K. Huang, C. J. Houtman, N. Labbé, D. P. Harper, C. Maravelias, T. Runge and J. A. Dumesic, Sci. Adv., 2017, 3, e1603301 CrossRef.
- E. C. Achinivu, B. W. Blankenship, N. R. Baral, H. Choudhary, R. Kakumanu, M. Mohan, E. E. K. Baidoo, C. D. Scown, A. George, B. A. Simmons and J. Gladden, Chem. Eng. J., 2023, 147824 Search PubMed.
- A. G. Cruz, C. Scullin, C. Mu, G. Cheng, V. Stavila, P. Varanasi, D. Xu, J. Mentel, Y.-D. Chuang, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2013, 6, 52 CrossRef CAS.
- R. Parthasarathi, J. Sun, T. Dutta, N. Sun, S. Pattathil, N. V. S. N. Murthy Konda, A. G. Peralta, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2016, 9, 160 CrossRef.
- E. Pucéat, B. Reynard and C. Lécuyer, Chem. Geol., 2004, 205, 83–97 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5su00266d |
| This journal is © The Royal Society of Chemistry 2025 |