Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Powering the sustainable future: a review of emerging battery technologies and their environmental impact
Peeyush
Phogat
 *ab,
Subhadeepa
Dey
a and
Meher
Wan
a
*ab,
Subhadeepa
Dey
a and
Meher
Wan
a
aCSIR–National Institute of Science Communication and Policy Research, Pusa, New Delhi, India. E-mail: email-peeyush.phogat@gmail.com
bResearch Lab for Energy Systems, Department of Physics, Netaji Subhas University of Technology, Dwarka, New Delhi, India
First published on 6th June 2025
Abstract
This review paper provides a comprehensive analysis of various battery technologies, categorizing them into primary (non-rechargeable), secondary (rechargeable), specialty, and emerging battery types. It delves into the key properties of these batteries, including energy density, cycle life, cost, environmental impact, and their suitability for different applications. The review highlights the environmental implications of each battery type, focusing on the sustainability of materials and manufacturing processes. Furthermore, it discusses potential future trends in battery technology, including advancements in solid-state batteries, nanotechnology, recycling techniques, and alternative chemistries. The paper concludes by summarizing key findings and emphasizing the importance of ongoing research and innovation in the field to meet the increasing global demand for efficient and sustainable energy storage solutions.
Sustainability spotlightBatteries have become indispensable in modern technology, powering everything from portable electronics to large-scale renewable energy storage systems. As the global demand for energy-efficient and sustainable solutions continues to grow, advancements in battery technologies are pivotal in shaping the future of energy storage. Traditional primary and secondary batteries have dominated the market for decades, but limitations in energy density, cycle life, and environmental sustainability have driven the search for innovative alternatives. Emerging battery technologies, such as solid-state, graphene, and sodium-ion batteries, promise breakthroughs in performance and sustainability. This review offers a comparative analysis of various battery types, highlighting their strengths, limitations, and environmental impacts. By examining key parameters such as energy density, cost, and recyclability, this paper underscores the need for further innovation in materials and design to meet the evolving demands of industries ranging from consumer electronics to electric vehicles. Moreover, the environmental implications of current technologies are scrutinized, emphasizing the urgent need for greener solutions. The findings presented here provide valuable insights for researchers, policymakers, and industry stakeholders, guiding future developments in energy storage systems that align with global sustainability goals. |
1. Introduction
Batteries play a crucial role in the modern technological landscape, acting as the primary energy source for an ever-expanding range of applications. Their development over the past two centuries has led to dramatic improvements in energy storage capabilities, making possible innovations in electronics, electric vehicles (EVs), and renewable energy systems.1–4 The demand for efficient, reliable, and environmentally sustainable batteries has never been higher, driven by the global push for cleaner energy and the proliferation of portable electronic devices.5,6 Understanding the history, categories, and importance of different battery technologies is key to selecting the right battery for specific applications and pushing the field forward through continued research.The history of battery development dates back to the early 19th century when Alessandro Volta invented the voltaic pile in 1800, considered the first true battery. This rudimentary device consisted of alternating layers of zinc and copper, separated by cardboard soaked in saltwater, which generated electricity through chemical reactions. Volta's invention laid the groundwork for the electrochemical power storage technologies we rely on today. Over the following decades, various improvements were made to battery technologies, leading to the development of the Daniell cell in 1836, which provided more reliable and steady current compared to the voltaic pile. By the late 1800s, the lead–acid battery, invented by Gaston Planté in 1859, revolutionized energy storage with its rechargeable capabilities. It was the first rechargeable battery to see widespread use and remains relevant in automotive and stationary energy storage applications today. Following this, the development of the dry cell in the late 19th century, which used a paste electrolyte rather than liquid, provided the foundation for modern batteries. This design eliminated the leakage problems associated with early batteries and made portable power possible. As the 20th century progressed, innovations such as the nickel–cadmium (NiCd) battery and the nickel–metal hydride (NiMH) battery added to the portfolio of rechargeable batteries, each with improvements in cycle life and energy density.7,8 However, the most transformative development came in the 1980s with the commercialization of lithium-ion (Li-ion) batteries. Introduced by Sony in 1991, Li-ion batteries provided significant improvements in energy density and cycle life, along with the advantage of being lighter than previous technologies. Today, Li-ion batteries dominate a wide array of applications, from smartphones and laptops to electric vehicles and renewable energy storage systems. In modern technology, batteries have become indispensable.9–12 The power everything from the smallest electronic devices, such as smartwatches and hearing aids, to large-scale energy storage systems that stabilize power grids. The significance of batteries extends to every sector, particularly in transportation with the rise of electric vehicles (EVs). As global efforts to reduce greenhouse gas emissions intensify, EVs and battery energy storage are critical to transitioning from fossil fuels to renewable energy sources like solar and wind.13,14 Additionally, in consumer electronics, batteries allow for the portability and convenience that drive technological advancements. Furthermore, batteries are becoming increasingly important in military applications, space exploration, and medical devices, where reliability and long-lasting power are essential. Given this wide range of applications, the ongoing development of new battery technologies is vital to ensuring future energy needs are met efficiently and sustainably.
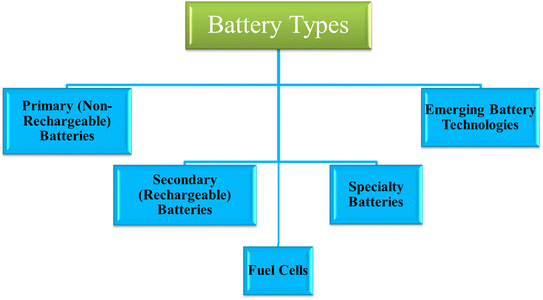
Batteries can be broadly categorized into three main types: primary (non-rechargeable), secondary (rechargeable), and specialty batteries. Additionally, emerging battery technologies represent a fourth category that focuses on cutting-edge developments with the potential to surpass current technologies in performance, sustainability, and scalability. Also there is Fuel cell which is also a type of energy conversion/generation as depicted in Fig. 1.
Primary batteries (non-rechargeable): primary batteries are designed for single-use applications where recharging is either impractical or unnecessary. These batteries are commonly found in devices like remote controls, flashlights, and medical equipment such as pacemakers.15 The most widely used types of primary batteries include alkaline, zinc–carbon, and lithium primary batteries. Alkaline batteries, in particular, are the most common type of household battery due to their relatively low cost, availability, and sufficient energy density for most low-power applications. The limitation of primary batteries lies in their inability to be recharged, which results in waste and environmental impact when disposed of improperly. Although primary batteries have lower energy density and a shorter lifespan than rechargeable batteries, they are still preferred in applications where long-term use is less important or in devices where recharging is impractical.16
Secondary batteries (rechargeable): secondary batteries are rechargeable, making them ideal for applications where repeated use is necessary. The most common types of secondary batteries include lead–acid, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), and lithium-ion (Li-ion) batteries. Among these, lithium-ion batteries have gained widespread adoption due to their high energy density, long cycle life, and relatively low self-discharge rate. Rechargeable batteries are critical in applications such as electric vehicles, smartphones, laptops, and renewable energy storage systems. Li-ion batteries, in particular, have enabled the growth of electric vehicles and portable electronic devices by providing a reliable and lightweight power source with a relatively long lifespan.17 These batteries also support the transition to renewable energy by storing excess power generated by solar and wind systems, ensuring a consistent energy supply even when sunlight or wind is not available. Despite their advantages, secondary batteries face challenges in terms of cost, environmental impact, and energy density. For example, lead–acid batteries, though still widely used, are bulky and have a relatively low energy density compared to modern alternatives. NiCd batteries are durable but suffer from the “memory effect”, which can reduce their efficiency over time.18 NiMH batteries have partially addressed this issue but still lag behind Li-ion batteries in terms of energy density and charge efficiency. Thus, comparing the performance and sustainability of secondary batteries is essential to optimizing their use for various applications.19
Fuel cells are energy conversion devices that generate electricity through an electrochemical reaction, typically between hydrogen and oxygen, without combustion.20 Unlike traditional batteries, fuel cells continuously produce power as long as they have a fuel supply. They are known for their high energy efficiency, low environmental impact (producing water as the main byproduct), and scalability for various applications, from portable electronics to large-scale power generation.21 Fuel cells are especially promising for clean energy transitions, offering an alternative to fossil fuels with potential in transportation, backup power systems, and renewable energy integration.
Specialty batteries: specialty batteries are designed for specific, often demanding, applications that require unique performance characteristics. These batteries are used in extreme environments, such as high-temperature conditions, or in applications where energy density and durability are critical, such as in aerospace or medical devices.22 Some examples of specialty batteries include silver–zinc batteries, thermal batteries, and reserve batteries. Specialty batteries offer distinct advantages over standard primary and secondary batteries in terms of performance under specialized conditions. For example, silver–zinc batteries have high energy density and are often used in military applications and space missions where both reliability and energy output are critical. However, specialty batteries tend to be more expensive and less widely used than primary or secondary batteries, which limit their application to niche markets.23
Emerging battery technologies: emerging battery technologies represent the forefront of research and development in the energy storage field. These include solid-state batteries, sodium-ion batteries, lithium–sulfur batteries, and flow batteries.24 Each of these technologies aims to address some of the limitations associated with existing battery types, such as energy density, safety, cost, and environmental sustainability. Solid-state batteries, for example, replace the liquid electrolyte found in conventional Li-ion batteries with a solid electrolyte, which significantly improves safety and energy density. Sodium-ion batteries offer the potential for a more abundant and less expensive alternative to lithium-based batteries, though they currently lag behind in terms of energy density.25 Lithium–sulfur batteries promise a higher energy density than traditional Li-ion batteries, but they face challenges related to stability and cycle life. Flow batteries, which store energy in liquid electrolytes that flow through a cell, offer scalability for large-scale energy storage but are typically more expensive and complex. As these technologies continue to develop, their potential to revolutionize the battery industry grows. Comparing these emerging technologies against established primary, secondary, and specialty batteries is crucial for identifying the most promising solutions for future energy storage needs.
The comparison of different battery types is essential for several reasons. First, batteries are central to the functioning of numerous devices and systems, from portable electronics to electric vehicles and renewable energy storage systems. Choosing the right battery technology can significantly impact the efficiency, cost, and environmental footprint of these systems. Therefore, understanding the strengths and limitations of each battery type is key to making informed decisions for specific applications. One of the most important criteria for comparing batteries is energy density, which refers to the amount of energy a battery can store relative to its weight or volume. Higher energy density is particularly important in applications like electric vehicles and portable electronics, where reducing weight and size while maintaining performance is critical.26 Cycle life, or the number of charge–discharge cycles a battery can undergo before its capacity significantly degrades, is another critical factor, especially in rechargeable batteries used in applications like EVs and renewable energy systems. Longer cycle life reduces the need for battery replacements, lowering overall costs and environmental impact.
Another crucial property to compare is the environmental impact of each battery type. Batteries, especially those containing heavy metals like lead or cadmium, can pose significant environmental hazards if not disposed of properly.27,28 Even more environmentally friendly batteries, such as lithium-ion, have sustainability issues related to the extraction of raw materials, energy consumption during production, and recycling challenges. Thus, environmental considerations must be taken into account alongside performance characteristics.29 With the growing demand for efficient and sustainable energy storage, comparing battery technologies has never been more important. The rise of electric vehicles, the global push for renewable energy, and the increasing need for portable power solutions all drive the search for the most effective, reliable, and environmentally friendly battery technologies.30 This paper seeks to contribute to that effort by comparing the key properties of primary, secondary, specialty, and emerging battery technologies, providing insights into their potential applications and future development directions. Table 1 shows a comparative summary of key properties of different battery types.
| Battery type | Energy density (W h kg−1) | Shelf life | Cycle life (cycles) | Cost | Environmental impact |
|---|---|---|---|---|---|
| Primary (non-rechargeable) | 100–300 | 5–10 years | N/A | Low | High (disposal issues, limited recycling options) |
| Secondary (rechargeable) | 150–300 | 1–3 years | 500–2000+ | Medium-high | Moderate (varies by type, recycling possible) |
| Specialty | 200–400 | 10–20 years | 100–500 | High | Low to moderate (depends on specific application) |
| Emerging technologies | 300–500 | 5–15 years | 1000–5000+ | High | Low to moderate (focus on sustainability and recycling) |
2. Primary (non-rechargeable) batteries
Primary batteries, also known as non-rechargeable batteries, are single-use energy storage devices commonly used in various everyday applications. These batteries are designed for devices where long-term energy needs are low or where recharging is not practical. Although they cannot be recharged once depleted, primary batteries offer several advantages, such as low cost, ease of use, and wide availability. There are several types of primary batteries, each with distinct chemistries, applications, and performance characteristics. This section reviews the key types of primary batteries: Alkaline, Zinc–Carbon, Lithium, Silver Oxide, Zinc–Air, Mercury, and Copper–Zinc batteries, as illustrated in Fig. 2.Alkaline batteries: alkaline batteries are the most common type of primary battery, widely used in household devices such as remote controls, toys, and flashlights. They operate through a reaction between zinc and manganese dioxide, using an alkaline electrolyte (usually potassium hydroxide).31 Alkaline batteries are known for their relatively high energy density and long shelf life, typically lasting between five and ten years.32 They offer reliable performance for low-drain applications and are available in various sizes, including AA, AAA, C, and D cells.33 Alkaline batteries are affordable and disposable, although they contribute to environmental concerns when discarded improperly due to limited recyclability.
Zinc–carbon batteries: zinc–carbon batteries, one of the oldest types of primary batteries, operate on a similar principle to alkaline batteries but use an acidic electrolyte (ammonium chloride or zinc chloride). They are typically cheaper but offer lower energy density and shorter shelf life compared to alkaline batteries.34 Zinc–carbon batteries are often used in low-drain applications, such as clocks or small radios, where their lower performance is sufficient. Their limited lifespan and energy output have led to a decline in their usage, being replaced by more efficient alkaline batteries in most consumer applications.35
Lithium batteries: lithium primary batteries are known for their high energy density and long shelf life, often exceeding ten years.36 They are commonly used in applications requiring a reliable, long-lasting power source, such as smoke detectors, medical devices, and military equipment. Lithium batteries employ lithium as the anode material, which enables high voltage output and stability over time. Their lightweight nature and superior performance in extreme temperatures make them ideal for portable and specialized electronics.37 However, lithium batteries are more expensive compared to alkaline and zinc–carbon batteries, which limits their use to specific high-performance applications.
Silver oxide batteries: silver oxide batteries are compact and offer excellent energy density and stable output voltage, making them ideal for small, high-drain devices such as wristwatches, calculators, and hearing aids. They use silver oxide as the cathode material and zinc as the anode, with a potassium hydroxide or sodium hydroxide electrolyte. While they deliver reliable performance and long life, the high cost of silver limits their use to premium applications.38 Silver oxide batteries are also highly recyclable due to their valuable silver content, making them more environmentally friendly compared to other primary batteries.
Zinc–air batteries: zinc–air batteries are unique in that they use oxygen from the air as a reactant, which reduces their weight and increases energy density.39 These batteries are commonly used in hearing aids, where their small size and long operational life are particularly advantageous. Zinc–air batteries have a limited shelf life once activated because they rely on oxygen entering the cell, but their high energy-to-weight ratio makes them ideal for specialized uses. Recycling options for zinc–air batteries are limited, but they are considered relatively low in environmental impact compared to other primary battery types.
Mercury batteries: mercury batteries, once widely used in medical and military applications due to their stability and long life, have been largely phased out due to environmental concerns. These batteries used mercury oxide as the cathode and zinc as the anode, offering a steady voltage output and long shelf life. However, mercury is highly toxic, and the improper disposal of these batteries can lead to environmental contamination.40 As a result, their production has been banned or severely restricted in many countries, and they have been replaced by more environmentally friendly alternatives, such as silver oxide and zinc–air batteries.
Copper–zinc batteries: copper–zinc batteries, also known as Daniell cells, were among the earliest types of batteries developed in the 19th century. Though largely obsolete in modern consumer electronics, they hold historical significance for their role in early electrochemical research and development. The copper–zinc chemistry is now used in some specialized industrial applications and educational demonstrations but is rarely seen in commercial primary battery markets today.41
2.1. Energy density
Energy density, a crucial property of batteries, measures the amount of energy a battery can store relative to its weight or volume, typically expressed in watt-hours per kilogram (W h kg−1). Higher energy density implies that a battery can deliver more energy for a given size, making it an essential parameter for selecting batteries in various applications. In this section, we review and compare the energy densities of different primary battery types, including Alkaline, Zinc–Carbon, Lithium, Silver Oxide, Zinc–Air, Mercury, and Copper–Zinc batteries.Alkaline batteries are widely recognized for their higher energy density compared to other common primary batteries. With energy densities typically ranging between 100 and 300 W h kg−1, they perform well in devices requiring moderate power for extended periods.42 The relatively high energy density of alkaline batteries stems from their use of manganese dioxide as the cathode and zinc as the anode, coupled with an alkaline electrolyte such as potassium hydroxide. This chemical setup provides a higher potential difference and energy output than older systems like zinc–carbon batteries. Alkaline batteries are commonly used in household devices, such as remote controls, toys, and flashlights, where their energy density ensures prolonged usage before replacement. However, their energy density, while competitive in the primary battery market, falls short compared to more advanced systems like lithium and silver oxide batteries, limiting their utility in high-energy applications.
Zinc–carbon batteries represent one of the earliest and most widely used types of primary batteries but suffer from a relatively low energy density, typically around 40–70 W h kg−1.43,44 This lower energy density is due to the materials used in their construction, which include zinc as the anode and manganese dioxide as the cathode, with an acidic electrolyte such as ammonium chloride or zinc chloride.45 While these materials provide a cost-effective solution for low-drain applications, such as clocks or small radios, they do not deliver the energy output needed for more power-hungry devices. The lower energy density of zinc–carbon batteries means they require more frequent replacements compared to alkaline or lithium counterparts. Despite their affordability, the decline in usage of zinc–carbon batteries in favor of more energy-efficient technologies like alkaline and lithium batteries reflects their decreasing relevance in modern electronics.
Lithium primary batteries are known for their exceptional energy density, which typically ranges between 150 and 300 W h kg−1, with some specialized types reaching even higher values. Lithium batteries use lithium as the anode, which offers several advantages, including a high electrochemical potential and light weight, contributing to their superior energy density compared to other primary battery types. This high energy density makes lithium batteries ideal for applications requiring long-lasting power, such as smoke detectors, medical devices, and portable electronics. Their performance in extreme temperatures further enhances their appeal for military and aerospace applications.46,47 While more expensive than other primary batteries, lithium batteries' combination of energy density, reliability, and lightweight properties make them indispensable in high-performance and critical applications. Compared to alkaline and zinc–carbon batteries, lithium batteries offer significantly more energy for the same weight, making them the preferred choice where compact and long-lasting power is essential. Silver oxide batteries offer one of the highest energy densities among primary batteries, typically ranging from 130 to 160 W h kg−1.48 They use silver oxide as the cathode and zinc as the anode, with an alkaline electrolyte. This configuration provides a stable voltage and high energy density, making silver oxide batteries particularly useful in small, high-drain devices such as watches, calculators, and hearing aids. The high energy density of silver oxide batteries allows for compact cell sizes while still delivering reliable power over extended periods. However, their cost is significantly higher than other primary batteries, due to the use of silver, which limits their application to niche markets that demand precision and long life. Despite their high cost, the superior energy density of silver oxide batteries ensures their continued use in applications where space and energy efficiency are critical.49
Zinc–air batteries are unique in their construction, as they rely on oxygen from the air as a reactant, rather than storing all the reactants internally. This design gives them an exceptionally high energy density, typically ranging from 200 to 400 W h kg−1, depending on the application.51 The use of zinc as the anode and oxygen from the air as the cathode reduces the overall weight of the battery while maximizing energy output. Zinc–air batteries are commonly used in hearing aids, where their lightweight design and high energy density are highly advantageous. Their ability to provide a high energy-to-weight ratio makes them competitive with other advanced primary batteries like lithium. However, zinc–air batteries have a limited shelf life once activated, as they begin to degrade upon exposure to air. Despite this limitation, their high energy density ensures their continued relevance in specialized low-power applications, particularly in the medical field.52–54 The image provides a comprehensive analysis of Zinc–Air Battery (ZAB) components and their performance: Fig. 3a schematic of a ZAB showing the zinc electrode, a porous air electrode with layers consisting of MnO2-based catalyst, nickel foam (current collector), and a gas diffusion layer connected to a battery tester for performance evaluation. Fig. 3b the anode structure, where iron fibers (IF) are adhered to a copper substrate, is displayed, emphasizing the composite surface used in the battery. Fig. 3(c–f) SEM images of the Zn/IF surface at various deposition potentials: (c) −1.60 V vs. Hg/HgO showing granular deposits, (d) −1.55 V vs. Hg/HgO with smoother surface features, (e) −1.50 V vs. Hg/HgO exhibiting more distinct crystal structures, (f) −1.45 V vs. Hg/HgO showing well-defined plate-like crystals, each imaged at 200× magnification. Fig. 3g graph displaying the Coulombic Efficiency (CE) and Round-Trip Efficiency (RTE) over 200 charge/discharge cycles for ZABs with Zn/IF and Zn/NF anodes. The Zn/IF anode shows slightly lower efficiency performance compared to Zn/NF over the cycling period.
 | ||
| Fig. 3 (a and b) Working and thin film deposition of Zinc–Air Battery (ZAB) with Zn/IF (Zinc/Iron Fibers) and Zn/NF (Zinc/Nickel Foam), (c and d) morphology of Zinc–Air Battery (ZAB) with Zn/IF (Zinc/Iron Fibers) and Zn/NF (Zinc/Nickel Foam) and (g) performance of Zinc–Air Battery (ZAB) with Zn/IF (Zinc/Iron Fibers) and Zn/NF (Zinc/Nickel Foam) reproduced from ref. 50 copyright © 2020 by the authors. Licensee MDPI, Basel, Switzerland. | ||
Fig. 4 provides a comparison of the theoretical energy density (in W h L−1) and specific energy (in W h kg−1) of different metal-air battery chemistries. These values are calculated by considering the mass and volume of the discharge product, open-circuit voltage (OCV), and charge transferred during the electrochemical reaction. Silicon–Oxygen (Si/O2): exhibits the highest energy density (9930 W h L−1) and specific energy (3750 W h kg−1), making it a potential future technology for high-performance applications. Lithium–Oxygen (Li/O2): another promising candidate with an energy density of 7990 W h L−1 and specific energy of 3460 W h kg−1, often studied for use in electric vehicles and portable electronics. Aluminum–Oxygen (Al/O2): offers an energy density of 6790 W h L−1 and specific energy of 2790 W h kg−1, balancing between lightweight and high energy. Magnesium–Oxygen (Mg/O2): has a similar energy density (6670 W h L−1) but slightly higher specific energy (2850 W h kg−1), making it a potential option for energy storage. Zinc–Oxygen (Zn/O2): widely researched, with a lower energy density of 6100 W h L−1 and specific energy of 1090 W h kg−1, often used for hearing aids and energy backup systems. Sodium–Oxygen (Na/O2): the lowest energy density (4430 W h L−1) and specific energy (1580 W h kg−1) among the listed batteries, researched for potential large-scale energy storage solutions.
 | ||
| Fig. 4 Theoretical energy density and specific energy of commonly researched metal–air batteries reproduced from ref. 55 copyright © 2018 by the authors. Licensee MDPI, Basel, Switzerland. | ||
Mercury batteries, once popular for their stability and long life, have energy densities ranging between 130 and 200 W h kg−1.56 They used mercury oxide as the cathode and zinc as the anode, with a highly stable voltage output. While mercury batteries offered excellent energy density and reliable performance in various applications, their use has been largely phased out due to environmental concerns related to mercury toxicity. Mercury batteries were commonly used in medical devices, military equipment, and portable electronics before environmental regulations restricted their use. Despite their high energy density, the environmental risks posed by mercury have led to their replacement by more environmentally friendly alternatives, such as silver oxide and lithium batteries. In terms of energy density, mercury batteries outperformed zinc–carbon and alkaline batteries, but their environmental impact has resulted in their obsolescence. Copper–zinc batteries, also known as Daniell cells, are one of the earliest battery chemistries and have relatively low energy densities, typically around 30–60 W h kg−1. While they were historically significant in the development of electrochemical technologies, their low energy density limits their modern applications. Copper–zinc batteries operate through a reaction between copper and zinc electrodes, with a salt bridge or porous separator. While they provided a stable voltage and were used in early electrical applications, their energy density pales in comparison to more modern chemistries such as lithium or zinc–air. Today, copper–zinc batteries are mostly used in educational demonstrations and some industrial applications but are no longer a common choice for consumer electronics.
When comparing the energy densities of these various primary batteries, lithium and zinc–air batteries stand out as the top performers, offering the highest energy densities and making them ideal for applications where weight and energy efficiency are critical. Alkaline and silver oxide batteries also provide competitive energy densities, with silver oxide batteries being particularly useful in small, high-drain devices. Zinc–carbon and copper–zinc batteries, while historically important, offer the lowest energy densities and are being replaced by more efficient alternatives in most applications. Lastly, while mercury batteries once provided a balance of high energy density and long life, environmental concerns have led to their decline in favor of more sustainable options.
2.2. Shelf life
When comparing the shelf life of various primary batteries, it is important to understand that this property significantly impacts the practical application and usability of these batteries in different devices. The shelf life refers to the duration a battery can be stored without significant degradation in its capacity or performance before use. Below is a comparison of the shelf life of Alkaline, Zinc–Carbon, Lithium, Silver Oxide, Zinc–Air, Mercury, and Copper–Zinc batteries.Alkaline batteries are among the most widely used primary batteries, known for their relatively long shelf life, typically ranging from 5 to 10 years.57,58 Their chemical stability makes them ideal for long-term storage, as they retain most of their charge over time. This long shelf life, combined with moderate cost and widespread availability, makes alkaline batteries a popular choice for everyday devices like remote controls, flashlights, and toys. Despite their longer storage capabilities, alkaline batteries may experience slight capacity loss over time, especially when stored in less-than-optimal conditions such as high temperatures.
Zinc–carbon batteries, on the other hand, have a considerably shorter shelf life compared to alkaline batteries, typically lasting between 2 to 3 years.59 These batteries are more susceptible to self-discharge, meaning they lose their stored energy more rapidly over time, even when not in use. As a result, Zinc–carbon batteries are less suitable for devices that are used infrequently or stored for long periods. Their lower cost makes them an economical choice for low-drain devices, but their shorter shelf life often limits their practicality in scenarios where long-term storage is necessary.60
Lithium primary batteries have an exceptionally long shelf life, often exceeding 10 years, with some types lasting as long as 15 years. This is one of the key reasons lithium batteries are preferred for critical devices that require a reliable and long-lasting power source, such as smoke detectors, medical equipment, and military electronics. Lithium's low self-discharge rate contributes to its superior shelf life, and these batteries maintain a high percentage of their initial capacity even after many years of storage. Additionally, lithium batteries perform well in extreme temperatures, further enhancing their appeal for long-term storage.
Silver oxide batteries, commonly used in small electronics like watches and calculators, have a shelf life of approximately 3 to 5 years. While this is not as long as lithium or alkaline batteries, it is still respectable given their high energy density and steady voltage output over time. Silver oxide batteries are designed for devices requiring a stable power source and high precision, where small size and reliable performance are prioritized. Though their shelf life is moderate, silver oxide batteries are highly valued for their specific use cases in microelectronics.
Zinc–air batteries are unique in that their shelf life is highly dependent on their exposure to air. When sealed, zinc–air batteries can be stored for up to 3 years without significant degradation. However, once exposed to air and activated, their lifespan shortens dramatically to a few weeks or months, depending on the application. This characteristic is particularly important for devices like hearing aids, where the battery needs to be replaced frequently after use. Despite their shorter operational life once activated, zinc–air batteries are valued for their high energy density relative to their size and weight.61,62
Mercury batteries, though now largely phased out due to environmental concerns, were once known for their long shelf life, often exceeding 10 years. Their stable voltage output and durability made them ideal for applications in medical and military devices. However, due to the toxic nature of mercury and the potential environmental hazards posed by improper disposal, mercury batteries have been banned or heavily restricted in most parts of the world. As a result, modern applications that once relied on mercury batteries have transitioned to more environmentally friendly alternatives like silver oxide or zinc–air batteries.
Copper–zinc batteries, or Daniell cells, are largely obsolete in modern commercial and consumer applications, and their shelf life is relatively short compared to modern battery technologies. Historically, these batteries were not designed for long-term storage, and their practical usage was limited by rapid degradation. As a result, they are now mainly used for educational demonstrations or specialized industrial purposes rather than everyday applications. The short shelf life and relatively low energy density of copper-zinc batteries make them impractical for modern storage and usage needs.63–65
In summary, lithium batteries offer the longest shelf life among these primary battery types, followed by alkaline and mercury batteries. Silver oxide and zinc–air batteries have moderate shelf lives, with zinc–air batteries experiencing a sharp decline after activation. Zinc–carbon batteries have the shortest shelf life, making them less suitable for long-term storage, while copper–zinc batteries are largely irrelevant for modern consumer needs. This comparison highlights how shelf life can influence battery selection, depending on the application and storage requirements.
2.3. Cost
Table 2 offers a clear comparison of battery costs, which varies significantly based on factors such as energy density, specialized applications, and environmental impact.| Battery type | Cost comparison | Remarks |
|---|---|---|
| Alkaline batteries | Low to medium | Widely available, affordable for everyday use. Generally cheaper than lithium and silver oxide batteries |
| Zinc–carbon batteries | Very low | Cheapest option, but offers lower energy density and shorter lifespan. Suitable for low-drain applications |
| Lithium batteries | High | More expensive due to higher energy density, long shelf life, and specialized use. Used in high-performance devices |
| Silver oxide batteries | High | Premium-priced due to small size, high energy density, and stable output voltage. Commonly used in watches and medical devices |
| Zinc–air batteries | Low to medium | Affordable, especially for small devices like hearing aids. Moderate cost due to specialized applications |
| Mercury batteries | Very high (obsolete/restricted) | Historically expensive, but now banned in most countries due to environmental hazards |
| Copper–zinc batteries | Low (obsolete/not in commercial use) | No longer in widespread use; primarily of historical interest. Not relevant for modern consumer electronics |
2.4. Leakage resistance
Leakage resistance is an important property in primary batteries, referring to the battery's ability to prevent electrolyte leakage, which can damage devices and compromise safety. Leakage can occur due to chemical reactions inside the battery that cause pressure build-up or corrosion of the battery casing. In this review, we compare the leakage resistance of various primary battery types, including Alkaline, Zinc–Carbon, Lithium, Silver Oxide, Zinc–Air, Mercury, and Copper–Zinc batteries.Alkaline batteries are widely known for their superior leakage resistance compared to other primary battery types. These batteries use potassium hydroxide as an electrolyte, which can cause corrosion if it leaks. However, modern alkaline batteries are designed with improved sealing technologies, including better casing materials and internal seals, that minimize the risk of leakage. Many manufacturers have developed leak-proof designs that provide a warranty against leakage under normal usage conditions. This enhanced leakage resistance is one of the reasons why alkaline batteries are preferred for household applications, especially in devices that may not be checked regularly, such as remote controls or smoke detectors. However, if an alkaline battery is left inside a device for an extended period after depletion, there is still a risk of leakage, especially if the battery casing has been compromised due to internal pressure build-up.
Zinc–carbon batteries, one of the earliest types of primary batteries, have a poorer leakage resistance compared to alkaline batteries. These batteries use an acidic electrolyte, typically zinc chloride or ammonium chloride, which is more prone to leakage as it can corrode the zinc casing over time.66 Zinc–carbon batteries are more susceptible to leakage, especially when they are left in devices after they are depleted, or if they are used in high-drain applications for which they are not suited. The risk of leakage increases with age and depletion, as the internal pressure may rise, causing the casing to rupture or crack. For this reason, Zinc–carbon batteries are often considered less reliable in terms of leakage resistance, and they are gradually being replaced by alkaline batteries in most consumer applications.67
Lithium primary batteries have excellent leakage resistance, making them highly reliable for long-term use. These batteries are typically used in devices where battery replacement is difficult or infrequent, such as smoke detectors, medical devices, or military applications. Lithium batteries use non-aqueous electrolytes, which reduces the risk of corrosion compared to aqueous electrolytes like those used in alkaline or Zinc–carbon batteries.68,69 Additionally, lithium batteries are designed with robust seals and casings to withstand extreme conditions, including temperature fluctuations, which further enhances their leakage resistance. The high energy density of lithium batteries also means they last much longer than other primary batteries, reducing the need for frequent replacement and the associated risk of leakage. Overall, lithium batteries offer some of the best leakage resistance available in primary battery technology, making them a top choice for critical applications.
Silver oxide batteries are known for their stable performance and excellent leakage resistance, especially in small, high-drain devices like watches, hearing aids, and medical instruments. These batteries use an alkaline electrolyte (potassium hydroxide or sodium hydroxide), but their construction is designed to minimize leakage risks. Silver oxide batteries are typically well-sealed, with a durable casing that resists corrosion even in humid or high-temperature environments. Moreover, the high purity of materials used in silver oxide batteries contributes to their stability and leakage resistance.70,71 While these batteries are generally safe from leakage during their operational life, it is still important to remove them from devices once they are fully discharged to prevent any chance of corrosion or leakage over time. Given their relatively high cost and excellent performance, silver oxide batteries are commonly used in applications where reliability and long life are critical, and leakage resistance is a significant advantage.
Zinc–air batteries present a unique case in terms of leakage resistance. These batteries rely on oxygen from the air to function, which means they have air vents that expose the internal components to the environment. While this design allows for a high energy density relative to the battery's size, it also increases the risk of electrolyte leakage, especially if the battery is exposed to moisture or humid conditions. The electrolyte in zinc–air batteries is usually a potassium hydroxide solution, which can cause corrosion and leakage if it escapes the cell. However, most zinc–air batteries are used in hearing aids or other small devices where their short lifespan means they are replaced frequently, reducing the risk of long-term leakage. Nevertheless, care should be taken to store these batteries in dry conditions, and they should be removed from devices if not in use for extended periods to prevent leakage.
Mercury batteries, which were once widely used in military and medical applications, have been largely phased out due to environmental concerns associated with mercury toxicity. These batteries used a mercury oxide cathode and a zinc anode, with an alkaline electrolyte (potassium hydroxide or sodium hydroxide). Mercury batteries had excellent leakage resistance due to their stable chemical composition and robust construction. The use of mercury in the cathode helped stabilize the battery's internal chemistry, reducing the risk of pressure build-up and leakage. However, due to the toxic nature of mercury, these batteries posed significant environmental and health risks if they were improperly disposed of, leading to their eventual ban in most countries. Although they had superior leakage resistance, their environmental impact outweighed their benefits, and they have been replaced by safer alternatives such as silver oxide and zinc–air batteries.72–75
Copper–zinc batteries, also known as Daniell cells, are one of the earliest forms of electrochemical cells. While they are not widely used today in commercial applications, they have historical significance. These batteries generally have poor leakage resistance, primarily because their construction is not optimized for modern-day standards of containment. Copper–zinc batteries use a liquid electrolyte, often sulfuric acid or a similar corrosive substance, which can easily leak if the cell is not properly sealed. In early designs, the risk of leakage was a major limitation, and this, combined with their relatively low energy density and the advent of more efficient battery technologies, has made copper–zinc batteries largely obsolete in contemporary usage. The leakage resistance of primary batteries varies significantly depending on the battery chemistry and construction. Alkaline and lithium batteries offer the best leakage resistance, making them ideal for long-term applications. Zinc–carbon and zinc–air batteries, on the other hand, have higher risks of leakage due to their chemical composition and design, though they are still used in specific applications where cost or weight considerations are more important. Silver oxide batteries strike a balance between performance and leakage resistance, especially in small, high-drain devices, while mercury batteries, though once known for their excellent leakage resistance, are no longer in use due to environmental concerns. Finally, copper–zinc batteries, while historically significant, offer poor leakage resistance and are not commonly used today. Table 3 will provide a structured comparison of commonly used cathode materials in rechargeable batteries, highlighting their performance characteristics.
| Cathode material | Battery type | Theoretical capacity (mA h g−1) | Energy density (W h kg−1) | Cycle life (cycles) | Rate capability | Cost ($ per kg) | Environmental impact |
|---|---|---|---|---|---|---|---|
| Lithium cobalt oxide (LiCoO2) | Li-ion | 274 | 150–200 | 500–1000 | Moderate | High | Toxic cobalt extraction |
| Lithium iron phosphate (LiFePO4) | Li-ion | 170 | 90–120 | 2000–7000 | High | Moderate | Environmentally safer |
| Lithium manganese oxide (LiMn2O4) | Li-ion | 148 | 100–150 | 300–700 | High | Moderate | Manganese dissolution limits lifespan |
| Nickel manganese cobalt (NMC) | Li-ion | 180–220 | 150–250 | 1000–2000 | High | High | Expensive due to cobalt |
| Nickel cobalt aluminum (NCA) | Li-ion | 200–250 | 200–300 | 1000–2000 | Moderate | High | High energy density but expensive |
| Sulfur (S) | Li–S | 1675 | 400–600 | 100–500 | Low | Low | High solubility of polysulfides |
| Vanadium pentoxide (V2O5) | Flow batteries | 350 | 50–80 | 5000+ | High | Moderate | High recyclability |
| Sodium vanadium phosphate (Na3V2(PO4)3) | Na-ion | 117 | 90–120 | 1000–3000 | High | Low | Sustainable and abundant |
| Prussian blue analogues (PBA) | Na-ion | 150–170 | 80–140 | 1000–2000 | Moderate | Low | Non-toxic and abundant |
2.5. Discharge rate
The discharge rate is a critical property of primary batteries, affecting how quickly they release stored energy to power devices. The discharge rate is influenced by the battery's chemistry and design, and it determines the suitability of each battery type for different applications. In this review, we compare the discharge rates of Alkaline, Zinc–Carbon, Lithium, Silver Oxide, Zinc–Air, Mercury, and Copper–Zinc batteries.Alkaline batteries have a moderate discharge rate, making them suitable for a wide range of low-to-moderate drain devices like remote controls, clocks, and flashlights. They maintain consistent performance under light loads but may struggle with high-drain applications. However, their improved performance compared to older zinc–carbon batteries makes them a popular choice for everyday electronics.76 Alkaline batteries tend to maintain stable voltage throughout the majority of their discharge cycle but can experience a significant drop-off toward the end of their life.
Zinc–carbon batteries exhibit a relatively high discharge rate, which makes them less efficient for long-term use in devices that require steady power.77 They are suitable for low-drain applications such as wall clocks and basic remote controls, where their lower cost justifies their limited lifespan and higher discharge rate. When used in high-drain applications, zinc–carbon batteries experience a rapid voltage drop, which reduces their overall efficiency compared to other battery chemistries like alkaline or lithium. Discharging of Zn–Cu and Zn–CO2 batteries as depicted in Fig. 5(a to d).
 | ||
| Fig. 5 (a) Discharge curves of LATSP-based Zn–Cu battery, (b) discharge curves of Nafion-based Zn–Cu battery reproduced from ref. 78 copyright © 2014, The Author(s), (c) discharge/charge profiles at current densities from 0.2 to 0.5, 1.0, 2.0, 5.0, and 10.0 mA cm−2 reproduced from ref. 79 copyright © 2020 Wiley-VCH GmbH, (d) charge/discharge of Zn–CO2 battery reproduced from ref. 80 copyright © 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Lithium primary batteries excel in terms of discharge rate, especially under high-drain conditions. They deliver a stable and high voltage throughout their lifespan, making them ideal for high-performance devices such as cameras, medical instruments, and portable electronics. Lithium batteries can handle both low and high-drain applications with consistent efficiency, outperforming alkaline and zinc–carbon batteries. Additionally, they maintain a steady discharge rate even in extreme temperatures, further expanding their usability across various applications.81
Silver oxide batteries have a relatively low discharge rate, allowing them to perform well in small, high-drain devices such as watches and hearing aids. Their discharge curve is generally flat, providing a stable voltage over time, which is particularly valuable in precision electronics.82 Although silver oxide batteries are more expensive than zinc–carbon or alkaline batteries, their ability to maintain a low discharge rate over a long period makes them highly reliable for specialized applications.
Zinc–air batteries, which rely on oxygen from the air as a reactant, have a low-to-moderate discharge rate. Their performance is well-suited to devices like hearing aids that require consistent, long-lasting power over time. Once activated by exposure to air, these batteries deliver a steady voltage until their energy is depleted. However, their performance can degrade if exposed to air for extended periods without use, limiting their practical shelf life after activation.
Mercury batteries, though largely phased out due to environmental concerns, offered an exceptionally low discharge rate, making them ideal for applications requiring long-term, stable power, such as pacemakers and military equipment. Mercury batteries had a flat discharge curve, delivering consistent voltage throughout their lifespan. Despite their excellent discharge properties, their environmental toxicity led to their replacement by safer alternatives like silver oxide and lithium batteries.
Copper–zinc batteries, historically used in early electrochemical research, exhibit a relatively high discharge rate compared to modern primary batteries. While they are not commonly used today, their performance in delivering rapid energy made them useful in laboratory experiments and early electrical circuits. Modern equivalents like alkaline and lithium batteries far surpass copper–zinc batteries in terms of efficiency and discharge stability.
2.6. Environmental impact
The environmental impact of primary batteries varies depending on their chemical composition and disposal practices. Alkaline batteries are widely used but pose moderate environmental concerns due to the use of non-toxic materials like zinc and manganese dioxide. They are often disposed of in landfills, where they contribute to waste, but they are relatively safe compared to older battery types. Zinc–carbon batteries, though cheap, have a higher environmental impact due to the use of acidic electrolytes and lower recyclability. They often end up in landfills, contributing to pollution. Lithium batteries are more environmentally friendly due to their long life and recyclability, though improper disposal can lead to environmental hazards, including chemical leaks and fire risks. Silver oxide batteries are considered environmentally safe due to the high recyclability of silver, a valuable resource. Their impact is low, especially in high-drain applications. Zinc–air batteries have a relatively low environmental footprint, as they use oxygen from the air and do not contain harmful heavy metals. However, their recyclability remains limited. Mercury batteries, once highly toxic, have been phased out due to the severe environmental risks of mercury, including contamination of water and soil. Copper–zinc batteries, historically significant, pose low environmental risks but are rarely used today, minimizing their overall impact. Table 4 comprises all the properties for all the type of batteries in primary batteries.| Battery type | Energy density (W h kg−1) | Shelf life | Cost | Environmental impact | Recyclability |
|---|---|---|---|---|---|
| Alkaline | 100–300 | 5–10 years | Low | Moderate (landfill contribution, limited recycling) | Limited |
| Zinc–carbon | 40–70 | 2–3 years | Very low | High (limited recyclability, contributes to pollution) | Low |
| Lithium | 150–300 | 10+ years | High | Moderate (potential chemical leaks, recyclable) | High |
| Silver oxide | 130–160 | 3–5 years | High | Low (recyclable due to silver content, low waste) | High |
| Zinc–air | 100–300 | 1–3 years | Medium | Low (uses oxygen, relatively safe but limited recycling) | Moderate |
| Mercury | 100–200 | 10+ years | High | Very high (toxic mercury contamination, banned in many regions) | Low (phase-out in progress) |
| Copper–zinc | 50–100 | 1–2 years | Low | Low (minimal usage, low environmental impact) | Low |
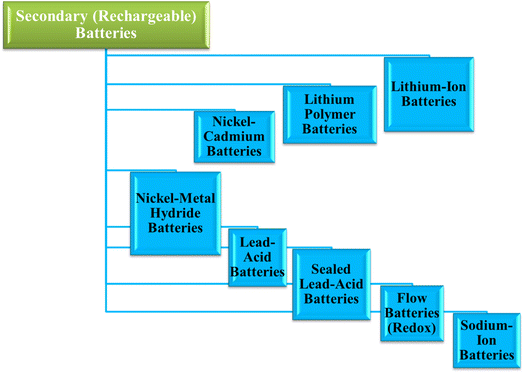
3. Secondary (rechargeable) batteries
Secondary batteries, also known as rechargeable batteries, are designed for repeated charge and discharge cycles, making them ideal for applications requiring long-term energy storage. Their ability to be recharged multiple times makes them more sustainable than primary batteries, contributing to a lower environmental footprint. The following section reviews the key types of secondary batteries: Lithium-Ion (Li-ion), Lithium Polymer (Li-Po), Nickel–Cadmium (NiCd), Nickel–Metal Hydride (NiMH), Lead–Acid, Sealed Lead-Acid (SLA), Flow (Redox), and Sodium-Ion batteries, as shown in Fig. 6.Lithium-ion (Li-ion) batteries: lithium-ion batteries are one of the most widely used secondary batteries, known for their high energy density, low self-discharge rate, and long cycle life. They are commonly found in consumer electronics, electric vehicles, and renewable energy storage systems. Li-ion batteries operate through the movement of lithium ions between the anode and cathode during charging and discharging. Despite their popularity, Li-ion batteries have safety concerns due to the risk of thermal runaway and are sensitive to high temperatures. They are, however, recyclable, and significant research is being conducted to improve their sustainability.83–85
Lithium polymer (Li-Po) batteries: lithium polymer batteries are a variant of Li-ion batteries, utilizing a polymer electrolyte instead of a liquid one. This allows for a more flexible form factor, making Li-Po batteries ideal for devices where space is a concern, such as smartphones, drones, and other portable electronics. While they offer a similar energy density and cycle life to Li-ion batteries, they are generally more expensive to manufacture. Li-Po batteries are also prone to swelling and potential leakage, which raises safety concerns similar to those of Li-ion batteries, though their widespread use continues to grow.
Nickel–cadmium (NiCd) batteries: nickel–cadmium batteries were once the go-to choice for rechargeable battery applications due to their robustness and long cycle life. NiCd batteries are highly durable and can function in a wide range of temperatures, making them suitable for industrial applications. However, they suffer from a “memory effect,” which can reduce their overall capacity if not fully discharged before recharging. Additionally, cadmium is highly toxic, posing significant environmental hazards if not disposed of properly. Consequently, NiCd batteries have been largely replaced by more environmentally friendly alternatives.
Nickel–metal hydride (NiMH) batteries: nickel–metal hydride batteries have emerged as a safer and more environmentally friendly alternative to NiCd batteries. They offer a higher energy density than NiCd batteries and are widely used in consumer electronics, electric vehicles, and hybrid vehicles. NiMH batteries are less affected by the memory effect, and they are easier to recycle compared to their NiCd counterparts. However, they have a higher self-discharge rate and a shorter cycle life than Li-ion batteries, which limits their use in high-performance applications.86–88
Lead–acid batteries: lead–acid batteries are one of the oldest types of rechargeable batteries and are still widely used today in automotive applications, backup power systems, and industrial machinery. They are known for their reliability, low cost, and high current output. However, lead–acid batteries have a relatively low energy density and are bulky, making them unsuitable for portable applications. The lead content in these batteries poses significant environmental hazards, requiring careful disposal and recycling processes. Despite their limitations, lead–acid batteries remain in demand due to their low cost and ease of manufacturing.
Sealed lead-acid (SLA) batteries: sealed lead-acid batteries are a maintenance-free version of traditional lead–acid batteries. They are designed to prevent leakage of the electrolyte, making them safer and easier to use in various applications, such as uninterruptible power supplies (UPS), medical equipment, and security systems. While they share the same environmental concerns as standard lead–acid batteries, SLA batteries offer improved safety and convenience.
Flow batteries (Redox): flow batteries, also known as redox flow batteries, operate by storing energy in liquid electrolytes that flow through a cell. These batteries are scalable and can store large amounts of energy, making them ideal for grid storage and large-scale renewable energy systems. Flow batteries have a long cycle life and can be recharged almost indefinitely. However, they have low energy density compared to other secondary batteries, limiting their use to stationary applications. Their environmental impact is relatively low, and research is ongoing to improve their efficiency and reduce costs.
Sodium-ion batteries: sodium-ion batteries are emerging as a promising alternative to Li-ion batteries due to the abundance and low cost of sodium. These batteries operate similarly to Li-ion batteries, using sodium ions to store and release energy. While they have a lower energy density than Li-ion batteries, they are more sustainable and environmentally friendly. Sodium-ion batteries are still in the development phase, but they hold potential for large-scale energy storage systems due to their scalability and lower cost.
3.1. Cycle life
The cycle life of secondary batteries refers to the number of complete charge–discharge cycles a battery can undergo before its capacity falls to a specified percentage of its original value, typically 80%. Understanding the cycle life is crucial when evaluating the performance and longevity of batteries in various applications. Below is a comparative analysis of the cycle life of key types of secondary batteries.Lithium-ion (Li-ion) batteries are known for their excellent cycle life, typically ranging from 500 to 1500 cycles, depending on the specific chemistry and usage conditions.89 In high-end applications such as electric vehicles (EVs), they may achieve even more cycles with careful battery management systems (BMS) in place.90 Li-ion batteries retain a high percentage of their capacity over numerous cycles, which makes them ideal for applications like portable electronics, EVs, and renewable energy storage. However, their cycle life can degrade rapidly under conditions of high heat or improper charging, and over time, performance tends to diminish due to lithium-ion plating and other degradation mechanisms. Overall, their relatively high cycle life, combined with high energy density, makes them one of the most widely used secondary batteries today. Fig. 7a: depicts the cycling performance of NCR cells at varying depths of discharge (DOD) of 100%, 75%, 50%, and 25%. The plot shows the charge–discharge capacity (Q) over multiple cycles. The 80% limit, indicating the capacity degradation point, is marked by the dash-dotted line. The cells are charged at a C/2 rate and discharged at 1C. A sharp decline in capacity is observed as the DOD increases, with higher DOD resulting in more rapid capacity fading.
 | ||
| Fig. 7 (a) Cycling performance of NCR cells at different depths of discharge: 100% (●), 75% (○), 50% (▲), and 25% (□) with charge at C/2 and discharge at 1C, highlighting capacity retention up to the 80% limit reproduced from ref. 11 copyright © 2015 Elsevier Ltd. (b–e) SEM images of negative active material (NAM) with various carbon additives after formation: (b) without carbon, (c) with 1.0 wt% 3D-RGO, (d) with 1.0 wt% AC, and (e) with 1.0 wt% ACET. (f) Initial discharge capacity of lead-acid test cells with different carbon additives across various charge/discharge rates reproduced from ref. 99 © 2017 Elsevier B.V. | ||
Lithium polymer (Li-Po) batteries, a variant of Li-ion technology, generally offer a cycle life in the range of 300 to 600 cycles.91 Their cycle life is somewhat shorter than that of Li-ion batteries due to the characteristics of the polymer electrolyte, which degrades more quickly under high stress. Li-Po batteries are preferred in applications that require lightweight, compact batteries with flexible shapes, such as smartphones, drones, and wearable devices. Although they offer a slightly lower cycle life than Li-ion batteries, their lightweight design and compactness often outweigh this disadvantage. Like Li-ion batteries, their performance can be significantly affected by overcharging, deep discharging, and exposure to high temperatures.
Nickel–cadmium (NiCd) batteries are known for their long cycle life, which can range from 1000 to 1500 cycles, and in some cases, even more.92 Their ability to deliver a consistent voltage over many cycles makes them particularly suitable for industrial applications and power tools. NiCd batteries are highly durable and perform well under extreme temperatures, which further enhances their cycle life. However, they suffer from a “memory effect”, where partial discharges can cause the battery to “remember” a lower capacity if not fully discharged regularly. This issue can reduce the effective cycle life unless proper discharge practices are followed.93 Despite their long cycle life, environmental concerns due to the toxicity of cadmium have led to the gradual replacement of NiCd batteries by more eco-friendly options like Nickel–Metal Hydride (NiMH).
Nickel–Metal Hydride (NiMH) batteries typically offer a cycle life ranging from 500 to 1000 cycles. While their cycle life is lower than that of NiCd batteries, they do not suffer from the same memory effect, making them easier to maintain. NiMH batteries have become popular in consumer electronics, hybrid vehicles, and other applications where energy density and environmental considerations are important. Although their self-discharge rate is higher than that of Li-ion batteries, they offer a more eco-friendly alternative to NiCd due to the absence of toxic cadmium. The slightly shorter cycle life, coupled with higher self-discharge, limits their usage in high-demand applications where Li-ion batteries are more suitable.94,95
Lead–acid batteries have a relatively low cycle life compared to other secondary batteries, generally ranging between 200 to 500 cycles. Their cycle life is highly dependent on the depth of discharge (DoD); shallow discharges significantly extend their life, while deep discharges reduce it. Lead–acid batteries are widely used in automotive applications and backup power systems, where their low cost and ability to deliver high current are valued over longevity.96–98 Despite their lower cycle life, they remain in use because of their reliability and affordability. However, lead–acid batteries have the disadvantage of being bulky and environmentally hazardous due to the lead content. Fig. 7b–e: present SEM micrographs of negative active material (NAM) samples with different carbon additives after formation: Fig. 7b: NAM without carbon shows a rough, irregular surface structure. Fig. 7c: NAM with 1.0 wt% 3D-RGO (3D Reduced Graphene Oxide) shows a more defined and structured morphology. Fig. 7d: NAM with 1.0 wt% AC (Activated Carbon) exhibits a dense and granular structure. Fig. 7e: NAM with 1.0 wt% ACET (Activated Carbon/Expanded Tubes) reveals a well-defined, fibrous morphology, enhancing conductive pathways. Fig. 7f: displays the initial discharge capacity of lead–acid test cells using different carbon additives in NAM. The graph shows a comparison of cycling performance for cells without carbon, with 3D-RGO, AC, and ACET across different charge/discharge rates (C20, C10, C5). The cells with 3D-RGO show the highest initial discharge capacity and better cycling performance compared to the others.
Sealed Lead-Acid (SLA) batteries are a variation of traditional lead–acid batteries, and while they are maintenance-free and leak-proof, their cycle life remains similar, at 200 to 500 cycles. Like traditional lead–acid batteries, their cycle life is strongly dependent on the DoD. SLA batteries are commonly used in uninterruptible power supplies (UPS), emergency lighting, and other stationary applications. While they offer convenience in terms of maintenance, their limited cycle life and lower energy density make them less ideal for modern, high-demand applications.
Flow batteries, or redox flow batteries, have an exceptionally long cycle life, often exceeding 5000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This makes them highly suitable for large-scale energy storage systems, such as grid storage for renewable energy sources like solar and wind. Flow batteries are unique in that their energy is stored in liquid electrolytes, allowing for virtually unlimited recharge cycles without significant degradation of capacity. The long cycle life is one of their key advantages, though they suffer from lower energy density compared to other battery types, which restricts their use to stationary applications.
000 cycles. This makes them highly suitable for large-scale energy storage systems, such as grid storage for renewable energy sources like solar and wind. Flow batteries are unique in that their energy is stored in liquid electrolytes, allowing for virtually unlimited recharge cycles without significant degradation of capacity. The long cycle life is one of their key advantages, though they suffer from lower energy density compared to other battery types, which restricts their use to stationary applications.
Sodium-ion batteries are an emerging technology with promising cycle life characteristics, typically expected to range from 500 to 2000 cycles.100,101 While still in the developmental stage, they offer a more sustainable alternative to Li-ion batteries, given the abundance of sodium compared to lithium. Sodium-ion batteries have slightly lower energy density than Li-ion batteries, but their cycle life is expected to improve with ongoing research. They are being developed for applications where sustainability and cost-effectiveness are critical, such as grid energy storage. Though not yet widely commercialized, their potential for long cycle life positions them as a future contender in the battery market. Fig. 8a: shows the voltage profiles of the TiO2 anode at different current rates using an EC/DEC-based electrolyte. The specific capacity (Scapacity) varies with the current rates of 0.1, 0.2, 0.5, 1, and 2 A g−1, showing a distinct drop in capacity with increasing current, especially beyond 0.2 A g−1. Fig. 8b: displays the voltage profiles of the TiO2 anode at the same current rates as in Fig. 8a, but using a diglyme-based electrolyte. As with the EC/DEC-based electrolyte, higher current rates reduce the specific capacity, though the overall profile shape appears slightly different due to the electrolyte's characteristics. The 0.1 A g−1 profile is shown for the third cycle, while the rest are from the first cycle at each respective current rate. Fig. 8c: illustrates the rate performance comparison between EC/DEC-based and diglyme-based electrolytes. Both electrolytes show a reduction in specific capacity as the current rate increases, with EC/DEC consistently delivering a higher capacity across all rates. Fig. 8d: plots the specific capacity (Scapacity) of the TiO2 anode against the current (i) for different anatase TiO2-based sodium-ion battery (SIB) anodes reported in literature. These include pure anatase, carbon-coated anatase, hollow spheres, hollow nanospheres, nanorods, and carbon-coated nanorods. The TiO2 anode's performance is compared with these literature values, showing the relative effectiveness of various morphologies and coatings. Fig. 8e: demonstrates the long-term cyclic performance of the TiO2 anode at a current rate of 0.1 A g−1 using both EC/DEC and diglyme-based electrolytes. The EC/DEC-based electrolyte exhibits a higher capacity retention over 500 cycles compared to the diglyme-based electrolyte, with marked differences at other current rates (0.01, 0.0335, 0.08, 0.168, 0.173, and 0.335 A g−1) as well.
 | ||
| Fig. 8 (a and b) Voltage profiles of the TiO2 anode at various current rates with (a) EC/DEC-based and (b) diglyme-based electrolytes. (c) Rate performance comparison between the two electrolytes. (d) Comparison of specific capacity performance with anatase TiO2-based sodium-ion battery (SIB) anodes reported in the literature, and (e) cyclic performance at 0.1 A g−1 for EC/DEC and diglyme-based electrolytes over 500 cycles reproduced from ref. 102 copyright © 2019, The Author(s). | ||
In summary, flow batteries offer the longest cycle life, making them ideal for large-scale energy storage, while NiCd batteries also provide an extended cycle life but are limited by their environmental impact. Li-ion and Li-Po batteries, though slightly more limited in cycle life, are highly favored for portable applications due to their energy density and performance. Lead–acid and SLA batteries, despite their short cycle life, remain essential in low-cost, high-current applications. Sodium-ion batteries represent an exciting future for sustainable, cost-effective energy storage with a competitive cycle life.
Table 5 will provide a structured comparison of widely used anode materials in rechargeable batteries, highlighting their performance characteristics.
| Anode material | Battery type | Theoretical capacity (mA h g−1) | Energy density (W h kg−1) | Cycle life (cycles) | Rate capability | Cost ($ per kg) | Environmental impact |
|---|---|---|---|---|---|---|---|
| Graphite (C) | Li-ion, Na-ion | 372 | 150–250 | 1000–3000 | Moderate | Low | Abundant, minimal impact |
| Silicon (Si) | Li-ion | 3579 | 400–600 | 100–500 | Low | High | Expansion issues affect lifespan |
| Lithium titanate (Li4Ti5O12, LTO) | Li-ion | 175 | 80–120 | 5000–10000 | High | High | Non-toxic, but expensive |
| Hard carbon | Na-ion | 300–400 | 100–200 | 1000–3000 | Moderate | Moderate | Sustainable alternative |
| Tin (Sn)-based anodes | Li-ion, Na-ion | 993 (Li), 847 (Na) | 250–400 | 500–1000 | Low | Moderate | Structural degradation limits use |
| Titanium dioxide (TiO2) | Li-ion | 330 | 150–200 | 3000+ | High | Low | Safe and stable |
| Sodium titanate (Na2Ti3O7) | Na-ion | 178 | 100–150 | 2000–5000 | Moderate | Low | Sustainable and abundant |
| Metallic lithium (Li metal) | Li-metal, solid-state batteries | 3860 | 500–700 | <500 | Low | Very high | High reactivity, dendrite formation |
3.2. Energy density and specific capacity
To provide a suitable comparison for the most important property of secondary battery is to provide a table. Table 6 a comparison table summarizing the energy density and specific capacity of various secondary battery types:| Battery type | Energy density (W h kg−1) | Specific capacity (mA h g−1) |
|---|---|---|
| Lithium-ion (Li-ion) | 150–250 | 150–200 |
| Lithium polymer (Li-Po) | 100–200 | 140–180 |
| Nickel–cadmium (NiCd) | 45–80 | 40–60 |
| Nickel–metal hydride (NiMH) | 60–120 | 100–150 |
| Lead–acid | 30–50 | 40–60 |
| Sealed lead-acid (SLA) | 30–50 | 40–60 |
| Flow batteries (redox) | 20–80 | 25–50 |
| Sodium-ion | 100–150 | 80–120 |
Lithium-ion (Li-ion) batteries stand out as the best option when considering energy density and specific capacity, making them ideal for applications like consumer electronics, electric vehicles, and energy storage. Their high energy density (150–250 W h kg−1) and specific capacity (150–200 mA h g−1) allow for more compact and efficient energy storage. Lithium polymer (Li-Po) batteries, while similar to Li-ion in energy characteristics, are generally less efficient in terms of energy density and specific capacity, but their flexible form factor makes them preferable for space-constrained applications like drones or slim devices. Nickel–Cadmium (NiCd) and Nickel–Metal Hydride (NiMH) batteries have significantly lower energy density compared to Li-ion batteries. NiMH batteries perform better than NiCd in terms of specific capacity and are more environmentally friendly, making them suitable for hybrid vehicles and backup power systems.103–105 Lead–acid and Sealed Lead-Acid (SLA) batteries offer very low energy density, which limits their use to automotive and backup power applications. They are bulky and have a poor energy-to-weight ratio, but they remain popular due to their low cost and high power output for short bursts of energy. Flow Batteries (Redox) offer a much lower energy density and specific capacity but are valued for their scalability and use in large-scale energy storage, particularly in renewable energy systems. Sodium-ion batteries provide a good balance between cost and energy density, though they currently lag behind Li-ion in performance. However, due to the abundance of sodium, they are emerging as a cost-effective solution for large-scale energy storage. Lithium-ion (Li-ion) remains the best choice for most high-performance applications due to its superior energy density and specific capacity, especially where compactness and efficiency are critical. Flow batteries are preferable for large-scale energy storage due to their scalability, while sodium-ion batteries may become more favorable as sustainable energy storage technologies continue to evolve.
3.3. Self-discharge rate
Self-discharge rate is an important parameter for secondary batteries, as it determines how quickly a battery loses its charge when not in use. Different types of secondary batteries have varying self-discharge rates, affecting their efficiency and usability in certain applications. Lithium-ion batteries are known for their low self-discharge rate, typically around 1–3% per month. This makes them highly efficient for long-term use in applications like consumer electronics, electric vehicles, and renewable energy storage. Their minimal self-discharge rate contributes to their popularity, as they retain their charge for extended periods when not in use. Lithium Polymer batteries have a similar self-discharge rate to Li-ion batteries, around 1–3% per month.106 Although their energy density and form factor make them ideal for compact devices like smartphones and drones, their low self-discharge rate ensures that they maintain high efficiency. However, Li–Po batteries may experience higher rates of degradation when exposed to extreme temperatures. Nickel–cadmium batteries have a relatively high self-discharge rate, around 10–15% per month (Fig. 9a). This limits their efficiency for long-term applications, as they lose a significant amount of charge when not used regularly.107 Despite their durability and ability to perform under harsh conditions, the high self-discharge rate and environmental concerns regarding cadmium have led to a decline in their use.Nickel–metal hydride batteries suffer from a high self-discharge rate, typically 30% or more per month, making them less efficient than other battery types. This rapid loss of charge, especially when stored for long periods, limits their use in applications where consistent, long-term energy storage is required. However, they remain a popular choice for hybrid vehicles and consumer electronics, where they are frequently recharged. Traditional lead–acid batteries have a moderate self-discharge rate of around 4–6% per month (Fig. 9b).108 Although not as efficient as Li-ion batteries, they are still suitable for automotive and backup power applications due to their reliability and low cost. Their lower energy density, however, limits their use in portable devices. Sealed lead-acid batteries share a similar self-discharge rate to traditional lead–acid batteries, around 3–5% per month. While they are more maintenance-free than their traditional counterparts, the moderate self-discharge rate is manageable for applications like uninterruptible power supplies (UPS) and security systems.
Flow batteries, or redox batteries, have a significantly lower self-discharge rate, often close to 0% due to the separation of the energy storage and conversion processes. This unique feature makes them ideal for large-scale, long-term energy storage systems, such as renewable energy integration and grid stabilization, where minimal energy loss is crucial. Sodium-ion batteries are still under development, but their self-discharge rate is expected to be slightly higher than that of Li-ion batteries, likely around 5–10% per month.109–112 This moderate rate makes them less efficient for long-term storage but still attractive due to their low cost and environmental benefits. In terms of self-discharge rate, Flow batteries exhibit the lowest, almost negligible rate, making them the best choice for long-term energy storage in grid-scale applications. Li-ion and Li-Po batteries are excellent for consumer electronics and portable applications due to their low self-discharge rate. NiCd and NiMH batteries, on the other hand, suffer from significantly higher self-discharge, making them less ideal for long-term storage applications. Lead–acid and SLA batteries have moderate self-discharge rates, suitable for backup power and automotive applications. For efficiency in long-term storage, flow batteries are the best, but for compact and portable use, Li-ion batteries stand out as the top choice.
3.4. Charge time
The charge time of secondary batteries is a crucial performance metric, influencing their usability in various applications, from consumer electronics to grid-scale energy storage systems. Different battery chemistries exhibit varying charge times due to their internal composition, energy density, and technological limitations. Lithium-ion batteries are known for their relatively fast charge times, which typically range from 1 to 4 hours depending on the specific application and charger capacity. This fast charge capability is due to the high energy density and advanced design of Li-ion cells, which allow for efficient energy transfer.113–117 In high-performance applications, such as electric vehicles (EVs) and power tools, fast-charging technology can reduce this time to under an hour. However, rapid charging can increase the risk of overheating and reduce the overall cycle life if not carefully managed. Advanced battery management systems (BMS) are crucial in maintaining a balance between fast charging and battery longevity.118Lithium polymer batteries share many characteristics with Li-ion batteries, including their relatively fast charge time, typically between 1 and 3 hours. However, due to their use of a polymer electrolyte, Li-Po batteries can be designed in various shapes and sizes, making them ideal for applications where space is constrained.119–121 Li-Po batteries are frequently used in smartphones, drones, and other portable electronics where quick charging is advantageous. While they have a similar charge time to Li-ion batteries, they are more sensitive to overcharging and require precise voltage control to avoid swelling or leakage.122
Nickel–cadmium batteries generally have longer charge times compared to Li-ion and Li-Po batteries, with a typical range of 5 to 8 hours.123 This longer charge time is due to the chemical properties of cadmium, which allows for slower energy absorption. While NiCd batteries are durable and can endure a large number of charge cycles, their slow charge rate is a disadvantage in applications requiring quick turnaround. Fast-charging NiCd batteries are available but can significantly reduce the battery's cycle life, and they are prone to the memory effect, where incomplete discharges lead to reduced capacity over time. Nickel–metal hydride batteries generally have a charge time of 3 to 6 hours, placing them between NiCd and Li-ion batteries in terms of charge speed. NiMH batteries are less prone to the memory effect than NiCd batteries, making them more convenient for applications requiring frequent recharging, such as household electronics and hybrid vehicles.124 However, their self-discharge rate is higher than that of Li-ion batteries, meaning they lose charge more quickly when not in use. Fast-charging options are available for NiMH batteries, but like NiCd batteries, fast charging can reduce their lifespan.
Lead–acid batteries are known for their slow charge times, typically ranging from 8 to 16 hours. This long charge time is one of the major disadvantages of lead–acid technology, making it less suitable for applications requiring frequent or rapid recharging. Lead–acid batteries are commonly used in automotive applications and backup power systems, where slow charge times are acceptable due to the battery's low cost and high reliability. Advanced charging methods, such as multi-stage charging, can slightly reduce the charge time, but the technology's limitations are inherent in its chemical structure. High-current charging can cause excessive heat and reduce the battery's lifespan. Sealed Lead-Acid (SLA) batteries have similar charge times to traditional lead–acid batteries, typically taking between 8 and 12 hours to fully charge. While they offer the advantage of being maintenance-free and leak-proof, their slow charge time limits their usefulness in applications requiring quick energy replenishment.125–127 SLA batteries are often used in emergency power supplies, medical devices, and security systems, where their long charge time is acceptable given their reliability and low cost. Fast-charging SLA batteries are available, but they often come with trade-offs in terms of reduced cycle life and higher maintenance requirements.
Flow batteries, also known as redox flow batteries, have a unique design where the energy is stored in liquid electrolytes that are pumped through a membrane. This allows for a long cycle life and high efficiency in large-scale energy storage applications, such as grid storage for renewable energy. However, flow batteries typically have long charge times, ranging from 10 to 20 hours depending on the system configuration. While they are not suitable for applications requiring quick charge–discharge cycles, their long charge time is offset by their ability to store large amounts of energy and their almost infinite charge–discharge cycle capacity.
Sodium-ion batteries are an emerging technology and offer a promising alternative to Li-ion batteries due to the abundance and low cost of sodium. The charge time for sodium-ion batteries is currently longer than that of Li-ion batteries, typically ranging from 3 to 6 hours.128–131 However, ongoing research is focused on reducing the charge time while maintaining the energy density and safety of the batteries. Sodium-ion batteries are still in the development stage, but they show potential for applications in large-scale energy storage systems due to their scalability and low cost.
Comparing these secondary batteries as illustrated in Fig. 10, lithium-ion (Li-ion) and lithium polymer (Li-Po) batteries stand out for their fast charge times, typically between 1 and 4 hours. This makes them ideal for consumer electronics, electric vehicles, and applications requiring frequent recharging. Li-ion batteries, in particular, offer a good balance between fast charging, energy density, and cycle life, making them the best choice for high-performance applications. Nickel–Cadmium (NiCd) and Nickel–Metal Hydride (NiMH) batteries offer slower charge times, with NiMH being slightly faster than NiCd. However, they are more suited to applications where long battery life and durability are prioritized over fast charging. For large-scale energy storage or backup power, lead–acid and Sealed Lead-Acid (SLA) batteries provide reliable energy at a low cost, though their slow charge times limit their use in applications requiring rapid energy replenishment. Flow batteries have the longest charge times, but they are ideal for stationary energy storage systems, where charge time is less important than storage capacity and cycle life. Sodium-ion batteries, though still under development, offer a promising balance between charge time and sustainability, making them a viable option for future large-scale applications. Lithium-ion batteries offer the best combination of fast charge time, energy density, and efficiency, making them the top choice for most portable and high-performance applications. For large-scale energy storage, flow batteries and emerging sodium-ion batteries may offer more sustainable solutions, despite their longer charge times.
3.5. Environmental impact
The environmental impact of secondary batteries varies significantly depending on their chemistry. Lithium-ion (Li-ion) batteries have a moderate environmental footprint due to mining and processing of lithium, cobalt, and other metals, although they are recyclable. Lithium polymer (Li-Po) batteries have similar environmental issues as Li-ion, but their flexible form factor adds manufacturing complexity, contributing slightly more to environmental concerns. Nickel–Cadmium (NiCd) batteries are highly toxic due to cadmium, a hazardous heavy metal, making them harmful to the environment if improperly disposed of. Nickel–Metal Hydride (NiMH) batteries are more environmentally friendly than NiCd, as they use less toxic materials and are easier to recycle, though they have a shorter lifespan. Lead–acid batteries are highly recyclable but pose environmental risks due to lead contamination if not properly handled. Sealed Lead-Acid (SLA) batteries mitigate some leakage risks but still carry the environmental burden of lead. Flow (Redox) batteries have a relatively low environmental impact due to their long life and scalability, but their low energy density makes them suitable only for stationary applications. Sodium-ion batteries are the most environmentally friendly due to the abundance and low toxicity of sodium, offering a sustainable alternative for future energy storage. In comparison, sodium-ion batteries are emerging as the most sustainable, while Li-ion batteries remain the best overall for energy density and wide applicability.4. Specialty batteries
Specialty batteries are designed for specific applications where traditional battery technologies may not be sufficient. These batteries often offer unique advantages such as higher safety, enhanced performance in extreme conditions, or innovative designs. Below, we review five types of specialty batteries—Solid-State Batteries, Molten Salt Batteries, Zinc–Bromine Flow Batteries, Thin-Film Batteries, and Paper Batteries—as shown in Fig. 11, which illustrates a flow chart of these battery types.Solid-state batteries: solid-state batteries use a solid electrolyte instead of the liquid or gel electrolytes found in conventional batteries like lithium-ion. This design improves safety by eliminating the risk of leakage or fire caused by volatile liquid electrolytes. Solid-state batteries also promise higher energy density and longer cycle life, making them a strong candidate for electric vehicles and portable electronics. However, they are currently expensive to manufacture, and scalability remains a challenge. Significant research is being conducted to make them more commercially viable. Their advantages in terms of safety and performance make them an exciting area of development in energy storage technologies.132–135
Molten salt batteries: molten salt batteries operate at high temperatures, using molten salts as the electrolyte. These batteries are typically used in large-scale energy storage systems for renewable energy, such as solar and wind power, due to their high energy capacity and low cost. They offer long cycle life and are highly efficient, particularly in large-scale grid storage applications. However, the requirement for high operating temperatures (usually above 300 °C) limits their usage to stationary systems, as cooling and insulation make them impractical for portable applications. Molten salt batteries also pose thermal management challenges and must be handled carefully to prevent thermal degradation.
Zinc–bromine flow batteries: zinc–bromine flow batteries are a type of redox flow battery where energy is stored in liquid electrolytes containing zinc and bromine ions. These batteries are ideal for large-scale energy storage systems because they offer excellent scalability, high cycle life, and long discharge times. One significant advantage is that they can be almost infinitely recharged without degradation. They are environmentally friendly due to the use of widely available, non-toxic materials, and they have a relatively low environmental footprint compared to lithium-ion or lead–acid batteries. However, their energy density is lower than conventional batteries, limiting their use to grid-scale applications.136–139
Thin-film batteries: thin-film batteries are ultra-compact, flexible batteries used primarily in medical devices, smart cards, and wearable electronics. These batteries use thin layers of materials as electrodes and electrolytes, allowing them to be produced in very small, flexible, and lightweight designs. Thin-film batteries offer moderate energy density and a long shelf life, but their capacity is limited, making them unsuitable for applications requiring high power. They are often used in specialized applications where form factor and flexibility are more critical than capacity. Thin-film batteries are also environmentally friendly, as they can be manufactured using non-toxic materials.
Paper batteries: paper batteries are a type of flexible, ultra-thin battery made from cellulose (paper) combined with carbon nanotubes or metal oxides to act as electrodes. These batteries are lightweight, biodegradable, and eco-friendly, making them an attractive option for low-power applications like medical devices and environmental sensors. While they do not offer high energy density or capacity compared to traditional batteries, their sustainability and ability to integrate into flexible electronics make them promising for future niche applications. However, paper batteries are still in the early stages of development, and their widespread adoption will depend on further improvements in energy capacity and production methods.
4.1. High-temperature tolerance
High-temperature tolerance is a critical factor in determining the suitability of batteries for specific applications. Batteries exposed to high temperatures tend to degrade faster, but some specialty battery types are specifically designed to handle these conditions effectively.140,141 Solid-state batteries exhibit excellent high-temperature tolerance due to their use of solid electrolytes instead of volatile liquid electrolytes. Unlike conventional batteries, which can suffer from thermal runaway—a condition where the electrolyte catches fire or explodes at high temperatures—solid-state batteries remain stable under high heat.142–144 This makes them safer for high-performance applications like electric vehicles and aerospace, where both heat resistance and safety are paramount. While they can tolerate higher temperatures than most lithium-ion batteries, sustained exposure to extreme heat can still cause some performance degradation. However, solid-state batteries are overall more reliable at elevated temperatures compared to many other battery types.Molten salt batteries are designed to operate at very high temperatures—typically above 300 °C—making them uniquely suited for large-scale, high-temperature environments such as grid energy storage or industrial settings. In these systems, the battery's electrolyte is in a molten state, which allows for efficient ion transport even at extreme temperatures.145 This type of battery thrives in high-heat conditions and performs optimally when kept within its high operating temperature range. The downside is that molten salt batteries are impractical for portable applications due to the need for continuous heat to maintain the molten state, as well as the risk of thermal degradation if the system's temperature drops too low. Fig. 12 is a table summarizing the effects of battery cell temperature on the performance and lifespan of lithium-ion batteries. It is divided into three main temperature zones: high, medium (optimal), and low, each with associated causes, consequences, and effects on battery performance. High temperatures lead to electrolyte decomposition, side reactions, reduced anode surface, and binder decomposition. Irreversible lithium loss, increased impedance, and loss of mechanical stability. Capacity fade and power fade. Medium temperature range offers optimal battery performance, ensuring maximum cycle life with minimal degradation. Low temperatures can cause lithium plating and electrolyte decomposition. Irreversible lithium loss and electrolyte loss. Capacity fade and power fade.
 | ||
| Fig. 12 Effects of battery temperature on performance reproduced from ref. 146 copyright © 2018 Elsevier B.V. | ||
Zinc–bromine flow batteries also perform well in high-temperature environments, although not to the extent of molten salt batteries (Fig. 13). These batteries operate with liquid electrolytes stored in external tanks, which helps in managing heat dissipation.147 They are often used in large-scale applications such as renewable energy storage, where they can endure fluctuating temperatures without significant degradation. However, extreme heat can still affect the efficiency of their chemical reactions, and while their high-temperature tolerance is good, it is not as exceptional as molten salt or solid-state batteries. Nonetheless, they are more versatile and scalable for grid-scale energy storage compared to the other types.148,149
 | ||
| Fig. 13 Schematic illustration of ZBRBs from device configuration, electrochemistry, material to performance evaluation reproduced from ref. 150 copyright © 2023, The Author(s). | ||
Thin-film batteries are highly specialized, and their high-temperature tolerance is limited. Designed primarily for low-power, compact, and flexible applications, these batteries are not typically used in high-temperature settings. Excessive heat can cause thin-film batteries to degrade rapidly, leading to reduced performance and lifespan.151–154 While they are ideal for wearable electronics and small medical devices, they are not suitable for environments where high heat is a consistent factor. Their primary strength lies in their flexibility and lightweight nature rather than thermal resistance.
Paper batteries as illustrated in Fig. 14 are another type of specialty battery that struggles with high temperatures. Made from biodegradable materials like cellulose combined with carbon or metal oxides, paper batteries are designed for low-power applications.155 While environmentally friendly, they are not built to withstand extreme temperatures, and high heat can compromise their integrity and reduce their overall performance. These batteries are most useful in applications where sustainability and flexibility are key concerns, but they are unsuitable for environments where thermal resistance is required.
 | ||
| Fig. 14 (a) Illustration of the water-activated paper battery: the electrochemical (EC) cell consists of a paper membrane placed between a zinc-based anode and a graphite-based air cathode. Carbon-based current collectors transfer charges from the EC cell to external circuits. The battery remains inactive until water, which acts as the electrolyte, is introduced, permeating the paper membrane. (b) Image of a single-cell battery: fabricated through stencil printing on filter paper, the battery is activated by submerging the wick in water or an aqueous solution. Wax is applied to the filter paper at the battery terminals to prevent unwanted electrochemical reactions of the lead wires and to enhance mechanical stability. (c) Photograph of a stencil-printed paper battery: designed to spell out the name of the research institution (Empa), this battery powers low-energy devices such as the LCD alarm clock shown. It consists of two electrochemical cells, separated by a water barrier (depicted in (d)), and connected in series as demonstrated in the (e) schematic cross-section, along with an overlaid equivalent circuit for ideal voltage sources reproduced from ref. 156 copyright © 2022, The Author(s). | ||
Fig. 14 contains several panels that illustrate the structure, function, and potential applications of a water-activated paper battery. In panel (a), the schematic of the electrochemical cell highlights the key components: a zinc anode (gray) on one side, an air cathode (light gray) on the opposite side, and a paper membrane (white) functioning as a separator. Water (blue) permeates the membrane, activating the cell. Once activated, the electrochemical reactions at the zinc anode (oxidation) and air cathode (reduction) generate an electric current, and the diagram includes the chemical reactions that occur at each electrode. Panel (b) features a photograph of a single-cell battery fabricated by stencil printing on filter paper. The image shows the activation wick, which can be dipped in water to start the battery. The battery terminals are connected to a paper-based cell with an active area of 1 cm2. In panel (c), the stencil-printed battery is demonstrated powering a low-power device, such as an LCD alarm clock, showing the battery's ability to run simple electronics upon water activation. Panel (d) displays a photograph of the stencil-printed battery design, which features the name of the research institution, Empa. This battery consists of two electrochemical cells separated by a water barrier, and the cells are connected in series, enabling the combined voltages to power a device. Finally, panel (e) presents a schematic cross-section of the battery structure with its two cells. Key components like the zinc anode, air cathode, paper membrane, and water are labeled. A layer of wax is applied to prevent undesired electrochemical reactions and provide mechanical stability. The overlaid circuit diagram shows how the cells are connected in series to create a complete battery system. Overall, the figure illustrates the construction, activation, and real-world application of a water-activated paper battery capable of powering low-energy devices like an LCD clock.
When comparing these batteries, molten salt batteries clearly outperform the others in terms of high-temperature tolerance, as they are specifically designed for operation at elevated temperatures. Solid-state batteries also exhibit strong high-temperature performance, though not as extreme as molten salt batteries, making them a more practical choice for applications like electric vehicles. Zinc–bromine flow batteries are well-suited for moderate high-temperature conditions, particularly in large-scale stationary applications, but they are not as effective in handling extreme temperatures as molten salt or solid-state batteries. Thin-film batteries and paper batteries, on the other hand, are not designed for high-temperature environments and are more suited for low-power, flexible, and eco-friendly applications.157–160 Applications requiring high-temperature tolerance, molten salt batteries are the best choice due to their ability to operate efficiently at temperatures exceeding 300 °C. Solid-state batteries come in second, offering good high-temperature performance with a focus on safety and energy density. Zinc–bromine flow batteries are suitable for moderate high-temperature applications, while thin-film and paper batteries are not designed for such conditions, instead excelling in low-power, flexible, and sustainable use cases. Each type of battery has its strengths, but when it comes to high-temperature tolerance, molten salt batteries are the clear winner.
4.2. Energy density and specific capacity
Energy density and specific capacity are critical factors that determine the performance and efficiency of batteries. Solid-state batteries boast the highest energy density and specific capacity among specialty batteries. With the elimination of liquid electrolytes, these batteries can store more energy in a smaller space. Solid-state batteries have an energy density of around 300–400 W h kg−1, which surpasses traditional lithium-ion batteries.161–163 Their high specific capacity makes them ideal for electric vehicles and portable electronics, where space and weight constraints are critical. Molten salt batteries have a relatively high specific capacity due to the use of molten salts as electrolytes, but their energy density (around 150–200 W h kg−1) is moderate compared to solid-state batteries. They excel in large-scale energy storage applications like grid storage, where high energy capacity over long periods is crucial, but their lower energy density limits their use in compact systems. Zinc–bromine flow batteries offer moderate energy density, typically around 70–80 W h kg−1, which is lower than both solid-state and molten salt batteries.164However, their specific capacity remains decent for large-scale, long-duration storage applications. They are not suitable for high-energy-density needs due to the bulkiness of the external electrolyte tanks. Thin-film batteries have low energy density and specific capacity, typically around 10–20 W h kg−1.165,166 They are designed for small, low-power devices, such as medical implants and RFID chips, where form factor, flexibility, and long shelf life are more important than energy density. Paper Batteries exhibit low energy density and specific capacity as well, similar to thin-film batteries. They are eco-friendly and flexible but not capable of storing large amounts of energy, limiting them to low-power, short-term applications like biosensors or small wearable electronics. When comparing these battery types, solid-state batteries clearly outperform the others in terms of both energy density and specific capacity, making them the best option for high-energy applications. Molten salt batteries follow, providing a balance of capacity and practicality for grid-scale storage. Zinc–bromine flow batteries are effective for long-term energy storage but fall short in energy density. Thin-film and paper batteries are more specialized for low-power, flexible applications and do not compete in high-energy-density scenarios.167Table 7 shows the comparison of energy density and specific capacity for speciality battery.
| Battery type | Energy density (W h kg−1) | Specific capacity | Ideal application |
|---|---|---|---|
| Solid-state batteries | 300–400 | High | Electric vehicles, portable electronics |
| Molten salt batteries | 150–200 | Moderate | Grid storage, large-scale energy systems |
| Zinc–bromine flow batteries | 70–80 | Moderate | Renewable energy storage |
| Thin-film batteries | 10–20 | Low | Medical implants, RFID chips |
| Paper batteries | 10–20 | Low | Biosensors, wearable electronics |
4.3. Durability in extreme conditions
The durability of batteries in extreme conditions is a crucial factor in determining their suitability for specialized applications such as aerospace, military, and large-scale renewable energy systems. Solid-state batteries are known for their exceptional safety and stability under extreme conditions.168 Unlike traditional batteries, they use a solid electrolyte, which eliminates the risk of leakage and combustion associated with liquid electrolytes, particularly under high-temperature or high-pressure conditions. This solid electrolyte can withstand much higher temperatures without degrading, making solid-state batteries an attractive option for applications that involve thermal extremes, such as electric vehicles or aerospace technologies.169 Furthermore, solid-state batteries exhibit excellent mechanical durability, as their solid electrolyte is less susceptible to physical damage compared to liquid or gel electrolytes. This allows them to perform well under conditions of high pressure or vibration, making them a reliable choice for rugged environments. However, the overall durability of solid-state batteries can be compromised by issues such as dendrite formation, where metallic lithium structures grow through the solid electrolyte, potentially causing short circuits. Advances in materials science, such as the development of more robust solid electrolytes, are needed to improve their long-term durability.Molten salt batteries are specifically designed to operate at high temperatures, typically above 300 °C, which gives them a unique advantage in extreme thermal conditions. Their high operating temperature is both a strength and a limitation: while it allows for efficient energy storage and release, the battery must be kept at these elevated temperatures to function, making it less suitable for portable applications or environments where maintaining such temperatures is impractical. Despite their reliance on high temperatures, molten salt batteries exhibit remarkable durability in these conditions, as the molten electrolyte remains stable and does not degrade easily.170–172 This makes them ideal for large-scale energy storage systems, particularly in renewable energy applications where they can be cycled repeatedly without significant loss of performance. However, in situations where rapid temperature fluctuations occur, the durability of molten salt batteries may be compromised, as extreme cooling can cause solidification of the electrolyte, leading to mechanical stress and potential failure. Additionally, the materials used in molten salt batteries, such as sodium and sulfur, are highly corrosive, requiring the use of specialized, corrosion-resistant materials for the battery casing and components. While these materials enhance the battery's durability in high-temperature environments, they also increase the overall cost and complexity of the system.
Zinc–bromine flow batteries are a type of redox flow battery that excels in large-scale, stationary energy storage applications. One of their primary advantages is their ability to operate in a wide range of temperatures without significant degradation, which makes them highly durable in both hot and cold environments.173–175 The flow of liquid electrolytes through the system allows for efficient heat dissipation, preventing the battery from overheating during operation. In terms of mechanical durability, zinc–bromine flow batteries are relatively resilient, as the liquid electrolytes can be replenished, and the system's components can be replaced or repaired with minimal disruption to overall performance.176 This makes them well-suited for applications where long-term durability is essential, such as grid energy storage or backup power systems. However, the durability of zinc–bromine flow batteries in extreme conditions is not without limitations. The bromine-based electrolyte can be highly corrosive, leading to potential material degradation over time. This requires careful management of the system's materials and components to ensure long-term durability. Additionally, while the battery performs well in moderate temperature extremes, its performance may decline in environments with rapid temperature changes or extreme cold, as the liquid electrolyte can become viscous or freeze, impairing the battery's efficiency.
Thin-film batteries are designed for specialized applications where size, weight, and flexibility are more critical than capacity. They are often used in wearable electronics, medical devices, and small-scale energy storage. While these batteries excel in terms of flexibility and form factor, their durability in extreme conditions is more limited compared to other specialty battery types. Thin-film batteries can tolerate moderate thermal extremes but are generally not designed for use in high-temperature or high-pressure environments. Their thin structure and the materials used in their construction make them more susceptible to damage from physical stress, such as vibration or impact, which can compromise their performance. In high-temperature conditions, the thin layers of the battery may degrade more quickly, reducing its overall lifespan.177,178 However, advances in thin-film battery technology, such as the use of more robust materials and better encapsulation techniques, are helping to improve their durability in challenging environments. While they may not be the most durable option for extreme conditions, their lightweight and flexible nature makes them well-suited for applications where these attributes are more important than high durability.
Paper batteries are an emerging technology that offers unique advantages in terms of sustainability and flexibility. Made from cellulose (paper) and carbon-based materials, these batteries are lightweight, biodegradable, and environmentally friendly. However, their durability in extreme conditions is currently limited, as the organic materials used in their construction are more prone to degradation than the materials used in other battery types. In high-temperature or high-pressure environments, paper batteries may lose their structural integrity, reducing their overall performance. Additionally, exposure to moisture can cause the cellulose to degrade, further limiting their durability in certain conditions. While paper batteries offer exciting potential for low-power applications, such as environmental sensors or medical devices, they are not yet suitable for use in extreme conditions where durability is a critical factor.
In comparing these specialty batteries, solid-state batteries stand out as the most durable option for extreme conditions, particularly in high-temperature or high-pressure environments. Their solid electrolyte provides stability and safety, making them an ideal choice for applications such as electric vehicles and aerospace technology. However, challenges like dendrite formation need to be addressed to maximize their durability. Molten Salt Batteries also perform well in extreme heat, making them ideal for large-scale, stationary energy storage, though their requirement for constant high temperatures limits their use in more versatile applications. Zinc–bromine flow batteries offer durability in a wider range of temperatures but require careful material management to prevent corrosion. They are best suited for grid-scale storage rather than portable applications. Thin-film batteries and paper batteries offer unique advantages in terms of flexibility and sustainability, respectively, but their durability in extreme conditions is more limited. Thin-film batteries may degrade under physical stress, while paper batteries are not yet equipped to handle high temperatures or pressures. For extreme conditions, solid-state batteries are the best choice, offering superior safety, stability, and durability. However, the specific application and environmental requirements will determine the most suitable battery technology.
4.4. Scalability
When considering scalability, different types of specialty batteries exhibit unique challenges and advantages, depending on their structure, materials, and intended applications. Solid-state batteries show immense potential due to their higher energy density and enhanced safety over traditional lithium-ion batteries, but their scalability is currently limited. The main challenge lies in manufacturing processes, which require advanced and expensive materials, as well as sophisticated production techniques. Mass-producing solid-state batteries at a competitive cost is still an obstacle. Companies are investing heavily in research to bring down production costs, improve manufacturing efficiency, and scale up for use in electric vehicles (EVs) and portable electronics.179–183 Once the manufacturing bottleneck is addressed, solid-state batteries could be highly scalable and revolutionize industries like transportation and consumer electronics.Fig. 15 shows a schematic of a zinc-bromine flow battery system, focusing on its performance characteristics and scalability. On the left side, the battery's internal operation is illustrated, highlighting the zinc electrode, carbon electrode, and the movement of zinc ions and bromine ions during the electrochemical process. On the right side, a graph presents the relationship between cell voltage and capacity for batteries connected both in series and in parallel. The red curve represents the series connection, which increases the cell voltage, while the blue curve shows the parallel connection, focusing on increasing capacity (mA h). The inset diagrams illustrate these connections, showing how scalability can be achieved by arranging battery cells either in series (to increase voltage) or in parallel (to increase capacity).
 | ||
| Fig. 15 Scalability of zinc–bromine flow battery system: schematic of cell components with performance factors and graphical comparison of series and parallel cell arrangements to enhance voltage and capacity, respectively reproduced from ref. 184 copyright © 2022 Elsevier B.V. All rights reserved. | ||
Molten salt batteries have significant scalability potential, especially for stationary energy storage systems such as those used for renewable energy grids. These batteries are generally composed of abundant, low-cost materials, making them a feasible option for large-scale applications. The primary challenge in scaling molten salt batteries is their high operating temperature (300 °C or more), which requires specialized thermal management systems, adding complexity and cost. While this limits their usage in portable devices or automotive applications, they are an ideal candidate for grid-scale energy storage. As renewable energy becomes more widespread, the demand for large-scale, long-duration energy storage solutions will enhance the scalability of molten salt batteries.
Zinc–bromine flow batteries are highly scalable due to their design. Since energy is stored in external tanks containing liquid electrolytes, the capacity of these batteries can be easily increased by enlarging the tanks.185,186 This makes zinc–bromine flow batteries ideal for large-scale energy storage systems, particularly in renewable energy grids. They offer long cycle life and can store large amounts of energy for extended periods without significant degradation. However, they have lower energy density compared to solid-state or lithium-ion batteries, which makes them unsuitable for portable or high-density applications. Despite this limitation, their scalability in stationary applications is exceptional, positioning them as a strong contender for grid energy storage.
Thin-film batteries are primarily suited for small-scale applications such as medical devices, wearables, and smart electronics. Their scalability for large-scale energy storage or high-demand applications is limited by their design, which prioritizes flexibility and compactness over capacity and power. While they can be produced on a small scale using relatively straightforward manufacturing techniques, their limited energy density makes them impractical for larger systems or heavy-duty applications. Thin-film batteries excel in niche markets that require specialized form factors, but their scalability is constrained when considering broader energy demands, such as those of EVs or grid storage.187
Paper batteries are one of the most environmentally friendly and sustainable battery technologies, as they are biodegradable and made from readily available materials like cellulose. However, their scalability is currently limited by both their early-stage development and their low energy density. While they show promise for small, low-power applications like medical implants, sensors, and flexible electronics, paper batteries are not yet viable for large-scale energy storage. Additionally, scaling up production to meet industrial demands presents challenges in achieving consistent quality and performance. Their potential is promising, but significant improvements in energy capacity and production efficiency are needed before they can be considered for wider commercial use.
Among these battery types, zinc–bromine flow batteries offer the best scalability for large-scale applications, particularly in renewable energy storage, due to their flexible design and long cycle life. Molten salt batteries also perform well in stationary energy storage systems but are limited by the need for high temperatures.188 Solid-state batteries show immense potential for high-demand applications like electric vehicles but face current challenges in scaling due to complex manufacturing processes. On the smaller end, thin-film batteries and paper batteries are better suited for specialized, small-scale applications and face scalability constraints for broader energy storage needs. In terms of overall scalability, zinc–bromine flow batteries appear to be the most scalable for grid-level storage, while solid-state batteries could dominate high-performance portable and automotive markets if manufacturing hurdles are overcome.
5. Emerging battery technologies
Emerging battery technologies hold the promise of overcoming the limitations of current battery systems, such as higher energy density, longer cycle life, faster charging times, and more environmentally friendly materials. These innovations are essential for advancing industries like electric vehicles (EVs), renewable energy storage, and consumer electronics. The key types of emerging battery technologies, as shown in Fig. 16, include Graphene Batteries, Silicon Anode Batteries, Quantum Batteries, and Sodium–Sulfur Batteries.Graphene batteries: graphene batteries represent a groundbreaking development in battery technology due to graphene's extraordinary properties. Graphene is a single layer of carbon atoms arranged in a hexagonal lattice, known for its high conductivity, strength, and lightweight structure. In battery applications, graphene can enhance the performance of both lithium-ion and supercapacitor systems. Graphene-based batteries promise faster charging times, increased capacity, and improved cycle life. This is due to graphene's high surface area, which allows more ions to interact with the electrode material. Additionally, graphene batteries are more thermally stable, reducing the risk of overheating and enhancing safety. These features make graphene batteries a promising candidate for electric vehicles, renewable energy systems, and high-performance electronics.189–191
Silicon anode batteries: silicon anode batteries are considered a significant improvement over traditional lithium-ion batteries that use graphite anodes. Silicon can store up to 10 times more lithium ions than graphite, which dramatically increases the energy density of these batteries. However, silicon's tendency to expand and contract during charge–discharge cycles presents a challenge, as it leads to mechanical degradation of the anode. Ongoing research is focused on solving these issues through advanced nanostructures and composite materials to stabilize silicon anodes. If these hurdles are overcome, silicon anode batteries could revolutionize the energy storage market by offering higher capacity, longer battery life, and faster charging. They are especially promising for electric vehicles and portable electronic devices that demand lightweight, high-energy-density power sources.192–194
Quantum batteries: quantum batteries are a futuristic concept that applies principles of quantum mechanics to energy storage. Unlike conventional batteries, which rely on classical electrochemical reactions, quantum batteries exploit quantum states, such as superposition and entanglement, to achieve nearly instantaneous charging. This emerging technology is still in its theoretical phase, but it has the potential to dramatically reduce charging times and increase energy efficiency. If successfully developed, quantum batteries could revolutionize sectors like EVs and grid storage, offering unprecedented speed and efficiency in energy delivery. However, much research is still needed before quantum batteries become commercially viable.
Sodium–sulfur batteries: sodium–sulfur (NaS) batteries have been under development for several decades, but recent advances have made them a more attractive option for large-scale energy storage. Sodium and sulfur are both abundant and inexpensive materials, making these batteries cost-effective and environmentally friendly compared to lithium-based systems.195,196 Sodium–sulfur batteries operate at high temperatures, which has traditionally been a limitation, but innovations in solid electrolytes are helping to overcome this issue. NaS batteries are known for their high energy density and long cycle life, making them well-suited for grid storage applications where large amounts of energy need to be stored and released over long periods. They are also being explored for integration with renewable energy systems due to their sustainability.
Emerging battery technologies like Graphene Batteries, Silicon Anode Batteries, Quantum Batteries, and Sodium–Sulfur Batteries represent the future of energy storage, addressing critical issues such as energy density, cycle life, cost, and environmental impact. These technologies, each with unique strengths and development challenges, have the potential to reshape industries by enabling more efficient, powerful, and sustainable energy storage solutions. Fig. 16 provides a visual flow chart illustrating these types of batteries and their interrelations. As research continues to advance, these emerging technologies could play a vital role in addressing the growing global demand for energy storage across various applications, from electric vehicles to large-scale renewable energy systems.
5.1. Energy density
Emerging battery technologies are pushing the limits of energy density, which is a key factor in determining the efficiency and performance of energy storage systems. Energy density, measured in watt-hours per kilogram (W h kg−1), represents the amount of energy a battery can store relative to its weight.Graphene batteries have attracted significant attention due to the exceptional properties of graphene, a one-atom-thick layer of carbon atoms arranged in a hexagonal lattice. Graphene's high electrical conductivity, large surface area, and lightweight structure contribute to improving the energy density of batteries. Current research suggests that graphene-based batteries can achieve energy densities in the range of 200–500 W h kg−1, depending on the specific design and configuration.197,198 Graphene can be used in both the cathode and anode of batteries, enhancing charge storage capacity and reducing internal resistance. While still in the experimental phase, graphene batteries hold great promise for electric vehicles, portable electronics, and grid storage, potentially surpassing the energy densities of traditional lithium-ion batteries.
Silicon anode batteries represent a significant improvement over conventional lithium-ion batteries, which typically use graphite anodes. Silicon has a much higher theoretical capacity for lithium-ion storage, offering energy densities of up to 4200 mA h g−1, which translates to approximately 350–600 W h kg−1 in practical terms.199–201 This makes silicon anode batteries one of the most energy-dense battery types currently under development. However, silicon expands significantly during charging, leading to mechanical stress and capacity degradation over time. Despite these challenges, advances in silicon anode technology, including the development of silicon nanostructures and composite materials, are improving the cycle life and stability of these batteries. Silicon anode batteries are likely to find applications in electric vehicles and portable electronics, where high energy density is essential for extended operation.
Quantum batteries, an emerging theoretical concept, hold the potential for groundbreaking improvements in energy storage. These batteries rely on the principles of quantum mechanics, such as quantum superposition and entanglement, to enhance energy storage and transfer efficiency. While quantum batteries are still in the early stages of development and their practical energy density is not yet fully realized, theoretical models suggest that quantum batteries could achieve energy densities far beyond traditional batteries. Some predictions estimate that quantum batteries could exceed 1000 W h kg−1, offering ultra-fast charging times and minimal energy loss.202,203 If realized, quantum batteries could revolutionize industries that require high energy density and quick recharge times, such as aerospace, electric vehicles, and renewable energy systems.
Sodium–sulfur (Na–S) batteries are another emerging technology that offers high energy density and sustainability advantages. Sodium is abundant and inexpensive compared to lithium, making sodium–sulfur batteries a cost-effective alternative for large-scale energy storage. Sodium–sulfur batteries typically have energy densities in the range of 150–250 W h kg−1, making them competitive with conventional lithium-ion batteries.204,205 These batteries operate at high temperatures, which can improve their energy efficiency, but also pose safety challenges related to thermal management. Sodium–sulfur batteries are primarily targeted at grid-scale energy storage, where energy density and cycle life are important, but not as critical as in portable applications. Their relatively lower energy density compared to graphene and silicon anode batteries limits their use in smaller, portable devices.
When comparing the energy densities of these emerging battery technologies, it is clear that silicon anode batteries offer the highest practical energy density, followed closely by graphene batteries. Quantum batteries hold the potential for even higher energy densities, but they are still in the conceptual stage and require significant development before they can be commercially viable. Sodium–sulfur batteries, while offering lower energy density, excel in terms of cost and sustainability, making them ideal for large-scale energy storage systems. In terms of which is the best, silicon anode batteries currently represent the most feasible and high-performing option for applications requiring high energy density, such as electric vehicles and portable electronics. However, graphene batteries offer substantial improvements in terms of charge time and durability, which could make them more suitable for applications where longevity and efficiency are critical. Quantum batteries, if developed successfully, could surpass all other technologies in terms of energy density, but they remain a future prospect. Sodium–sulfur batteries are best suited for stationary applications, where cost-effectiveness and sustainability outweigh the need for extreme energy density. The Table 8 shows a Comparison of the energy density.
| Battery type | Energy density (W h kg−1) | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| Graphene batteries | 200–500 | High conductivity, fast charge, lightweight | Still in experimental phase | Electric vehicles, grid storage, electronics |
| Silicon anode batteries | 350–600 | Highest energy density, long life potential | Mechanical stress from silicon expansion | Electric vehicles, portable electronics |
| Quantum batteries | >1000 (theoretical) | Ultra-fast charging, high theoretical energy density | Still theoretical, requires development | Aerospace, EVs, renewable energy systems |
| Sodium–sulfur batteries | 150–250 | Low cost, abundant materials, large-scale storage | Lower energy density, high operating temperature | Grid-scale energy storage, renewable systems |
5.2. Cycle life and charge/discharge efficiency
When reviewing emerging battery technologies, cycle life and charge/discharge efficiency are two critical factors that influence their potential applications and overall performance. Graphene batteries are gaining attention for their exceptional charge/discharge efficiency and long cycle life. Graphene's high electrical conductivity allows faster electron and ion transport, reducing energy losses during charging and discharging. As a result, graphene batteries exhibit excellent charge/discharge efficiency, often exceeding 90%. In terms of cycle life, they outperform traditional batteries, enduring thousands of cycles with minimal capacity loss.206–209 This makes them suitable for high-demand applications like electric vehicles and portable electronics. However, commercial scalability remains a challenge due to the cost of graphene production.Silicon anode batteries, often seen as an upgrade to traditional lithium-ion batteries, offer high energy density but struggle with cycle life. Silicon can store more lithium ions than conventional graphite anodes, which boosts the energy density, but the material expands significantly during charging.210–212 This leads to degradation of the anode over time, reducing the cycle life to a few hundred cycles unless advanced engineering techniques are used to mitigate this issue. Charge/discharge efficiency is moderately high, but frequent degradation due to expansion and contraction impacts long-term performance. Fig. 17 shows the cycle life of silicon anode in lithium ion batteries along with SEM images.
 | ||
Fig. 17 (a) Cycling comparison and (b) coulombic efficiency of the silicon anode in 1 M LiPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEC (3 FEC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolytes with different contents of DMAA additive and their respective SEM images reproduced from ref. 213 copyright © The Royal Society of Chemistry 2019. 1) electrolytes with different contents of DMAA additive and their respective SEM images reproduced from ref. 213 copyright © The Royal Society of Chemistry 2019. | ||
Quantum batteries are still in the conceptual and experimental phase, but they promise near-perfect charge/discharge efficiency. Leveraging quantum phenomena, these batteries could charge incredibly fast and achieve efficiencies close to 100%. However, their cycle life is still theoretical, as quantum batteries have not yet been fully realized for practical use. Early models suggest that they could last much longer than current batteries, but this remains to be proven with scalable prototypes.
Sodium–sulfur batteries, primarily used for large-scale energy storage, have a lower charge/discharge efficiency compared to the other technologies mentioned, typically ranging from 80–90%. Their cycle life, however, is excellent, often exceeding 2000 cycles. Sodium–sulfur batteries operate at high temperatures, which contributes to faster degradation compared to room-temperature technologies like graphene and silicon anode batteries. Despite these limitations, their low cost and abundance of sodium make them attractive for grid-scale energy storage.
When comparing these batteries, graphene batteries stand out with the best combination of long cycle life and high charge/discharge efficiency, making them ideal for applications where both durability and efficiency are crucial, such as electric vehicles. Silicon anode batteries offer high energy density but are limited by their shorter cycle life. Quantum batteries hold great promise with their near-perfect efficiency, but they are still in the early stages of development. Sodium–sulfur batteries, while having lower efficiency, are cost-effective for large-scale energy storage due to their long cycle life. For current practical use, graphene batteries are the most promising option due to their balance of high cycle life and charge/discharge efficiency. However, quantum batteries could surpass all other technologies in the future if they become commercially viable.
5.3. Sustainability
Emerging battery technologies are rapidly evolving to address sustainability concerns, including resource availability, environmental impact, and scalability. Graphene batteries leverage the extraordinary electrical conductivity and mechanical strength of graphene, a single layer of carbon atoms arranged in a hexagonal lattice. Graphene's sustainability stems from its abundance (as carbon is widely available) and its potential to increase the energy density and charging speed of batteries.214–217 Graphene-enhanced batteries could also reduce reliance on rare and expensive metals like lithium and cobalt. The manufacturing process, however, still faces challenges related to scalability and energy consumption, which can impact its environmental footprint. Despite these hurdles, graphene batteries hold strong potential for sustainability, especially if eco-friendly production methods are further developed.Silicon anode batteries are an advancement over traditional lithium-ion batteries, replacing the graphite anode with silicon. Silicon can store up to ten times more lithium than graphite, increasing the energy density of batteries. Since silicon is the second-most abundant element on Earth, this technology promises a more sustainable solution to energy storage. However, silicon anode batteries face challenges due to the expansion and contraction of silicon during charging cycles, leading to shorter battery life. Research is ongoing to stabilize the anode and make the batteries more durable. While silicon anode batteries offer significant potential for sustainability, particularly in terms of material availability, the technological challenges must be resolved to fully realize their potential.
Quantum batteries represent a futuristic approach to energy storage, leveraging quantum mechanics to store and release energy. The sustainability of quantum batteries is largely theoretical at this stage, but they hold the potential for virtually instantaneous charging and vastly improved energy efficiency. Quantum batteries could revolutionize the energy storage sector by eliminating inefficiencies inherent in current battery technologies. However, they are still in the early stages of development, and their environmental and material impact remains largely unknown. The promise of quantum batteries lies in their ability to drastically reduce energy consumption during charging and extend the lifespan of batteries, potentially making them a highly sustainable option in the long run.
Sodium–Sulfur (Na–S) batteries are a molten–salt technology that utilizes abundant materials like sodium and sulfur, making them a highly sustainable alternative to lithium-ion batteries. Na–S batteries have been used in grid-scale energy storage due to their long cycle life and the low cost of materials. However, they require high operating temperatures (around 300–350 °C) to maintain the molten state, which raises concerns about energy consumption and safety. Despite these challenges, sodium–sulfur batteries offer a promising solution for large-scale, cost-effective energy storage, particularly in renewable energy systems. Their sustainability is bolstered by the abundance and recyclability of sodium and sulfur, although improvements in operating conditions are necessary for broader adoption.
When comparing these emerging battery technologies, graphene batteries stand out for their potential to increase energy density and charging speed while reducing reliance on rare metals. However, the production of graphene is still energy-intensive and needs improvement. Silicon Anode batteries offer a more immediate solution with a significant boost in energy density, but their sustainability is hindered by technological challenges like anode degradation. Quantum batteries, though theoretical, could offer unparalleled efficiency, but their real-world sustainability remains speculative until further developed. Sodium–sulfur batteries, while highly sustainable in terms of material availability, are hampered by the high operating temperatures required, which reduce their overall efficiency. In terms of sustainability, sodium–sulfur batteries appear to be the most promising for large-scale applications due to their use of abundant materials and low cost, particularly for stationary energy storage. However, graphene batteries may eventually surpass them if production methods improve, offering higher energy density and faster charging for consumer electronics and electric vehicles. Silicon anode batteries are an attractive alternative for the medium term but require further research to stabilize their performance. Quantum batteries hold immense long-term potential but are still too early in their development to be compared definitively. Ultimately, the “best” battery depends on the specific application, with sodium–sulfur batteries currently leading in sustainability for grid storage and graphene batteries showing potential for broader uses in the future. Table 9 provide a compressive trade offs in different application.
| Application | Recommended battery type | Advantages | Limitations |
|---|---|---|---|
| Electric vehicles (EVs) | Lithium-ion (Li-ion), lithium polymer (Li-Po), sodium-ion | High energy density, long cycle life, fast charging | Expensive, resource-intensive (Li, Co), degradation over time |
| Grid energy storage | Lead–acid, flow batteries, sodium-ion | Cost-effective, long cycle life, scalable | Low energy density, slow charge/discharge, large footprint |
| Consumer electronics | Lithium-ion (Li-ion), lithium polymer (Li-Po) | Compact, lightweight, high energy density | Limited cycle life, potential safety risks (thermal runaway) |
| Medical devices (pacemakers, hearing aids) | Silver oxide, zinc-air | Long shelf life, stable voltage output | Low energy density, non-rechargeable (except advanced designs) |
| Aerospace and defense | Specialty batteries (lithium-thionyl chloride, silver–zinc) | High-temperature tolerance, reliability | High cost, limited availability |
| Wearable devices & IoT | Lithium-ion (Li-ion), solid-state, graphene batteries | Lightweight, flexible, fast charging | High cost, emerging technology, scalability challenges |
6. Comparison and analysis
Table 10 below provides a comparative analysis of various battery types, examining their performance across key properties such as energy density, cycle life, cost, and environmental impact. This analysis highlights the trade-offs involved in different applications, showing how certain battery types excel in specific areas (e.g., energy density or cost-effectiveness), while others are more suited for sustainable use or durability. Understanding these performance trade-offs is essential for selecting the most appropriate battery technology for a given application, whether it's for portable electronics, electric vehicles, or grid-scale energy storage. Table 11 on the other hand provides a quick-reference comparison of the major battery technologies currently dominating the energy storage market. Highlighting key strengths and weaknesses that influence battery selection in various industries and help benchmark emerging technologies (e.g., Sodium-Ion, Graphene, Solid-State, Flow Batteries) against conventional ones.| Battery type | Energy density (W h kg−1) | Cycle life (cycles) | Cost | Environmental impact | Best applications |
|---|---|---|---|---|---|
| Alkaline batteries | 100–120 | Non-rechargeable | Low | Moderate | Portable electronics, flashlights |
| Zinc–carbon batteries | 60–80 | Non-rechargeable | Very low | Moderate | Low-power devices, toys |
| Lithium batteries | 150–200 | Non-rechargeable | High | Moderate to high | High-drain devices, cameras |
| Silver oxide batteries | 150–200 | 100–300 | High | Moderate | Watches, hearing aids |
| Zinc–air batteries | 300–400 | 300–500 | Low to moderate | Moderate | Hearing aids, cameras |
| Mercury batteries | 100–150 | Non-rechargeable | High | High (toxic) | Specialized medical devices |
| Copper–zinc batteries | 80–120 | Non-rechargeable | Low | Moderate | Low-drain devices |
| Lithium-ion (Li-ion) | 150–200 | 1000–2000 | High | Moderate | Electric vehicles, smartphones |
| Lithium polymer (Li-Po) | 150–200 | 300–500 | High | Moderate | Drones, portable devices |
| Nickel–cadmium (NiCd) | 40–60 | 500–1000 | Low | High (due to cadmium) | Power tools, emergency lighting |
| Nickel-metal hydride (NiMH) | 60–120 | 500–1000 | Moderate | Lower than NiCd | Hybrid vehicles, consumer electronics |
| Lead-acid batteries | 30–50 | 300–500 | Very low | High (lead content) | Automotive, backup power systems |
| Sealed lead-acid (SLA) | 30–50 | 300–500 | Low | High (lead content) | UPS systems, emergency lighting |
| Flow batteries (redox) | 15–40 | 1000–10,000 | Moderate to high | Low | Grid energy storage |
| Sodium-ion batteries | 100–140 | 1000–2000 | Low | Low | Grid storage, large-scale applications |
| Sodium–sulfur batteries | 150–240 | 2500+ | Low | Low | Grid-scale energy storage |
| Graphene batteries | 250–400 | 3000+ | High | Low | Future EVs, high-performance devices |
| Silicon anode batteries | 200–300 | 500–1000 | Moderate to high | Moderate | Electric vehicles, consumer electronics |
| Quantum batteries | Theoretical (high potential) | Unknown | Unknown | Unknown | Future applications, possibly high-efficiency |
| Battery type | Pros | Cons | Common applications |
|---|---|---|---|
| Lithium-ion (Li-ion) | High energy density | Expensive raw materials | Electric vehicles (EVs) |
| Long cycle life | Risk of thermal runaway | Portable electronics | |
| Fast charging | Limited recyclability | Grid storage | |
| Sodium-ion (Na-ion) | Abundant and low-cost materials | Lower energy density than Li-ion | Grid storage |
| Safer, non-flammable | Still under research (limited commercialization) | Stationary energy storage | |
| Lead–acid | Low cost | Heavy and bulky | Automotive starter batteries |
| Reliable in standby applications | Short cycle life | Backup power (UPS) | |
| Fully recyclable | Slow charging | Industrial power backup | |
| Nickel–cadmium (NiCd) | Robust, works well in extreme temperatures | Toxicity concerns (cadmium is hazardous) | Aviation and power tools |
| Long cycle life | High self-discharge rate | Medical devices | |
| High charge/discharge efficiency | Memory effect reduces capacity over time | Emergency power backup | |
| Nickel–metal hydride (NiMH) | Higher capacity than NiCd | More expensive than NiCd | Hybrid vehicles |
| Environmentally safer (no cadmium) | High self-discharge rate | Consumer electronics | |
| Solid-state | Improved safety (non-flammable) | Expensive manufacturing costs | Next-gen EV batteries |
| Higher energy density than Li-ion | Still in early-stage development | Wearable tech | |
| Sodium–sulfur (Na–S) | High energy efficiency | High operating temperature required | Grid-scale energy storage |
| Long cycle life | Complex thermal management needed | Renewable energy storage | |
| Graphene-based | Ultra-fast charging (seconds) | Expensive production process | Next-gen portable devices |
| Extremely lightweight | Limited commercial availability | High-performance electronics | |
| Flow batteries | Scalable for large-scale storage | Bulky and low energy density | Grid energy storage |
| Long lifespan | High initial installation cost | Industrial backup power |
7. Conclusion and future research directions
The future of battery technology is poised for significant transformation, driven by the increasing demand for efficient, sustainable, and high-performance energy storage solutions. One potential trend is the continued advancement in solid-state batteries, which promise enhanced safety, energy density, and longer cycle life compared to conventional lithium-ion batteries. Researchers are exploring various solid electrolytes, such as sulfides and oxides, to improve ionic conductivity and reduce manufacturing costs. Nanotechnology also presents exciting opportunities, enabling the development of batteries with superior performance characteristics through materials like graphene and silicon, which can enhance energy density and charge rates. Additionally, the integration of recycling technologies is becoming increasingly important as the industry strives for sustainability. Future research should focus on developing efficient recycling processes for lithium-ion and other battery types, aiming to recover valuable materials while minimizing environmental impact. This includes optimizing the design of batteries for easier disassembly and material recovery.Artificial intelligence (AI) and machine learning are set to revolutionize battery development by enabling faster design, testing, and optimization processes. These technologies can analyze vast amounts of data to predict battery performance under various conditions, accelerating the identification of new materials and configurations that can improve energy density, cycle life, and charging rates. Furthermore, the exploration of alternative battery chemistries such as sodium-ion, magnesium-ion, and lithium–sulfur batteries can potentially alleviate resource scarcity and environmental concerns associated with lithium extraction. Research into these alternative chemistries can lead to the discovery of low-cost and abundant materials, ultimately contributing to the establishment of a more sustainable battery ecosystem.
Lastly, there is a growing emphasis on smart battery systems that incorporate advanced monitoring and management technologies. Future innovations may include batteries equipped with sensors and communication systems that provide real-time data on performance, health, and environmental conditions. Such advancements could significantly enhance the reliability and efficiency of battery systems in applications ranging from consumer electronics to electric vehicles and renewable energy integration.
In summary, the evolution of battery technology is characterized by a dynamic interplay of innovation and increasing demand for energy solutions that meet the needs of modern society. This review has highlighted the various types of batteries, their properties, applications, and environmental impacts, providing a comprehensive understanding of the current landscape of battery technology. The insights gained underscore the importance of selecting appropriate battery technologies based on specific application requirements, balancing factors such as energy density, cycle life, cost, and sustainability. The implications for industries and technology are profound, as advancements in battery technology can significantly enhance the efficiency of electric vehicles, renewable energy systems, and portable electronics. As industries increasingly adopt electrification and renewable energy sources, the demand for high-performance batteries will continue to rise. This shift is likely to accelerate investments in research and development, leading to breakthroughs that will drive down costs and improve performance across various applications.
Moreover, the pursuit of sustainability in battery technology is essential for mitigating environmental concerns associated with battery production and disposal. By focusing on innovative materials, recycling processes, and alternative chemistries, researchers can contribute to creating a circular economy in the battery industry. Final thoughts on the future of battery technology indicate a promising landscape where continued research and innovation will pave the way for next-generation energy storage solutions. The integration of advanced technologies such as AI, smart monitoring systems, and sustainable materials will not only enhance battery performance but also foster a more environmentally responsible approach to energy storage. As the world transitions towards a more sustainable energy future, the development of cutting-edge battery technologies will play a pivotal role in shaping the way we generate, store, and utilize energy. Table 12 provides a systematically difference and analysis of battery types.
| Battery type | Energy density (W h kg−1) | Cycle life (number of cycles) | Shelf life | Cost ($ per kW per h) | Environmental impact | Key applications |
|---|---|---|---|---|---|---|
| Primary batteries | 80–300 | Not rechargeable | 5–15 years | Low | Moderate (disposal concerns) | Remote sensors, medical devices, military applications |
| Secondary batteries | 100–700 | 500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2–10 years | Medium | High (mining, recycling challenges) | EVs, consumer electronics, grid storage |
| Specialty batteries | 150–600 | 1000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5–20 years | High | Moderate (varies by chemistry) | Aerospace, high-temperature applications, submarines |
| Emerging batteries | 200–800 | 2000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10+ years | Medium to high | Low (sustainable materials in development) | Next-gen EVs, smart grids, wearable tech |
Ethical approval
All the authors have worked in accordance with ethical standard and approved by Netaji Subhas University of Technology.Data availability
All the data is presented in the manuscript. No supplementary data is needed.Author contributions
Peeyush Phogat: conceptualization, data Collection, writing – original draft and formal analysis. Subhadeepa Dey: formal analysis. Meher Wan: supervision, writing – review & editing.Conflicts of interest
It is declared that this article is original and written by the stated authors. There is no conflict of interest between the authors.Acknowledgements
This work is funded through OLP project by Council of Scientific And Industrial Research–National Institute Of Science Communication and Policy Research (CSIR–NIScPR). The authors extend their gratitude to the Director of CSIR-NIScPR for providing the necessary resources. In preparing this work, the authors utilized ChatGPT (version 3.5/4) to refine the language and correct grammatical errors. After employing this tool, the authors carefully reviewed and edited the content as required and accept full responsibility for the publication's content.References
- H. Li, M. Xu, Z. Zhang, Y. Lai and J. Ma, Engineering of polyanion type cathode materials for sodium-ion batteries: toward higher energy/power density, Adv. Funct. Mater., 2020, 30, 2000473, DOI:10.1002/adfm.202000473.
- C. Jin, L. Zhou, L. Fu, J. Zhu, D. Li and W. Yang, The acceleration intermediate phase (NiS and Ni3S2) evolution by nanocrystallization in Li/NiS2 thermal batteries with high specific capacity, J. Power Sources, 2017, 352, 83–89, DOI:10.1016/j.jpowsour.2017.03.119.
- K. Matsumoto, J. Hwang, S. Kaushik, C. Y. Chen and R. Hagiwara, Advances in sodium secondary batteries utilizing ionic liquid electrolytes, Energy Environ. Sci., 2019, 12, 3247–3287, 10.1039/c9ee02041a.
- Y. Liu, N. Zhang, L. Jiao, Z. Tao and J. Chen, Ultrasmall Sn nanoparticles embedded in carbon as high-performance anode for sodium-ion batteries, Adv. Funct. Mater., 2015, 25, 214–220, DOI:10.1002/adfm.201402943.
- X. Guo, Z. Wang, Z. Deng, X. Li, B. Wang and X. Chen, et al., Water contributes to higher energy density and cycling stability of Prussian blue analogue cathodes for aqueous sodium-ion batteries, Chem. Mater., 2019, 31, 5933–5942, DOI:10.1021/acs.chemmater.9b02269.
- Y. Shi, Y. Chen, L. Shi, K. Wang, B. Wang and L. Li, et al., An Overview and Future Perspectives of Rechargeable Zinc Batteries, Small, 2020, 16(23), 2000730, DOI:10.1002/smll.202000730.
- E. Rudnik and M. Nikiel, Hydrometallurgical recovery of cadmium and nickel from spent Ni-Cd batteries, Hydrometallurgy, 2007, 89(1–2), 61–71 CrossRef CAS.
- L. Fan, L. Tang, H. Gong, Z. Yao and R. Guo, Carbon-nanoparticles encapsulated in hollow nickel oxides for supercapacitor application, J. Mater. Chem., 2012, 22(32), 16376–16381 RSC.
- S. L. Yang, B. H. Zhou, M. Lei, L. P. Huang, J. Pan and W. Wu, et al., Sub-100 nm hollow SnO2@C nanoparticles as anode material for lithium ion batteries and significantly enhanced cycle performances, Chin. Chem. Lett., 2015, 26(10), 1293–1297, DOI:10.1016/j.cclet.2015.05.051.
- J. Feng, S. Xiong, Y. Qian and L. Yin, Synthesis of nanosized cadmium oxide (CdO) as a novel high capacity anode material for Lithium-ion batteries: Influence of carbon nanotubes decoration and binder choice, Electrochim. Acta, 2014, 129, 107–112, DOI:10.1016/j.electacta.2014.02.085.
- H. de Vries, T. T. Nguyen and B. Op het Veld, Increasing the cycle life of lithium ion cells by partial state of charge cycling, Microelectron. Reliab., 2015, 55(11), 2247–2253 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0026271415301505.
- M. A. Hope, B. L. D. Rinkel, A. B. Gunnarsdóttir, K. Märker, S. Menkin and S. Paul, et al., Selective NMR observation of the SEI–metal interface by dynamic nuclear polarisation from lithium metal, Nat. Commun., 2020, 11, 2224, DOI:10.1038/s41467-020-16114-x.
- C. Abbey and G. Joos, Supercapacitor Energy Storage for Wind Energy Applications, IEEE Trans. Ind. Appl., 2007, 43(3), 769–776 Search PubMed.
- T. J. T. Ugli, The Importance of Alternative Solar Energy Sources and the Advantages and Disadvantages of Using Solar Panels in this Process, Int. J. Ind. Syst. Eng., 2019, 3(4), 32–35 Search PubMed.
- X. Chen, X. Liu, Q. Le, M. Zhang, M. Liu and A. Atrens, A comprehensive review of the development of magnesium anodes for primary batteries, J. Mater. Chem. A, 2021, 9(21), 12367–12399, 10.1039/D1TA01471D.
- A. Silva, M. Liu and M. Moghaddam, Power-Management Techniques for Wireless Sensor Networks and Similar Low-Power Communication Devices Based on Nonrechargeable Batteries, J. Comput. Network. Commun., 2012, 2012(1), 757291, DOI:10.1155/2012/757291.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Functional Materials for Rechargeable Batteries, Adv. Mater., 2011, 23(15), 1695–1715, DOI:10.1002/adma.201003587.
- J. B. Goodenough, Rechargeable batteries: challenges old and new, J. Solid State Electrochem., 2012, 16(6), 2019–2029, DOI:10.1007/s10008-012-1751-2.
- J. B. Goodenough, Evolution of Strategies for Modern Rechargeable Batteries, Acc. Chem. Res., 2013, 46(5), 1053–1061, DOI:10.1021/ar2002705.
- L. Carrette, K. A. Friedrich and U. Stimming, Fuel Cells: Principles, Types, Fuels, and Applications, ChemPhysChem, 2000, 1(4), 162–193, DOI:10.1002/1439-7641(20001215)1:4%3C162::AID-CPHC162%3E3.0.CO.
- A. Boudghene Stambouli and E. Traversa, Fuel cells, an alternative to standard sources of energy, Renew. Sustain. Energy Rev., 2002, 6(3), 295–304 CrossRef . https://www.sciencedirect.com/science/article/pii/S1364032101000156.
- V. Toniazzo, New separators for industrial and specialty lead acid batteries, J. Power Sources, 2002, 107(2), 211–216 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775301010734.
- H. Tsukamoto, High reliability lithium rechargeable batteries for specialties, IEEE Aero. Electron. Syst. Mag., 2003, 18(1), 21–23 CrossRef.
- T. Versteeg, M. J. Baumann, M. Weil and A. B. Moniz, Exploring emerging battery technology for grid-connected energy storage with Constructive Technology Assessment, Technol. Forecast. Soc. Change, 2017, 115, 99–110 CrossRef . https://www.sciencedirect.com/science/article/pii/S0040162516303481.
- J. Li and S. Passerini, Introduction to the special Issue: Focus review - New and emerging battery technologies, J. Power Sources, 2021, 484, 229333 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775320316219.
- S. Dörfler, S. Walus, J. Locke, A. Fotouhi, D. J. Auger and N. Shateri, et al., Recent Progress and Emerging Application Areas for Lithium–Sulfur Battery Technology, Energy Technol., 2021, 9(1), 2000694, DOI:10.1002/ente.202000694.
- M. C. McManus, Environmental consequences of the use of batteries in low carbon systems: The impact of battery production, Appl. Energy, 2012, 93, 288–295 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0306261911008580.
- S. Yoda and K. Ishihara, The advent of battery-based societies and the global environment in the 21st century, J. Power Sources, 1999, 81–82, 162–169 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775398002109.
- R. Bird, Z. J. Baum, X. Yu and J. Ma, The Regulatory Environment for Lithium-Ion Battery Recycling, ACS Energy Lett., 2022, 7(2), 736–740, DOI:10.1021/acsenergylett.1c02724.
- Z. Yang, H. Huang and F. Lin, Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy, Adv. Energy Mater., 2022, 12(26), 2200383, DOI:10.1002/aenm.202200383.
- U. Köhler, C. Antonius and P. Bäuerlein, Advances in alkaline batteries, J. Power Sources, 2004, 127(1), 45–52 CrossRef . https://www.sciencedirect.com/science/article/pii/S0378775303009352.
- C. Chakkaravarthy, A. K. A. Waheed and H. V. K. Udupa, Zinc—air alkaline batteries — A review, J. Power Sources, 1981, 6(3), 203–228 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/0378775381800274.
- M. F. Almeida, S. M. Xará, J. Delgado and C. A. Costa, Characterization of spent AA household alkaline batteries, Waste Manag., 2006, 26(5), 466–476 CrossRef CAS PubMed . https://www.sciencedirect.com/science/article/pii/S0956053X05001212.
- E. Sayilgan, T. Kukrer, G. Civelekoglu, F. Ferella, A. Akcil and F. Veglio, et al., A review of technologies for the recovery of metals from spent alkaline and zinc–carbon batteries, Hydrometallurgy, 2009, 97(3), 158–166 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0304386X0900053X.
- F. Ferella, I. De Michelis and F. Vegliò, Process for the recycling of alkaline and zinc–carbon spent batteries, J. Power Sources, 2008, 183(2), 805–811 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775308010379.
- M. S. Whittingham, Lithium Batteries and Cathode Materials, Chem. Rev., 2004, 104(10), 4271–4302, DOI:10.1021/cr020731c.
- B. Scrosati and J. Garche, Lithium batteries: Status, prospects and future, J. Power Sources, 2010, 195(9), 2419–2430 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775309020564.
- A. P. Karpinski, S. J. Russell, J. R. Serenyi and J. P. Murphy, Silver based batteries for high power applications, J. Power Sources, 2000, 91(1), 77–82 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775300004894.
- Y. Li and H. Dai, Recent advances in zinc–air batteries, Chem. Soc. Rev., 2014, 43(15), 5257–5275, 10.1039/C4CS00015C.
- A. J. Salkind and S. Ruben, in Mercury Batteries for Pacemakers and Other Implantable Devices BT - Batteries for Implantable Biomedical Devices, ed. B. B. Owens, Springer US, Boston, MA, 1986, pp. 261–274, DOI:10.1007/978-1-4684-9045-9_9.
- G. Kasiri, J. Glenneberg, A. Bani Hashemi, R. Kun and F. La Mantia, Mixed copper-zinc hexacyanoferrates as cathode materials for aqueous zinc-ion batteries, Energy Storage Mater., 2019, 19, 360–369 CrossRef . https://www.sciencedirect.com/science/article/pii/S2405829718314570.
- L. Deng, F. Wu, X. Gao and W. Wu, Development of a LiFePO4-based high power lithium secondary battery for HEVs applications, Rare Met., 2020, 39, 1457–1463, DOI:10.1007/s12598-014-0316-1.
- X. Feng, M. Ouyang and X. Liu, Thermal runaway mechanism of lithium ion battery for electric vehicles: a review, Energy Storage Mater., 2018, 10, 246–267, DOI:10.1016/j.ensm.2017.05.013.
- J. B. Goodenough and K. Park, ChemInform abstract: the Li–ion rechargeable battery: a perspective, ChemInform, 2013, 44 DOI:10.1002/chin.201320273.
- L. Lu, X. Han and J. Li, A review on the key issues for lithium–ion battery management in electric vehicles, J. Power Sources, 2013, 226, 272–288, DOI:10.1016/j.jpowsour.2012.10.060.
- A. Senyshyn, M. J. Mühlbauer, O. Dolotko and H. Ehrenberg, Low-temperature performance of Li–ion batteries: the behavior of lithiated graphite, J. Power Sources, 2015, 282, 235–240, DOI:10.1016/j.jpowsour.2015.02.008.
- R. Xiong and W. Shen, Advanced Battery Management Technologies for Electric Vehicles, Wiley, Chichester, 2019, DOI:10.1002/9781119481652.
- R. Xiong, H. Chen, C. Wang and F. Sun, Towards a smarter hybrid energy storage system based on battery and ultracapacitor - a critical review on topology and energy management, J. Clean. Prod., 2018, 202, 1228–1240, DOI:10.1016/j.jclepro.2018.08.134.
- J. Choi and D. Aurbach, Promise and reality of post-lithium-ion batteries with high energy densities, Nat. Rev. Mater., 2016, 1, 16013, DOI:10.1038/natrevmats.2016.13.
- R. Khezri, K. Jirasattayaporn, A. Abbasi, T. Maiyalagan, A. A. Mohamad and S. Kheawhom, Three-Dimensional Fibrous Iron as Anode Current Collector for Rechargeable Zinc–Air Batteries, Energies, 2020, 13, 1429 CrossRef CAS.
- D. Linden and T. B. Reddy, Handbook of Batteries, McGraw-Hill Companies Inc., New York, 2001 Search PubMed.
- C. J. Rydh, Environmental Assessment of Vanadium Redox and Lead-acid Batteries for Stationary Energy Source, J. Power Sources, 1999, 80, 21–29, DOI:10.1016/S0378-7753(98)00249-3.
- Z. Wen, J. Cao, Z. Gu, X. Xu, F. Zhang and Z. Lin, Research On Sodium Sulfur Battery For Energy Storage, Solid State Ionics, 2008, 179, 1697–1701, DOI:10.1016/j.ssi.2008.01.070.
- M. Alahmad, H. Hess, M. Mojarradi, W. West and J. Whitacre, Battery switch array system with application for JPL's rechargeable micro-scale batteries, J. Power Sources, 2008, 177, 566–578, DOI:10.1016/j.jpowsour.2007.11.053.
- S. Clark, A. Latz and B. Horstmann, A Review of Model-Based Design Tools for Metal-Air Batteries, Batteries, 2018, 4, 5 CrossRef.
- T. Amietszajew, E. McTurk, J. Fleming and R. Bhagat, Understanding the limits of rapid charging using instrumented commercial 18650 high-energy Li-ion cells, Electrochim. Acta, 2018, 263, 346–352, DOI:10.1016/j.electacta.2018.01.076.
- C. J. Bae, A. Manandhar, P. Kiesel and A. Raghavan, Monitoring the strain evolution of lithium-ion battery electrodes using an optical fiber Bragg grating sensor, Energy Technol., 2016, 4, 851–855, DOI:10.1002/ente.201500514.
- N. Bouchhima, M. Gossen, S. Schulte and K. P. Birke, Lifetime of self-reconfigurable batteries compared with conventional batteries, J. Energy Storage, 2018, 15, 400–407, DOI:10.1016/j.est.2017.11.014.
- S. Dey, Z. A. Biron, S. Tatipamula, N. Das, S. Mohon and B. Ayalew, Model-based real-time thermal fault diagnosis of Lithium-ion batteries, Control Eng. Pract., 2016, 56, 37–48, DOI:10.1016/j.conengprac.2016.08.002.
- S. Drake, M. Martin, D. Wetz, J. Ostanek, S. Miller and J. Heinzel, Heat generation rate measurement in a Li-ion cell at large C-rates through temperature and heat flux measurements, J. Power Sources, 2015, 285, 266–273, DOI:10.1016/j.jpowsour.2015.03.008.
- X. Feng, M. Fang, X. He, M. Ouyang, L. Lu and H. Wang, Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry, J. Power Sources, 2014, 255, 294–301, DOI:10.1016/j.jpowsour.2014.01.005.
- J. Fleming, T. Amietszajew, J. Charmet, A. J. Roberts, D. Greenwood and R. Bhagat, The design and impact of in-situ and operando thermal sensing for smart energy storage, J. Energy Storage, 2019, 22, 36–43, DOI:10.1016/j.est.2019.01.026.
- W. Han, T. Wik, A. Kersten, G. Dong and C. Zou, Next-generation battery management systems: dynamic reconfiguration, IEEE Ind. Electron. Mag., 2020, 14, 20–31, DOI:10.1109/MIE.2020.3002486.
- A. Hegyi, P. Kiesel and A. Raghavan, Time- and wavelength-multiplexed wavelength shift detection for high-resolution, low-cost distributed fiber-optic sensing, J. Lightwave Technol., 2017, 35, 4234–4241, DOI:10.1109/JLT.2017.2736503.
- C. Heubner, M. Schneider, C. Lämmel, U. Langklotz and A. Michaelis, In-operando temperature measurement across the interfaces of a lithium-ion battery cell, Electrochim. Acta, 2013, 113, 730–734, DOI:10.1016/j.electacta.2013.08.091.
- C.-Y. Lee, S.-J. Lee, M.-S. Tang and P.-C. Chen, In situ monitoring of temperature inside lithium-ion batteries by flexible micro temperature sensors, Sensors, 2011, 11, 9942–9950, DOI:10.3390/s111009942.
- C.-Y. Lee, S.-J. Lee, Y.-H. Chen, M.-Y. Chung, K.-C. Han and Y.-M. Chang, In-situ monitoring of temperature and voltage in lithium-ion battery by embedded flexible micro temperature and voltage sensor, J. Electrochem. Sci., 2013, 8, 2968–2976, DOI:10.1016/S1452-3981(23)14365-3.
- P. Phogat, S. Rai, Shreya, R. Jha and S. Singh, High-performance self-powered electrochemical photodetectors based on co-precipitation and hydrothermally synthesized HgS nanoparticles, J. Mater. Sci.: Mater. Electron., 2024, 35(22), 1524, DOI:10.1007/s10854-024-13299-5.
- D. Kumari, Shreya, P. Phogat, Dipti, S. Singh and R. Jha, Enhanced electrochemical behavior of C@CdS Core-Shell heterostructures, Mater. Sci. Eng., B, 2024, 301, 117212 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0921510724000394.
- C.-Y. Lee, H.-C. Peng, S.-J. Lee, I.-M. Hung, C.-T. Hsieh and C.-S. Chiou, A flexible three-in-one microsensor for real-time monitoring of internal temperature, voltage and current of lithium batteries, Sensors, 2015, 15, 11485–11498, DOI:10.3390/s150511485.
- Z. Li, J. Zhang, B. Wu, J. Huang, Z. Nie and Y. Sun, Examining temporal and spatial variations of internal temperature in large-format laminated battery with embedded thermocouples, J. Power Sources, 2013, 241, 536–553, DOI:10.1016/j.jpowsour.2013.04.117.
- T. Jindal, P. Phogat, Shreya, S. Singh and R. Jha, Electrochemical and optical comparison of Cr3+, Co2+, Ag1+, Hg1+ and Pb4+ doped WO3 as a thin layer working electrode for electrochemical sensing, Appl. Phys. A, 2024, 130(7), 512, DOI:10.1007/s00339-024-07666-6.
- Shreya, P. Phogat, R. Jha and S. Singh, Carbon nanospheres-induced enhanced capacitive dynamics in C/WS2/WO3 nanocomposites for high-performance electrochemical capacitors, Mater. Sci. Eng., B, 2024, 304, 117390 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0921510724002198.
- S. Yadav, Shreya, P. Phogat, R. Jha and S. Singh, Hydrothermal synthesis and characterization of tin telluride Hydrothermal synthesis and characterization of tin telluride, RP Cur. Tr. Appl. Sci., 2024, 3, 14–19 Search PubMed.
- P. Phogat, Shreya, R. Jha and S. Singh, Optical and Microstructural Study of Wide Band Gap ZnO@ZnS Core-Shell Nanorods to be Used as Solar Cell Applications, in Recent Advances in Mechanical Engineering, ed. B. Sethuraman, P. Jain and M. Gupta, Springer Nature Singapore, Singapore, 2023, pp. 419–429 Search PubMed.
- N. Loeffler, J. Zamory, N. Laszczynski, I. Doberdo, G.-T. Kim and S. Passerini, Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder, J. Power Sources, 2014, 248, 915–922, DOI:10.1016/j.jpowsour.2013.10.018.
- N. Martiny, A. Rheinfeld, J. Geder, Y. Wang, W. Kraus and A. Jossen, Development of an all kapton-based thin-film thermocouple matrix for in situ temperature measurement in a lithium ion pouch cell, IEEE Sens. J., 2014, 14, 3377–3384, DOI:10.1109/JSEN.2014.2331996.
- X. Dong, Y. Wang and Y. Xia, Re-building Daniell Cell with a Li-ion exchange Film, Sci. Rep., 2014, 4(1), 6916, DOI:10.1038/srep06916.
- D. Du, S. Zhao, Z. Zhu, F. Li and J. Chen, Photo-excited Oxygen Reduction and Oxygen Evolution Reactions Enable a High-Performance Zn–Air Battery, Angew. Chem., Int. Ed., 2020, 59(41), 18140–18144, DOI:10.1002/anie.202005929.
- J. Xie, X. Wang, J. Lv, Y. Huang, M. Wu and Y. Wang, et al., Reversible Aqueous Zinc–CO2 Batteries Based on CO2–HCOOH Interconversion, Angew. Chem., Int. Ed., 2018, 57(52), 16996–17001, DOI:10.1002/anie.201811853.
- M. S. K. Mutyala, J. Zhao, J. Li, H. Pan, C. Yuan and X. Li, In-situ temperature measurement in lithium ion battery by transferable flexible thin film thermocouples, J. Power Sources, 2014, 260, 43–49, DOI:10.1016/j.jpowsour.2014.03.004.
- M. Nascimento, M. S. Ferreira and J. L. Pinto, Real time thermal monitoring of lithium batteries with fiber sensors and thermocouples: a comparative study, Measurement, 2017, 111, 260–263, DOI:10.1016/j.measurement.2017.07.049.
- J. Song, M. Noked, E. Gillette, J. Duay, G. Rubloff and S. B. Lee, Activation of a MnO2 cathode by water-stimulated Mg2+ insertion for a magnesium ion battery, Phys. Chem. Chem. Phys., 2015, 17, 5256–5264, 10.1039/c4cp05591h.
- A. Ponrouch, C. Frontera, F. Barde and M. R. Palacin, Towards a calcium-based rechargeable battery, Nat. Mater., 2016, 15, 169–172, DOI:10.1038/nmat4462.
- L. Stievano, I. Meatza, J. Bitenc, C. Cavallo, S. Brutti and M. A. Navarra, Emerging calcium batteries, J. Power Sources, 2021, 482, 228875, DOI:10.1016/j.jpowsour.2020.228875.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A, 1976, 32, 751–767, DOI:10.1107/s0567739476001551.
- R. D. Shannon and C. T. Prewitt, Effective ionic radii in oxides and fluorides, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 1969, 25, 925–946, DOI:10.1107/s0567740869003220.
- D. S. Jacks and M. Stuermer, What drives commodity price booms and busts?, Energy Econ., 2020, 85, 104035, DOI:10.1016/j.eneco.2018.05.023.
- M. Nascimento, S. Novais, M. S. Ding, M. S. Ferreira, S. Koch and S. Passerini, Internal strain and temperature discrimination with optical fiber hybrid sensors in Li-ion batteries, J. Power Sources, 2019, 410, 1–9, DOI:10.1016/j.jpowsour.2018.10.096.
- A. Othonos, Fiber Bragg gratings, Rev. Sci. Instrum., 1997, 68, 4309–4341, DOI:10.1063/1.1148392.
- M. Parhizi, M. Ahmed and A. Jain, Determination of the core temperature of a Li-ion cell during thermal runaway, J. Power Sources, 2017, 370, 27–35, DOI:10.1016/j.jpowsour.2017.09.086.
- G. Pereira, M. McGugan and L. P. Mikkelsen, Method for independent strain and temperature measurement in polymeric tensile test specimen using embedded FBG sensors, Polym. Test., 2016, 50, 125–134, DOI:10.1016/j.polymertesting.2016.01.005.
- A. Raghavan, P. Kiesel, L. W. Sommer, J. Schwartz, A. Lochbaum and A. Hegyi, Embedded fiber-optic sensing for accurate internal monitoring of cell state in advanced battery management systems part 1: cell embedding method and performance, J. Power Sources, 2017, 341, 466–473, DOI:10.1016/j.jpowsour.2016.11.104.
- Y. J. Rao, Recent progress in applications of in-fibre Bragg grating sensors, Opt. Lasers Eng., 1999, 31, 297–324, DOI:10.1016/S0143-8166(99)00025-1.
- T. Waldmann, G. Bisle, B.-I. Hogg, S. Stumpp, M. A. Danzer and M. Kasper, Influence of cell design on temperatures and temperature gradients in lithium-ion cells: an in operando study, J. Electrochem. Soc., 2015, 162, A921–A927, DOI:10.1149/2.0561506jes.
- P. Wang, X. Zhang, L. Yang, X. Zhang, M. Yang and H. Chen, Real-time monitoring of internal temperature evolution of the lithium-ion coin cell battery during the charge and discharge process, Extreme Mech. Lett., 2016, 9, 459–466, DOI:10.1016/j.eml.2016.03.013.
- C.-Y. Wang, G. Zhang, S. Ge, T. Xu, Y. Ji and X.-G. Yang, Lithium-ion battery structure that self-heats at low temperatures, Nature, 2016, 529, 515–518, DOI:10.1038/nature16502.
- Z. Wei, J. Zhao, H. He, G. Ding, H. Cui and L. Liu, Future smart battery and management: advanced sensing from external to embedded multi-dimensional measurement, J. Power Sources, 2021, 489, 229462, DOI:10.1016/j.jpowsour.2021.229462.
- Q. Long, G. Ma, Q. Xu, C. Ma, J. Nan and A. Li, et al., Improving the cycle life of lead-acid batteries using three-dimensional reduced graphene oxide under the high-rate partial-state-of-charge condition, J. Power Sources, 2017, 343, 188–196 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775317300563.
- Z. Wei, J. Hu, H. He, Y. Yu and J. Marco, Embedded distributed temperature sensing enabled multistate joint observation of smart lithium-ion battery, IEEE Trans. Ind. Electron., 2023, 70, 555–565, DOI:10.1109/TIE.2022.3146503.
- M. Xiao and S.-Y. Choe, Theoretical and experimental analysis of heat generations of a pouch type LiMn2O4/carbon high power Li-polymer battery, J. Power Sources, 2013, 241, 46–55, DOI:10.1016/j.jpowsour.2013.04.062.
- K. Li, J. Zhang, D. Lin, D.-W. Wang, B. Li and W. Lv, et al., Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes, Nat. Commun., 2019, 10(1), 725, DOI:10.1038/s41467-019-08506-5.
- P. Phogat, Shreya, R. Jha and S. Singh, in Optical and Microstructural Study of Wide Band Gap ZnO@ZnS Core–Shell Nanorods to Be Used as Solar Cell Applications BT – Recent Advances in Mechanical Engineering, ed. B. Sethuraman, P. Jain and M. Gupta, Springer Nature Singapore, Singapore, 2023, pp. 419–429 Search PubMed.
- Shreya, P. Phogat, R. Jha and S. Singh, Electrochemical analysis of hydrothermally synthesized 2D/1D WS2/WO3 nanocomposites for solar cell application, J. Phys. Chem. Solids, 2024, 192, 112110 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0022369724002452.
- P. Phogat, Shreya, R. Jha and S. Singh, Electrochemical analysis of thermally treated two dimensional zinc sulphide hexagonal nano-sheets with reduced band gap, Phys. Scr., 2023, 98(12), 125962, DOI:10.1088/1402-4896/ad0d93.
- G. Yang, C. Leitao, Y. H. Li, J. Pinto and X. F. Jiang, Real-time temperature measurement with fiber Bragg sensors in lithium batteries for safety usage, Measurement, 2013, 46, 3166–3172, DOI:10.1016/j.measurement.2013.05.027.
- X.-G. Yang, G. Zhang and C.-Y. Wang, Computational design and refinement of self-heating lithium ion batteries, J. Power Sources, 2016, 328, 203–211, DOI:10.1016/j.jpowsour.2016.08.028.
- G. Zhang, L. Cao, S. Ge, C.-Y. Wang, C. E. Shaffer and C. D. Rahn, In situ measurement of radial temperature distributions in cylindrical Li-ion cells, J. Electrochem. Soc., 2014, 161, A1499–A1507, DOI:10.1149/2.0051410jes.
- P. Phogat, Shreya, R. Jha and S. Singh, Harnessing ZnO morphologies in energy application and sustainable development, Phys. Scr., 2024, 99(10), 102004, DOI:10.1088/1402-4896/ad7990.
- P. Phogat, A. Rai, Shreya, R. Jha and S. Singh, Effect of microwave, ultraviolet and ultrasonic treatment on crystal size and particle size of ZnS quantum dots as a working thin layer for solar cells, Indian J. Phys., 2024, 691–704, DOI:10.1007/s12648-024-03304-2.
- P. Phogat, Shreya, R. Jha and S. Singh, Impedance Study of Zinc Sulphide Quantum Dots via One Step Green Synthesis, Mater. Sci. Forum, 2023, 1099, 119–125 Search PubMed . https://www.scientific.net/MSF.1099.119.
- Shreya, P. Phogat, R. Jha and S. Singh, Emerging advances and future prospects of two dimensional nanomaterials based solar cells, J. Alloys Compd., 2024, 175063 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0925838824016505.
- P. Phogat, Shreya, R. Jha and S. Singh, in The Role of Nanotechnology in Space Exploration and Colonization BT – Nanotechnology in Societal Development, ed, S. C. George and B. Tawiah, Springer Nature Singapore, Singapore, 2024, pp. 395–437, DOI:10.1007/978-981-97-6184-5_12.
- Dipti, P. Phogat, Shreya, D. Kumari and S. Singh, Fabrication of tunable band gap carbon based zinc nanocomposites for enhanced capacitive behaviour, Phys. Scr., 2023, 98(9), 95030, DOI:10.1088/1402-4896/acf07b.
- P. Phogat, Shreya, R. Jha and S. Singh, Photocatalytic Performance of ZnO@ZnS Core–Shell Heterostructures for Malachite Green and Rhodamine B Dye Degradation, Phys. Status Solidi, 2024, 22, 2400404, DOI:10.1002/pssa.202400404.
- P. Phogat, Shreya, R. Jha and S. Singh, Synthesis and characterization of C@CdS core-shell structures for high-performance capacitors, Next Mater., 2024, 5, 100246 CrossRef . https://www.sciencedirect.com/science/article/pii/S2949822824001436.
- Shreya, P. Phogat, R. Jha and S. Singh, Microwave-synthesized γ-WO3 nanorods exhibiting high current density and diffusion characteristics, Transition Met. Chem., 2023, 48(3), 167–183, DOI:10.1007/s11243-023-00533-y.
- C. Zhang, K. Li and J. Deng, Real-time estimation of battery internal temperature based on a simplified thermoelectric model, J. Power Sources, 2016, 302, 146–154, DOI:10.1016/j.jpowsour.2015.10.052.
- S. Zhu, J. Han, H.-Y. An, T.-S. Pan, Y.-M. Wei and W.-L. Song, A novel embedded method for in-situ measuring internal multi-point temperatures of lithium ion batteries, J. Power Sources, 2020, 456, 227981, DOI:10.1016/j.jpowsour.2020.227981.
- C. Zou, X. Hu, Z. Wei, T. Wik and B. Egardt, Electrochemical estimation and control for lithium-ion battery health-aware fast charging, IEEE Trans. Ind. Electron., 2017, 65, 6635–6645, DOI:10.1109/TIE.2017.2772154.
- E. Sanchez-Díez, E. Ventosa, M. Guarnieri, A. Trovò, C. Flox and R. Marcilla, et al., Redox flow batteries: status and perspective towards sustainable stationary energy storage, J. Power Sources, 2021, 481, 228804, DOI:10.1016/j.jpowsour.2020.228804.
- L. F. Arenas, A. Loh, D. P. Trudgeon, X. Li, C. Ponce de León and F. C. Walsh, The characteristics and performance of hybrid redox flow batteries with zinc negative electrodes for energy storage, Renewable Sustainable Energy Rev., 2018, 90, 992–1016, DOI:10.1016/j.rser.2018.03.016.
- M. Wu, Y. Jing, A. A. Wong, E. M. Fell, S. Jin and Z. Tang, et al., Extremely stable Anthraquinone Negolytes synthesized from common precursors, Chem, 2020, 6, 1432–1442, DOI:10.1016/j.chempr.2020.03.021.
- Y. Lv, Z. Yuan, X. Li, G. Yu, Z. Yang and T. Xu, Organic electrolytes for pH-neutral aqueous organic redox flow batteries, Adv. Funct. Mater., 2022, 32, 2108777, DOI:10.1002/adfm.202108777.
- Shreya, P. Phogat, R. Jha and S. Singh, Enhanced Electrochemical Performance and Charge-Transfer Dynamics of 2D MoS2/WO3 Nanocomposites for Futuristic Energy Applications, ACS Appl. Nano Mater., 2024, 7(8), 8593–8611, DOI:10.1021/acsanm.3c06017.
- P. Phogat, Shreya, R. Jha and S. Singh, Synthesis of Novel ZnO Nanoparticles with Optimised Band Gap of 1.4 eV for High-Sensitivity Photo Electrochemical Detection, Mater. Today Sustain., 2024, 27, 100823 Search PubMed . https://www.sciencedirect.com/science/article/pii/S2589234724001593.
- P. Phogat, Shreya, R. Jha and S. Singh, Supercapacitive studies of hybrid materials based on cadmium deuteroxide chloride (CdDOCl) with activated carbon, J. Mater. Sci., 2024, 11757–11780, DOI:10.1007/s10853-024-09874-0.
- Y. Ji, M. A. Goulet, D. A. Pollack, D. G. Kwabi, S. Jin and D. Porcellinis, et al., A phosphonate-functionalized Quinone redox flow battery at near-neutral pH with record capacity retention rate, Adv. Energy Mater., 2019, 9, 1900039, DOI:10.1002/aenm.201900039.
- J. Luo, B. Hu, C. Debruler, Y. Bi, Y. Zhao and B. Yuan, et al., Unprecedented capacity and stability of ammonium Ferrocyanide Catholyte in pH neutral aqueous redox flow batteries, Joule, 2019, 3, 149–163, DOI:10.1016/j.joule.2018.10.010.
- X. L. Lv, P. Sullivan, H. C. Fu, X. X. Hu, H. Liu and S. Jin, et al., Dextrosil-Viologen: a robust and sustainable Anolyte for aqueous organic redox flow batteries, ACS Energy Lett., 2022, 7, 2428–2434, DOI:10.1021/acsenergylett.2c01198.
- A. Hollas, X. Wei, V. Murugesan, Z. Nie, B. Li and D. Reed, et al., A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries, Nat. Energy, 2018, 3, 508–514, DOI:10.1038/s41560-018-0167-3.
- D. Wang, X. Gao, Y. Chen, L. Jin, C. Kuss and P. G. Bruce, Plating and stripping calcium in an organic electrolyte, Nat. Mater., 2018, 17, 16–20, DOI:10.1038/nmat5036.
- A. Shyamsunder, L. E. Blanc, A. Assoud and L. F. Nazar, Reversible calcium plating and stripping at room temperature using a borate salt, ACS Energy Lett., 2019, 4, 2271–2276, DOI:10.1021/acsenergylett.9b01550.
- K. V. Nielson, J. Luo and T. L. Liu, Optimizing calcium electrolytes by solvent manipulation for calcium batteries, Batteries Supercaps, 2020, 3, 766–772, DOI:10.1002/batt.202000005.
- Z. L. Xu, J. Park, J. Wang, H. Moon, G. Yoon and J. Lim, et al., A new high-voltage calcium intercalation host for ultra-stable and high-power calcium rechargeable batteries, Nat. Commun., 2021, 12, 3369, DOI:10.1038/s41467-021-23703-x.
- X. Deng, L. Li, G. Zhang, X. Zhao, J. Hao and C. Han, et al., Anode chemistry in calcium ion batteries: a review, Energy Storage Mater., 2022, 53, 467–481, DOI:10.1016/j.ensm.2022.09.033.
- T. Lv and L. Suo, Water-in-salt widens the electrochemical stability window: thermodynamic and kinetic factors, Curr. Opin. Electrochem., 2021, 29, 100818, DOI:10.1016/j.coelec.2021.100818.
- C. Han, H. Li, Y. Li, J. Zhu and C. Zhi, Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure, Nat. Commun., 2021, 12, 2400, DOI:10.1038/s41467-021-22698-9.
- X. Xu, M. Duan, Y. Yue, Q. Li, X. Zhang and L. Wu, et al., Bilayered Mg0.25V2O5·H2O as a stable cathode for rechargeable Ca-Ion batteries, ACS Energy Lett., 2019, 4, 1328–1335, DOI:10.1021/acsenergylett.9b00830.
- B. Hu, M. Hu, J. Luo and T. L. Liu, A stable, low permeable TEMPO Catholyte for aqueous Total organic redox flow batteries, Adv. Energy Mater., 2022, 12, 2102577, DOI:10.1002/aenm.202102577.
- M. Huang, S. Hu, X. Yuan, J. Huang, W. Li and Z. Xiang, et al., Five-membered-heterocycle bridged Viologen with high voltage and superior stability for flow battery, Adv. Funct. Mater., 2022, 32, 2111744, DOI:10.1002/adfm.202111744.
- F. R. Brushett, M. J. Aziz and K. E. Rodby, On lifetime and cost of redox-active organics for aqueous flow batteries, ACS Energy Lett., 2020, 5, 879–884, DOI:10.1021/acsenergylett.0c00140.
- H. Fu, C. Zhang, H. Wang, B. Du, J. Nie and J. Xu, et al., Stable aqueous redox flow battery assembled in air atmosphere employing an anionic terpolymer as active cathode material, J. Power Sources, 2022, 545, 231905, DOI:10.1016/j.jpowsour.2022.231905.
- J. Winsberg, C. Stolze, A. Schwenke, S. Muench, M. D. Hager and U. S. Schubert, Aqueous 2,2,6,6-Tetramethylpiperidine-N-oxyl Catholytes for a high-capacity and high current density oxygen-insensitive hybrid flow battery, ACS Energy Lett., 2017, 2, 411–416, DOI:10.1021/acsenergylett.6b00655.
- I. A. Shkrob, L. A. Robertson, Z. Yu, R. S. Assary, L. Cheng and L. Zhang, et al., Crowded electrolytes containing redoxmers in different states of charge: solution structure, properties, and fundamental limits on energy density, J. Mol. Liq., 2021, 334, 116533, DOI:10.1016/j.molliq.2021.116533.
- S. Arora, Selection of thermal management system for modular battery packs of electric vehicles: A review of existing and emerging technologies, J. Power Sources, 2018, 400, 621–640 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775318308796.
- Z. Rhodes, J. R. Cabrera-Pardo, M. Li and S. D. Minteer, Electrochemical advances in non-aqueous redox flow batteries, Isr. J. Chem., 2021, 61, 101–112, DOI:10.1002/ijch.202000049.
- D. Xu, C. Zhang, Y. Zhen, Y. Zhao and Y. Li, A high-rate nonaqueous organic redox flow battery, J. Power Sources, 2021, 495, 229819, DOI:10.1016/j.jpowsour.2021.229819.
- X. Zhang, X. G. Wang, Z. Xie and Z. Zhou, Recent progress in rechargeable alkali metal-air batteries, Green Energy Environ., 2016, 1, 4–17, DOI:10.1016/j.gee.2016.04.004.
- N. S. Alghamdi, M. Rana, X. Peng, Y. Huang, J. Lee and J. Hou, et al., Zinc–Bromine Rechargeable Batteries: From Device Configuration, Electrochemistry, Material to Performance Evaluation, Nano-Micro Lett., 2023, 15(1), 209, DOI:10.1007/s40820-023-01174-7.
- C. S. Li, Y. Sun, F. Gebert and S. L. Chou, Current progress on rechargeable magnesium–air battery, Adv. Energy Mater., 2017, 7, 1700869, DOI:10.1002/aenm.201700869.
- R. D. McKerracher, C. Ponce de León, R. G. A. Wills, A. A. Shah and F. C. Walsh, A review of the iron-air secondary battery for energy storage, Chempluschem, 2015, 80, 323–335, DOI:10.1002/cplu.201402238.
- S. Monaco, F. Soavi and M. Mastragostino, Role of oxygen mass transport in rechargeable Li/O2 batteries operating with ionic liquids, J. Phys. Chem. Lett., 2013, 4, 1379–1382, DOI:10.1021/jz4006256.
- F. Poli, L. K. Ghadikolaei and F. Soavi, Semi-empirical modeling of the power balance of flow lithium/oxygen batteries, Appl. Energy, 2019, 248, 383–389, DOI:10.1016/j.apenergy.2019.04.133.
- I. Ruggeri, C. Arbizzani and F. Soavi, Carbonaceous catholyte for high energy density semi-solid Li/O2 flow battery, Carbon, 2018, 130, 749–757, DOI:10.1016/j.carbon.2018.01.056.
- A. Poulin, X. Aeby and G. Nyström, Water activated disposable paper battery, Sci. Rep., 2022, 12(1), 11919, DOI:10.1038/s41598-022-15900-5.
- S. Rai, Shreya, P. Phogat, R. Jha and S. Singh, Wide absorption spectrum and rapid response time of PEC photodetectors based on MoS2–Se nanocomposites, Chem. Eng. Commun., 2024, 1–16, DOI:10.1080/00986445.2024.2362799.
- A. Rai, P. Phogat, Shreya, R. Jha and S. Singh, Microwave Assisted Zinc Sulphide Quantum Dots for Energy Device Applications, MATEC Web Conf., 2024, 393, 01011, DOI:10.1051/matecconf/202439301011.
- P. Phogat, Shreya, R. Jha and S. Singh, Phase Transition of Thermally Treated Polyhedral Nano Nickel Oxide with Reduced Band Gap, MATEC Web Conf., 2024, 393, 01001, DOI:10.1051/matecconf/202439301001.
- Shreya, P. Phogat, R. Jha and S. Singh, Electrochemical study of cerium and iron doped MoO3 nanoparticles showing potential for supercapacitor application, Next Mater., 2024, 5, 100260 CrossRef . https://www.sciencedirect.com/science/article/pii/S2949822824001576.
- P. Pei, Z. Ma, K. Wang, X. Wang, M. Song and H. Xu, High performance zinc air fuel cell stack, J. Power Sources, 2014, 249, 13–20, DOI:10.1016/j.jpowsour.2013.10.073.
- X. Han, X. Li, J. White, C. Zhong, Y. Deng and W. Hu, et al., Metal–air batteries: from static to flow system, Adv. Energy Mater., 2018, 8, 1801396, DOI:10.1002/aenm.201801396.
- W. Yu, W. Shang, P. Tan, B. Chen, Z. Wu and H. Xu, et al., Toward a new generation of low cost, efficient, and durable metal–air flow batteries, J. Mater. Chem. A, 2019, 7, 26744–26768, 10.1039/C9TA10658H.
- Y. Kim, M. Künzel, D. Steinle, X. Dong, G. T. Kim and A. Varzi, et al., Anode-less seawater batteries with a Na-ion conducting solid-polymer electrolyte for power to metal and metal to power energy storage, Energy Environ. Sci., 2022, 15, 2610–2618, 10.1039/D2EE00609J.
- S. M. Hwang, J. S. Park, Y. Kim, W. Go, J. Han and Y. Kim, et al., Rechargeable seawater batteries—from concept to applications, Adv. Mater., 2019, 31, 1804936, DOI:10.1002/adma.201804936.
- Y. Kim, G. T. Kim, S. Jeong, X. Dou, C. Geng and Y. Kim, et al., Large-scale stationary energy storage: seawater batteries with high rate and reversible performance, Energy Storage Mater., 2019, 16, 56, DOI:10.1016/j.ensm.2018.04.028.
- M. K. Debe, Electrocatalyst approaches and challenges for automotive fuel cells, Nature, 2012, 486, 43–51, DOI:10.1038/nature11115.
- A. Varzi, K. Thanner, R. Scipioni, D. Lecce, J. Hassoun and S. Dörfler, et al., Current status and future perspectives of lithium metal batteries, J. Power Sources, 2020, 480, 228803, DOI:10.1016/j.jpowsour.2020.228803.
- D. Y. Voropaeva, E. Y. Safronova, S. A. Novikova and A. B. Yaroslavtsev, Recent progress in lithium-ion and lithium metal batteries, Mendeleev Commun., 2022, 32, 287–297, DOI:10.1016/j.mencom.2022.05.001.
- S. Sharma, P. Phogat, R. Jha and S. Singh, Electrochemical and Optical Properties of Microwave Assisted MoS 2 Nanospheres for Solar Cell Application, Int. J. Smart Grid Clean Energy Prospect, 2023, 12(3), 66–72 CrossRef.
- A. Verma, Shreya, P. Phogat, N. L. Singh and R. Jha, Synthesis of Acid Co-Precipitated WO3 Nanoflowers and Its Characterization: Structural, Optical, and Morphological Insights, J. Eng. Technol., 2024, 9(8), 4893–4897 Search PubMed . https://everant.org/index.php/etj/article/view/1490.
- P. Phogat, Shreya, R. Jha and S. Singh, Design and performance evaluation of 2D nickel oxide nanosheet thin film electrodes in energy storage devices, Indian J. Phys., 2024, 1679–1690, DOI:10.1007/s12648-024-03415-w.
- H. Wang, Z. Yu, X. Kong, S. C. Kim, D. T. Boyle and J. Qin, et al., Liquid electrolyte: the nexus of practical lithium metal batteries, Joule, 2022, 6, 588–616, DOI:10.1016/j.joule.2021.12.018.
- H. Yuan, X. Ding, T. Liu, J. Nai, Y. Wang and Y. Liu, et al., A review of concepts and contributions in lithium metal anode development, Mater. Today, 2022, 53, 173–196, DOI:10.1016/j.mattod.2022.01.015.
- H. Zhang and Y. Qi, Investigating lithium metal anodes with nonaqueous electrolytes for safe and high-performance batteries, Sustain. Energy Fuels, 2022, 6, 954–970, 10.1039/d1se01739j.
- L. T. Gao, P. Huang and Z.-S. Guo, Critical role of pits in suppressing Li dendrites revealed by continuum mechanics simulation and in situ experiment, J. Electrochem. Soc., 2022, 169, 060522, DOI:10.1149/1945-7111/ac7668.
- V. Venturi and V. Viswanathan, Thermodynamic analysis of initial steps for void formation at lithium/solid electrolyte interphase interfaces, ACS Energy Lett., 2022, 7, 1953–1959, DOI:10.1021/acsenergylett.2c00550.
- S. Zhang, G. Yang, X. Li, Y. Li, Z. Wang and L. Chen, Controlled lithium deposition, Front. Energy Res., 2022, 10 DOI:10.3389/fenrg.2022.837071.
- P. Phogat, Shreya, R. Jha and S. Singh, Diffusion Controlled Features of Microwave Assisted ZnS/ZnO Nanocomposite with Reduced Band Gap, ECS J. Solid State Sci. Technol., 2023, 12(3), 034004, DOI:10.1149/2162-8777/acc426.
- P. Phogat, Shreya, R. Jha and S. Singh, Chalcogenide Nanocomposites for Energy Materials, J. Eng. Technol., 2024, 9(7), 4580–4606 Search PubMed . https://everant.org/index.php/etj/article/view/1433.
- Shreya, P. Phogat, R. Jha and S. Singh, Elevated Refractive Index of MoS2 Amorphous Nanoparticles with a Reduced Band Gap Applicable for Optoelectronics BT – Recent Advances in Mechanical Engineering, in Recent Advances in Mechanical Engineering, ed. B. Sethuraman, P. Jain and M. Gupta, Springer Nature Singapore, Singapore, 2023, pp. 431–439 Search PubMed.
- T. Kumar, Shreya, P. Phogat, V. Sahgal and R. JHA, Surfactant-Mediated Modulation of Morphology and Charge Transfer Dynamics in Tungsten Oxide Nanoparticles, Phys. Scr., 2023, 98(8), 085936, DOI:10.1088/1402-4896/ace566.
- V. Sahgal, P. Phogat, Shreya, T. Kumar, R. Jha and S. Singh, Design strategies for morphologically ramified ZnO–ZnS core shell nano flowers for magnified electrochemical studies, Indian J. Phys., 2024, 4351–4372, DOI:10.1007/s12648-024-03196-2.
- J. S. Cha, J. Lee, N.-U. Seo, D. K. Kim, Y.-C. Kang and J. H. Yang, Scalable design of zinc-bromine battery in 3-dimensional honeycomb lattice for superior low-cost battery, J. Power Sources, 2023, 553, 232243 CrossRef CAS . https://www.sciencedirect.com/science/article/pii/S0378775322012204.
- K. Leung, L. C. Merrill and K. L. Harrison, Galvanic corrosion and electric field in lithium anode passivation films: effects on self-discharge, J. Phys. Chem. C, 2022, 126, 8565–8580, DOI:10.1021/acs.jpcc.1c10602.
- S. Zhu and J. Chen, Dual strategy with Li-ion solvation and solid electrolyte interphase for high coulombic efficiency of lithium metal anode, Energy Storage Mater., 2022, 44, 48–56, DOI:10.1016/j.ensm.2021.10.007.
- D. Tewari, S. P. Rangarajan, P. B. Balbuena, Y. Barsukov and P. P. Mukherjee, Mesoscale anatomy of dead lithium formation, J. Phys. Chem. C, 2020, 124, 6502–6511, DOI:10.1021/acs.jpcc.9b11563.
- D. He, J. Lu, G. He and H. Chen, Recent advances in solid-electrolyte interphase for Li metal anode, Front. Chem., 2022, 10 DOI:10.3389/fchem.2022.916132.
- H. Song, Y. Li, F. Tian and C. Wang, Electrolyte optimization and interphase regulation for significantly enhanced storage capability in ca-metal batteries, Adv. Funct. Mater., 2022, 32 DOI:10.1002/adfm.202200004.
- Z. J. Baum, R. E. Bird, Y. Xiang and J. Ma, Lithium-ion battery recycling-overview of techniques and trends, ACS Energy Lett., 2022, 7, 712–719, DOI:10.1021/acsenergylett.1c02602.
- K. Berger, J.-P. Schöggl and J. Rupert, Baumgartner. Digital battery passports to enable circular and sustainable value chains: conceptualization and use cases, J. Clean. Prod., 2022, 353, 131492, DOI:10.1016/j.jclepro.2022.131492.
- L. Komsiyska, T. Buchberger, S. Diehl, M. Ehrensberger, C. Hanzl and C. Hartmann, et al., Critical review of intelligent battery systems: challenges, implementation, and potential for electric vehicles, Energies, 2021, 14, 5989, DOI:10.3390/en14185989.
- M. Lamagna, D. Groppi, M. M. Nezhad and G. Piras, a comprehensive review on digital twins for smart energy management systems, Int. J. Energy Prod. Manag., 2021, 6, 323–334, DOI:10.2495/EQ-V6-N4-323-334.
- T. Christen and M. W. Carlen, Theory of Ragone plots, J. Power Sources, 2000, 91, 210–216, DOI:10.1016/S0378-7753(00)00474-2.
- K. Kitoh and H. Remoto, 100 Wh large size Li-ion batteries and safety tests, J. Power Sources, 1999, 81–82, DOI:10.1016/S0378-7753(99)00125-1.
- B. Hariprakash, S. K. Martha and A. Shukla, Monitoring sealed automotive lead-acid batteries by sparse-impedance spectroscopy, Proc. Indian Acad. Sci. Chem. Sci., 2003, 115, 465–472, DOI:10.1007/BF02708238.
- S. Li, M. Jiang, Y. Xie, H. Xu, J. Jia and J. Li, Developing high-performance lithium metal anode in liquid electrolytes: challenges and Progress, Adv. Mater., 2018, 30 DOI:10.1002/adma.201706375.
- R. Mogi, M. Inaba, S.-K. Jeong, Y. Iriyama, T. Abe and Z. Ogumi, Effects of some organic additives on lithium deposition in propylene carbonate, J. Electrochem. Soc., 2002, 149, A1578, DOI:10.1149/1.1516770.
- L. Dong, Y. Liu, D. Chen, Y. Han, Y. Ji and J. Liu, et al., Stabilization of high-voltage lithium metal batteries using a sulfone-based electrolyte with bi-electrode affinity and LiSO2F-rich interphases, Energy Storage Mater., 2022, 44, 527–536, DOI:10.1016/j.ensm.2021.10.045.
- D. Wu, J. He, J. Liu, M. Wu, S. Qi and H. Wang, et al., Li2CO3/LiF-rich Heterostructured solid electrolyte interphase with superior Lithiophilic and Li+-transferred characteristics via adjusting electrolyte additives, Adv. Energy Mater., 2022, 12 DOI:10.1002/aenm.202200337.
- Y. Lu, Z. Tu, J. Shu and L. A. Archer, Stable lithium electrodeposition in salt-reinforced electrolytes, J. Power Sources, 2015, 279, 413–418, DOI:10.1016/j.jpowsour.2015.01.030.
- N. W. Li, Y. X. Yin, C. P. Yang and Y. G. Guo, An artificial solid electrolyte interphase layer for stable lithium metal anodes, Adv. Mater., 2016, 28, 1853–1858, DOI:10.1002/adma.201504526.
- J. Niu, M. Wang, T. Cao, X. Cheng, R. Wu and H. Liu, et al., Li metal coated with Li3PO4 film via atomic layer deposition as battery anode, Ionics (Kiel), 2021, 27, 2445–2454, DOI:10.1007/s11581-021-04030-z.
- W. Wang, Y. Yuan, J. Wang, Y. Zhang, C. Liao and X. Mu, et al., Enhanced electrochemical and safety performance of lithium metal batteries enabled by the atom layer deposition on PVDF-HFP separator, ACS Appl. Energy Mater., 2019, 2, 4167–4174, DOI:10.1021/acsaem.9b00383.
- Z. Yao, Y. Kang, M. Hou, J. Huang, J. Zhang and B. Yang, et al., Promoting homogeneous interfacial Li+ migration by using a facile N2 plasma strategy for all-solid-state lithium-metal batteries, Adv. Funct. Mater., 2022, 32 DOI:10.1002/adfm.202111919.
- L. Fan, H. L. Zhuang, L. Gao, Y. Lu and L. A. Archer, Regulating Li deposition at artificial solid electrolyte interphases, J. Mater. Chem. A, 2017, 5, 3483–3492, 10.1039/c6ta10204b.
- L. Ma, M. S. Kim and L. A. Archer, Stable artificial solid electrolyte interphases for lithium batteries, Chem. Mater., 2017, 29, 4181–4189, DOI:10.1021/acs.chemmater.6b03687.
- C. Jin, O. Sheng, J. Luo, H. Yuan, C. Fang and W. Zhang, et al., 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries, Nano Energy, 2017, 37, 177–186, DOI:10.1016/j.nanoen.2017.05.015.
- Z. Lu, Z. Zhang, X. Chen, Q. Chen, F. Ren and M. Wang, et al., Improving Li anode performance by a porous 3D carbon paper host with plasma assisted sponge carbon coating, Energy Storage Mater., 2018, 11, 47–56, DOI:10.1016/j.ensm.2017.09.011.
- L. Y. Qi, L. Shang, X. Chen, L. Ye, W. Zhang and P. Feng, et al., A versatile strategy to fabricate 3D conductive frameworks for lithium metal anodes, Adv. Mater. Interfaces, 2018, 5 DOI:10.1002/admi.201800807.
- V. Raj, N. P. B. Aetukuri and J. Nanda, Solid state lithium metal batteries – issues and challenges at the lithium-solid electrolyte interface, Curr. Opin. Solid State Mater. Sci., 2022, 26, 100999, DOI:10.1016/j.cossms.2022.100999.
- L. Fan, S. Wei, S. Li, Q. Li and Y. Lu, Recent Progress of the solid-state electrolytes for high-energy metal-based batteries, Adv. Energy Mater., 2018, 8 DOI:10.1002/aenm.201702657.
- G. Zhu, S. Yang, Y. Wang, Q. Qu and H. Zheng, Dimethylacrylamide, a novel electrolyte additive, can improve the electrochemical performances of silicon anodes in lithium-ion batteries, RSC Adv., 2019, 9(1), 435–443, 10.1039/C8RA07988A.
- S. Rai, Shreya, P. Phogat, R. Jha and S. Singh, Hydrothermal synthesis and characterization of selenium-doped MoS2 for enhanced optoelectronic properties, MATEC Web Conf., 2024, 393, 01008, DOI:10.1051/matecconf/202439301008.
- A. Sharma, Shreya, P. Phogat, R. Jha and S. Singh, Electrochemical behavior of carbon/nickel sulfide nanocomposite thin films for advanced energy applications, Next Nanotechnol., 2024, 6, 100080 CrossRef . https://www.sciencedirect.com/science/article/pii/S294982952400041X.
- Shreya, P. Phogat, S. Singh and R. Jha, Reduction mechanism of hydrothermally synthesized wide band gap ZnWO4 nanorods for HER application, MATEC Web Conf., 2024, 393, 01004, DOI:10.1051/matecconf/202439301004.
- S. Tak, S. Grewal, Shreya, P. Phogat, Manisha and R. Jha, et al., Mechanistic Insights and Emerging Trends in Photocatalytic Dye Degradation for Wastewater Treatment, Chem. Eng. Technol., 2024, e202400142, DOI:10.1002/ceat.202400142.
| This journal is © The Royal Society of Chemistry 2025 |