Open Access Article
Open Access ArticleSurfactant-free gold nanoparticles synthesized in alkaline water–ethanol mixtures: leveraging lower grade chemicals for size control of active nanocatalysts†
Frederik
Jæger‡
,
Albert Abildtrup
Pedersen‡
,
Peter Stensgaard
Wacherhausen‡
,
Aleksandra
Smolska‡
 and
Jonathan
Quinson
and
Jonathan
Quinson
 *
*
Nanomaterials Engineering for Sustainable Technologies (NEST) Group, Biological and Chemical Engineering Department, Aarhus University, 40 Åbogade, 8200 Aarhus, Denmark. E-mail: jquinson@bce.au.dk
First published on 5th June 2025
Abstract
Low(er) purity chemicals are often detrimental for nanomaterial synthesis. Here, lower grade chemicals, e.g. lower grade water, are used to achieve size control over surfactant-free gold nanoparticles simply obtained at room temperature in alkaline ethanol–water mixtures. The nanoparticles are active catalysts, e.g. for water treatment with the example of the reduction of 4-nitrophenol. The simple strategy reported to obtain size-controlled gold nanoparticles opens new door for the holistic development of sustainable nanotechnologies.
Sustainability spotlightNanotechnologies can address several of the UN's Sustainable Development Goals (SDGs), provided green synthesis methods of nanomaterials are developed. In contrast to a widespread preference for high purity and expensive chemicals, we show the benefits of using lower purity chemicals to develop more sustainable syntheses of nanomaterials. As an example, gold nanoparticles with a relatively fine size control are simply obtained at room temperature in water–ethanol mixtures without surfactants under only slightly alkaline conditions. The nanomaterials are relevant nanocatalysts for water treatment. The research aligns with UN SGDs: industry, innovation and infrastructure (SGD 9), clean water and sanitation (6). The technology is also ultimately relevant for affordable and clean energy (SDG 7) and the concept for education purposes (SDG 4). |
Introduction
Nanomaterials and nanoparticles (NPs) are promising materials with outstanding size-dependent properties relevant for catalysis, energy conversion, water/air treatment, sensing, optics, medicine, and many more.1–4 The full exploitation of the unique properties of NPs relies on their careful preparation, processing and combination with other (nano)materials.5,6 The holistic development of more sustainable nanotechnologies calls for the development of more sustainable and more affordable preparation methods of nanomaterials.7,8 Unfortunately, the development of alternative and more sustainable preparation methods of nanomaterials is far from trivial. Indeed, the formation and production of NPs, and therefore the resulting properties of the NPs, can strongly depend on multiple parameters such as the nature of the chemicals and/or processes used,9 but also the purity of the related chemicals and solvents.10,11In the quest to develop more sustainable and green technologies,7 using commercially available solvents12 or relatively low grade and lower purity solvents and chemicals,13 are attractive sustainable options, for instance, using by-products of waste-generating technologies.14 It is worth stressing that these lower grade chemicals are also often cheaper and therefore more likely to be relevant for scale up. Water in particular is a to-be-preferred solvent,15 although it is known that the purity of water can strongly impact the outcome of NP syntheses.10 An example is the synthesis of gold (Au) nanoparticles (NPs), where high purity water is highly recommended.10,16 The need for high purity water is even more important in so-called surfactant-free (SF) colloidal syntheses, defined elsewhere,17 where electrostatic interactions play a key role in the NP stabilization.4,13,17
In particular, recently reported SF syntheses of precious metal NPs performed in alkaline water–ethanol mixtures with high water contents (e.g. 80 v% water) present the benefits: (i) to require low energy (low temperature processes);18,19 (ii) to require few and benign chemicals, e.g. no need for fossil-fuels-derived surfactants and yet the resulting colloids are (iii) stable for months;13,18,20 (iv) to be easily implemented at laboratory scale and yet are scalable;18,19 (iv) to limit waste by limiting post-treatment of the materials obtained in non-viscous media (as opposed to alternative using polyols that require waste-generating washing steps typically in acids);21 (vi) to allow simple recycling of the solvent.19 These features overall lead to a better use of the precious metals leading for instance to more active (electro)catalysts.17–19,21,22
Motivation
However, at the practical level, e.g. at large scale, high purity water might be too expensive to develop sustainable and truly green technologies. This consideration pushed us to explore alternatives to the state-of-the-art use of high purity water (mQ, Milli-Q® Millipore Water, 18.2 MΩ cm) for the synthesis of Au NPs. In most cases, syntheses performed in lower purity water do not lead to stable Au NPs colloids.10,13,16 Here, rather than completely ruling out the possibility to use low(er) purity water, we exploit the features of non-ideal case synthesis scenarios to achieve a fine size control over SF Au NPs obtained in alkaline ethanol–water mixtures at room temperature (RT) by tuning the amount of lower purity water. We also prefer the use of lower purity gold precursor and low purity stock solution of ethanol. It is also shown how the NPs obtained using relatively low grade chemicals are readily active catalysts for the reduction of 4-nitrophenol (4-NP) for water treatment23 and to study and exploit various size-dependent effects at the nanoscale.Experimental
A detailed account of the experimental methods, metrics and how the selected strategy complies with the principles of sustainable research is provided in ESI.†7,8,24,25General synthesis
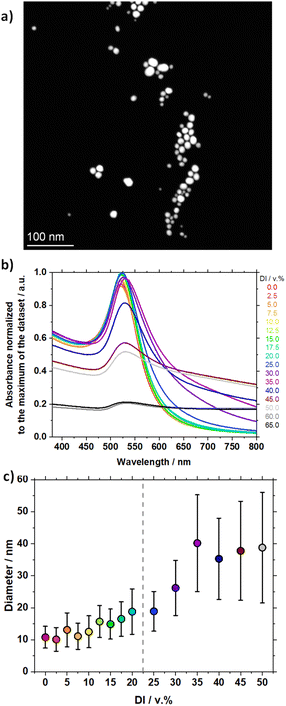
An alkaline mixture of an alcohol and water leads to the formation of alkoxides that can play the role of reducing agents for the room temperature reduction of AuIII in HAuCl4 to Au0 in NPs.18,26 Adding concentrated HAuCl4 to an alkaline mixture of water and ethanol leads to the appearance of a grey color that then turns blue, deep purple, purple and finally red, see Fig. S1.† The red colour indicates the formation of spherical Au NPs ca. 10–20 nm in size. This synthesis has largely been documented elsewhere,13,18,27,28 and typical STEM and UV-vis data for the obtained materials using 0.5 mM HAuCl4, 2 mM NaOH in 80 v% mQ water and 20 v% ethanol are reported in Fig. 1a and b (in red), respectively. The synthesis can easily be performed with relatively lower grade HAuCl4, Fig. 1a, without significant benefits to use a higher grade precursor, see Fig. S2.† The synthesis is sensitive to the amount of base,18,29 but tuning the NaOH/Au molar ratio does not lead to a simple nor fine size control, as illustrated in Fig. S3.†Size control
To assess the effect of the content of DI water, samples with 0.0 to 65.0 v% DI water were prepared (still with 20 v% ethanol and using otherwise mQ). Using higher amounts of DI water does not lead to any improvement to the synthesis but rather leads to unstable colloidal dispersions.13 The UV-vis spectra of the obtained colloidal Au NPs are reported in Fig. 1b. When a controlled amount of DI water is added for the reactions, the UV-vis spectra of the Au NPs show distinctive features as a function of the DI water content, further detailed in Fig. S4.† At lower DI water amounts, a clear spr peak is observed around 520 nm and the absorption at high wavelengths is low. At higher DI water amounts the spectra show a spr at higher wavelengths and the absorption at high wavelengths is more pronounced.The shift of the position of the surface plasmon resonance (λspr) towards larger wavelengths as the amount of DI water increases, illustrated in Fig. 1b and detailed in Fig. S4b,† indicates a size increase that is confirmed by STEM analysis and illustrated in Fig. 1c. STEM micrographs and size distribution are provided in Fig. S5,† and summarized in Table S2.† The Au NP sizes, for which the corresponding amount of DI water is given in parenthesis, are 10.8 ± 3.4 nm (0.0 v%), 10.1 ± 3.7 nm (2.5 v%), 13.1 ± 5.3 nm (5.0 v%), 11.1 ± 4.1 nm (7.5 v%), 12.5 ± 5.0 nm (10.0 v%), 15.7 ± 5.0 nm (12.5 v%), 14.9 ± 4.7 nm (15.0 v%), 16.5 ± 5.4 nm (17.5 v%), 18.8 ± 7.1 nm (20 v%), 18.9 ± 6.2 nm (25.0 v%), 26.2 ± 8.6 nm (30 v%), 40.2 ± 15.1 nm (35.0 v%), 35.3 ± 12.7 nm (40.0 v%), 37.8 ± 15.5 nm (45.0 v%) and 38.8 ± 17.3 nm (50 v%). The relative standard deviation is in the range 30–40% which is commonly observed for SF Au NPs.18,20 The samples obtained with 60.0 v% and 65.0 v% did not lead to stable colloids, in agreement with the relatively low absorbance observed in UV-vis measurements in Fig. 1b, and were not characterized by STEM.
In terms of morphology, below ca. 25 v% of DI water, the Au NPs are rather spherical, whereas for higher DI water contents, the Au NPs start showing less defined forms and worm-like structures illustrated in Fig. S5.† This ‘cut-off’ around 20–25 v% is also observed considering other indicators such as the Aspr/A450 values, A380/A800, A650/Aspr and A400 values detailed in Sections S2 and S4 in ESI.†
The stability factor A380/A800 decreases as the amount of DI water increases, and the stability factor A650/Aspr increases as the amount of DI water increases, which in both cases shows a decrease in stability as the content in DI water increases, as reported in Fig. S4.† Equally, the relative yield evaluated by the UV-vis absorbance at 400 nm is relatively constant up to ca. 35 v% DI water and decreases for higher DI water content. Despite the negative effect of DI water, suggesting the strong sensitivity of the SF Au NPs to electrostatic interactions, small amount of DI water up to ca. 20–25 v% leads to a relative fine control on the Au NP size.
Robustness
Batch to batch variations are to be expected when it comes to NP synthesis,10,30 also illustrated in Fig. S2.† The trends detailed above are reproducible, as detailed in ESI† where we report experiments performed by less experienced researchers (bachelor level, trained for only few days, see Section S6†) and using higher purity ethanol (see Section S7†). In all cases, the use of higher amounts of DI water leads to larger NPs and above ca. 20–25 v% the NPs tend to not be spherical anymore. Although the kinetics of formation were not studied in detail, it was generally observed that the time required to observe a colour change towards a blue or red colour was longer as the amount of DI water increased. This is in agreement with our experience that the apparently slower formation of the Au NP leads to larger NPs, previously documented using controlled light environments and lights with different wavelengths.27Achievements
To date, strategies to achieve size control in the detailed RT SF colloidal synthesis of Au NPs using mono-alcohols as the source of the reducing agents were: (i) to add methanol to ethanol keeping the total amount of mono-alcohol constant,18 where larger NPs are obtained as the methanol content increases, unfortunately methanol is a relatively toxic chemical;15 (ii) tune the NaOH/Au molar ratio, where too high or too low values around 4 lead to larger NPs, however the size control is relatively limited;18,29 (iii) use controlled light environment, where the use of lights with lower wavelengths lead to smaller NPs, however this approach requires specific equipment and a limited throughput (limited to the number of lamps with controlled wavelengths available);27 (iv) induced the synthesis in different ways; where sono-chemistry leads to smaller size Au NPs compared to stirring and manual shaking to even larger NPs;30 however this approach requires performing the synthesis in different approaches and can be subjected to significant batch to batch variations.30 An alternative fifth and relatively simpler strategy is here reported (v) by controlling the content of DI water, with the benefits that the same stock solution of DI water is used, the relative size of the different Au NPs is well and finely controlled compared to previous approaches. The fine size control is attributed to the destabilization of the SF Au NPs that increases as the amount of DI water increases and that favours the formation of larger NPs.Polyols-based syntheses
Glycerol31 and ethylene glycol32 are suitable alternative to ethanol but their high viscosity prevents a simple work-up of the as-prepared Au NPs.21 In Fig. S11† are reported the effect of adding DI water to mQ water for the synthesis using glycerol or ethylene glycol. The effect of DI water to control the size of the Au NPs is still observed but less pronounced than for ethanol-mediated synthesis. This is in line with our previous observation that the synthesis using glycerol is generally more robust, i.e. less sensitive to the variations of base concentration and/or alcohol contents and/or light conditions.27 Using ethylene glycol leads to synthesis a bit more sensitive to such parameters, but still less sensitive than ethanol-mediated syntheses. It is here shown that the sensitivity to DI water increases in the order glycerol < ethylene glycol < ethanol. This observation, together with previous results on the sensitivity of the synthesis to the variations of other parameters,27 might account for the preferred use of polyols to date. The higher viscosity of the polyols is expected to favour the stabilization of the NPs, which could in part explain those results.Stability
The SF Au NPs prepared in 80 v% mQ are stable for months as illustrated in Fig. S12.† The study overtime (weeks) of the SF colloidal dispersions confirms the trend that higher DI water contents lead to less stable colloids. This is especially clear considering the A400 values that indicate that the amount of Au0 in the colloidal dispersions33 decreases over time, which can be correlated to the sedimentation of the Au NPs, Fig. S13.† A simple shake of the solution shows that the sedimented NPs re-disperse rather well. As the amount of DI water decreases, the dispersions are more stable and even stable for years in the case of 0.0 v% DI water as documented elsewhere.27,30The stability of the SF NPs is attributed to electrostatic stabilization. This argument is in line with zeta-potential measurements leading to values between −15 or −78 mV when the analysis is performed by inputting the properties of water or ethanol in the analysis software.18 These low values correlate well with the stability observed over time. A further argument for stabilization by electrostatic is that the cations used influence the stability in the decreasing order of stability Li+ > Na+ > K+.18 The negative values from zeta-potential measurements could then suggest a role of the Cl− anion in the stabilisation. We also observed that adding NaCl can lead to destabilization of the NPs although the NP remain stable for a relatively large range of NaCl concentrations.30 Along those considerations, it cannot be excluded that pH is also likely to have an influence on the stability of the NPs, although an accurate measurement can be complex here, as detailed in ESI.† Preliminary results in this direction suggest that the pH drops upon adding HAuCl4 from ca. 9–10 and stabilizes at a value typically around 4–5. This is in agreement with the expected equation of the reaction taking place, detailed elsewhere.18 However, using a pH probe often leads to destabilization of the colloids, which actually indirectly strongly suggests an electrostatic stabilization of the colloids. Finally, it cannot be excluded that oxidation products of ethanol such as acetaldehyde can also play a role in the stabilization, although surface species on the Au NPs remain challenges to probe and this is beyond the scope of this report.
The likely role of electrostatic stabilization explains why the size and stability of the NPs is sensitive to potential impurities10 and to the conductivity of the media used for synthesis which increases as the amount of DI water used increases, as detailed in ESI,† see for instance Fig. S14.†
Size effects
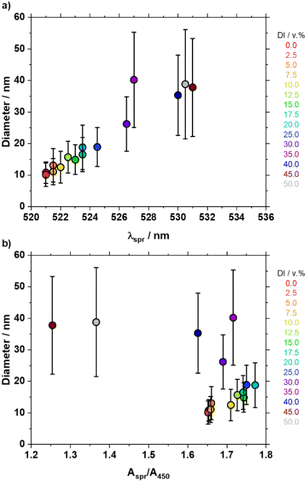
A first benefit of the size control achieved is to correlate various optical properties of the NPs to their size, illustrated in Fig. 2. As expected, lower λspr and lower Aspr/A450 values relate to smaller Au NP sizes.34 However a closer look at the UV-vis spectra and Aspr/A450 values show that very low values, e.g. below 1.6, are not due to smaller size NPs but rather non-spherical NPs which is confirmed by STEM (samples with DI water amounts larger than 25 v%) as well as the relatively large corresponding λspr values. This explains why the Aspr/A450 value first increases as the amount of DI water increases (size effect), and then decreases as the amount of DI water keeps increasing (morphology effect) while the λspr values keep increasing, see Fig. S4 and S6.† In contrast, the relationship lower λspr values – smaller NP size is rather well maintained for all the samples in this study, see also Fig. S10.† Regarding other metrics, higher A380/A800 and lower A650/Aspr, also relate to smaller size and spherical Au NPs that leads to more stable colloids.Catalysis
Au NPs obtained by the present RT SF strategy in alkaline water–ethanol mixtures are convenient and readily active catalysis demonstrated previously for electrocatalytic reactions, such as the ethanol oxidation reaction (EOR), relevant for energy conversion in direct alcohol fuel cells.18,21,30 Here we explore the relevance of such NPs for water treatment. As an illustration and as a model system largely reported in the literature,23,35,36 the reduction of 4-NP in presence of NaBH4 was also performed. The reaction can easily be monitored by following the decrease in the absorption at 400 nm in UV-vis spectra,37,38 as illustrated in Fig. S15a and detailed in Section S1.† The results are summarized in Table S5 in ESI† and details on the challenges to benchmark this reaction are provided elsewhere38 and discussed in ESI, e.g. Fig. S15 and Section S11.† Presentation of the data including repeats and different normalization is proposed in Fig. S16–S18.†The advantage of preparing size-controlled Au NPs is to perform studies of size-dependent effects that are usually pronounced at the nanoscale. The samples selected had a size of 10.8 ± 3.4 nm, 13.1 ± 5.3 nm, 12.5 ± 5.0, 15.7 ± 5.0, 18.8 ± 7.1 nm and 26.2 ± 8.6 nm and were obtained with 0.0 v%, 5.0 v%, 10.0. v%, 12.5 v%, 20.0 v% and 30.0 v% of DI water, respectively, as illustrated in Fig. 3 and see also S17.† As the NP size increases, the TOF for 4-NP reduction decreases, as expected from previous literature.39 Similar results were obtained using NPs prepared using higher purity ethanol as stock solution of the alcohol, see Fig. S17.† Although the presence of alcohol in several v% was shown to slow down or even suppress the reduction of 4-NP,40 it is here shown that traces of ethanol from the synthesis of the Au NPs are not a challenge. A control experiment using directly HAuCl4 – that can form NPs in presence of NaBH4 – did not lead to a high TOF,41, see Fig. S16,† stressing the optimal strategy to use pre-formed NPs for the reaction. In previous reports, Au NPs prepared using only NaBH4, water and a gold precursor were shown to be very active for the 4-NP reduction, in part due to the small size of the Au NPs obtained around 5 nm.37 Au NPs were prepared by this method in the size range of 10 nm to allow a fair comparison. The Au NPs prepared using NaBH4 showed lower TOF values that those estimated for the SF Au NPs obtained using ethanol–water mixtures with 0% DI water, or similar values to those obtained using up to 12.5 v% DI water despite the larger size of the latest. Overall, the results stress the promising catalytic activity of the here prepared SF Au NPs.
 | ||
| Fig. 3 TOF% as a function of the NP diameter for Au NPs prepared using different amounts of DI water or NaBH4, as indicated. | ||
Conclusions
SF Au NPs are easily obtained at RT in alkaline water–ethanol mixtures. The synthesis is relatively green and (i) is performed with simple equipment, (ii) with low energy requirement (RT), (iii) requires only relatively benign chemicals, (iv) generates no or little waste during synthesis and/or for further use of the NPs given that low boiling point solvents are used.It is shown that there is no need for high purity chemicals for a successful synthesis. Low(er) grade HAuCl4, ethanol and water can be used to produce the Au NPs. The results stress the importance of controlling water purity for the RT SF Au NPs in alkaline water–ethanol mixtures. While it is typically considered as a disadvantage to use low(er) purity water, it is here used to develop an alternative strategy to those reported to date to tune the NP size and achieve a relatively fine size control. Controlling the DI water content in mQ water and 20 v% ethanol enables to achieve a size control in the range 10–30 nm. Increasing the DI water content leads to larger Au NPs.
The obtained Au NPs are readily relevant to study size effects in catalysis, for example for the 4-NP reduction. The smaller NPs lead to the more active catalysts for the 4-NP reduction. The NPs obtained by the proposed method are more active than NPs obtained using for instance harmful chemicals such as NaBH4.
The unique combination of a simple synthesis easily implementable by researchers without prior expertise in nanomaterial synthesis, that allows a simple yet fine control over Au NP size, and that leads to readily active NPs for catalysis, opens a range of opportunities. The presented synthesis leads to nanomaterials relevant for nanomaterial science,42 catalysis,43 water/air treatment,44 energy conversion,45 addressing climate change,46 sensing,47 optics48 and/or medicine,49 and the concept is relevant for education.50 It is expected that the simple and sustainable synthetic strategy reported will enable the holistic development of more sustainable nanotechnologies.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: AS, JQ; data curation: all authors; formal analysis: all authors; funding acquisition: JQ; investigation: all authors; methodology: AS, JQ; project administration: JQ; resources: JQ; supervision: AS, JQ; validation: FJ, AAP, PSW, AS; visualization: all authors; writing – original draft: JQ; writing – review and editing: all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Espen D. Bøjesen, iNano, Aarhus University, Denmark, is thanked for facilitating access to the Talos F200X. This research benefited from the support of the Aarhus University Research Foundation (AUFF-E-2022-9-40). JQ and AS thank the Independent Research Fund (DFF) for support via a DFF-Green grant (Light-SCREEN, 3164-00128B).References
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. B. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- D. S. Zhang, B. Goekce and S. Barcikowski, Chem. Rev., 2017, 117, 3990 Search PubMed.
- C. A. Charitidis, P. Georgiou, M. A. Koklioti, A.-F. Trompeta and V. Markakis, Manuf. Rev., 2014, 1, 11 Search PubMed.
- S. Reichenberger, G. Marzun, M. Muhler and S. Barcikowski, ChemCatChem, 2019, 11, 4489 CrossRef CAS.
- J. Quinson, S. Kunz and M. Arenz, ChemCatChem, 2021, 13, 1692 CrossRef CAS.
- J. Du, J. Quinson, A. Zana and M. Arenz, ACS Catal., 2021, 11, 7584 CrossRef CAS.
- J. E. Hutchison, ACS Sustainable Chem. Eng., 2016, 4, 5907 Search PubMed.
- L. M. Gilbertson, J. B. Zimmerman, D. L. Plata, J. E. Hutchison and P. T. Anastas, Chem. Soc. Rev., 2015, 44, 5758 Search PubMed.
- P. Lettenmeier, J. Majchel, L. Wang, V. A. Saveleva, S. Zafeiratos, E. R. Savinova, J. J. Gallet, F. Bournel, A. S. Gago and K. A. Friedrich, Chem. Sci., 2018, 9, 3570 RSC.
- L. M. Liz-Marzan, C. R. Kagan and J. E. Millstone, ACS Nano, 2020, 14, 6359 CrossRef CAS.
- N. El Amri and K. Roger, J. Colloid Interface Sci., 2020, 576, 435 Search PubMed.
- J. Quinson, S. B. Simonsen, L. Theil Kuhn and M. Arenz, Sustainable Chem., 2021, 2, 1 Search PubMed.
- J. Quinson, Gold Bull., 2024, 57, 27 Search PubMed.
- R. Parveen and G. Tremiliosi, RSC Adv., 2016, 6, 95210 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288 Search PubMed.
- C. D. De Souza, B. R. Nogueira and M. Rostelato, J. Alloys Compd., 2019, 798, 714 Search PubMed.
- J. Quinson, S. Kunz and M. Arenz, ACS Catal., 2023, 13, 4903 Search PubMed.
- J. Quinson, O. Aalling-Frederiksen, W. L. Dacayan, J. D. Bjerregaard, K. D. Jensen, M. R. V. Jørgensen, I. Kantor, D. R. Sørensen, L. Theil Kuhn, M. S. Johnson, M. Escudero-Escribano, S. B. Simonsen and K. M. Ø. Jensen, Chem. Mater., 2023, 35, 2173 CrossRef CAS PubMed.
- J. Quinson, S. Neumann, T. Wannmacher, L. Kacenauskaite, M. Inaba, J. Bucher, F. Bizzotto, S. B. Simonsen, L. T. Kuhn, D. Bujak, A. Zana, M. Arenz and S. Kunz, Angew. Chem., Int. Ed., 2018, 57, 12338 CrossRef CAS PubMed.
- M. Varga and J. Quinson, ChemistrySelect, 2025, 10, e202404819 CrossRef CAS.
- J. Quinson, T. M. Nielsen, M. Escudero-Escribano and K. M. Ø. Jensen, Colloids Surf., A, 2023, 675, 131853 CrossRef CAS.
- F. Bizzotto, J. Quinson, J. Schröder, A. Zana and M. Arenz, J. Catal., 2021, 401, 54 Search PubMed.
- P. Zhao, X. Feng, D. Huang, G. Yang and D. Astruc, Coord. Chem. Rev., 2015, 287, 114 CrossRef CAS.
- H. H. Duan, D. S. Wang and Y. D. Li, Chem. Soc. Rev., 2015, 44, 5778 RSC.
- T. Freese, N. Elzinga, M. Heinemann, M. M. Lerch and B. L. Feringa, RSC Sustain., 2024, 2, 1300 RSC.
- J. F. Gomes, A. C. Garcia, E. B. Ferreira, C. Pires, V. L. Oliveira, G. Tremiliosi-Filho and L. H. S. Gasparotto, Phys. Chem. Chem. Phys., 2015, 17, 21683 RSC.
- D. R. Rasmussen, N. Lock and J. Quinson, ChemSusChem, 2025, 18, e202400763 CrossRef.
- J. Quinson, Inorganics, 2023, 11, 140 CrossRef CAS.
- T. Jensen, J. Saugbjerg, M. Henriksen and J. Quinson, Colloids Surf., A, 2024, 702, 135125 Search PubMed.
- D. Panagopoulos, A. Asghari Alamdari and J. Quinson, Mater. Today Nano, 2025, 29, 100600 CrossRef CAS.
- R. Parveen, S. Ullah, R. Sgarbi and G. Tremiliosi, Colloids Surf., A, 2019, 565, 162 CrossRef CAS.
- D. R. Rasmussen, M. F. Nielsen and J. Quinson, Chemistry, 2023, 5, 900 CrossRef CAS.
- T. Hendel, M. Wuithschick, F. Kettemann, A. Birnbaum, K. Rademann and J. Polte, Anal. Chem., 2014, 86, 11115 CrossRef CAS PubMed.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79, 4215 Search PubMed.
- Y. Mejía and N. Bogireddy, RSC Adv., 2022, 12, 18661 Search PubMed.
- T. K. Das and N. C. Das, Int. Nano Lett., 2022, 12, 223 CrossRef CAS.
- C. Deraedt, L. Salmon, S. Gatard, R. Ciganda, R. Hernandez, J. Ruiz and D. Astruc, Chem. Commun., 2014, 50, 14194 RSC.
- N. E. Larm, N. Bhawawet, J. A. Thon and G. A. Baker, New J. Chem., 2019, 43, 17932 RSC.
- H. S. Devi, N. R. Singh, H. P. Singh and T. D. Singh, J. Environ. Chem. Eng., 2015, 3, 2042 CrossRef CAS.
- V. Lomonosov, J. Asselin and E. Ringe, React. Chem. Eng., 2022, 7, 1728 Search PubMed.
- X. Wu, X. Wu, J. Shen and H. Zhang, RSC Adv., 2014, 4, 49287 Search PubMed.
- M. A. Wall, S. Harmsen, S. Pal, L. H. Zhang, G. Arianna, J. R. Lombardi, C. M. Drain and M. F. Kircher, Adv. Mater., 2017, 29, 1605622 CrossRef.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- R. D. Neal, R. A. Hughes, P. Sapkota, S. Ptasinska and S. Neretina, ACS Catal., 2020, 10, 10040 CrossRef CAS.
- Y. Y. Zhang, J. G. Wang, X. F. Yu, D. R. Baer, Y. Zhao, L. Q. Mao, F. Y. Wang and Z. H. Zhu, ACS Energy Lett., 2019, 4, 215 CrossRef CAS.
- M. Miola, X. M. Hu, R. Brandiele, E. T. Bjerglund, D. K. Gronseth, C. Durante, S. U. Pedersen, N. Lock, T. Skrydstrup and K. Daasbjerg, J. CO2 Util., 2018, 28, 50 CrossRef CAS.
- L. Qin, G. M. Zeng, C. Lai, D. L. Huang, P. A. Xu, C. Zhang, M. Cheng, X. G. Liu, S. Y. Liu, B. S. Li and H. Yi, Coord. Chem. Rev., 2018, 359, 1 CrossRef CAS.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Marago and M. A. Iati, J. Condens. Matter Phys., 2017, 29, 203002 CrossRef PubMed.
- J. B. Vines, J. H. Yoon, N. E. Ryu, D. J. Lim and H. Park, Front. Chem., 2019, 7, 167 Search PubMed.
- J. Quinson, J. Chem. Educ., 2023, 100, 3612 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5su00213c |
| ‡ Equally contributing authors. |
| This journal is © The Royal Society of Chemistry 2025 |